Abstract
The fine structure of the eggshell of blow fly, Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae), was examined using scanning and transmission electron microscopy. Eggs, 1.09±0.07 mm in length and 0.25±0.05 mm in width, bore a relatively wide plastron that extending along almost the entire length. The polygonal pattern of chorionic sculpture was indistinct. The ultrathin section indicated a multi-layered eggshell having an exochorion, outer endochorion, pillars, an inner endochorion, innermost chorionic layer, and a wax layer. This study provides new information about the fine morphology of blow flies eggs. A key to differentiate the eggs of forensically important flies in Thailand is given.
Keywords : eggshell, ultrastructure, Lucilia cuprina, forensic entomology
Introduction
Lucilia (=Phaenicia) cuprina (Wiedemann, 1830) (Diptera: Calliphoridae) is a fly of medical and veterinary importance, not only as an ectoparasite, but also because it causes myiasis in humans and other mammals, particularly sheep (Zumpt 1965; Stevens and Wall 1997; Tellam et al. 2001; Colditz et al. 2002). It has recently been claimed to be forensically important, since L. cuprina was found associated with corpses and could be used in forensic investigations (Smith 1986; Goff 2000; Byrd and Castner 2001; Greenberg and Kunich 2002). In Thailand, the larvae of this species have been found in human corpses in Chiang Mai, northern Thailand (KL Sukontason, unpublished data). Systematically, L. cuprina has been classified in the Family Calliphoridae, Subfamily Calliphorinae, and Tribe Luciliini (Kurahashi et al. 1997).
The presence in corpses of fly eggs, larva or puparia, as well as other arthropods can be used in forensic investigation. For example, the presence of only fly eggs in a corpse can used to estimate a short postmortem interval (Smith 1986; Lord 1990; Anderson 1999; Byrd and Castner 2001; Anderson 2004). However, an essential first step is the species identification of fly eggs. The identity of fly eggs has been performed using light microscopy (Sukontason et al. 2004a) or scanning electron microscopy (SEM) (Kitching 1976; Greenberg and Szyska 1984; Greenberg and Singh 1995). In this study, the fine structure of the eggshell of L. cuprina is presented using SEM and transmission electron microscopy (TEM). A key is also provided to differentiate L. cuprina eggs from other forensically important fly species in Thailand.
Materials and Methods
The eggs of L. cuprina were obtained from the laboratory colony maintained at the Department of Parasitology, Faculty of Medicine, Chiang Mai University. The rearing procedure was modified by using the technique of Haskell (1990) at room temperature (average, 24–28°C). Fresh pork liver was provided as a larval food source and oviposition site.
For the SEM process, eggs were washed several times using normal saline solution to remove any pork liver tissue residue. The specimens were fixed with 2.5% glutaraldehyde in phosphate buffer solution (PBS) at a pH of 7.4 at 4°C for 24 h. They were then rinsed twice with PBS at 10-min intervals. The rinsed eggs were then treated with 1% osmium tetroxide at room temperature for one day for post-fixation. This was followed by rinsing the eggs twice with PBS and dehydrating with increasing concentrations of alcohol as follows: 30, 50, 70, 80 and 90%. The eggs remained in each concentration of alcohol for 12 h during each step of the dehydration process. The eggs were then placed in absolute alcohol for two 12 h periods followed by acetone for two 12 h periods. Finally, the eggs were subjected to critical point drying in order to complete the dehydration process. In order to view the eggs, they were first attached to aluminum stubs with double-stick tape so they could be coated with gold in a sputter-coating apparatus before being viewed with a JEOL-JSM840A scanning electron microscope (JOEL, www.jeol.com).
The procedure for the TEM process was the same as that for SEM until the eggs were placed in absolute alcohol for two 12 h periods. After that, they were placed in acetone for 2 h before transferring to a ratio of resin:acetone 1:3 for 24 h, 1:1 for 24 h and 3:1 for 24 h, followed by resin for 2, 3 h periods. Egg specimens were embedded in Spurr's resin by placing them into a plastic block, and incubating at 70 °C for 24 h. Section of the eggs was made with a glass knife on an Ultramicrotome (Leica, www.leica-microsystems.com). The ultra-thin section was stained with uranyl acetate and lead citrate; and observed under the JEOL 1200.
