Abstract
Background:
Solutions of vancomycin for oral administration are not available commercially in Canada or the United States but are needed for patients who cannot swallow capsules.
Objective:
To evaluate the stability of vancomycin solutions stored in unit-dose cups and plastic bottles under refrigeration (4°C) and at room temperature (25°C) for up to 75 days.
Methods:
Vancomycin 25 mg/mL in Ora-Sweet vehicle and water (1:1 ratio by volume) was dispensed into opaque blue polyethylene unit-dose cups with aluminum seal (14 replicates) or amber plastic prescription bottles (6 replicates). Seven cups and 3 bottles were refrigerated (4°C), and the remainder of the containers were stored at room temperature (25°C). At the time of preparation and at 15, 30, 40, 50, 63, and 75 days, 3 aliquots were collected from one of the cups and from every bottle and were stored frozen (−85°C) until the time of analysis. Physical characteristics were evaluated at each time point, including measurement of pH and visual assessment of colour and precipitation. After thawing, the samples were analyzed in triplicate by a validated stability-indicating high-performance liquid chromatography method. A solution was considered stable if 90% of the initial concentration of vancomycin was maintained.
Results:
No notable changes in colour, taste, or pH were observed in vancomycin solutions stored in the unit-dose cups at 4°C or 25°C or in the plastic bottles stored at 4°C over the 75-day study period. Starting on day 63, a white precipitate was observed in the solutions stored in plastic bottles at 25°C, but there were no notable changes in taste or pH during the 75-day period. The 95% confidence interval of the slope of the curve relating concentration to time, determined by linear regression, indicated that vancomycin solutions stored in cups or bottles at 4°C would maintain at least 93.6% of the initial vancomycin concentration for 75 days and that solutions stored at 25°C would maintain at least 90.0% of the initial concentration for 30 days (cups) or 26 days (bottles), with 95% confidence.
Conclusions:
Vancomycin 25 mg/mL stored in unit-dose cups or plastic bottles at 4°C was stable for at least 75 days, whereas solutions stored in cups or bottles at 25°C are expected to be stable for 30 or 26 days, respectively.
Keywords: vancomycin, high-pressure liquid chromatography, stability
RÉSUMÉ
Contexte:
Les solutions de vancomycine pour administration orale ne sont pas offertes commercialement au Canada ou aux États-Unis, mais elles sont requises pour les patients qui sont incapables d’avaler des capsules.
Objectif:
Évaluer la stabilité de solutions de vancomycine préparées dans des godets pour dose unitaire et des flacons de plastique, et conservées au réfrigérateur (4 °C) et à la température ambiante (25 °C) pendant un maximum de 75 jours.
Méthodes:
Des solutions de vancomycine à 25 mg/mL dans un excipient composé de parties égales (rapport de 1:1 par volume) d’Ora-Sweet et d’eau ont été conditionnées dans des godets pour dose unitaire en polyéthylène bleu opaque scellés par un opercule d’aluminium (14 échantillons) ou des flacons pour médicaments d’ordonnance en plastique ambré (6 échantillons). Sept godets et trois flacons ont été conservés au réfrigérateur (4 °C) et le reste des contenants ont été conservés à la température ambiante (25 °C). Au moment de la préparation et à 15, 30, 40, 50, 63 et 75 jours, trois aliquotes ont été prélevées d’un des godets et de chaque flacon, puis congelées (85 °C) jusqu’au moment de l’analyse. Les propriétés physiques ont été évaluées à chaque point dans le temps, notamment le pH, la couleur et la présence de précipité. Après avoir été décongelés, les échantillons ont été analysés en triple à l’aide d’une épreuve validée par chromatographie liquide haute performance mesurant la stabilité. Les solutions étaient considérées stables si elles conservaient 90 % de la concentration initiale de vancomycine.
