Abstract
Cystic lesions in a variety of organs are being increasingly recognized as an incidental finding on cross-sectional imaging. These lesions can be benign, premalignant or malignant. When these cystic lesions are small it can be difficult to characterize them radiologically. However, with appropriate clinical history and knowledge of typical imaging features of cystic pancreatic lesions this can enable accurate diagnosis and thus guide appropriate treatment. In this review, we provide an overview of the most common types of cystic lesions and their appearance on computer tomography, magnetic resonance imaging and ultrasound. We will also discuss the follow up and management strategies of these cystic lesions.
Keywords: Cystic pancreatic lesions, Follow up management, Imaging
INTRODUCTION
Cystic lesions of the pancreas can be malignant or benign, occur in a wide range of sizes, and may or may not cause clinical symptoms. These lesions are often identified incidentally on cross-sectional imaging obtained for other reasons. The correct characterization of any cystic pancreatic lesion is critical in determining appropriate management.
Methods currently available for imaging cystic pancreatic lesions include contrast-enhanced computed tomography (CECT), ultrasound (US), endoscopic US (EUS), magnetic resonance imaging (MRI), and positron emission tomography (PET)/CT. However, the utility of PET/CT is still under investigation. EUS and transabdominal US have the disadvantage of being operator-dependent, but EUS is able to visualize cystic lesions in real time, and biopsy and cyst fluid analysis can be performed simultaneously. EUS is able to visualize cystic lesions in real time, and can guide biopsy; its primary limitations include its invasive nature, and the fact that results are operator-dependent. Transabdominal ultrasonography, is non-invasive, but offers limited resolution because of the depth of tissue penetration needed, and is therefore limited in patients with a large body habitus, and importantly the pancreas may be obscured by overlying bowel gas. Calcifications can be better seen on CECT; although septations may or may not be well seen. Septations are better seen on MRI and cyst contents can be identified due to better soft tissue contrast, however, detection of mucin is equivocal and spatial resolution is lower than that of CT. In this article we will discuss commonly seen pancreatic cystic lesions and their appearance on imaging.
CLASSIFICATION OF PANCREATIC CYSTIC LESIONS
Pancreatic cystic lesions can be divided into primary and secondary cystic lesions. Primary cystic lesions include pseudocysts, serous cystadenomas (SCAs), various mucin-containing cysts such as mucinous non-neoplastic cysts, mucinous cystadenomas, mucinous cystadenocarcinomas, intraductal papillary mucinous neoplasms, pseudopapillary tumors of the pancreas and lymphoepithelial cysts. Secondary cystic lesions are solid neoplasms that have undergone cystic changes such as primary ductal adenocarcinoma, and neuroendocrine tumors. In this article we will discuss primary cystic lesions. Secondary cystic lesions and metastatic lesions, such as renal cell carcinoma, that show cystic changes will not be covered.
In the past, the majority of pancreatic cystic lesions were thought to be pseudocysts, however, this is being re-revaluated given the use of thin section imaging. Hydatid cysts of the pancreas are rare, but should be considered in countries where this disease is endemic[1]. Primary pancreatic cystic tumors fall into one of three major groups; serous tumors, mucinous tumors and solid pseudopapillary tumors (SPT).
Most cystic pancreatic tumors that are incidentally diagnosed are asymptomatic and small[2]. As these cystic lesions grow larger they cause symptoms due to mass effect and the symptoms are vague and poorly localized. For example, intraductal papillary mucinous neoplasms (IPMNs) may cause epigastric pain which may mimic chronic pancreatitis[3].
Pancreatic pseudocysts
Pseudocysts have been reported to comprise 70% of all cystic lesions[4], however, that is being challenged because of the numerous instances of small cystic lesions being seen on cross-sectional imaging in patients without a history of pancreatitis. Pseudocysts occur following an episode of pancreatitis due to leakage of pancreatic enzymes, causing fat necrosis and hemorrhage. Pseudocysts are more often seen in alcoholics and can be associated with abdominal trauma. Hemorrhagic components may be seen in pseudocysts, and may be associated with intermittent or massive GI bleeding, which in turn is associated with increased mortality[5]. These fluid collections lack an epithelial lining and have a fibrotic wall[6]. While pseudocysts do not have a malignant potential, they may mimic malignant neoplasms such as mucinous neoplasms.
On imaging, pseudocysts typically are most commonly unilocular, without internal septations or mural nodules. Pseudocysts typically communicate with the pancreatic duct[4] but this is often not identifiable on cross-sectional imaging. On CECT (Figure 1), pseudocysts look like fluid collections and usually have an imperceptible or minimally visible wall, but the appearance can be variable[7]. On US, pseudocysts are usually hypoechoic with increased through transmission and may have internal debris. Cystic lesions with the characteristic appearance of pseudocysts, and a clinical history of recent pancreatitis, can typically be followed. Pseudocysts usually resolve over time, whereas neoplasms will persist or show interval growth. Importantly, for a pseudocyst to be considered a diagnostic possibility, a history of pancreatitis should be present; if such as history is absent, strong consideration should be given to the workup of such lesions as possible neoplasms.
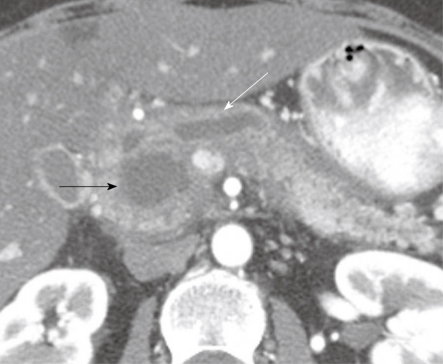
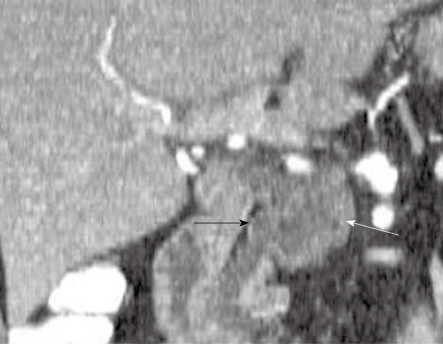
Figure 1.
