Abstract
Fasting-induced suppression of thyroid hormone levels is an adaptive response to reduce energy expenditure in both humans and mice. This suppression is mediated by the hypothalamic-pituitary-thyroid axis through a reduction in TRH levels expressed in neurons of the paraventricular nucleus of the hypothalamus (PVN). TRH gene expression is positively regulated by leptin. Whereas decreased leptin levels during fasting lead to a reduction in TRH gene expression, the mechanisms underlying this process are still unclear. Indeed, evidence exists that TRH neurons in the PVN are targeted by leptin indirectly via the arcuate nucleus, whereas correlative evidence for a direct action exists as well. Here we provide both in vivo and in vitro evidence that the activity of hypothalamic-pituitary-thyroid axis is regulated by both direct and indirect leptin regulation. We show that both leptin and α-MSH induce significant neuronal activity mediated through a postsynaptic mechanism in TRH-expressing neurons of PVN. Furthermore, we provide in vivo evidence indicating the contribution of each pathway in maintaining serum levels of thyroid hormone.
Leptin, independent of α-MSH, directly acts on thyrotropin-releasing hormone-expressing neurons in paraventricular nucleus and prevents the fasting induced suppression of the hypothalamic-pituitary-thyroid axis.
As a major regulator of serum thyroid hormone (TH) levels, the hypophysiotropic TRH neurons of the paraventricular nucleus (PVN) play an important role in the control of energy homeostasis (1). These neurons have a primary role in the regulation of TH production through release of TRH at the median eminence (ME) to stimulate pituitary TSH release. TSH then stimulates the thyroid gland to produce THs T4 and T3. TH, in turn, regulates the activity of TRH neurons in the hypothalamus by a classical negative feedback pathway (2,3,4). TRH neurons also receive neuronal projections from arcuate proopiomelanocortin (POMC) and neuropeptide Y (NPY) neurons (5,6,7,8,9), from brainstem catecholaminergic neurons (4,10,11), and from neurochemically uncharacterized neurons in the dorsomedial nucleus of hypothalamus (12).
During starvation, significant changes occur in adrenal, gonadal, and thyroidal axes (13). Under this condition, the hypothalamic-pituitary-thyroid (HPT) axis is profoundly depressed, resulting in decreases in basal metabolic rate and energy expenditure, presumably serving as a survival mechanism. This suppression is mediated by TRH neurons in the PVN, in which TRH gene expression is positively regulated by leptin. Thus, the leptin-deficient ob/ob mouse, although profoundly obese, mimics the metabolic profile of starvation (14).
The adipostatic hormone leptin was demonstrated as the primary signaling molecule that communicates information about decreasing long-term energy stores to the HPT axis (15). In this study, a 48-h fast in mice was found to significantly reduce total serum T4 levels. Replacement of serum leptin levels in the fasted animals partially restored serum T4 levels to normal levels. It also significantly restored hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes to normal levels of functioning. More recent experiments have been able to restore serum T4 levels toward normal with more physiological leptin replacement regimens (16). In two separate studies of human subjects with weight loss from conditions such as hypothalamic amenorrhea, recombinant leptin therapy was demonstrated to elevate levels of free T3 and T4 to pre-weight-loss levels (17,18).
Most reports have argued that leptin primarily regulates hypophysiotropic TRH neurons indirectly, through leptin action on the POMC and NPY/agouti-related protein (AgRP) expressing neurons of the arcuate nucleus of the hypothalamus (ARC). These neurons project to TRH neurons of the PVN (9,19) and release α-MSH and AgRP to control the level of activity of the melanocortin-4 receptor (MC4-R) and, in turn, expression and release of TRH. There are significant data supporting this hypothesis. First, leptin receptor is expressed on both POMC and NPY/AgRP neurons in the ARC, and leptin administration activates c-fos and phospho-signal transducer and activator of transcription (pSTAT) more densely in the ARC than the PVN (7,20,21,22). Ablation of the ARC by monosodium glutamate treatment removes the effects of fasting on the HPT axis (23). Central administration of α-MSH activates TRH neurons in multiple experimental systems, whereas AgRP and NPY both inhibit TRH neurons (6,9,19,24,25). Indeed, the effect of starvation on the HPT axis can be replicated by central administration of AgRP (19).
However, a series of observations also suggest that central melanocortin signaling, involving the ARC projections to TRH neurons, is not essential for normal regulation of the HPT axis. Mice and humans with deficient MC4-R signaling do not exhibit hypothyroidism (26,27). Indeed, the MC4-R knockout mouse does not exhibit the hypothyroidism, hypercorticosteronism, or hypogonadism seen in the leptin-deficient mouse (19). Although the response of the MC4-R knockout mouse has not been reported, NPY knockout mice exhibit normal declines of T4 levels after a fast (28). Conflicting observations regarding the role of melanocortin signaling in thyroid axis function have also been reported. POMC knockout mice have been reported to exhibit both hypo- and hyperthyroidism (29,30). Additionally, although young AgRP−/− mice appear euthyroid, at 10 months of age, they exhibit hyperthyroidism, with an approximate 20% increase in total basal T3 and T4 (31).
Importantly, data also exist to support a direct action of leptin on TRH neurons of the PVN (7,22). Leptin receptor mRNA is expressed in TRH PVN neurons, and leptin potently up-regulates TRH biosynthesis in fetal hypothalamic cultures, even in the presence of the melanocortin antagonist SHU9119 (32). Additionally, STAT3, a direct target of leptin and other cytokines, is recruited to the TRH promoter with leptin administration in vivo, demonstrated by chromatin immunoprecipitation (ChIP) analysis of hypothalamic chromatin (33).
The experiments described above, however, are correlative and do not directly demonstrate functional leptin receptors on TRH PVN neurons. To directly characterize the ability of TRH PVN neurons to respond to leptin and other hypothalamic neuropeptides, we have used the transgenic TRH-Cre × Rosa-26-GFP mouse to create a slice preparation allowing direct electrophysiological recordings from TRH-expressing neurons and then investigated the mechanism of action of α-MSH, leptin, and NPY on these cells. Furthermore, by monitoring serum levels of total T4 in groups of mice subjected to fasting, we demonstrate the relative contributions of direct and indirect leptin action on the regulation of serum TH levels.
Results
To directly study properties of TRH-expressing neurons in the PVN, we used transgenic mice that express Cre-recombinase driven by the TRH promoter, constructed by inserting a bacterial artificial chromosome engineered to contain the Cre-recombinase sequence into TRH exon II (34). We then crossed the founder TRH-Cre mice to reporter Rosa-26-GFP mice (The Jackson Laboratory, Bar Harbor, ME) to generate offspring that produce green fluorescent protein (GFP) in TRH-expressing neurons. As reported previously, the TRH-Cre-expressing transgene was shown to be transcriptionally regulated in PVN neurons by TH levels in parallel with endogenous TRH mRNA (34). Additionally, GFP-expressing neurons in the PVN in these animals also expressed TRH mRNA. However, not all GFP-immunoreactive neurons were positive for TRH mRNA in the PVN (34). Previous reports had not quantified the colocalization of TRH-GFP immunoreactivity and TRH mRNA in these animals. Therefore, using dual-label immunohistochemistry (IHC)/in situ hybridization (ISH), we quantified the percentage of GFP-immunoreactive neurons that also contained TRH mRNA across the regions of the PVN containing hypophysiotropic TRH neurons (n = 3 mice; Table 1). We observed that 46.9–60.2% of GFP-positive neurons along the PVN express detectable TRH mRNA. Representative images of each region of the PVH are presented in Supplemental Fig. 1 (published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). We thus used TRH-Cre × Rosa-26-GFP mice as a means to directly visualize TRH-expressing neurons for the neuroanatomical and electrophysiological analyses described here.