Résultats:
Aucun changement notable de la couleur, du goût ou du pH n’a été observé dans les solutions de vancomycine conservées dans des godets pour dose unitaire à 4 °C ou à 25 °C ou dans des flacons de plastique à 4 °C pendant la période d’étude de 75 jours. Au 63e jour, on a observé la formation d’un précipité blanc dans les solutions conservées dans des flacons de plastique à 25 °C, mais aucun changement notable du goût ou du pH pendant la période de 75 jours. L’intervalle de confiance à 95 % de la pente de la courbe de la concentration en fonction du temps, déterminée par régression linéaire, a montré que les solutions de vancomycine préparées dans des godets ou des flacons entreposés à 4 °C conserveraient au moins 93,6 % de la concentration initiale de vancomycine pendant 75 jours et que les solutions entreposées à 25 °C conserveraient au moins 90,0 % de la concentration initiale pendant 30 jours (godets) ou 26 jours (flacons), avec un niveau de confiance de 95 %.
Conclusions:
Les solutions de vancomycine à 25 mg/mL conservées dans des godets pour dose unitaire ou des flacons de plastique à 4 °C demeuraient stables pendant au moins 75 jours, alors que celles conservées dans des godets ou des flacons à 25 °C devraient demeurer stables respectivement pendant 30 et 26 jours.
[Traduction par l’éditeur]
Keywords: vancomycine, chromatographie liquide haute performance, stabilité
INTRODUCTION
Orally administered vancomycin is indicated for the treatment of Clostridium difficile infections and is often used as the first-line agent in severe cases.1 With the rising incidence of C. difficile infection in the health care setting, usage of oral vancomycin has also increased.2 Typically, oral vancomycin is administered in capsule form. However, vancomycin in a liquid form is required for patients who have nasogastric and other feeding tubes and also for many patients in the elderly and pediatric populations.
Vancomycin as an oral solution is not available commercially in Canada or the United States. Currently, pharmacists must prepare the oral liquid extemporaneously from the injectable or oral capsule form. An unpublished survey conducted within the Fraser Health region revealed that pharmacy staff members were preparing oral vancomycin liquid from the injectable form (i.e., powder requiring reconstitution in vials) and supplying it as patient-specific prescriptions in small volumes with short expiry dates. Without published stability information, pharmacists were recommending storage in the refrigerator anywhere from 4 days to 14 days. This represented significant workload and logistic challenges in both the pharmacy and the patient care areas. From the patients’ perspective, the oral solution was very unpalatable. Although it is possible to improve palatability by mixing a dose of the drug with flavouring (syrups) or acidic juices, these would have to be added immediately before administration, which would contribute to inconvenience on the part of the patient or caregiver.3 Pharmacy staff were unable to find stability information for any palatable oral liquid formulation that would allow it to be prepared in advance and stored before administration; all existing information indicated that the sweetener had to be added immediately before administration.
This study examined the physical characteristics and chemical stability (defined as maintenance of more than 90% of initial concentration) of extemporaneously prepared vancomycin oral solution 25 mg/mL in a mixture of equal volumes of Ora-Sweet vehicle and water, stored in 2 types of containers (unit-dose polyethylene cups and plastic bottles) at 4°C and 25°C throughout a 75-day study period.
METHODS
Preparation of Vancomycin
Stock solutions of vancomycin 25 mg/mL were prepared by reconstituting commercially available vancomycin for injection (10 g/vial) (Pharmaceutical Partners of Canada, Richmond Hill, Ontario; lot 204369, expiry December 2009) with sterile water for injection (Baxter Corporation, Mississauga, Ontario; lot W9F15AO, expiry January 2011) and then diluting with a mixture of equal volumes of Ora-Sweet vehicle (Paddock Laboratories Inc, Minneapolis, Minnesota; lot 9085961, expiry February 2012) and distilled water (Ripple FX Water Inc, New Westminster, British Columbia; lot 9147102, expiry February 27, 2010). Portions of this preparation were transferred to fourteen 15-mL opaque blue polyethylene unit-dose cups with aluminum seal (Medical Packaging Inc, Ringoes, New Jersey; lot FD15MB0705) and six 50-mL amber polyvinyl chloride (recycle code 3) prescription bottles (Richards Packaging Inc, RIGO Products Division, Gloucester, Ontario). The volume dispensed was 5 mL into each unit-dose cup and 25 mL into each bottle. Half of the unit-dose cups and half of the plastic bottles were kept at room temperature (25°C) and the remainder of the containers were stored in the refrigerator (4°C).
