Abstract
Existing evidence suggests that the organization of cognitive functions may differentiate during development. We investigated two key components of executive functions, memory maintenance and inhibitory control, by applying latent factor models appropriate for examining developmental differences in functional associations among aspects of cognition. Two-hundred and sixty-three children (aged 4 to 14 years) were administered tasks that required maintaining rules in mind or inhibiting a prepotent tendency to respond on the same side as the stimulus. Memory maintenance and inhibitory control were not separable in children of 4–7 or 7–9.5 years, but were differentiated in an older group (9.5–14.5 years).
Psychometric research generally emphasizes the stability of the structure of cognitive abilities (see Carroll, 1993; Sternberg, 1994, for reviews). In contrast, multivariate research on cognitive development in children and older adults highlights developmental differences in the structure of cognitive abilities and their functional organization (Cattell, 1971; Flavell, 1982; Garrett, 1946; Horn, 1968; Jones & Conrad, 1933). One of the main hypotheses concerning changes in the functional organization of cognitive abilities during child development is the differentiation hypothesis (e.g., Garrett, 1946). It postulates that the structure of intelligence develops from a relatively unified, general ability in childhood to more differentiated, specific cognitive abilities by early adulthood. Heinz Werner (1957) generalized the notion of differentiation to the orthogenetic principle, denoting that development proceeds from a state of relative globality to a state of increasing differentiation and hierarchical integration. The notion of developmental changes in specificity and differentiation of cognitive processes was later extended to account for cognitive changes across the lifespan, suggesting that the organization of cognitive processes de-differentiates in old age. Indeed, considerable evidence for such de-differentiation has been found (Baltes, Cornelius, Spiro, Nesselroade, & Willis, 1980; Schaie, 1962; for recent evidence, see de Frias et al., 2007; Li et al., 2004).
Methodologically, developmental differentiation refers to the observation that the degree of correlation across different intellectual abilities, often represented as the prominence of a general factor (g) of intellectual functioning, decreases from childhood to adulthood (e.g., Carroll, 1993). This empirical observation is assumed to reflect the decreasing influence of domain-general processing constraints and the increasing importance of specialized mechanisms and knowledge across development (Baltes, Lindenberger, & Staudinger, 2006; Deary et al., 1996; cf. Spearman, 1927).
This study provides evidence for the developmental differentiation of two cognitive functions that depend on the prefrontal cortex (PFC), working memory and inhibitory control, in the course of child development. Both working memory and inhibitory control are key components of executive functions, broadly defined as processes that control (e.g., regulate and coordinate) goal-directed behavior and are often linked to the functioning of the PFC (Diamond, 2006; Miller & Cohen, 2001; Miyake & Shah, 1999; Stuss & Benson, 1986). Working memory has been conceptualized differently by various researchers. Notwithstanding the conceptual differences, a general consensus holds that working memory refers to the ability to briefly hold information in mind (memory maintenance) while also performing mental operations on that information (e.g., Baddeley, 2007; Baddeley & Hitch, 1974; Cowan, 1995; Just & Carpenter, 1992; Petrides, 1994; Shah & Miyake, 1996; Smith & Jonides, 1999). In adults, memory maintenance appears to rely more heavily on ventrolateral (inferior) PFC (except at supra-span levels) and manipulation of its contents appears to rely more on dorsolateral PFC (D’Esposito, Postle, Ballard, Lease, 1999; D’Esposito, Postle, & Rypma, 2000; Rypma, Berger, & D’Esposito, 2002; Rypma & D’Esposito, 1999).
Inhibitory control refers to the ability to suppress inappropriate response or attentional tendencies in order to act appropriately (Carlson, Moses, Hix, 1998; Dempster, 1992; Diamond, 1989, 2006; Moutier, Plagne-Cayeux, Melot, Houdé, 2006; Nigg, 2000). It, too, relies on lateral PFC, albeit usually more posteriorly within lateral PFC (Casey et al., 1997; Durston et al., 2002; Leung & Cai, 2007; Xue, Aron, & Poldrack, 2008). When memory maintenance and inhibition are compared directly within the same study (e.g., Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001; McNab et al., 2008), both processes recruit several overlapping regions. However, within the circuitry, some regions are more critically involved in the resolution of interferences, whereas others are more involved in the resolution of an increase in maintenance load, supporting the notion that the two processes are distinct but interrelated. In this article, we focus on the information-maintenance aspect of working memory (henceforth termed “memory maintenance”) and inhibitory control and investigate whether developmental differentiation occurs between these executive functions.
Empirical studies in the psychometric tradition have often investigated the developmental differentiation hypothesis with measures of intellectual abilities that do not map onto specific cognitive mechanisms such as working memory or inhibitory control. Evidence from this line of research is mixed. In a classical review of about 60 factor analytic studies, Reinert (1970) reported a trend toward increasing differentiation of abilities with age. However, in a more recent review, Carroll (1993) failed to find strong evidence in support of differentiation. Results from a handful of recent cross-sectional studies investigating this topic are consistent with Carroll’s finding, as the proportion of variance accounted for by a general factor was found to remain unchanged across age groups (e.g., Bickley, Keith, & Wolfle, 1995; Deary et al., 1996; Juan-Espinosa, Cuevas, Escorial, & Garcia, 2006; Juan-Espinosa, Garcia, Colom, & Abad, 2000; Juan-Espinosa et al., 2002). An exception to this is a study conducted by Tideman and Gustafsson (2004). Employing multiple-group confirmatory factor-analytic techniques, the authors found that latent factors of verbal and non-verbal intelligence were less highly correlated in older age groups (6- to 7-year-olds) than in younger children (3- to 4-year-olds), consistent with the developmental differentiation hypothesis.
To date, there is a small but growing body of research that directly examines the functional organization of basic cognitive mechanisms (as opposed to intelligence constructs) across child development. Gathercole and colleagues (Gathercole, Pickering, Ambridge, Wearing, 2004; Alloway, Gathercole, & Pickering, 2006) examined whether the structure of working memory (according to the Baddeley multi-component model) changes during child development. In general, the authors found that the basic structure of working memory components is largely in place by 4 years of age. However, the domain-specific visuospatial construct and the domain-general processing construct show stronger links in younger children (4–6 years old) compared to older children. In a recent lifespan study, Li et al. (2004) examined correlations among a variety of basic processes (ranging from visual search, response competition, long-term and short-term memory search, and choice reactions) and psychometric intelligence (i.e., fluid and crystallized intelligence) in a lifespan sample covering the ages of 6 to 89 years. It was found that correlations between fluid and crystallized intelligence and the constituent cognitive processes were stronger at either end of the lifespan than in young adulthood. These results support the longstanding theoretical conjecture (Baltes et al., 1980; Schaie, 1962) that intellectual abilities and cognitive processes are more undifferentiated in childhood, gradually become more specialized and reach a more differentiated state in adolescence and adulthood, and then undergo de-differentiation during old age. Given that higher-order intellectual functions are supported by basic cognitive mechanisms, we decided to examine whether increasing differentiation can be observed in the functional organization of two key components of executive functions: memory maintenance and inhibitory control.
Recent progress in understanding the neural bases of cognitive development and the emerging emphasis on cross-level integrations provide increasing opportunities for linking cognitive differentiation to neural changes (Churchland & Sejnowski, 1988; Li & Lindenberger, 1999; Li, Lindenberger, & Sikström, 2001; Lindenberger, Li, & Bäckman, 2006; Munakata, Casey, & Diamond, 2004; Nelson et al., 2002). Specifically, the development of memory maintenance and inhibitory control, the two mechanisms investigated here, appears to be closely linked to the development of PFC. Converging evidence from anatomical, neurochemical, and functional studies suggests that PFC and the functional circuits in which it participates undergo profound changes and reorganization over age well into adolescence and young adulthood (e.g., Bens, 2002; Casey, Galvan, & Hare, 2005; Diamond, 2002; Elbert, Heim, & Rockstroh, 2001; Giedd et al., 1999; Gogtay et al., 2004; Lenroot & Giedd, 2006; Paus, 2005; Sowell, Thompson, Holmes, Batth, et al., 1999; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999).
