Abstract
The differentiation and function of cumulus cells depend upon oocyte-derived paracrine factors, but studies on the estrogen receptor knockout mice suggested that estrogen also participates in these processes. This study investigates the possible coordination of estrogen and oocytes in the development and function of cumulus cells using cumulus expansion and the expression of transcripts required for expansion as functional endpoints. Preantral granulosa cell-oocyte complexes developed in vitro with 17β-estradiol (E2) exhibited increased levels of cumulus expansion and Has2 transcripts, encoding hyaluronan synthase 2, compared with those developed without E2. Moreover, cumulus cell-oocyte complexes (COCs) isolated from antral follicles and maintained in culture without E2 exhibited reduced cumulus expansion and Has2 mRNA levels compared with freshly isolated COCs. Exogenous E2, provided during the maintenance culture, alleviated these deficiencies. However, when oocytes were removed from COCs, E2 supplementation did not maintain competence to undergo expansion; the presence in culture of either fully grown oocytes or recombinant growth differentiation factor 9 (GDF9) was required. Recombinant bone morphogenetic protein 15, but not fibroblast growth factor 8, augmented the GDF9 effect. Oocytes or GDF9 suppressed cumulus cell levels of Nrip1 transcripts encoding nuclear receptor-interacting protein 1, a potential inhibitor of estrogen receptor signals. Therefore, E2 and oocyte-derived paracrine factors GDF9 and bone morphogenetic protein 15 coordinate to promote the development of cumulus cells and maintain their competence to undergo expansion. Furthermore, suppression of Nrip1 expression in cumulus cells by oocyte may be one mechanism mediating cross talk between oocyte and E2 signals that promotes follicular development.
Oocyte-derived paracrine factors GDF9 and BMP15 coordinate with estradiol for the development of cumulus cells and the maintenance of cumulus cell competence to undergo expansion.
Ovarian follicular development requires precise coordination of extra- and intrafollicular signals (1). Oocytes play a critical role in this coordination by producing several growth factors (2,3,4,5), including bone morphogenetic protein 6 and 15 (BMP6 and -15), growth differentiation factor 9 (GDF9), and fibroblast growth factor 8 (FGF8) (6,7,8,9,10,11,12). A major step in folliculogenesis is the formation of an antrum that divides the granulosa cells into two subpopulations: mural granulosa cells that line the follicular wall and cumulus cells that are closely associated with the oocyte. Although FSH is necessary for the development of antral follicles, differentiation of cumulus cells is dependent upon oocyte-derived paracrine factors (13,14).
In addition to the oocyte signals, estrogen signals are required for normal follicular development (15,16). Disrupting the estrogen signal by deletion of the gene encoding estrogen receptor 2 (ESR2, also known as estrogen receptor β), the predominant estrogen receptor expressed by ovarian granulosa cells, results in female subfertility with fewer and smaller litters (17); attenuated follicular development (17,18,19); and reduced ovulation rate responding to exogenous-gonadotropin treatment (17,20).
Expansion (mucification) of the cumulus oophorus is a prerequisite process for normal ovulation (21). Cumulus expansion in vivo is induced by the LH surge the signal of which is mediated by epidermal growth factor (EGF)-like peptides (EGFLPs) produced by mural granulosa cells (22,23,24). In vitro, cumulus expansion can be induced by treating cumulus cell-oocyte complexes (COCs) with either EGF or FSH (25). Normal cumulus expansion requires cumulus cell expression of transcripts encoding HAS2, PTGS2, PTX3, and TNFAIP6 because genetic deletion of Ptgs2, Ptx3, or Tnfaip6 (26,27,28,29), or RNA interference-mediated silencing of Has2 transcripts result in defective cumulus expansion (30) (Fig. 1A).
Figure 1.
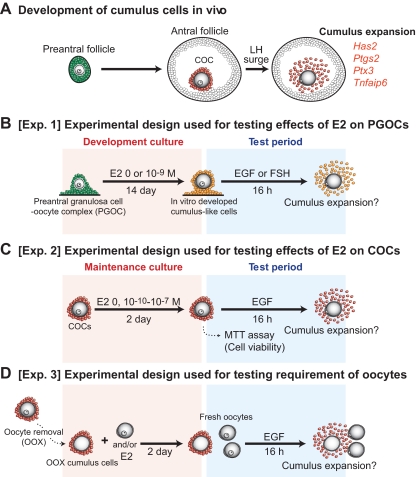
Model of follicular development (A) and experimental designs (B–D). A, Granulosa cells of preantral follicles (green) differentiate into cumulus cells (red) during preantral to antral follicular transition and acquire the competence to undergo expansion. The preovulatory LH surge induces expansion of COCs, which is associated with increased levels of the cumulus expansion-related transcripts (Has2, Ptgs2, Ptx3, and Tnfaip6). B, To determine whether E2 play a role in preantral granulosa cells to develop competent to undergo expansion, PGOCs isolated from 8-d-old mice were grown in vitro with or without exogenous supplementation of E2 for 14 d. The in vitro developed cumulus-like cells (yellow) were treated with EGF (10 ng/ml) to assess competence to undergo cumulus expansion in vitro. C, Experimental design used to test effects of E2 on cumulus cell competence to undergo cumulus expansion. COCs were isolated from the large antral follicles and cultured with or without E2 for 2 d. After the maintenance culture period, the complexes were treated with EGF (10 ng/ml) to assess competence to undergo cumulus expansion. D, To address whether oocytes are required for E2 to exert its effects on maintenance of cumulus cell competence to undergo expansion, oocytes were removed from COCs, and resulting OOX cumulus cell complexes were cultured with E2 and/or oocytes (2 oocytes/μl), or oocyte-derived paracrine factors for 2 d. After the maintenance culture period, the OOX complexes were treated with EGF to assess competence to undergo expansion. Exp., Experiment.
Oocyte-associated granulosa cells become competent to undergo cumulus expansion during transition from preantral to antral follicles (Fig. 1A) (25). Participation of oocyte-derived paracrine factors is required for this transition (13). The oocyte-derived factors (ODFs) that promote the transition of preantral granulosa cells to cumulus cells are probably BMP15 and GDF9 because cumulus cells of Bmp15−/− or Bmp15−/− /Gdf9+/− mice are deficient in undergoing normal cumulus expansion and expression of Has2 and Ptgs2 transcripts (9,31). In addition, the ODFs are also required for maintenance of cumulus cell phenotype, because removing oocytes from COCs results in mural granulosa cell-like phenotype, which is indicated by an increased expression of Lhcgr mRNA encoding LH receptor, a marker of mural granulosa cells (32).
