Scientific Abstract
Magnetic resonance imaging (MRI) and postmortem neuropathological studies have implicated the cerebellum in the pathophysiology of autism. Controversy remains, however, concerning the nature and the consistency of cerebellar alterations. MRI studies of the cross-sectional area of the vermis have found both decreases and no difference in autism groups. Volumetric analysis of the vermis, which is less prone to “plane of section artifacts” may provide a more reliable assessment of size differences but few such studies exist in the literature. Here we present the results of a volumetric analysis of the structure of the whole cerebellum and its components in children and adolescents with autism spectrum disorders. Structural MRI’s were acquired from 62 male participants (7.5 to 18.5 years-old) who met criteria for the following age-matched diagnostic groups: low functioning autism, high functioning autism, Asperger syndrome, and typically-developing children. When compared to controls, the midsagittal area of the vermis, or of subgroups of lobules, was not reduced in any of the autism groups. However, we did find that total vermis volume was decreased in the combined autism group. When examined separately, the vermis of only the high functioning autism group was significantly reduced compared to typically-developing controls. Neither IQ nor age predicted the size of the vermis within the autism groups. There were no differences in the volume of individual vermal lobules or cerebellar hemispheres. These findings are discussed in relation to the pathology of autism and to the fairly common alterations of vermal morphology in various neurodevelopmental disorders.
Keywords: Asperger, MRI, developmental delays, vermis, neurodevelopmental disorder
Introduction
Autism is a neurodevelopmental disorder characterized by impaired social interaction, deficits in communication, and restricted activities or interests (American Psychiatric Association, 1994). The potential role of the cerebellum in the pathophysiology of autism spectrum disorders has been explored by many groups (Table 1). Subject ages and clinical characteristics in these studies vary widely and conclusions concerning the type and consistency of cerebellar pathology remain controversial. Some studies suggest that cognitive functions that are mediated, in part, by the cerebellum, such as imitation, are impaired in autism (Rogers, et al., 2003, Williams, et al., 2001).
Table 1.
Main findings on cerebellar structure in autism spectrum disorder
| First Author, Year | Age Range | ASD Group | Comparison Group(s) | Exclusion Criteria | Measurement | Primary Finding(s) | Statistical Significance |
|---|---|---|---|---|---|---|---|
| Courchesne E, 1988 | 6–30 years | 18 ASD 16 Male 2 Female |
12 Typically–developing (TD) 9 Male 3 Female |
Mental retardation Comorbid neurological conditions |
Area of vermal lobules I–V, V–VII, VIII ROI |
Reduced area of VI–VII | I–V, p>0.55 VI–VII, p=0.003 VIII, p>0.55 |
| Piven J, 1992 | 18–53 years | 15 ASD 1 subject, 8 years |
15 matched for IQ 15 matched for age and SES |
Seizure history Female |
Area of vermal lobules I–V, VI–VII, fourth ventricle, midsagittal brain area ROI |
Reduced area of VI–VII when compared to age-matched but not IQ-matched group No differences in the area of fourth ventricle Larger brain area in HFA |
Used ratio measures No difference after a multivariance anaylsis was adjusted for brain area, age and IQ |
| Holttum JR. 1992 | 11–42 years | 18 HFA | 18 TD matched for age, gender, SES, IQ, race | Mental retardation Comorbid neurological conditions Female |
Area of vermal lobules I–V, VI–VII, VIII, fourth ventricle ROI |
No differences found | I–V, p=0.34 VI–VII, p=0.31 VIII–X, p=0.15 |
| Kleiman MD, 1992 | 2.7–16.8 years | 13 ASD 3 Seizure history 10 Seizure history absence |
28 controls 11 Seizure history 17 Seizure history absence |
History of ataxia, tremor or clumsiness | Area of vermal lobules I–V, VI–VII Fourth ventricle height Computer-assisted planimetry |
No differences found in comparison of seizure history groups No differences found in comparison of absence of seizure history groups |
I–V, p=0.99 VI–VII, p=0.45 Fourth ventricle, p=0.17 |
| Courchesne E, 1994 | 2–40 years | 32 ASD 25 Male 7 Female |
41 TD 34 Male 7 Female |
Fragile X syndrome | Area of vermal lobules I–V, V–VII ROI |
Reduced area of VI–VII | I–V, p=0.71 VI–VII, p=0.031 |
| Hashimoto, T, 1995 | 3 month s-20 years | 102 ASD 76 Male 26 Females |
122 TD | Hyperactivity disorder | Area of vermal lobules I–V, VI–VII, VIII–X Area of brainstem: midbrain, pons, medulla oblongata ROI |
Accelerated growth of I–V, VI–VII, and pons Reduced area of vermis and brainstem structures |
Vermis, p=0.001 in ASD and TD Brainstem, p=0.001 in ASD and TD |
| Schaefer GB, 1996 | 0–90 years | 13 ASD | 125 TD 89 Neurogenetic Disorders |
None | Area of vermal lobules I–V, VI–VII, VIII–X | Reduced area of VI–VII in some Neurogenetic Disorder groups with and without autistic traits | VI–VII, p<0.05 in Retts syndrome and Chiari malformations |
| Piven J, 1997 | 12–29 years | 35 ASD 26 Male 9 Female |
36 TD 20 Male 16 Female |
Comorbid neurological conditions | Area of lobules I–V, VI–VII Volume of total cerebellum ROI |
No area difference in I–V, VI–VII Larger cerebellar volume without correction for TBV |
I–V, p=0.59 VI–VII, p=0.89 Uncorrected cerebellar volume, p=0.0002 Corrected cerebellar volume for TBV and IQ, p>0.05 |
| Manes F, 1999 | 6–21 years | 27 LFA | 17 matched for mental age | CARS Score >30 in comparison group IQ tested in only 4 of the comparison group and 1 LFA |
Area of I–V, VI–VII, VIII–X as a proportion of intracranial area ROI |
No difference in area | I–V, p=0.87 VI–VII, p=0.68 VIII–X, p=0.92 |
