Abstract
Background
Generalized Social Phobia (GSP) involves the fear/avoidance of social situations while Generalized Anxiety Disorder (GAD) involves an intrusive worry about everyday life circumstances. It remains unclear whether these, highly comorbid, conditions represent distinct disorders or alternative presentations of a single underlying pathology. In this study, we examined stimulus-reinforcement based decision-making in GSP and GAD.
Methods
Twenty unmedicated patients with GSP, sixteen unmedicated patients with GAD and nineteen age, IQ, and gender matched healthy comparison individuals completed the Differential Reward/ Punishment Learning Task (DRPLT). In this task, the subject chooses between two objects associated with different levels of reward or punishment. Thus, response choice indexes not only reward/ punishment sensitivity but also sensitivity to reward/ punishment level according to between-object reinforcement distance.
Results
We found that patients with GAD committed a significantly greater number of errors compared to both the patients with GSP and the healthy comparison individuals. In contrast, the patients with GSP and the healthy comparison individuals did not differ in performance on this task.
Conclusions
These results link GAD with an anomalous non-affective based decision-making. Further, they are indicative that GSP and GAD are associated with distinct pathophysiologies.
INTRODUCTION
Generalized social phobia (GSP) and generalized anxiety disorder (GAD) are two highly prevalent anxiety disorders that are characterized by significant chronicity and disability (Heinrichs and Hofmann, 2001, Schneier, 2003, Stein and Kean, 2000). They are also significantly co-morbid. GSP involves the fear/ avoidance of social situations whereas GAD involves intrusive and largely uncontrollable worry about everyday life circumstances. While both have considerable social and economic costs, relatively little is known about their neurobiological basis (Charney, 2004, Mathew et al., 2001, Stein et al., 2002). In particular, it remains unclear whether the two disorders are associated with overlapping or distinct neurocognitive impairments. Thus, the high comorbidity indicated by cross-sectional and longitudinal studies indicate a relatively subtle distinction between the two disorders at the descriptive level (Bruce et al., 2001, Pine et al., 1998). Conversely, the relative specificity indicated in family aggregation (Coelho et al., 2007), and pharmacological response (Blanco et al., 2003, Liebowitz et al., 2005, Rickels and Rynn, 2002) in the two disorders suggest dissociation. However, no study has directly compared the response to non-social affective stimuli in GSP and GAD.
There has been some work examining the response to social affective stimuli in the two disorders. This work has linked GSP with an atypical response to social stimuli. At the neural level GSP has been linked with atypical responses in regions including the amygdala to facial expression stimuli (Birbaumer et al., 1998, Blair et al., 2008b, Phan et al., 2006, Straube et al., 2004), public speeches (Lorberbaum et al., 2004), and self-referential criticism (Blair et al., 2008a). Behaviorally, patients with social phobia display a pattern of hypervigilance to (Coles and Heimberg, 2005, Mogg et al., 2004) and avoidance of (Chen et al., 2002, Mogg et al., 1987) socially threatening stimuli. Relatively little work has examined the response to social stimuli in GAD. However, this work has similarly indicated some circuitry anomalies, implicating in particular prefrontal gyrus (Blair et al., 2008b, Hoehn-Saric et al., 2004, Monk et al., 2006, Wu et al., 1991). Importantly, a handful of neuroimaging and behavioral studies have recently directly compared the two disorders’ response to social affective stimuli (Becker et al., 2001, Blair et al., 2008b, Turk et al., 2005). These studies have all shown differential responses in GSP and GAD, suggesting that the two disorders might be associated with dissociable neurocognitive profiles for social processing.
Little work has examined the response to non-social affective stimuli in the two disorders. In particular, little focus has been given to non-social processing in GSP. However, one study reported impaired aversive conditional discrimination ability in patients with social phobia during an eyelid conditional discrimination learning task (Sachs et al., 2003). Patients with GAD for their part report chronic, uncontrollable worry even in the absence of specific threats (Borkovec et al., 1991, Brown et al., 1994, Craske et al., 1989) and it is possible that this constant worry can negatively impact upon reward and punishment based decision-making abilities. In line with this, there is data suggesting that patients with GAD report lower levels of problem-solving confidence than comparison individuals (although the empirical data on this is mixed, see Roemer et al., 2002). Given this, it would be interesting to determine whether patients with GSP and GAD show impairment during stimulus-reinforcement based decision-making. Performance on the paradigm chosen, the differential reward/ punishment learning (DRPLT) task (Blair et al., 2006a, Blair et al., 2006b) is impaired in individuals with psychopathy, shown to be impaired in stimulus-reinforcement learning in the context of aversive conditioning tasks as well as other stimulus-reinforcement based decision-making tasks such as the passive avoidance learning paradigm (e.g., Newman and Kosson, 1986). Moreover, fMRI work (Blair et al., 2006b) has shown that the task recruits ventromedial prefrontal cortex (vmPFC), a region previously implicated in the representation of reinforcement information. In addition, this fMRI work showed that decision conflict (between-object reinforcement distance) is associated with activity in dorsomedial prefrontal cortex, a region implicated in conflict resolution in other paradigms (see e.g., Botvinick et al., 2004). Importantly, these regions of prefrontal cortex may be implicated in the pathophysiology of GAD (Blair et al., 2008b, Nutt, 2001, Stein et al., 2002, Whalen et al., 2007).