The physical characteristics of the solutions were evaluated qualitatively at the time of preparation and at 15, 30, 40, 50, 63, and 75 days. As samples were collected throughout the study period, one individual (D.D.) measured the pH and visually examined the solutions for changes in colour against white and black backgrounds and to check for formation of precipitate. The same person (D.D.) also tasted the solutions. At each time point, 3 aliquots from one of the unit-dose cups at each temperature and 1 aliquot from each plastic bottle at each temperature were collected for determination of pH. The pH meter (model 8000, VWR, Mississauga, Ontario) was calibrated with commercially available standards at the beginning of each testing session. Immediately after the physical observations, three 1.5-mL samples from the selected unit-dose cup at each storage temperature were transferred into 2-mL polypropylene vials (Nalgene Company, Rochester, New York; lot 735276294). Similarly, one 1.5-mL sample from every bottle at both temperatures was transferred into a 2-mL polypropylene vial. As such, there were 3 samples for each combination of temperature and type of storage container on each study day. All vials were immediately stored at −85°C until analysis by a validated, stability-indicating HPLC method with ultraviolet (UV) detection.
Preparation of Stocks, Standards, and Standard Curve
Standards were prepared as follows. Vancomycin (Sigma-Aldrich, Oakville, Ontario; lot 1418285) was reconstituted to a concentration of 10 mg/mL in water and was further diluted with water to concentrations of 8, 6, 4, 3, 2, 1, and 0.5 mg/mL to allow construction of the standard curve. The internal standard metronidazole (Sigma-Aldrich, Oakville, Ontario; lot 095K0693) was prepared by dilution in water to a concentration of 1 mg/mL. The standards were prepared by combining 0.5 mL of each stock solution and 0.5 mL of metronidazole 1 mg/mL. The final concentrations of vancomycin in the standard samples injected onto the chromatograph were 4, 3, 2, 1.5, 1, 0.5, and 0.25 mg/mL. The final concentration of internal standard (metronidazole) was 0.5 mg/mL. To prevent injection of impurities onto the column, each standard was passed through a 0.45-μm microfilter before injection (Acrodisc GHP syringe filter, Gelman, Ann Arbor, Michigan; lot 21692572).
A 7-point calibration curve was prepared, with a blank (water) at the beginning of each run to prevent carry-over from one run to the next. The range of this calibration curve encompassed the diluted test concentration of vancomycin (1.25 mg/mL, as described in the next section). The calibration curve was generated by least-squares regression of the peak area ratio of vancomycin to internal standard and the concentration of each vancomycin standard. The precision of the assay was evaluated by intraday and interday validation methods. Intraday variability was determined by running stocks with concentrations of 2.5, 1.25, 0.62, and 0.31 mg/mL in quadruplicate throughout a single day, whereas interday variability was determined by running stocks with the same concentrations (as in the testing for intraday variability) in quadruplicate daily for 4 days. The means, standard deviations (SDs), coefficients of variation (CVs), and accuracy were calculated. Acceptable limits were defined a priori as up to 10% (for coefficients of variation) and 90% or above (for accuracy).
Preparation of Study Samples
The vancomycin study samples were thawed, mixed (by vortex for 10 s), and diluted to 2.5 mg/mL in HPLC-grade water (Fisher Scientific, Richmond, British Columbia; lot 092106). A 0.5-mL aliquot of each sample was then diluted with 0.5 mL of internal standard (metronidazole 1 mg/mL). The final theoretical concentrations were 1.25 mg/mL for vancomycin and 0.5 mg/mL for the internal standard. Each sample was passed through a 0.45-μm microfilter before withdrawal of a 10-μL sample for injection onto the column.