| Hardan AY, 2001 | 12–52 years | 22 ASD | 22 TD matched for IQ, age, race, SES, gender | Comorbid development al or neurological conditions Seizure history Female |
Volume of cerebellum and vermis Area of vermal lobules I–V, VI–VII, VIII–X ROI |
Larger cerebellar volume with correction for TBV No differences in vermal area |
Cerebellar volume, p=0.03 Cerebellar hemispheres, p=0.05 Vermis, p=0.23 |
| Courchesne E, 2001 | 2–16 | 60 ASD | 52 TD | Female Comorbid conditions PDD |
Gray and white matter volumes Area of vermal lobules I–V, VI–VII, VIII–X |
From 2 – 3 years, greater white matter in ASD Less gray matter in ASD at all ages Reduced VI–VII at all ages |
White matter volume, p<0.001 Gray matter volume, p<0.01 VI–VII area, p<0.05 one-tailed |
| Sparks BF, 2002 | 3–4.5 years | 45 ASD 38 Male 7 Female |
26 TD 14 DD 16 PDD-NOS |
Comorbid neurological conditions | Volume of cerebellum Cavalieri grid |
Larger cerebellar volume was proportional to TBV | Uncorrected cerebellar volume for ASD v. TD, p=0.03 Corrected for ASD v. TD, p>0.05 |
| Herbert MR, 2003 | 7–11 years | 17 ASD | 15 TD | Mental retardation Seizure history History of head injury Sensorimotor deficits Encephalopathy Female |
Volume of gray and white matter in total brain and cerebellum Voxel-based morphometry |
Larger cerebellar volume was proportional to TBV | Uncorrected cerebellar volume, p=0.009 Corrected cerebellar volume for TBV, p>0.05 |
| Kaufmann WE, 2003 | 3–9 years | 39 ASD 10 Idiopathic autism 16 Down Syndrome + autism 13 Fragile X + autism |
22 TD 11 Down Syndrome only 9 Fragile X only Matched for age |
History of mental health issues Female |
Area of vermal lobules I–V, VI–VII, VIII–X Vermal measures are expressed as ratios of intracranial area ROI |
Reduced area of VI–VII and VIII–X in FX + autism and DS + autism Reduced area of VI–VII in idiopathic autism VI–VII:intracranial area dependent on autism status only in FX |
DS:VI–VII, p=0.010 VIII–X, p=0.003 FX:VI–VII, p=0.003 VIII–X, p=0.086 ASD: VI–VII, p=0.052 Intracranial area in DS and DS + autism compared to TD, p=0.0001 |
| McAlonan GM, 2005 | 8–14 years | 17 HFA 16 Male 1 Female |
17 TD 16 Male 1 Female |
Co-morbid psychiatric or medical conditions Mental retardation History of head injury Fragile X syndrome |
Volume of gray and white matter and CSF in total brain and cerebellum Voxel-based morphometry |
Reduced total gray matter in ASD Increased CSF volume in ASD Decreased white matter in cerebellum in ASD |
Total Gray Matter, p=0.004 CSF, p=0.008 Total White Matter, NS Whole Brain Volume, NS |
| Palmen SJMC, 2005 | 7–15 years | 21 HFA, medication-naive | 21 TD | Epilepsy, head trauma, and other neurological illness Mental retardation |
Volume of gray and white matter and CSF in total brain and cerebellum Semi-automated method |
Larger cerebellar volume was proportional to TBV Larger CSF volume disproportionate to TBV |
Uncorrected cerebellar volume, p= 0.032 Uncorrected lateral ventricle p=0.032 Uncorrected third ventricle p=0.001 |
| Hazlett HC, 2005 | 1.5–3 years | 51 ASD | 14 TD 11 DD |
Epilepsy, Fragile X syndrome, head trauma, and other neurological illness | Volume of gray and white matter and CSF in total brain and cerebellum Estimation Maximization Software |
Cerebellar volume was not enlarged compared to TD and DD | NS differences in total volume, GM, or WM |
| Rojas DC, 2006 | 7–44 years | 24 ASD | 23 TD | Female Fragile X |
Voxel-based morphometry | Reduced left and right hemispheric lobule VII Reduced lobules VIII–IX along midline |
Reduced regions, p<0.05 with small volume corrections |
| Hallahan B, 2009 | 18–58 years | 80 ASP (71 Male) 28 ASD (21 Male) 6 PDD- NOS (4 Male) |
60 TD (53 Male) | Comorbid medical condition, head injury, psychosis, genetic disorder associated with autism | Volume of intracrainal space, cortical lobes, cerebellum, ventricular CSF, and peripheral CSF ROI |
Reduced cerebellum volume in all groups Increased peripheral CSF in all groups |
Reduced cerebellum and increased peripheral CSF, p<0.05 with intracranial volume as a covariate |
| Webb SJ, 2009 | 36–58 month s | 45 ASD (38 Male) | 26 TD (18 Male) 14 DD (6 Male) |
Comorbid medical condition, perinatal trauma, genetic disorder | Volume of the cerebrum and cerebellum; area of vermis lobules I–V, VI–VII, VIII–X | Reduced area of lobules I–V and VI–VII compared to TD DD reduced areas and volumes compared to TD |
Reduced regions, p<0.001 with age, gender and cerebellar volume as covariates |
Abbreviations: ASD-autism spectrum disorder; DD-developmental delay; DS-Down syndrome; FX-Fragile X syndrome; ROI-region of interest; TBV-total brain volume; TD-typically developing
Previous MRI studies have found that the midsagittal area of the vermis, particularly lobules VI–VII, is reduced in idiopathic autism and autism with co-morbid conditions, such as Down syndrome (Courchesne, et al., 1994, Courchesne, et al., 1988, Hashimoto, et al., 1995, Kaufmann, et al., 2003, Schaefer, et al., 1996). Other studies, however, have found no evidence of vermal hypoplasia in autism (Holttum, et al., 1992, Kleiman, et al., 1992, Manes, et al., 1999). A recent meta-analysis of the issue of vermal hypoplasia (Stanfield et al., 2008) concluded that the area of lobules I–V and VI–VII of the vermis are reduced in individuals with autism compared to controls (Stanfield, et al., 2008). Moreover, the authors suggested that the vermal reduction was negatively associated with age and IQ.
Postmortem neuropathological studies also suggest that the cerebellum may be abnormal in autism; lower Purkinje cell density has been reported for both the vermis and hemispheres (Bailey, et al., 1998, Kemper and Bauman, 2002). However, Whitney et al. (2008) recently suggested that only about half of postmortem autism cases show a lower density of Purkinje cells (Whitney, et al., 2008).