While GSP often presents as an isolated condition, GAD typically is seen with comorbid anxiety disorders, particularly GSP, complicating attempts to compare the two disorders. Prior studies from our group have combined subjects with GAD who are comorbid for GSP and subjects with GAD who are not comorbid for GSP in a single group (Blair et al., 2008b, McClure et al., 2007, Monk et al., 2006). These prior studies have informed our decisions about classification in the current study where subjects were separated into three groups: (1) Patients with GSP but no other current anxiety disorder; (2) patients with GAD including those with or without GSP (referred to in the paper as patients with GAD); and (3) healthy comparison (HC) individuals. This patient-grouping scheme in the current and prior studies reflects our hypothesis that the presentation of GSP in the context of co-occuring GAD reflects a process whereby generalized worry extends to social settings, distinct from the development of isolated or “pure” GSP, in the context of minimal worries in non-social settings.
By comparing the performance of patients with GSP, GAD and HC individuals on the DRPL task (Blair et al., 2006a, Blair et al., 2006b) we tested our primary hypothesis: patients with GAD exhibit distinct, atypical responding from patients with GSP and HC individuals during reward and punishment based decision-making.
METHODS AND MATERIALS
Subjects
20 patients with GSP, 16 patients with GAD, and 19 HCs participated in the study. Samples were group-matched on age, gender, and IQ as assessed by the matrix reasoning and verbal subtests of the Wechsler Abbreviated Scale of Intelligence (WASI) (see Table 1). Subjects in the GSP patient group met the criteria for generalized social phobia, and the subjects in the GAD group met the criteria for generalized anxiety disorder according to the DSM-IV (1994) criteria based on the Structural Clinical interview for DSM-IV Axis I disorders (SCID) (First et al., 1997) and an confirmative interview by a board-certified psychiatrist (DSP). All patients with any other co-occurring anxiety disorder were excluded, as were patients with severe major depressive disorder (MDD). Other patients with MDD were included if the evaluating psychiatrist determined the severity of the MDD to be milder than that of the anxiety disorder, such that the anxiety disorder was deemed the primary condition in need of clinical attention. Only one of 36 patients exhibited comorbid MDD. As in our prior work, the GAD group (N=16) was made up of patients with GAD who were comorbid for GSP (N=9) as well as “pure cases” who were not (N=7) (Blair et al., 2008b, McClure et al., 2007, Monk et al., 2006). Beyond one MDD case, in the GSP group, none of the 35 other patients had any other current Axis 1 diagnosis.
Table 1.
Subject Characteristics: Standard Error in parenthesis.
| GSP (n=20) | GAD (n=16) | HC (n=19) | P | ||
|---|---|---|---|---|---|
| Age (SE; range) | 35.3 (2.45; 20–51) | 41.6 (2.75; 22–55) | 34.1 (2.52; 23–61) | 0.11 | |
| Gender | 9M/11F | 7M/9F | 6M/13F | 0.70 | |
| Race | |||||
| Caucasian | 14 | 13 | 15 | ||
| African-American | 3 | 2 | 4 | ||
| Asian | 3 | 1 | - | ||
| IQ | 121.8 (2.70) | 114.1 (3.02) | 119.6 (2.77) | 0.16 | |
| LSAS | 68.6 (5.17) | 47.6 (6.79) | 19.6 (3.99) | 0.000 | |
| STAI-T | 49.0 (.60) | 47.7 (2.37) | - | - | |
| BAI | 8.7 (1.57) | 7.9 (1.34) | 2.6 (0.60) | 0.002 | |
| IDS | 14.4 (1.97) | 16.1 (1.83) | 7.1 (1.02) | 0.001 | |
Key to Table 1: M = Male; F = Female; LSAS = Liebowitz Social Anxiety Scale; STAI-T = The Spielberger Trait Anxiety Inventory; BAI = Beck’s Anxiety Inventory; IDS = Inventory of Depressive Symptoms.
All subjects were required to be currently medication-free (no regular use of psychotropic medication within 2 weeks or fluoxetine/ benzodiazepine within 8 weeks of the study). Comparison individuals were excluded if they had current/past history of any psychiatric illness. All subjects were in good physical health, as confirmed by a complete physical exam. Subjects provided written informed consent for participation in the study. The consent forms were approved by the NIMH IRB. The patients with GSP and GAD reported significantly greater depression, social anxiety and general anxiety than the HC individuals1. However, the patient groups did not differ on these measures (see Table 1).
The Differential Reward/ Punishment Learning Task (DRPLT)
The DRPLT consists of 10 images each depicting a different object (house, cup, fork, duck, pineapple, necklace, raccoon, door, torch, or shoe) from the Snodgrass and Vanderwart picture set (Snodgrass and Vanderwart, 1980). To prevent systematic task interference from any existing valence attached to the objects (e.g., pineapple might have a preexisting positive valence), each object was randomly assigned a value automatically by the E-Prime program running the task (−900, −700, −500, −300, −100, 100, 300, 500, 700, or 900) before the subject began (i.e., not every subject received 900 points for choices of the shoe).