Evidence suggests that the competence to undergo cumulus expansion is also controlled by factors other than those emanating from oocytes (15,16,17,18,19,20). Estrogen is one of such factors, because the reduced ovulation rate in Esr2-deficient mice is attributed, at least in part, to defective cumulus expansion and lower levels of Ptgs2 transcripts (19,20,33). Therefore, EG is required for multiple stages of follicular development. How estrogen signaling interacts with other factors to affect follicular growth and development of specific follicular cell types, such as cumulus cells, is not well resolved.
Therefore, to determine whether there is coordination of oocyte and estrogen signals during follicular development, the effect of 17β-estradiol (E2) and oocytes on the function of cumulus cells was examined using EGF-induced cumulus expansion and expression of transcripts encoding HAS2, PTGS2, PTX3, and TNFAIP6 as functional endpoints. First, we asked whether E2 promotes the development of the cumulus cell phenotype in cultured preantral granulosa cell-oocyte complexes (PGOCs). Then, we examined the effect of E2 on the maintenance of cumulus cell competence to undergo expansion and the requirement of oocytes on the E2 effects using COCs isolated from antral follicles. The results of these studies revealed that E2 and oocyte signals cooperate to promote development and maintenance of the cumulus cell phenotype, specifically, acquisition and maintenance of competence to undergo cumulus expansion.
Results
E2 promotes acquisition of competence to undergo expansion by cumulus cells developed from PGOCs in vitro
Cumulus cells are derived from preantral granulosa cells, and the development of the cumulus cell phenotype in vitro is promoted by oocytes even in the absence of FSH (13). To address whether estrogen signaling, in addition to the oocyte signals, promotes the development of the cumulus cell phenotype from PGOCs in vitro (Experiment 1; Fig. 1B), PGOCs were isolated from 8-d-old mice (not competent to undergo expansion), and were developed in vitro for 14 d with or without 10−9 m of exogenous E2 supplementation (Fig. 1B; development period). After the 14-d in vitro development period, the in vitro-grown complexes were transferred to the medium supplemented with EGF to induce cumulus expansion (test period). Levels of cumulus expansion-related transcripts, Has2, Ptgs2, Ptx3, and Tnfaip6 mRNA were measured by real-time PCR analysis after 6 h of EGF treatment, and the cumulus expansion index (34) was assessed after 14–16 h of the treatment (Fig. 2).
Figure 2.
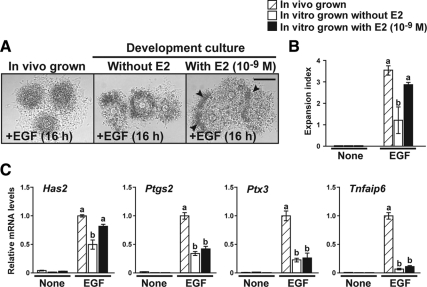
Exogenous E2 supplementation promoted acquisition of competence to undergo expansion by PGOCs developed in vitro. PGOCs were cultured with or without exogenous E2 supplementation, and their competence to undergo expansion (A and B) and expression levels of the cumulus expansion-related transcripts (C) were assessed. In vivo grown complexes (i.e. COCs freshly isolated from large antral follicles) were used as control groups (in vivo grown). Arrowheads indicate clumps of unexpanded cells. Bar, 200 μm. The values indicated by different alphabetical letters (a and b) are significantly different (P < 0.05).
PGOCs grown in vitro without exogenous E2 supplementation did not develop full competence to undergo expansion or express full levels of the cumulus expansion-related transcripts when compared with those grown in vivo (i.e. COCs freshly isolated from large antral follicles) (Fig. 2). The in vitro-grown complexes exhibited incomplete expansion, and most of the cumulus cells remained as clumps of unexpanded cells (Fig. 2A). E2 supplementation during the in vitro development culture period promoted acquisition of competence to undergo expansion; however, some of the cells that were located at the most outside of complexes remained unexpanded (Fig. 2A, arrowheads). Expression levels of Has2 transcripts were increased when compared with those expressed by complexes grown without E2 (Fig. 2C); however, levels of Ptgs2, Ptx3, and Tnfaip6 transcripts were not significantly promoted with E2 supplementation during the culture. Therefore, E2 can promote acquisition of competence to undergo expansion by cumulus cells developed in vitro; however, other factors must be required for complete acquisition of the cumulus cell phenotype.
E2 maintains competence of cumulus cells to undergo expansion in vitro
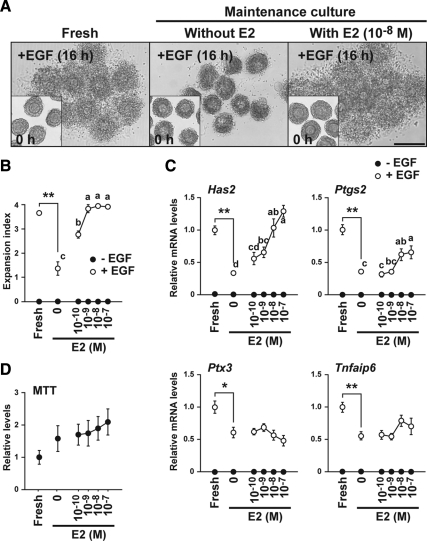
To address whether E2 maintains cumulus cell competence to undergo expansion (Experiment 2; Fig. 1C), COCs isolated from large antral follicles (fully competent to undergo cumulus expansion) were cultured with or without E2 for 2 d (maintenance culture period), and then they were assessed for their ability to undergo expansion and expression of cumulus expansion-related transcripts in vitro (test period) (Fig. 3) (34). Freshly isolated COCs without maintenance culture were treated with or without EGF as control groups (Fig. 3, indicated as “Fresh”). The overall viable cell numbers of the COCs, assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay, were not significantly different between freshly isolated COCs and COCs cultured with or without exogenous E2 supplementation (Fig. 3D).
Figure 3.
Exogenous E2 supplementation maintained cumulus cell competence to undergo expansion in vitro. Effects of exogenous E2 supplementation on viable cell number (D), maintenance of cumulus cell competence to undergo expansion (A and B), and expression levels of the cumulus expansion-related transcripts (C) were assessed. Freshly isolated COCs without maintenance culture treated with or without EGF were used as control groups (Fresh). A, Representative photographs of COCs before induction of cumulus expansion are shown as insets (0 h). Bar, 200 μm. Asterisks denote a significant difference between the two indicated groups (*, P < 0.05; **, P < 0.01). The values indicated by different alphabetical letters (a, b, c, and d) are significantly different (P < 0.05).