At the functional level, neuroimaging studies suggest that cognitive development is accompanied by changes in patterns of brain activation, including enhanced activation in critical regions, attenuation in others, as well as shifts in lateralization (e.g., Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002; de Haan, Pascalis, & Johnson, 2002; Durston et al., 2006; Johnson, 2000, 2001). These effects taken together usually result in more focal and less diffuse brain activation from childhood to adulthood. Also, the activation pattern seen during one type of cognitive task shows less overlap with the pattern of neural activation while performing a different task. Both of these patterns are consistent with a change from less differentiated neural mechanisms to more differentiated function. When differentiation first starts to occur, focal activation is quite intense, but with increasing efficiency the key neural region needs to work less hard. Accumulating evidence suggests that functional circuitry (within-region as well as between regions) and cortical dynamics are constantly undergoing reorganization in adapting to influences such as environmental stimulation, experience, brain development, and deterioration or damage (e.g., Li, 2003; Mueller, Brehmer, von Oertzen, Li, & Lindenberger, 2008; Mueller, Gruber, Klimesch, & Lindenberger, submitted; Theoret et al., 2005).
AIMS OF THIS STUDY
We postulate that the increasing specialization of PFC neural circuits over time, in conjunction with normative developmental experiences, promotes the differentiation of PFC-dependent cognitive functions. In early childhood, the operation and expression of basic cognitive mechanisms are assumed to depend strongly on system-general constraints. With maturation, these system-general constraints are relaxed through increased specification and specialization of neural circuits, which in turn support greater complexity in cognitive structure and diversity in the operations of cognitive mechanisms.
The analyses reported here are based on data originally reported by Davidson, Amso, Ander-son, and Diamond (2006). In contrast to that publication, which, for the most part, examined age differences separately for each of the different tasks, here we use multivariate statistical techniques to examine age differences in the relations among tasks. In the original study, 325 participants (from 4 to 13 years of age and young adults) were tested on a computerized battery of tests designed to independently manipulate (a) the maintenance and manipulation of information held in mind and (b) inhibitory control, to examine the development, interrelations, and possible independence of these two domains of function. Specifically, the battery included tasks designed to vary demands on retaining and processing information that is held in mind and/or inhibiting irrelevant information and/or prepotent responses, in single-task as well as in task-switching contexts (the latter requiring more cognitive flexibility). Although only behavioral data were presented in the Davidson et al. (2006) article, the battery of tasks was designed to be suitable for use in the MR scanner environment and those results have been presented elsewhere (Diamond, O’Craven, & Savoy, 1998).
Differences in the developmental trajectories of information maintenance and inhibitory control aspects of executive functions have been reported (Diamond, 2002; Huizinga, Dolan, & van der Molen, 2006; Luciana, Conklin, Hooper, & Yarger, 2005). In the original article, Davidson et al. (2006) found that 4-year-old children were already able to retain two conditional rules in mind, and that the relative increase in difficulty of holding more rules in mind did not change over age. Inhibitory control, on the other hand, was especially difficult for younger children as they showed (a) an inhibitory cost completely absent in adults (a cost in both speed and accuracy of exercising inhibition in single-task blocks of incongruent trials versus single-task blocks of congruent trials), (b) that inhibitory cost (the difference in performance on congruent and incongruent blocks) was greater for children of 4–9 years of age than was the differences in performance due to increasing the memory load from two to six items, and (c) the younger the child, the greater the spatial incompatibility effect in variants of the Simon task (reflecting the cost of inhibiting the pull to respond on the same side as the stimulus). The age difference in performance was greatest in the most difficult condition, in which inhibition and memory maintenance were taxed in a task-switching context (Davidson et al., 2006; see Kray, Eber, & Lindenberger, 2004; Kray & Lindenberger, 2000, for parallel findings in aging and across the lifespan). In addition to examining age differences in mean performance level, Davidson et al. examined raw correlations between the memory maintenance and inhibitory control measures. To their surprise, the correlations turned out to be high, despite their hypothesis that the two constructs would be independent.
ANALYTICAL STRATEGY
By using statistical models separating specific variance from variance common to all indicators of a given construct, this study examines the hypothesis that inhibitory control and the maintenance aspect of working memory develop from a relatively unitary executive function construct at younger ages into distinct mechanisms at older ages. As discussed by Burgess (1997), interpretation of raw correlations among executive function tasks (as was done by Davidson et al., 2006) may be problematic due to issues associated with task impurity, low reliability, and measurement error. A multivariate latent variable approach (i.e., confirmatory factor analysis) that simultaneously accounts for measurement error and extracts common variance among the tasks provides better estimates of interrelations among postulated constructs (Horn & McArdle, 1992; Nesselroade & Thompson, 1995). Thus, the present analysis extends the original work of Davidson et al. (2006) by representing both the mean and covariance structures between memory maintenance and inhibitory control as two aspects of executive functions at the latent (error-free) level separately for three age groups, thereby allowing for a direct test of the developmental differentiation hypothesis.
In an early observation, Nesselroade (1970) postulated that “the universe of behavior is not constant for different age levels and therefore the manifest nature of the factor in behavioral measures will change” (pp. 199–200). According to this notion of differential manifestation, the same ability may manifest itself in different ways as development unfolds (see also, Kagan, 1980). The analytical strategies of structural equation modeling applied in child development and aging research are typically more appropriate for examining individual differences in amount (quantity) rather than in form (quality) (cf. Nesselroade, Gerstorf, Hardy, & Ram, 2007). In particular, invariant loading patterns across groups (i.e., invariance in form) are seen as prerequisites before factor comparisons across groups can be considered legitimate (see e.g., Meredith, 1993). This view on invariance assumes that the behavioral manifestations of unobserved abilities are necessarily the same across development and there can only be quantitative difference.
Here, we follow a relatively novel strategy for identifying and scaling latent variables that allows for different manifestations of latent factors in different age groups (for a similar application in aging research, see Tucker-Drob & Salthouse, 2008). The basic strategy was introduced by Little, Slegers, and Card (2006) and designated as the effects-coding method. The strategy entails constraining the set of indicator intercepts to sum to zero for each construct and the set of loadings for each construct to sum to the number of unique indicators. As a consequence, the estimated latent variances and latent means meaningfully reflect the observed metric of the indicators, optimally weighted by the degree to which each indicator represents the underlying latent construct. Little et al. (2006) noted that this strategy adheres to Nesselroade’s (2007) idiographic method. Within the invariant configuration of construct-indicator mappings, differences in the relative pattern of factor loadings across groups capture the idiosyncratic manifestation of a construct while simultaneously yielding latent variable estimates for group comparison. In the context of the current study, decreases over age in the correlation between factors indicate increasing differentiation at the level of process interrelations with age.
METHODS
We restrict the description of measures and procedures to variables that are of primary relevance to the analysis reported here (for more details of the original study see Davidson et al., 2006).
Participants
Adults in the original sample were excluded from the present analyses because of the small sample size (N = 20), which would have prevented use of confirmatory factor analysis. This left us with 263 participants with complete data, aged 4 to 14 years. For the multiple-group analysis, the sample was equally divided into three age groups: youngest (N = 90; Mage = 5.2 years; age range = 4.0–6.7 years), middle (N = 90; Mage = 8.05; age range = 6.8–9.45 years), and oldest (N = 83; Mage = 11.14; age range = 9.5–14.6 years). Having three age groups was based on theoretical considerations that it is important to keep the separation between the middle and oldest group as developmental difference in PFC integrity was found between pre-adolescent and adolescence periods (e.g., Giedd et al., 1999).