To differentiate L. cuprina eggs from other forensically important flies, five species of flies were included in this study, namely Callliphoridae Chrysomya megacephala, Chrysomya rufifacies, and Chrysomya nigripes; Muscidae, Musca domestica, Synthesiomyia nudiseta; and Phoridae, Megaselia scalaris. The eggs were either processed by the SEM or stained with one percent of potassium permanganate solution, as previously described by Sukontason et al. 2004a. Terminology used for of describing fly eggshell followed Margaritis (1985).
Results and Discussion
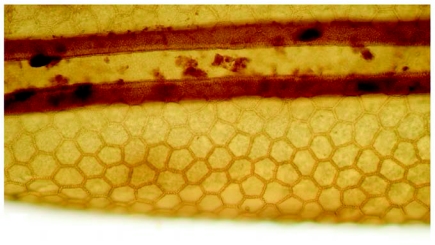
The eggs of L. cuprina were creamy-white, elongated and 1.09±0.07 mm in length, 0.25±0.05 mm in width (n = 50). The plastron originated from the anterior end near the micropyle, and extended dorsally along almost the entire length (Figure 1A). The width of the plastron was 0.022±0.006 mm (n = 50), representing ≈8.8% of the width of the eggs. The plastron adjacent to the micropyle was slightly bifurcated (Figure 1B), whereas that along the hatching line (Figure 1B, 1C, stars) was upright. The chorionic sculpture had a polygonal pattern (pentagonal or hexagonal) with indistinct boundary (Figure 1C, arrow). Under TEM observation, a section of the eggshell reveals a multi-layered surface with the outermost exochorion, outer endochorion, a layer of vertical pillars between the irregular space of aeropyles (star), inner endochorion, innermost chorionic layer, and wax layer (Figure 2).
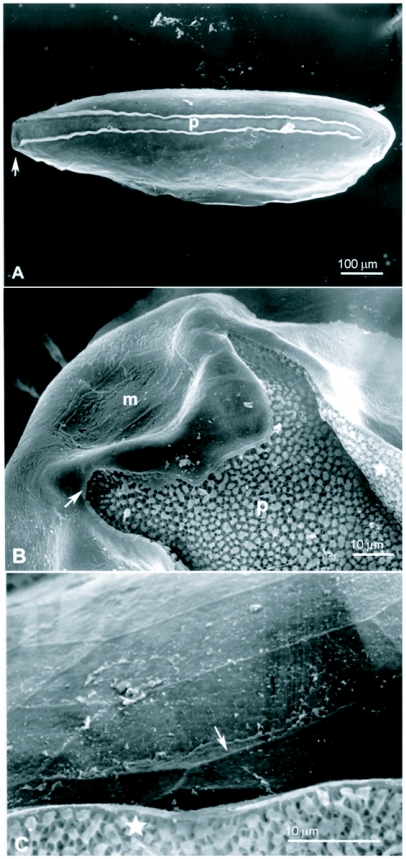
Figure 1.
Scanning electron micrographs of egg of L. cuprina. A (Above) Whole egg showing the wide plastron region (p) extending along almost the entire length. Arrow indicates anterior end bearing the micropyle. B (Middle) Plastron (p) near micropyle (m) showing slight bifurcation. Arrow indicates the end of bifurcation; star indicates upright plastron region along the hatching line. C (Lower) Chorionic sculpture that has a smooth surface inside the indistinct polygonal patterns (arrow). Star indicates the upright plastron region along the hatching line.
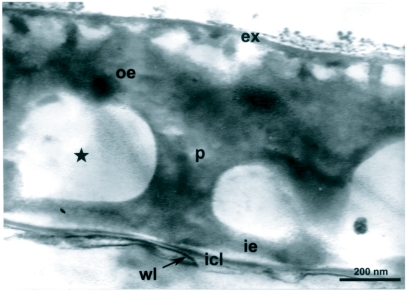
Figure 2.