Table 1.
GFP-immunoreactive neuron colocalization with TRH mRNA in PVN
| PVN | Anterior | Mid | Posterior |
|---|---|---|---|
| Bregma range | −0.55 to −0.65 | −0.75 to −0.95 | −1.05 to −1.25 |
| GFP neurons coexpressing TRH (%) | 51.9 ± 6.2 | 60.2 ± 3.8 | 46.9 ± 2.7 |
Percentage of GFP-immunoreactive neurons that colocalize with TRH mRNA across the PVN obtained from TRH-Cre × Rosa-GFP mice (n = 3 mice) via dual-label IHC/ISH. Regions of the PVN span the hypophysiotropic TRH neurons. Sections were quantified by counting the number of GFP neurons also positive for TRH mRNA. Per mouse, the average percentage for each region (anterior, mid, and posterior) was attained from one to four sections. Data are presented as sample means (± sem) for each region of the PVN. Images representative of each region of the PVN are presented in Supplemental Fig. 1.
Neuroanatomical characterization of TRH-GFP neurons
The neuronal population in the PVN is heterogeneous and includes neurosecretory cells projecting to the ME and magnocellular neurosecretory cells that project to the posterior pituitary (PP). Additionally, the dorsal parvocellular subdivision of the PVN sends projections to brainstem centers (35), and this region also contains many TRH neurons (36). By using neuroanatomical tools, we first sought to characterize the relative distribution of these TRH cell types in the TRH-Cre × Rosa-26-GFP transgenic mice.
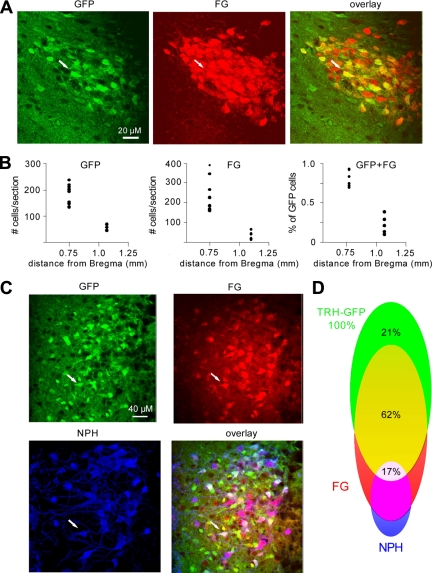
To identify TRH-GFP neurons of PVN projecting to ME, a circumventricular region outside the blood-brain area, we examined their ability to take up the retrograde dye, fluorogold (FG) from systemic circulation (37). Because FG does not cross transsynaptically to neighboring neurons, labeling by this dye identifies neurons that contain axonal projections to the brain areas where the blood-brain barrier is permeable. We tested this by injecting FG ip into TRH-Cre × Rosa-26-GFP transgenic mice, allowing 2–3 d for neuronal uptake before perfusing them for double-staining fluorescence IHC using antibodies against FG and GFP (38,39). We quantitated the distribution of cells labeled with GFP, FG, or both in anterior (binned between −0.55 and −0.9 mm from Bregma) and posterior (binned between −0.9 and −1.25 mm from Bregma) PVN (Fig. 1, A and B). Our data indicate that the number of GFP cells counted per section was significantly higher in anterior PVN (mean ± sem, 188.5 ± 13.1) than in posterior PVN (58.4 ± 5.2). The number of cells labeled with FG immunoreactivity per section was also significantly higher in anterior PVN (249.1 ± 33.9) and decreased in the anterior-posterior direction, reaching zero in the most posterior sections of the PVN (24.0 ± 3.6). Furthermore, the percentage of GFP neurons colabeling with FG in anterior PVN were 79 ± 4% and declined in posterior parts of the PVN to 21 ± 6% (n = 3 mice; Fig. 1, A and B). These data confirm that a significant fraction of TRH-GFP neuroendocrine neurons, projecting outside the blood-brain barrier, are located in anterior PVN.
Figure 1.
Single-plane confocal images from 35-μm-thick brain sections indicate that a large fraction of TRH-GFP neurons in the PVN are labeled by ip injections of FG and not immunoreactive to neurophysin (NPH). Sections were cut frozen by a sledge microtome and stained free floating. A, Panels show the same portion of the PVN indicating immunoreactivity to GFP, FG, and overlay; B, frequency and distribution of staining for TRH-GFP, FG, and fraction of GFP-expressing neurons that labeled with FG in mouse PVN along the anterior-posterior axis; C, panels show the same portion of the PVN indicating immunoreactivity to GFP, FG, NPH, and overlay; D, fractions of TRH-GFP neurons that do not co-stain with FG (green), co-stain with FG (yellow), and co-stain with FG and NPH (white) and the fraction of NPH-, FG-positive neurons that do not express TRH-GFP (magenta) in mouse PVN.
Magnocellular neurosecretory neurons of the PVN send axonal projections to PP and are exposed to elements circulating in the blood. We therefore identified this neuronal population with triple-staining fluorescence IHC, using antibody against the magnocellular marker neurophysin, a precursor of oxytocin and vasopressin, as well as GFP and FG in TRH-Cre × Rosa-26-GFP mice that had been injected with FG, as described above. We hypothesized that TRH-GFP neurons that have taken up FG and do not express neurophysin are putative TRH-releasing parvocellular neurosecretory neurons projecting to ME. The TRH-GFP neurons that labeled with FG and antineurophysin are putative magnocellular neurosecretory neurons that send projections to PP. Our results (Fig. 1, C and D) indicate that in anterior PVN, where we observed a significant number of neurons labeling with FG, approximately 17% of TRH-GFP neurons that have taken up FG were also immunoreactive with neurophysin antibody, suggesting that they are putative magnocellular neurosecretory neurons presumably projecting to PP. Approximately 62% of TRH-GFP neurons labeled with FG but did not express neurophysin immunoreactivity, suggesting that these are putative TRH-releasing parvocellular neurons presumably projecting to ME. We also note that approximately 21% of GFP-positive neurons did not label for neurophysin or FG, a population that could possibly include the putative preautonomic TRH-positive cells of the PVN thought to project to brainstem (40).