Part of the ambiguity related to cerebellar pathology in autism may also be due to limitations in the strategy employed in early MRI analyses. For example, most studies measured the midsagittal area of the vermis as well as defined lobule groups (Courchesne, et al., 1988, Gaffney, et al., 1987, Holttum, et al., 1992). The cross-sectional area measurement is dependent on only a single slice through the vermis. Since slight variations in the orientation of the head can profoundly affect the size of the vermal region, so called “plane of section” artifacts may be easily introduced. The midsaggital area measurement also does not assess more laterally placed portions of the vermis. Recent volumetric studies of the cerebellum have not addressed the question of vermal abnormalities (Hazlett, et al., 2005, McAlonan, et al., 2005, Palmen, et al., 2005) but examined total volume and tissue class volume differences.
The goal of the present study was to carry out a comprehensive MRI volumetric analysis of the cerebellum in male children and adolescents with low and high functioning autism, Asperger syndrome and typically-developing controls. Traditional midsaggital measurements were carried out with careful alignment of the brain. In addition, the volume of the vermis, vermal lobule groups and cerebellar hemispheres were measured.
Methods
Participants
A parent or guardian for each study participant gave informed consent, and children with typical cognitive development gave their assent, to participate in these studies as approved by the Institutional Review Board of the University of California, Davis. Study participants were recruited through local advocacy groups and the M.I.N.D. Institute clinic. Seventy-two male volunteers between the ages of 7.5 and 18.5 years participated in the study. All participants were healthy volunteers who met criteria in one of four diagnostic groups: low functioning autism (LFA, n=19), high functioning autism (HFA, n=19), Asperger syndrome (ASP, n=16), and typically-developing controls (CON, n=18).
Diagnostic assessments were conducted at the M.I.N.D. Institute clinic. The Autism Diagnostic Interview (ADI-R) (Lord, et al., 1994) and Autism Diagnostic Observation Schedule (ADOS-G) (DiLavore, et al., 1995, Lord, et al., 2000) were administered by a clinician (B.L.G.J.), who had previously obtained reliability with an author of these measures (C. Lord). An IQ exam was administered to all participants. Depending on verbal ability, the appropriate test was used from the following: the Wechsler Intelligence Scale for Children, the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) or the non-verbal Leiter International Performance Scale-Revised (Roid and Miller, 1997). A full scale IQ of 70 divided the high and low functioning autistic groups. Exclusionary criteria included diagnosis of Fragile X, seizure disorder, tuberous sclerosis, a primary diagnosis of obsessive-compulsive disorder, bipolar disorder, or any other major neurological illness. Details of the diagnostic assessments are available in a previous publication that included this cohort of subjects (Schumann, et al., 2004).
Neuroimaging
A parent or guardian for each participant was present throughout the duration of the scan in an adjacent waiting room. Those study participants requiring anesthesia to undergo MRI were imaged at the UC Davis Hospital on a 1.5T GE Signa NV/I system (LFA, n=19; HFA, n=13; ASP=7). All remaining participants were scanned at the UC Davis Research Imaging Center on a 1.5T GE Signa NV/I system (HFA, n=6; ASP, n=9; CON, n=18). These systems were calibrated prior to scan acquisition and similar image acquisition on both scanners was experimentally validated (Lotspeich, et al., 2004).
The protocol for scanning each participant included a three-dimensional coronal SPGR series (TR: 35 ms, TE: 6 ms, FOV: 24 cm, matrix: 256×256, section thickness: 1.5 mm, number of slices: 124, total scan time: 14:24 min), which was used for the volumetric assessment of the cerebellum. In addition, a two-dimensional sagittal T1 spin echo, two-dimensional PD/T2 interleaved double echo, and a diffusion tensor sequence were collected on all participants for other analyses.
Upon review of the images, ten participants were excluded from the study due to excessive movement, distorted images resulting from orthodontics, or additional diagnostic information that precluded the series from being used (LFA, n=1; HFA, n=4; ASP=1; CON, n=4). Within each diagnostic group, excluded participants did not differ from included participants with respect to age, IQ, or symptom severity.
Structural analysis
Each coronal SPGR series was imported into ANALYZE 6.0 (Robb, et al., 1989) and converted to cubic voxel dimensions of 0.9375 mm using a cubic spline interpolation algorithm. Images were reoriented along an axis through the anterior and posterior commissures. Measurements of total cerebral volume used in the current study were described in a previous report (Schumann, et al., 2004). Briefly, each series of images was edited manually to remove non-brain structures, the brainstem, and the cerebellum. Using a Gaussian cluster multispectral thresholding tool, the ventricles were defined and excluded. Total cerebral volume was calculated from a mask of the remaining brain tissue.
Prior to volumetric analyses, the midsagittal area of the cerebellar vermis was measured (Fig. 1.A). The vermis was outlined on a single section that approximated as closely as possible the midline of the brain. The vermis was subdivided into lobule groups including lobules I–V, VI–VII, and VIII–X along the primary and prepyramidal fissures.
Figure 1.
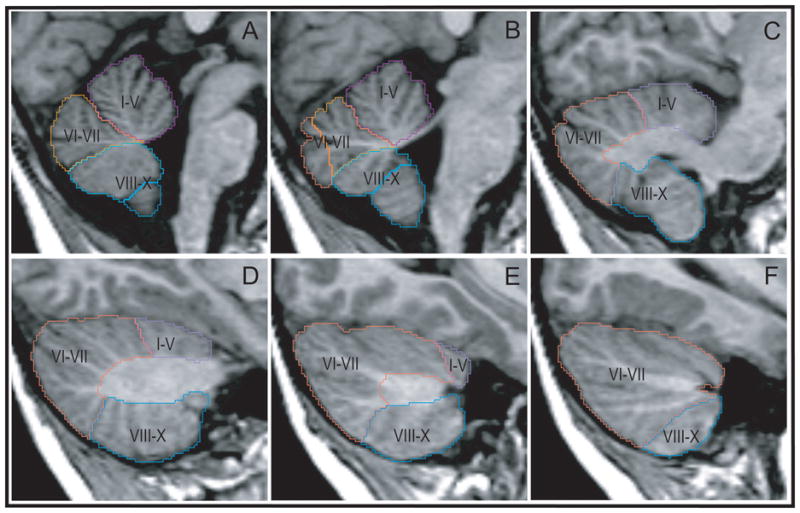
Sagittal series of MRI sections illustrating lobar segmentation of the cerebellum. Panels are arranged from midsagittal (top left) to lateral (bottom right). Lobule groups: I–V, lobules one through five; VI–II, lobules six through seven; VIII–X, lobules eight through ten. Light colored profiles are of vermal lobule groups; darker colors indicate hemispheric lobule groups.
