Abstract
c-Jun N-terminal kinase (JNK) signaling is an important contributor to stress-induced apoptosis, but it is unclear whether JNK and its isoforms (JNK1, JNK2, and JNK3) have distinct roles in cerebral ischemia. Here we show that JNK1 is the major isoform responsible for the high level of basal JNK activity in the brain. In contrast, targeted deletion of Jnk3 not only reduces the stress-induced JNK activity, but also protects mice from brain injury after cerebral ischemia–hypoxia. The downstream mechanism of JNK3-mediated apoptosis may include the induction of Bim and Fas and the mitochondrial release of cytochrome c. These results suggest that JNK3 is a potential target for neuroprotection therapies in stroke.
Stroke is the third largest cause of death in the United States (1). Currently, the only approved therapy for acute cerebral ischemia is the administration of tissue plasminogen activator (tPA) within 3 h of the initial onset (2). After the 3-h window, the risk of induced cerebral hemorrhage by tPA outweighs its benefits and creates the need for additional neuroprotection therapies. The design of future neuroprotective therapies depends on better understanding the mechanism of neuronal death in the ischemic penumbra area, a hypoperfused and hypoxic region surrounding the core of infarction (3, 4). A large body of literature indicates apoptosis within the penumbra area, which may be the result of complex signal transduction events (5, 6). Of these, the c-Jun NH2-terminal kinase (JNK) signaling pathway is a leading candidate mechanism in ischemic apoptosis (7).
JNK, also called stress-activated protein kinase (SAPK), is activated by external noxious stimuli through a kinase cascade (8, 9). Once activated, JNK phosphorylates serine-63 and -73 residues of c-Jun and increases the transcription activity of the AP-1 complex (10, 11). JNK signaling also targets the mitochondria and regulates the release of cytochrome c (12–14). Although an important role of JNK signaling in stress-induced apoptosis was demonstrated in a variety of neuronal and nonneural systems, its function in cerebral ischemia is unclear (7). Histologic analysis suggested JNK signaling induction in the peri-infarct area, but biochemical studies showed a high level of basal JNK activity and only slight increase after middle cerebral artery occlusion in the rodent brain (15–18). It is also uncertain whether JNK isoforms have preferential functions in cerebral ischemia. Among the three JNK isoforms encoded by different genes, JNK1 and JNK2 are present in most tissues, whereas JNK3 is selectively expressed in the nervous system and in the heart (19–21). Previous studies showed that targeted disruption of the neural-specific Jnk3 gene, but not Jnk1 or Jnk2, rendered mice highly resistant to glutamate excitotoxicity (22). In contrast, the Jnk1/Jnk2 compound mutation leads to neural tube defects and embryonic lethality (20, 23). These studies indicate the functional diversity of JNK isoforms and suggest that JNK3 may have a preferential role in stress-induced neuronal apoptosis.
Materials and Methods
The generation Jnk1-, Jnk2-, and Jnk3-null mice have been reported (22, 24–25). One-month-old C57BL/6 wild-type and Jnk2- and Jnk3-null mice that had been crossed to the C57BL/6 strain for seven generations were used for brain injury and biochemical studies. Mice were anethestized by isoflurane and subjected to unilateral occlusion of the common carotid artery. After recovery from anesthesia, mice were challenged by hypoxia inside a controlled atmosphere chamber (model 855-AC, PlasLabs, Lansing, MI) infused with 7.5% oxygen. An oxygen analyzer (model 600, ESD) was used to monitor the oxygen concentration inside the chamber. At the end of hypoxia treatment, mice were returned to the animal facility and observed daily. The animal procedures were approved by the Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for Care and Use of Laboratory Animals. For detailed description regarding histology, biochemical, and oxygen-glucose deprivation assays, please refer to the Supporting Text, which is published as supporting information on the PNAS web site.