Electrophysiological characterization of TRH-GFP neurons of PVN
Previous studies in the rat have associated certain electrogenic membrane properties of PVN neurons with their postrecording morphological findings and neurophysin immunoreactivity by labeling neurons with biocytin, delivered via the electrode during electrophysiological recordings. They have revealed that certain electrogenic membrane properties, including low threshold potentials (LTS), delayed onset to action potential firing, action potential width, adaptation of spike frequency, and phasic bursting, were differentially associated with certain subtypes of PVN neurons (39,41,42). The electrogenic membrane properties were then used to categorize PVN neurons into type I, II, and III subtypes. Type I and II cells are magnocellular and parvocellular neurosecretory neurons retrospectively, whereas type III cells are nonsecretory cells thought to project to the brainstem.
These electrogenic criteria have not been verified in mouse PVN, nor have they been investigated within specific neuronal populations of the PVN, such as the TRH-expressing cells. We used the statistical population data from the neuroanatomical investigations described above to correlate the distribution of electrogenic properties to that of the neuroanatomical properties of TRH-GFP neurons in PVN. Then, using electrogenic criteria to categorize TRH-GFP neurons of the PVN, we subsequently probed for a subtype-specific neuronal response to exogenously applied neuropeptides.
First, we identified the electrogenic membrane properties of randomly chosen TRH-GFP neurons during each recording before examining responses to neuropeptides and used these properties to predict neuronal subtype in PVN (Supplemental Fig. 2 and Table 1). Based on expression of bursting LTS, our results indicate that around 8% of TRH-GFP neurons are type III, putative preautonomic neurons that project to the brainstem, and the recordings obtained from this set of neurons were excluded from further analysis in this study. From 92% of neurons that did not display bursting LTS, based on the expression of delayed onset to generation of action potential firing, 35% were identified as type I, and 65% were identified as type II (Supplemental Table 1). The percentage of TRH-GFP neurons classified as type I and II based on electrogenic properties is consistent with the subdivision of neurophysin-positive (∼21%) or -negative (∼62%) FG-labeled TRH-GFP neurons in PVN and thus further supports that type I TRH-GFP cells represent putative PP projecting magnocellular neurosecretory neurons and that type II TRH-GFP cells represent putative ME projecting parvocellular neurosecretory TRH-expressing neurons.
We next examined the effects of neuropeptides on both type I and II TRH-GFP neurons of PVN. Our analysis showed no significant difference in direction or magnitude of neuronal responses to α-MSH, leptin, or NPY between type I and II neurons (Supplemental Table 2). Thus, recordings from both types of neurons were compiled to form the data sets presented below.
α-MSH increases firing activity of TRH-GFP neurons of the PVN
Because previous reports have shown that activation of MC4-R signaling can affect the firing activity of hypothalamic neurons (43,44), we hypothesized that α-MSH would affect firing activity of TRH-expressing neurons in the PVN. To test this, we examined changes in action potential firing frequency and membrane potentials of these neurons induced by bath application of this peptide.
By injecting −20 to 0 pA constant DC current, under current clamp mode, the cells were held between −55 and −50 mV to allow spontaneous firing of action potentials and changes in membrane potential. Under these circumstances, the activity of neurons was monitored for 15–25 min in the control solution to reach a stationary state of activity before bath applying 250 nm α-MSH, submaximal reported concentrations (45), lasting for 7–11 min, and then switching back to control solution. Upon termination of recording, we measured and averaged frequency of firing activity and membrane potentials 4–8 min in control and the same period in the presence of the peptide immediately before washing out the peptide (Fig. 2, A and B).
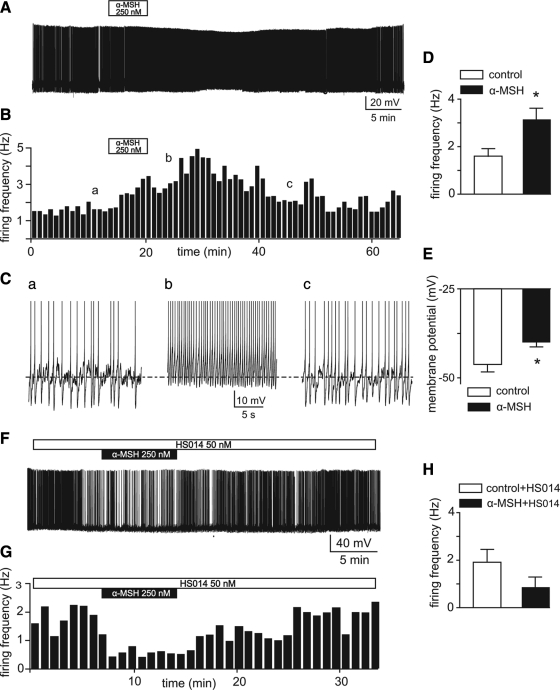
Figure 2.
α-MSH activates firing activity of TRH-GFP neurons in the PVN through MC4-R signaling. A, Voltage trace of a whole-cell recording from a TRH-GFP neuron firing spontaneous action potentials. Note that bath application of 250 nm α-MSH generates reversible increases in frequency of firing associated with a depolarization. B, Frequency histogram of this recording. C, Traces obtained from different time points of the same recording (indicated by a–c on the histogram) expanded in time scale to clearly demonstrate changes in firing rate and membrane potential in association with applications of the peptide. D and E, Means ± sem of action potential firing frequency (D) and membrane potentials (E) in control and in 250–350 nm α-MSH. *, P < 0.0001. F, Voltage trace of whole-cell recording indicates that bath application of 250 nm α-MSH fails to increase firing activity in neurons pretreated with 50 nm HS014. G, Frequency histogram of this effect. H, Bar graphs indicate the means ± sem of effects of 250 nm α-MSH on five neurons pretreated with 50 nm HS014 (n = 5; P > 0.05 by paired t test).
Our data, obtained under these circumstances, indicate that in 32 of 35 PVN neurons tested, bath application of 250 nm α-MSH activated action potential firing of these neurons. However, upon application of α-MSH to 35 cells, one cell did not display any measureable response, and one cell displayed inhibition of firing activity associated with hyperpolarization. Nevertheless, for the purpose of analysis, we included all the neurons recorded and compared the frequency of action potential firing and changes in membrane potential in control conditions with application of α-MSH. Our results indicate that α-MSH potently increased (to more than 90%) firing activity of these neurons from 1.6 ± 0.3 Hz in control to 3.2 ± 0.5 Hz (P < 0.0001, by paired t test; Fig. 2, A–D). Furthermore, this increase in firing induced by α-MSH was associated with depolarization of membrane potentials in these neurons. This peptide induced around 6 mV depolarization of membrane potential of the cells tested from −46.2 ± 2.1 mV in control to −40 ± 1.3 mV (P < 0.0001, paired t test; Fig. 2, C and E). The responses of these neurons to α-MSH reached the peak, defined as the highest measured values of firing frequency, in 15 ± 3 min after commencement of drug application.
Monitored up to 40 min during washout, measured by these parameters of cellular activity, about 85% of the effect of α-MSH was washed out in 27 ± 2 min (n =14; Fig. 2, B and C, panel c). Parameters at this point were not significantly different from the control state (P > 0.05; n =14). These results indicate that α-MSH predominantly, potently, and reversibly augments firing activity of the TRH-GFP neurons in the PVN.