The whole cerebellar volume was also measured. The total volume was segmented into a medullary core (the central white matter and deep nuclei of the cerebellum), the hemispheres (cortex and white matter), and the vermis (midline region of the cerebellum) (Fig. 2.A). The vermis and hemispheres were separately subdivided into lobules I–V, VI–VII, and VIII–X (Fig. 1 and Fig. 2.B). All structures were manually defined by a set of raters who achieved greater than 0.96 inter- and intra-rater reliability on each of the structures. The MRI Atlas of the Cerebellum (Schmahmann, et al., 2000) and The Human Cerebellum (Angevine, et al., 1961) were closely consulted in the development of the region of interest tracing protocols. Detailed protocols for analysis of all of the cerebellar structures are provided in the Supplemental Materials.
Figure 2.
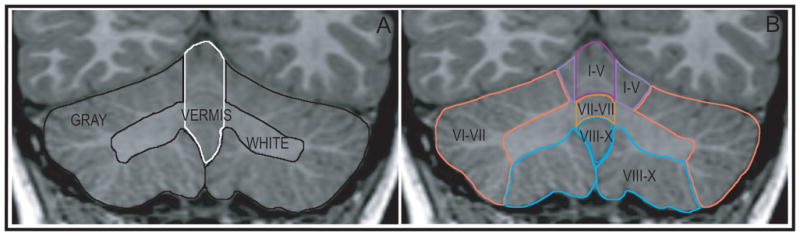
Coronal sections illustrating segmentation into whole cerebellar structures (A) and lobar structures (B). GRAY includes the cortex of the hemispheres; WHITE includes the medullary core and deep nuclei.
Statistical analyses
All statistical analyses were conducted with SPSS 16.0 (SPSS Inc., Chicago, Illinois). Prior to analysis of the anatomical data, age and IQ were compared between groups by an analysis of variance (ANOVA) to detect any group differences. Tukey’s post hoc test followed up on any main effects.
Due to the small and uneven sample size of the groups, there was a potential for the data to be distributed non-parametrically or adversely influenced by outliers. Tests of kurtosis and skewness were conducted on each anatomical measure to determine which type of analysis of variance should be used. The data were sufficiently normally distributed and only one measure, medullary core, was skewed with a right-handed tail.
Since all of the data were normally distributed, univariate and multivariate general linear models (GLM) were used to compare anatomical structures between groups. Age and total cerebral volume were entered as covariates in each analysis. Simple contrasts were made with the typically-developing group as the reference category. Tests were conducted at each anatomical level. Univariate GLM was applied to vermis area and a multivariate GLM was applied to the analysis of the area of vermal lobule groups. A univariate GLM was used for the cerebellum volume. Separate multivariate GLM were conducted for major parts of the cerebellum, and the lobule groups on the left and right hemispheres and vermis. A significance level of a two-tailed alpha of 0.05 was selected a priori. These analyses were repeated for comparison of the collective autism spectrum group to the typically-developing group without specific contrasts.
Subregions of the cerebellum were also analyzed as a ratio to the total cerebellar volume (i.e. normalized). Multivariate GLM, with simple contrasts, were repeated for the normalized volumes (the major parts of the cerebellum, and the lobule groups of the left hemisphere, right hemisphere, and the vermis).
The potential relationships between age and IQ and the anatomical measures within each group were evaluated by linear regression. A regression analysis was also performed for vermis volume in which vermis area was a regressor. This regression tested the degree to which the midsagittal area measurement predicted the volume measurement. A Pearson’s correlation analysis between total cerebellar volume and total cerebral volume determined whether the cerebellum was proportional to the cerebrum.
Results
Age and IQ measures
Group demographics are summarized in Table 2. There was no difference in the mean age of the groups at the time of MRI acquisition. There was a significant group effect for full scale IQ (p<0.001). As expected, post hoc tests indicated that full scale IQ for the low functioning autism group was lower than all other groups (p<0.01). The full scale IQ for high functioning autism and Asperger syndrome were also lower than the group of typically-developing controls (p<0.01 and p<0.05, respectively). Both verbal and performance IQ were compared between the high functioning autism, Asperger syndrome and control groups. The verbal IQ for high functioning autism was lower than Asperger syndrome (p<0.05) and controls (p<0.05). The performance IQ for the high functioning group was lower than for the control group (p<0.01).
Table 2.
Participant Demographics
| LFA (n=18) | HFA (n=15) | ASP (n=15) | CON (n=14) | |
|---|---|---|---|---|
| Age in years | 13.1 (3.0) | 11.7 (3.2) | 12.3 (3.2) | 12.5 (3.1) |
| Full scale IQ | 56 (10)** | 88 (16)** | 97 (17)* | 113 (12) |
| Verbal IQ | n/a | 86 (21)** | 105 (23) | 110 (14) |
| Performance IQ | n/a | 92 (13)* | 98 (32) | 115 (14) |
p<0.05,
p<0.01 when compared to CON
Midsagittal area of the vermis
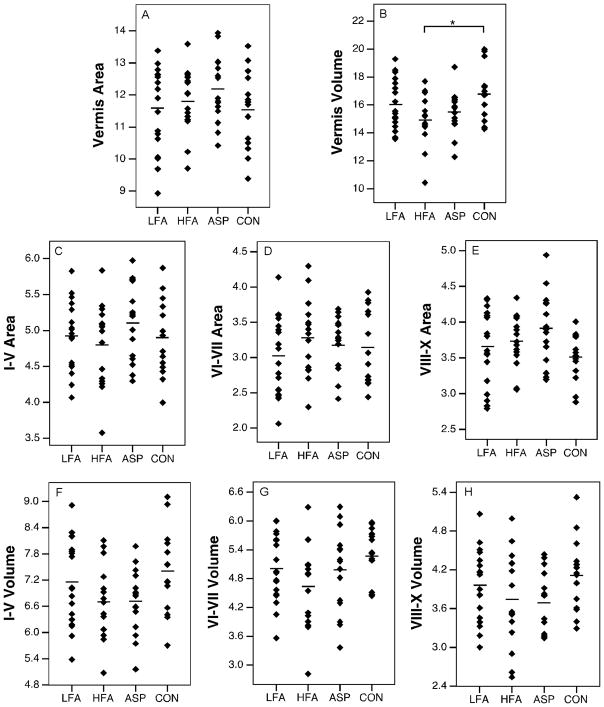
Uncorrected areal measurements are summarized in Table 3. When all autism groups were analyzed together, total vermis area was not reduced relative to controls (p=0.37) (Fig. 3). Neither were there significant differences when the vermis was broken down into lobule groups (I–V, VI–VII, VIII–X) (p>0.067). When autism groups were separated into low functioning autism, high functioning autism, and Asperger syndrome, similar findings were obtained. The vermis area was not reduced in any of the autism groups (p=0.38) (Fig. 4.A) and the areas of lobule groups were not significantly different between groups (p>0.11) (Fig. 4.C–E). Age and IQ did not predict the area of the vermis in any of the groups (for all groups, beta<0.416, p>0.15).