Transmission electron micrograph of the eggshell of L. cuprina showing a multi-layered surface with the outermost exochorion (ex), outer endochorion (oe), and layer of vertical pillars (p) between the irregular space of aeropyles (star), inner endochorion (ie), innermost chorionic layer (icl), wax layer (wl).
A key to simplify the identification of egg of L. cuprina from the other forensically important species in Thailand was summarized for morphological comparison as follows (Table 1).
Table 1.
Egg identification key.
The eggshell structure of L. cuprina conforms to general ultrastructural patterns shown by SEM micrographs of blow fly species (Kitching 1976; Erzinclioglu 1989; Liu and Greenberg 1989; Peterson and Newman 1991; Greenberg and Singh 1995; Sukontason et al. 2004b), by bearing the dorsal plastron, micropyle and polygonal pattern of chorionic sculpture. The relatively wide plastron and slight bifurcation of the plastron near the micropyle of L. cuprina eggs are similar to that found in Lucilia species, e.g., Phaenicia sericata, Phaenicia coeruleiviridis and Phaenicia illustris (Greenberg and Singh 1995). These authors indicated the difficulty in differentiating between them, however, a slight bifurcation of the plastron near the micropyle of L. cuprina differed from the marked bifurcation of L. ibis (Greenberg and Kunich 2002). Hence, this feature would be partially useful in future for differentiating eggs of the Lucilia species that exist in Thailand and other countries in Asia, e.g. L. porphyrina, L. papuensis, L. sinensis, L. bismarckensis, L. calviceps, L. fumicosta, L. hainanensis and L. salazarae (Tumrasvin et al. 1979; Kurahashi et al. 1997; Kurahashi and Magpayo 2000; Kurahashi 2001).
Figure 3.
Scanning electron micrograph of egg of M. scalaris. Whole egg showing the wide plastron region flanked with prominent flanges.
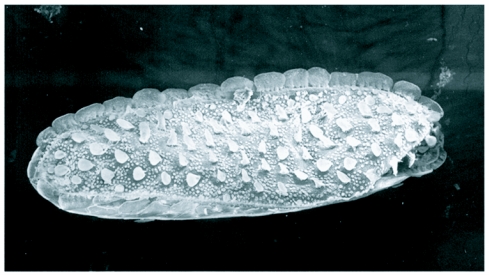
Figure 4.
Egg of S. nudiseta after being stained with 1% potassium permanganate solution for 1 min. Whole egg showing wide plastron area with polygonal pattern and upright hatching line.
Figure 5.
Egg of C. megacephala after being stained with 1% potassium permanganate solution for 1 min. Whole egg showing narrow plastron area lengthen almost the entire length.
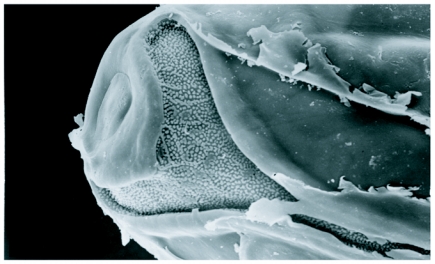
Figure 6.
Scanning electron micrograph of egg of C. megacephala displaying “Y-shape” of plastron area near micropyle.
Figure 7.
Egg of C. nigripes after being stained with 1% potassium permanganate solution for 1 min. Lower half egg showing swollen of the boundary of polygonal pattern on chorion and upright hatching line (dark brown lines).
Figure 8.
Egg of M. domestica after being stained with 1% potassium permanganate solution for 1 min. Whole egg showing wide plastron area almost the entire length and slightly swollen hatching line.
The chorionic ultrastructure has been used as one of the taxonomic characters for differentiating between fly eggs (Kitching 1976; Feliciangeli et al. 1993; Colwell et al. 1999; Sukontason et al. 2004a). The indistinct boundary of the hexagonal pattern of L. cuprina presented herein, used for incorporation with other features, may help to differentiate eggs from other Lucilia species.