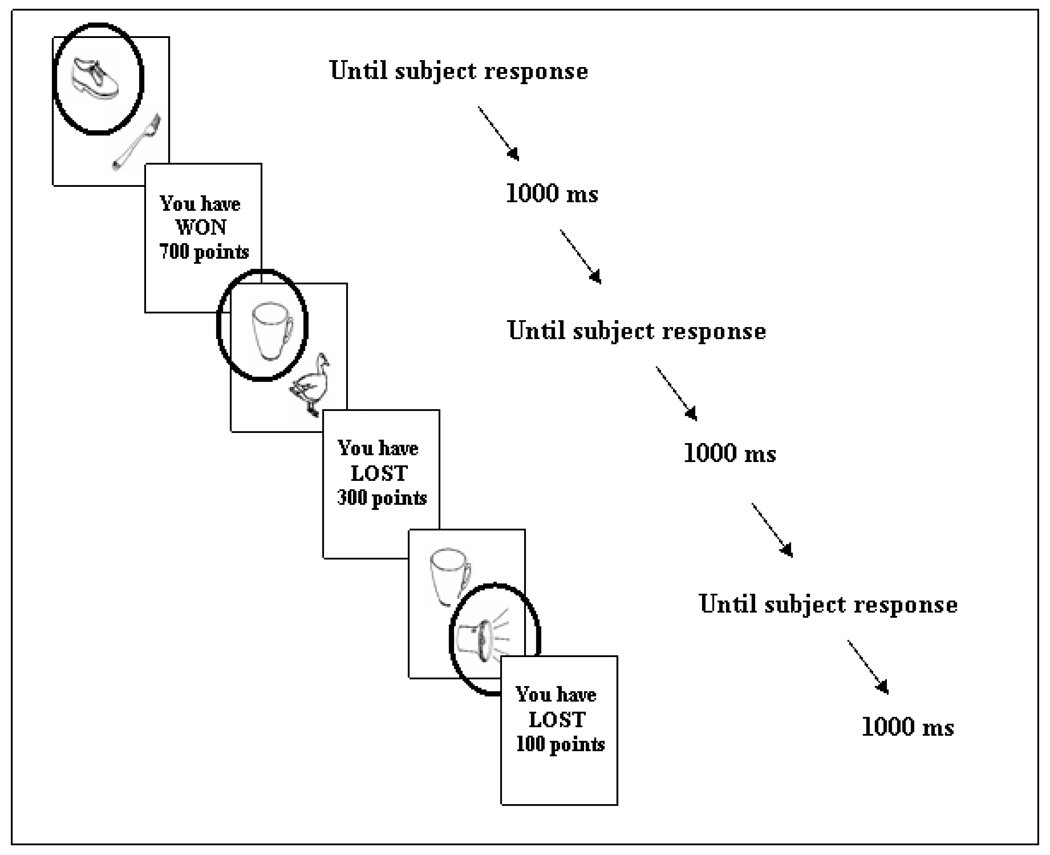
During the task, the objects were presented together in pairs, appearing in two of four different locations positioned around the middle of the screen. At the viewing distance of 60 cm, the visual angle was 10 arc min horizontally and 12 arc min vertically. The subject was told that on each trial one of the two objects must be chosen, and that some objects would result in losing points and that some objects would result in winning points. Following object selection (with the click of a mouse) its assigned value was revealed. Thus, choosing the object assigned the value 100 resulted in the feedback: ‘You have WON 100 points’. Conversely, choosing the object assigned the value –100, resulted in the feedback: ‘You have LOST 100 points’. Feedback stayed on the screen for 1000 ms and was then replaced by the two objects for the subsequent trial (see Figure 1 for session diagram). There was no time limit for making a response. At the end of the study subjects were told how many points they had accumulated.
Figure 1.
Session diagram. In this example, the randomly assigned values for the shoe, cup, and torch are 700, −300, and −100 respectively. Circle indicates that object was selected.
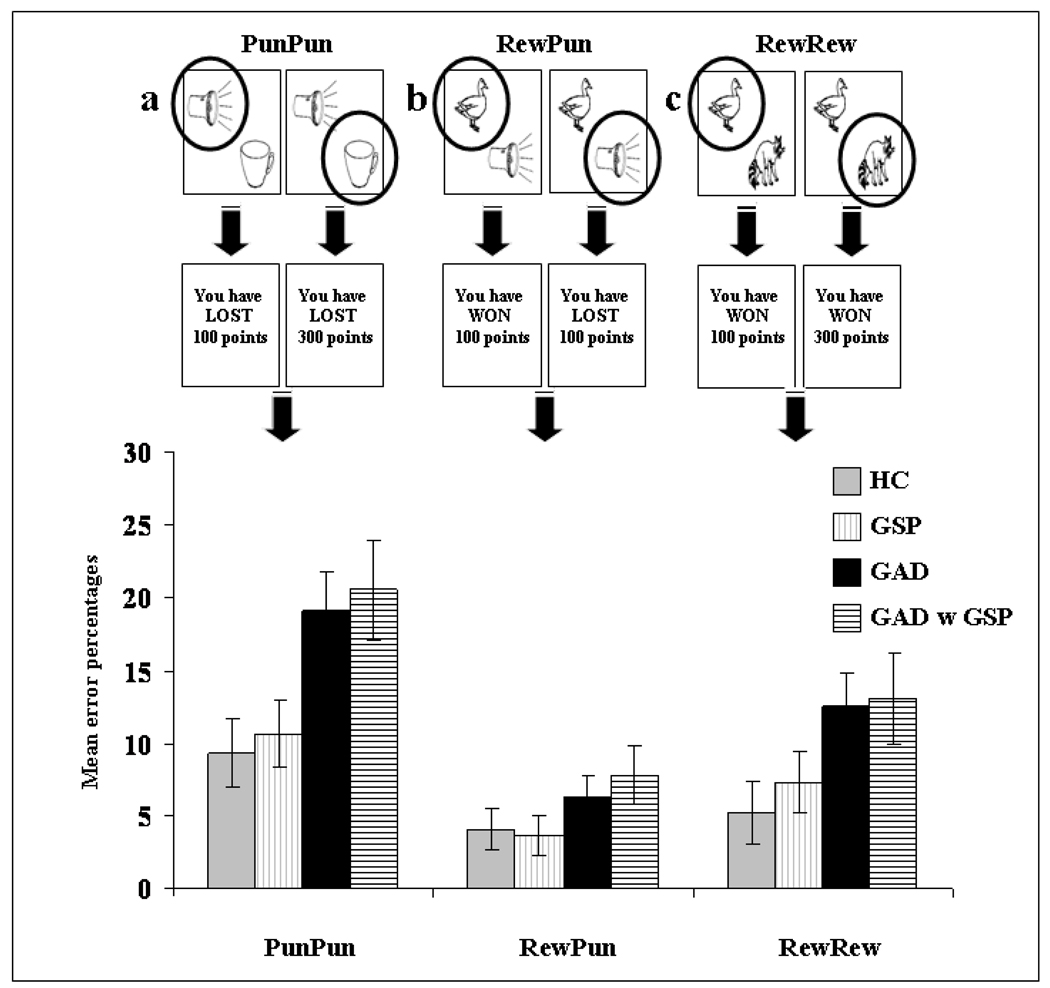
