Abstract
Mutations in the HNF1A gene cause maturity-onset diabetes of the young type 3, one of the most common genetic causes of non-insulin-dependent (type 2) diabetes mellitus. Although the whole-body Hnf1a-null mouse recapitulates the low insulin levels and high blood glucose observed in human maturity-onset diabetes of the young type 3 patients, these mice also suffer from Laron dwarfism and aminoaciduria, suggesting a role for hepatocyte nuclear factor 1α (Hnf1α) in pathophysiologies distinct from non-insulin-dependent (type 2) diabetes mellitus. In an effort to identify pathways associated with inactivation of Hnf1α, an ultraperformance liquid chromatography coupled to mass spectrometry-based metabolomics study was conducted on urine samples from wild-type and Hnf1a-null mice. An increase in phenylalanine metabolites is in agreement with the known regulation of the phenylalanine hydroxylase gene by Hnf1α. This metabolomic approach also identified urinary biomarkers for three tissue-specific dysfunctions previously unassociated with Hnf1α function. 1) Elevated indolelactate coupled to decreased xanthurenic acid also indicated defects in the indole and kynurenine pathways of tryptophan metabolism, respectively. 2) An increase in the neutral amino acid proline in the urine of Hnf1a-null mice correlated with loss of renal apical membrane transporters of the Slc6a family. 3) Further investigation into the mechanism of aldosterone increase revealed an overactive adrenal gland in Hnf1a-null mice possibly due to inhibition of negative feedback regulation. Although the phenotype of the Hnf1a-null mouse is complex, metabolomics has opened the door to investigation of several physiological systems in which Hnf1α may be a critical regulatory component.
Metabolomic analysis of the MODY3 Hnf1α-null mouse reveals alterations in renal handling of amino acids and the hypothalamic-pituitary-adrenal axis.
Hepatocyte nuclear factor 1α (Hnf1α), expressed in liver, kidney, pancreas, and intestine, is a homeodomain-containing transcription factor that has an important role in glucose homeostasis. Humans with inactivating mutations in the HNF1A gene develop maturity-onset diabetes of the young type 3 (MODY3), one of several monogenic causes of non-insulin-dependent (type 2) diabetes mellitus (1). These patients are characterized by reduced insulin secretion and elevated blood glucose levels, clinical characteristics that are replicated in two separate Hnf1a-null mouse models (2,3). Defective insulin secretion in the Hnf1a-null mouse results from impaired mitochondrial function and reduced glucose-stimulated insulin secretion due to reduced expression of genes such as glucose transporter 2 (Glut2) and insulin (Ins-1) (4,5,6). However, beyond its role in maintaining glucose homeostasis, Hnf1α also regulates a variety of other essential metabolic processes including cholesterol and bile acid homeostasis as well as numerous solute carrier (SLC) transporters, all of which have been identified using the Hnf1a-null mouse model.
Hnf1a-null mice are hypercholesterolemic and have fatty livers primarily due to defects in liver and intestinal cholesterol transport as well as increased de novo cholesterol synthesis (7). Hnf1a-null mice also exhibit phenylketonuria (PKU) due to loss of the Hnf1α-responsive gene (8,9), phenylalanine hydroxylase (Pah) (3,10). Hnf1α also regulates proline metabolism by controlling proline oxidase and dehydrogenase expression (11). More recently, it has become evident that Hnf1α regulates the expression of many transporters located in the liver, kidney, intestine, and pancreas. In characterization of the Hnf1a-null mouse, Pontoglio et al. (10) noted defects in the reabsorption of many amino acids, resembling the human disease renal Fanconi syndrome. A previous study characterized the expression of many members of the organic anion transporter (Oat), organic anion-transporting polypeptide, ATP-binding cassette sub-family C, and ATP-binding cassette, sub-family B transporter families in Hnf1a-null mice (12). The down-regulation of Oats in the kidney was further characterized and Hnf1α binding to the promoters of Oat1 and Oat3 confirmed (13,14). Most recently, Hnf1α/β was shown to regulate expression of the human sodium-dependent vitamin C transporter-1 (SLC23A1) in cell culture (15). With alteration in many transporter families, it is likely that Hnf1a-null mice suffer from deficiencies in dietary nutrients as well as toxicity and wasting from improper renal handling.
Metabolomics, the global unbiased analysis of metabolic changes in response to genetic or external stimuli within a biofluid, is an ideal method to obtain an instantaneous picture of the physiological impact of Hnf1α deletion in an easily obtainable biofluid such as urine. In this study, the Hnf1a-null mouse was used as a model of the naturally occurring human diabetic disease MODY3 in an effort to identify biomarkers that may provide a phenotypic profile distinct to HNF1A deletion within the non-insulin-dependent (type 2) diabetes mellitus population as well as uncover alterations in physiological pathways that may be masked by the diabetic phenotype. The data establish a role for Hnf1α in phenylalanine metabolism as well as novel effects on amino acid transport, tryptophan metabolism, and the hypothalamic-pituitary-adrenal axis.
Results
Metabolomic analysis of mouse urine
Several physiological parameters were examined before the analysis of urinary metabolomic data to confirm the Hnf1a-null phenotype as well as to assess kidney function known to be altered in diabetic states. Hnf1a-null mice have slightly larger kidneys, although they appear histologically normal, as previously reported (3). The larger kidneys may account for the 1.7-fold increase in diuresis [urine production (microliters)/body mass (grams) per 24 h] calculated in Hnf1a-null mice in this study (Table 1). The glomerular filtration rate was not determined. Increases in serum alkaline phosphatase, alanine aminotransferase, and bilirubin are indicative of the known fatty liver disease in this mouse model. Serum measurements of kidney function (phosphorous, blood urea nitrogen, and amylase), although trending toward an increase in Hnf1a-null mice, were not statistically significant.
Table 1.
Physiological characteristics and serum chemistry of Hnf1a-null mice
| Wild-type | Hnf1a-null | Ratioa | P value | |
|---|---|---|---|---|
| Body mass (g) | 23.3 ± 0.62 | 11.0 ± 0.97 | 0.47 | <0.0001 |
| Liver/body mass (mg/g) | 51.9 ± 0.87 | 145 ± 4.35 | 2.80 | <0.0001 |
| Kidney/body mass (mg/g) | 9.56 ± 0.14 | 13.2 ± 0.87 | 1.38 | <0.0001 |
| Adrenal gland/body mass (mg/g) | 0.13 ± 0.01 | 0.36 ± 0.03 | 2.77 | <0.0001 |
| Diuresis (μl/g · 24 h) | 43.6 ± 3.30 | 74.7 ± 20.0 | 1.71 | 0.02 |
| Serum chemistry analysis | ||||
| Na+ (mmol/liter) | 154.3 ± 2.84 | 148.7 ± 3.18 | 0.96 | 0.25 |
| K+ (mmol/liter) | 7.57 ± 0.20 | 6.50 ± 0.26 | 1.16 | 0.02 |
| Glucose, fed (mg/dl) | 180.0 ± 8.10 | 239.7 ± 18.80 | 1.33 | 0.02 |
| Phosphorous (mg/dl) | 9.30 ± 0.25 | 8.53 ± 0.57 | 0.92 | 0.23 |
| Blood urea nitrogen (mg/dl) | 28.75 ± 1.65 | 32.33 ± 1.86 | 1.12 | 0.21 |
| Amylase (U/liter) | 1318 ± 79.23 | 1603 ± 81.42 | 1.21 | 0.06 |
| Ca2+ (mg/dl) | 10.95 ± 0.12 | 10.53 ± 0.32 | 0.96 | 0.22 |
| Globulin (g/dl) | 1.95 ± 0.03 | 1.57 ± 0.03 | 0.81 | 0.0003 |
| Total protein (g/dl) | 6.10 ± 0.08 | 5.30 ± 0.21 | 0.86 | 0.01 |
| Alanine aminotransferase (U/liter) | 87.25 ± 13.96 | 224.0 ± 68.57 | 2.57 | 0.07 |
| Alkaline phosphatase (U/liter) | 156.8 ± 13.0 | 870.3 ± 63.6 | 5.55 | <0.0001 |
| Albumin (g/dl) | 4.12 ± 0.05 | 3.73 ± 0.18 | 0.90 | 0.06 |
| Total bilirubin (mg/dl) | 0.23 ± 0.03 | 0.90 ± 0.35 | 3.91 | 0.07 |
Ratio of Hnf1a-null value to wild-type value.
