Abstract
Pediatric brain tumors are significant causes of morbidity and mortality. It has been hypothesized that they derive from self-renewing multipotent neural stem cells. Here, we tested whether different pediatric brain tumors, including medulloblastomas and gliomas, contain cells with properties similar to neural stem cells. We find that tumor-derived progenitors form neurospheres that can be passaged at clonal density and are able to self-renew. Under conditions promoting differentiation, individual cells are multipotent, giving rise to both neurons and glia, in proportions that reflect the tumor of origin. Unlike normal neural stem cells, however, tumor-derived progenitors have an unusual capacity to proliferate and sometimes differentiate into abnormal cells with multiple differentiation markers. Gene expression analysis reveals that both whole tumors and tumor-derived neurospheres express many genes characteristic of neural and other stem cells, including CD133, Sox2, musashi-1, bmi-1, maternal embryonic leucine zipper kinase, and phosphoserine phosphatase, with variation from tumor to tumor. After grafting to neonatal rat brains, tumor-derived neurosphere cells migrate, produce neurons and glia, and continue to proliferate for more than 4 weeks. The results show that pediatric brain tumors contain neural stem-like cells with altered characteristics that may contribute to tumorigenesis. This finding may have important implications for treatment by means of specific targeting of stem-like cells within brain tumors.
Primary malignant central nervous system tumors are the most frequent form of solid malignancy in children (1). A heterogeneous group of tumors, some of the most common histological types are astrocytomas (52%), primitive neuroectodermal tumors (PNETs), including medulloblastoma (21%), and high-grade gliomas (19%) (2). Despite advances in therapy, morbidity and mortality remain high (3).
The cellular origin of pediatric brain tumors (PBTs) is unclear. One possibility is that they arise by transformation of proliferating neural stem cells (NSCs) (4–6), cells with the ability to self-renew and differentiate into neurons and glia (7). There are several lines of indirect evidence in support of this hypothesis. First, PBTs often contain multiple cell types, suggestive of an origin from a cell with multilineage potential (8). Second, many PBTs appear to arise from the ventricular zone, the location of NSCs (9–11). Third, both PBTs and NSCs express nestin, an intermediate filament characteristic of several progenitors (12–14). Fourth, PBTs often express genes that regulate proliferation and self-renewal of normal NSCs (15–17) and mutations in genes that normally regulate neural stem cell proliferation are frequently found in PBTs. Finally, forced expression of oncogenes in neural stem and progenitors cells in mice produces tumors that are similar to primary human tumors (6).
If PBTs contain cells with stem cell properties, an important question is whether these cells also have abnormal properties that are responsible for the aberrant and persistent growth of the tumor (18). In models of breast cancer and acute myelogenous leukemia, “cancer stem cells” have been isolated and repassaged into experimental animals to form new tumors, providing strong evidence that these cells are the root cause of the tumor (19, 20). Such individual cells are capable of self-renewal, proliferation, and differentiation to create the complex heterogeneous tumor. It is unknown whether PBTs contain such cancer stem cells, and, if so, whether such cells are derived from neural stem cells.
In the current study, we asked whether PBTs contained progenitor cells with characteristics similar to those of NSCs. We have isolated and characterized multipotent, self-renewing cells from tumor samples, here referred to as “tumor-derived progenitors,” which have both similarities to and differences from normal NSCs (21). Cells derived from PBTs were able to produce proliferating neurospheres that could be passaged at clonal density and differentiated into cells with defining antigenic characteristics of neurons and glia. These neurospheres expressed many genes characteristic of NSC-derived spheres. Furthermore, like normal neurospheres, tumor-derived spheres migrated and continued to proliferate when transplanted into neonatal rat brain. Unlike NSCs, however, these tumor-derived progenitors were more long-lived and often gave rise to abnormal dual-phenotype cells. Our data suggest that PBTs arise from cells with many of the characteristics of NSCs but with abnormal ability to propagate and differentiate. These studies also raise the possibility that some tumor-derived cells may be cancer stem cells with the ability to generate PBTs.
Materials and Methods
Tissue Collection and Grading. PBT and nontumor human brain specimens from patients undergoing neocortical resections for intractable epilepsy were obtained within 30 min of surgical resection in accordance with protocols approved by institutional review boards at University of California Los Angeles Medical Center and the California Institute of Technology. The epilepsy surgery tissue was taken from the lateral ventricular surface and immediately adjacent tissues. Tumors were graded at the University of California Los Angeles Medical Center by the attending neuropathologist in accordance with World Health Organization (WHO) established guidelines (22).
Neurosphere Culture. Tumor and neurosphere cultures were performed as according to Svendsen et al. (23) with some modifications. A detailed protocol is found in Supporting Methods, which is published as supporting information on the PNAS web site. Briefly, tissue was washed, minced, digested with trypsin, dissociated, and passed through a series of cell strainers. Cells were seeded in growth medium supplemented with basic fibroblast growth factor (20 ng/ml), epidermal growth factor (20 ng/ml), and leukemia inhibitory factor (20 ng/ml) at a density of 100,000 cells per ml. Clonal cultures were plated at a density of 1,000 cells per ml in mouse neurosphere-conditioned medium (24), a density that has been demonstrated to produce almost entirely clonal neurospheres (25).