All objects were paired with each other, leading to three task conditions: Reward/ Reward (RewRew), Punishment/ Punishment (PunPun) and Reward/ Punishment (RewPun). In the RewRew condition, both objects were associated with reward. In the PunPun condition, both objects were associated with punishment. Finally, in the RewPun condition, one object was associated with reward and one object was associated with punishment; see Figure 2.
Figure 2.
Breakdown of group performance by decision form (a) PunPun; (b) RewPun, and (c) RewRew. The patients with GAD committed significantly more errors across decision form than the GSP and HC groups. There was no significant group by decision form interaction. Error bars represent standard error.
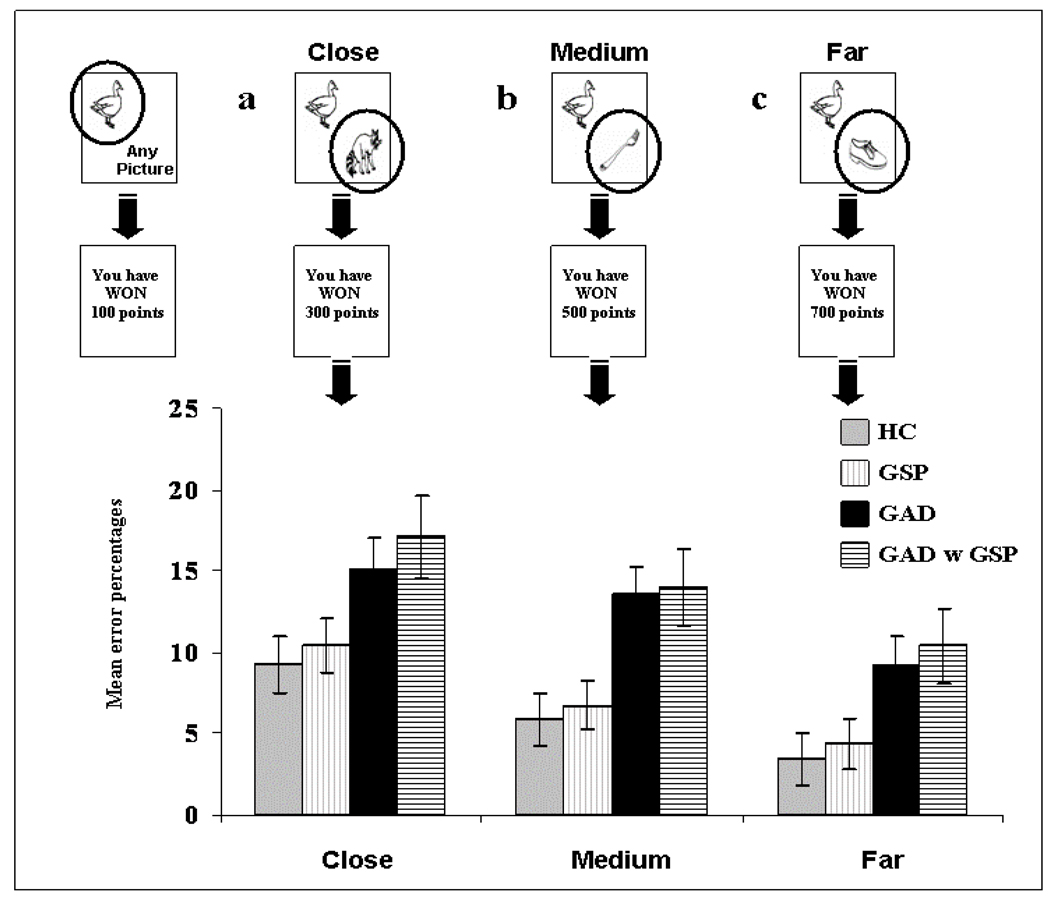
In addition to the three overall task conditions, the task also involved different between-object reinforcement distances (e.g., 100 vs. 300; 100 vs. 900). The point distance was divided into three different between-object reinforcement distances: Close, Medium, Far. So, for example, the “close” between-object reinforcement distance trials involved two objects associated with values that were close together in value (e.g., [−900 v −700] [900 v 700] [100 v −100]. In contrast, the “far” between-object reinforcement distance trials involved two objects associated with values that were far apart in value (e.g., [−900 v −100] [900 v 100] [900 v −900]); see Figure 3. The task therefore involved a 3 (Group: GSP, GAD, HC) by 3 (Decision form: RewRew, PunPun, RewPun) by 3 (Distance: Close, Medium, Far) design. The task was programmed in VisualBasic and presented on a Dell Inspiron 4150 laptop.