COCs maintained without exogenous E2 supplementation exhibited a reduced degree of cumulus expansion and lower expression levels of the cumulus expansion-related transcripts when compared with those of freshly isolated COCs (P < 0.05) (Fig. 3, A–C). Importantly, EGF stimulation significantly promoted expression levels of the cumulus expansion-related transcripts in COCs maintained without E2 when compared with those not stimulated with EGF, but the levels were not the same as those of freshly isolated COCs stimulated with EGF. Exogenous E2 supplementation during the maintenance culture period prevented, in a dose-dependent manner, loss of competence to undergo expansion and expression levels of Has2 mRNA. E2 concentration of 10−8 m efficiently maintained COC competence to undergo expansion and Has2 mRNA expression to the same levels of those by freshly isolated COCs. COCs maintained with E2 expressed significantly higher levels of Ptgs2 mRNA when compared with those by COCs maintained without E2, but the levels were not the same as those of freshly isolated COCs. E2 supplementation did not promote full expression of Ptx3 and Tnfaip6 mRNA by COCs. This suggests that the observed expression levels of Ptgs2, Ptx3, and Tnfaip6 transcripts in COCs maintained with 10−8 m E2 are sufficient for producing expansion. Furthermore, after the EGF treatment (3, 6, 9, and 12 h), the levels of Ptgs2, Ptx3, and Tnfaip6 transcripts in COCs that had been maintained with E2 never reached the levels expressed by fresh COCs (data not shown).
Factors produced by fully grown oocytes are required for enabling cumulus expansion and expression of the cumulus expansion-related transcripts (25,35,36). One explanation for the reduced levels of cumulus expansion and expression of these transcripts during maintenance culture could be insufficient production of these factors by cultured oocytes, rather than loss of competence to undergo expansion by cumulus cells. However, COCs maintained without exogenous E2 were not competent to undergo cumulus expansion or full expression of these transcripts even in the presence of freshly isolated oocytes during the test period (Supplemental Fig. 1 published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Furthermore, oocytes isolated from COCs after maintenance culture without E2 were fully capable of inducing expansion in fresh oocytectomized (OOX) cumulus cell complexes (data not shown). Thus, oocytes in these COCs, which have been cultured for 2 d, are still competent to produce factors required for cumulus expansion and cumulus cell expression of the specific transcripts.
Together, these results indicate that estrogen signals are required for maintaining cumulus cell competence to undergo expansion and expression of normal levels of Has2 mRNA in vitro. However, in addition to E2, other factors must be required to maintain competence to fully express Ptgs2, Ptx3, and Tnfaip6 transcripts by cumulus cells.
Oocyte-derived BMP15 and GDF9 are required for the E2 effects
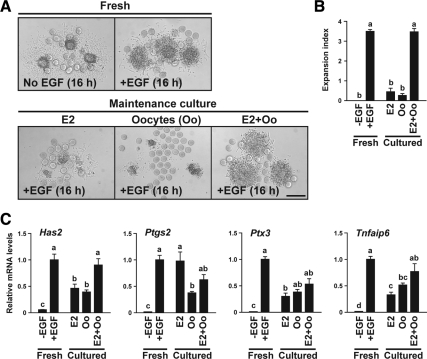
To test whether oocytes are required for the E2-mediated maintenance of cumulus cell competence to undergo expansion (Fig. 1D), oocytes were microsurgically removed from COCs (oocytectomy, OOX) (35), and OOX cumulus cells were cultured with E2 and/or oocytes (maintenance culture period). After the maintenance culture, OOX cumulus cells were assessed for their competence to undergo expansion by coculturing them during the test period with freshly isolated full-grown oocytes and EGF.
OOX cumulus cells maintained with either E2 or oocytes alone were not competent to undergo expansion (Fig. 4, A and B). In OOX cumulus cells maintained with E2 alone, expression levels of the cumulus expansion-related transcripts, except for Ptgs2 mRNA, were significantly lower than those in fresh OOX cumulus cells (Fig. 4C). Supplying both exogenous E2 and oocytes during the maintenance period abrogated the loss of competence to undergo expansion and maintained cumulus cell ability to express full levels of Has2 transcripts (Fig. 4C). OOX cumulus cells maintained with E2 alone exhibited relatively high levels of Ptgs2 mRNA that were comparable with those in fresh OOX cumulus cells (Fig. 4C).
Figure 4.
Oocytes are required for E2 maintenance of cumulus cell competence to undergo expansion. OOX cumulus cell complexes were cultured with E2 and/or oocytes (Oo), and their competence to undergo expansion (A and B) and expression levels of the cumulus expansion-related transcripts (C) were assessed. OOX cumulus cells without maintenance culture were used as control groups (Fresh). Note that oocytes are present in micrographs of all groups because they are added during the test period whether or not they were present during the maintenance culture. Bar, 200 μm. The values indicated by different alphabetical letters (a, b, and c) are significantly different (P < 0.05).
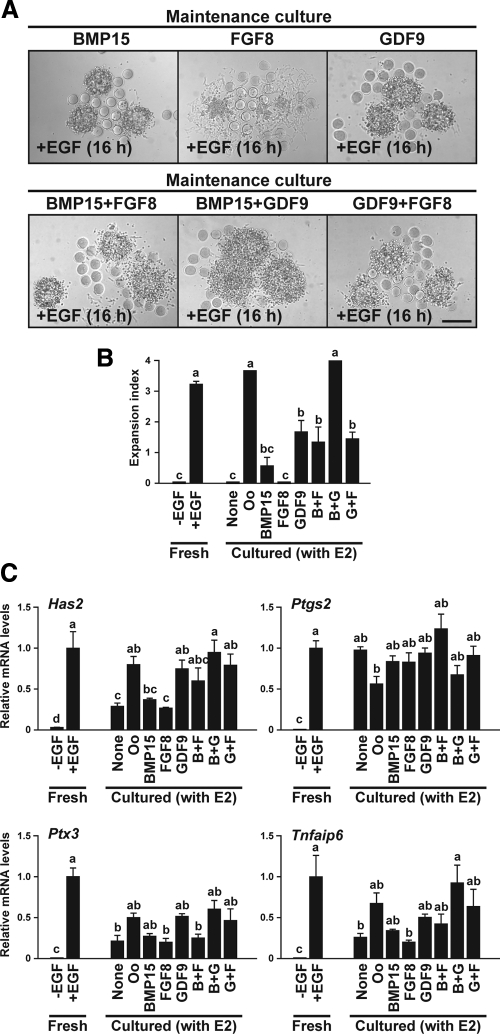
To test oocyte factors that might be required for E2 maintenance of competence to undergo cumulus expansion, the competence of OOX cumulus cells to undergo expansion was assessed after they were maintained with exogenous E2, supplemented with recombinant BMP15, FGF8, GDF9, singly or in combination (experimental design shown in Fig. 1D). Supplementation with either BMP15 or FGF8 alone during the maintenance culture period did not effectively maintain the competence of OOX cumulus cells to undergo expansion (Fig. 5, A and B). Competence to undergo expansion was partially maintained by GDF9 alone or in combination with FGF8, and by BMP15 in combination with FGF8. The combination of BMP15 and GDF9 was the most potent in maintaining OOX expansion ability, returning it to the level of OOX cells maintained with oocytes (Fig. 5, A and B). Interestingly, OOX cumulus cells maintained with GDF9 alone expressed similar levels of the cumulus expansion-related transcripts as those maintained with oocytes, but the degree of expansion was not comparable unless cotreated with BMP15, which has little effect on levels of cumulus expansion-related transcripts (Fig. 5C). This suggests that, in addition to expression of these cumulus expansion-related transcripts, other processes augmented by addition of BMP15 are required for full expansion. These results strongly suggest that the combination of E2 and oocyte-derived BMP15 and GDF9 are required for the maintenance of competence to undergo cumulus expansion.