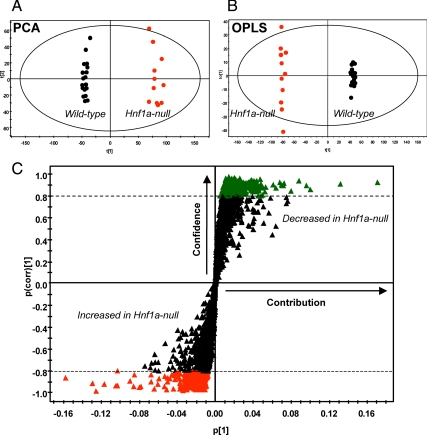
Urine samples collected from male wild-type and Hnf1a-null mice were analyzed by ultraperformance liquid chromatography coupled to electrospray ionization quadrupole time-of-flight mass spectrometer (UPLC-ESI-QTOFMS) operating in positive ionization mode. The mass to charge ratio (m/z) and retention time and abundance data generated were subjected to principal components analysis (PCA) and orthogonal projection to latent structures (OPLS) multivariate data analysis. A two-component PCA distinguished wild-type from Hnf1a-null mice (Fig. 1A). Further analysis of the data matrix by the supervised method of OPLS resulted in a robust model. Again, separation between wild-type and Hnf1a-null mice was clear in the scores scatter plot (Fig. 1B). The loadings S-plot generated from OPLS is a convenient way of visualizing those ions with the highest contribution to the separation between wild-type and Hnf1a-null mice (p[1]) in relation to their correlation to the model (p(corr)[1]). Those ions that increased in Hnf1a-null mice compared with wild-type mice are located in the lower left quadrant, whereas those that are significantly decreased in the Hnf1a-null mice are located in the upper right quadrant (Fig. 1C). Ions with a p(corr) higher than 0.8 were identified by searching the web-based databases Madison-Qingdao Metabolomics Consortium Database and MetaboAnalyst (16,17). Confirmation of ion identity was obtained by comparison of retention time and mass fragmentation pattern to authentic standards (see Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Identified markers that were increased in the Hnf1a-null mice are listed in Table 2. Many of the highly correlating ions were Na+ or K+ adducts or dimers of the parent compounds. For simplicity, only parent compounds or the highest correlating in-source fragment are shown in Table 2.
Figure 1.
PCA and OPLS plots demonstrating separation of wild-type and Hnf1a-null mice. Urine samples from wild-type and Hnf1a-null mice were subjected to UPLC-ESI-QTOFMS. A and B, The MarkerLynx data matrix was then used to generate PCA (A) and OPLS (B) models with accompanying scores plots. t[1] and t[2] correspond to principal components 1 and 2, respectively. C, The OPLS model was then used to generate a loadings S-plot showing ions important to the clustering of samples. The upper right quadrant shows those ions depleted in the Hnf1a-null mouse, and the lower left quadrant shows those ions increased in Hnf1a-null mouse urine.
Table 2.
Summary of identifiable ions increased in Hnf1a-null mice
| p(corr) | Ret. time (min) |
m/z (ESI+)
|
Mass error (ppm) | Empirical formula [M+H]+ | Identity | |
|---|---|---|---|---|---|---|
| Observed | Calculated | |||||
| −0.982 | 4.88 | 361.201 | 361.202 | −2.77 | C21H29O5 | Aldosterone |
| −0.979 | 4.16 | 377.197 | 377.196 | 2.65 | C21H29O6 | 18-Oxocortisola |
| −0.972 | 3.47 | 206.081 | 206.082 | −4.85 | C11H12NO3 | dl-3-Indolelactate |
| −0.922 | 1.10 | 131.050 | 131.050 | 0 | C9H7Ob | l-Phenylalanineb |
| −0.911 | 2.61 | 194.081 | 194.082 | −5.15 | C10H12NO3 | N-Phenylacetylglycine |
| −0.891 | 5.24 | 363.219 | 363.217 | 5.51 | C21H29O5 | DHOPA |
| −0.858 | 5.34 | 361.204 | 361.202 | 5.54 | C21H29O5 | HDOPA |
| −0.871 | 3.35 | 131.051 | 131.050 | 7.63 | C9H7Ob | N-Acetyl-l-phenylalanineb |
p(corr), The modeled correlation or confidence.
Putative identification only. Authentic standards were not available for confirmation.
In-source fragment; putative empirical formula of fragment provided.
The highest correlating ion elevated in Hnf1a-null mice is the steroid hormone aldosterone, which increased 4-fold in Hnf1a-null mice as measured by ELISA (Table 3). The second most important ion is putatively identified as 18-oxocortisol (authentic standard not available). Further inspection of the elevated ion pool revealed the presence of the corticosterone metabolites 11beta,20-dihydroxy-3-oxo-pregn-4-en-21-oic acid (DHOPA) and 11beta-hydroxy-3,20-dioxopregn-4-en-21-oic acid (HDOPA).
Table 3.
Steroid hormone levels in urine and serum
| Wild type | Hnf1a-null | Ratioa | P value | |
|---|---|---|---|---|
| Urine | ||||
| Aldosterone (ng/ml) | 2.76 ± 0.27 | 8.09 ± 1.18 | 2.93 | 0.0009 |
| Corticosterone (ng/ml) | 128.9 ± 12.18 | 575.1 ± 53.7 | 4.46 | <0.0001 |
| Serum | ||||
| Aldosterone (ng/ml) | 23.4 ± 4.19 | 43.8 ± 7.33 | 1.87 | 0.029 |
| Corticosterone (ng/ml) | 14.51 ± 2.48 | 72.21 ± 6.15 | 4.97 | <0.0001 |
| DHEA (ng/ml) | 1.31 ± 0.01 | 0.24 ± 0.13 | 0.18 | 0.015 |
| DHEAS (ng/ml) | 19.0 ± 3.47 | 5.25 ± 0.88 | 0.28 | 0.0018 |
| Progesterone (ng/ml) | 229 ± 18.8 | 663 ± 208 | 2.90 | 0.0325 |
Ratio of Hnf1a-null value to wild-type value.
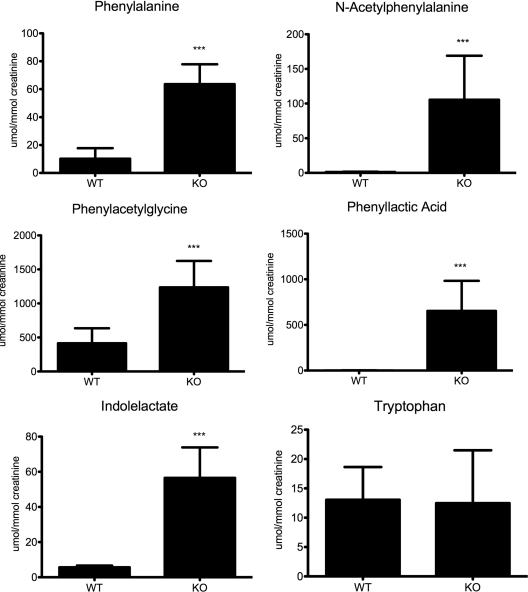
dl-3-Indolelactate was also highly correlated to the model in Hnf1a-null mice. The 10-fold increase in dl-3-indolelactate was not mirrored in urinary tryptophan concentration (Fig. 2). The intermediate indole pyruvate could not be resolved by UPLC-ESI-QTOFMS.
Figure 2.