Results
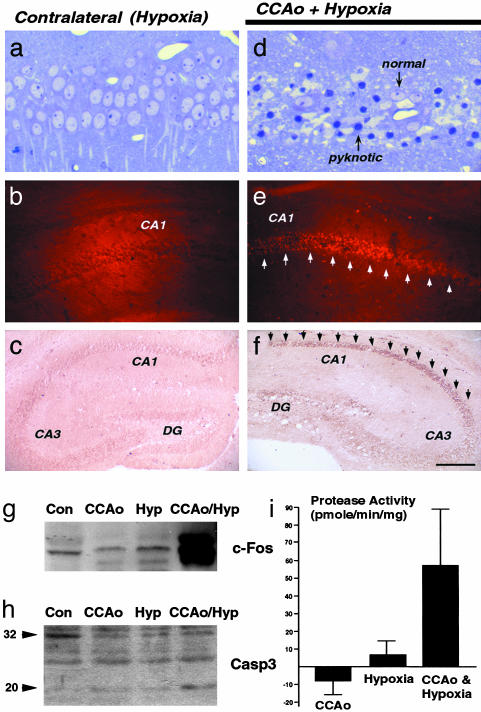
Induction of JNK Signaling by Cerebral Ischemia–Hypoxia. Cumulative evidence indicates that apoptosis primarily occurs in the penumbra area, whereas rapid energy depletions inside the ischemic core causes acute necrosis (26). Because the penumbra area is characterized by moderate levels of ischemia and hypoxia (27), we set out to test whether Levine's procedure (28, 29), unilateral occlusion of the common carotid artery followed by hypoxia, mimics the stress inside the penumbra to induce neuronal apoptosis in postneonate mice. The original report by Levine stated that brain damage occurs only when adult rats are subjected to combined ischemia and anoxia (28). Similarly, we did not find brain lesion caused by unilateral occlusion of the common carotid artery (n > 20) or on the side of brain subjected only to hypoxia (Fig. 1a, n > 200). In contrast, the combination of common carotid artery ligation and hypoxia resulted in numerous pyknotic nuclei in the CA1-hippocampal subfield, which correlated with positive terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)-staining and intense immunoreactivity for the 20-kDa active fragment of caspase-3 (Fig. 1 a–f) (30). In addition, immunoblotting analysis showed that ischemia and hypoxia have a synergistic effect on the induction of c-Fos (Fig. 1g) and the conversion of the inactive 32-kDa pro-caspase-3 to the 20-kDa active form (Fig. 1h). Similarly, only the combined stress of ischemia and hypoxia resulted in significant increase of the caspase-3-like protease activity (Fig. 1i). These results showed that Levine's procedure causes caspase-3-mediated, ischemia/hypoxia-dependent apoptosis in the hippocampus of adult mice.
Fig. 1.
Cerebral ischemia–hypoxia induces caspase-3 activation and neuronal apoptosis. (a and d) Plastic sections stained by Toluidine blue show pyknotic nuclei in the CA1 hippocampal subfield after unilateral common carotid artery occlusion (CCAo) and hypoxia (d) but not on the contralateral, hypoxic hippocampus (a). (b and e) Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) stain also labels the CA1 hippocampal neurons after combined ischemic and hypoxic stress (e) but not on the contralateral side (b). (c and f) Immunocytochemistry for activated p20 caspase-3 fragment selectively labels the hippocampus after combined ischemia–hypoxia challenge (f) but not the contralateral side (c). (g and h) Immunoblot shows the induction of c-Fos protein (g) and cleavage of procaspase-3 (h) in hippocampal extracts from the ischemia–hypoxia-challenged mice (CCAo/Hyp) but not from unstressed mice (Con) or mice challenged by ischemia (CCAo) or hypoxia alone (Hyp). (i) The enzymatic assay using Ac-DEVD-7-amino-4-methyl coumarin (amc) as substrate shows an increased caspase-3-like protease activity in the hippocampal extracts from the ischemia–hypoxia-challenged hippocampi. (Scale bar: 40 μm in a and d, 1.2 mm in b and e, and 2.4 mm in c and f.)
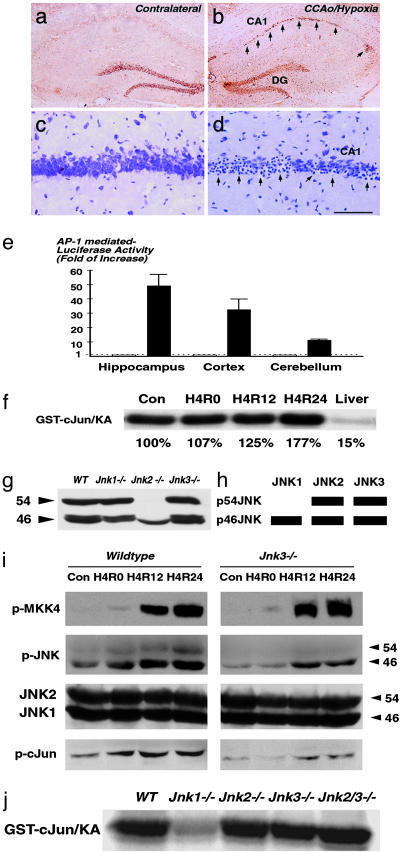
We next tested whether the JNK signaling pathway is activated in the Levine's model of cerebral ischemia–hypoxia. Expression of c-Jun correlated with pyknotic nuclei was found in the CA1 hippocampal subfield at 24 h after cerebral ischemia–hypoxia, suggesting the induction of JNK signaling (Fig. 2 a–d). We then used transgenic AP-1-luc mice carrying the reporter gene luciferase that is driven by four repeated AP-1 DNA-binding sequences to assess the JNK signaling activity (31). A 49.6-fold increase of luciferase activity in the hippocampus and a 32.3-fold increase in the cortex were observed in the AP-1-luc mice at 24 h after the cerebral ischemia–hypoxia challenge. In contrast, the luciferase activity was only increased 10.5-fold in the cerebellum where the vertebral artery provides the primary blood supply (Fig. 2e). These two assays suggested that cerebral ischemia–hypoxia induces JNK signaling. However, an in vitro kinase assay using whole hippocampus extracts only showed a modest 77% increase of total JNK activity at 24 h after cerebral ischemia–hypoxia (Fig. 2f, H4R24). The stress-induced JNK signaling might be obscured by a high level of basal activity in the brain (Fig. 2f).