Table 3.
Midsagittal Area Data
| LFA (n=18) | HFA (n=15) | ASP (n=15) | ASD (n=48) | CON (n=14) | |
|---|---|---|---|---|---|
| Vermis | 11.59 (1.3) | 11.81 (1.0) | 12.19 (1.0) | 11.8 (1.1) | 11.54 (1.2) |
| I–V | 4.92 (.47) | 4.79 (.58) | 5.10 (.54) | 4.9 (.50) | 4.89 (.54) |
| VI–VII | 3.02 (.55) | 3.28 (.54) | 3.17 (.41) | 3.2 (.50) | 3.14 (.53) |
| VIII–X | 3.66 (.53) | 3.73 (.36) | 3.91 (.50) | 3.8 (.50) | 3.50 (.32) |
Mean (standard deviation) of volumes measured in square centimeters.
Figure 3.
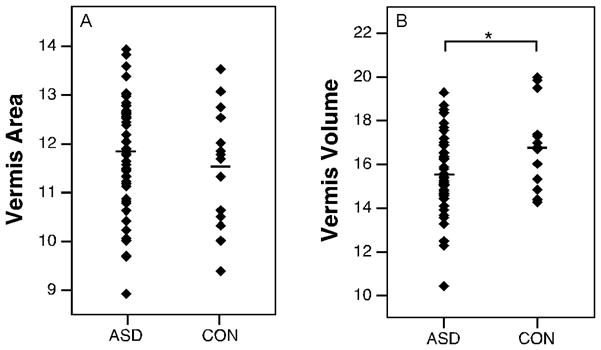
Scatter plots with means of volumes (cm3) and areas (cm2) of vermis structures collapsed across autism spectrum disorder groups. ASD, autism spectrum disorder; CON, typically-developing. (* p<0.05)
Figure 4.
Scatter plots with means (indicated by horizontal lines) of volumes (cm3) and areas (cm2) of vermal structures: total vermis area and volume (top panel), vermis area of lobule groups (middle panel), vermis volume of lobules groups (bottom panel). ASP, Asperger syndrome; HFA, high functioning autism; LFA, low functioning autism. (* p<0.05)
Cerebellum and major subregions
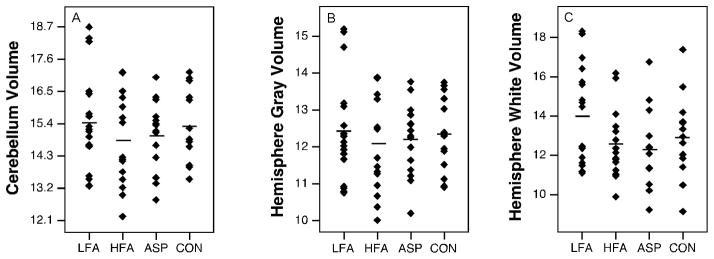
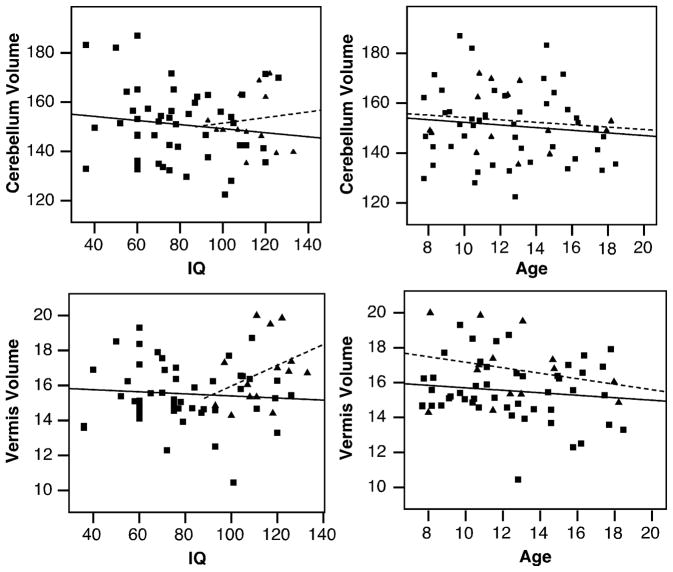
Volume measurements are summarized in Table 4. Total cerebellar volume did not differ between the diagnostic groups (p=0.509) (Fig. 5.A). In an analysis of the major subregions of the cerebellum (right and left hemispheres, medullary core and vermis) across each diagnostic group, only the vermis showed a main effect of group (raw: p=0.016, pEta2=0.166, normalized: p=0.143). The high functioning autism group in particular had a smaller vermis volume than the control group (raw: p=0.002; normalized: p=0.022) (Fig. 4.B). When data for the collective autism group was compared to the typically-developing group, the vermis volume was significantly smaller (raw: p=0.019, pEta2=0.091; normalized: p=0.039, pEta2=0.071) (Fig. 3.B). Raw and normalized volumes were compared for the lobule groups (I–V, VI–VII, and VIII–X) of the vermis (Fig. 4.F–H). None of the lobule groups were reduced in low functioning autism, high functioning autism, or Asperger syndrome (for all comparisons, p>0.097). When diagnostic groups were collapsed, no differences were found in these structures (for all comparisons, p>0.062). The effects of age and IQ on the volume measurements were tested (Fig. 6). These were not significant regressors for cerebellar volume in any of the groups (for all groups, beta<0.315, p>0.22). No significant relationships between age and IQ and vermis volume were found (for all groups, beta<0.361, p>0.137).
Table 4.