Immunocytochemistry of Neurospheres. Immunocytochemistry of neurosphere cultures was performed as described (24). Differentiation of early passage (≈4 weeks) spheres was induced by plating onto coverslips precoated with poly-l-lysine (Sigma) in Neurobasal medium (GIBCO–Invitrogen) supplemented with B-27 and in the absence of added basic fibroblast growth factor or epidermal growth factor. After 7 days, the neurospheres were fixed in 4% paraformaldehyde and immunostained with rabbit anti-nestin (1:200; Chemicon) or rabbit anti-musashi (1:200; Chemicon) for neural stem and progenitor cells, mouse anti-TuJ1 (1:500; Berkeley Antibodies) or anti-Hu (1:300; Molecular Probes) for neurons, rabbit anti-glial fibrillary acidic protein (GFAP; 1:500; DAKO) for astrocytes, and anti-O4 (1:40; Chemicon) for oligodendrocytes, followed by Alexa fluorophore-conjugated secondary antibodies (1:2,000; Molecular Probes). In some cases, nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI).
Statistical Analysis. Statistical analysis of cell counts was performed as described in Supporting Methods.
Immunohistochemistry of Whole Tumor Sections. Formalin-fixed, paraffin-embedded tissue specimens from tumors BT1, -2, -3, and -5 were sectioned at 8 μm, mounted on Superfrost Plus slides (Fisher), deparaffinized, and processed for antigen retrieval by microwave heating as described (26).
BrdUrd-Labeling of Neurospheres. Neurospheres were cultured in proliferative medium containing 2 μM BrdUrd (5-bromo-2′-deoxyuridine) for 14 h. The cells were fixed in 4% phosphate-buffered paraformaldehyde solution. Staining with anti-BrdUrd antibody (Becton Dickinson) was performed according to the supplier's suggestions, and fluorescent secondary antibody was used.
Semiquantitative RT-PCR. Semiquantitative RT-PCR was performed with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard as described in Supporting Methods.
Transplantation of Neurosphere Cells into Neonatal Rat Brain. Transplantation of 50,000 cells into the neostriata of neonatal rats was performed according to the methods of Uchida et al. (27) with modifications as described in Supporting Methods.
Immunohistochemical Analysis of Transplanted Rat Brain. Four weeks after transplantation, tissue was prepared for immunocytochemical analysis as described in Supporting Methods and stained with antibodies directed against human nuclei, neurons, astrocytes, and Ki-67.
Results
Twenty-two PBTs were used in the current studies: 10 gliomas (including 2 glioblastomas), 6 medulloblastomas, 5 primitive neuroectodermal tumors, and 1 ependymoma. For detailed analysis of cell fate and gene expression, we studied a subgroup of 6 tumors from 5 pediatric patients, aged 5 months to 6 years [designated tumors 1–5 (BT1–BT5); Table 1]. These were a midline anaplastic astrocytoma (World Health Organization grade III astrocytic tumor; BT1), two cerebellar medulloblastomas (BT2 and BT3), one glioblastoma multiforme (World Health Organization grade IV astrocytic tumor; BT4), and one desmoplastic medulloblastoma (BT5), distinguished from medulloblastoma by marked neuronal differentiation (22).
Table 1. Tumor and patient characteristics.
| Tumor | Patient age | Diagnosis | Site of tumor |
|---|---|---|---|
| BT1 | 5 years | Anaplastic astrocytoma | Midline hypothalamus |
| BT2 | 5 months | Medulloblastoma | Cerebellum |
| BT3 | 6 years | Medulloblastoma | Cerebellum |
| BT4 | 6 years | Glioblastoma multiforme | Temporal lobe |
| BT5 | 15 months | Desmoplastic medulloblastoma | Cerebellum |
Brain Tumor Cells Have the Ability to Form Neurospheres. Dissociated tumor cells were assayed for their ability to form neurospheres by using the same methods as described previously for human NSCs (23, 27). All 22 primary brain tumors studied produced proliferating neurospheres. Large numbers of small spheres ≈4–10 cells in diameter were observed in culture flasks between 3 and 7 days after seeding the cells at 100,000 per ml. Within 2 weeks, most spheres had increased their diameters ≈5- to 10-fold (Fig. 1A). Cells from a local recurrence of one tumor (BT4, glioblastoma multiforme) also gave rise to spheres in culture. Neurospheres could be passaged multiple times by mechanical dissociation of large spheres and reseeding in fresh proliferative medium every 2–3 weeks and could be maintained for 16 weeks or more.
Fig. 1.