A 49-year-old woman with a history of epigastric and lower abdominal pain accompanied by abnormal liver function studies. Axial contrast-enhanced computed tomography scan of the abdomen shows cystic change (black arrow) within the pancreas with associated biliary ductal dilation (white arrow). When correlated with the patient’s history and endoscopic ultrasound with FNA findings, this was consistent with a pseudocyst.
A complication that can be seen with pseudocysts is that of pseudoaneurysms, which occur when pancreatic enzymes erode adjacent vessels. Pseudoaneurysms are at risk of rupture and hemorrhage. The splenic, gastroduodenal and superior pancreaticoduodenal arteries are at greatest risk of pseudoaneurysm formation[8] in the setting of pancreatitis.
Simple cysts
True cystic lesions (Figure 2) of the pancreas[9,10], which are lined by epithelium, are seen in patients with von Hippel Lindau disease, cystic fibrosis or polycystic kidney disease[11] and have an imaging appearance similar to that of simple cysts seen in the liver and kidneys[10]. On CECT, simple cysts have a thin wall and have a Hounsfield value equal to that of fluid. On US they are anechoic with posterior acoustic enhancement and on color Doppler evaluation they do not show vascularity.
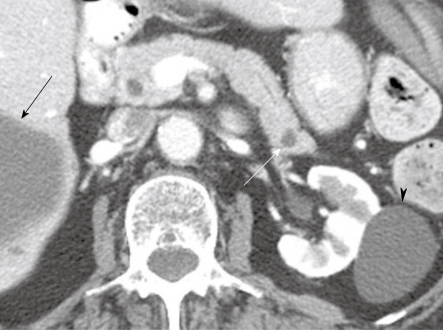
Figure 2.
An 80-year-old woman with a history of hypertension, congestive heart failure and colon cancer. Axial contrast-enhanced computed tomography of the abdomen shows a small low attenuation lesion in the pancreatic tail consistent with a cyst (white arrow), a cyst within the kidney (arrowhead) and a cyst within the liver (black arrow).
Mucinous cystic neoplasms
Mucinous cystic neoplasms (MCNs) are usually solitary, range from 6-35 cm in size, are generally found in the body and tail of the pancreas and account for 10% of cystic neoplasms seen in the pancreas[12]. These tumors typically have a thick wall and are multilocular[3]. They do not communicate with the main pancreatic duct except through fistulae[3,13]. MCNs have < 6 locules which are usually > 2 cm in size. The internal contents of the cyst may be hemorrhagic, necrotic or consist of mucinous material. MCNs typically have internal nodules, which histological may harbor high-grade dysplasia or invasive carcinoma[13]. MCNs of the pancreas resemble MCNs of the ovary and are seen in women of reproductive age (> 95%) (mean age, 45 years)[13-16]. The cyst wall has two layers. The inner layer of cells secretes mucin and the outer layer of cells resembles ovarian stroma. Calcifications may be noted in the periphery of the tumor or in the capsule in 10%-25% of cases[7]. These patients may present with vague abdominal pain, weight loss and anorexia.
MCNs can appear on imaging as a single large cyst with multiple locules, a thick outer wall, septations, and enhancing intramural nodules. Sometimes calcifications can be present on the outer wall[3], and the wall may enhance on the delayed phase. On MRI, the fluid within the cyst may have a low signal on T1-weighted images (T1WI) and a high signal on T2WI, however, increased signal on T1WI has also been observed[17]. It is not possible on imaging to exclude malignancy but the presence of enhancing nodules increases the likelihood[16].
Mucinous cystadenocarcinomas
Mucinous cystadenocarcinomas (Figure 3) are part of a continuum of mucinous cystadenomas. Importantly, it is not possible to differentiate benign mucinous cystadenomas from malignant mucinous cystadenocarcinomas. Nevertheless, a variety of signs, including intracystic nodules, irregular wall thickening, or size > 3-4 cm all increase concern for malignant mucinous cystadenocarcinoma[14].
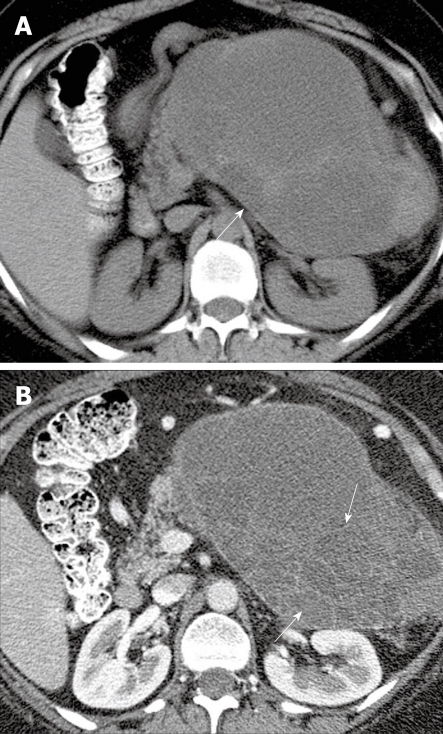
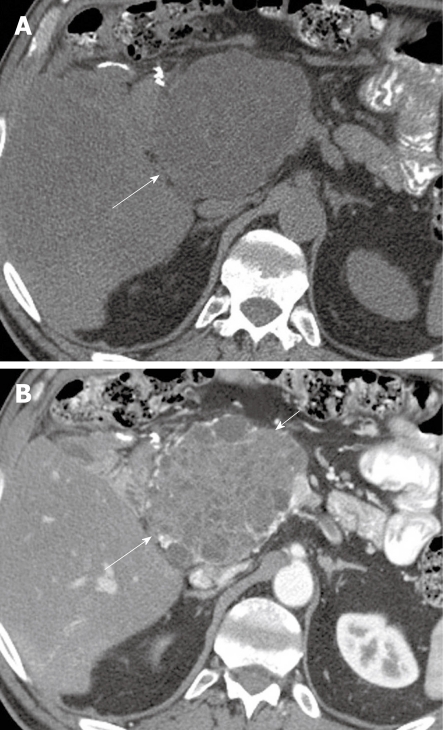
Figure 3.