Figure 3.
Breakdown of group performance by between-object reinforcement distance (a) Close; (b) Medium, and (c) Far. The patients with GAD committed significantly more errors across distances than the GSP and HC groups. There was no significant group by distance interaction. Error bars represent standard error.
On any trial, choosing the superior object over the more inferior object was scored as ‘correct’. Thus, on RewRew trials where both objects represented a gain (e.g., 100 and 300), choosing the object representing the greater gain of 300 would be correct. On PunPun trials where both objects represented a loss (e.g., −100 and −300), choosing the object representing the smaller loss of −100 would be correct. Finally, on RewPun trials where one object represented a gain and one a loss (e.g., −100 and 100), choosing the object representing a gain of 100 would be correct. The data points pertaining to the first time any object was presented were excluded from the analysis. As there were more RewPun trials than RewRew and PunPun trials, subjects’ error scores were converted into error percentages as a function of the number of trials within each of the three decision forms.
Procedure
Each subject was tested individually in a quiet interview room. Each subject was presented with the DRPLT within a larger neurocognitive test battery. The duration of the DRPLT was approximately 22 min for each subject.
RESULTS
A 3 (Group: GSP; GAD; HC) by 3 (Decision form: RewRew; PunPun; RewPun) by 3 (Distance: Close; Medium; Far) ANOVA was conducted on the data. Critically, the main effect of group was significant (F(2,52)=4.70; p<0.025; η2p=0.15). Patients with GAD committed significantly more errors on this task relative to the patients with GSP and the HCs (p<0.025 & 0.01 respectively). However, the patients with GSP and HCs did not differ significantly (F<1; ns); (M[GAD]=12.65; s.e.=1.65), (M[GSP]=7.19; s.e.=1.48), (M[HC]=6.21; s.e.=1.52); see Figures 2 and 3.
There was also a significant main effect of decision form (F(2,104)=19.48; p<0.001; η2p=0.27). Subjects, as a whole, committed a significantly greater number of errors on PunPun trials relative to RewRew trials and on RewRew trials relative to RewPun trials (p<0.01 & 0.05 respectively); (M[PunPun]=13.03; s.e.=1.39), (M[RewRew]=8.36; s.e.=1.26), (M[RewPun]=4.66; s.e.=0.83). The main effect of distance was also significant (F(2,104)=43.00; p<0.001; η2p=0.45), with the linear contrast being significant (F(1,52)=89.93; p<0.001; η2p=0.63); the number of errors increased as the between-object reinforcement distance decreased. Subjects made significantly more errors on decisions close, relative to medium in distance, and on decisions medium, relative to far in distance (p<0.001 for both); (M[close]=11.61; s.e.=1.02), (M[medium]=8.74; s.e.=0.94), (M[far]=5.70; s.e.=0.94). However, there was no significant decision form by group, distance by group, or decision by distance by group interaction.
Patients with GAD and GSP
Nine of the patients with GAD also presented with GSP. The error rates for these 9 patients are also depicted in Figures 2 and 3. As can be seen, their data parallels that shown by the patients with GAD rather than those with GSP. To confirm this impression, we conducted a second 2 (Group: GAD with GSP; GSP) by 3 (Decision form: RewRew; PunPun; RewPun) by 3 (Distance: Close; Medium; Far) ANOVA. The results of this ANOVA were consistent with the results from our main ANOVA. Thus, as before the main effect of group was significant (F(1,27)=5.29; p<0.05; η2p=0.16), with the patients with GAD with GSP committing significantly more errors on this task relative to the patients with GSP only. In addition, as before there was no significant decision form by group, distance by group, or decision by distance by group interaction; see Figures 2 & 3.
Stratification of performance by group
We performed a preliminary stratification of performance by group to determine whether the patients with GSP as a group were more likely to show notably impaired performance (defined as a total error rate greater than 1 sd above the HC mean) and less likely to show superior performance (defined as a total error rate less than 1 sd above the HC mean). This revealed that in contrast to 11% of the HCs and 15% of the patients with GSP, 31% of the patients with GAD showed notably impaired performance on the task. Moreover, in contrast to 32% of the HCs and 30% of the patients with GSP only 12.5% of the patients with GAD showed superior performance on this task.