HPLC Instrumentation
The HPLC instrumentation consisted of a delivery pump, an automatic injector system equipped with a 200-mL injector, a Symmetry RP18 3.0 × 100 mm column (Waters Ltd, Mississauga, Ontario; lot W13541F-011) with a Symmetry RP18 3.9 × 20 mm guard column (Waters Ltd; lot 239392051) and a UV detector set at 247 nm. The mobile phase consisted of a 26:74 (v/v) mixture of methanol (Fisher Scientific, Richmond, British Columbia; lot 092106) and a 0.01 mol/L solution of ammonium acetate (Fluka-Sigma-Aldrich, Oakville, Ontario; lot 1386428) at pH 3.0. All solvents were HPLC grade, and all were filtered before use. The flow rate was set at 1.0 mL/min. The assay was developed in the authors’ laboratory on the basis of previous published work.4–8
Accelerated Degradation of Vancomycin
A solution of vancomycin 25 mg/mL (in water) was prepared, mixed with 1N sodium hydroxide, adjusted to a final vancomycin concentration of 2.5 mg/mL (and 0.1N sodium hydroxide), and boiled for 10 min. Another vancomycin solution, in Ora-Sweet and water, was incubated at 100°C for 18 h. After the samples had cooled to room temperature, a 0.5 mL aliquot from each was combined with an equal volume of internal standard, and the combined solution was filtered and injected onto the column. The chromatograms obtained for the preparations of degraded vancomycin were compared with chromatograms obtained from the standard curve to determine any changes in concentration, retention time, and peak shape.
Statistical Analysis
The means, SDs, and CVs were calculated for samples analyzed in triplicate or quadruplicate, as applicable. For each study day, the percentage of initial vancomycin concentration remaining was calculated for each sample. Stability was defined as maintenance of at least 90% of the initial vancomycin concentration. The percentage of vancomycin remaining at 75 days was calculated from the concentration at 75 days, as determined by linear regression, and the concentration observed on day 0, according to the following formula: concentration at 75 days ÷ concentration on day 0 × 100%. The 95% confidence interval (CI) of the amount remaining on the last study day (day 75) was calculated from the lower limit of the 95% CI of the slope of the curve relating concentration to time, determined by linear regression, via computer analysis, according to the following formula: lower limit of the 95% CI of the concentration at 75 days ÷ concentration on day 0 × 100%. If the percentage remaining at 75 days was not at least 90% of the initial concentration, then the time during which the solution would maintain at least 90% of its initial concentration was calculated according to the following formula: 10 ÷ lower limit of the 95% CI of the slope of the curve relating concentration to time (determined by linear regression).
RESULTS
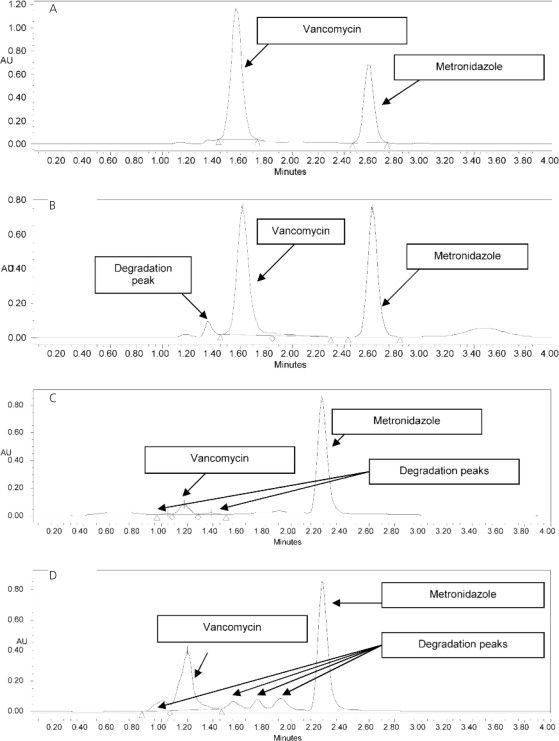
Regression analysis of the peak area ratios of vancomycin to internal standard versus vancomycin standard concentrations demonstrated linearity over the range of the concentrations, with coefficient of determination (r2) greater than 0.998 (n = 4). The intraday and interday CVs were less than 10% and therefore within acceptable limits: 2.7% and 5.5%, respectively, at 0.31 mg/mL; 7.22% and 7.37%, respectively, at 0.62 mg/mL; 2.17% and 8.76%, respectively, at 1.25 mg/mL; and 0.70% and 5.24%, respectively, at 2.5 mg/mL. The intraday and interday accuracies were at least 90% and therefore also within acceptable limits: 96.19% and 99.77%, respectively, at 0.31 mg/mL; 92.22% and 92.22%, respectively, at 0.62 mg/mL; 92.13% and 92.20%, respectively, at 1.25 mg/mL; and 94.57% and 96.17%, respectively, at 2.5 mg/mL. The lower limit of quantitation was 0.25 mg/mL, and the lower limit of detection was 0.0025 mg/mL. The retention times were 1.57 min for vancomycin and 2.59 min for metronidazole (Figures 1A and 1B).