A 48-year-old woman with left upper quadrant pain. A: Axial non-contrast computed tomography (CT) scan of the abdomen shows a heterogeneous mass (arrow) in the left upper quadrant; B: Axial contrast-enhanced CT of the abdomen shows enhancing septa (arrows) within the mass.
Intraductal papillary mucinous neoplasms
Ohashi et al[18] first described IPMNs in 1982[19]. These tumors are more commonly seen in males, with presentation typically in the seventh to ninth decade of life. Patients may present with epigastric abdominal pain, which is frequently exacerbated by food[3]. The presentation of pain can resemble that of pancreatitis and is due to blockage of the pancreatic ducts by mucin in the main duct form of this disease. Other symptoms and signs include weight loss, fever and jaundice[20].
IPMNs are characterized by whether they involve the main pancreatic duct or side branches[12]. Prognosis depends on the location of the tumor[21]. Only 5%-10% of the time do these involve the entire pancreas; such tumors are usually multifocal[22]. There are three main types of IPMNs: the main duct type, side branch duct type and combined/mixed[23]. The mucin produced by these tumors accumulates within ducts causing cystic dilation of the ducts. Histologically tumors may range from hyperplasia to invasive carcinoma[24]. The risk of malignancy in IPMNs increases with increasing caliber of the main pancreatic duct, visualization of mucin at the ampulla of Vater, and the clinical presence of jaundice and/or diabetes[25-27].
Main duct type IPMN
The main duct type of IPMN arises from the epithelium of the main pancreatic duct. IPMN is classified by the World Health Organization (WHO) based on the degree of epithelial dysplasia: adenoma, borderline tumor, and carcinoma (either in situ or invasive)[28]. Histologically they differ from mucinous cystic tumors in that they lack ovarian-type stroma. IPMNs are more commonly seen in men with a mean age at presentation of 65 years[13]. The pathognomic feature of a main duct IPMN is visible mucin extruding from the ampulla of Vater on endoscopic retrograde cholangiopancreatography[13]. The tumors may be papillary or polypoid in appearance, and arise from pancreatic ductal epithelium which are transformed to mucinous cells[12,29]. The tumor nodule may show cell atypia, ranging from slight dysplasia to frank invasive carcinoma with the likelihood of invasive cancer increasing significantly when the size of the main duct increases to 1 cm or more in diameter. The time to progress from benign to malignant disease is thought to range from 5 to 7 years[12,30]. Approximately 60%-92% of cases demonstrate invasive carcinoma[29,31] on histologic examination.
The appearance of main duct IPMN on CT (Figure 4) depends on its location. The entire main pancreatic duct is dilated if the tumor is present in the head of the pancreas and segmental dilation of the duct can be seen if the tumor is present in the body. However, as disease progresses the entire duct often becomes dilated[13,32]. Pancreatic atrophy is often present secondary to duct obstruction and often consequent episodes of pancreatitis occur. These recurrent episodes of pancreatitis will often cause a loss of the bright T1 signal of the pancreas on non-contrast images and delayed uptake of contrast best seen on delayed images, thought to indicate the presence of fibrosis[33], ultimately resulting in glandular atrophy and dysmorphic calcifications. In the setting of main duct IPMN, the features of particular concern for malignancy or invasive carcinoma include main duct dilation > 1.5 cm in diameter, enhancing nodules, diffuse or multifocal involvement of the pancreatic duct, presence of a soft tissue mass, or bile duct obstruction. Main duct IPMNs even without the above-mentioned features are considered premalignant and are usually resected[9,10].
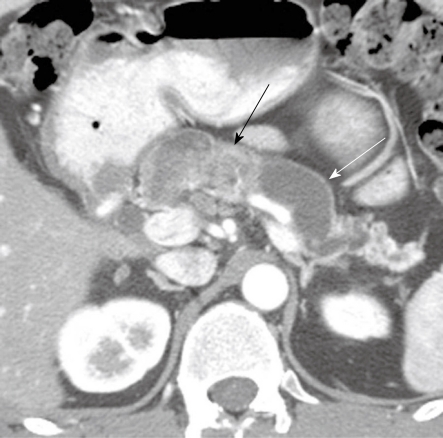
Figure 4.
A 48-year-old man presenting with pancreatitis and exocrine pancreatic insufficiency and a mass in the pancreas representing diffuse intraductal papillary mucinous neoplasm of the pancreas. Axial contrast-enhanced computed tomography of the abdomen shows dilated main pancreatic duct (white arrow) due to mucin production and an enhancing mass in the main pancreatic duct (black arrow) representing a main duct intraductal papillary mucinous neoplasm.
Branch duct IPMNs
Branch-type IPMNs are usually indolent and are found in younger patients compared to main duct IPMNs. They are considered to have a lower malignant potential than main duct forms of IPMN but can evolve to invasive tumors; therefore, close attention to size (less than or greater than 30 mm) and imaging characteristics (wall thickening, internal nodules) is important as these are predictive of invasive tumor[34]. The prevalence of cancer in branch duct forms of IPMNs has been reported to be 6%-46%[9,28,35]. Tumors > 30 mm are at higher risk for invasive cancer compared to simple appearing cystic side branch lesions < 30 mm where the likelihood of invasive cancer is much lower[35,36]. On cross-sectional imaging, thin section CECT (Figure 5) or heavily T2 weighted MRI such as MRCP, communication of the cystic side branch IPMN may be identified with pancreatic ducts and is a useful diagnostic sign[37]. Side branch IPMNs may be unilocular or have the appearance of “a bunch of grapes.” Mucin is secreted by this tumor can extrude into the pancreatic ducts. These tumors can be multifocal, as all pancreatic ductal epithelium may be at risk for developing malignancy[35]. These tumors may mimic a SCA; however, the communication with the main pancreatic duct helps differentiate between the two entities. The ductal communication is reportedly better seen on T2WI MRI sequences than CECT[23,38].