Quantification of phenylalanine and tryptophan metabolites. After confirmation by tandem mass spectrometry fragmentation pattern comparison to authentic standards, metabolite concentrations were determined by triple-quadrupole mass spectrometry and normalized to millimoles of creatinine. Significant increases in metabolite concentration in Hnf1a-null mouse urines are indicated (***, P ≤ 0.0005). KO, Knockout; WT, wild type.
The list of ions increased in Hnf1a-null mouse urine is replete with metabolites related to phenylalanine metabolism. This finding is consistent with the known regulation of the phenylalanine hydroxylase (Pah) gene by Hnf1α (8,10). The concentrations of the top markers were obtained by triple-quadrupole mass spectrometry (Fig. 2). N-Phenylacetylglycine was the most abundant marker quantitated, reaching levels of 1200 μmol/mmol creatinine in the Hnf1a-null mice compared with 415 μmol/mmol creatinine in wild-type mice (P < 0.0001). In wild-type mice, l-phenylalanine had an average concentration of 10.22 μmol/mmol creatinine compared with 60 μmol/mmol creatinine in Hnf1a-null mice (P < 0.0001). The phenylalanine metabolites dl-3-phenyllactic acid and N-acetylphenylalanine, which result primarily from bacterial metabolic activity, were nearly undetectable in wild-type mice (1.274 and 1.228 μmol/mmol creatinine, respectively). dl-3-Phenyllactic acid increased to 653.4 μmol/mmol creatinine in Hnf1a-null mice (P < 0.0001), and N-acetylphenylalanine in Hnf1a-null mice was measured at 105.4 μmol/mmol creatinine (P < 0.0001).
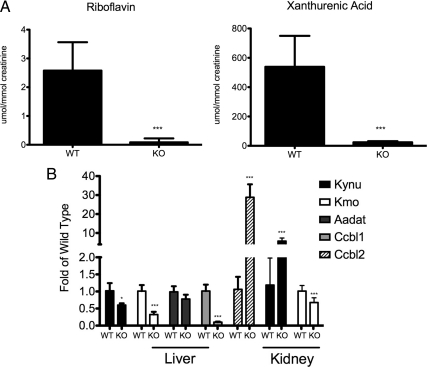
Identification of ions decreased in Hnf1a-null mice proved somewhat more difficult than elevated markers. Two ions were identified, riboflavin (m/z = 377.1477+) and xanthurenic acid (m/z = 206.0449+) and quantified (Fig. 3A). Riboflavin, present at very low concentrations in wild-type urine (2.577 μmol/mmol creatinine) was undetectable in most Hnf1a-null urine samples with an average measurable concentration at 0.085 μmol/mmol creatinine (P < 0.0001). The xanthurenic acid concentration decreased from 538.5 μmol/mmol creatinine in wild-type mice to 23.83 μmol/mmol creatinine in Hnf1a-null mice (P < 0.0001). No change in either kynurenine or kynurenic acid was found in the urine of Hnf1a-null mice (data not shown).
Figure 3.
The kynurenine pathway of tryptophan metabolism is inhibited in Hnf1a-null mice. A, Two ions decreased in Hnf1a-null mouse urine were identified as riboflavin and xanthurenic acid. Metabolite concentrations are normalized to millimoles of creatinine. B, Gene expression of selected enzymes involved in the kynurenine pathway of tryptophan metabolism was assessed in the liver and kidney by QPCR analysis. All values are normalized to β-actin expression and are represented as fold of the wild-type value for each gene analyzed. Significant changes in Hnf1a-null mice are indicated (*, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005). KO, Knockout; WT, wild type.
Changes in tryptophan metabolism in the liver and kidney
The decrease of xanthurenic acid in the urine of Hnf1a-null mice is suggestive of a defect in the metabolism of tryptophan to quinolinic acid. Gene expression was assessed for several of the enzymes involved in this pathway in both kidney and liver. Given that there was no change in the levels of kynurenic acid, kynurenine, or tryptophan in the urine of Hnf1a-null mice, gene expression analysis focused on those enzymes involved in the conversion of kynurenine to xanthurenic acid. The gene Kmo encodes kynurenine 3-hydroxylase and is responsible for the hydroxylation of kynurenine to 3-hydroxykynurenine (3-HK). Kmo transcript was significantly decreased in both the liver and kidney of Hnf1a-null mice with the liver having the larger fold change (Fig. 3B). Kynureninase (Kynu) converts 3-HK to 3-hydroxyanthranilic acid as well as kynurenine to anthranilic acid. Kynu was decreased 40% in the liver (P = 0.011) but induced 8-fold in the kidney (P = 0.0006). Three genes (Aadat, Ccbl1, and Ccbl2) encode kynurenine aminotransferases (Kats) that convert kynurenine to kynurenic acid or 3-HK to xanthurenic acid. Aadat (KatII) was unchanged in the liver. However, Ccbl1 (KatI) transcript was decreased nearly 90% in Hnf1a-null mice (P < 0.0001), whereas conversely, Ccbl2 (KatIII) transcript was increased 30-fold in the Hnf1a-null mice (P = 0.0002).
Alteration of amino acid transporters in the kidney
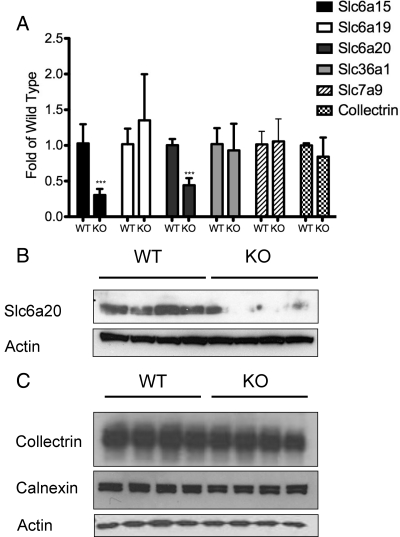
The identification of renal Fanconi syndrome in Hnf1a-null mice as well as the regulation of the amino acid transporter-associated protein collectrin by Hnf1α led us to investigate the expression of several kidney apical neutral amino acid transporters. There are two B0 transporters: neutral amino acid transporter 1 (B0AT1) encoded by the gene Slc6a19 and neutral amino acid transporter 2 (B0AT2) encoded by Slc6a15. Slc6a15 expression was decreased 60% in Hnf1a-null mice (P < 0.0001), whereas Slc6a19 expression was unaffected (Fig. 4A). The IMINO system transporter Slc6a20 (XT3, IMINO) RNA expression was reduced 50% in the Hnf1a-null mice (P < 0.0001). Of note was the lack of regulation of collectrin in the kidney of Hnf1a-null mice. Slc36a1 (proton amino acid transporter 1), a glycine, proline, and alanine transporter, expression was unaffected by Hnf1α disruption. Similarly, there was no change in the expression of the cationic amino acid exchanger, Slc7a9 (b0,+AT). Quantitative PCR (QPCR) results were confirmed by Western blot for Slc6a20 and collectrin (Fig. 4, B and C). Calnexin expression was used as a marker for general effects on membrane protein expression.
Figure 4.
Decrease in the expression of renal apical neutral amino acid transporters in Hnf1a-null mice. A, Gene expression analysis by QPCR of several transporters important for renal reuptake of amino acids. All values are normalized to β-actin expression and are represented as fold of the wild-type value for each gene analyzed. Significant changes in Hnf1a-null mice are indicated (*, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005). B and C, Western blot analysis of kidney membrane extracts from four individual wild-type and in Hnf1a-null mice for Slc6a20 (B) and collectrin (C). Actin and calnexin were used as loading controls. KO, Knockout; WT, wild type.
The metabolomic data collected allowed for a directed search of metabolites associated with neutral amino acid apical reabsorption. B0AT1 is a general neutral amino acid transporter, whereas B0AT2 preferentially transports branched-chain amino acids and proline. Slc6a20 is a Na+- and Cl−-dependent transporter primarily of imino and N-methylated amino acids. Urinary proline was increased 3-fold in Hnf1a-null mice (from 7.42 to 22.12 μmol/mmol; Fig. 5). The N-methylated amino acid glycine betaine was similarly increased from 32.31 to 73.08 μmol/mmol creatinine, a 2.26-fold increase. However, the imino acid pipecolic acid was decreased 60% from 81.11 to 34.23 μmol/mmol creatinine in urine of Hnf1a-null mice.