Figure 5.
Oocyte (Oo)-derived BMP15 and GDF9 are required for E2 maintenance of cumulus cell competence to undergo expansion. OOX cumulus cell complexes were cultured with E2 and recombinant BMP15 (500 ng/ml), FGF8 (100 ng/ml), GDF9 (500 ng/ml), or combination of them, and their competence to undergo expansion (A and B) and expression levels of the cumulus expansion-related transcripts (C) were assessed. OOX cumulus cells without maintenance culture were used as control groups (Fresh). Bar, 200 μm. The values indicated by different alphabetical letters (a, b, and c) are significantly different (P < 0.05).
Oocyte-derived GDF9 and BMP15 suppress expression of Nrip1 transcript in cumulus cells, which encodes a potential inhibitor of estrogen receptor signals
Estrogen signals in granulosa cells are mediated by the nuclear receptor, ESR2 (19,20). Signals of nuclear receptors, including estrogen receptors, are controlled by multiple coregulators. Nuclear receptor-interacting protein 1 (NRIP1), also known as receptor interacting protein 140 (RIP140), is one of the negative regulators of nuclear receptor signals (37,38). Interestingly, Nrip1-deficient mice are infertile because of anovulation partially due to defective expansion of cumulus cells and reduced expression of Has2, Ptgs2, and Tnfaip6 mRNA (39,40,41).
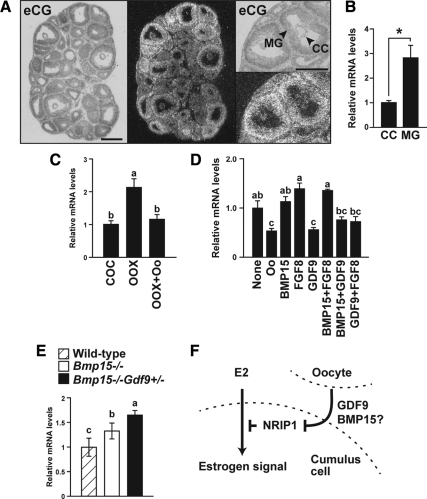
In our previous microarray data [http://www.ncbi.nlm.nih.gov/geo/ (GEO accession no. GSE7225)] (42), we found that expression of Nrip1 is significantly elevated in cumulus cells of Bmp15−/− or Bmp15−/− /Gdf9+/− mice compared with those of wild-type mice. This was confirmed herein by real-time PCR analysis; cumulus cells of Bmp15−/− mice expressed significantly higher levels of Nrip1 mRNA than those in wild-type mice, and its expression was further increased in cumulus cells of Bmp15−/− /Gdf9+/− mice (P < 0.05) (Fig. 6E). In situ hybridization using ovaries of equine choriogonadotropin-primed wild-type mice showed that Nrip1 transcripts were localized to granulosa cells of antral follicles as reported previously (39) (Fig. 6A). Expression levels of Nrip1 transcripts in cumulus cells seemed to be lower than those in mural granulosa cells by in situ hybridization (Fig. 6A), and this was confirmed by RT-PCR analysis comparing Nrip1 mRNA levels between freshly isolated wild-type cumulus cells and mural granulosa cells (Fig. 6B). These results suggest that oocytes, probably by secreting BMP15 and GDF9, suppress Nrip1 expression in cumulus cells in vivo.
Figure 6.
Oocytes suppress Nrip1 mRNA expression in cumulus cells. A, Localization of Nrip1 transcripts was determined by in situ hybridization. Bar, 300 μm. B, Freshly isolated cumulus cells (CC) express significantly lower levels of Nrip1 transcripts compared with mural granulosa cells (MG). *, P < 0.05. C, Oocytes (Oo) suppress Nrip1 mRNA expression in cumulus cells in vitro. D, Effects of recombinant proteins on Nrip1 mRNA levels in OOX cumulus cells were assessed by real-time PCR. E, Cumulus cells of Bmp15−/− and Bmp15−/− /Gdf9+/− mice express significantly higher levels of Nrip1 transcripts. The values indicated by different alphabetical letters (a, b, and c) are significantly different (P < 0.05). F, Hypothesis depicting possible role of NRIP1 in cumulus cells in mediating the coordination of estrogen and oocyte-drived signals (please see text for details). eCG, Equine choriogonadotropin.
To test this possibility in vitro, the effect of oocyte removal (OOX) from wild-type COCs on the levels of Nrip1 transcripts was determined. Cumulus cells were cultured as intact COCs, OOX cumulus cells, or OOX cumulus cells cocultured with denuded fully grown oocytes, and expression levels of Nrip1 transcripts in cumulus cells were examined after 20 h of culture (Fig. 6C). Levels of Nrip1 transcripts were up-regulated in OOX cumulus cells, when compared with those in cumulus cells cultured as intact COCs, and this increase was prevented by coculturing OOX cumulus cells with oocytes (Fig. 6C). Therefore, oocytes suppress expression of Nrip1 transcripts in cumulus cells in vitro.