Because α-MSH is also a ligand for the MC3-R, expressed in the hypothalamus, we next decided to test whether the observed excitatory effect of α-MSH on TRH-GFP neurons is in fact mediated through MC4-R signaling. We tested this hypothesis by examining the effects of α-MSH on firing activity of these neurons, as described above, in hypothalamic slices that were pretreated with 50 nm HS014 (46), a specific antagonist of MC4-R (Fig. 2, F and G). Our results obtained from recordings performed under these circumstances indicate that bath applications of 250 nm α-MSH failed to increase neuronal activity in the presence of HS014 in five neurons tested (n = 5; P > 0.5, paired t test, from 1.9 ± 0.5 Hz in control to 0.8 ±0.4 Hz in α-MSH; Fig. 2, F–H). The blockade of the excitatory effects of α-MSH by HS014 suggests that this peptide is acting through MC4-R to induce the responses. Some inhibitory effect of α-MSH in the presence of HS014 was also observed, although it was not statistically significant.
The excitatory effect of melanocortin agonist is mediated through postsynaptic mechanisms
We next examined whether the observed excitatory effects of α-MSH on TRH-GFP neurons are direct actions on postsynaptic cells or mediated through presynaptic actions on other neurons. This was determined by examining the effects of bath application of α-MSH on these neurons as described above in the absence of ionotropic glutamate and γ-aminobutyric acid(A) [GABAa]-mediated synaptic transmission by pretreating hypothalamic slices with 1 mm kynurenic acid (KYN) and 200 μm picrotoxin (PIC) (Fig. 3, A and B).
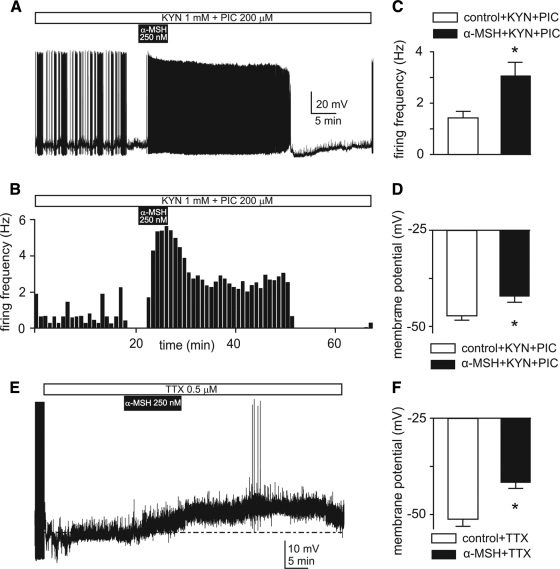
Figure 3.
The excitatory effects of α-MSH on TRH-GFP neurons is mediated by postsynaptic mechanisms. A, Voltage trace recording from a spontaneously firing neuron indicates that effects of 250 nm α-MSH persist in a hypothalamic slice pretreated with 1 mm kynurenic acid (KYN) and 200 μm picrotoxin (PIC) to block ionotropic glutamate and GABA(A) neurotransmission. Note that α-MSH increases frequency of firing as well as duration of bursts of firing. B, Frequency histogram quantifies these effects. C and D, Means ± sem of frequency of firing (C) and membrane potentials (D) in control and during applications of 250–350 nm α-MSH recorded from neurons pretreated with KYN and PIC. *, P < 0.001 (C) and P < 0.01 (D), paired t test. E, Voltage trace recording of a TRH-GFP neuron indicates that application of 250 nm α-MSH generates depolarization of membrane potential in the presence of 0.5 μm TTX. Note that the depolarization elicited a few Ca2+-mediated action potentials. F, Bar graphs show means ± sem of the effects of 250–350 nm α-MSH on membrane potentials in TTX-pretreated neurons (n = 19). *, P < 0.0001.
In 17 neurons tested under these circumstances, bath application of 250 nm α-MSH significantly increased (about 100%) the frequency of action potential firing from 1.5 ± 0.3 Hz in control to 3.1 ± 0.5 Hz (P < 0.001, paired t test; Fig. 3, A–C). This increase in firing activity was associated with around 5 mV depolarization of membrane potentials from −47.2 ± 1.4 mV in control to −42.1 ± 1.9 mV in the presence of α-MSH (P < 0.01, paired t test; Fig. 3D).
Although ionotropic glutamate and GABAa mediate principal fast neurotransmitter signal across the synapse in the mammalian central nervous system, a role of other, particularly slower, neurotransmitters in mediating the observed excitatory effects α-MSH cannot be ruled out. We hence decided to examine effects of this peptide in the absence of all types of action potential-driven synaptic transmission by pretreating hypothalamic slices with 0.5 μm tetrodotoxin (TTX), a blocker of Na+-dependent action potentials, 15–20 min before application of α-MSH (Fig. 3E).
Under these circumstances, bath application of TTX blocked spontaneous Na+-mediated action potential firing of these neurons. After 15–20 min of bath application of 0.5 μm TTX, we added 250 nm α-MSH as described above. We then compared changes in membrane potentials induced by application of the peptide. Our results obtained from 19 TRH-GFP neurons indicate that α-MSH induced significant depolarization of membrane potentials from −51.1 ± 1.9 mV in control solution to −41.6 ± 1.5 mV in the presence of peptide (P < 0.0001, by paired t test; Fig. 3, E and F). Furthermore, the magnitude of α-MSH-induced depolarization in these neurons was not different from experiments in which cells were not pretreated with TTX (P > 0.05, unpaired t test). These findings provide evidence indicating that the excitatory effects of α-MSH are mediated through postsynaptic mechanisms in TRH-GFP neurons of the PVN.
NPY inhibits TRH-GFP neurons
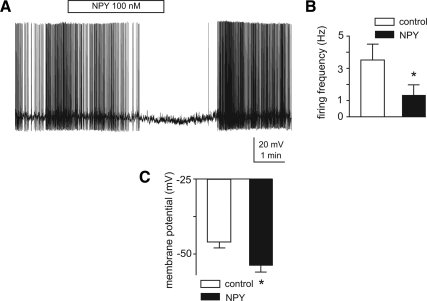
Most MC4-R-expressing brain nuclei receive projections from both POMC and NPY/AgRP ARC neurons and are thus potentially regulated by NPY. We thus investigated whether the orexigenic peptide NPY can regulate activity of TRH-GFP neurons by examining its effects on action potential firing activity of these neurons. Under current clamp recording as described above, we compared the firing activity of TRH-GFP-expressing neurons in control and in the presence of bath-applied 100 nm NPY (Fig. 4A). We observed that this peptide potently and reversibly diminished action potential firing activity of eight neurons tested from 3.5 ± 1.0 Hz in control to 1.4 ± 0.6 Hz in the presence of the peptide (P < 0.05; Fig. 4, A and B). Furthermore, this effect on firing activity was associated with significant hyperpolarization of membrane potentials from −46.3 ± 2.04 mV in control to −54.3 ± 2.2 mV in the presence of NPY (P < 0.005; Fig. 4, A and C). These data provide evidence supporting an inhibitory role of NPY on activity of TRH-expressing neurons in the PVN.