Volumetric Data
| LFA (n=18) | HFA (n=15) | ASP (n=15) | ASD (n=48) | CON (n=14) | |
|---|---|---|---|---|---|
| Total Cerebral Volume | 1237 (160) | 1219 (107) | 1181 (85) | 1195 (201) | 1190 (78) |
| Total Cerebellar Volume | 154.4 (16.7) | 148.8 (15.8) | 149.8 (11.6) | 151.0 (14.9) | 152.4 (11.8) |
| Cerebellar Subregions | |||||
| Vermis | 16.0 (1.8) | 14.9 (1.8) * | 15.5 (1.5) | 15.5 (1.7) * | 16.8 (1.9) |
| Right Hemisphere | 62.3 (6.9) | 60.0 (6.8) | 60.6 (4.9) | 61.0 (6.2) | 61.6 (5.2) |
| Left Hemisphere | 62.1 (7.1) | 60.8 (6.7) | 61.4 (4.9) | 61.5 (6.2) | 61.8 (5.0) |
| Core | 14.0 (2.5) | 12.6 (1.8) | 12.2 (1.9) | 13.0 (1.7) | 12.9 (2.1) |
| Vermal Lobules | |||||
| I–V | 7.16 (.99) | 6.70 (.86) | 6.71 (.75) | 6.87 (.89) | 7.4 (.97) |
| VI–VII | 5.01 (.71) | 4.63 (.89) | 4.97 (.86) | 4.88 (.82) | 5.27 (.58) |
| VIII–X | 3.96 (.56) | 3.74 (.74) | 3.69 (.48) | 3.81 (.60) | 4.11 (.57) |
| Right Hemisphere Lobules | |||||
| I–V | 5.30 (1.6) | 5.03 (1.2) | 5.57 (1.1) | 5.30 (1.34) | 5.33 (1.5) |
| VI–VII | 41.2 (4.7) | 40.1 (5.3) | 39.7 (3.1) | 40.39 (4.45) | 41.5 (2.8) |
| VIII–X | 15.7 (2.5) | 14.8 (1.7) | 15.4 (2.1) | 15.26 (2.10) | 14.9 (2.3) |
| Left Hemisphere Lobules | |||||
| I–V | 5.31 (1.1) | 5.01 (1.2) | 5.17 (1.3) | 5.17 (1.17) | 4.91 (1.3) |
| VI–VII | 40.4 (5.6) | 39.0 (5.2) | 40.2 (2.7) | 39.89 (4.68) | 40.7 (2.4) |
| VIII–X | 16.4 (1.7) | 16.7 (1.9) | 15.8 (2.9) | 16.29 (2.17) | 15.9 (2.6) |
Mean (standard deviation) of areas measured in cubic centimeters.
p<0.05, when compared to CON
Figure 5.
Scatter plots with means of volumes (cm3) of cerebellum and gray and white matter of the hemispheres. (* p<0.05)
Figure 6.
Linear regression of total cerebellum and vermis volumes for IQ and age variables. Solid line, ASD; Dashed line, CON.
Correlation analyses indicated that cerebellar and cerebral volumes were highly associated (Pearson=0.295, p=0.02). Across all subjects, vermis volume was not predicted by the midsagittal area of the vermis (beta=0.086, p=0.509).
Discussion
We have carried out a comprehensive MRI analysis of the whole cerebellum and its subregions in children 7.5 to 18.5 years of age with autism spectrum disorder. Carefully conducted midsagittal areal measurement of the vermis did not reveal any differences between the autism groups and controls. We also found that the total cerebellar volume did not differ between those with autism and typically-developing controls. However, the volume of the vermis, but not any particular lobule group, was reduced in the autism spectrum group. Somewhat surprisingly, the reduction in vermal volume was most prominent in the high functioning autism group. This difference was not due to differences in IQ, as IQ was not a significant predictor of vermal volume.
Comparison with previous findings
Hypoplasia of the vermis, especially of lobules VI–VII, has been highlighted as a prominent component of the neuropathology of autism (Courchesne et al, 1988). However, as indicated in Table 1, this finding is inconsistently observed across studies. In fact, even the Courchesne group has suggested that subgroups of individuals with autism demonstrate either hypoplasia or hyperplasia of the vermis (Courchesne et al., 1994). Our area measurements of the vermis in a sample of 48 children and adolescents with autism did not detect any reliable differences in comparison to typically developing controls. Given the substantial heterogeneity in reports related to vermal area in autism (Stanfield, et al., 2008 - Table 1), the conclusion that vermal hypoplasia is not consistently seen across all individuals with autism appears to be warranted. It will be interesting to determine what phenotypes of autism may be more consistently associated with vermal hypoplasia.
Although we did not find a difference in the midsagittal area of the vermis, we did find evidence for a decreased volume of the vermis in the autism spectrum group. Individual comparisons of the diagnostic groups indicated that this difference was driven primarily by a smaller vermis in the individuals with autism and IQ greater than 70 (high functioning autism group). In a previous study of cerebellar volumes carried out in autistic males with a broader age range (12–52), Hardan et al. (2001) found that hemispheric and total cerebellar volumes were enlarged, but vermal volume was not different from controls (cross sectional area of the vermis was not different in this study either). The authors concluded that the enlargement of the cerebellum was in line with a more general increase in brain size that they and others had observed. Our cohort of males (aged 8.5–17.5) did not demonstrate an overall increased brain volume, though the volume of the cerebellum was highly correlated with the volume of the cerebrum. The surprising finding in our study was that the high functioning autism group was the group that had a reduction in vermal volume. Even in this group, however, there was no difference in the midline area measurement. This must mean that the more laterally situated portions of the vermis were smaller in this group. We have no good explanation for why a vermal volume reduction was observed in the high functioning autistic group and not in either those individuals with low functioning autism or Asperger syndrome, with the caveat that the low functioning autism group also exhibited varying levels of mental retardation, which may confound their neuropathological profile. Additionally, the sample size in the current study is insufficient to handle the inherent heterogeneity of the autism cohort to parcellate sub-phenotypes of autism or isolate incidental findings.