Tumor-derived progenitors form neurospheres in culture that give rise to both neuronal and glial cells. Neurospheres from one tumor, BT1, were cultured at medium (A–D) and clonal (E–H) densities. (A) A typical primary neurosphere. (B and C) Undifferentiated primary neurospheres expressed high levels of nestin protein (B, green) and low levels of β-III-tubulin (C, red) and GFAP (C, green). (D) Expression of β-III-tubulin and GFAP, after 7 days of differentiation on substrate. (E) Nestin expression in undifferentiated clonal neurosphere cells. (F) Musashi-1 (green) expression in undifferentiated clonal neurospheres. (G) β-III-tubulin (red) and GFAP (green) expression in a differentiated clonal neurosphere. Some cells (arrows) expressed both markers. (H) Hu (green) expression in a differentiated neurosphere. Some nuclei were counterstained with DAPI (F and H, blue). (Scale bar in H = 30 μm in A, G, and H, 60 μm in B–F.)
We next asked whether tumor-derived progenitors maintained the differentiative capacity of NSCs by examining the types of molecular markers expressed by neurospheres grown under both proliferative and differentiating conditions with immunocytochemistry. For most tumors, undifferentiated tumor spheres in proliferation medium contained many cells expressing the neural progenitor marker nestin (28) (Fig. 1B; except BT3) and relatively few cells expressing the neuronal marker β-III-tubulin and/or the astrocytic marker GFAP (Fig. 1C). These characteristics persisted even in long-term cultures (10–16 weeks).
Under differentiating conditions, cell division in the spheres slowed dramatically and attached cells migrated on the substrate. Immunocytochemstry revealed an increase in the proportion of cells expressing β-III-tubulin and/or GFAP. Differentiated neurospheres produced numerous cells resembling neurons and astrocytes, with many cells extending processes on the substrate (Fig. 1D).
Tumor-Derived Progenitors Can Be Serially Recloned. Self-renewal and multipotency are critical features of neural and other stem cells. We next tested whether individual cells derived from these neurospheres had the ability to form new neurospheres that subsequently differentiated into multiple cell types. Primary neurospheres were dissociated into single-cell suspensions and reseeded at clonal density in proliferative medium. After 1 day, only individual cells and no clusters could be observed, verifying their clonal origin. In all cases, clonally derived neurospheres were visible within 2–4 weeks after reseeding, suggesting that neurospheres contain individual stem-like cells with the ability to self-renew and form neurosphere colonies.
Immunocytochemistry was used to compare the molecular markers expressed by clonal neurospheres grown under proliferation and differentiation conditions. Undifferentiated clonal spheres from four of five tumors expressed nestin in a large percentage of their cells (>45%) with little variability between clones (Figs. 1E and 2 A, B, D, and E). However, nestin protein expression was undetectable in BT3, a medulloblastoma (Fig. 2C). In addition, tumor-derived spheres expressed musashi-1, another marker of NSCs (Fig. 1F) (29). After transfer to differentiating conditions for 7 days, the proportion of nestin-expressing cells declined in clonal spheres derived from three of the four tumors (Fig. 2 A, B, and D); it remained unchanged only in BT5, a desmoplastic medulloblastoma (Fig. 2E).
Fig. 2.
Neurospheres derived from multiple types of tumors give rise to cells expressing neuronal and glial markers in various proportions. (Left) Average count of cells expressing nestin, TuJ1 alone, GFAP alone, or both markers in clonal neurospheres (NS) from BT1–5 (A–E) before (white) and after (black) differentiation. (Right) Fates of clonal neurospheres (NS) after differentiation. Markers used are TuJ1 for neurons (N) and GFAP for astrocytes (A).
We quantitatively compared the cell types produced by spheres before and after differentiation by using cell type-specific markers. Clonal neurospheres gave rise to cells with neuronal and glial properties. Under differentiation conditions, the majority of clones from each tumor gave rise to cells resembling both neurons and glia, with many cells per sphere expressing β-III-tubulin and/or GFAP (Fig. 1G). The percentage of β-III-tubulin+ cells in clonal spheres increased significantly after transfer to differentiation conditions in all tumors except BT5, a desmoplastic medulloblastoma (Fig. 2 Left). The percentage of GFAP+ cells in clonal spheres increased significantly after differentiation in all tumors except BT4, a glioblastoma multiforme (Fig. 2 Left). Differentiated clonal spheres expressed the neuronal HuC/D antigen in proportions similar to their expression of β-III-tubulin (Fig. 1H). In contrast, the oligodendrocyte marker O4 could not be detected by immunocytochemistry in clonal or high-density neurospheres. These results show that tumors contain progenitor cells that are multipotent, giving rise to cells with neuronal and glial characteristics.
Despite their all being multipotent, the percentage of differentiated cell types produced varied considerably from one tumor to another (Fig. 2 Right). BT5 produced only neurospheres that differentiated into both neurons and glia. The remaining tumors gave rise to a mixture of clones, some of which formed multiple cell types and others that formed only neuron-like cells.