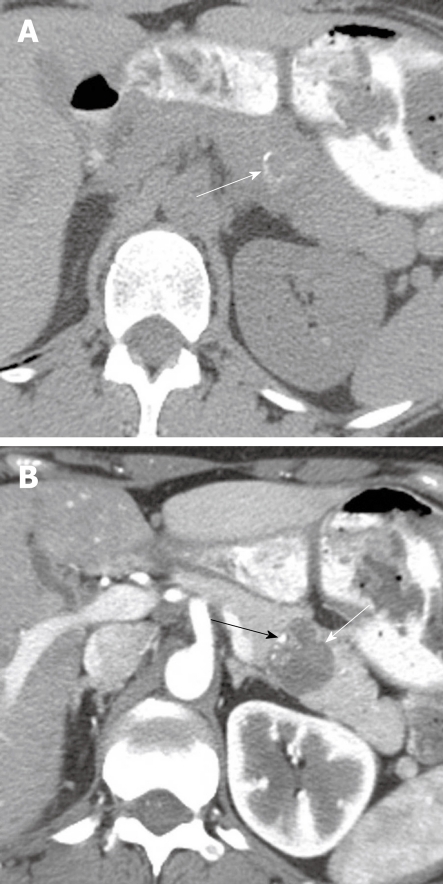
Figure 5.
A 57-year-old man with cysts in the pancreas. Coronal reformatted contrast-enhanced computed tomography scan of the abdomen shows a cystic lesion in the pancreas (white arrow) which communicates with the main pancreatic duct (black arrow).
Combined IPMN
In a combined IPMN, the main pancreatic duct and the side branches are dilated. The main duct dilation of > 15 mm is a predictor of malignancy, whereas ductal dilation of 11 mm may be seen in benign or borderline IPMNs[30]. The presence of nodules within the duct or enhancement of the main pancreatic duct walls are some of the signs which suggest malignant mixed-type IPMNs and are similar to the features seen in the main duct IPMN[30].
Imaging follow-up of IPMN
CECT and/or MRI can be used to follow up IPMNs. PET/CT is an evolving modality for detecting malignancy in cystic pancreatic lesions. In a study of 17 malignant cystic lesions by Sperti et al[39], the sensitivity, specificity, positive and negative predictive value, and accuracy of 18-FDG PET and CT in detecting malignant tumors were 94%, 94%, 89%, 97%, and 94% and 65%, 88%, 73%, 83%, and 80%, respectively, however, these patients already had findings suspicious for malignancy based on CECT findings alone. According to international guidelines developed during the Eleventh Congress of the International Association of Pancreatology held in Sendai, Japan, from July 11 through 14, 2004, the small, simple appearing IPMNs and mucinous cystic lesions can be followed by imaging if the cystic lesion is simple (i.e. no internal nodularity, no wall thickening) and < 1 cm, at an interval of 6-12 mo if 1-2 cm, and every 6 mo if 2-3 cm[9]. Findings suggestive of invasive features such as internal nodularity, wall thickening, changes in adjacent pancreatic parenchyma, etc. would be indications for biopsy and/or potential resection. Depending on the patient’s age, MRI can be considered for follow-up to reduce radiation exposure. Given the risk of malignancy throughout the pancreas, patients who have undergone resection of an IPMN, even if benign on pathology, should have surveillance of the remaining pancreas[9].
Serous cystadenomas
SCAs account for up to 30% of pancreatic cystic neoplasms. They can arise from any part of the pancreas but are commonly seen in the body and tail. SCAs are more commonly seen in women, in their sixth decade of life[13,40] and may produce non-specific symptoms, such as epigastric abdominal pain, and weight loss, if they are large. They are classified into two categories: microcystic SCAs (mutilocular) and oligocystic lesions (unilocular). SCAs are usually < 5 cm in diameter, with a median size of 25-30 mm. These tumors have a well-defined lobulated contour. On cytology they have clear or eosinophil rich cytoplasm. These tumors can be associated with von Hippel-Lindau (VHL) disease[32,41], which is a syndrome consisting of hemangioblastomas of the retina and central nervous system and pheochromocytomas. The VHL gene can also be seen in sporadic cases of SCA. Even though SCAs are predominantly benign, a meta analysis of 673 lesions was found to suggest that the risk of, malignancy in these tumors is approximately < 3%, and this may be an overestimate as all incidentally found SCAs have not been reported in the literature[32,42-44].
Microcystic SCAs
Microcystic SCAs, also known as glycogen rich adenomas, are multilocular. Usually the number of locules is > 6 and the size of these locules ranges from 0.1 to 2.0 cm and they may have a calcified stellate scar[32,45]. These are cystic masses and on non-contrast CT examination (Figure 6), their attenuation is < 20 HU, but they do enhance due to the presence of fibrovascular septa[32,45]. They are best described as having a “honeycomb” appearance. Since these tumors enhance they can easily be mistaken for solid masses if the cystic locules are very small or are very poorly visualized, therefore mimicking lesions such as neuroendocrine neoplasms on cross-sectional imaging.
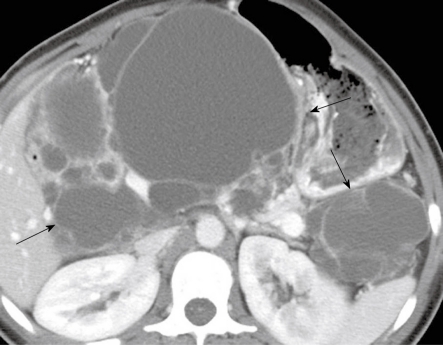
Figure 6.
A 62-year-old man with history of epigastric discomfort. A: Axial non-contrast computed tomography (CT) of the abdomen shows a mass in the pancreatic head (long arrow); B: Axial contrast-enhanced CT of the abdomen shows enhancement within the mass (long arrow) and cystic components (short arrow) suggestive of a microcystic serous cystadenoma.
In such circumstances, MRI may be helpful in demonstrating tiny cystic locules as these usually have a characteristic high T2 signal. These tumors do not communicate with the main pancreatic duct[46]. Prominent calcifications, if present, are readily identifiable on CT, but may show a signal void on MR images. A central scar with calcification, while highly suggestive of a SCA is seen in only 10%-30% of cases. On sonographic evaluation these tumors typically have the appearance of solid echogenic masses due to the many interfaces produced by the numerous cysts.
Oligocystic SCA
Oligocystic SCAs (Figure 7) are rare tumors and may be unilocular or multilocular but have much fewer and larger locules compared to the more common microcystic form of SCA. The individual cysts may be > 2 cm and only one locule is seldom seen. These lesions therefore can mimic MCNs on imaging[3,47] or even pseudocysts.