Multiple regression analyses examining potential relationships between individual subject variables and DRPT total score
An initial multiple regression analysis was conducted to evaluate whether there was a relationship between the individual subject variables and total score on the DRPT. The predictor variables were: age, gender, IQ as well as scores on the BAI, IDS and LSAS while the criterion variable was total score on the DRPT. This multiple regression revealed a weak trend for a linear relationship between the criterion variable and the entire set of predictor variables, F(6,54)=1.95; p<.01. The sample multiple correlation coefficient was 0.202. Just under 10% of the variance in total score on the DRPT was accounted for by these variables. However, with the exception of IQ, none of these variables had a significant relationship with DRPT total score. In contrast, a second multiple regression analysis examining only IQ and group revealed a significant linear relationship between total score on the DRPT and these two predictor variables, F(6,54)=7.44: p<.001. The sample multiple correlation coefficient was 0.222; 19.3% of the variance in total score on the DRPT was accounted for by these variables. Moreover, both of these variables had a significant relationship with DRPT total score (p<0.02 in both cases).
Correlational analyses examining potential relationships between depressive symptomatology and DRPT scores
Correlational analyses were used to examine the relationship between level of depressive symtomatology as assessed by scores on the IDS and performance on the DRPLT in the GAD group. There was no significant correlation between IDS and total DPRLT score (rPearson’s=.328; p=215). In addition, there was no significant correlation between IDS score and performance on the DRPLT subcomponents: RewRew, PunPun, and RewPun (rPearson’s range=.209–.279; p range=.294–.364), indicating that level of depressive symptomatology (at least in this sample) does not modulate performance on the DRPLT.
DISCUSSION
The current study compared stimulus-reinforcement based decision-making in GSP, GAD and HC individuals. The patients with GAD committed significantly more errors on our reward and punishment based decision-making task across all trial conditions, relative to patients with GSP and HC individuals. In contrast, there were no significant performance differences between patients with GSP and HC individuals.
Recent studies have indicated that GSP and GAD might be associated with differential responses to social affective stimuli (Becker et al., 2001, Blair et al., 2008b, Turk et al., 2005). In contrast, the response in GSP and GAD to non-social affective stimuli has not been directly compared. However, the observation of significant impairment in the patients with GAD on the current reward and punishment based decision-making task is consistent with previous self reports of poor problem orientation and pathological “conceptual planning” in patients with GAD (see Roemer et al., 2002). As can be seen in Figures 2 and 3, the patients with GAD made more errors in the current study relative to patients with GSP and HCs across decision forms and between-object reinforcement distances. Previous work with the current task has suggested that it is reliant on appropriate representation of reinforcement information in ventromedial prefrontal cortex and the recruitment of dorsal anterior cingulate cortex to resolve response conflicts engendered by different reinforcement values being associated with different objects (Blair et al., 2006b). In line with this, neuroimaging work with patients with GAD have implicated frontal pathology (Blair et al., 2008b, Hoehn-Saric et al., 2004, Monk et al., 2006, Wu et al., 1991).
These data suggest two possible reasons for the impairment seen in the patients with GAD in the current study. First, regions implicated in GAD may be dysfunctional in the representation of reinforcement information and, as such, lead to impairment on the current task. Second, it is possible that on-going worry in these patients may have interfered with their task performance. That is, the patients with GAD may have been attending to features of their foci or worry rather than representations necessary for successful task performance. Importantly, these two positions can be distinguished in our ongoing follow-up study. If patients with GAD show impairment in the representation of reinforcement information, we can predict reduced representation of this information relative to patients with GSP and HCs in an fMRI implementation of this paradigm (Blair et al., 2006b). Alternatively, if task performance relates to pathological worry, it can be predicted that the patients with GAD will show increased activity in regions previously implicated in worry, that will not be differentially affected by decision type of reinforcement distance. We are currently testing these predictions.
Very little work has investigated whether patients with GSP show impairment in the processing of non-social affective stimuli. One study has reported impaired aversive conditional discrimination ability in patients with GSP relative to HCs during an eyelid conditional discrimination learning task (Sachs et al., 2003). In contrast, we found no significant impairment in patients with GSP on the DRPLT. There are several possible reasons for the inconsistency between the current results and those of Sachs et al. (2003). For example, the Sachs et al. (2003) study involved patients with non-generalized social phobia whereas the current study involved patients with generalized social phobia. It has been suggested that the impairments seen in non-generalized and generalized social phobia may differ (Boone et al., 1999, Vriends et al., 2007). Moreover, there are clear task differences between the two studies. Thus, while a firm conclusion cannot be made in the absence of additional data, it appears that patients with GSP may be unimpaired in processing of non-social affective stimuli
The current data suggest that GSP and GAD might be associated with different neurocognitive impairments. Few studies have previously directly compared the two disorders. However, other recent behavioral work has also revealed significant group differences (Becker et al., 2001, Turk et al., 2005). Moreover, a recent neuroimaging study observed that while individuals with GSP showed significantly greater amygdala responses to fearful expressions relative to HCs, patients with GAD showed significantly reduced amygdala responses relative to HCs. In addition, and in line with the current results, the neural responses of patients with GAD comorbid for GSP did not differ from those of patients with “pure” GAD but did significantly differ from those of patients with GSP only.
The use of points as a secondary reinforcer in this study rather than other more salient reinforcers such as money could be criticized. However, it should be noted that fMRI work shows that very similar regions, in particular vmPFC (e.g., Blair et al., 2006b), are involved in the representation and anticipation of point gain as in financial gain (Knutson and Cooper, 2005). Indeed, it should be noted that vmPFC is also implicated in the representation and anticipation of primary reinforcers such as food (O'Doherty et al., 2001). As such while money may be a more salient secondary reinforcer than points gained, their computational implications appear similar.