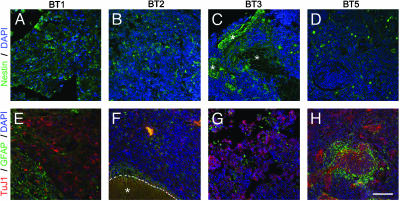
Properties of Clonally Derived Spheres Recapitulate Characteristics of the Parental Tumors. To determine whether the above findings recapitulate the properties of the parental tumors, we examined nestin, GFAP, and TuJ1 staining of four tumors (Fig. 3). The staining patterns were broadly similar to those observed in tumor-derived spheres. For example, BT1 expressed high levels of nestin within the tumor (Fig. 3A) as well as in neurospheres. Many cells in this same tumor expressed either TuJ1or or GFAP (Fig. 3E). BT2 (Fig. 3 B and F) also expressed all three markers but at lower levels than BT1, both in the parent tumor and in the neurospheres. BT3 had little nestin staining in the tumor (although nestin is obvious in the adjacent normal brain; Fig. 3C); this low level of nestin parallels that observed in the neurospheres (compare Figs. 2C and 3C). With respect to TuJ1 and GFAP (Fig. 3G), BT3 had strong reactivity for both, as did the neurospheres (Fig. 2C). BT5 showed good correlation between the amount of TuJ1 and GFAP staining in both the tumors (Fig. 3H) and neurospheres (Fig. 2E); in contrast, nestin expression did correlate well between tumor and neurosphere in this one particular case (Figs. 3D and 2E). Taken together, these data indicate that the tumor-derived spheres, even after weeks in culture, differentiate similarly to the original tumors from which they were derived in the majority of cases.
Fig. 3.
Immunohistochemical characteristics of original tumor samples. Paraffin-embedded sections were labeled with antibodies to nestin (green; A–D) or TuJ1 (red; E–H) to recognize neurons or GFAP (green; E–H) to recognize glia. Area denoted by asterisk in C and F delineates normal brain tissue adjacent to the tumor. (Scale bar in H = 60 μm in A–H.)
Tumor-Derived Neurospheres Express NSC-Related Genes. We used semiquantitative RT-PCR analysis to determine whether neurospheres cultured from PBTs expressed multiple genes enriched in neural and other stem cells, and to compare expression among proliferating and differentiating tumor progenitors as well as the parent tumor. Primers were designed for the following human genes: CD133, a cell surface protein expressed on all fetal human NSCs (27); musashi-1 (msi1) (30); Sox2, an early transcription factor expressed in NSCs and the developing neural tube (31, 32); and bmi-1, a Polycomb Group gene required for self-renewal and proliferation of normal and leukemic hematopoietic stem cells (33, 34). We also examined the expression of two genes that we have identified from mouse neural progenitors by a subtractive microarray screening approach: melk, maternal embryonic leucine zipper kinase and PSP, phosphoserine phosphatase (24, 35, 36). Both of these genes are highly and selectively expressed in murine central nervous system germinal zones and are present in both neural progenitors.
All tumors as well as cells derived from the ventricular zone of normal brain had detectable gene expression of CD133, Sox2, melk, PSP, and bmi-1 (Table 2). Those genes expressed in both tumor-derived and normal neurospheres were also expressed in their parental tissues, suggesting that gene expression observed in neurospheres is not an artifact of cell culture. Sox2, melk, and bmi-1 were expressed in all whole tumor, normal brain, and undifferentiated neurosphere samples. Expression of some genes, most noticeably PSP, was reduced or lost in spheres, as compared with whole tumor or ventricular zone-containing tissue. This finding suggests either that uncultured tissues expressed these genes at higher levels than their neurosphere-initiating cells and their progeny or that these genes were down-regulated as a consequence of cell culture. Neurospheres from BT1 and BT4, both glial tumors of different pathological grades, expressed nearly all stem cell-related genes, but BT3 and BT5, both medulloblastomas, had significantly lower or absent detectable expression of those genes, indicating that cells in glial tumors might have a closer molecular relation to NSCs. msi1 expression was variable but clearly present in spheres derived from two of the five tumors. In 11 instances, expression of specific genes in the tumor-derived spheres was reduced after growth factor withdrawal, consistent with our prediction that tumor-derived progenitors would produce differentiated cells at the expense of multipotent progenitor cells.