Figure 7.
A 26-year-old woman with a history of von Hippel-Lindau syndrome. Axial contrast-enhanced computed tomography scan shows cystic lesions in the pancreas (black arrows) representing oligocystic serous cyst adenomas.
SCAs both oligocystic and microcystic can cause symptoms requiring resection. The growth rate of these tumors depends on their size seen at initial diagnosis. Tumors < 4 cm grow at a rate of 0.6 cm/year and tumors > 4 cm grow 0.12 cm/year[47,48], suggesting that resection is appropriate for large lesions, as larger lesions are more likely to be symptomatic[47,48].
Solid pseudopapillary tumors
SPTs were previously known by several other names including solid and cystic papillary epithelial neoplasms of the pancreas, papillary cystic neoplasms, Hamoudi tumors, or Frantz tumors. In 1996 they were renamed SPTs of the pancreas by the WHO[49]. SPTs are less common than IPMNs, SCAs and MCNs. They are usually seen in the second or third decade of life and occur more commonly in women than in men[50,51]. SPTs can have both neuroendocrine and epithelial components. They occur entirely in the pancreas, have no corollary in other organ systems and are reportedly of low malignant potential[52]. When these solid tumors undergo degeneration they develop cystic spaces[52]. Histologically, blood, necrotic debris, and foamy macrophages are found in the cystic areas[7,32]. These tumors commonly measure > 10 cm at presentation, can occur anywhere within the pancreas and can have eccentric calcification[53]. On cross-sectional imaging (Figure 8) these tumors are solid except for cystic components due to tumor degeneration. These tumors are well circumscribed, and on T2WI have slightly high signal intensity, although tumors which are predominantly cystic will have a high T2 signal intensity, which follows a fluid signal. These tumors can show a blood fluid levels. Blood in the cystic spaces can have a high signal on non-contrast T1 weighted images. These tumors can be differentiated from neuroendocrine tumors as they enhance progressively, whereas the latter shows arterial phase enhancement although the appearance of both tumor types is variable and considerable overlap can occur. Features suggestive of a benign histology include being well-encapsulated, smoothly lobulated, and the presence of rim calcifications[53]. Features raising concern for malignancy include disruption of the capsule and an eccentric lobulated margin with focal nodular calcification or amorphous/scattered calcifications[53]. Nodal metastases are rare, however peritoneal, cutaneous and hepatic metastases can occur following excision of SPT[52].
Figure 8.
A 30-year-old woman with increasing abdominal discomfort and bloating. A: Axial non contrast computed tomography (CT) of the abdomen shows a solid mass in the pancreatic tail containing curvilinear calcification (arrow); B: Axial contrast-enhanced CT of the abdomen shows a hypoattenuating mass (white arrow) in the pancreas containing eccentric calcifications (black arrow) consistent with a solid papillary epithelial neoplasm.
Considerations for imaging surveillance
The natural history of cystic pancreatic lesions is still unknown and selective observation is emerging[9,10,54,55]. CT and MRI are currently being used in assessment of pancreatic cystic lesions. CT is probably more widely used as it requires less scan time, offers higher spatial resolution, and is frequently the most accessible. While typically requiring a longer imaging time, MRI with MRCP can identify communication of the cystic lesion with the main pancreatic duct, however with currently available thin section imaging, CT can perform in a similar manner[45]. CT alone has an average accuracy of 61% in differentiating benign (43%) or potentially malignant (57%, papillary mucinous neoplasms, mucinous cystic neoplasms, cancer)[56]. The accuracy of MDCT is better than MRI in classifying cysts as mucinous or non mucinous at 71%-84.2% vs 39.5%-44.7%, respectively, whereas the accuracy of the two techniques in characterizing cysts into nonaggressive and aggressive categories is relatively similar (MDCT vs MRI, 75%-78% vs 78%-86%) respectively[57]. MRI has the additional advantage of higher inherent soft tissue contrast, and it does not utilize ionizing radiation, however in a recent study even though the sensitivity of MRCP for detection of morphologic characteristic was slightly better than that of MDCT, but the difference was not statistically significant[57]. FDG-PET/CT has been used to differentiate malignant from a benign cystic lesions depending on its metabolic activity but is still under investigation[39]. The reported negative predictive value, of 18-FDG PET in detecting malignant cystic tumors is 97%[39]. The high negative predictive value suggests that, when cystic lesion and is not metabolically active it is benign.
Indeterminate cysts should be followed as enlarging cysts have a higher probability for malignancy, with risk also increasing with increasing age (> 70 years)[58,59]. Cysts 3 cm or greater, even without the presence of a solid component, can have in situ cancer or invasive malignancy in up to 3% of cases[2,54,60,61]. If a cyst is not characterized on cross-sectional imaging sufficiently, then evaluation under EUS should be considered, with biopsy of nodules, if present, and aspiration of cyst contents, to test for such factors as mucin, amylase and tumor marker levels including CEA, can be obtained to differentiate mucinous (and therefore potentially malignant) from non mucinous lesions, regardless of lesion size.
Because of the risk of malignancy in mucinous cystic lesions or indeterminate cysts a more conservative approach is necessary[9]. Since asymptomatic cystic lesions < 3 cm in size, without wall thickening, without main duct dilation (≤ 6 mm), or mural nodules have a low risk of malignancy, these patients can be managed conservatively by follow-up with imaging. One algorithm suggested in the literature for lesions meeting the criteria described, is yearly follow-up if a lesion is < 10 mm, 6-12 mo follow-up for lesions between 10 and 20 mm, and for lesions > 2 cm 6-mo follow-up[9].
Surgical resection has been suggested for cases where the main pancreatic duct is > 6 mm in diameter, lesion size is > 30 mm, or intramural nodules are seen, given that the patient is a good candidate with reasonable life expectancy[9,10,61]. Alternatively, biopsy can be obtained under EUS to assess whether a lesion is mucinous or not. Mucinous lesions with such features typically proceed to biopsy.