Procedures
Participants completed six tasks designed to vary demands on memory load, inhibitory control, or both. All tasks were computer administered, including stimulus presentation and response recording. Participants were instructed to hold a button box with both hands and to respond with their thumbs. For each trial, one stimulus appeared either at the left, right, or middle of the screen. Depending on the conditional rules associated with the stimuli for each task (e.g., if stimulus A, press left; if stimulus B, press right), participants had to decide whether to press the left or right button. To adjust for the robust effect of age differences in processing speed, stimulus presentation time was 2,500 msec for the youngest age group, whereas stimulus presentation time was 750 msec for the middle and oldest age groups. Stimulus durations were established based on pilot testing. Differences in stimulus duration across the groups affected mean performance but not the correlations among tasks within each group and thus should not pose a concern for the present analyses. The current analysis focused on factor structure instead of mean performance, we refer the readers to the original publication (i.e., Davidson et al., 2006) for results of mean performance.
Each task began with instructions followed by a short practice block consisting of four or six trials. Achieving more than 75% correct performance on the practice trials and being able to verbally explain the conditional rules of the task to the experimenter were the criteria for indicating that a participant had obtained a good enough understanding of the task to proceed to testing. Participants were allowed to repeat the practice block if necessary to achieve that criterion. In the following sections, each of the six tasks is described.
Tasks
Abstract shapes
This memory task consisted of two conditions that varied in set size, involving either two or six abstract shapes. At the beginning of each block, participants were taught a rule for each shape (i.e., whether they should press the left or right button when that abstract shape appeared). The stimuli were presented in the center of the screen so there should have been no preferential activation of the right or left hand. The Abstract Shapes task taxed memory maintenance but not inhibition. Participants first completed the two-shape condition, followed by the six-shape condition. There were two blocks of 20 trials for each of these two conditions. For each condition, the dependent measure was the proportion of correct responses over total responses.
Dots—Incongruent
Two types of dots (striped or solid) were used as stimuli for this task. In each trial, a stimulus appeared randomly on the left or right side of the screen. For half of the participants a solid dot indicated they should respond on the side opposite to the location of the dot (i.e., spatially incompatible trials). For a striped dot they were to respond on the same side as the dot. For the other half of participants, these instructions were reversed. All participants started with an initial block of 20 compatible trials (i.e., respond on the same side as the dot, called the Dots—Congruent condition). Since this condition placed little demand on either inhibition or working memory, it is not considered here. This condition was followed by a block of 20 incompatible trials (Dots—Incongruent). Participants had to inhibit the prepotent tendency to respond on the same side as the stimulus in order to respond correctly (Lu & Proctor, 1995; Georgopoulos, 1994; Georgopoulos, Lurito, Petrides, Schwartz, & Massey, 1989). The dependent measure was the proportion of correct responses in this block.
Dots—Mixed
This condition was presented after the Dots—Congruent and Dots—Incongruent conditions. Here, both types of dots (striped and solid) were used as stimuli. Before the task started, participants were reminded again about the conditional rule associated with each of the two dots. Either a striped or solid dot appeared randomly on the left or right of the computer screen, yielding spatially compatible and incompatible trials. Participants had to hold in mind the two rules, mentally calculate whether same-side or opposite-side meant press left or press right on a given trial, inhibit the irrelevant rule, and (on spatially incompatible trials) inhibit the tendency to press the button on the same side as the stimulus. This task heavily taxed both inhibition and working memory. There were 20 trials. The dependent measure was the proportion of correct responses on spatially-incompatible trials in this block.
Pictures
This inhibitory control task was a classic Simon task. A color picture of either a frog or butterfly was presented on the left or right of the computer screen. Participants were instructed to press the left button when they saw a butterfly, and to press the right button when they saw a frog. Stimuli were presented randomly on the left or right of the screen over the block of 20 trials, yielding spatially compatible and incompatible trials. To minimize the demands on memory, small pictures of the stimuli were attached next to the correct buttons on the response box. Thus, the Pictures task is seen as taxing inhibition on spatially incompatible trials, but not taxing memory. The dependent measure was the proportion of correct responses on spatially incompatible trials.
Arrows
In this measure of inhibitory control, an arrow (either pointing straight down or toward the opposite side at a 45-degree angle) was presented randomly at the left or right of the computer screen. When an arrow pointing straight down appeared on the screen, participants were to press the button on the same side as the arrow (spatially compatible trials). When an arrow pointing diagonally toward the opposite side appeared, participants were to press the button on the side opposite the arrow (spatially incompatible trials). Twenty trials were administered. The Arrows task is seen as taxing inhibition of the tendency to respond on the same side as the stimulus for trials with a diagonal arrow, but it is not seen as taxing memory because no arbitrary, abstract association needed to be held in mind; all participants had to do was look at the stimulus and it indicated where to respond. The dependent measure was the proportion of correct responses on spatially incompatible trials.
Overview of Statistical Analyses
Trials in which a response was given in less than 200 msec (anticipatory response) were considered too fast to be meaningful and thus were excluded from the analyses. The mean proportion of such non-valid responses ranged from 10–17% across tasks. Significant age-specific and task-specific effects on anticipatory responses were observed (see Davidson et al., 2006). This was resolved by taking proportional measures of accuracy. We focused on accuracy instead of reaction time because reaction time measures are known to reflect a strong processing speed factor (e.g., Fry & Hale, 1996; Li et al., 2004; Salthouse, 1996). The correlations between reaction time measures are high in all age groups and produce factors that are highly correlated, precluding the possibility of detecting age differences in differentiation, unless a wide lifespan age range is covered (e.g., Li et al., 2004).
For each measure, outliers were identified within each group as having a z-score of more than 3.29 (p < .001, two-tailed test). Only .01 percent of the data was considered as outliers (distributed equally across the groups) and extreme scores were replaced with the next most extreme score within the acceptable distribution. Descriptive data for all tasks are listed in Table 1. Most variables showed acceptable distributions in each age group (i.e., no extreme skewness or kurtosis). The reliability of each measure was examined with the Spearman-Brown reliability coefficient estimated from split-half reliabilities between odd- and even-numbered trials of the corresponding task. This procedure yielded satisfactory values for reliability for most tasks: .69 for Arrows, .74 for Dots—Incongruent, .70 for Dots—Mixed, .87 for 2-Abstract-Shape, .75 for 6-Abstract-Shape, and .55 for Pictures.
TABLE 1.
Means, Standard Deviations, Kurtosis, and Skewness for Each Measure Within Each Age Group
| 2-Abstract-Shapes | 6-Abstract-Shapes | Dots—Incongruent | Arrows | Dots—Mixed | Pictures | |
|---|---|---|---|---|---|---|
| Youngest group | ||||||
| Mean | 0.90 | 0.81 | 0.89 | 0.75 | 0.69 | 0.88 |
| Standard Deviation | 0.10 | 0.14 | 0.13 | 0.26 | 0.31 | 0.13 |
| Kurtosis | 0.46 | −0.90 | 2.86 | 0.18 | −0.39 | 0.67 |
| Skewness | −1.16 | −0.32 | −1.73 | −1.05 | −0.82 | −1.07 |
| Middle group | ||||||
| Mean | 0.87 | 0.76 | 0.90 | 0.68 | 0.74 | 0.81 |
| StandardDeviation | 0.09 | 0.12 | 0.11 | 0.24 | 0.20 | 0.16 |
| Kurtosis | 3.94 | 0.38 | 0.89 | −0.54 | 0.75 | 0.51 |
| Skewness | −1.72 | −0.70 | −1.25 | −0.58 | −0.93 | −0.87 |
| Oldest group | ||||||
| Mean | 0.92 | 0.81 | 0.95 | 0.80 | 0.84 | 0.89 |
| Standard Deviation | 0.06 | 0.14 | 0.06 | 0.19 | 0.15 | 0.11 |
| Kurtosis | 1.12 | −0.18 | 0.64 | 2.48 | −0.23 | −0.11 |
| Skewness | −1.20 | −0.79 | −1.24 | −1.44 | −0.85 | −0.83 |
The youngest age group received a longer presentation time than the other age groups.