Figure 4.
NPY potently and reversibly inhibits firing activity of TRH-GFP neurons. A, Voltage trace recording from a spontaneously firing TRH-GFP-expressing neuron indicates that 100 nm NPY potently and reversibly inhibits its firing activity. Note that decrease in firing frequency is associated with hyperpolarization of membrane potentials. B and C, Bar graphs indicate means ± sem of inhibitory effects of 100 nm NPY on action potential firing frequency (B) and membrane potentials (C) of eight TRH-GFP neurons tested. *, P < 0.001.
Leptin increases firing activity of TRH-GFP neurons
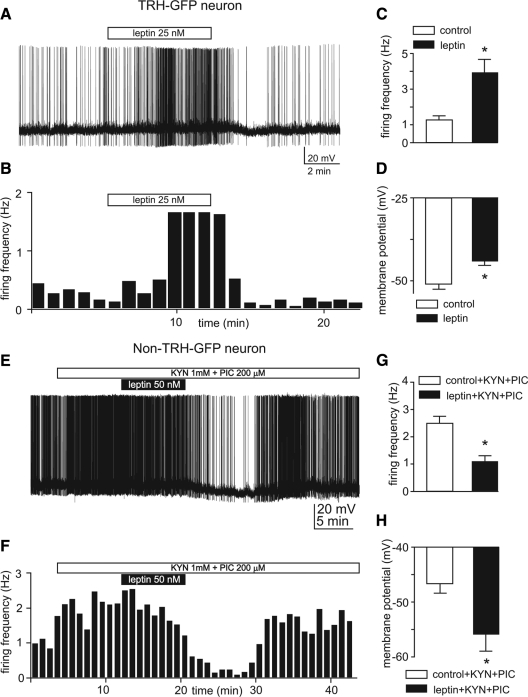
Despite detection of activation of pSTAT3, a leptin receptor signaling molecule, in TRH PVN neurons after administration of leptin (7,32,33), it is not known whether STAT3 directly affects the activity of TRH-expressing neurons in mouse PVN to regulate activity of the HPT axis. Here we addressed this question by directly testing effects of leptin on TRH-GFP neurons of the PVN in hypothalamic slices. We tested this hypothesis by examining effects of bath applications of submaximal in vitro concentrations, 25–50 nm, of this peptide on action potential firing activity and membrane potentials of these neurons. Performed under these conditions, we tested response to leptin of 30 TRH-GFP PVN neurons by comparing the average action potential firing frequency for 4–7 min in control with averages of the same duration obtained in the presence of the hormone immediately before commencement of washout (Fig. 5, A and B). Under these conditions, applications of 25–50 nm leptin augmented firing activity of 29 of 30 neurons tested. One cell responded to leptin by hyperpolarization and a decrease in firing activity. However, for the purpose of analysis, we considered all neurons recorded from, and we observed that applications of 25–50 nm leptin increased action potential firing frequency of these neurons from 1.2 ± 0.2 to 3.9 ± 0.7 Hz (P < 0.005 by paired t test; Fig. 5, A–C). Furthermore, this increase in firing activity was associated with depolarization of membrane potentials from −50.7 ± 1.6 mV in control to −43.8 ± 1.3 mV in the presence leptin (P < 0.01, paired t test; Fig. 5, A and D). These effects of leptin reached the peak magnitude, measured by highest observed values, in 9 ± 1 min after commencement of application of the peptide (data not shown). We continued monitoring the activity of neurons for at least up to 30 min after beginning of washout. We observed that the neuronal firing activity returned to 72% of observed maximal response in 20 ± 3 min after beginning of washout (data not shown). These results indicate that leptin potently and reversibly activates neuronal firing of TRH neurons in mouse PVN. To examine whether leptin also acts on adjacent non-TRH-expressing neurons of PVN, we examined effects of this peptide on firing activity and membrane potentials of non-GFP neurons in the same hypothalamic slices under whole-cell recording in the presence of 1 mm KYN and 200 μm picrotoxin, as described elsewhere. Our results indicate that bath application of 25–50 nm leptin in fact decreased action potential firing frequency of non-GFP neurons (from 2.5 ± 0.2 Hz in the control to 1.1 ± 0.19 Hz, P < 0.001; n = 9). This decrease in firing activity was associated with hyperpolarization of membrane potentials from −46.5 ± 1.7 mV in control to −55.9 (P < 0.05; n = 9; Fig. 5, E–H).
Figure 5.
Leptin activates action potential firing activity of TRH-GFP neurons. A, Voltage trace of whole-cell recording from a spontaneously firing TRH-GFP neuron indicates that bath application of 25 nm leptin induces an increase in firing activity associated with depolarization of membrane potential. B, Frequency histogram of this effect. C and D, Bar graphs indicate means ± sem of effects of 25–50 nm leptin on action potential firing frequency (C) and membrane potentials (D) of 30 neurons tested. *, P < 0.005 (C) and P < 0.1 (D). E–H, Leptin inhibits action potential firing activity of non-TRH-GFP-expressing neurons; E, voltage trace of whole-cell recording from a non-TRH-GFP-expressing neuron indicates that bath application of 50 nm leptin induces inhibition of firing activity associated with hyperpolarization of membrane potential; F, frequency histogram of this effect; G and H, bar graphs indicate means ± sem of effects of 25–50 nm leptin on action potential firing frequency (G) and membrane potentials (H) of nine neurons tested. *, P < 0.001 (G) and P < 0.05 (H).
The effects of leptin are mediated through postsynaptic mechanisms
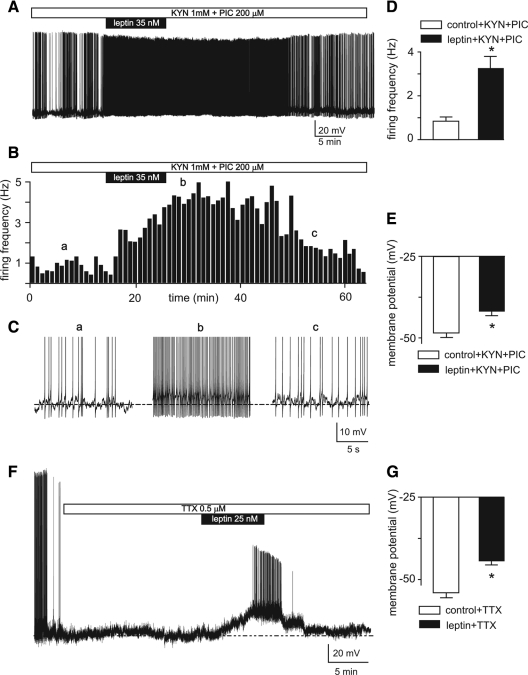
We tested the hypothesis that leptin’s actions were mediated postsynaptically by leptin receptors on TRH neurons by examining effects of bath applications of leptin on TRH-GFP neurons in the absence of ionotropic glutamate and GABA(A) neurotransmission by pretreating hypothalamic slices with 1 mm KYN and 200 μm PIC (Fig. 6, A–C). Our results obtained from 14 neurons tested under these conditions indicate that applications of 25–50 nm leptin increased firing activity of these neurons from 0.8 ± 0.2 Hz in control to 3.3 ± 0.6 Hz (P < 0.005 by paired t test; Fig. 6, A–D). Furthermore, the increase in firing activity induced by leptin was associated with depolarization of membrane potentials from −48.2 ± 1.3 mV in control to −41.6 ± 1.3 mV in the presence of leptin (n = 14; P < 0.001, paired t test; Fig. 6, C and E).