Following surgery of a histopathologically confirmed benign IPMN, it has been suggested that patients should be followed yearly with either CT or MRI for the first 5 years and thereafter imaged if symptomatic[9]. It has also been suggested that in patients in whom invasive carcinoma is identified that they be imaged every 6 mo for the first 2 years, and to have yearly follow-up thereafter[35], as patients with invasive carcinoma can recur locally or develop distant metastases to the liver and/or lung[9,35]. It is notable that there are no clear guidelines regarding the length of follow-up in any of these groups of patients.
CONCLUSION
When a cystic lesion of the pancreas is discovered incidentally on routine cross-sectional imaging, it is important to correlate this with clinical history to accurately characterize the lesion. For example, a prior history of acute pancreatitis would likely suggest a pseudocyst. When a cystic lesion communicates with pancreatic ducts this may suggest IPMN. If such a lesion is associated with dilatation of the main pancreatic duct, or there is primarily dilatation of the main pancreatic duct, then a main duct type of mixed form of IPMN is a consideration. In menstruating females, a solitary, multilocular mass should raise concern for a mucinous cystadenoma/cystadenocarcinoma. In contrast, a cystic lesion in an elderly female with a honeycomb appearance, particularly if it contains a central scar that may be calcified, is indicative of a SCA. However, while characteristically benign, patients with SCAs > 4 cm have a risk of involvement of invasive structures and may be considered candidates for surgical resection.
Finally, there is a growing consensus that asymptomatic small (< 3 cm), simple appearing cystic lesions (no wall thickening, no internal nodules, no invasive features, no evidence of main duct dilation, etc.) can probably be followed because of the low risk of invasive malignancy. In contrast, if suspicious features are present, a more aggressive approach, including cyst aspiration, biopsy of solid components and potential surgical resection need to be considered. In conclusion, the radiologist plays an important role in the identification, characterization, monitoring and triaging of cystic lesions of the pancreas for appropriate treatment planning.
Footnotes
Peer reviewer: Yasunori Minami, MD, PhD, Division of Gastroenterology and Hepatology, Department of Internal Medicine, 377-2 Ohno-higashi Osaka-sayama Osaka 589-8511, Japan
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM
References
- 1.Safioleas MC, Moulakakis KG, Manti C, Kostakis A. Clinical considerations of primary hydatid disease of the pancreas. Pancreatology. 2005;5:457–461. doi: 10.1159/000086548. [DOI] [PubMed] [Google Scholar]
- 2.Lahav M, Maor Y, Avidan B, Novis B, Bar-Meir S. Nonsurgical management of asymptomatic incidental pancreatic cysts. Clin Gastroenterol Hepatol. 2007;5:813–817. doi: 10.1016/j.cgh.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 3.Salvia R, Festa L, Butturini G, Tonsi A, Sartori N, Biasutti C, Capelli P, Pederzoli P. Pancreatic cystic tumors. Minerva Chir. 2004;59:185–207. [PubMed] [Google Scholar]
- 4.Singhal D, Kakodkar R, Sud R, Chaudhary A. Issues in management of pancreatic pseudocysts. JOP. 2006;7:502–507. [PubMed] [Google Scholar]
- 5.Stabile BE, Wilson SE, Debas HT. Reduced mortality from bleeding pseudocysts and pseudoaneurysms caused by pancreatitis. Arch Surg. 1983;118:45–51. doi: 10.1001/archsurg.1983.01390010035009. [DOI] [PubMed] [Google Scholar]
- 6.Grace PA, Williamson RC. Modern management of pancreatic pseudocysts. Br J Surg. 1993;80:573–581. doi: 10.1002/bjs.1800800508. [DOI] [PubMed] [Google Scholar]
- 7.Kim YH, Saini S, Sahani D, Hahn PF, Mueller PR, Auh YH. Imaging diagnosis of cystic pancreatic lesions: pseudocyst versus nonpseudocyst. Radiographics. 2005;25:671–685. doi: 10.1148/rg.253045104. [DOI] [PubMed] [Google Scholar]
- 8.Flati G, Salvatori F, Porowska B, Talarico C, Flati D, Proposito D, Talarico E, Carboni M. Severe hemorrhagic complications in pancreatitis. Ann Ital Chir. 1995;66:233–237. [PubMed] [Google Scholar]
- 9.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 10.Katz DS, Friedel DM, Kho D, Georgiou N, Hines JJ. Relative accuracy of CT and MRI for characterization of cystic pancreatic masses. AJR Am J Roentgenol. 2007;189:657–661. doi: 10.2214/AJR.07.2772. [DOI] [PubMed] [Google Scholar]
- 11.Leung RS, Biswas SV, Duncan M, Rankin S. Imaging features of von Hippel-Lindau disease. Radiographics. 2008;28:65–79; quiz 323. doi: 10.1148/rg.281075052. [DOI] [PubMed] [Google Scholar]
- 12.Lack EE. Pathology of the pancreas, gallbladder, extrahepatic biliary tract, and ampullary region. New York: Oxford University Press; 2003. [Google Scholar]
- 13.Campbell F, Azadeh B. Cystic neoplasms of the exocrine pancreas. Histopathology. 2008;52:539–551. doi: 10.1111/j.1365-2559.2007.02856.x. [DOI] [PubMed] [Google Scholar]
- 14.Crippa S, Salvia R, Warshaw AL, Domínguez I, Bassi C, Falconi M, Thayer SP, Zamboni G, Lauwers GY, Mino-Kenudson M, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247:571–579. doi: 10.1097/SLA.0b013e31811f4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goh BK, Tan YM, Chung YF, Chow PK, Cheow PC, Wong WK, Ooi LL. A review of mucinous cystic neoplasms of the pancreas defined by ovarian-type stroma: clinicopathological features of 344 patients. World J Surg. 2006;30:2236–2245. doi: 10.1007/s00268-006-0126-1. [DOI] [PubMed] [Google Scholar]