Table 2. Gene expression analysis.
| Gene | BT1 | BT2 | BT3 | BT4 | BT5 | Normal |
|---|---|---|---|---|---|---|
| Whole tumors | ||||||
| CD133 | ND | ± | ++ | + | ++ | + |
| msi1 | ND | ++ | – | + | ++ | ++ |
| Sox2 | ND | ND | ++ | ++ | + | + |
| melk | ND | +++ | + | +++ | ++ | +++ |
| PSP | ND | +++ | +++ | ++ | ++ | ++ |
| bmi-1 | ND | ND | ND | +++ | +++ | +++ |
| Undifferentiated neurospheres | ||||||
| CD133 | + | ± | – | + | – | – |
| msi1 | ++ | – | – | ++ | ± | ++ |
| Sox2 | ++ | ++ | ND | ++ | ± | + |
| melk | +++ | ++ | + | +++ | + | +++ |
| PSP | +++ | + | – | ++ | – | – |
| bmi-1 | +++ | +++ | ND | +++ | ND | + |
| Differentiated neurospheres | ||||||
| CD133 | + | – | ++ | – | – | – |
| msi1 | ++ | – | ND | – | ± | ++ |
| Sox2 | ++ | ++ | ± | – | – | ± |
| melk | +++ | + | – | ++ | ± | + |
| PSP | – | +++ | – | – | – | – |
| Bmi-1 | +++ | +++ | ND | +++ | +++ | ± |
Semiquantitative RT-PCR of pediatric brain tumors (BT1–5) and normal human brain was used to analyze expression of stem cell markers. ND, not determined; –, no signal; ±, minor signal; +, weak signal; ++, moderate signal; +++, strong signal.
All tumor-derived neurospheres maintained high bmi-1 expression both under proliferative and after differentiative conditions. In contrast, normal neurospheres had significantly less bmi-1 expression, which further declined after differentiation.
Tumor-Derived Neurospheres Incorporate into Rat Brain. To test whether tumor-derived progenitors possess the ability to migrate and differentiate after transplantation into rodent brain, similar to normal human progenitors (27, 37), 50,000 dissociated cells from neurospheres derived from BT4 at passage 4 were injected into the neostriata of neonatal rats. The data shown in Fig. 5, which is published as supporting information on the PNAS web site, demonstrate that the transplanted cells incorporate into the brain, have the characteristics of migrating neurons (38), differentiate into neuronal and glial elements, and continue to divide as demonstrated by expression of Ki-67, a marker of cycling cells (39).
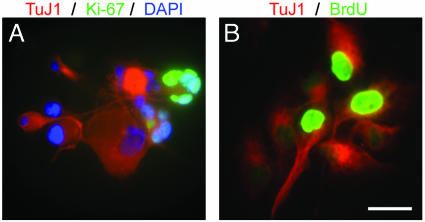
Tumor-Derived Neurospheres Are Different from Normal Neurospheres. We compared the properties of tumor-derived neurospheres with those of normal neurospheres cultured from the brain tissue of pediatric epilepsy surgery patients. The data suggest that tumor-derived progenitors are not derived from contaminating normal NSCs. Tumor-derived neurospheres could be maintained for at least 4 months with no obvious change in their proliferative properties, whereas normal neurospheres grown under identical conditions persisted no longer than 1 month in culture. Tumor-derived spheres demonstrated unusual proliferation even in apparently differentiated cells. We observed cells that expressed both β-III-tubulin and Ki-67, suggesting that cells with the appearance of differentiated neurons were dividing (BT4; Fig. 4A). Moreover, ≈50% of β-III-tubulin+ cells derived from this tumor incorporated BrdUrd after a 14-h pulse, indicating that a significant proportion of tumor-derived neurons divided over a brief time (Fig. 4B).
Fig. 4.
Tumor-derived neurospheres give rise to neurons that proliferate aberrantly. (A) Clonal neurosphere derived from the BT4 tumor, double-labeled for β-III-tubulin (red) and Ki-67 (green). Nuclei were counterstained with DAPI (blue). (B) Double-label for BrdUrd (green), visualized after a 14-h pulse and TuJ1 (red). (Scale bar in B = 15 μm in A and B.)
Another distinctive feature of tumor-derived progenitors was that they gave rise not only to neurons and glia but also to cells that expressed both β-III-tubulin and GFAP (Fig. 2). Such dual-fate cells were common and represented a significant fraction of the population. β-III-tubulin+ GFAP+ cells were often larger than other cells in the same sphere (Fig. 1G, arrows). In two tumors, BT1 and BT5, the percentage of β-III-tubulin+ GFAP+ cells exceeded the percentage of cells expressing β-III-tubulin or GFAP alone (Fig. 2). Previous studies have reported similar abnormal cells derived from adult brain tumors (40).
Discussion
In the present study, we asked whether pediatric brain tumors contain neural stem-like cells that may be responsible for their formation. Our results show that tumor-derived cells have the ability to form neurospheres and can be propagated for prolonged times in culture. This property is a general property of all of the 22 tumors examined in this study. We demonstrate that tumor-derived progenitors and NSCs express many of the same genes and proteins, and they share some common characteristics, including self-renewal and multipotency. However, the types of progeny arising from neurospheres vary from tumor to tumor and, to some extent, recapitulate the properties of their tumor of origin.