Our TEM observation on L. cuprina eggs corresponds with the multi-layered eggshell of blow flies, e.g. Lucilia sericata, Calliphora erythrocephala (Hinton 1960), Cochliomyia hominivorax (Peterson and Newman 1991) and Chrysomya nigripes (Sukontason et al. 2004b). The large perforation of aeropyles in the middle layer and wide plastron enable the efficient distribution system of gases for the developing oocytes (Hinton 1960; Margaritis 1985; Ma et al. 2002).
Acknowledgements
We thank two anonymous reviewers, and the editor, for helpful comments on the manuscript. This work received support from the Faculty of Medicine Research Fund, Chiang Mai University.
References
- Anderson GS. Wildlife forensic entomology: determining time of death in two illegally killed black bear cubs. Journal of Forensic Science. 1999;44:856–859. [PubMed] [Google Scholar]
- Anderson GS. Determining time of death using blow fly eggs in the early postmortem interval. International Journal of Legal Medicine. 2004;118:240–241. doi: 10.1007/s00414-004-0443-6. [DOI] [PubMed] [Google Scholar]
- Byrd JH, Castner JL. Insects of forensic importance. In: Byrd JH, Castner JL, editors. Forensic Entomology: The Utility of Arthropods in Legal Investigations. Florida: CRC Press; 2001. pp. 43–79. [Google Scholar]
- Colditz IG, Watson DL, Eisemann CH, Tellam RL. Production of antibodies to recombinant antigens from Lucilia cuprina following cutaneous immunisation of sheep. Veterinary Parasitology. 2002;104:345–350. doi: 10.1016/s0304-4017(01)00648-3. [DOI] [PubMed] [Google Scholar]
- Colwell DD, Baird CR, Lee B, Milton K. Scanning electron microscopy and comparative morphometrics of eggs from six bot fly species (Diptera: Oestridae). Journal of Medical Entomology. 1999;36:803–810. doi: 10.1093/jmedent/36.6.803. [DOI] [PubMed] [Google Scholar]
- Erzinclioglu YZ. The value of chorionic structure and size in the diagnosis of blowfly eggs. Medical and Veterinary Entomology. 1989;3:281–285. doi: 10.1111/j.1365-2915.1989.tb00229.x. [DOI] [PubMed] [Google Scholar]
- Feliciangeli MD, Castejon OC, Limongi J. Egg surface ultrastructure of eight New World phlebotomine sand fly species (Diptera: Psychodidae). Journal of Medical Entomology. 1993;30:651–656. doi: 10.1093/jmedent/30.4.651. [DOI] [PubMed] [Google Scholar]
- Goff ML. A Fly for the Prosecution. How Insect Evidence Helps Solve Crimes. Cambridge, MA: Harvard University Press; 2000. [Google Scholar]
- Greenberg B, Kunich JC. Entomology and the Law. Flies as Forensic Indicators. Cambridge, United Kingdom: Cambridge University Press; 2002. [Google Scholar]
- Greenberg B, Singh D. Species identification of calliphorid (Diptera) eggs. Journal of Medical Entomology. 1995;32:21–6. doi: 10.1093/jmedent/32.1.21. [DOI] [PubMed] [Google Scholar]
- Greenberg B, Szyska ML. Immature stages and biology of fifteen species of Peruvian Calliphoridae (Diptera). Annals of the Entomological Society of America. 1984;77:488–517. [Google Scholar]
- Haskell NH. Procedures in the entomology laboratory. In: Catts EP, Haskell NH, editors. Entomology & Death: A Procedural Guide. Clemson, SC: Joyce's Print Shop Inc; 1990. pp. 111–123. [Google Scholar]
- Hinton HE. Plastron respiration in the eggs of blowflies. Journal of Insect Physiology. 1960;4:176–183. [Google Scholar]