Figure 6.
The excitatory effects of leptin on TRH-GFP neurons are mediated through postsynaptic mechanisms. A, Voltage trace recording indicates that 35 nm leptin generates an increase in firing activity associated with depolarization of membrane potential in a TRH-GFP neuron pretreated with 1 mm KYN and 200 μm PIC. B, Frequency histogram of this effect. C, Insets of the voltage trace (shown in A) expanded in time scale obtained from different time points (indicated by a–c) to clearly demonstrate the effects of leptin on firing activity and membrane potential. D and E, Bar graphs indicate means ± sem of effects of 25–50 nm leptin on frequency of firing (D) and membrane potentials (E) of 14 neurons pretreated with KYN and PIC. *, P < 0.001. F, Voltage trace of recording from a TRH-GFP neuron indicates that 25 nm leptin induces significant depolarization of membrane potential in the presence of TTX. Note that leptin-induced depolarization generated Ca2+-mediated action potentials. G, Bar graphs of means ± sem of depolarizing effects of 25–50 nm leptin on 34 neurons tested. *, P < 0.0001.
Although these results provide evidence suggesting that the observed excitatory effect of leptin is not mediated by ionotropic glutamate and GABA(A), a role of other neurotransmitters in mediating this response could not be ruled out. We hence decided to examine whether the observed excitatory effects of leptin are mediated by any action potential-driven synaptic activity. We tested this hypothesis by examining the effects of leptin in hypothalamic slices pretreated with TTX to block Na+-dependent action potentials (Fig. 6F). Our results obtained under these conditions from 34 TRH-GFP neurons in PVN pretreated with TTX indicate that leptin causes significant and reversible depolarization of membrane potentials in all neurons tested from −54.1± 1.5 mV in control to −44. 4 ± 1.3 mV (n = 34; P < 0.0001, by paired t test; Fig. 6G). These findings provide evidence indicating that the excitatory effect of leptin on the TRH-GFP neurons of the PVN is mediated by postsynaptic mechanisms.
Contribution of the direct and indirect actions of leptin on regulation of TH release
Given that 97% of TRH-GFP PVN neurons were potently and postsynaptically activated by leptin, we sought to investigate the contribution of the direct and indirect pathways of leptin action on regulation of serum T4 levels (Fig. 7). We compared serum total T4 levels in groups of mice that received one of five different experimental treatments (for details see Materials and Methods).
Figure 7.
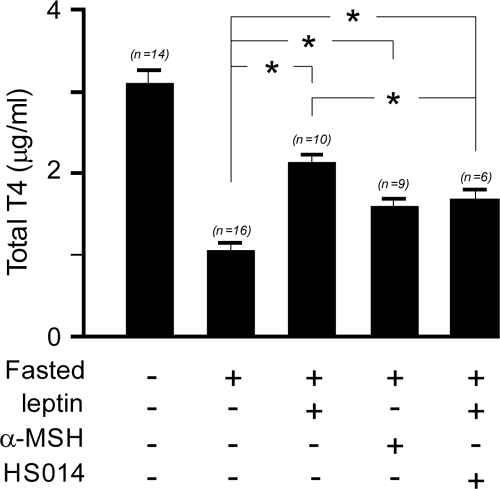
Regulation of levels of serum total T4 by both melanocortin-dependent and -independent pathways of leptin action. The bar graphs indicate serum total T4 levels from 10- to 12-wk-old male C57B/6J mice at the end of 36 h of different treatments as indicated below each column. *, P < 0.05 (one-way ANOVA, Bonferroni’s multiple-comparison test, n values are indicated above each column).
The first group (n = 14) had free access to food and was injected intracerebroventricularly (icv) with 2 μl artificial cerebrospinal fluid (aCSF) containing 0.1% BSA and ip with 100 μl saline every 6 h for the duration of the experiment. The second (n = 9), third (n = 10), fourth (n = 6), and fifth (n = 16) groups were fasted for 36 h beginning at 2000 h on the first day and ending between 0800 and 0900 h on the third day. These groups were then injected as follows: group 2, icv with 750 ng α-MSH in 2 μl aCSF; group 3, ip with leptin 1 mg/kg; group 4, ip with leptin 1 mg/kg and icv with 10 nmol of MC4-R antagonist HS014 in 2 μl aCSF; and group 5, icv with 2 μl aCSF and ip with 100 μl saline, respectively, every 6 h. Our results indicate that means (± sem) of serum total T4 levels in each of the treatment groups, α-MSH (1.59 ± 0.10 μg/ml, n = 9), leptin (2.14 ± 0.09 μg/ml, n = 10), and leptin and HS014 (1.65 ± 0.145 μg/ml, n = 6) were significantly higher compared with the fasted control group (1.06 ± 0.09 μg/ml, n = 16) and lower compared with control fed group (3.11 ± 0.16 μg/ml, n = 14; P < 0.05, one-way ANOVA, Bonferroni’s multiple-comparison test). Leptin was more potent (70% of fed state) than α-MSH alone (52% of fed state) in restoring serum total T4 levels (P < 0.05, one-way ANOVA, Bonferroni’s multiple-comparison test), and both were stimulatory compared with total T4 levels in the fasted control group (34% of fed group).
Furthermore, cotreatment with the potent MC4-R blocker HS014 diminished but did not fully block leptin action in restoring total T4 (P < 0.05, one-way ANOVA, Bonferroni’s multiple-comparison test), indicating existence of a functional melanocortin-independent pathway of leptin action. Collectively, these findings support functional melanocortin-dependent and -independent actions of leptin in regulating activity of TRH PVN neurons and the thyroid axis.
Discussion
The HPT axis is a critical component of the starvation response, and thus it is important to understand precisely how leptin communicates the starvation signal to this axis. The TRH-Cre × Rosa-26-GFP mouse was demonstrated to express GFP in TRH neurons (34) and thus provide the first model system for facile characterization of the electrophysiological properties of TRH-expressing neurons in the PVN. As described, the electrophysiological recordings described here were made from TRH-GFP neurons from the mid-anterior PVN. By dual ISH/IHC, approximately 52–60% of these GFP-positive PVN neurons expressed detectable TRH mRNA (Table 1). This is possibly an underestimate of the percentage of GFP-positive PVN cells in this animal that are functional TRH neurons, because lower levels of TRH mRNA are expressed in the basal euthyroid state (34). Thus, a possibility is that some TRH neurons may be detectable by IHC for GFP, but below the threshold of detection by ISH for TRH mRNA.