Fig. 2.
Cerebral ischemia–hypoxia-induced JNK signaling activity. (a–d) Nissl and immunostain show induced expression of c-Jun (b) in the CA1 hippocampal subfield correlated with pyknotic nuclei (d) after combined ischemia and hypoxia. (e) Gene expression assay using the transgenic AP-1-luciferase mice shows induced AP-1 transcriptional activity in the hippocampus and cortex after cerebral ischemia–hypoxia. (f) In vitro kinase assay shows a high level of basal JNK activity in the brain compared to the liver, and a 77% increase of the total JNK activity at 24 h after ischemia and hypoxia (H4R24). (g and h) Immunoblot analysis using JNK1/JNK2-specific antibody in wild-type and Jnk1-, Jnk2-, and Jnk3-null mice (g) reveals the long and short-form composition of JNK1 and JNK2 in the brain (h). The position of JNK3 was deduced previously by using an in-gel kinase assay (22). (i) Immunoblot analysis of phosphorylated MKK4, JNK, c-Jun, and JNK1/JNK2 in wild-type and Jnk3-null mice at different times after cerebral ischemia–hypoxia. (j) In vitro kinase assay of the basal JNK activity in wild-type and Jnk1-, Jnk2-, and Jnk3-null, and Jnk2/Jnk3 double-null mice. The basal JNK activity in Jnk1-null mice was reduced to 35% of the level in the wild-type mice. (Scale bar: 2 mm in a and b and 200 μm in c and d.)
Isoform-Specific Functions of JNK Signaling in Ischemia–Hypoxia. JNK is encoded by three different genes, Jnk1, Jnk2, and Jnk3, but it is not known whether individual JNK isoforms are preferentially involved in the basal versus stress-induced activity in the brain (21). To address this issue, we first used a JNK1/JNK2-speciifc monoclonal anitibody to examine the mice lacking individual Jnk gene to determine the long (p54) versus short (p46) form composition of JNK1 and JNK2 in the brain. This analysis revealed that JNK1 exists mainly as p46JNK and JNK2 exists as p54JNK and p46JNK (Fig. 2g). We previously determined that JNK3 contributes to both p46JNK and p54JNK (22). Therefore, p54JNK is comprised of JNK2 and JNK3, and p46JNK contains JNK1, JNK2, and JNK3 in the mouse brain (Fig. 2h).
We next used antibodies against phosphorylated mitogen-activated protein (MAP) kinase kinase 4 (MKK4), JNK, and c-Jun to examine the JNK signaling pathway after ischemia–hypoxia (Fig. 2i). This analysis revealed that MKK4, an upstream kinase that activates JNK (32), was highly phosphorylated at 12 and 24 h after cerebral ischemia–hypoxia in both wild-type and Jnk3-null mice. In contrast, the levels of stress-induced p46JNK and p54JNK phosphorylation were greatly reduced or absent, respectively, in the Jnk3-null mice. In addition, the level of c-Jun phosphorylation after ischemia–hypoxia was also markedly reduced in the Jnk3-null mice (Fig. 2i). Finally, we measured the basal JNK activity in the brain from the wild-type, Jnk1-null, Jnk2-null, Jnk3-null, and Jnk2/Jnk3 double-null mice. This analysis revealed the basal JNK activity in the Jnk1-null mice is markedly reduced to ≈35% of the level in the wild-type mice (Fig. 2j).
Together, these analyses suggest the following preferential roles of JNK isoforms in the brain. First, JNK1 is primarily responsible for the high level of basal JNK activity in the brain. Second, JNK3 is important for stress-induced JNK signaling activity because the deletion of Jnk3 results in reduced phosphorylation of the downstream effector c-Jun. Third, JNK2 appears to have a lesser role in the stress-induced activity because the deletion of Jnk3 almost completely eliminated the phosphorylation of p54JNK after cerebral ischemia–hypoxia.
A Critical Role of JNK3 in Ischemia–Hypoxia-Induced Brain Injury. To assess the function of stress-induced JNK3 activity in cerebral ischemia–hypoxia, we compared the responses of wild-type, Jnk2-null, and Jnk3-null mice in the Levine model of stroke (Fig. 3a). To avoid the complication of the diverse genetic background (33), Jnk2- and Jnk3-null mice used in the present study were crossed to the C57BL/6 strain for seven generations.
Fig. 3.