Among the characteristics in common between tumor-derived spheres and normal neural stem cells is the expression of specific genes, including CD133, musashi-1, Sox2, melk, PSP, bmi-1, and nestin. Only one of the tumors we studied, a medulloblastoma (BT3), failed to express nestin in most cells in undifferentiated clonal neurospheres. Interestingly, BT3 was the least neural stem cell-like of all of the tumors we tested, giving rise to a low percentage of multipotent neurospheres that lacked expression of most known stem cell genes. Sox2, a gene known to play a role in maintenance of the neural progenitor state (41) was expressed by all tumors and neurospheres tested. In addition, tumor-derived spheres express other markers that are specifically enriched in NSCs, such as maternal embryonic leucine zipper kinase and phosphoserine phosphatase (35, 36).
Although tumor-derived progenitors have many similarities to NSCs and to each other, it is important to note that differences exist between them and NSCs as well as between tumor-derived progenitors from different tumors. For example, unlike normal neurospheres, cancer-derived neurospheres undergo aberrant proliferation and differentiation. Clonally derived spheres from an individual tumor generally gave rise to similar percentages of neuron- and glial-like cells, suggesting that each tumor contained a fundamental type of stem cell. However, the degree of differentiation and types of cells produced differed from tumor to tumor, possibly reflecting a fundamental difference in the progenitor cells of different tumors.
One finding of our study is that the tumor-derived neurospheres tend to differentiate into an array of progeny with the same general profile as the parental tumor. Immunohistochemical analysis of the original tumor revealed striking similarities with the corresponding neurospheres, suggesting that the presence of diverse cell types in the tumors is not simply a result of the presence of “host” cells within the tumor mass, but rather is a property of the tumor itself.
Our finding that PBTs contain neural stem-like cells is consistent with the hypothesis that at least some PBTs are derived from transformed NSCs in the central nervous system. It has previously been hypothesized that medulloblastomas and primitive neuroectodermal tumors (PNETs) are derived from multipotent progenitor cells (8). Our data suggest that these tumors are somewhat similar to NSCs but more strongly suggest that pediatric astrocytomas and high-grade gliomas are derived from either NSCs or progenitor cells. Previous studies have demonstrated that adult high-grade gliomas can form multiple differentiated cell types (42), and that some cells can form neurospheres with multiple differentiated cell types (40). It is unknown whether the originating cells of PNETs, medulloblastomas and gliomas, may be the same type of cell, such as a NSC, but resulting from different transformation events, or whether the glial tumors arise from a cell at a later stage of differentiation. In the latter case, a tumor may arise from a glial cell that is caused to dedifferentiate by a transforming event or from a more gliogenic, but still multipotent, stem cell. In support of the latter hypothesis is the strong evidence to indicate that there is a lineage of cells that progresses from primitive cells within the neural tube to radial glia to GFAP-expressing “astrocytes” that all retain the fundamental properties of NSCs: self-renewal and multipotency (11, 43). Additionally, it is possible that medulloblastomas arise not from a true NSC, but rather from a different type of progenitor, such as a cerebellar granule cell. In any of the alternatives presented above, the ultimate result would still be that the tumors themselves contain multipotent self-renewing stem-like cells.
The mechanism whereby normal NSCs may become malignant is not clear. Our data demonstrate that normal and tumor-derived spheres express bmi-1, with sphere expression being maintained even after withdrawal of mitogen, in contrast to what was observed in normal neural progenitors. This gene has been demonstrated to be important for self-renewal of both leukemic and normal hematopoietic stem cells (33, 34). It is possible that the high level and persistent expression of bmi-1 in tumor cells indicates a greater capacity for self-renewing divisions.
Our data raise the possibility, but do not prove, that tumor-derived progenitors are cancer stem cells for PBTs. While we have shown that at least some tumor-derived progenitors, like normal NSCs, are able to migrate, proliferate, and differentiate when transplanted into developing rat brains, it is not yet known whether the tumor-derived progenitors also have the ability to form tumors in an animal model.
Knowledge of the developmental origin of PBTs has important implications for therapy. Our results demonstrate that these tumors contain cells that are multipotent and self-renewing and are consistent with the hypothesis that these tumors are derived from progenitor cells with stem-cell like properties. These observations suggest that therapies for treating PBTs should include methods for targeting and elimination of the stem cell population.
Note Added in Proof. After the final revision of this manuscript was submitted, another paper also demonstrating the presence of multipotent progenitors in brain tumors was published (44).
Supplementary Material
Acknowledgments
We are grateful to Simon Bababeygy, Benjamin Rafii, Miguel Minera, and Alexandra Lowry for laboratory assistance and patient recruitment, Keith Tatsukawa for performing animal surgeries, Lori Shoemaker for supplying neurosphere-conditioned medium, Gary Mathern, Dennis Chute, and Beth Johnson for brain specimens, and Bud Saxton and Marcos Paiva for their guidance and support. We thank Drs. Jeffrey Twiss and Paul Mischel for helpful comments on the manuscript. This work was supported by U.S. Public Health Service Grant NS42287 (to M.B.-F.), National Institute of Mental Health Grant MH65756 (to H.I.K.), and the Jonsson Comprehensive Cancer Center at the University of California, Los Angeles. H.D.H. was supported by the McCallum Fund at California Institute of Technology, Medical Scientist Training Program Grant GM08042, and the Aesculapians Fund of the David Geffen School of Medicine at the University of California, Los Angeles.