- Kitching RL. The immature stages of the Old-World screw-worm fly, Chrysomya bezziana Villeneuve, with comparative notes on other Australasian species of Chrysomya. Bulletin of Entomological Research. 1976;66:195–203. [Google Scholar]
- Kurahashi H. The blow flies recorded from Sri Lanka, with description of two new species (Diptera, Calliphoridae). Japanese Journal of Systematic Entomology. 2001;7:241–254. [Google Scholar]
- Kurahashi H, Benjaphong N, Omar B. Blow flies (Insecta: Diptera: Callihporidae) of Malaysia and Singapore. The Raffles Bulletin of Zoology. 1997:1–88. (Suppl 5) [Google Scholar]
- Kurahashi H, Magpayo FR. Blow flies (Insecta: Diptera: Callihporidae) of the Philippines. The Raffles Bulletin of Zoology. 2000:1–78. (Suppl 9) [Google Scholar]
- Liu D, Greenberg B. Immature stage of some flies of forensic importance. Annals of the Entomological Society of America. 1989;82:80–93. [Google Scholar]
- Lord WD. Case histories of the use of insects in investigations. In: Catts EP, Haskell NH, editors. Entomology and Death; A Procedural Guide. Clemson, SC: Joyce's Print Shop Inc; 1990. pp. 9–37. [Google Scholar]
- Ma PWK, Baird S, Ramaswamy SB. Morphology and formation of the eggshell in the tarnished plant bug, Lygus lineloaris (Palisot de Beauvois) (Hemiptera: Miridae). Arthropods Structure and Development. 2002;31:131–146. doi: 10.1016/s1467-8039(02)00019-1. [DOI] [PubMed] [Google Scholar]
- Margaritis LH. Structure and physiology of the eggshell. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. I. Embryogenesis and Reproduction. Oxford: Pergamon Press; 1985. pp. 153–230. [Google Scholar]
- Peterson RD, Newman SM., Jr Chorionic structure of the egg of the screwworm Cochliomyia hominivorax (Diptera: Calliphoridae). Journal of Medical Entomology. 1991;28:152–160. doi: 10.1093/jmedent/28.1.152. [DOI] [PubMed] [Google Scholar]
- Smith KGV. A Manual of Forensic Entomology. London: British Museum of Natural History; 1986. [Google Scholar]
- Stevens J, Wall R. The evolution of ectoparasitism in the genus Lucilia (Diptera: Calliphoridae). International Journal for Parasitology. 1997;27:51–59. doi: 10.1016/s0020-7519(96)00155-5. [DOI] [PubMed] [Google Scholar]
- Sukontason K, Sukontason KL, Piangjai S, Boonchu N, Kurahashi H, Hope M, Olson JK. Identification of forensically important fly eggs using a potassium permanganate staining technique. Micron. 2004a;35:391–395. doi: 10.1016/j.micron.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Sukontason K, Sukontason KL, Boonchu N, Chaiwong T, Piangjai S. Ultrastructure of eggshell of Chrysomya nigripes Aubertin (Diptera: Calliphoridae). Parasitology Research. 2004b;93:151–154. doi: 10.1007/s00436-004-1102-z. [DOI] [PubMed] [Google Scholar]
- Tellam RL, Eisemann CH, Vuocolo T, Casu R, Jarmey J, Bowles V, Pearson R. Role of oligosaccharides in the immune response of sheep vaccinated with Lucilia cuprina larval glycoprotein, peritrophin-95. International Journal for Parasitology. 2001;31:798–809. doi: 10.1016/s0020-7519(01)00195-3. [DOI] [PubMed] [Google Scholar]
- Tumrasvin W, Kurahashi H, Kano R. Studies on medically important flies in Thailand. VII. Report on 42 species of calliphorid flies, including the taxonomic keys (Diptera: Calliphoridae). Bulletin of Tokyo Medical and Dental University. 1979;26:243–272. [PubMed] [Google Scholar]
- Zumpt F. Myiasis in Man and Animals in the Old World. London, UK: Butterworths; 1965. [Google Scholar]