- 16.Goh BK, Tan YM, Yap WM, Cheow PC, Chow PK, Chung YF, Wong WK, Ooi LL. Pancreatic serous oligocystic adenomas: clinicopathologic features and a comparison with serous microcystic adenomas and mucinous cystic neoplasms. World J Surg. 2006;30:1553–1559. doi: 10.1007/s00268-005-0749-7. [DOI] [PubMed] [Google Scholar]
- 17.Nishihara K, Kawabata A, Ueno T, Miyahara M, Hamanaka Y, Suzuki T. The differential diagnosis of pancreatic cysts by MR imaging. Hepatogastroenterology. 1996;43:714–720. [PubMed] [Google Scholar]
- 18.Ohashi K, Murakami Y, Murayama M, Taketoshi T, Ohta T, Ohashi I. Four cases of mucin-secreting cancer of the pancreas on specific findings of the papilla of Vater. Prog Dig Endosc. 1982;20:348–351. [Google Scholar]
- 19.Sessa F, Solcia E, Capella C, Bonato M, Scarpa A, Zamboni G, Pellegata NS, Ranzani GN, Rickaert F, Klöppel G. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425:357–367. doi: 10.1007/BF00189573. [DOI] [PubMed] [Google Scholar]
- 20.Lubezky N, Ben-Haim M, Nakache R, Lahat G, Blachar A, Brazowski E, Santo E, Klausner JM. Clinical presentation can predict disease course in patients with intraductal papillary mucinous neoplasm of the pancreas. World J Surg. 2010;34:126–132. doi: 10.1007/s00268-009-0269-y. [DOI] [PubMed] [Google Scholar]
- 21.Terris B, Ponsot P, Paye F, Hammel P, Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P, Fléjou JF. Intraductal papillary mucinous tumors of the pancreas confined to secondary ducts show less aggressive pathologic features as compared with those involving the main pancreatic duct. Am J Surg Pathol. 2000;24:1372–1377. doi: 10.1097/00000478-200010000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Salvia R, Crippa S, Falconi M, Bassi C, Guarise A, Scarpa A, Pederzoli P. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut. 2007;56:1086–1090. doi: 10.1136/gut.2006.100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, Sandrasegaran K, Akisik F, Howard TJ, Nakeeb A, et al. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg. 2008;12:101–109. doi: 10.1007/s11605-007-0367-9. [DOI] [PubMed] [Google Scholar]
- 24.D'Angelica M, Brennan MF, Suriawinata AA, Klimstra D, Conlon KC. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shima Y, Mori M, Takakura N, Kimura T, Yagi T, Tanaka N. Diagnosis and management of cystic pancreatic tumours with mucin production. Br J Surg. 2000;87:1041–1047. doi: 10.1046/j.1365-2168.2000.01496.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Ogawa Y, Chijiiwa K, Tanaka M. Mucin-hypersecreting tumors of the pancreas: assessing the grade of malignancy preoperatively. Am J Surg. 1996;171:427–431. doi: 10.1016/S0002-9610(97)89624-9. [DOI] [PubMed] [Google Scholar]
- 27.Traverso LW, Peralta EA, Ryan JA Jr, Kozarek RA. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426–432. doi: 10.1016/s0002-9610(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 28.Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD, Vege SS, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? A study of 147 patients. Am J Gastroenterol. 2007;102:1759–1764. doi: 10.1111/j.1572-0241.2007.01224.x. [DOI] [PubMed] [Google Scholar]
- 29.Balzano G, Zerbi A, Di Carlo V. Intraductal papillary mucinous tumors of the pancreas: incidence, clinical findings and natural history. JOP. 2005;6:108–111. [PubMed] [Google Scholar]
- 30.Manfredi R, Graziani R, Motton M, Mantovani W, Baltieri S, Tognolini A, Crippa S, Capelli P, Salvia R, Pozzi Mucelli R. Main pancreatic duct intraductal papillary mucinous neoplasms: accuracy of MR imaging in differentiation between benign and malignant tumors compared with histopathologic analysis. Radiology. 2009;253:106–115. doi: 10.1148/radiol.2531080604. [DOI] [PubMed] [Google Scholar]
- 31.Salvia R, Fernández-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, Pederzoli P, Warshaw AL. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685; discussion 685-687. doi: 10.1097/01.sla.0000124386.54496.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adsay NV. Cystic neoplasia of the pancreas: pathology and biology. J Gastrointest Surg. 2008;12:401–404. doi: 10.1007/s11605-007-0348-z. [DOI] [PubMed] [Google Scholar]
- 33.Zhang XM, Shi H, Parker L, Dohke M, Holland GA, Mitchell DG. Suspected early or mild chronic pancreatitis: enhancement patterns on gadolinium chelate dynamic MRI. Magnetic resonance imaging. J Magn Reson Imaging. 2003;17:86–94. doi: 10.1002/jmri.10218. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas. 2004;28:282–288. doi: 10.1097/00006676-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino-Kenudson M, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133:72–79; quiz 309-310. doi: 10.1053/j.gastro.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sugiyama M, Abe N, Yamaguchi Y, Yamato T, Koyama H, Tokuhara M, Masaki T, Mori T, Atomi Y. Endoscopic pancreatic stent insertion for treatment of pseudocyst after distal pancreatectomy. Gastrointest Endosc. 2001;53:538–539. doi: 10.1067/mge.2001.111564. [DOI] [PubMed] [Google Scholar]
- 37.Procacci C, Megibow AJ, Carbognin G, Guarise A, Spoto E, Biasiutti C, Pistolesi GF. Intraductal papillary mucinous tumor of the pancreas: a pictorial essay. Radiographics. 1999;19:1447–1463. doi: 10.1148/radiographics.19.6.g99no011447. [DOI] [PubMed] [Google Scholar]
- 38.Song SJ, Lee JM, Kim YJ, Kim SH, Lee JY, Han JK, Choi BI. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: comparison of multirow-detector CT and MR imaging using ROC analysis. J Magn Reson Imaging. 2007;26:86–93. doi: 10.1002/jmri.21001. [DOI] [PubMed] [Google Scholar]