Figure 5.
Urinary concentrations of Slc6a transporter substrates are elevated in Hnf1a-null mice. The concentration of proline, glycine betaine, and pipecolic acid was determined by triple-quadrupole mass spectrometry in wild-type and Hnf1α-null mouse urine. Values are normalized to millimoles of creatinine. Significant changes from wild type are indicated (*, P ≤ 0.05; **, P ≤ 0.005). KO, Knockout; WT, wild type.
Adrenal gland dysfunction in Hnf1a-null mice
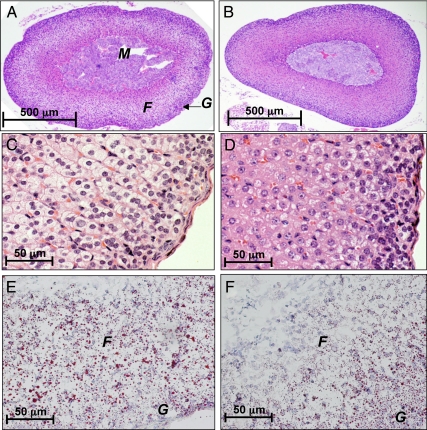
The steroid hormone aldosterone was the most important ion increased in the urine of Hnf1a-null mice. This result prompted a closer inspection of the adrenal glands, the site of aldosterone synthesis. The mass of adrenal glands was nearly 3-fold higher in Hnf1a-null mice (Table 1). Histological examination revealed eosinophilia of the cortical cells as well as increased activity in the zona glomerulosa indicated by darker purple staining of nuclei (Fig. 6D). Cells that appear to be atrophying populate the zona fasciculata. Oil red O staining indicates abundant neutral lipids in the cortex of wild-type mice, whereas Hnf1a-null adrenal gland is depleted of lipids (Fig. 6, E and F).
Figure 6.
Adrenal gland exhaustion in Hnf1a-null mice. Adrenal glands from wild-type (panels A, C, and E) and Hnf1a-null (panels B, D, and F) mice were collected for histological analysis. The medulla (M), zona fasiculata (F), and zona glomerulosa (G) are indicated in panel A. Panels A–D are hematoxylin and eosin staining of formalin-fixed and paraffin-embedded sections shown at ×40 (A and B) and ×400 (C and D) magnification. Frozen sections were used for oil red O staining and shown at ×400 magnification (E and F). Panels are representative of three individual animals assessed for each genotype.
The metabolomic and histological data suggest increased adrenal activity in Hnf1a-null mice. The elevation in aldosterone was confirmed by ELISA in both serum and urine samples (Table 3). Furthermore, serum and urine corticosterone levels were dramatically increased as well as serum progesterone. In contrast, the levels of serum dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) were reduced in Hnf1a-null mice. The significant increase in cholesterol-derived steroids suggested possible up-regulation of synthetic enzymes in the adrenal gland, in particular the P450 side-chain cleavage enzyme (P450scc/Cyp11a1). However, QPCR analysis showed no change in Cyp11a1 or in the corticosterone and aldosterone synthetic enzymes, Cyp11b1 and Cyp11b2, respectively (Fig. 7A). Although DHEA and DHEAS levels were both low in the Hnf1a-null mice, the ratio of DHEAS to DHEA was 1.5 times higher than in wild-type mice. The conversion of DHEA to DHEAS is accomplished by the sulfotransferase Sult2a1 primarily in the liver. Sult2a1, induced 20-fold in Hnf1a-null liver, may contribute to the higher DHEAS ratio in Hnf1a-null mice (Fig. 7B).
Figure 7.
A, Assessment of genes involved in steroid synthesis. QPCR analysis of RNA from adrenal glands of wild-type and Hnf1a-null mice; B, expression of Sult2a1 was assessed in the liver by QPCR; C, QPCR analysis of genes involved in the hypothalamic-pituitary-adrenal gland axis. All values are normalized to β-actin expression and are represented as fold of the wild-type value for each gene analyzed. Significant changes in Hnf1a-null mice are indicated (*, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.0005). KO, Knockout; WT, wild type.
The adrenal gland is stimulated to produce and release corticosteroids by the anterior pituitary hormone ACTH. ACTH is a cleavage product of the pituitary precursor polypeptide proopiomelanocortin (POMC). ACTH binds to melanocortin receptors in the zona fasiculata of the adrenal cortex to stimulate steroid hormone production. The corticotropes of the pituitary are stimulated to produce ACTH by the hypothalamic hormone CRH. In a negative feedback loop, the glucocorticoids produced in the adrenal gland then inhibit further CRH and POMC secretion through the actions of the glucocorticoid receptor (GR). Pomc gene expression in the pituitary was increased an average of 4-fold in Hnf1a-null mice (Fig. 7C). However, Crh gene expression in the hypothalamus was unchanged. Additionally, the expression of the ACTH receptor Mc2r was not significantly altered in the adrenal gland. GR expression in the hypothalamus was reduced 35%, whereas pituitary expression was unaltered.
Discussion
Hnf1α is a critical regulator of glucose metabolism as demonstrated in human populations by the occurrence of MODY3. Although much research has focused on the role of Hnf1α in the liver and pancreas in relation to diabetes, Hnf1α also regulates the expression of many enzymes involved in endo- and xenobiotic metabolism as well as metabolite transporters. The phenotype of the Hnf1a-null mouse is complex, and metabolomics has opened the door to investigation of several physiological systems in which Hnf1α may be a critical component (Fig. 8).
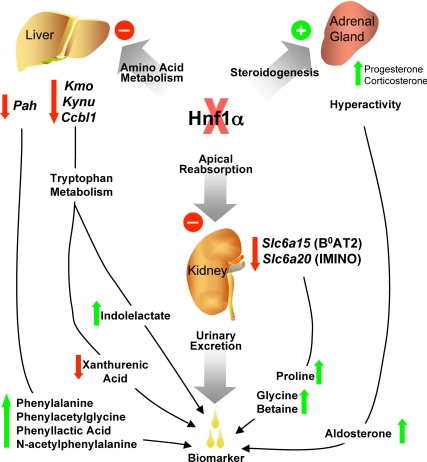
Figure 8.
Summary of metabolomic findings in Hnf1a-null mice. Identification of biomarkers in the urine of Hnf1a-null mice enabled discovery of several altered biochemical and physiological pathways. Hyperactive adrenal glands produce excess progesterone leading to increase in aldosterone. Several amino acid pathways are altered in Hnf1a-null mice, principally those of phenylalanine and tryptophan. Reduced phenylalanine hydroxylase (Pah) enzyme inhibits conversion of phenylalanine to tyrosine, thereby causing a build-up of phenylalanine and its bacterial metabolites. Reduction in mRNA transcripts of enzymes key in the kynurenine pathway of tryptophan metabolism may lead to the observed decrease in urinary xanthurenic acid. Additionally, this blockage in the kynurenine pathway may result in increased clearance of tryptophan through the secondary indole pathway. The renal apical transporters B0AT2 (Slc6a15) and IMINO (Slc6a20) are decreased in Hnf1a-null mice, thereby contributing to urinary loss of proline and glycine betaine. All of these markers are excreted in the urine, an easily obtainable biofluid in which to find clinically useful biomarkers for diagnosis of disease.