Figure 1.
A: Chromatogram of a sample obtained at time 0 and stored for 85 days at −85°C shows vancomycin peak at 1.57 min and internal standard (metronidazole) peak at 2.59 min. B: Chromatogram of a sample obtained on day 75 of storage at room temperature and then stored for 10 days at −85°C shows an additional small degradation peak at 1.35 min. C: Chromatogram of vancomycin solution (in water) after degradation with 1N sodium hydroxide and boiling for 10 minutes shows 2 noninterfering peaks at 1.04 min and 1.38 min and a 93% reduction in the vancomycin peak. D: Chromatogram of vancomycin solution (in Ora-Sweet vehicle and water) after degradation by incubation at 100°C for 18 h shows 4 noninterfering peaks at 1.00, 1.48, 1.75, and 1.89 min and a 58% reduction in the vancomycin peak. AU = absorbance units.
When the first vancomycin solution (in water) was subjected to degradation, the vancomycin peak was reduced by 93% relative to the standard curve, and non-interfering peaks were detected at 1.04 and 1.38 min (Figure 1C). When the vancomycin solution in Ora-Sweet and water was subjected to degradation, the vancomycin peak was reduced by 58%, and non-interfering peaks were detected at 1.00, 1.48, 1.75, and 1.89 min (Figure 1D). Thus, the HPLC method was deemed capable of indicating stability.
There were no notable changes in colour, taste, or pH for vancomycin solutions stored in unit-dose cups at 4°C and 25°C or in plastic bottles at 4°C throughout the 75-day period. In the vancomycin solutions stored in plastic bottles at 25°C, a white precipitate was observed starting on day 63, but there were no notable changes in taste or pH during the 75-day period. On day 0, the mean pH ± SD was 3.69 ± 0.006 for solutions in unit-dose cups to be stored at 4°C, 3.67 ± 0.006 for solutions in unit-dose cups to be stored at 25°C, 3.69 ± 0.010 for solutions in plastic bottles to be stored at 4°C, and 3.68 ± 0.012 solutions in plastic bottles to be stored at 25°C. On day 75, the mean pH ± SD was 3.85 ± 0.117 for solutions stored in unit-dose cups at 4°C, 4.06 ± 0.219 for solutions stored in unit-dose cups at 25°C, 3.82 ± 0.156 for solutions stored in plastic bottles at 4°C, and 4.02 ± 0.193 solutions stored in plastic bottles at 25°C. The solutions had a sweet-and-sour taste throughout the period, with subjective palatability (based on a single taster) of 3.5 out of 5 points.
The 95% CI of the slope determined by linear regression indicated that all vancomycin solutions stored in unit-dose cups or plastic bottles at 4°C would maintain at least 93.6% of the initial vancomycin concentration for 75 days (Table 1). However, the solutions stored in unit-dose cups and plastic bottles at 25°C were predicted to maintain only 74.6% and 71.0%, respectively, of the initial concentration on day 75 (Table 2). On the basis of the lower limit of the 95% CI, vancomycin solutions stored at room temperature in unit-dose cups or plastic bottles were predicted to maintain at least 90.0% of initial concentration for 30 and 26 days, respectively, with 95% confidence (Table 2).
Table 1.
Vancomycin Remaining after up to 75 Days of Storage at 4°C in Unit-Dose Cups and in Plastic Bottles
|
Storage Container; Mean Concentration ± SD (% of Original)† |
||||
|---|---|---|---|---|
| Study day | Unit-Dose Cups | Plastic Bottles | ||
| Initial concentration* (mg/mL) | 24.69 ± 0.170 | (100.0) | 25.77 ± 1.028 | (100.0) |
| 15 | 25.49 ± 1.247 | (103.2) | 25.73 ± 1.899 | (99.8) |
| 30 | 25.77 ± 0.705 | (104.4) | 24.65 ± 2.232 | (95.7) |
| 40 | 24.86 ± 0.612 | (100.7) | 25.35 ± 1.331 | (98.4) |
| 50 | 25.11 ± 0.960 | (101.7) | 25.41 ± 0.910 | (98.6) |
| 63 | 24.62 ± 0.747 | (99.7) | 25.11 ± 0.615 | (97.4) |
| 75 | 25.03 ± 0.620 | (101.4) | 26.37 ± 0.791 | (102.3) |
| % remaining on day 75 by linear regression‡ | 99.0 | 100.9 | ||
| Lower limit of 95% CI for % remaining§ | 93.6 | 94.0 | ||
CI = confidence interval, SD = standard deviation.