Abbreviations: PBTs, pediatric brain tumors; NSCs, neural stem cells; GFAP, glial fibrillary acidic protein; DAPI, 4′,6-diamidino-2-phenylindole.
References
- 1.Smith, M. A., Freidlin, B., Ries, L. A. & Simon, R. (1998) J. Natl. Cancer Inst. 90, 1269-1277. [DOI] [PubMed] [Google Scholar]
- 2.Sklar, C. A. (2002) J. Pediatr. Endocrinol. Metab. 15, Suppl. 2, 669-673. [DOI] [PubMed] [Google Scholar]
- 3.MacDonald, T. J., Rood, B. R., Santi, M. R., Vezina, G., Bingaman, K., Cogen, P. H. & Packer, R. J. (2003) Oncologist 8, 174-186. [DOI] [PubMed] [Google Scholar]
- 4.Brustle, O. & McKay, R. D. (1995) J. Neurooncol. 24, 57-59. [DOI] [PubMed] [Google Scholar]
- 5.Holland, E. C. (2000) Toxicol. Pathol. 28, 171-177. [DOI] [PubMed] [Google Scholar]
- 6.Holland, E. C., Celestino, J., Dai, C., Schaefer, L., Sawaya, R. E. & Fuller, G. N. (2000) Nat. Genet. 25, 55-57. [DOI] [PubMed] [Google Scholar]
- 7.Morrison, S. J. (2001) Curr. Biol. 11, R7-R9. [DOI] [PubMed] [Google Scholar]
- 8.Valtz, N. L., Hayes, T. E., Norregaard, T., Liu, S. M. & McKay, R. D. (1991) New Biol. 3, 364-371. [PubMed] [Google Scholar]
- 9.Sutton, L. N., Phillips, P. & Lange, B. (1992) Neurosurg. Clin. N. Am. 3, 821-837. [PubMed] [Google Scholar]
- 10.Koos, W. T. & Horaczek, A. (1985) Acta Neurochir. Suppl. (Wien) 35, 1-5. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Buylla, A. & Garcia-Verdugo, J. M. (2002) J. Neurosci. 22, 629-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tohyama, T., Lee, V. M., Rorke, L. B., Marvin, M., McKay, R. D. & Trojanowski, J. Q. (1992) Lab. Invest. 66, 303-313. [PubMed] [Google Scholar]
- 13.Rorke, L. B., Trojanowski, J. Q., Lee, V. M., Zimmerman, R. A., Sutton, L. N., Biegel, J. A., Goldwein, J. W. & Packer, R. J. (1997) Brain Pathol. 7, 765-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Almqvist, P. M., Mah, R., Lendahl, U., Jacobsson, B. & Hendson, G. (2002) J. Histochem. Cytochem. 50, 147-158. [DOI] [PubMed] [Google Scholar]
- 15.Gilbertson, R., Hernan, R., Pietsch, T., Pinto, L., Scotting, P., Allibone, R., Ellison, D., Perry, R., Pearson, A. & Lunec, J. (2001) Genes Chromosomes Cancer 31, 288-294. [DOI] [PubMed] [Google Scholar]
- 16.Taipale, J. & Beachy, P. A. (2001) Nature 411, 349-354. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler-Reya, R. & Scott, M. P. (2001) Annu. Rev. Neurosci. 24, 385-428. [DOI] [PubMed] [Google Scholar]
- 18.Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. (2001) Nature 414, 105-111. [DOI] [PubMed] [Google Scholar]
- 19.Bonnet, D. & Dick, J. E. (1997) Nat. Med. 3, 730-737. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. (2003) Proc. Natl. Acad. Sci. USA 100, 3983-3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds, B. A. & Weiss, S. (1992) Science 255, 1707-1710. [DOI] [PubMed] [Google Scholar]
- 22.Kleihues, P., Louis, D. N., Scheithauer, B. W., Rorke, L. B., Reifenberger, G., Burger, P. C. & Cavenee, W. K. (2002) J. Neuropathol. Exp. Neurol. 61, 215-225, and discussion 226-229. [DOI] [PubMed] [Google Scholar]
- 23.Svendsen, C. N., ter Borg, M. G., Armstrong, R. J., Rosser, A. E., Chandran, S., Ostenfeld, T. & Caldwell, M. A. (1998) J. Neurosci. Methods 85, 141-152. [DOI] [PubMed] [Google Scholar]
- 24.Geschwind, D. H., Ou, J., Easterday, M. C., Dougherty, J. D., Jackson, R. L., Chen, Z., Antoine, H., Terskikh, A., Weissman, I. L., Nelson, S. F. & Kornblum, H. I. (2001) Neuron 29, 325-339. [DOI] [PubMed] [Google Scholar]