Steps of Confirmatory Factor Analysis
Structural equation modeling analyses were implemented in M-plus (Muthén & Muthén, 1998–2006). We first attempted to achieve a tenable multiple-group solution (Horn & McArdle, 1992; Vandenberg & Lance, 2000). A three-group model was fit, with the equality constraints mentioned above imposed. Having established a tenable two-factor model, a sequence of increasingly strict sets of equality constraints was imposed across age groups to examine the extent of measurement and factorial invariance. The latent factors were regressed onto age to control for potential group differences in the contribution of age-induced collinearities to the factor structure.
All structural models were computed using maximum likelihood estimation. Several indices of model fit were considered. Models were considered a good fit with χ2/df of less than 2, comparative fit index of more than .95 (CFI; Bentler, 1990), and a root-mean-square error of approximation of less than .08 including .05 with its 90% confidence interval (RMSEA; Browne & Cudeck, 1992; Steiger, 1990). Nested models were compared by testing the significance of the difference in χ2, with the degrees of freedom being equal to the difference in the number of free parameters between the two models. The threshold for statistical significance was specified at the .05 level.
RESULTS
Goodness-of-fit test results of the models are presented in Table 2. First, a one-factor model was simultaneously fit to the data of all age groups (Model 1). All tasks loaded on a general factor and parameters including factor loadings, factor variance, factor means, indicator intercepts, and unique variance (residuals) were estimated. As can be seen in Table 2, the fit of Model 1 was not acceptable, χ2 = 65.03, χ2/df =1.55, CFI = 0.89, and RMSEA = 0.08 (90% CI = .04 to .12). Thus, within the common metric space of all age groups, a single factor model did not adequately capture the complexity of the data.
TABLE 2.
Summary of Model Fitting Procedure
| Competing models | χ2 | df | CFI | RMSEA (90% CI) | Nested comparison | Δχ2 | Δdf |
|---|---|---|---|---|---|---|---|
| 1. One factor | 65.03 | 42 | .89 | .08 (.04– .12) | |||
| 2. Two factors, configural invariance | 55.84 | 36 | .91 | .08 (.03– .12) | |||
| 3. Full Metric Invariance (all factor loadings equal across groups) | 73.15 | 44 | .86 | .09 (.05– .12) | 3 vs. 2 | 17.31* | 8 |
| 3a. Partial Metric Invariance (Inhibitory Control loadings equal across groups) | 71.41 | 42 | .86 | .09 (.05– .12) | 3a vs. 2 | 15.57* | 6 |
| 3b. Partial Metric Invariance (Memory Maintenance loadings equal across groups) | 58.31 | 38 | .91 | .08 (.03– .12) | 3b vs. 2 | 2.47 | 2 |
| 4a. Correlation of youngest group = 1 | 58.60 | 39 | .91 | .08 (.03– .11) | 4a vs. 3b | 0.29 | 1 |
| 4b. Correlation of middle group = 1 | 58.71 | 39 | .91 | .08 (.03– .11) | 4b vs. 3b | 0.34 | 1 |
| 4c. Correlation of oldest group =1 | 62.91 | 39 | .89 | .08 (.04– .12) | 4c vs. 3b | 4.61* | 1 |
| 4d. Correlation of youngest group = Correlation of middle group | 58.99 | 39 | .91 | .08 (.03– .11) | 4d vs. 3b | 0.68 | 1 |
| 4e. Correlation of all groups equal | 63.61 | 40 | .89 | .08 (.04– .12) | 4e vs. 4d | 4.62* | 1 |
See text for detailed description of the models. df: degrees of freedom for χ2 test; CFI: comparative fit index; RMSEA: root mean square error of approximation.
p < .05.
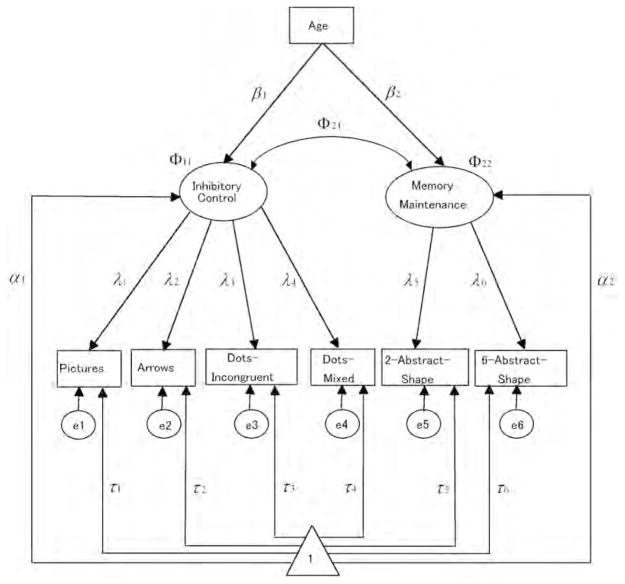
Second, a model with two correlated factors was fit. We postulated two factors: (a) memory maintenance (as measured by 2-Abstract-Shapes and 6-Abstract-Shapes, assuming that the common variance between the two memory tasks reflects the ability to hold rules in mind) and (b) inhibitory control (as measured by Dots—Incongruent, Dots—Mixed, Pictures, and Arrows, assuming that the common variance among these four tasks reflects the ability to inhibit stimulus-congruent response tendencies). The loading patterns were fixed to be the same across all groups (configural invariance), while the factor loadings, factor variances, unique variance parameters (i.e., error variances), factor means, and intercepts of indicators were allowed to vary across the three age groups (see Figure 1). Model 2 provided a better fit to the data than Model 1, χ2 = 55.84, χ2/df = 1.55, CFI = .91, and RMSEA = .08 (90% CI = .03 to .12). The correlation estimated for the youngest group was .98, for the middle-aged group it was 0.81, and it was 0.32 for the oldest group. This model provides initial support to the hypothesis that memory maintenance and inhibitory control differentiate with increasing age.
FIGURE 1.
Two-factor model estimated for the three age groups. Note that the following constraints were imposed: λ1 = 4 − λ2 − λ3 − λ4; λ5 = 2 − λ6; τ1 + τ2 + τ3 + τ4 = 0; τ5 + τ6 = 0. The triangle refers to a constant that equals 1 for the estimation of means.
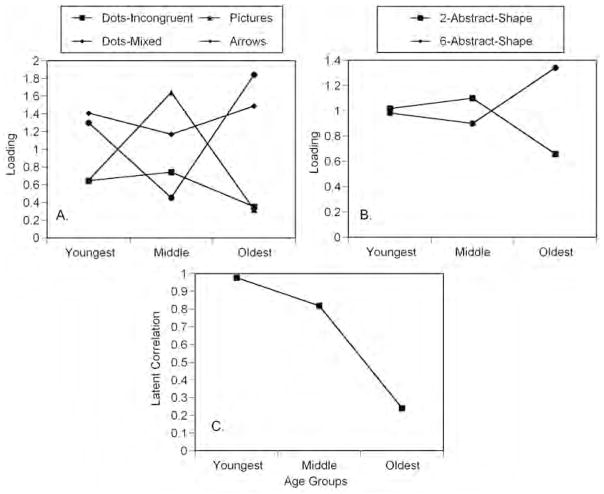
We next proceeded from configural to metric measurement invariance by imposing equality constraints on the unstandardized factor loadings (Model 3). Model 3 was nested within Model 2, and a direct comparison showed that the assumption of equal factor loadings was not tenable, Δχ2 = 17.31, Δdf = 8, p = .03. In other words, constraining all factor loadings to be equal across age groups led to a significant decrement in fit. An examination of the factor loading pattern revealed that the inhibitory control factor manifested itself differently across the three age groups (see Figure 2 Panel A), whereas memory maintenance showed relatively similar loading patterns across age (see Figure 2 Panel B). Hence, we examined whether partial metric invariance across the three age groups was tenable (Models 3a and 3b). As expected, holding the factor loadings of the memory maintenance factor equal across groups but allowing factor loadings for inhibitory control to vary freely provided an acceptable representation of the data (but not vice versa). Therefore, the corresponding model (i.e., Model 3b in Table 2) was retained for subsequent analyses. The common variances of the two factors reliably differed from zero in all three age groups (for all six nested comparisons, p < .05), which is a precondition for testing hypotheses regarding their intercorrelations.