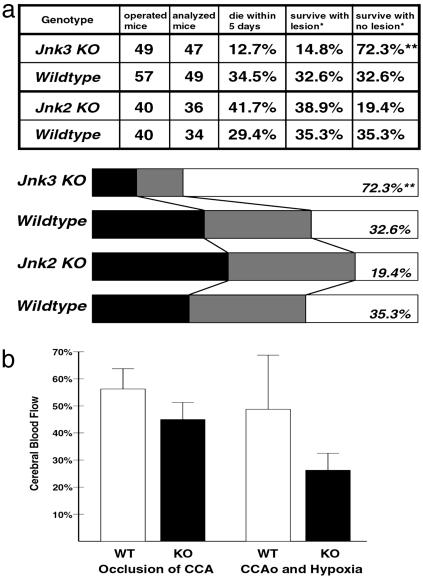
JNK3 deficiency protects against ischemia and hypoxia-induced brain injury. (a) A significantly greater proportion of Jnk3-null mice survived after cerebral ischemia–hypoxia and showed no detectable brain damage than the Jnk2-null and wild-type mice. Black bar, animals die within 5 days; gray bar, animals survive with CA1 brain lesion; white bar, animals survive with no obvious brain lesion. **, P < 0.001 compared to the wild-type mice by the χ2 method. (b) Changes of the cerebral blood flow measured by laser-Doppler flowmetry show comparable levels of initial reduction upon the occlusion of the common carotid artery (CCA) between wild-type and Jnk3-null mice, and further decrease at the end of the hypoxia challenge.
A total of 47 Jnk3-null and 34 Jnk2-null mice were subjected to Levine's model of stroke. They were compared to 49 and 34 age-matched wild-type mice, respectively (Fig. 3a). A total of 29.4–34.5% of wild-type mice died within 5 days after the challenge; the majority of these mice showed tilting of the body toward the occlusion side, repetitive circling before death, and massive brain infarct by necropsy. A total of 32.6–35.3% of wild-type mice survived the ischemia–hypoxia challenge and showed brain damage in histology analysis. Another 32.6–35.3% of wild-type mice survived and had no obvious brain damage as analyzed by Nissl's staining. The outcomes of Jnk2-null mice did not differ significantly from those of wild-type mice. In contrast, the percentage of Jnk3-null mice that survived and exhibited no apparent brain damage was significantly higher than that in the wild-type mice (72.3%, P < 0.002 by the χ2 analysis).
To test whether there are significant differences in the cerebrovascular factors between wild-type and Jnk3-null mice, laser-Doppler flowmetry was used to compare the change of cerebral blood flow in the middle cerebral artery (MCA) region in wild-type and Jnk3-null mice (Fig. 3b). Upon occlusion of the common carotid artery, the cerebral blood flow in the MCA region dropped to 55.9% (SD, 9.2%) in the wild-type mice (n = 20) and 45.0% (SD, 6.0%) in the Jnk3-mutants (n = 10). This difference is not statistically significant. At the end of hypoxia challenge, the cerebral blood flow in the MCA region remained diminished in both Jnk3-null (mean, 25.8%; SD, 6%) and wild-type mice (mean, 49.2%; SD, 20.2%), although wider variation of the cerebral blood flow level was noticed in the wild-type mice.
The comparable levels of initial decrease of the cerebral blood flow between wild-type and Jnk3-null mice, followed by further reduction after hypoxia in Jnk3-null mice, suggests that the resistance to brain damage is most likely not caused by better preservation of the cerebral blood flow in the Jnk3-mutant mice. This scenario is consistent with the observation of an equivalent level of MKK4 phosphorylation at 12 and 24 h after hypoxia, suggesting the upstream stimuli of JNK signaling were comparable between wild-type and Jnk3-null mice (Fig. 2i).
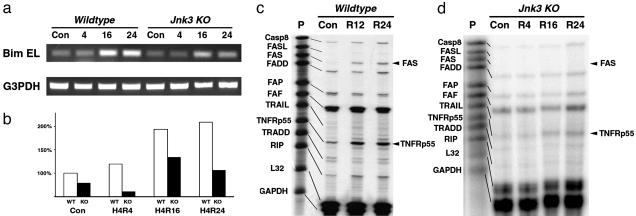
Transcriptional Targets of JNK3 Signaling in Ischemia–Hypoxia. Previous studies suggest that Bim, a BH3-only proapoptotic BCL2 protein, is an important transcriptional target of JNK signaling (34, 35). To test whether Bim is induced by JNK3 signaling after cerebral ischemia–hypoxia, total hippocampal RNA at varying times after the stroke insult was extracted for RT-PCR analysis (Fig. 4 a and b). The RT-PCR analysis indicated the amount of Bim EL transcripts increased ≈2-fold after ischemia–hypoxia in the wild-type mice (194% at 16 and 209% at 24 h, respectively). In contrast, the absolute level and the fold of induction of Bim EL after ischemia–hypoxia were both greatly reduced in the Jnk3-null mice (134% at 16 h and 106% at 24 h of the baseline level of the wild-type mice). These results suggest the proapoptotic protein Bim may be one of the downstream targets of JNK3 signaling in ischemic apoptosis.