Amino acid metabolism
The regulation of phenylalanine hydroxylase by Hnf1α is well documented, and increased urinary phenylalanine was detected in the initial characterization of two separate Hnf1a-null mouse models (3,10). In the present study, OPLS identified the phenylalanine metabolites N-acetylphenylalanine, phenylacetylglycine (present as phenylacetylglutamine in humans), and phenyllactic acid as well as phenylalanine itself as significantly elevated ions in Hnf1a-null mouse urine. All three phenylalanine metabolites are known to be present in human PKU urine samples (18,19,20). Elevated urinary phenylalanine and phenyllactic acid have been identified in the mouse model of PKU, the Pahhph-5 mouse (21,22). This is the first confirmation of the correlation between phenylalanine hydroxylase deficiency and elevated urinary N-acetylphenylalanine in the mouse. Phenylacetylglycine, as well as being a marker for PKU, has recently been associated with drug-induced phospholipidosis in rats (23,24). Although phospholipidosis has not been specifically identified in Hnf1a-null mice, the enlarged fatty livers and disruption of fatty acid catabolism and transport are suggestive of such a phenotype occurring (25). Additionally, several phospholipase genes, including phospholipase A1, are down-regulated in Hnf1a-null liver as assessed by microarray analysis (data not shown).
There are four major pathways of tryptophan metabolism: kynurenine, serotonin, tryptamine, and indole, listed in relative importance under normal physiological conditions (26). Hnf1a-null mice excrete reduced amounts of xanthurenic acid, thus suggesting a defect in the kynurenine pathway. Indeed, gene expression analysis revealed a significant reduction in the expression of kynurenine 3-hydroxylase (Kmo) in the liver. Perhaps of more significance to the formation of xanthurenic acid was the nearly complete loss of expression of Ccbl1, encoding the enzyme KatI. Analysis of the first 2 kb of the Ccbl1 promoter by transcription factor binding prediction software did not reveal any Hnf1α binding sites, thus leaving open the question whether this gene is a direct target of Hnf1α. It is not possible to definitively conclude KatI is the major enzyme in liver responsible for xanthurenic acid generation, but given that there is no change in Aadat (KatII) and an increase in Ccbl2 (KatIII) expression, the data are suggestive of this conclusion. It is interesting to note that KatIII can transaminate phenylalanine nearly as well as tryptophan or kynurenine but is only weakly active toward 3-HK (27), suggesting that the large increase in KatIII expression may be a compensatory mechanism to aid in the elimination of phenylalanine. The reduction of Kmo and Kynu in the liver indicates down-regulation of the kynurenine pathway leading to nicotinamide production.
The possibility that the decrease in xanthurenic acid in the urine of Hnf1a-null mice is due to a defect in basolateral transporters of the kidney proximal tubule cannot be overlooked. Oat1 and -3 have been shown to transport both kynurenic and xanthurenic acid (28), and mouse and human Oat1 and Oat3 expression is controlled by Hnf1α and -β (12,13,14). Thus, the decrease in urinary xanthurenic acid might be due to impaired transport from the blood across the basolateral membrane into the collecting ducts of the proximal tubules. Although this is a possibility, given the complications of this mouse being a whole-body knockout, the fact that urinary kynurenic acid levels are unchanged argues more toward a defect in metabolism rather than transport.
If the kynurenine pathway of tryptophan metabolism is impaired in liver, this may contribute to the observed increase in indolelactate. Indolelactate is a degradation product of the indole pathway of tryptophan metabolism resulting from bacterial metabolism of the intermediate indolepyruvate. Indolepyruvate may be formed directly from tryptophan or through the tryptamine pathway of tryptophan metabolism. Thus, indolelactate increase may simply be a secondary mechanism to prevent tryptophan overload resulting from defective kynurenine metabolism. An additional possibility is that increased indolelactate is a marker of fatty liver. Chronic alcohol consumption results in fatty alcoholic liver disease. Among many of the metabolic changes occurring, reduced conversion of tryptophan to kynurenine has been identified after chronic alcohol consumption (26). Additionally, 2 wk treatment with the peroxisome proliferator activated receptor-α ligand Wy-14,643 decreases urinary xanthurenic acid in mice (29). Peroxisome proliferator activated receptor-α ligand treatment in mice leads to increased hepatic lipid metabolism and hepatomegaly (30). Finally, a metabolomic analysis of early insulin resistance in mice found serum kynurenine and liver nicotinic acid adenine dinucleotide-P reduced (31). Although the etiology of the fatty liver and specific mechanisms of tryptophan metabolism inhibition may be different between the three models, there may be a consistent pattern of metabolic endpoints indicative of fatty liver diseases and/or insulin resistance.
Gene expression analysis suggests a generalized down-regulation of the kynurenine pathway of tryptophan metabolism. Interestingly, the other metabolite identified as being reduced in Hnf1a-null mice, riboflavin, may also contribute to the reduction in xanthurenic acid and increase in indolelactate. Riboflavin, also known as vitamin B2, is a water-soluble vitamin and a component of the cofactors flavin adenine dinucleotide and flavin mononucleotide (reviewed in Ref. 32). Therefore, a deficiency in riboflavin decreases biochemical reactions requiring flavoproteins including the electron transport chain, fatty acid oxidation, vitamin B6 utilization, retinoic acid production, oxidized glutathione reduction, and metabolism of tryptophan to niacin (vitamin B3). Kynurenine 3-hydroxylase is a flavin-dependent monooxygenase, and thus, reduced xanthurenic acid might result from the combination of reduced Kmo gene expression and enzymatic activity. Kynurenine 3-hydroxylase hydroxylation of kynurenine is also one of the first steps in nicotinamide synthesis. An increase in indolelactate formation may be a compensatory mechanism to deal with the reduced capacity of the kynurenine pathway enzymes facing a lack of flavin adenine dinucleotide and nicotinamide adenine dinucleotide. The cause of riboflavin deficiency in Hnf1a-null mice is difficult to identify due to this model being a whole-body knockout. Because riboflavin is obtained from the diet, the simplest explanation would be a defect in riboflavin uptake in the small intestine. A specific riboflavin transporter has not been identified in the mouse, and only recently, the riboflavin transporters 1 and 2 have been cloned in human and rat (33,34). Because Hnf1α is known to regulate many intestinal and kidney transporters, Hnf1α may be a critical regulator of the as yet unidentified mouse riboflavin transporter.
Renal apical transport
Precedence for Hnf1α regulation of transporters is well established in the liver, kidney, and intestine. Thus, it is likely that the aminoaciduria observed in Hnf1a-null mice is due to defects in renal reabsorption. Hnf1a-null mice have a 3-fold higher concentration of the neutral amino acid proline in urine. The primary transporters responsible for proline reuptake are Slc6a19 (B0AT1), Slc6a15 (B0AT2), Slc6a20 (XT3), and Slc36a1. The significant decreases in Slc6a15 and Slc6a20 kidney expression suggest specific regulation of these genes by Hnf1α in the kidney leading to increased urinary proline. The N-methylated amino acid glycine betaine was also increased, although to a lesser degree than proline. Of the transporters studied, XT3 is known to transport N-methylated amino acids, thus correlating Slc6a20 expression to glycine betaine reabsorption in vivo. Additionally, the lysine degradation product pipecolic acid binds with an equal affinity as proline to XT3 (35). However, pipecolic acid levels in the urine were greatly reduced in the Hnf1a-null mouse. The decrease is likely reflective of alterations in lysine degradation because the metabolic pathways of several amino acids including phenylalanine and tryptophan are disrupted in Hnf1a-null mice.
Of great surprise was the relatively normal expression of collectrin RNA and protein. Hnf1α was previously shown in this mouse model to be necessary for collectrin expression in pancreatic islet cells (36). In contrast, we observed only a slight reduction in collectrin protein expression in the kidney. Collectrin-deficient mice have reduced renal apical transporters and suffer from severe aminoaciduria (37,38). Hnf1β is more highly expressed in the kidney than Hnf1α, and Hnf1b-null mice as well as mutations in the human gene cause severe kidney diseases (reviewed in Ref. 39). Thus, it is likely that Hnf1β regulates collectrin expression in the kidney and Hnf1α is responsible for regulation in the pancreas. The small decrease noted in collectrin protein may be due to Hnf1α regulation in specific cell types of the kidney.