To identify the oocyte factors that suppress Nrip1 expression in cumulus cells, the effects of recombinant BMP15, FGF8, GDF9, or combinations of them, on Nrip1 mRNA expression in OOX cumulus cells were determined (Fig. 6D). GDF9 alone was as effective as oocytes in suppressing Nrip1 expression in OOX cumulus cells (Fig. 6D). Combination with BMP15 or FGF8 did not further promote this suppressive effect of GDF9. In addition, BMP15 or FGF8 alone or together had little effect on Nrip1 mRNA expression in OOX cumulus cells in vitro. These results indicate that oocyte-derived GDF9 suppresses Nrip1 mRNA expression in cumulus cells before the LH surge. Furthermore, although an acute effect of BMP15 on the steady-state level of Nrip1 mRNA in cumulus cells in vitro was not detected, the chronic absence of BMP15 in Bmp15 mutant mice significantly affected the levels of Nrip1 mRNA in vivo (Fig. 6E).
Discussion
This study assessed the coordination of oocyte-derived paracrine factors and estrogen in the development and maintenance of the cumulus cell phenotype using cumulus expansion and expression of expansion-related transcripts as endpoints. E2 maintained cumulus cell competence to undergo expansion and promoted development of preantral granulosa cells to become competent to undergo expansion in vitro. Importantly, paracrine signals from oocytes, synergistic effects of BMP15 and GDF9, were required for these effects of the E2 signal. Therefore, oocytes and intrafollicular estrogen signals are coordinated in a manner that promotes and maintains development and function of cumulus cells.
Estrogen is required for acquisition and maintenance of cumulus cell competence to undergo expansion. This is reflected by the phenotype of Esr2-null mice, in which cumulus cells are not fully competent to undergo cumulus expansion (19,20,33). The reduced fertility in Esr2-null female mice is attributed primarily to insufficient ovulation because of reduced response of preovulatory follicles to the LH surge (19,20,43). Although downstream actions of LH receptor are mediated by cAMP, direct activation of adenylyl cyclase by forskolin only partially rescued ovulatory response of Esr2-null follicles grown in vitro (44). This suggests that events upstream of the LH receptor-mediated ovulatory signal, such as competence to undergo cumulus expansion, may also be attenuated in Esr2-null follicles in addition to the reduced downstream responses to LH and thereby contribute to ovulatory failure. Consistent with this idea, Couse et al. (20) have shown that granulosa cells of Esr2-deficient mice are less sensitive to FSH-induced differentiation, indicating that ESR2-mediated estrogen signal is required for proper differentiation of granulosa cells in vivo.
Several findings support the idea that oocyte-derived paracrine factors are involved in both the generation of E2 and response of cumulus cells to E2. Oocytes and recombinant GDF9 promote the production of E2 by mouse cumulus cells and rat granulosa cells, respectively (45,46). Furthermore, our previous microarray study (42) showed significantly lower levels of Cyp19a1 transcripts encoding CYP19A1 (aromatase) in Bmp15−/−/Gdf9+/− cumulus cells in vivo, suggesting a possibility that estrogen production in Bmp15−/−/Gdf9+/− follicles may be attenuated. In addition, oocyte-derived BMP15 and GDF9 are required for the E2 effects on maintenance of cumulus cell competence to undergo expansion in vitro (present study), and cumulus cells of Bmp15−/− or Bmp15−/− /Gdf9+/− mice are deficient in competence to undergo complete expansion (9,31). Therefore, it is possible that the reduced competence of Bmp15−/−/Gdf9+/− cumulus cells to undergo cumulus expansion might be attributed, at least in part, to a defective E2 signal.
Exogenous E2 maintained and promoted acquisition of competence to undergo expansion and full expression of Has2 transcripts; however, Ptgs2, Ptx3, and Tnfaip6 transcript levels were not affected by exogenous E2 supplementation. This suggests that factors, in addition to E2 and oocyte-derived paracrine factors, are required for full development of the cumulus cell phenotype. Interestingly, OOX cumulus cells maintained with E2 alone (without oocytes) expressed relatively higher levels of Ptgs2 transcripts when compared with those maintained with oocytes alone or both oocytes and E2 (Fig. 4C). One possible explanation for this observation is that cumulus cells maintained without oocytes may lose characteristics of cumulus cells and differentiate into a mural granulosa cell-like phenotype, because mural granulosa cells are capable in expressing high levels of Ptgs2 transcripts responding to EGF receptor signals (23). Furthermore, some granulosa cells of the in vitro-grown PGOCs that were located most distal from the oocytes remained unexpanded (Fig. 2A, arrowheads), indicating that these cells did not develop full competence to undergo expansion. It is possible that these cells may have differentiated toward mural granulosa cell-like phenotype due to the lower concentrations of oocyte signals because of the distance from the oocytes.
How do oocyte and estrogen signals cooperate to maintain the competence to undergo cumulus expansion? The finding that Nrip1 transcript levels in cumulus cells were suppressed by oocyte-derived paracrine factors, GDF9 and BMP15, suggests that NRIP1 may participate in the coordination of estrogen and oocyte signals during follicular development. NRIP1 was originally identified as a hormone-dependent coregulator of estrogen receptor 1 (ESR1) signaling (47). Subsequent studies have shown that NRIP1 interacts with and represses transcriptional activities of many other nuclear receptors including androgen receptor and estrogen-related receptors (for review see Ref. 38). Although few studies have been conducted to assess the involvement of NRIP1 in ESR2 signaling, association of NRIP1 with ESR2 was reported in rat uterus (48). Therefore, we hypothesize that suppression of Nrip1 expression in cumulus cells by oocyte-derived GDF9 and BMP15 may be critical for appropriate E2 signaling in granulosa cells (Fig. 6F). Reduction in oocyte-derived signals, e.g. in Bmp15−/−/Gdf9+/− follicles, may result in increased NRIP1 activity and therefore reduced cumulus cell response to E2 signals. Interestingly, Nrip1-deficient mice are infertile due to failure in ovulation, which can be attributed, in part, to defective cumulus expansion (39,40,41). This suggests that excess levels of E2 signals, due to the loss of inhibitory activity of NRIP1, may have negative effects on cumulus cell competence to undergo expansion. In fact, we observed that a high concentration of E2 (10−7 m) during the in vitro development of PGOCs resulted in defective cumulus expansion and expression of the cumulus expansion-related transcripts (our unpublished results). Therefore, intrafollicular estrogen signaling must be tightly regulated for the normal development of ovarian follicles. Further studies addressing the effects of NRIP1 on E2 function in cumulus cells are warranted. Interestingly, NRIP1 is also directly involved in expression of Areg transcripts encoding an EGFLP, amphiregulin, in COCs after the LH surge (49). Therefore, NRIP1 may be a key regulator of granulosa cell development and function both before and after the LH surge.