Interestingly, vermal hypoplasia in general as well as specifically in lobules VI–VII has been found in other neurodevelopmental disorders (Ciesielski, et al., 1997, Soto-Ares, et al., 2003). Kaufmann and colleagues, for example, examined vermis area in autism with and without the presence of Down syndrome or fragile X syndrome (Kaufmann, et al., 2003). Reductions in lobules VI–VII were found in groups with single diagnoses and in the dual diagnosis of autism and Down syndrome. Similar outcomes were observed in another study in which vermal hypoplasia was found in both neurogenetic disorders with and without autistic traits (Schaefer, et al., 1996). Other studies looking at developmental disabilities, such as non-specific mental retardation and juveniles treated with radiation and chemotherapy, also report vermis size reduction when compared to controls, particularly in lobules VI–VII (Ciesielski, et al., 1997, Soto-Ares, et al., 2003). In fact, several studies of non-autism neurodevelopmental disorders, including Dandy-Walker syndrome (Aldinger et al., 2009), attention deficit/hyperactivity disorder (Curatolo et al., 09), fetal alcohlol syndrome (Astley et al., 09) and chromosome 22q deletion syndrome (Bish et al., 2006) find vermal hypoplasia, which indicates that this form of neuropathology is not specific to autism but a common feature of atypical development.
IQ and Age Correlates
Previous studies have suggested that reductions in the volume of the vermis in individuals with autism may be related to IQ or age. Early on, IQ was suggested to be a major factor in vermis reductions (Piven, et al., 1992). A recent meta-analysis found that lower IQ does appear to be associated with greater reduction in the area of vermal lobules VI–VII in autistic groups (Stanfield, et al., 2008). In the current study, which included individuals with a broad range of IQ scores, regression analyses indicated that IQ was not significantly related to the vermal area or volume. We also found that none of the cerebellar measures were correlated with age in either the control or autism groups. The human brain typically undergoes a rapid period of growth in the first five years of life, followed by gradual growth into adolescence (Dekaban and Sadowsky, 1978, Giedd, et al., 1996, Knickmeyer, et al., 2008). Other studies of cerebellar volume in the age range we measured have also not reported a correlation with age (Herbert, et al., 2003, Palmen, et al., 2005).
Conclusion
In this study of children and adolescents with autism spectrum disorders, we did not replicate the finding of a reduced area of vermal lobules VI–VII. We did however, observe a decrease of overall vermal volume in this population; this finding was driven primarily by observations from the high functioning autism group. This came in the context of no global cerebellar volume changes in the autism spectrum group. Since the vermis appears to be vulnerable to a variety of neurodevelopmental disorders and insults, it is not surprising that it is also pathological in some individuals with autism. However, given the current finding in the context of previous studies, this form of neuropathology is neither a specific nor a sensitive biological signature of autism.
Supplementary Material
Acknowledgments
We thank Meridith Brandt for participant recruitment and scheduling at UC Davis, John Ryan for his assistance in carrying out MRI acquisition, and Kristine Strohbin, Carolynn Nolte and Jonathan Lee for assisting in anatomical segmentation. We also wish to thank the study participants and their families for their contribution.
Grant Sources: NIH MH41479, MH01832, MH01142, MH50047, NS16980, and HD31715 andby the UC Davis M.I.N.D. Institute.
Works Cited
- Aldinger KA, Lehmann OJ, Hudgins L, Chizhikov VV, Bassuk AG, Ades LC, Krantz ID, Dobyns WB, Millen KJ. FoxC1 is required for normal cerebellar development and is a major contributor to chromosome 6p25.3 Dandy-Walker malformation. Nat Genet. 2009;41(9):1037–1042. doi: 10.1038/ng.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angevine JB, Mancall EL, Yakovlev P. The Human Cerebellum: An Atlas of Gross Topography in Serial Sections. Great Britain: J&A Churchill Ltd; 1961. [Google Scholar]
- Astley SJ, Aylward EH, Olson HC, Kerns K, Brooks A, Coggins TE, Davies J, Dorn S, Gendler B, Jirikowic T, Kraegel P, Maravilla K, Richards T. Magnetic Resonance Imaging Outcomes From a Comprehensive Magnetic Resonance Study of Children With Fetal Alcohol Spectrum Disorders. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.01004.x. epub Jul 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Luthert P, Dean A, Harding B, Janota I, Montgomery M, Rutter M, Lantos P. A clinicalpathological study of autism. Brain. 1998;121:889–905. doi: 10.1093/brain/121.5.889. [DOI] [PubMed] [Google Scholar]
- Bish JP, Pendyal A, Ding L, Ferrante H, Nguyen V, McDonald-McGinn D, Zackai E, Simon TJ. Specific cerebellar reductions in children with chromosome 22q11.2 deletion syndrome. Neurosci Lett. 2006;22(399):245–248. doi: 10.1016/j.neulet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Ciesielski K, Harris R, Hart B, Pabst H. Cerebellar hypoplasia and frontal lobe cognitive deficits in disorders of early childhood. Neuropsychologia. 1997;35(5):643–655. doi: 10.1016/s0028-3932(96)00119-4. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Yeung-Courchesne R, Press G, Hesselink J, Jernigan T. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318(21):1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Townsend J, Saitoh O. The brain in infantile autism: posterior fossa structures are abnormal. Neurology. 1994;44(2):214–223. doi: 10.1212/wnl.44.2.214. [DOI] [PubMed] [Google Scholar]
- Curatolo P, Paloscia C, D’Agati E, Moavero R, Pasini A. The neurobiology of attention deficit/hyperactivity disorder. Eur J Paediatr Neurol. 2009;13(4):299–304. doi: 10.1016/j.ejpn.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Dekaban A, Sadowsky D. Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Ann Neurol. 1978;4(4):345–356. doi: 10.1002/ana.410040410. [DOI] [PubMed] [Google Scholar]
- DiLavore P, Lord C, Rutter M. The pre-linguistic autism diagnostic observation schedule. J Autism Dev Disord. 1995;25(4):355–379. doi: 10.1007/BF02179373. [DOI] [PubMed] [Google Scholar]