Nominal concentration was 25 mg/mL.
Measured concentration, reported as mean ± standard deviation of 3 replicate analyses of 3 samples, with percent of initial (measured) concentration in parentheses.
Calculated from concentration at day 75 as determined by linear regression and concentration observed at time 0, according to the following formula: concentration at day 75 ÷ concentration at time 0 x 100.
Calculated from lower limit of 95% CI of the slope of the curve relating concentration to time, determined by linear regression, according to the following formula: lower limit of 95% CI of concentration at day 75 ÷ concentration at time 0 x 100.
Table 2.
Vancomycin Remaining after up to 75 Days of Storage at 23°C in Unit-Dose Cups and in Plastic Bottles
|
Storage Container; Mean Concentration ± SD (% of Original)† |
||||
|---|---|---|---|---|
| Study day | Unit-Dose Cups | Plastic Bottles | ||
| Initial concentration* (mg/mL) | 25.23 ± 1.029 | (100.0) | 24.74 ± 0.817 | (100.0) |
| 15 | 24.96 ± 0.203 | (98.9) | 25.16 ± 1.686 | (101.7) |
| 30 | 25.13 ± 0.491 | (99.6) | 23.43 ± 0.597 | (94.7) |
| 40 | 25.63 ± 1.231 | (101.6) | 22.69 ± 0.647 | (91.7) |
| 50 | 25.45 ± 0.450 | (100.9) | 23.02 ± 0.642 | (93.0) |
| 63 | 22.65 ± 0.516 | (89.8) | 22.71 ± 1.107 | (91.8) |
| 75 | 21.95 ± 0.333 | (87.0) | 19.69 ± 1.146 | (79.6) |
| %% remaining on day 75 by linear regression‡ | 87.9 | 81.9 | ||
| Lower limit of 95% CI for % remaining§ | 74.6 | 71.0 | ||
| Time predicted to maintain at least 90.0% of initial concentration¶ | 30 days | 26 days | ||
CI = confidence interval, SD = standard deviation.
Nominal concentration was 25 mg/mL.
Measured concentration, reported as mean ± standard deviation of 3 replicate analyses of 3 samples, with percent of initial (measured) concentration in parentheses.
Calculated from concentration at day 75 as determined by linear regression and concentration observed at time 0, according to the following formula: concentration at day 75 ÷ concentration at time 0 x 100.
Calculated from lower limit of 95% CI of the slope of the curve relating concentration to time, determined by linear regression, according to the following formula: lower limit of 95% CI of concentration at day 75 ÷ concentration at time 0 x 100.
Calculated according to the following formula: 10 ÷ lower limit of the 95% CI of the slope of the curve relating concentration to time (determined by linear regression).
DISCUSSION
The lack of a commercially available vancomycin solution for oral administration poses problems for adults and children who are unable to swallow capsules. Until the time of this study, vancomycin oral liquid was prepared on an “as needed” basis by the pharmacy staff at Fraser Health. These solutions were given short expiration dates, and refrigeration was specified for storage. To the authors’ knowledge, there are no published stability studies for vancomycin oral solutions prepared in equal volumes of Ora-Sweet and distilled water.
In the serial analysis of samples reported here, no notable changes in colour, taste, or pH were observed for vancomycin solutions stored in unit-dose cups at 4°C and 25°C and in plastic bottles at 4°C throughout the 75-day period. A white precipitate was observed starting on day 63 in the plastic bottles stored at 25°C, but there were no notable changes in taste or pH for these samples during the 75-day period. Although the measures of physical characteristics (aside from pH) were qualitative, all observations were documented by the same individual throughout the study period, which eliminated interobserver bias.
On the basis of the 95% CI of the slope determined by linear regression, it is predicted that all vancomycin solutions stored in cups or bottles at 4°C will maintain at least 93.6% of their initial vancomycin concentration for 75 days, whereas solutions stored at 25°C will maintain at least 90.0% of their initial concentration for 30 days (cups) and 26 days (bottles), with 95% confidence.