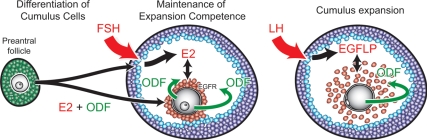
Evidence emerging from this and earlier studies indicates that oocyte-derived paracrine signals (ODFs) coordinate with the other follicular signals to regulate cumulus cell development and function in several ways (Fig. 7). First, ODFs coordinate with estrogen to promote the development of preantral granulosa cells to the cumulus cell phenotype, specifically the competence to undergo expansion and express the expansion-related transcripts. Once this phenotype is established in antral follicles, ODFs and estrogen function to maintain this phenotype until the preovulatory surge of LH triggers the cascade of processes that induce cumulus expansion. In addition, ODFs promote the expression of EGF receptors by cumulus cells that potentiate the response to EGFLPs produced by mural granulosa cells in response to the LH signal (50,51). Moreover, ODFs promote E2 production by cumulus cells (45). Finally, ODFs are required to enable cumulus cells to respond to post-LH-dependent EGFLPs and undergo expansion (25,35).
Figure 7.
Coordination of oocyte-derived paracrine factors (ODFs) and other intrafollicular factors in cumulus cell development and function. ODFs coordinate with E2 to promote the preantral granulosa cell-to-cumulus cell phenotype and then the maintenance of this phenotype in antral follicles. In antral follicles, ODFs promote the expression of EGF receptors (EGFR) in cumulus cells to prepare for the LH-dependent generation of EGFLPs that induce cumulus expansion (50,51). After initial stimulation by EGFLPs, the cumulus cells also produce EGFLPs to amplify the signal (23). ODFs also promote the production of E2 by cumulus cells (45). Finally, ODFs are required to enable cumulus expansion in response to stimulation by EGFLPs (25,35).
ODFs coordinate with other follicular signals in other ways during follicular development. For example, LH signaling is suppressed in cumulus cells by oocyte-derived BMP15 and/or GDF9 through suppression of Lhcgr mRNA expression, which encodes LH receptor (32,52,53). This is important to prevent cumulus cells from undergoing precocious luteinization. In addition, oocyte-derived BMP signals suppress FSH signaling, at least in part by suppressing expression of Fshr mRNA encoding FSH receptor (10,53,54). Finally, ODFs coordinate with estrogen signals in amplifying FSH signals during granulosa cell development in diethylstilbestrol-treated rat preantral granulosa cells in vitro (55). In addition to this previous study (55), the present study has shown a novel interaction of oocytes and estrogen signals in development and maintenance of cumulus cell competence to undergo expansion. Oocyte-derived GDF9 and BMP15 participate in this coordination.
Our earlier studies using reaggregated chimeric ovaries containing oocytes and somatic cells mismatched in their stages of development showed that oocytes orchestrate follicular development. The rates of development of both cumulus and mural granulosa cells were accelerated by mid-growth stage oocytes reaggregated with the somatic cells from newborn ovaries (56). The mechanisms for this dramatic orchestration involve effects of oocytes on both granulosa cell metabolism (5,57) and granulosa cell lineage to produce the cumulus cell phenotype (13,14). We conclude from the present and previous studies that coordination with other well-defined follicular regulators, such as estrogen and FSH, is crucial for these functions of oocytes.
Materials and Methods
Mice
B6SJLF1 mice were produced and raised in the research colony of the investigators at The Jackson Laboratory and used for all experiments unless otherwise noted. All animal protocols were approved by the Administrative Panel on Laboratory Animal Care at The Jackson Laboratory, and all experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Isolation and culture of PGOCs
Experimental designs are illustrated in Fig. 1. To test the effect of E2 supplementation on development of cumulus cells in vitro (Experiment 1), PGOCs were isolated from ovaries of 8-d-old mice using collagenase digestion as described previously (13,58). PGOCs without adherent interstitial cells (theca) were grown in vitro as described previously (13,36,59) on collagen-coated membranes for 14 d in the basic culture medium supplemented with 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium, and 1 mg/ml fetuin with or without 10−9 m E2 supplementation (Development Culture) (Fig. 1B).
Isolation and culture of COCs
Experimental designs are illustrated in Fig. 1. Bicarbonate-buffered MEMα (Life Technologies, Inc., Gaithersburg, MD) with Earles’ salts, supplemented with 75 μg/ml penicillin G, 50 μg/ml streptomycin sulfate, 0.23 mm pyruvate, and 3 mg/ml BSA (Sigma-Aldrich Co., St. Louis, MO) was used as a basic culture medium. COCs and fully grown oocytes were isolated from 22- to 24-d-old B6SJLF1 mice injected with 5 IU of equine chorionic gonadotropin 44–48 h earlier as reported previously (11). OOX cumulus cells were produced by microsurgically removing oocytes from the COCs (11,35).
To test the effect of E2 supplementation on ability of COCs to undergo cumulus expansion (Experiment 2), COCs were first maintained with or without E2 (10−10 to 10−7 m) for 2 d before the induction of cumulus expansion (test period) (Fig. 1C). All media used in the maintenance cultures, but not in the test cultures, were supplemented with the phosphodiesterase inhibitor milrinone (10 mm; Sigma-Aldrich) to arrest oocytes at the germinal vesicle stage. After the maintenance culture period, the viability of the cultured COCs were assessed by MTT assay using TACS MTT Cell Proliferation Assay Kit (Trevigen, Inc., Gaithersburg, MD) according to manufacturer’s instruction. To test a role for oocytes (Experiment 3), OOX cumulus cells were maintained with or without denuded fully grown oocytes (two oocytes/μl) in the basic culture medium supplemented with milrinone, and with or without 10−8 m E2 before testing competence to undergo cumulus expansion (Fig. 1D). In some experiments, medium was supplemented with recombinant human BMP15 (500 ng/ml) (12,60), mouse GDF9 (500 ng/ml) (12), and/or mouse FGF8B (100 ng/ml, Sigma). These concentrations of supplemented recombinant proteins are the maximally effective doses when tested for their ability to induce cumulus expansion (GDF9) or expression of transcripts encoding glycolytic enzymes (BMP15 and FGF8B) (data not shown) (12). All the cultures were maintained at 37 C in a modular incubation chamber (Billups Rothenberg, Del Mar, CA) infused with 5% O2, 5% CO2, and 90% N2.