Fig. 4.
JNK3 deficiency reduces the expression of Bim and Fas after cerebral ischemia–hypoxia. (a and b) RT-PCR analysis shows attenuated induction of Bim EL in Jnk3-null mice after cerebral ischemia–hypoxia (a). The expression of GAPDH is used for normalization. Quantification analysis showed a 94% and 109% increase of Bim EL expression at 16 and 24 h after ishcemia–hypoxia in wild-type mice, compared to Jnk3-null mice showing only 34% and 6% increases at the same times (b). (c and d) Ribonuclease protection assay revealed an induction of the Fas and TNFRp55 transcripts in wild-type mice, but not in the Jnk3-null mice after cerebral ischemia–hypoxia.
A previous study indicated that Fas ligand (FasL) is also a downstream target of JNK signaling (36). We used a multiprobe ribonuclease protection assay (RPA) kit to examine this possibility but detected little increase of FasL. In contrast, there was a prominent induction of Fas receptor (229% of the baseline level at 24 h) and p55 tumor necrosis factor receptor TNFRp55 (198% at 24 h) in the wild-type mice (Fig. 4c). These results are consistent with a previous report of induction of Fas in neurons after cerebral ischemia (37). Interestingly, Jnk3-null mice showed minimal induction of Fas receptor, suggesting that Fas receptor may be downstream target of JNK3 signaling in apoptosis after cerebral ischemia–hypoxia (Fig. 4d).
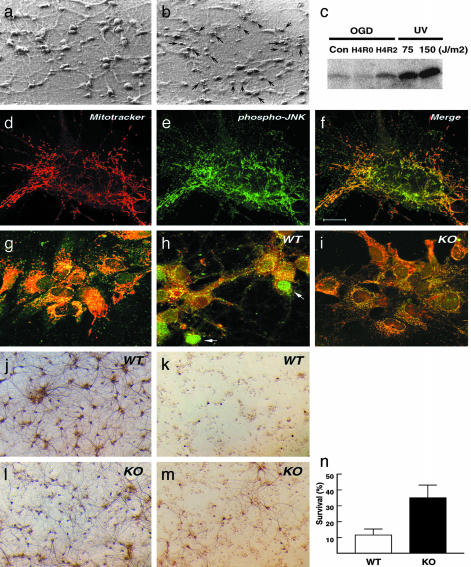
Mitochondrial Targeting of JNK3 Signaling in Oxygen-Glucose Deprivation. To further test the role of JNK3 signaling in ischemic apoptosis, we isolated embryonic hippocampal neurons from the wild-type and Jnk3-null mice and challenged them with oxygen-glucose deprivation (OGD), an in vitro model of cerebral ischemia (38, 39). Hippocampal neurons were exposed to 1% oxygen in the glucose-free medium for 4 h and then reoxygenated in the conditioned growth medium. By 24 h after reoxygenation, the stressed hippocampal neurons showed prominent nuclear condensation and degradation of the neurites (Fig. 5 a and b). Previous studies established that OGD causes caspase-3-mediated apoptosis and intense c-Jun phosphorylation (38–40). Similarly, we detected a rapid induction of the JNK activity by OGD-reoxygenation in primary cultures of mouse cardiomyocytes that have a low level of basal JNK activity (Fig. 5c). Together, these results suggest that OGD induces the JNK signaling.
Fig. 5.
JNK3 deficiency protects against OGD. (a and b) OGD causes condensed nuclei of hippocampal neurons and degradation of neurites. (c) Kinase assay shows that OGD induces the JNK activity in mouse cardiomyocytes. (d–f) Double labeling of mitochondria (d, red, MitoTracker red), phosphorylated JNK (e, green, FITC-conjugated secondary antibody), and the merged image (f) shows that phosphorylated JNK is largely located around the mitochondria. (g–i) Merged images of cytochrome c (green, FITC-conjugated secondary antibody) and mitochondria (red, MitoTracker red) show diffused cytochrome c distribution in the wild-type neurons at 6 h after OGD (h), but not in unchallenged (g) or Jnk3-deficient neurons after OGD (i). (j and k) Immunostain of mitogen-activated protein 2 (MAP2)-positive wild-type neurons with (k) or without (j) OGD challenge. (l and m) Immunostain of MAP2-positive Jnk3-null neurons with (m) or without (l) OGD challenge. (n) Quantification of three sets of cultures shows an average of 36.5% survival of Jnk3-deficient hippocampal neurons (SD, 6.4%) compared to 11.0% survival of wild-type neurons (SD, 2.6%) at 24 h after OGD. (Scale bar: 10 μm in d–f, 20 μm in g–i, and 400 μm in j–m.)