FIGURE 2.
Estimates of factor loading and intercorrelations plotted according to age group. Panel A shows age trends in factor loadings for indicators of Inhibitory Control. Panel B shows age trends in factor loadings for indicators of Memory Maintenance. Panel C shows estimates of factor intercorrelations.
Finally, we examined group differences in factor interrelations, which are shown in Figure 2, Panel C, to address the main hypothesis of this study. First, we examined separately for each age group whether the correlation between memory maintenance and inhibitory control could be fixed to one (cf. van der Sluis, Dolan, & Stoel, 2005), indicating an undifferentiated general executive functioning factor instead of two distinct memory maintenance and inhibitory control factors. In the resulting models (Model 4a, 4b, 4c), the model fit was significantly worse only when the correlation of the oldest children group was fixed to one Δχ2 = 4.61, Δdf = 1, p < .05. In the last step of testing, we examined whether the correlations of the three age groups could be constrained to be equal. The resulting model (Model 4d) showed significantly worse fit Δχ2 =4.62, Δdf = 1, p < .05, indicating that the correlation between the two factors is significantly different across the age groups.
DISCUSSION
The goal of this study was to examine whether two components of executive functions, memory maintenance and inhibitory control, differentiate from childhood to early adolescence. Using multiple-group confirmatory factor analysis, we found: (a) that the correlation between memory maintenance and inhibitory control differs reliably from 1.0 in early adolescence, but not in younger children; (b) that the correlation between memory maintenance and inhibitory control is significantly lower in young teenagers than in younger children.
Our findings appear counter to some recent studies that failed to find support for developmental differentiation (e.g., Deary et al., 1996; e.g., Juan-Espinosa et al., 2006). The present study and those studies differ in several important ways, however. First, the main analytical strategies of the previous studies were either to examine the manifest (i.e., raw) correlations, or to compare the amount of variance extracted by the first unrotated factor in a principal component analysis. Neither of those methods separates unique variance from common variance. The confirmatory factor analysis utilized in the current study ensured that the constructs of interest were error-free (see also Alloway et al., 2006; Gathercole et al., 2004; Tideman & Gustafsson, 2004). In particular, the scaling method adapted from Little et al. (2006) is a suitable addition to the multivariate methodological toolbox of developmental psychology, as it allows the relative pattern of factor loadings, and hence the behavioral manifestation of cognitive abilities, to change with age (cf. Kagan, 1980; Nesselroade, 1970; Nesselroade et al., 2007). This nonarbitrary yet flexible scaling method provides valuable indications of the nature of the developmental change process. For example, we observed that the factor-loading pattern of memory maintenance remained relatively similar across the ages investigated, whereas the factor-loading pattern of inhibitory control differed by age. Furthermore, inhibitory control has been shown to be particularly poor in young children and undergo prolonged development across childhood compared to working memory maintenance as defined in this study (e.g., Davidson et al., 2006; Diamond 1995, 2002; but see Huizinga et al., 2006), indicating that age-related differences in covariance pattern of the ability structures occurs concomitantly with age-related changes in the mean pattern (i.e., performance level). Based on these findings, we suggest that the increasing differentiation of executive functions from childhood to early adolescence is driven by the protracted development of inhibitory control.
The second reason that our findings appear counter to other findings is that many previous studies made use of psychometric tests of intellectual abilities (e.g., Wechsler scale or Differential Aptitude Test), and predominantly assessed complex forms of cognition such as language, numerical ability, and reasoning, each of which requires multiple cognitive abilities. In the present study, tasks were designed from a cognitive neuroscience perspective with the explicit goal of assessing basic cognitive functions that are building blocks for more complex forms of cognition. Thus, the abilities measured by the current task battery may have been more sensitive to developmental differentiation than measures conceived within the psychometric research tradition.
Our findings are relevant to the debate over whether working memory and inhibitory control are essentially one function or represent separable cognitive mechanisms. Some researchers view holding information in mind and exercising inhibitory control as closely related but separate processes, both being components of executive function (e.g., Diamond, 2006; Gernsbacher & Faust, 1991; Levy & Anderson, 2002), or as two separate sub-processes of working memory (e.g., Conway & Engle, 1994; Hasher, Stoltzfuz, Zacks, & Rypma, 1991; Kane & Engle, 2002). According to an alternative view, often adopted in neural network modeling, there is no need for a separate inhibitory function beyond working memory (e.g., Cohen, Dunbar, & McClelland, 1990; Kimberg & Farah, 1993; Miller & Cohen, 2001; Munakata, 2001). The increasing differentiation between memory maintenance and inhibitory control observed here suggests the presence of two mechanisms after early childhood and that the specificity of these two mechanisms increases from childhood to adolescence.
The development of working memory and inhibitory control as key components of executive functions has been of great interest to developmental psychology and developmental cognitive neuroscience, as these mechanisms are considered cornerstones of adaptive self-regulation, higher-order cognition, and goal-directed behavior (Camos, 2008; Dempster, 1992; Harnishfeger & Bjorklund, 1993; Houdé & Tzourio-Mazoyer, 2003; St Clair-Thompson & Gathercole, 2006; Unsworth & Engle, 2007) important for success in school and in life (e.g., Diamond, Barnett, Thomas, Munro, 2007). A child’s capacity to retain and work with information held in mind (i.e., working memory) and to suppress inappropriate attention or actions in favor of appropriate ones (i.e., inhibitory control) continues to develop across childhood and adolescence (see Diamond, 2006, for a review). The functioning and development of various aspects of working memory, inhibition, and cognitive flexibility have been shown to depend closely on the structure, neuro-chemistry, and functional activity of PFC and its networks of functional connections (Arnstein & Robbins, 2002; Bunge & Wright, 2007; Casey et al., 1995; Crone, Donohue, Honomichl, Wendelken, & Bunge, 2006; Miller & Cohen, 2001; Perlstein, Dixit, Noll, & Cohen, 2003).
Based on a dataset with a wide age range spanning early childhood to adolescence, the results from our analysis indicate that the construct of executive functions is not differentiated in children before seven years of age. In parallel, functional changes in the neural basis for cognitive control and executive functioning appear to be characterized by an early lack of differentiation that gives way to increasingly focal activation later in childhood and then decreasingly intense activation of the focal regions during late adolescence as the key regions come to function more efficiently (e.g., Brown et al., 2005; Casey et al., 2005; Durston et al., 2006). Thus, we hypothesize that (a) the undifferentiated memory maintenance and inhibitory control observed in younger children in the present analyses corresponds to highly overlapping patterns of neural activation documented by others for tasks requiring different components or combinations of executive function in young children; (b) the increasing differentiation between memory maintenance and inhibitory control from early childhood to late childhood/early adolescence observed in the present analyses corresponds to increasingly focal and less overlapping neural activation patterns for the cognitive functions with age; and (c) further specialization and partial automatization of these cognitive processes in the course of later development and learning reported by others corresponds to decreasing neural activation of these focal regions and increasing recruitment of other regions of the brain that can subserve task performance more efficiently.