Induction and evaluation of cumulus expansion in vitro
The cultured COCs or in vitro-grown PGOCs were washed thoroughly in the basic culture medium and were cultured in the basic culture medium supplemented with 5% serum with or without 10 ng/ml EGF (BD Biosciences, San Jose, CA) to test for the ability to undergo cumulus expansion and express expansion-related transcripts. As control groups, freshly isolated COCs (without the maintenance culture) treated with or without EGF were included for the analyses (indicated as “Fresh” in the figures). Some samples were taken for analysis of transcript levels after 6 h of EGF treatment. To determine expansion indexes, average expansion index values were calculated using 10 COCs, three to five OOX cumulus cells or three in vitro-grown PGOCs as one independent experiment, and these experiments were repeated at least three times. Cumulus expansion index was assessed after 14–16 h of culture (34).
Real-time RT-PCR
To assess the steady-state levels of transcripts, real-time RT-PCR was conducted as reported previously (12). Nrip1 PCR primers used were 5′-AACAGCCTTCTCAGCTTCCTTTC-3′ and 5′-TCATCTTTCGTTGCTCACCAAA-3′ (GenBank accession no. NM_173440). The other PCR primers used (Has2, Ptgs2, Ptx3, Tnfaip6, and Rpl19) were reported previously (30). The results were first normalized to the levels of transcripts encoding a housekeeping gene, ribosomal protein L19 (Rpl19), by the 2−ΔΔCt method (61), and presented as the transcript levels relative to those of a standard sample. Only one product of the appropriate size was identified by agarose gel electrophoresis for each set of primers, and PCR products were sequenced to verify sequence identity. The reactions in each of at least three independent experiments were conducted at least in duplicate.
In situ hybridization
An antisense fragment of Nrip1 cDNA (810 bp, accession no. NM_173440) was amplified by PCR using primers 5′-CTCGTACTCCAATTGCGTTCCC-3′ and 5′-ATCTCACAACCAGGAAGTCCCG-3′. The PCR product was cloned, sequenced, and used for in vitro transcription to make [33P]CTP-labeled riboprobes for in situ hybridization as reported previously (11). No signal was detected with a sense probe (data not shown).
Statistical analysis
A standard t test or the Tukey-Kramer HSD test was used for paired or multiple comparisons, respectively, using computer software JMP (SAS Institute, Inc., Cary, NC). A P value < 0.05 was considered statistically significant. The results are presented as mean ± sem of at least three independent experiments.
Supplementary Material
Acknowledgments
We thank Drs. Mary Ann Handel and Anne Peaston (The Jackson Laboratory, ME) for their helpful suggestions during preparation of this paper.
Footnotes
This work was supported by Grants HD23839 and HD21970 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and CA34196 from National Institutes of Health (to K.S., Y.Q.S., K.W., J.J.E.) and HD33438 (to Q.L., M.M.M.).
Present address for K.S.: Laboratory of Applied Genetics, Graduate School of Agricultural and Life Science, University of Tokyo, Tokyo, Japan.
Disclosure Summary: The authors have nothing to declare.
First Published Online November 3, 2010
Abbreviations: BMP, Bone morphogenetic protein; COC, cumulus cell-oocyte complex; E2, 17β-estradiol; EGF, epidermal growth factor; EGFLP, EGF-like peptide; ESR2, estrogen receptor 2; FGF, fibroblast growth factor; GDF, growth differentiation factor; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NRIP1, nuclear receptor-interacting protein 1; ODF, oocyte-derived factor; OOX, oocytectomized; PGOC, preantral granulosa cell-oocyte complex.
References
- Edson MA, Nagaraja AK, Matzuk MM 2009 The Mammalian ovary from genesis to revelation. Endocr Rev 30:624–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ 2001 Oocyte control of ovarian follicular development and function in mammals. Reproduction 122:829–838 [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Burns KH, Viveiros MM, Eppig JJ 2002 Intercellular communication in the mammalian ovary: oocytes carry the conversation. Science 296:2178–2180 [DOI] [PubMed] [Google Scholar]
- Sugiura K, Eppig JJ 2005 Society for Reproductive Biology Founders’ Lecture 2005. Control of metabolic cooperativity between oocytes and their companion granulosa cells by mouse oocytes. Reprod Fertil Dev 17:667–674 [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Eppig JJ 2009 Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med 27:32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM 1996 Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature 383:531–535 [DOI] [PubMed] [Google Scholar]
- Valve E, Penttilä TL, Paranko J, Härkönen P 1997 FGF-8 is expressed during specific phases of rodent oocyte and spermatogonium development. Biochem Biophys Res Commun 232:173–177 [DOI] [PubMed] [Google Scholar]
- Galloway SM, McNatty KP, Cambridge LM, Laitinen MP, Juengel JL, Jokiranta TS, McLaren RJ, Luiro K, Dodds KG, Montgomery GW, Beattie AE, Davis GH, Ritvos O 2000 Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat Genet 25:279–283 [DOI] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM 2001 Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol 15:854–866 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Moore RK, Shimasaki S 2001 Biological function and cellular mechanism of bone morphogenetic protein-6 in the ovary. J Biol Chem 276:32889–32895 [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ 2007 Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development 134:2593–2603 [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ 2009 Fibroblast growth factors and epidermal growth factor cooperate with oocyte-derived members of the TGFβ superfamily to regulate Spry2 mRNA levels in mouse cumulus cells. Biol Reprod 81:833–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ 2007 Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol 305:300–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, Wigglesworth K, Eppig JJ 2007 Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 120:1330–1340 [DOI] [PubMed] [Google Scholar]
- Couse JF, Korach KS 1999 Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- Drummond AE 2006 The role of steroids in follicular growth. Reprod Biol Endocrinol 4:16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Weihua Z, Mäkinen S, Mäkelä S, Saji S, Warner M, Gustafsson JA, Hovatta O 2002 A role for the androgen receptor in follicular atresia of estrogen receptor β knockout mouse ovary. Biol Reprod 66:77–84 [DOI] [PubMed] [Google Scholar]
- Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS 2005 In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER)α and ERβ null mice indicate a role for ERβ in follicular maturation. Endocrinology 146:2817–2826 [DOI] [PubMed] [Google Scholar]
- Couse JF, Yates MM, Deroo BJ, Korach KS 2005 Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology 146:3247–3262 [DOI] [PubMed] [Google Scholar]
- Chen L, Russell PT, Larsen WJ 1993 Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev 34:87–93 [DOI] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M 2004 EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303:682–684 [DOI] [PubMed] [Google Scholar]
- Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS 2006 Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol 20:1352–1365 [DOI] [PubMed] [Google Scholar]