Recent studies suggest that JNK signaling regulates the release of cytochrome c under cellular stress (12–14). The mitochondrial effect of JNK signaling may be indirectly mediated by the expression of Bim that subsequently translocates to the mitochondria (34, 35). Alternatively, JNK may directly target the mitochondria and modulate the release of cytochrome c. To test this possibility, we performed double labeling to examine the distribution of activated JNK after OGD (Fig. 5 d–f). This analysis revealed a large fraction of activated JNK in the proximity of the mitochondria, suggesting that activated JNK may directly target the mitochondria.
To test the function of JNK signaling in OGD, the response of wild-type and Jnk3-null hippocampal neurons to OGD were compared. First, the distribution of cytochrome c colabeled with a mitochondrial dye (MitoTracker red CMXRos) was examined at 12 h after reoxygenation. This analysis revealed that cytochrome c is largely retained inside the mitochondria in the unchallenged wild-type neurons and in Jnk3-null neurons after OGD (Fig. 5 g and i). In contrast, wild-type neurons showed diffused cytochrome c distribution after OGD (Fig. 5h). Secondly, the number of mitogen-activated protein 2 (MAP2)-positive (a marker for neurons) wild-type and Jnk3-null hippocampal neurons were counted at 24 h after OGD to compare neuronal survival (Fig. 5 j–m). This analysis showed an average of 11% survival of wild-type neurons (SD, 2.6%), whereas Jnk3-null neurons showed three times higher percentage of survival (mean, 36.5%; SD, 6.4%) in three sets of experiments (Fig. 5n). Together, these results suggest a critical role of JNK3 in apoptosis and the release of cytochrome c from mitochondria after OGD.
Discussion
There has long been a paradox of JNK signaling in cerebral ischemia. Immunocytochemical studies showed induced expression of c-Jun in the ischemic brain, suggesting the activation of JNK signaling (15–17). In contrast, biochemical analysis revealed a high level of basal JNK activity and only slight increase of JNK activity after cerebral ischemia (17, 18). In the present study, we showed that the paradox is in part due to preferential functions of JNK isoforms in the basal versus stress-induced signaling activity. We showed that JNK1 is the major isoform responsible for the high level of basal JNK activity in the brain, because the deletion of Jnk1 reduces the basal JNK activity to ≈35% of the whole brain lysates. Similar findings of a JNK1-mediated high level of constitutive JNK activity in the cerebellar granule cells were also reported (41). Because several studies suggested that JNK signaling could be cytoprotective in some experimental paradigms (8), future studies are needed to determine whether the largely JNK1-mediated basal JNK activity in the brain has neuroprotective effects. The present study showed that targeted disruption of the Jnk3 gene not only reduced the downstream effector c-Jun phosphorylation, but also remarkably protected mice from brain injury after cerebral ischemia–hypoxia. These results suggest that JNK3 is a critical component of the stress-induced JNK signaling and neuronal apoptosis.
Past studies indicated that the downstream mechanisms of JNK signaling in apoptosis include transcriptional induction of Bim (by withdrawal of nerve growth factor in sympathetic neurons) (34, 35), FasL (by nerve growth factor and potassium deprivation in PC12 cells and cerebellar granule cells, respectively) (36), and release of cytochrome c from the mitochondria (by UV irradiation in fibroblasts) (12–14). Because these mechanisms were elucidated in a variety of distinct systems, it is important to examine whether they are also induced by JNK in ischemic apoptosis. Our results confirmed the induction of Bim EL, a proapoptotic BH3-only BCL2 family member, by JNK3 signaling after cerebral ischemia–hypoxia. In contrast, the present study revealed robust JNK3-dependent transcription of Fas and TNFRp55, but not FasL, after cerebral ischemia–hypoxia. These results suggest that JNK signaling elicits different downstream mechanisms to execute apoptosis under different conditions. Moreover, our results are consistent with previous studies showing the induced expression of Fas in neurons after cerebral and spinal ischemia (37, 42). The expression of death receptors (Fas and p55TNFR) on the cell surface may render ischemic neurons susceptible to the Fas and tumor necrosis factor-mediated apoptosis induced by the influx of inflammatory cells to the brain (43, 44). Finally, we showed that OGD, an in vitro model of cerebral ischemia, triggers JNK signaling and the absence of JNK3 provides better retention of cytochrome c inside the mitochondria and higher resistance to OGD. Together, these results suggest that JNK signaling may induce neuronal cell death by both transcriptional induction of death-promoting genes and modulation of the mitochondrial apoptosis pathways (8).
In conclusion, the present study implicates the JNK3 isoform in neuronal apoptosis following cerebral ischemia and hypoxia. JNK3 is therefore a promising target for future neuroprotective therapy in acute stroke.