We would like to mention four limitations of the present study that are pertinent for future investigations. First, our analyses were based on age group as a categorical variable. There may be differences within each age group that a finer-scale analysis might reveal and/or any discontinuity between younger and older children might not exactly correspond to our age-group split (based on equal numbers of subjects per group). It would be desirable to examine age differences in latent construct correlations in a more continuous fashion, that is, as a function of years of age (or of other, more proximal indicators of development such as white matter growth). Sample size restrictions did not permit us to examine that here.
Second, the two Abstract-Shape measures of working memory assessed only memory maintenance, not the manipulation of contents held in mind. Future studies should examine the developmental differentiation of memory manipulation, memory maintenance, and inhibitory control (cf. Huizinga et al., 2006). Third, the number of trials for some of the tasks was relatively low (particularly the inhibitory control tasks). We proceeded with the analysis as there was no difference in the number of available trials across age groups, and the analysis method used in this study is suited for dealing with task impurity, low reliability, and measurement error. However, the limitation in number of trials in the tasks needs to be considered, especially for planning of future studies. Finally, our interpretation of results rests on the assumption that inter-individual differences in cross-sectional data approximate patterns of development at the within-person level. Clearly, however, developmental differentiation is a within-person phenomenon, and is best observed within individuals over time using longitudinal designs (Molenaar, Huizenga, & Nesselroade, 2003). Thus, it remains to be seen whether the present results represent a valid approximation to within-person changes in the organization of memory maintenance and inhibitory control. At this point, using confirmatory factor analysis, we found that the mechanisms of memory maintenance and inhibitory control, as assessed by inter-individual differences within different age groups, reliably become more differentiated from early childhood (beginning at around 5 years) to early adolescence (mean of 11 years). This appears to be driven by changes in inhibitory control with age. Future investigations should assess individual children longitudinally and investigate the neural bases for the cognitive changes investigated here.
Contributor Information
Yee Lee Shing, Max Planck Institute for Human Development, Center for Lifespan Psychology Berlin, Germany.
Ulman Lindenberger, Max Planck Institute for Human Development, Center for Lifespan Psychology Berlin, Germany.
Adele Diamond, Department of Psychiatry, University of British Columbia, Vancouver, Canada and Division of Child & Adolescent Psychiatry, BC Children’s Hospital, Vancouver, Canada.
Shu-Chen Li, Max Planck Institute for Human Development, Center for Lifespan Psychology Berlin, Germany.
Matthew C. Davidson, Department of Psychology, University of Massachusetts, Amherst, Massachusetts
References
- Alloway TP, Gathercole SE, Pickering SJ. Verbal and visuospatial short-term and working memory in children: Are they separable? Child Development. 2006;77(6):1698–1716. doi: 10.1111/j.1467-8624.2006.00968.x. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Robbins TW. Neurochemical modulation of prefrontal cortical function in humans and animals. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 51–84. [Google Scholar]
- Baddeley A. Working memory, thought, and action. Oxford: Oxford University Press; 2007. [Google Scholar]
- Baddeley A, Hitch GJ. Working memory. In: Bower GA, editor. The psychology of learning and motivation. New York: Academic Press; 1974. pp. 47–89. [Google Scholar]
- Baltes PB, Cornelius SW, Spiro A, Nesselroade JR, Willis SL. Integration versus differentiation of fluid/crystallized intelligence in old age. Developmental Psychology. 1980;16(6):625–635. [Google Scholar]
- Baltes PB, Lindenberger U, Staudinger UM. Lifespan theory in developmental psychology. In: Damon W, Lerner RM, editors. Handbook of child psychology: Vol. 1. Theoretical models of human development. New York: Wiley; 2006. pp. 569–664. [Google Scholar]
- Bens FM. The development of prefrontal cortex: The maturation of neurotransmitter systems and their interactions. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: MIT Press; 2002. pp. 79–92. [Google Scholar]
- Bentler PM. Fit indices, Lagrange multipliers, constraint changes, and incomplete data in structural models. Multivariate Behavioral Research. 1990;25:163–172. doi: 10.1207/s15327906mbr2502_3. [DOI] [PubMed] [Google Scholar]
- Bickley PG, Keith TZ, Wolfle LM. The three-stratum theory of cognitive abilities: Test of the structure of intelligence across the life span. Intelligence. 1995;20:309–328. [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cerebral Cortex. 2005;15:275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. Sociological Methods & Research. 1993;21:230–258. [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JDE. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JDE. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124:2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wright SB. Neurodevelopmental changes in working memory and cognitive control. Current Opinion in Neurobiology. 2007;17(2):243–250. doi: 10.1016/j.conb.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Burgess PW. Theory and methodology in executive function research. In: Rabbitt P, editor. Methodology of frontal and executive function. East Sussex: Psychology Press; 1997. pp. 81–111. [Google Scholar]
- Camos V. Low working memory capacity impedes both efficiency and learning of number transcoding in children. Journal of Experimental Child Psychology. 2008;99(1):37–57. doi: 10.1016/j.jecp.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ, Hix HR. The role of inhibitory processes in young children’s difficulties with deception and false belief. Child Development. 1998;69(3):672–691. [PubMed] [Google Scholar]
- Carroll JB. Human cognitive abilities: A survey of factor-analytic studies. New York: Cambridge University Press; 1993. [Google Scholar]
- Casey BJ, Castellanos FX, Giedd JN, Marsh WL, Hamburger SD, Schubert AB, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Galvan A, Hare TA. Changes in cerebral functional organization during cognitive development. Current Opinions in Neurobiology. 2005;15:239–244. doi: 10.1016/j.conb.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Cattell RB. Abilities: Their structure, growth, and action. Boston, MA: Houghton Mifflin; 1971. [Google Scholar]
- Churchland PS, Sejnowski TJ. Perspectives on cognitive neuroscience. Science. 1988;242:741–745. doi: 10.1126/science.3055294. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the stroop effect. Psychological Review. 1990;97(3):332–361. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- Conway ARA, Engle AK. Working memory and retrieval: A resource-dependent inhibition model. Journal of Experimental Psychology: General. 1994;123:354–373. doi: 10.1037//0096-3445.123.4.354. [DOI] [PubMed] [Google Scholar]
- Cowan N. Attention and memory: An integrated framework. New York: Oxford University Press; 1995. [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA. Brain regions mediating flexible rule use during development. Journal of Neuroscience. 2006;26(43):11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson MC, Amso D, Anderson LC, Diamond A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuro-psychologia. 2006;44:2037–2078. doi: 10.1016/j.neuropsychologia.2006.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Lövdén M, Lindenberger U, Nilsson LG. Revisiting the dedifferentiation hypothesis with longitudinal multi-cohort data. Intelligence. 2007;35(4):381–392. [Google Scholar]
- de Haan M, Pascalis O, Johnson MH. Specialization of neural mechanisms underlying face recognition in human infants. Journal of Cognitive Neuroscience. 2002;14:199–209. doi: 10.1162/089892902317236849. [DOI] [PubMed] [Google Scholar]
- Deary IJ, Egan V, Gibson GJ, Austin EJ, Brand CR, Kellaghan T. Intelligence and the differentiation hypothesis. Intelligence. 1996;23:105–132. [Google Scholar]