- 39.Sperti C, Pasquali C, Decet G, Chierichetti F, Liessi G, Pedrazzoli S. F-18-fluorodeoxyglucose positron emission tomography in differentiating malignant from benign pancreatic cysts: a prospective study. J Gastrointest Surg. 2005;9:22–28; discussion 28-29. doi: 10.1016/j.gassur.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Wargo JA, Fernandez-del-Castillo C, Warshaw AL. Management of pancreatic serous cystadenomas. Adv Surg. 2009;43:23–34. doi: 10.1016/j.yasu.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 41.Mohr VH, Vortmeyer AO, Zhuang Z, Libutti SK, Walther MM, Choyke PL, Zbar B, Linehan WM, Lubensky IA. Histopathology and molecular genetics of multiple cysts and microcystic (serous) adenomas of the pancreas in von Hippel-Lindau patients. Am J Pathol. 2000;157:1615–1621. doi: 10.1016/S0002-9440(10)64799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schneider C, Reck T, Greskötter KR, Köckerling F, Gall FP. [Cystic pancreatic tumors] Langenbecks Arch Chir. 1993;378:281–287. doi: 10.1007/BF00183965. [DOI] [PubMed] [Google Scholar]
- 43.Grieshop NA, Wiebke EA, Kratzer SS, Madura JA. Cystic neoplasms of the pancreas. Am Surg. 1994;60:509–514; discussion 514-515. [PubMed] [Google Scholar]
- 44.Strobel O, Z'graggen K, Schmitz-Winnenthal FH, Friess H, Kappeler A, Zimmermann A, Uhl W, Büchler MW. Risk of malignancy in serous cystic neoplasms of the pancreas. Digestion. 2003;68:24–33. doi: 10.1159/000073222. [DOI] [PubMed] [Google Scholar]
- 45.Sahani DV, Kadavigere R, Saokar A, Fernandez-del Castillo C, Brugge WR, Hahn PF. Cystic pancreatic lesions: a simple imaging-based classification system for guiding management. Radiographics. 2005;25:1471–1484. doi: 10.1148/rg.256045161. [DOI] [PubMed] [Google Scholar]
- 46.Martin DR, Semelka RC. MR imaging of pancreatic masses. Magn Reson Imaging Clin N Am. 2000;8:787–812. [PubMed] [Google Scholar]
- 47.Tseng JF. Management of serous cystadenoma of the pancreas. J Gastrointest Surg. 2008;12:408–410. doi: 10.1007/s11605-007-0360-3. [DOI] [PubMed] [Google Scholar]
- 48.Tseng JF, Warshaw AL, Sahani DV, Lauwers GY, Rattner DW, Fernandez-del Castillo C. Serous cystadenoma of the pancreas: tumor growth rates and recommendations for treatment. Ann Surg. 2005;242:413–419; discussion 419-421. doi: 10.1097/01.sla.0000179651.21193.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams AL, Siegal GP, Jhala NC. Solid pseudopapillary tumor of the pancreas: a review of salient clinical and pathologic features. Adv Anat Pathol. 2008;15:39–45. doi: 10.1097/PAP.0b013e31815e5237. [DOI] [PubMed] [Google Scholar]
- 50.Faraj W, Jamali F, Khalifeh M, Hashash J, Akel S. Solid pseudopapillary neoplasm of the pancreas in a 12-year-old female: case report and review of the literature. Eur J Pediatr Surg. 2006;16:358–361. doi: 10.1055/s-2006-924642. [DOI] [PubMed] [Google Scholar]
- 51.Choi SH, Kim SM, Oh JT, Park JY, Seo JM, Lee SK. Solid pseudopapillary tumor of the pancreas: a multicenter study of 23 pediatric cases. J Pediatr Surg. 2006;41:1992–1995. doi: 10.1016/j.jpedsurg.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 52.Tipton SG, Smyrk TC, Sarr MG, Thompson GB. Malignant potential of solid pseudopapillary neoplasm of the pancreas. Br J Surg. 2006;93:733–737. doi: 10.1002/bjs.5334. [DOI] [PubMed] [Google Scholar]
- 53.Chung YE, Kim MJ, Choi JY, Lim JS, Hong HS, Kim YC, Cho HJ, Kim KA, Choi SY. Differentiation of benign and malignant solid pseudopapillary neoplasms of the pancreas. J Comput Assist Tomogr. 2009;33:689–694. doi: 10.1097/RCT.0b013e31818f2a74. [DOI] [PubMed] [Google Scholar]
- 54.Allen PJ, D'Angelica M, Gonen M, Jaques DP, Coit DG, Jarnagin WR, DeMatteo R, Fong Y, Blumgart LH, Brennan MF. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg. 2006;244:572–582. doi: 10.1097/01.sla.0000237652.84466.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walsh RM, Vogt DP, Henderson JM, Zuccaro G, Vargo J, Dumot J, Herts B, Biscotti CV, Brown N. Natural history of indeterminate pancreatic cysts. Surgery. 2005;138:665–670; discussion 670-671. doi: 10.1016/j.surg.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 56.Fisher WE, Hodges SE, Yagnik V, Morón FE, Wu MF, Hilsenbeck SG, Raijman IL, Brunicardi FC. Accuracy of CT in predicting malignant potential of cystic pancreatic neoplasms. HPB (Oxford) 2008;10:483–490. doi: 10.1080/13651820802291225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sainani NI, Saokar A, Deshpande V, Fernández-del Castillo C, Hahn P, Sahani DV. Comparative performance of MDCT and MRI with MR cholangiopancreatography in characterizing small pancreatic cysts. AJR Am J Roentgenol. 2009;193:722–731. doi: 10.2214/AJR.08.1253. [DOI] [PubMed] [Google Scholar]
- 58.Lee SH, Shin CM, Park JK, Woo SM, Yoo JW, Ryu JK, Yoon YB, Kim YT. Outcomes of cystic lesions in the pancreas after extended follow-up. Dig Dis Sci. 2007;52:2653–2659. doi: 10.1007/s10620-006-9634-y. [DOI] [PubMed] [Google Scholar]
- 59.Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651–657; discussion 657-659. doi: 10.1097/01.sla.0000124299.57430.ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Handrich SJ, Hough DM, Fletcher JG, Sarr MG. The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol. 2005;184:20–23. doi: 10.2214/ajr.184.1.01840020. [DOI] [PubMed] [Google Scholar]
- 61.Sahani DV, Saokar A, Hahn PF, Brugge WR, Fernandez-Del Castillo C. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology. 2006;238:912–919. doi: 10.1148/radiol.2382041806. [DOI] [PubMed] [Google Scholar]