The information from this study will allow for a variety of storage and distribution scenarios. Furthermore, vancomycin liquid prepared from the injectable form is less expensive than vancomycin solutions prepared from capsules. Specifically, the expiry date of 30 days for vancomycin oral liquid at room temperature is expected to significantly change manufacturing, storage, and distribution practices at the authors’ institution. Packaging vancomycin in the unit-dose cups will result in further improvements in patient safety, quality control, and operational efficiency.
One limitation of this study relates to freezing of the samples at −85°C until the time of batch analysis. It was assumed that vancomycin degradation would not occur at this low temperature and that there would be no volume losses due to freeze-drying during storage. It was also assumed that errors due to serial analysis would be greater than any errors associated with batch analysis. Furthermore, time 0 samples stored for 85 days at −85°C showed no degradation products and contained the expected concentration of vancomycin 25 mg/mL (see Figure 1A), yet samples stored for a shorter period of time at room temperature (e.g., 75 days) showed an additional degradation peak (see Figure 1B). These contrasting results provide evidence that storage at −85°C had no effect on the estimate of stability of vancomycin.
Before the study began, several recipes were tried, with various combinations of Ora-Plus, Ora-Sweet SF (sugar-free), and Ora-Sweet (all produced by Paddock Laboratories). The first 2 of these products caused clumping and were therefore abandoned in favour of Ora-Sweet. This sweetening vehicle was used in the current recipe because it not only dissolves well with reconstituted vancomycin for injection and distilled water but also improves the taste.
CONCLUSIONS
According to serial qualitative, pH, and HPLC analyses, vancomycin solutions 25 mg/mL stored in unit-dose cups or plastic bottles at 4°C were stable over a 75-day period, whereas those stored at 25°C are expected to be stable for up to 30 days (cups) and 26 days (bottles).
Acknowledgments
We thank Dr Shallen Letwin, Linda Morris, Holly Gibson, and Sherry Moseanko for their assistance with project logistics.
References
- 1.Gerding DN, Muto CA, Owens RC., Jr Treatment of Clostridium difficile infection. Clin Infect Dis. 2008;46(Suppl 1):S32–S42. doi: 10.1086/521860. [DOI] [PubMed] [Google Scholar]
- 2.Henrick TJ, Krakower D, Bitton A, Yokoe DS. Clinical risk factors for severe Clostridium difficile–associated disease. Emerg Infect Dis. 2009;15(3):415–422. doi: 10.3201/eid1503.080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaczowka R, editor. Alberta Health Services – Calgary Health Region pharmacy compounding manual. Calgary (AB): Alberta Health Services, Calgary Pharmacy; 2009. Vancomycin; p. 227. [Google Scholar]
- 4.Wood MJ, Lund R, Beavan M. Stability of vancomycin in plastic syringes measured by high performance liquid chromatography. J Clin Pharmacol Ther. 1995;20(6):319–325. doi: 10.1111/j.1365-2710.1995.tb00705.x. [DOI] [PubMed] [Google Scholar]
- 5.Galanti LM, Hecq JD, Vanbeckbergen D, Jamart J. Long term stability of vancomycin hydrochloride in intravenous infusions. J Clin Pharmacol Ther. 1997;22(5–6):353–356. doi: 10.1111/j.1365-2710.1997.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 6.de Jesus-Valle MJ, Gonzales-Lopez F, Snachez-Navarro A. Development and validation of an HPLC method for vancomycin and its application to a pharmacokinetic study. J Pharm Biomed Anal. 2008;48(3):835–839. doi: 10.1016/j.jpba.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 7.Ensom MHH, Decarie D, Leung K, Montgomery C. Stability of hydromorphone–ketamine solutions in glass bottles, plastic syringes, and IV bags for pediatric use. Can J Hosp Pharm. 2009;62(2):112–118. doi: 10.4212/cjhp.v62i2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trissel LA, Xu QA, Zhang Y, Saenz CA, Ingram DS. Stability of ciprofloxacin and vancomycin hydrochloride in auto dose infusion system bags. Hosp Pharm. 2001;36(11):1170–1173. [Google Scholar]