- Gaffney GR, Tsai LY, Kuperman S, Minchin S. Cerebellar structure in autism. American journal of diseases of children. 1987;141(12):1330–1332. doi: 10.1001/archpedi.1987.04460120096044. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Hardan A, Minshew N, Harenski K, Keshavan M. Posterior fossa magnetic resonance imaging in autism. Journal of American Acadamey of Child and Adolescent Psychiatry. 2001;40(6):666–672. doi: 10.1097/00004583-200106000-00011. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Tayama M, Murakawa K, Yoshimoto T, Miyazaki M, Harada M, Kuroda Y. Development of the brainstem and cerebellum in autistic patients. Journal of Autism and Developmental Disorders. 1995;25(1):1–18. doi: 10.1007/BF02178163. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe M, Gerig G, Smith RG, Provenzale J, Ross A, Gilmore J, Piven J. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Archives of General Psychiatry. 2005;62(12):1366–1376. doi: 10.1001/archpsyc.62.12.1366. [DOI] [PubMed] [Google Scholar]
- Herbert MR, Ziegler DA, Deutsch CK, O’Brien LM, Lange N, Bakardjiev A, Hodgson J, Adrien KT, Steele S, Makris N, Kennedy D, Harris GJ, Caviness V. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- Holttum J, Minshew N, Sanders R, Phillips N. Magnetic resonance imaging of the posterior fossa in autism. Biological Psychiatry. 1992;32(12):1091–1101. doi: 10.1016/0006-3223(92)90189-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cooper KL, Mostofsky SH, Capone GT, Kates WR, Newschaffer CJ, Bukelis I, Stump MH, Jann AE, Lanham DC. Specificity of cerebellar vermian abnormalities in autism: a quantitative magnetic resonance imaging study. J Child Neurol. 2003;18(7):463–470. doi: 10.1177/08830738030180070501. [DOI] [PubMed] [Google Scholar]
- Kemper TL, Bauman ML. Neuropathology of infantile autism. Molecular Psychiatry. 2002;7(Suppl 2):S12–13. doi: 10.1038/sj.mp.4001165. [DOI] [PubMed] [Google Scholar]
- Kleiman M, Neff S, Rosman N. The brain in infantile autism: are posterior fossa structures abnormal? Neurology. 1992;42(4):753–760. doi: 10.1212/wnl.42.4.753. [DOI] [PubMed] [Google Scholar]
- Knickmeyer RC, Gouttard S, Kang C, Evans D, Wilber K, Smith JK, Hamer RM, Lin W, Gerig G, Gilmore JH. A structural MRI study of human brain development from birth to 2 years. Journal of Neuroscience. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–689. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook E, Leventhal B, DiLavore P, Pickles A, Rutter M. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- Lotspeich L, Kwon H, Schumann C, Fryer S, Goodlin-Jones B, Buonocore M, Lammers C, Amaral D, Reiss A. Investigation of neuroanatomical differences between autism and Asperger syndrome. Arch Gen Psychiatry. 2004;61(3):291–298. doi: 10.1001/archpsyc.61.3.291. [DOI] [PubMed] [Google Scholar]
- Manes F, Piven J, Vrancic D, Nanclares V, Plest C, Starkstein S. An MRI Study of the Corpus Callosum and Cerebellum in Mentally Retarded Autistic Individuals. Journal of Neuropsychiatry and Clinical Neuroscience. 1999;11(4):470–474. doi: 10.1176/jnp.11.4.470. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Yip L, Murphy DG, Chua SE. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain. 2005;128(Pt 2):268–276. doi: 10.1093/brain/awh332. [DOI] [PubMed] [Google Scholar]
- Palmen SJ, Hulshoff Pol HE, Kemner C, Schnack HG, Durston S, Lahuis BE, Kahn RS, Van Engeland H. Increased gray-matter volume in medication-naive high-functioning children with autism spectrum disorder. Psychol Med. 2005;35(4):561–570. doi: 10.1017/s0033291704003496. [DOI] [PubMed] [Google Scholar]
- Piven J, Nehme E, Simon J, Barta P, Pearlson G, Folstein S. Magnetic resonance imaging in autism: measurement of the cerebellum, pons, and fourth ventricle. Biol Psychiatry. 1992;31(5):491–504. doi: 10.1016/0006-3223(92)90260-7. [DOI] [PubMed] [Google Scholar]
- Robb R, Hanson D, Karwoski R, Larson A, Workman E, Stacy M. Analyze: a comprehensive, operator-interactive software package for multidimensional medical image display and anaylsis. Comput Med Imaging Graph. 1989 doi: 10.1016/0895-6111(89)90285-1. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn SL, Stackhouse T, Wehner E. Imitation performance in toddlers with autism and those with other developmental disorders. J Child Psychol Psychiatry. 2003;44(5):763–781. doi: 10.1111/1469-7610.00162. [DOI] [PubMed] [Google Scholar]
- Roid G, Miller L. Leiter International Performance Scale-revised. Illinois: Stoelting; 1997. [Google Scholar]
- Schaefer GB, Thompson JN, Bodensteiner JB, McConnell JM, Kimberling WJ, Gay CT, Dutton WD, Hutchings DC, Gray SB. Hypoplasia of the cerebellar vermis in neurogenetic syndromes. Ann Neurol. 1996;39(3):382–385. doi: 10.1002/ana.410390316. [DOI] [PubMed] [Google Scholar]
- Schmahmann J, Doyon J, Toga A, Petrides M, Evans A. MRI Atlas of the Human Cerebellum. San Diego, CA: 2000. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Hamstra J, Goodlin-Jones BL, Lotspeich LJ, Kwon H, Buonocore MH, Lammers CR, Reiss AL, Amaral DG. The Amygdala Is Enlarged in Children But Not Adolescents with Autism; the Hippocampus Is Enlarged at All Ages. Journal of Neuroscience. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Ares G, Joyes B, Lemaitre MP, Vallee L, Pruvo JP. MRI in children with mental retardation. Pediatric Radiology. 2003;33:334–345. doi: 10.1007/s00247-003-0891-z. [DOI] [PubMed] [Google Scholar]
- Stanfield AC, McIntosh AM, Spencer MD, Philip R, Gaur S, Lawrie SM. Towards a neuroanatomy of autism: a systematic review and meta-analysis of structural magnetic resonance imaging studies. Eur Psychiatry. 2008;23(4):289–299. doi: 10.1016/j.eurpsy.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Sparks BF, Friedman SD, Shaw DW, Giedd J, Dawson G, Dager SR. Cerebellar vermal volumes and behavioral correlates in children with autism spectrum disorder. Psychiatry Res. 2009;172(1):61–67. doi: 10.1016/j.pscychresns.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Whitney ER, Kemper TL, Bauman ML, Rosene DL, Blatt GJ. Cerebellar Purkinje Cells are Reduced in a Subpopulation of Autistic Brains: A Stereological Experiment Using Calbindin-D28k. Cerebellum. 2008;7(3):406–416. doi: 10.1007/s12311-008-0043-y. [DOI] [PubMed] [Google Scholar]
- Williams JH, Whiten A, Suddendorf T, Perrett DI. Imitation, mirror neurons and autism. Neurosci Biobehav Rev. 2001;25(4):287–295. doi: 10.1016/s0149-7634(01)00014-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.