To further characterize these cells, we examined the coexpression of GFP with FG and neurophysin (Fig. 1). Our analysis of all GFP neurons within the PVN has shown that these neurons are distributed in about a 3.2:1 ratio in anterior PVN to posterior PVN.
Furthermore, over 90% of GFP-positive neurons labeled with systemic administration of FG, indicating axonal projection to ME or PP, were in the anterior PVN. These findings are in agreement with a recent paper suggesting that TRH-expressing neurons relevant to the function of HPT axis are located in anterior PVN (7). By analyzing the colocalization of TRH, FG, and GFP, we were also able to determine the percentage of FG-positive neurosecretory cells that were magnocellular, pituitary-projecting cells. First, PVN cells labelled with GFP were overwhelmingly labeled with FG (79% of total GFP cells). In all, 21% of PVN GFP cells appeared nonsecretory, 61% were neurosecretory cells projecting to the ME, and 18% were neurosecretory cells projecting to the PP. In agreement with these results, our investigation of the electrogenic membrane properties of these neurons indicate that most of the neurons in the anterior PVN (55%) express characteristics of type II, putative parvocellular neurosecretory neurons of PVN, as described previously in rat PVN (41).
Results from our physiological and anatomical investigations collectively indicate that the majority of the GFP-expressing neurons lie in the anterior part of the PVN and include functionally relevant parvocellular neurosecretory neurons projecting to ME and presumably regulating the activity of the HTP axis. Hence, our randomly chosen neurons for electrophysiological recordings comprised mostly cells located in the anterior part of the PVN.
Our investigation of responses of a large number of neurons to bath applications of α-MSH indicated that 91% of neurons (32 of 35) responded by activation of neuronal firing (Fig. 2), in agreement with a previous report of effects of melanotan-II, a synthetic analog of α-MSH, on MC4-R-expressing neurons in PVN (43). Furthermore, we demonstrated that this response was mediated by postsynaptic mechanisms in TRH-GFP neurons because the observed excitatory effects of α-MSH persisted in the absence of ionotropic or action potential-dependent synaptic transmission (Fig. 3). Both pre- and postsynaptic actions of α-MSH have been previously reported in other electrophysiological preparations (44).
One neuron of 35 responded to α-MSH by inhibition of action potential firing activity. We surmise that the inhibitory effects on that neuron could have been mediated by presynaptic inputs, because in the absence of ionotropic synaptic transmission, none of the 17 neurons tested responded to α-MSH by inhibition of firing activity. In fact, it has been reported that bath application of the α-MSH analog melanotan-II increases magnitude of evoked inhibitory postsynaptic currents recorded from certain rat PVN neurons (47).
α-MSH in the presence of HS014, a selective antagonist of MC4-R (46), failed to cause excitation in any of the neurons tested, indicating that the observed excitatory response in our study is mediated by MC4-R signaling. In fact, the average response in the presence of the antagonist, although not statistically significant, indicated a slight decrease in neuronal activity. Because α-MSH is also a ligand for the MC3-R, which can act as an inhibitory autoreceptor in some cases, we surmise that this inhibitory trend observed by α-MSH could possibly be mediated by MC3-R signaling through other neurons (48). Alternatively, HS014 may be acting as an inverse agonist to reduce constitutive activity of the MC4-R. In contrast to the actions of α-MSH, NPY potently inhibited the firing frequency of anterior TRH-GFP neurons, reducing firing frequency by 61%, averaged over eight neurons tested (Fig. 2). This observation argues that each individual TRH-GFP neuron is coordinately regulated by both NPY and α-MSH.
Our results indicate that leptin increases the firing activity of approximately 97% of TRH-GFP neurons (29 of 30) tested in anterior PVN (Fig. 3). Furthermore, this increase in firing activity persisted in the absence of ionotropic or action potential-dependent synaptic transmission, ruling out the possibility that the observed responses to leptin are mediated indirectly, through leptin-responsive neurons in the ARC or elsewhere. However, a role of activity-independent synaptic transmission, by leptin receptor expressed on presynaptic neurons, such as POMC and NPY neurons, is not ruled out by these observations. These findings suggest existence of postsynaptic mechanisms involved in mediating the observed response to leptin, in agreement with a previous study of effects of leptin on randomly selected neurons in rat PVN (49). It is important to note that these data argue that detection of leptin-responsive neurons by IHC for leptin receptor mRNA, c-fos detection, or pSTAT detection may thus be underdetecting the actual population of leptin-responsive neurons in the central nervous system.
In the current study, GFP-expressing neurons mostly in the anterior PVN were selected to examine responses of leptin as well as α-MSH. The results suggest that most TRH neurons in anterior PVN respond by increase in firing activity in response to either of these adiposity signals. In a previous study, Perello et al. (7) suggested that α-MSH-responsive neurons in PVN are different from leptin-responsive neurons in this nucleus. However, the fact that greater than 91 and 97% of anterior TRH-GFP neurons respond to α-MSH and leptin, respectively, makes this hypothesis unlikely. Nonetheless, we also tested effects of both α-MSH and leptin consecutively on nine neurons. All these neurons responded to both α-MSH and leptin by activation of firing frequency and induction of membrane depolarization (data not shown). These results strongly suggest that the majority of TRH neurons in the anterior PVN are capable of responding to both leptin and α-MSH, providing evidence supporting both direct and indirect pathways as mechanisms by which leptin regulates the HPT axis. Furthermore, these data demonstrate that each neurosecretory TRH PVN neuron integrates signals from peripheral leptin, α-MSH, and NPY to determine the set-point of the HPT axis. Measurement of serum total T4 levels in fasted animals treated with leptin, α-MSH, or leptin plus the potent melanocortin antagonist HS014 support the idea that leptin regulates TRH neurons and the HPT axis in a multimodal fashion, regulating release of α-MSH, AgRP, NPY, and GABA onto TRH neurons via activation of leptin receptors on ARC POMC and NPY/AgRP neurons and regulating TRH cells directly via leptin receptors on TRH neurons. It is possible to imagine specific physiological roles for these two leptin signaling pathways. For example, the ARC and PVN leptin responses may serve to detect different magnitudes in changes in serum leptin. Mice with leptin receptor deleted specifically from the PVN, currently under construction, may be used to probe this hypothesis.
Materials and Methods
Generation of TRH-Cre mice
All mice used in this study for characterization of responses of TRH-expressing neurons were TRH-Cre transgenic mice generated on an FVB background (34) crossed with Rosa-26-GFP reporter mice on a B6:126 background (004077; The Jackson Laboratory) to generate offspring that express GFP protein in neurons that express the Cre-recombinase. Both male and female mice are used in this study and housed in a rodent facility on a 12-h light, 12-h dark cycle, with lights on at 0700 h (Pacific standard time), with ad libitum access to water and food (Lab Diet 5001, Rodent Diet; PMI Nutrition International, LLC, Brentwood, MO). All mice are weaned at least 6 d before recording and decapitated at age 26–60 d between 1130 and 1200 h, approximately 4 h, 30 min after lights on. All animal experiments are conducted in accordance with Society for Neuroscience Policies on the Use of Animals and Humans in Neuroscience Research and were approved by the university Animal Care and Use Committee.