Hypothalamic-pituitary-adrenal axis
The production of steroid hormones in the adrenal gland is primarily regulated by the hypothalamic-pituitary-adrenal axis. In humans, primary hypercortisolism, or Cushing’s syndrome, is defined as elevated cortisol of any etiology, whereas Cushing’s disease (secondary hypercortisolism) is specific to elevated cortisol due to high ACTH secretion from the pituitary (often a pituitary adenoma). The pituitary gland in Hnf1a-null mice was histologically similar to wild-type mice (data not shown), indicating a pituitary adenoma or similar abnormality is not the cause of elevated corticosterone and aldosterone. Additionally, plasma ACTH levels were normal or lower in Hnf1a-null mice compared with wild-type (Supplemental Fig. 2). The observed 4-fold increase in Pomc transcript in the pituitary of Hnf1a-null mice is contradictory to the plasma ACTH values. It is unknown why the increased Pomc transcript did not translate to increased ACTH. One possibility is that the prohormone cleavage enzyme, which cleaves the prohormone POMC into ACTH, has reduced activity in the Hnf1a-null mice or simply that the 4-fold RNA increase is not sufficient to elicit downstream biological changes in this mouse model. One possibility for the increased corticosterone and aldosterone production would be that the adrenal gland is compensating for excess steroid loss associated with the increased diuresis in Hnf1a-null mice. However, the serum to urine ratio of corticosterone and aldosterone is unaffected in the Hnf1a-null mice, indicating diuresis/kidney function is not the cause. Thus, Hnf1a-null mice present with ACTH-independent Cushing’s syndrome, and the adrenal gland is the site of the defect in the hypothalamic-pituitary-adrenal axis. Whether this adrenal defect results from increased sensitivity of the adrenal gland to ACTH or other signaling pathways within the adrenal gland remains to be determined.
Regardless of the mechanism of corticosterone and aldosterone elevation, these two steroid hormones have significant impact on the physiological state of the body. Aldosterone is an antidiuretic mineralocorticoid acting through the mineralocorticoid receptor (MR) to increase Na+ reabsorption into the blood, whereas K+ is pumped out to the urine. The glucocorticoid corticosterone has been shown to have similar effects on the Na+ and K+ flux by stimulating both GR and MR. Potassium levels were slightly reduced in the serum of Hnf1a-null mice, whereas Na+ was unchanged (Table 1). This suggests that the kidney is not responsive to the actions of aldosterone and corticosterone. Hnf1α is involved with the regulation and/or activation of many nuclear receptors and coactivators. Indeed, GR transcript was reduced 36% in Hnf1a-null hypothalamus compared with wild type. Hnf1α was previously shown to regulate GR expression in the liver (40). The authors argued that the 25% reduction in GR mRNA in the liver was sufficient to alter the expression of various GR-mediated genes. No studies have been conducted investigating the role of Hnf1α on MR activity. Thus, it is possible that functional Hnf1α is necessary for steroid hormone activation of GR and MR in the kidney. The growth retardation in the Hnf1a-null mice has been attributed to a GH-insensitivity syndrome linked to the reduced liver expression of IGF-I (3). However, given the adrenal gland malfunction identified in this study, it is clear that many developmental and growth pathways are altered in this mouse model.
The Hnf1a-null mouse serves as a model of the human diabetic disease MODY3. Although diabetic nephropathy occurs in nearly all cases of MODY5 (HNF1B mutation), the occurrence is less prevalent in MODY3. The aminoaciduria and hyperadrenal gland observed in the Hnf1a-null mouse may only reveal themselves clinically after a prolonged diabetic state in humans or represent species-specific regulatory roles for Hnf1α. One study of human urine samples linked generalized aminoaciduria with the co-occurrence of glucosuria in MODY3 as well as type 1 and type 2 diabetic patients (41). Although serum levels of phenylalanine and many other amino acids are not changed in MODY3 patients, the urinary concentrations of phenylalanine were increased 2.52-fold, the largest increase of the amino acids measured in MODY3 urine (42,43). Proline, tryptophan, and other metabolites assessed in our study were not measured in these human studies. Thus, defects in phenylalanine hydroxylase activity are likely species specific, whereas the effects of HNF1A mutations on renal apical transport in humans require further study.
Currently, the only way to identify which of the nine types of MODY a patient has is through DNA sequencing. There are differences in clinical response to treatment among the MODY disorders. The phenotypes of the knockout mice for the various MODY mutations are distinct from one another and are a valuable resource for investigating molecular and physiological consequences of loss of these genes. Comparison of their metabolomes may provide a unique biomarker, or set of markers, present in a rapid, noninvasive biofluid such as urine to assist in the diagnosis of human MODY disorders.
Materials and Methods
Reagents
All authentic standards were purchased from Sigma Chemical Co. (St. Louis, MO) with the exception of HDOPA and DHOPA, which were previously synthesized as described (29). All reagents were of the highest grade available.
Animals
The Hnf1a-null mouse line was described previously (3). Male wild-type (n =18) and knockout (Hnf1a-null, n =11) littermates at 8 wk of age were used for this study. All animals were maintained in a National Cancer Institute animal facility under standard 12-h light, 12-h dark cycle with access to food (NIH31 standard chow) and water ad libitum. All animal handling and procedures were approved by the National Cancer Institute Animal Care and Use Committee.
Sample collection
Urine samples were collected from individually housed mice in glass mini-metabowls (Jencons, Lutterworth, UK) over a 24-h period. The same food and water provided in colony housing cages was used in the metabowls to prevent fluctuations in dietary metabolites. Urine was stored at −80 C until analyzed. Immediately after removal from metabowls, mice were weighed, and serum was collected by retroorbital bleeding. After CO2 asphyxiation, tissues were harvested, weighed, and either fixed in formalin or frozen in liquid N2.
UPLC-ESI-QTOFMS analysis
Urine (1:5 dilution) was extracted with 50% acetonitrile containing 0.5 μm of the internal standard chlorpropamide and centrifuged at 18,000 × g for 15 min at 4 C to remove precipitated protein and other particulates. Samples (5 μl/injection) were subjected to reverse-phase chromatography on a 50- × 2.1-mm ACQUITY 1.7 μm BEH C18 column (Waters Corp., Milford, MA) using an ACQUITY UPLC system (Waters Corp.) with a gradient mobile phase comprising 0.1% formic acid and acetonitrile containing 0.1% formic acid. A 0.5-ml/min flow rate was maintained in a 10-min run. The eluent was introduced directly into a Waters Q-TOF Premier mass spectrometer by electrospray ionization operating in positive ionization mode (ESI+). For mass spectrometry scanning, the data were acquired in the centroid mode from 100–850 m/z. To confirm the identity of markers, authentic standards were compared with urine samples for retention time and tandem mass spectrometry fragmentation pattern when collision energies ranging from 15–35 V were applied.
Data processing and multivariate analysis
Centroided and integrated chromatographic mass data from 50–850 m/z were processed by MarkerLynx (Waters) to generate a multivariate data matrix. Pareto-scaled MarkerLynx matrices including information on sample identity were analyzed by PCA and OPLS using SIMCA-P +12 (Umetrics, Kinnelon, NJ). The OPLS loadings scatter S-plot was used to determine those ions that contributed significantly to the separation between wild-type and Hnf1a-null mice. The identity of ions with a correlation of 0.8 or higher to the model was further investigated.
Quantification of urinary metabolites
Metabolite concentrations were determined using an ACQUITY UPLC system coupled to a Xevo TripleQuad mass spectrometer (Waters). Chromatography was as described for UPLC-ESI-QTOFMS analysis. Serial dilution calibration curves (25–0.2 μm) were generated for each authenticated marker. Ten urine samples each from wild-type and Hnf1a-null mice were diluted (20- to 500-fold) in 50% acetonitrile containing the internal standard chlorpropamide (0.5 μm). The mass spectrometer was operated in MRM mode, and optimal transition energies for each metabolite were monitored (see Supplemental Table 1). Each metabolite concentration is expressed as micromoles per millimole creatinine.