Supplementary Material
Acknowledgments
We thank M. Kofron for assistance with the confocal laser microscope imaging. R.J.D. and R.A.F. are investigators of the Howard Hughes Medical Institute. This work was supported by the Howard Hughes Medical Institute (R.J.D. and R.A.F.), the National Institutes of Health (C.-Y.K. and P.R.), the American Heart Association (P.R.), and the Atorvastatin Research Award (C.-Y.K.).
Abbreviations: JNK, c-Jun NH2-terminal kinase; OGD, oxygen–glucose deprivation; FasL, Fas ligand.
References
- 1.Legos, J. J., Tuma, R. F. & Barone, F. C. (2002) Exp. Opin. Invest. Drugs 11, 603-614. [DOI] [PubMed] [Google Scholar]
- 2.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group (1995) N. Engl. J. Med. 333, 1581-1587. [DOI] [PubMed] [Google Scholar]
- 3.Astrup, J., Siesjo, B. K. & Symon, L. (1981) Stroke 12, 723-725. [DOI] [PubMed] [Google Scholar]
- 4.Hossmann, K.-A. (1994) Ann. Neurol. 36, 557-565. [DOI] [PubMed] [Google Scholar]
- 5.Graham, S. H. & Chen, J. (2001) J. Cereb. Blood Flow Metab. 21, 99-109. [DOI] [PubMed] [Google Scholar]
- 6.Sharp, F. R., Lu, A., Tang, Y. & Millhorn, D. E. (2001) J. Cereb. Blood Flow Metab. 20, 1011-1032. [DOI] [PubMed] [Google Scholar]
- 7.Irving, E. A. & Bamford, M. (2002) J. Cereb. Blood Flow Metab. 22, 631-647. [DOI] [PubMed] [Google Scholar]
- 8.Davis, R. J. (2000) Cell 103, 239-252. [DOI] [PubMed] [Google Scholar]
- 9.Chang, L. & Karin, M. (2001) Nature 410, 37-40. [DOI] [PubMed] [Google Scholar]
- 10.Derijard, B., Hibi, M., Wu, I. H., Barrett, T., Su, B., Deng, T., Karin, M. & Davis, R. J. (1994) Cell 76, 1025-1037. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakis, J. M., Banerjee, P., Nikolakaki, E., Dai, T., Rubie, E. A., Ahmad, M. F., Avruch, J. & Woodgett, J. R. (1994) Nature 369, 156-160. [DOI] [PubMed] [Google Scholar]
- 12.Tournier, C., Hess, P., Yang, D. D., Xu, J., Turner, T. K., Nimnual, A., Bar-Sagi, D., Jones, S. N., Flavell, R. A. & Davis, R. J. (2000) Science 288, 870-874. [DOI] [PubMed] [Google Scholar]
- 13.Kharbanda, S., Saxena, S., Yoshida, K., Pandey, P., Kaneki, M., Wang, Q., Cheng, K., Chen, Y. N., Campbell, A., Sudha, T., et al. (2000) J. Biol. Chem. 275, 322-327. [DOI] [PubMed] [Google Scholar]
- 14.Lei, K., Nimnual, A., Zong, W. X., Kennedy, N. J., Flavell, R. A., Thompson, C. B., Bar-Sagi, D. & Davis, R. J. (2002) Mol. Cell. Biol. 22, 4929-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herdegen, T., Claret, F. X., Kallunki, T., Martin-Villalba, A., Winter, C., Hunter, T. & Karin, M. (1998) J. Neurosci. 18, 5124-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugino, T., Nozaki, K., Takagi, Y., Hattori, I., Hashimoto, N., Moriguchi, T. & Nishida, E. (2000) J. Neurosci. 20, 4506-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillardon, F., Spranger, M., Tiesler, C. & Hossmann, K.-A. (1999) Brain Res. Mol. Brain Res. 73, 138-143. [DOI] [PubMed] [Google Scholar]
- 18.Xu, X., Raber, J., Yang, D., Su, B. & Mucke, L. (1997) Proc. Natl. Acad. Sci. USA 94, 12655-12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin, J. H., Mohit, A. A. & Miller, C. A. (1996) Brain Res. Mol. Brain Res. 35, 47-57. [DOI] [PubMed] [Google Scholar]
- 20.Kuan, C. Y., Yang, D. D., Samanta Roy, D. R., Davis, R. J., Rakic, P. & Flavell, R. A. (1999) Neuron 22, 667-676. [DOI] [PubMed] [Google Scholar]
- 21.Gupta, S., Barrett, T., Whitmarsh, A. J., Cavanagh, J., Sluss, H. K., Derijard, B. & Davis, R. J. (1996) EMBO J. 15, 2760-2770. [PMC free article] [PubMed] [Google Scholar]