- Dempster FN. The rise and fall of the inhibitory mechanism: Toward a unified theory of cognitive development and aging. Developmental Review. 1992;12:45–75. [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain and Cognition. 1999;4:66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: Evidence from event-related fMRI studies. Experimental Brain Research. 2000;133:3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Diamond A. Evidence of robust recognition memory early in life even when assessed by reaching behavior. Journal of Experimental Child Psychology. 1995;59:419–456. doi: 10.1006/jecp.1995.1020. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 466–503. [Google Scholar]
- Diamond A. The early development of executive functions. In: Bialystok E, Craik F, editors. Lifespan cognition: Mechanisms of change. New York: Oxford University Press; 2006. pp. 70–95. [Google Scholar]
- Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, O’Craven KM, Savoy RL. Dorsolateral prefrontal cortex contributions to working memory and inhibition as revealed by fMRI. Society for Neuroscience Abstracts. 1998;24:1251. [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Developmental Science. 2006;9(1):1–20. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Yang Y, Ulug AM, Zimmerman RD, Casey BJ. A neural basis for the development of inhibitory control. Developmental Science. 2002;5:9–16. [Google Scholar]
- Elbert T, Heim S, Rockstroh B. Neural plasticity and development. In: Nelson CA, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge, MA: The MIT Press; 2001. pp. 191–202. [Google Scholar]
- Flavell JH. On cognitive development. Child Development. 1982;53:1–10. [Google Scholar]
- Fry AF, Hale S. Processing speed, working memory, and fluid intelligence: Evidence for a developmental cascade. Psychological Science. 1996;7:237–241. [Google Scholar]
- Garrett HE. A developmental theory of intelligence. American Psychologist. 1946;1:372–378. doi: 10.1037/h0056380. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Pickering SJ, Ambridge B, Wearing H. The structure of working memory from 4 to 15 years of age. Developmental Psychology. 2004;40(2):177–190. doi: 10.1037/0012-1649.40.2.177. [DOI] [PubMed] [Google Scholar]
- Georgopoulos A. Population activity in the control of movement. International Review of Neurobiology. 1994;37:103–119. doi: 10.1016/s0074-7742(08)60241-x. [DOI] [PubMed] [Google Scholar]
- Georgopoulos A, Lurito JT, Petrides M, Schwartz AB, Massey JT. Mental rotation of the neuronal population vector. Science. 1989;243:234–236. doi: 10.1126/science.2911737. [DOI] [PubMed] [Google Scholar]
- Gernsbacher MA, Faust ME. The mechanism of suppression: A component of general comprehension skill. Journal of Experimental Psychology. 1991;17:245–262. doi: 10.1037//0278-7393.17.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zigdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AD, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnishfeger KK, Bjorklund DF. The ontogeny of inhibition mechanisms: A renewed approach to cognitive development. In: Howe ML, Pasnak R, editors. Emerging themes in cognitive development. Vol I. Foundations. New York: Springer-Verlag; 1993. pp. 28–49. [Google Scholar]
- Hasher L, Stoltzfus ER, Zacks RT, Rypma B. Age and inhibition. Journal of Experimental Psychology. 1991;17:163–169. doi: 10.1037//0278-7393.17.1.163. [DOI] [PubMed] [Google Scholar]
- Horn JL. Organization of abilities and the development of intelligence. Psychological Review. 1968;75:242–259. doi: 10.1037/h0025662. [DOI] [PubMed] [Google Scholar]
- Horn JL, McArdle JJ. A practical and theoretical guide to measurement invariance in aging research. Experimental Aging Research. 1992;18(3):117–144. doi: 10.1080/03610739208253916. [DOI] [PubMed] [Google Scholar]
- Houdé O, Tzourio-Mazoyer N. Neural foundations of logical and mathematical cognition. Nature Reviews Neuroscience. 2003;4:507–514. doi: 10.1038/nrn1117. [DOI] [PubMed] [Google Scholar]
- Huizinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in infants: Elements of an interactive specialization framework. Child Development. 2000;71(1):75–81. doi: 10.1111/1467-8624.00120. [DOI] [PubMed] [Google Scholar]
- Johnson MH. Functional brain development in humans. Nature Reviews Neuroscience. 2001;2:475–483. doi: 10.1038/35081509. [DOI] [PubMed] [Google Scholar]
- Jones HE, Conrad HS. The growth and decline of intelligence: A study of a monogenous group between the ages of ten and sixty. Genetic Psychology Monographs. 1933;13:233–297. [Google Scholar]
- Juan-Espinosa M, Cuevas L, Escorial S, Garcia LF. Testing the indifferentiation hypothesis during childhood, adolescence, and adulthood. Journal of Genetic Psychology. 2006;167(1):5–15. doi: 10.3200/GNTP.167.1.5-15. [DOI] [PubMed] [Google Scholar]
- Juan-Espinosa M, Garcia LF, Colom R, Abad FJ. Testing the age related differentiation hypothesis through the Wechsler’s scales. Personality and Individual Differences. 2000;29:1069–1075. [Google Scholar]
- Juan-Espinosa M, Garcia LF, Escorial S, Rebollo I, Colom R, Abad FJ. Age dedifferentiation hypothesis: Evidence from the WAIS III. Intelligence. 2002;30(5):395–408. [Google Scholar]
- Just MA, Carpenter PA. A capacity theory of comprehension: Individual differences in working memory. Psychological Review. 1992;99:122–149. doi: 10.1037/0033-295x.99.1.122. [DOI] [PubMed] [Google Scholar]
- Kagan J. Perspectives on continuity. In: Brim OG, Kagan J, editors. Constancy and change in human development. Cambridge: Harvard University Press; 1980. pp. 26–74. [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin & Review. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Farah MJ. A unified account of cognitive impairments following frontal-lobe damage: The role of working-memory in complex, organized behavior. Journal of Experimental Psychology: General. 1993;122(4):411–428. doi: 10.1037//0096-3445.122.4.411. [DOI] [PubMed] [Google Scholar]
- Kray J, Eber J, Lindenberger U. Age differences in executive functioning across the lifespan: The role of verbalization in task preparation. Acta Psychologia. 2004;115:143–165. doi: 10.1016/j.actpsy.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kray J, Lindenberger U. Adult age differences in task switching. Psychology and Aging. 2000;15(1):126–147. doi: 10.1037//0882-7974.15.1.126. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Leung HC, Cai W. Common and differential ventrolateral prefrontal activity during inhibition of hand and eye movements. Journal of Neuroscience. 2007;27:9893–9900. doi: 10.1523/JNEUROSCI.2837-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BJ, Anderson MC. Inhibitory processes and the control of memory retrieval. Trends in Cognitive Sciences. 2002;6:299–305. doi: 10.1016/s1364-6613(02)01923-x. [DOI] [PubMed] [Google Scholar]
- Li SC. Biocultural orchestration of developmental plasticity across levels: The interplay between biology and culture in shaping the mind and behavior across the lifespan. Psychological Bulletin. 2003;129:171–194. doi: 10.1037/0033-2909.129.2.171. [DOI] [PubMed] [Google Scholar]
- Li S-C, Lindenberger U. Cross-level unification: A computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. In: Nilsson L-G, Markowitsch HJ, editors. Cognitive Neuroscience of Memory. Hogrefe & Huber; Göttingen, Germany: 1999. pp. 103–146. [Google Scholar]
- Li SC, Lindenberger U, Sikström S. Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences. 2001;5:479–486. doi: 10.1016/s1364-6613(00)01769-1. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, Hommel B, Aschersleben G, Prinz W, Baltes PB. Transformations in the couplings among intellectual abilities and constituent cognitive processes across the life span. Psychological Science. 2004;15:155–163. doi: 10.1111/j.0956-7976.2004.01503003.x. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Li SC, Bäckman L. Delineating brain-behavior mappings across the lifespan: Substantive and methodological advances in developmental neuroscience. Neuroscience and Biobehavioral Reviews. 2006;30(6):713–717. doi: 10.1016/j.neubiorev.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Little TD, Slegers DW, Card NA. A non-arbitrary method of identifying and scaling latent variables in SEM and MACS models. Structural Equation Modeling. 2006;13(1):59–72. [Google Scholar]
- Luciana M, Conklin HM, Hooper CJ, Yarger RS. The development of nonverbal working memory and executive control processes in adolescents. Child Development. 2005;76:697–712. doi: 10.1111/j.1467-8624.2005.00872.x. [DOI] [PubMed] [Google Scholar]