Serum chemistry analysis and steroid measurement
Serum chemistry analysis was performed using the Abaxis VetScan VS2 chemistry analyzer with the Comprehensive Diagnostic Profile reagent rotor (Abaxis Inc., Union City, CA). Serum and urinary corticosterone and aldosterone and serum progesterone levels were measured using enzyme immunoassay kits from Cayman (Ann Arbor, MI). Serum DHEA was measured using the Active enzyme immunoassay from Diagnostic Systems Laboratories, Inc. (Webster, TX). Serum DHEA-S was measured using an ELISA kit from Calbiotech (Spring Valley, CA).
Gene expression analysis
RNA was prepared from frozen liver, kidney, and adrenal gland using the QIAGEN (Valencia, CA) RNeasy mini kit. cDNA was synthesized from 1 μg total RNA using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). For QPCR analysis, primers crossed exon-exon junctions, and NCBI-BLAST searches confirmed sequence specificity. SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) was used for analysis on an Applied Biosystems Prism 7900HT system. Relative mRNA levels were calculated by the comparative threshold cycle method using GAPDH as the internal control.
Western blot analysis
Kidney membrane extracts were obtained by homogenizing 50 mg frozen kidney in 1 ml lysis buffer [5 mm Tris (pH 8.0), 2 mm EDTA, and protease inhibitors]. The homogenate was centrifuged twice at 500 × g for 15 min at 4 C. The supernatant was retained each time and then centrifuged at 50,000 × g for 15 min at 4 C. The pellet was resuspended in 75 mm Tris (pH 8.0), 12.5 mm MgCl2, 5 mm EDTA, and protease inhibitors. Protein concentration was determined using the bicinchoninic acid assay (Thermo Scientific, Waltham, MA). Twenty micrograms of extract was separated on a 4–15% Tris-HCl gel and transferred to polyvinylidene difluoride membrane. Membranes were incubated with antibodies against Slc6a20 (Santa Cruz Biotechnology Inc., Santa Cruz, CA), collectrin (Alexis Biochemicals, San Diego, CA), calnexin (Santa Cruz Biotechnology), and Actin (Abcam, Cambridge, MA).
Histological analysis
Adrenal glands were fixed in phosphate-buffered formalin for 18 h and then subjected to alcohol dehydration and xylene for paraffin embedding. Four-micrometer serial sections were made through the entire tissue and stained with hematoxylin and eosin for visualization. For Oil red O analysis of lipids, fresh adrenal gland tissue was frozen in O.C.T. compound (Tissue-Tek, Torrance, CA). Frozen tissue sections were then stained with fresh oil red O and counterstained with hematoxylin.
Statistical analysis
Statistical analysis of differences was performed using GraphPad Prism software (San Diego, CA). Unpaired Student’s t test was used to compare levels in wild-type with Hnf1a-null mice. P values <0.05 were considered significant.
Supplementary Material
Footnotes
This work was supported by the National Cancer Institute Intramural Research Program. J.A.B. was supported by the Pharmacology Research Associate Program administered through the National Institute of General Medical Sciences.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 13, 2010
Abbreviations: B0AT, Neutral amino acid transporter; DHEA, dehydroepiandrosterone; DHEAS, DHEA sulfate; DHOPA, 11beta,20-dihydroxy-3-oxo-pregn-4-en-21-oic acid; GR, glucocorticoid receptor; 3-HK, 3-hydroxykynurenine; Hnf1α, hepatocyte nuclear factor 1α; HDOPA, 11beta-hydroxy-3,20-dioxopregn-4-en-21-oic acid; Kat, kynurenine aminotransferase; MODY3, maturity-onset diabetes of the young type 3; MR, mineralocorticoid receptor; m/z, mass to charge ratio; Oat, organic anion transporter; OPLS, orthogonal projection to latent structures; PCA, principal components analysis; PKU, phenylketonuria; POMC, proopiomelanocortin; QPCR, quantitative PCR; SLC, solute carrier; UPLC-ESI-QTOFMS, ultraperformance liquid chromatography coupled to electrospray ionization quadrupole time-of-flight mass spectrometer.
References
- Yamagata K, Oda N, Kaisaki PJ, Menzel S, Furuta H, Vaxillaire M, Southam L, Cox RD, Lathrop GM, Boriraj VV, Chen X, Cox NJ, Oda Y, Yano H, Le Beau MM, Yamada S, Nishigori H, Takeda J, Fajans SS, Hattersley AT, Iwasaki N, Hansen T, Pedersen O, Polonsky KS, Bell GI, et al. 1996 Mutations in the hepatocyte nuclear factor-1α gene in maturity-onset diabetes of the young (MODY3). Nature 384:455–458 [DOI] [PubMed] [Google Scholar]
- Pontoglio M, Sreenan S, Roe M, Pugh W, Ostrega D, Doyen A, Pick AJ, Baldwin A, Velho G, Froguel P, Levisetti M, Bonner-Weir S, Bell GI, Yaniv M, Polonsky KS 1998 Defective insulin secretion in hepatocyte nuclear factor 1α-deficient mice. J Clin Invest 101:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Sauer B, Gonzalez FJ 1998 Laron dwarfism and non-insulin-dependent diabetes mellitus in the Hnf-1α knockout mouse. Mol Cell Biol 18:3059–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DQ, Screenan S, Munoz KN, Philipson L, Pontoglio M, Yaniv M, Polonsky KS, Stoffel M 2001 Loss of HNF-1α function in mice leads to abnormal expression of genes involved in pancreatic islet development and metabolism. Diabetes 50:2472–2480 [DOI] [PubMed] [Google Scholar]
- Dukes ID, Sreenan S, Roe MW, Levisetti M, Zhou YP, Ostrega D, Bell GI, Pontoglio M, Yaniv M, Philipson L, Polonsky KS 1998 Defective pancreatic β-cell glycolytic signaling in hepatocyte nuclear factor-1α-deficient mice. J Biol Chem 273:24457–24464 [DOI] [PubMed] [Google Scholar]
- Wang H, Antinozzi PA, Hagenfeldt KA, Maechler P, Wollheim CB 2000 Molecular targets of a human HNF1α mutation responsible for pancreatic β-cell dysfunction. EMBO J 19:4257–4264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih DQ, Bussen M, Sehayek E, Ananthanarayanan M, Shneider BL, Suchy FJ, Shefer S, Bollileni JS, Gonzalez FJ, Breslow JL, Stoffel M 2001 Hepatocyte nuclear factor-1α is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet 27:375–382 [DOI] [PubMed] [Google Scholar]
- Faust DM, Catherin AM, Barbaux S, Belkadi L, Imaizumi-Scherrer T, Weiss MC 1996 The activity of the highly inducible mouse phenylalanine hydroxylase gene promoter is dependent upon a tissue-specific, hormone-inducible enhancer. Mol Cell Biol 16:3125–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei XD, Kaufman S 1998 Identification of hepatic nuclear factor 1 binding sites in the 5` flanking region of the human phenylalanine hydroxylase gene: implication of a dual function of phenylalanine hydroxylase stimulator in the phenylalanine hydroxylation system. Proc Natl Acad Sci USA 95:1500–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontoglio M, Barra J, Hadchouel M, Doyen A, Kress C, Bach JP, Babinet C, Yaniv M 1996 Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell 84:575–585 [DOI] [PubMed] [Google Scholar]
- Kamiya A, Inoue Y, Kodama T, Gonzalez FJ 2004 Hepatocyte nuclear factors 1α and 4α control expression of proline oxidase in adult liver. FEBS Lett 578:63–68 [DOI] [PubMed] [Google Scholar]
- Maher JM, Slitt AL, Callaghan TN, Cheng X, Cheung C, Gonzalez FJ, Klaassen CD 2006 Alterations in transporter expression in liver, kidney, and duodenum after targeted disruption of the transcription factor HNF1α. Biochem Pharmacol 72:512–522 [DOI] [PubMed] [Google Scholar]