It should be noted that the current study did not find any relationship between DRPT total score and severity of symptomatology as indexed by the BAI, LSAS or IDS. We believe that this likely reflects that fact that the LSAS and IDS, and even the BAI, do not directly assess GAD symptom expression levels. It is possible that a self report measure more directly focused on expression of worry might reveal a relationship with DRPT total score.
It should also be noted that the current study provided an index of successful decision-making across conditions. However, it did not provide data on the subject’s learning rate. As yet, it remains unclear whether patients with GAD are slower to learn the reinforcement values associated with the different objects or whether, irrespective of learning rate, they are less able to use the information appropriately across time. Future work will address this issue.
Considering that a relatively small number of patients took part in the study, and that this is the first study that directly compared stimulus-reinforcement based decision-making in GSP and GAD our data must clearly be considered preliminary. Nevertheless, consistent with family-based and pharmacological data (Blanco et al., 2003, Liebowitz et al., 2005, Rickels and Rynn, 2002), our results do add to the initial other evidence that GSP and GAD might be dissociable. However, future work is clearly needed to further investigate this issue.
In short, in this study we found that patients with GAD committed significantly more errors relative to patients with GSP or HCs on a decision-making task that is sensitive to overall levels of reward and punishment as well as between-object reinforcement distances. In contrast, the patients with GSP and the healthy comparison individuals did not differ on this task. These results link GAD with an anomalous decision-making process, that we suggested might relate to an impairment in the representation of reinforcement information, or alternatively that on-going worry may have interfered with their task performance. We are currently examining these possibilities in a current on-going follow up study. Our results join other recent studies in tentatively suggesting that GSP and GAD may be dissociable disorders and may have implications for future treatment considerations of the two groups.
ACKNOWLEDGEMENTS
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health.
Footnotes
One GAD patient’s STAI-T, and one HCs LSAS, IDS, and BAI scores were not available.
The authors have no conflicts of interest or financial disclosures to report.
REFERENCE LIST
- Becker ES, Rinck M, Margraf J, Roth WT. The emotional Stroop effect in anxiety disorders: general emotional or disorder specificity? Journal of Anxiety Disorders. 2001;15:147–159. doi: 10.1016/s0887-6185(01)00055-x. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, Schneider F, Weiss U, Flor H. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9:1223–1226. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Blair K, Geraci M, Devido J, McCaffrey D, Chen G, Vythilingam M, Ng P, Hollon N, Jones M, Blair RJ, Pine DS. Neural response to self- and other referential praise and criticism in generalized social phobia. Archives of General Psychiatry. 2008a;65:1176–1184. doi: 10.1001/archpsyc.65.10.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Leonard A, Morton J, Blair RJR. Impaired decision making on the basis of both reward and punishment information in individuals with psychopathy. Personality and Individual Differences. 2006a;41:155–165. [Google Scholar]
- Blair KS, Marsh AA, Morton J, Vythilingham M, Jones M, Mondillo K, Pine DS, Drevets WC, Blair RJR. Choosing the lesser of two evils, the better of two goods: Specifying the roles of ventromedial prefrontal cortex and dorsal anterior cingulate cortex in object choice. Journal of Neuroscience. 2006b;26:11379–11386. doi: 10.1523/JNEUROSCI.1640-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair KS, Shaywitz J, Smith BW, Rhodes R, Geraci M, Jones M, McCaffrey D, Vythilingam M, Finger E, Mondillo K, Jacobs M, Charney D, Blair RJR, Drevets WC, Pine DS. Response to emotional expressions in Generalized Social Phobia (GSP) and Generalized Anxiety Disorder (GAD): Evidence for separate disorders. The American Journal of Psychiatry. 2008b;165:1193–1202. doi: 10.1176/appi.ajp.2008.07071060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Schneier FR, Schmidt A, Blanco-Jerez CR, Marshall RD, Sanchez-Lacay A, Liebowitz MR. Pharmacological treatment of social anxiety disorder: a meta-analysis. Depression and Anxiety. 2003;18:29–40. doi: 10.1002/da.10096. [DOI] [PubMed] [Google Scholar]
- Boone ML, McNeil DW, Masia CL, Turk CL, Carter LE, Ries BJ, Lewin MR. Multimodal comparisons of social phobia subtypes and avoidant personality disorder. Journal of Anxiety Disorders. 1999;13:271–292. doi: 10.1016/s0887-6185(99)00004-3. [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Shadick RN, Hopkins M. The nature of normal and pathological worry. New York, NY: Guilford Press; 1991. [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Science. 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown TA, DiNardo PA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV — Adult Version. San Antonio, TX: The Psychological Corporation; 1994. [Google Scholar]
- Bruce SE, Machan JT, Dyck I, Keller MB. Infrequency of "pure" GAD: impact of psychiatric comorbidity on clinical course. Depression and Anxiety. 2001;14:219–225. doi: 10.1002/da.1070. [DOI] [PubMed] [Google Scholar]