- Hsieh M, Zamah AM, Conti M 2009 Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med 27:52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ 2006 The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol 299:91–104 [DOI] [PubMed] [Google Scholar]
- Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R 1999 Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology 140:2685–2695 [DOI] [PubMed] [Google Scholar]
- Varani S, Elvin JA, Yan C, DeMayo J, DeMayo FJ, Horton HF, Byrne MC, Matzuk MM 2002 Knockout of pentraxin 3, a downstream target of growth differentiation factor-9, causes female subfertility. Mol Endocrinol 16:1154–1167 [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS 2003 Disrupted function of tumor necrosis factor-α-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology 144:4376–4384 [DOI] [PubMed] [Google Scholar]
- Fülöp C, Szántó S, Mukhopadhyay D, Bárdos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K 2003 Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 130: 2253–2261 [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Eppig JJ 2009 Targeted suppression of Has2 mRNA in mouse cumulus cell-oocyte complexes by adenovirus-mediated short-hairpin RNA expression. Mol Reprod Dev 76:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Wu X, O'Brien MJ, Pendola FL, Denegre JN, Matzuk MM, Eppig JJ 2004 Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol 276:64–73 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola F, Hirao Y 1997 Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod 56:976–984 [DOI] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Chesnel F 1993 Secretion of cumulus expansion enabling factor by mouse oocytes: relationship to oocyte growth and competence to resume meiosis. Dev Biol 158:400–409 [DOI] [PubMed] [Google Scholar]
- Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ 1990 FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor(s) secreted by the oocyte. Dev Biol 138:16–25 [DOI] [PubMed] [Google Scholar]
- Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ 1990 Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol 140:307–317 [DOI] [PubMed] [Google Scholar]
- Augereau P, Badia E, Balaguer P, Carascossa S, Castet A, Jalaguier S, Cavaillès V 2006 Negative regulation of hormone signaling by RIP140. J Steroid Biochem Mol Biol 102:51–59 [DOI] [PubMed] [Google Scholar]
- Augereau P, Badia E, Carascossa S, Castet A, Fritsch S, Harmand PO, Jalaguier S, Cavaillès V 2006 The nuclear receptor transcriptional coregulator RIP140. Nucl Recept Signal 4:e024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Leonardsson G, Rosewell I, Ann Jacobs M, Milligan S, Parker M 2000 The nuclear receptor co-repressor Nrip1 (RIP140) is essential for female fertility. Nat Med 6:1368–1374 [DOI] [PubMed] [Google Scholar]
- Leonardsson G, Jacobs MA, White R, Jeffery R, Poulsom R, Milligan S, Parker M 2002 Embryo transfer experiments and ovarian transplantation identify the ovary as the only site in which nuclear receptor interacting protein 1/RIP140 action is crucial for female fertility. Endocrinology 143:700–707 [DOI] [PubMed] [Google Scholar]
- Tullet JM, Pocock V, Steel JH, White R, Milligan S, Parker MG 2005 Multiple signaling defects in the absence of RIP140 impair both cumulus expansion and follicle rupture. Endocrinology 146:4127–4137 [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Wigglesworth K, O'Brien MJ, Affourtit JP, Pangas SA, Matzuk MM, Eppig JJ 2008 Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development 135:111–121 [DOI] [PubMed] [Google Scholar]
- Deroo BJ, Rodriguez KF, Couse JF, Hamilton KJ, Collins JB, Grissom SF, Korach KS 2009 Estrogen receptor beta is required for optimal cAMP production in mouse granulosa cells. Mol Endocrinol 23:955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez KF, Couse JF, Jayes FL, Hamilton KJ, Burns KA, Taniguchi F, Korach KS 2010 Insufficient luteinizing hormone-induced intracellular signaling disrupts ovulation in preovulatory follicles lacking estrogen receptor-β. Endocrinology 151:2826–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhyden BC, Cohen JN, Morley P 1993 Mouse oocytes regulate granulosa cell steroidogenesis. Endocrinology 133:423–436 [DOI] [PubMed] [Google Scholar]
- Vitt UA, Hayashi M, Klein C, Hsueh AJ 2000 Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod 62:370–377 [DOI] [PubMed] [Google Scholar]
- Cavaillès V, Dauvois S, L'Horset F, Lopez G, Hoare S, Kushner PJ, Parker MG 1995 Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO J 14:3741–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daverey A, Saxena R, Tewari S, Goel SK, Dwivedi A 2009 Expression of estrogen receptor co-regulators SRC-1, RIP140 and NCoR and their interaction with estrogen receptor in rat uterus, under the influence of ormeloxifene. J Steroid Biochem Mol Biol 116:93–101 [DOI] [PubMed] [Google Scholar]
- Nautiyal J, Steel JH, Rosell MM, Nikolopoulou E, Lee K, Demayo FJ, White R, Richards JS, Parker MG 2010 The nuclear receptor cofactor receptor-interacting protein 140 is a positive regulator of amphiregulin expression and cumulus cell-oocyte complex expansion in the mouse ovary. Endocrinology 151:2923–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ 2010 Mouse oocytes enable lh-induced maturation of the cumulus-oocyte complex via promoting EGF receptor-dependent signaling. Mol Endocrinol 24:1230–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M 2007 Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27:1914–1924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM 1999 Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 13:1035–1048 [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yamamoto S, Erickson GF, Shimasaki S 2001 Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem 276:11387–11392 [DOI] [PubMed] [Google Scholar]
- McMahon HE, Hashimoto O, Mellon PL, Shimasaki S 2008 Oocyte-specific overexpression of mouse bone morphogenetic protein-15 leads to accelerated folliculogenesis and an early onset of acyclicity in transgenic mice. Endocrinology 149:2807–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka F, Moore RK, Wang X, Sharma S, Miyoshi T, Shimasaki S 2005 Essential role of the oocyte in estrogen amplification of follicle-stimulating hormone signaling in granulosa cells. Endocrinology 146:3362–3367 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K, Pendola FL 2002 The mammalian oocyte orchestrates the rate of ovarian follicular development. Proc Natl Acad Sci USA 99:2890–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura K, Pendola FL, Eppig JJ 2005 Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol 279:20–30 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ 1996 Development in vitro of mouse oocytes from primordial follicles. Biol Reprod 54:197–207 [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O'Brien MJ, Pendola FL, Watanabe S 1998 Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod 59:1445–1453 [DOI] [PubMed] [Google Scholar]
- Li Q, Rajanahally S, Edson MA, Matzuk MM 2009 Stable expression and characterization of N-terminal tagged recombinant human bone morphogenetic protein 15. Mol Hum Reprod 15:779–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD 2001 Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.