- Saji T, Kikuchi R, Kusuhara H, Kim I, Gonzalez FJ, Sugiyama Y 2008 Transcriptional regulation of human and mouse organic anion transporter 1 by hepatocyte nuclear factor 1α/β. J Pharmacol Exp Ther 324:784–790 [DOI] [PubMed] [Google Scholar]
- Kikuchi R, Kusuhara H, Hattori N, Shiota K, Kim I, Gonzalez FJ, Sugiyama Y 2006 Regulation of the expression of human organic anion transporter 3 by hepatocyte nuclear factor 1α/β and DNA methylation. Mol Pharmacol 70:887–896 [DOI] [PubMed] [Google Scholar]
- Michels AJ, Hagen TM 2009 Hepatocyte nuclear factor 1 is essential for transcription of sodium-dependent vitamin C transporter protein 1. Am J Physiol Cell Physiol 297:C1220–C1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Lewis IA, Hegeman AD, Anderson ME, Li J, Schulte CF, Westler WM, Eghbalnia HR, Sussman MR, Markley JL 2008 Metabolite identification via the Madison Metabolomics Consortium Database. Nat Biotechnol 26:162–164 [DOI] [PubMed] [Google Scholar]
- Xia J, Psychogios N, Young N, Wishart DS 2009 MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res 37:W652–W660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellum E, Horn L, Thoresen O, Kvittingen EA, Stokke O 1986 Urinary excretion of N-acetyl amino acids in patients with some inborn errors of amino acid metabolism. Scand J Clin Lab Invest Suppl 184:21–26 [PubMed] [Google Scholar]
- Michals K, Matalon R 1985 Phenylalanine metabolites, attention span and hyperactivity. Am J Clin Nutr 42:361–365 [DOI] [PubMed] [Google Scholar]
- Woolf LI 1951 Excretion of conjugated phenylacetic acid in phenylketonuria. Biochem J 49:ix–x [PubMed] [Google Scholar]
- McDonald JD, Bode VC, Dove WF, Shedlovsky A 1990 Pahhph-5: a mouse mutant deficient in phenylalanine hydroxylase. Proc Natl Acad Sci USA 87:1965–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidell N, Hao L, Pasquali M, McDonald JD 2009 Carcinogenic effects in a phenylketonuria mouse model. PLoS One 4:e4292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls AW, Nicholson JK, Haselden JN, Waterfield CJ 2000 A metabonomic approach to the investigation of drug-induced phospholipidosis: an NMR spectroscopy and pattern recognition study. Biomarkers 5:410–423 [DOI] [PubMed] [Google Scholar]
- Delaney J, Neville WA, Swain A, Miles A, Leonard MS, Waterfield CJ 2004 Phenylacetylglycine, a putative biomarker of phospholipidosis: its origins and relevance to phospholipid accumulation using amiodarone treated rats as a model. Biomarkers 9:271–290 [DOI] [PubMed] [Google Scholar]
- Akiyama TE, Ward JM, Gonzalez FJ 2000 Regulation of the liver fatty acid-binding protein gene by hepatocyte nuclear factor 1α (HNF1α). J Biol Chem 275:27117–27122 [DOI] [PubMed] [Google Scholar]
- Badawy AA, Evans M 1974 Alcohol and tryptophan metabolism: a review. Alcohol Alcohol 9:97–116 [Google Scholar]
- Han Q, Robinson H, Cai T, Tagle DA, Li J 2009 Biochemical and structural properties of mouse kynurenine aminotransferase III. Mol Cell Biol 29:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn A, Ljubojevic M, Lorenz H, Schultz C, Ghebremedhin E, Ugele B, Sabolic I, Burckhardt G, Hagos Y 2005 Murine renal organic anion transporters mOAT1 and mOAT3 facilitate the transport of neuroactive tryptophan metabolites. Am J Physiol Cell Physiol 289:C1075–C1084 [DOI] [PubMed] [Google Scholar]
- Zhen Y, Krausz KW, Chen C, Idle JR, Gonzalez FJ 2007 Metabolomic and genetic analysis of biomarkers for peroxisome proliferator-activated receptor α expression and activation. Mol Endocrinol 21:2136–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ 1995 Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol 15:3012–3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LO, Hu YF, Wang L, Mitchell M, Berger A, Coleman RA 2010 Early hepatic insulin resistance in mice: a metabolomics analysis. Mol Endocrinol 24:657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depeint F, Bruce WR, Shangari N, Mehta R, O'Brien PJ 2006 Mitochondrial function and toxicity: Role of the B vitamin family on mitochondrial energy metabolism. Chem Biol Interact 163:94–112 [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Inoue K, Ohta KY, Fukatsu R, Maeda JY, Yoshida Y, Yuasa H 2009 Identification and Functional Characterization of Rat Riboflavin Transporter 2. J Biochem 145:437–443 [DOI] [PubMed] [Google Scholar]
- Yonezawa A, Masuda S, Katsura T, Inui K-i 2008 Identification and functional characterization of a novel human and rat riboflavin transporter, RFT1. Am J Physiol Cell Physiol 295:C632–C641 [DOI] [PubMed] [Google Scholar]
- Stevens BR, Wright EM 1985 Substrate specificity of the intestinal brush-border proline/sodium (IMINO) transporter. J Membr Biol 87:27–34 [DOI] [PubMed] [Google Scholar]
- Fukui K, Yang Q, Cao Y, Takahashi N, Hatakeyama H, Wang H, Wada J, Zhang Y, Marselli L, Nammo T, Yoneda K, Onishi M, Higashiyama S, Matsuzawa Y, Gonzalez FJ, Weir GC, Kasai H, Shimomura I, Miyagawa J, Wollheim CB, Yamagata K 2005 The HNF-1 target collectrin controls insulin exocytosis by SNARE complex formation. Cell Metab 2:373–384 [DOI] [PubMed] [Google Scholar]
- Danilczyk U, Sarao R, Remy C, Benabbas C, Stange G, Richter A, Arya S, Pospisilik JA, Singer D, Camargo SM, Makrides V, Ramadan T, Verrey F, Wagner CA, Penninger JM 2006 Essential role for collectrin in renal amino acid transport. Nature 444:1088–1091 [DOI] [PubMed] [Google Scholar]
- Malakauskas SM, Quan H, Fields TA, McCall SJ, Yu MJ, Kourany WM, Frey CW, Le TH 2007 Aminoaciduria and altered renal expression of luminal amino acid transporters in mice lacking novel gene collectrin. Am J Physiol Renal Physiol 292:F533–F544 [DOI] [PubMed] [Google Scholar]
- Igarashi P, Shao X, McNally BT, Hiesberger T 2005 Roles of HNF-1β in kidney development and congenital cystic diseases. Kidney Int 68:1944–1947 [DOI] [PubMed] [Google Scholar]
- Lin WY, Hu YJ, Lee YH 2008 Hepatocyte nuclear factor-1α regulates glucocorticoid receptor expression to control postnatal body growth. Am J Physiol Gastrointest Liver Physiol 295:G542–F551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham C, Ellard S, Nicholls AJ, Pennock CA, Allen J, James AJ, Satchell SC, Salzmann MB, Hattersley AT 2001 The generalized aminoaciduria seen in patients with hepatocyte nuclear factor-1α mutations is a feature of all patients with diabetes and is associated with glucosuria. Diabetes 50:2047–2052 [DOI] [PubMed] [Google Scholar]
- Bingham C, Bulman MP, Ellard S, Allen LI, Lipkin GW, Hoff WG, Woolf AS, Rizzoni G, Novelli G, Nicholls AJ, Hattersley AT 2001 Mutations in the hepatocyte nuclear factor-1β gene are associated with familial hypoplastic glomerulocystic kidney disease. Am J Hum Genet 68:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stride A, Pearson ER, Brown A, Gooding K, Castleden HA, Hattersley AT 2004 Serum amino acids in patients with mutations in the hepatocyte nuclear factor-1α gene. Diabet Med 21:928–930 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.