- Lu CH, Proctor RW. The influence of irrelevant location information on performance: A review of the Si-mon and spatial Stroop effects. Psychonomic Bulletin & Review. 1995;2:174–207. doi: 10.3758/BF03210959. [DOI] [PubMed] [Google Scholar]
- McNab F, Leroux G, Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: An fMRI, within-subjects investigation. Neuropsychologia. 2008;46(11):2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Meredith W. Measurement invariance, factor analysis and factor invariance. Psychometrika. 1993;58:525–543. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miyake A, Shah P. Models of working memory: Mechanisms of active maintenance and executive control. New York: Cambridge University Press; 1999. [Google Scholar]
- Molenaar PCM, Huizenga HM, Nesselroade JR. The relationship between the structure of inter-individual and intraindividual variability: A theoretical and empirical vindication of developmental systems theory. In: Staudinger UM, Lindenberger U, editors. Understanding human development: Dialogues with lifespan psychology. Dordrecht: Kluwer Academic Publishers; 2003. pp. 339–360. [Google Scholar]
- Moutier S, Plagne-Cayeux S, Melot AM, Houdé O. Syllogistic reasoning and belief-bias inhibition in school children: Evidence from a negative priming paradigm. Developmental Science. 2006;9(2):166–172. doi: 10.1111/j.1467-7687.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- Mueller V, Brehmer Y, von Oertzen T, Li S-C, Lindenberger U. Electrophysiological correlates of selective attention: A lifespan comparison. BMC Neuroscience. 2008;9(18) doi: 10.1186/1471-2202-9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller V, Gruber W, Klimesch W, Lindenberger U. Lifespan differences in cortical dynamics of auditory perception. Developmental Science. 2009;12:839–853. doi: 10.1111/j.1467-7687.2009.00834.x. [DOI] [PubMed] [Google Scholar]
- Munakata Y. Graded representations in behavioral dissociations. Trends in Cognitive Sciences. 2001;5(7):309–315. doi: 10.1016/s1364-6613(00)01682-x. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Casey BJ, Diamond A. Developmental cognitive neuroscience: Progress and potential. Trends in Cognitive Science. 2004;8:122–128. doi: 10.1016/j.tics.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus user’s guide. 4. Los Angeles, CA: Muthén & Muthén; 1998–2006. [Google Scholar]
- Nelson CA, Bloom FE, Cameron JL, Amaral D, Dahl RE, Pine D. An integrative, multidisciplinary approach to the study of brain-behavior relations in the context of typical and atypical development. Development and Psychopathology. 2002;14:499–520. doi: 10.1017/s0954579402003061. [DOI] [PubMed] [Google Scholar]
- Nesselroade JR. Application of multivariate strategies to problems of measuring and structuring long-term change. In: Goulet LR, Baltes PB, editors. Life-span developmental psychology: Research and theory. New York: Academic Press; 1970. pp. 193–211. [Google Scholar]
- Nesselroade JR. Factoring at the individual level: Some matters for the second century of factor analysis. In: Cudeck R, MacCallum RC, editors. Factor analysis at 100: Historical developments and future directions. Mahwah, NJ: Erlbaum; 2007. pp. 249–264. [Google Scholar]
- Nesselroade JR, Gerstorf D, Hardy SA, Ram N. Idiographic filters for psychological constructs. Measurement. 2007;5(4):217–235. [Google Scholar]
- Nesselroade JR, Thompson WW. Selection and related threats to group comparisons: An example comparing factorial structures of higher and lower ability groups of adult twins. Psychological Bulletin. 1995;117(2):271–284. doi: 10.1037/0033-2909.117.2.271. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: Views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9(2):60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Perlstein WM, Dixit NK, Carter CS, Noll DC, Cohen JD. Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biological Psychiatry. 2003;53(1):25–38. doi: 10.1016/s0006-3223(02)01675-x. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and working memory: Evidence from investigations of the effects of cortical excisions in nonhuman primates. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Amsterdam: Elsevier Science; 1994. pp. 59–84. [Google Scholar]
- Reinert G. Comparative factor analytic studies of intelligence throughout the human life-span. In: Goulet LR, Baltes PB, editors. Life-span developmental psychology. New York: Academic Press; 1970. pp. 467–484. [Google Scholar]
- Rypma B, Berger JS, D’Esposito M. The influence of working memory demand and subject performance on prefrontal cortical activity. Journal of Cognitive Neuroscience. 2002;14:721–731. doi: 10.1162/08989290260138627. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. The roles of prefrontal brain regions in components of working memory: Effects of memory load and individual differences. Proceedings of the National Academy of Sciences. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. The processing-speed theory of adult age differences in cognition. Psychological Review. 1996;103(3):403–428. doi: 10.1037/0033-295x.103.3.403. [DOI] [PubMed] [Google Scholar]
- Schaie KW. A field-theory approach to age changes in cognitive behavior. Vita Humana. 1962;5:129–141. doi: 10.1159/000269554. [DOI] [PubMed] [Google Scholar]
- Shah P, Miyake A. The separability of working memory resources for spatial thinking and language processing: An individual differences approach. Journal of Experimental Psychology: General. 1996;125:4–27. doi: 10.1037//0096-3445.125.1.4. [DOI] [PubMed] [Google Scholar]
- Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:1657–1661. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW. Localizing age-related changes in brain structure between childhood and adolescence using statistical parametric mapping. NeuroImage. 1999;9:587–597. doi: 10.1006/nimg.1999.0436. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spearman C. The abilities of man. London: MacMillan; 1927. [Google Scholar]
- St Clair-Thompson HL, Gathercole SE. Executive functions and achievements in school: Shifting, updating, inhibition, and working memory. Quarterly Journal of Experimental Psychology. 2006;59(4):745–759. doi: 10.1080/17470210500162854. [DOI] [PubMed] [Google Scholar]
- Steiger JH. Structural model evaluation and modification: An interval estimation approach. Multivariate Behavioral Research. 1990;25:173–180. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- Sternberg RJ. Encyclopedia of human intelligence. New York: Macmillan; 1994. [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- Theoret H, Halligan E, Kobayashi M, Fregni F, Tager-Flusberg H, Pascual-Leone A. Impaired motor facilitation during action observation in individuals with autism spectrum disorder. Current Biology. 2005;15:R84–R85. doi: 10.1016/j.cub.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Tideman E, Gustafsson JE. Age-related differentiation of cognitive abilities in ages 3–7. Personality and Individual Differences. 2004;36:1965–1974. [Google Scholar]
- Tucker-Drob EM, Salthouse TA. Adult age trends in the relations among cognitive abilities. Psychology and Aging. 2008;23(2):453–460. doi: 10.1037/0882-7974.23.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth N, Engle RW. On the division of short-term and working memory: An examination of simple and complex span and their relation to higher order abilities. Psychological Bulletin. 2007;133(6):1038–1066. doi: 10.1037/0033-2909.133.6.1038. [DOI] [PubMed] [Google Scholar]
- Van der Sluis S, Dolan CV, Stoel RD. A note on testing perfect correlations in SEM. Structural Equation Modeling. 2005;12(4):551–577. [Google Scholar]
- Vandenberg RJ, Lance CE. A review and synthesis of the measurement invariance literature: Suggestions, practices and recommendations for organizational research. Organizational Research Methods. 2000;3(1):4–69. [Google Scholar]
- Werner H. The concept of development from a comparative and organismic point of view. In: Harris DB, editor. The concept of development: An issue in the study of human behaviour. Minneapolis: Jones Press, Inc; 1957. pp. 125–148. [Google Scholar]
- Xue G, Aron AR, Poldrack RA. Common neural substrates for inhibition of spoken and manual responses. Cerebral Cortex. 2008;18:1923–1932. doi: 10.1093/cercor/bhm220. [DOI] [PubMed] [Google Scholar]