- 25.Groszer, M., Erickson, R., Scripture-Adams, D. D., Lesche, R., Trumpp, A., Zack, J. A., Kornblum, H. I., Liu, X. & Wu, H. (2001) Science 294, 2186-2189. [DOI] [PubMed] [Google Scholar]
- 26.Imam, S. A., Young, L., Chaiwun, B. & Taylor, C. R. (1995) Anticancer Res. 15, 1153-1158. [PubMed] [Google Scholar]
- 27.Uchida, N., Buck, D. W., He, D., Reitsma, M. J., Masek, M., Phan, T. V., Tsukamoto, A. S., Gage, F. H. & Weissman, I. L. (2000) Proc. Natl. Acad. Sci. USA 97, 14720-14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lendahl, U., Zimmerman, L. B. & McKay, R. D. (1990) Cell 60, 585-595. [DOI] [PubMed] [Google Scholar]
- 29.Sakakibara, S., Nakamura, Y., Yoshida, T., Shibata, S., Koike, M., Takano, H., Ueda, S., Uchiyama, Y., Noda, T. & Okano, H. (2002) Proc. Natl. Acad. Sci. USA 99, 15194-15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaneko, Y., Sakakibara, S., Imai, T., Suzuki, A., Nakamura, Y., Sawamoto, K., Ogawa, Y., Toyama, Y., Miyata, T. & Okano, H. (2000) Dev. Neurosci. 22, 139-153. [DOI] [PubMed] [Google Scholar]
- 31.Zappone, M. V., Galli, R., Catena, R., Meani, N., De Biasi, S., Mattei, E., Tiveron, C., Vescovi, A. L., Lovell-Badge, R., Ottolenghi, S. & Nicolis, S. K. (2000) Development (Cambridge, U.K.) 127, 2367-2382. [DOI] [PubMed] [Google Scholar]
- 32.Cai, J., Wu, Y., Mirua, T., Pierce, J. L., Lucero, M. T., Albertine, K. H., Spangrude, G. J. & Rao, M. S. (2002) Dev. Biol. 251, 221-240. [DOI] [PubMed] [Google Scholar]
- 33.Lessard, J. & Sauvageau, G. (2003) Nature 423, 255-260. [DOI] [PubMed] [Google Scholar]
- 34.Park, I. K., Qian, D., Kiel, M., Becker, M. W., Pihalja, M., Weissman, I. L., Morrison, S. J. & Clarke, M. F. (2003) Nature 423, 302-305. [DOI] [PubMed] [Google Scholar]
- 35.Easterday, M. C., Dougherty, J. D., Jackson, R. L., Ou, J., Paucar, A. A., Roobini, B., Dianati, M., Irvin, D. K., Tershikh, A. V., Weissman, I. L., et al. (2003) Dev. Biol., in press. [DOI] [PubMed]
- 36.Terskikh, A. V., Easterday, M. C., Li, L., Hood, L., Kornblum, H. I., Geschwind, D. H. & Weissman, I. L. (2001) Proc. Natl. Acad. Sci. USA 98, 7934-7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brustle, O., Choudhary, K., Karram, K., Huttner, A., Murray, K., Dubois-Dalcq, M. & McKay, R. D. (1998) Nat. Biotechnol. 16, 1040-1044. [DOI] [PubMed] [Google Scholar]
- 38.Yang, H., Mujtaba, T., Venkatraman, G., Wu, Y. Y., Rao, M. S. & Luskin, M. B. (2000) Proc. Natl. Acad. Sci. USA 97, 13366-13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gerdes, J., Schwab, U., Lemke, H. & Stein, H. (1983) Int. J. Cancer 31, 13-20. [DOI] [PubMed] [Google Scholar]
- 40.Ignatova, T. N., Kukekov, V. G., Laywell, E. D., Suslov, O. N., Vrionis, F. D. & Steindler, D. A. (2002) Glia 39, 193-206. [DOI] [PubMed] [Google Scholar]
- 41.Graham, V., Khudyakov, J., Ellis, P. & Pevny, L. (2003) Neuron 39, 749-765. [DOI] [PubMed] [Google Scholar]
- 42.Mischel, P. S., Shai, R., Shi, T., Horvath, S., Lu, K. V., Choe, G., Seligson, D., Kremen, T. J., Palotie, A., Liau, L. M., et al. (2003) Oncogene 22, 2361-2373. [DOI] [PubMed] [Google Scholar]
- 43.Imura, T., Kornblum, H. I. & Sofroniew, M. V. (2003) J. Neurosci. 23, 2824-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh, S. K., Clarke, I. D., Terasaki, M., Bonn, V. E., Hawkins, C., Squire, J. & Dirks, P. B. (2003) Cancer Res. 63, 5821-5828. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.