- 22.Yang, D. D., Kuan, C. Y., Whitmarsh, A. J., Rincon, M., Zheng, T. S., Davis, R. J., Rakic, P. & Flavell, R. A. (1997) Nature 389, 865-870. [DOI] [PubMed] [Google Scholar]
- 23.Sabapathy, K., Jochum, W., Hochedlinger, K., Chang, L, Karin, M. & Wagner, E. F. (1999) Mech. Dev. 89, 115-124. [DOI] [PubMed] [Google Scholar]
- 24.Dong, C., Yang, D. D., Wysk, M., Whitmarsh, A. J., Davis, R. J. & Flavell, R. A. (1998) Science 282, 2092-2095. [DOI] [PubMed] [Google Scholar]
- 25.Yang, D. D., Conze, D., Whitmarsh, A. J., Barrett, T., Davis, R. J., Rincon, M. & Flavell, R. A. (1998) Immunity 9, 575-585. [DOI] [PubMed] [Google Scholar]
- 26.Benchoua, A., Guegan, C., Couriaud, C., Hosseini, H., Sampaio, N., Morin, D. & Onteniente, B. (2001) J. Neurosci. 21, 7127-7134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Touzani, O., Roussel, S. & MacKenzie, E. T. (2001) Curr. Opin. Neurol. 14, 83-88. [DOI] [PubMed] [Google Scholar]
- 28.Levine, S. (1960) Am. J. Pathol. 36, 1-17. [PMC free article] [PubMed] [Google Scholar]
- 29.Rice, J. E., Vannucci, R. C. & Brierley, J. B. (1981) Ann. Neurol. 9, 131-141. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasan, A., Roth, K. A., Sayers, R. O., Shindler, K. S., Wong, A. M., Fritz, L. C. & Tomaselli, K. J. (1998) Cell Death Differ. 5, 1004-1016. [DOI] [PubMed] [Google Scholar]
- 31.Rincon, M., Derijard, B., Chow, C. W., Davis, R. J. & Flavell, R. A. (1997) Genes Funct. 1, 51-68. [DOI] [PubMed] [Google Scholar]
- 32.Derijard, B., Raingeaud, J., Barrett, T., Wu, I. H., Han, J., Ulevitch, R. J. & Davis, R. J. (1995) Science 267, 682-685. [DOI] [PubMed] [Google Scholar]
- 33.Fujii, M., Hara, H., Meng, W., Vonsattel, J. P., Huang, Z. & Moskowitz, M. A. (1997) Stroke 28, 1805-1810. [DOI] [PubMed] [Google Scholar]
- 34.Whitfield, J., Neame, S. J., Paquet, L., Bernard, O. & Ham, J. (2001) Neuron 29, 629-643. [DOI] [PubMed] [Google Scholar]
- 35.Putcha, G. V., Moulder, K. L., Golden, J. P., Bouillet, P., Adams, J. A., Strasser, A. & Johnson, E. M. (2001) Neuron 29, 615-628. [DOI] [PubMed] [Google Scholar]
- 36.Le-Niculescu, H., Bonfoco, E., Kasuya, Y., Claret, F. X., Green, D. R. & Karin, M. (1999) Mol. Cell. Biol. 19, 751-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsuyama, T., Hata, R., Yamamoto, Y., Tagaya, M., Akita, H., Uno, H., Wanaka, A., Furuyama, J. & Sugita, M. (1995) Brain Res. Mol. Brain Res. 34, 166-172. [DOI] [PubMed] [Google Scholar]
- 38.Nath, R., Probert, A., Jr., McGinnis, K. M. & Wang, K. K. (1998) J. Neurochem. 71, 186-195. [DOI] [PubMed] [Google Scholar]
- 39.Whitmarsh, A. J., Kuan, C. Y., Kennedy, N. J., Kelkar, N., Haydar, T. F., Mordes, J. P., Appel, M., Rossini, A. A., Jones, S. N., Flavell, R. A., et al. (2001) Genes Dev. 15, 2421-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao, G., Pei, W., Lan, J., Stetler, R. A., Luo, Y., Nagayama, T., Graham, S. H., Yin, X. M., Simon, R. P & Chen, J. (2000) J. Neurosci. 21, 4678-4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coffey, E. T., Smiciene, G., Hongisto, V., Cao, J., Brecht, S., Herdegen, T. & Courtney, M. J. (2002) J. Neurosci. 22, 4335-4345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsushita, K., Wu, Y., Qiu, J., Lang-Lazdunski, L., Hirt, L., Waeber, C., Hyman, B. T., Yuan, J. & Moskowitz, M. A. (2000) J. Neurosci. 20, 6879-6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gregersen, R., Lambertsen, K. & Finsen, B. (2000) J. Cereb. Blood Flow Metab. 20, 53-65. [DOI] [PubMed] [Google Scholar]
- 44.Ishibashi, N., Prokopenko, O., Reuhl, K. R. & Mirochnitchenko, O. (2002) J. Immunol. 168, 1926-1933. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.