- Charney DS. Discovering the neural basis of human social anxiety: a diagnostic and therapeutic imperative. The American Journal of Psychiatry. 2004;161:1–2. doi: 10.1176/appi.ajp.161.1.1. [DOI] [PubMed] [Google Scholar]
- Chen YP, Ehlers A, Clark DM, Mansell W. Patients with generalized social phobia direct their attention away from faces. Behaviour Research and Therapy. 2002;40:677–687. doi: 10.1016/s0005-7967(01)00086-9. [DOI] [PubMed] [Google Scholar]
- Coelho HF, Cooper PJ, Murray L. A family study of co-morbidity between generalized social phobia and generalized anxiety disorder in a non-clinic sample. Journal of Affective Disorders. 2007;100:103–113. doi: 10.1016/j.jad.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Coles ME, Heimberg RG. Recognition bias for critical faces in social phobia: a replication and extension. Behaviour Research and Therapy. 2005;43:109–120. doi: 10.1016/j.brat.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Craske MG, Rapee RM, Jackel L, Barlow DH. Qualitative dimensions of worry in DSM-III-R generalized anxiety disorder subjects and nonanxious controls. Behaviour Research and Therapy. 1989;27:397–402. doi: 10.1016/0005-7967(89)90010-7. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV. Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Heinrichs N, Hofmann SG. Information processing in social phobia: a critical review. Clinical Psychology Review. 2001;21:751–770. doi: 10.1016/s0272-7358(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Hoehn-Saric R, Schlund MW, Wong SH. Effects of citalopram on worry and brain activation in patients with generalized anxiety disorder. Psychiatry Research. 2004;131:11–21. doi: 10.1016/j.pscychresns.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Current Opinion in Neurology. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR, Ninan PT, Schneier FR, Blanco C. Integrating neurobiology and psychopathology into evidence-based treatment of social anxiety disorder. CNS Spectr. 2005;10 suppl13:1–11. discussion 12-3; quiz 14-5. [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- Mathew SJ, Coplan JD, Gorman JM. Neurobiological mechanisms of social anxiety disorder. The American Journal of Psychiatry. 2001;158:1558–1567. doi: 10.1176/appi.ajp.158.10.1558. [DOI] [PubMed] [Google Scholar]
- McClure EB, Adler A, Monk CS, Cameron J, Smith S, Nelson EE, Leibenluft E, Ernst M, Pine DS. fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology. 2007;191:97–105. doi: 10.1007/s00213-006-0542-9. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Weinman J. Memory bias in clinical anxiety. Journal of Abnormal Psychology. 1987;96:94–98. doi: 10.1037//0021-843x.96.2.94. [DOI] [PubMed] [Google Scholar]
- Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. Journal of Abnormal Psychology. 2004;113:160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Blair RJ, Chen G, Charney DS, Ernst M, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. The American Journal of Psychiatry. 2006;163:1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1986;95:252–256. [PubMed] [Google Scholar]
- Nutt DJ. Neurobiological mechanisms in generalized anxiety disorder. Journal of Clinical Psychiatry. 2001;62 Suppl 11:22–27. [PubMed] [Google Scholar]
- O'Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. Journal of Neurophysiology. 2001;85:1315–1321. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Rickels K, Rynn M. Pharmacotherapy of generalized anxiety disorder. Journal of Clinical Psychiatry. 2002;63 Suppl 14:9–16. [PubMed] [Google Scholar]
- Roemer L, Orsillo SM, Barlow DH. Anxiety and its disorders. The nature and treatment of anxiety and panic. New York: The Guilford Press; 2002. [Google Scholar]
- Sachs G, Anderer P, Doby D, Saletu B, Dantendorfer K. Impaired conditional discrimination learning in social phobia. Neuropsychobiology. 2003;47:66–72. doi: 10.1159/000070011. [DOI] [PubMed] [Google Scholar]
- Schneier FRFR. Social anxiety disorder. British Medical Journal. 2003;327:515. doi: 10.1136/bmj.327.7414.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Stein DJ, Westenberg HG, Liebowitz MR. Social anxiety disorder and generalized anxiety disorder: serotonergic and dopaminergic neurocircuitry. Journal of Clinical Psychiatry. 2002;63 Suppl 6:12–19. [PubMed] [Google Scholar]
- Stein MB, Kean YM. Disability and quality of life in social phobia: epidemiologic findings. The American Journal of Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Glauer M, Miltner WH. Brain activation to phobia-related words in phobic subjects. Neuroscience Letters. 2004;372:204–208. doi: 10.1016/j.neulet.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Turk CL, Heimberg RG, Luterek JA, Mennin DS, Fresco DM. Emotion Dysregulation in Generalized Anxiety Disorder: A Comparison with Social Anxiety Disorder. Cognitive Therapy and Research. 2005;29:89–106. [Google Scholar]
- Vriends N, Becker ES, Meyer A, Michael T, Margraf J. Subtypes of social phobia: are they of any use? Journal of Anxiety Disorders. 2007;21:59–75. doi: 10.1016/j.janxdis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Johnstone T, Somerville LH, Nitschke JB, Polis S, Alexander AL, Davidson RJ, Kalin NH. A Functional Magnetic Resonance Imaging Predictor of Treatment Response to Venlafaxine in Generalized Anxiety Disorder. Biological Psychiatry. 2007;63:858–863. doi: 10.1016/j.biopsych.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Buchsbaum MS, Hershey TG, Hazlett E, Sicotte N, Johnson JC. PET in generalized anxiety disorder. Biological Psychiatry. 1991;29:1181–1199. doi: 10.1016/0006-3223(91)90326-h. [DOI] [PubMed] [Google Scholar]