Preparation of slices for electrophysiology
Young adult (26–60 d old) TRH-GFP mice were generated by crossing TRH-Cre to Rosa-26-EGFP mice (The Jackson Laboratory). The offspring TRH-GFP mice were used in all electrophysiological experiments. They were deeply anesthetized with isoflurane before decapitation. The brain was entirely removed and immediately submerged in ice-cold, gassed (95% O2, 5% CO2) aCSF, containing (in mm) 126.2 NaCl, 3.1 KCl, 2 CaCl2, 1 MgCl2, 1 NaH2PO4, 26.2 NaHCO3, 10 glucose, and 16.2 sucrose (320 mosm/kg, pH 7.4 when gassed with 95% O2, 5% CO2 at room temperature). Brain blocks containing hypothalamus are then made by trimming the whole brains while immersed in oxygenated, near-freezing aCSF and glued to a dental-cement cast customized to the size of the block mounted on a plate with adjustable angle. Brain slices of 200 μm thickness were then cut at an angle range between 44° and 49° in reference to horizontal plane and transferred to a glass beaker containing oxygenated aCSF at 31 C. After an incubation period lasting at least 1 h, the slice is transferred to a recording chamber (∼1.0 ml in volume), where it is submerged and immobilized with nylon strands drawn taut across a C-shaped platinum wire (1 mm outer diameter) and perfused with warmed (31–32 C) oxygenated aCSF at a rate of 2–3 ml/min.
Electrophysiological recordings
Whole-cell current clamp recordings were performed to obtain information about action potential firing activity and membrane potentials using patch pipettes of 3.5-5 mΩ resistance when filled with a solution containing (in mm) 125 K gluconate, 8 KCl, 5 MgCl2, 10 HEPES, 5 NaOH, 4 Na2ATP, 0.4 Na3GTP, 15.4 sucrose, and 7 KOH, which results in a pH of approximately 7.23 and osmolality of 295–300 mosmol/kg.
For drugs, 1 mm KYN and 100–200 μm PIC(both from Sigma Chemical Co., St. Louis, MO) were directly dissolved in aCSF while being gassed. Recombinant murine leptin (purity 95–99% by SDS-PAGE) was purchased from A. F. Parlow (National Hormone and Peptide Program, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD) and stored at −80 C until stock solutions were made. Leptin was dissolved in sterile-filtered PBS (Invitrogen Corp., Carlsbad, CA; adjusted to pH 7.9 with NaOH) at 50 μm concentration and stored in aliquots at −80 C until use. Porcine NPY and acylated α-MSH amide (both from American Peptide Co. Inc., Sunnyvale, CA) and TTX and HS014 (both from Sigma) were dissolved in water as stock solutions and stored at −80 C at 1000 times higher than final concentrations. FG (Fluorochrome LLC, Denver, CO) was dissolved in 5% saline solution and stored in 4 C in the dark until final.
Data acquisition
Data are acquired at 10 kHz using a MultiClamp 700A amplifier (100–200 times gain; −3-dB filter frequency, 5 kHz) and Clampex 10.1 software (Axon Instruments, Union City, CA). Data are analyzed using Clampfit 10.1 (Axon Instruments), Mini Analysis Program version 5.6.28 (Synaptosoft, Decatur, GA), GraphPad Prism version 3.0 (GraphPad Software, Inc., San Diego, CA), and Excel 2007 (Microsoft Corp., Bellevue, WA).
Immunohistochemistry
Tissue fixation
Ad libitum-fed mice were deeply anesthetized and underwent tissue fixation via transcardial perfusion with 0.9% saline followed by ice-cold fixative (4% paraformaldehyde in 0.01 m PBS. Brains were postfixed for 4–6 h in the same fixative and were then stored overnight in 20% sucrose in PBS as a cryoprotectant before being frozen at −80 C until use.
Antibodies
The following primary and secondary antibody combinations were used: 1:100 mouse monoclonal antineurophysin antibody (PS 38, a kind gift of Dr. H. Gainer (50) for 24 h followed by 1:500 donkey antimouse Alexa 594 (Molecular Probes Inc., Eugene, OR). The expression of GFP was detected by 1:250 goat anti-GFP conjugated to biotin (ab6658; Abcam Antibody, Cambridge, MA) for 24 h followed by 1:1000 streptavidin-conjugated Alexa 488 (S32354; Molecular Probes). Each mouse used in relevant IHC experiments received 15 μg/g FG in 5% saline solution (Fluorochrome) ip 2–3 d before perfusion and fixation. The signal of FG was further amplified by incubating fixed slices with 1:20,000 rabbit anti-FG polycolonal antibody (AB153; Chemicon Inc., Temecula, CA) for 24 h and then followed by 1:500 donkey antirabbit Alexa 594 (Molecular Probes).
Dual-label ISH/IHC
Dual-label ISH/IHC procedures have been previously described (34). Briefly, brain sections from TRH-Cre × Rosa-26-GFP mice (n = 3) were mounted onto Superfrost Plus slides (Thermo Fisher Scientific Pittsburgh, PA). Prehybridization and hybridization steps used diethylpyrocarbonate-treated solutions. After prehybridization washes, 120 μl 35S-radiolabeled antisense TRH cRNA riboprobe diluted to 106 cpm/μl (51). After 16 h incubation at 57 C, posthybridization washes were performed to remove excess probe, and then slides were incubated in 0.3% H2O2 in PBS to block endogenous peroxide activity. Sections were blocked with 1% normal goat serum for 2 h and then incubated overnight at 4 C with gasketed coverslips in 1:1000 anti-GFP (Molecular Probes/Invitrogen). After washes, application of antirabbit biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) and Vectastain ABC (Vector) incubation, antibody staining was visualized with 0.04% 3,3′-diaminobenzidine and 0.1% H2O2 in PBS. mRNA signal was visualized as previously described (34), and light-field images were captured using the Zeiss Axioimager.Z1 with Axiovision version 4.5 software. To quantify GFP-positive neurons also containing TRH mRNA, sections were quantified by counting the total number of GFP neurons and then the number of GFP neurons also positive for TRH mRNA. Per mouse, the average percentage for each region (anterior, mid, and posterior) was attained from one to four sections. Data are presented as sample means (± sem) for each region of the PVN. Representative sections of each region of the PVN are shown in Supplemental Fig. 1 from a previously unpublished transgenic animal.
Animal preparation for injections
The mice were implanted with a 23-gauge stainless steel guide cannula (Small Parts Inc., Seattle, WA) into the lateral cerebral ventricle under stereotaxic control (coordinates from Bregma anterior to posterior, −0.45mm; lateral, 1.1mm to right; dorsal to ventral, −2.4mm) through a burr hole in the skull. The cannula was secured to the skull first with super glue and then dental cement and temporarily occluded with a stylus. Animals were weighed daily, and any animal showing signs of illness or weight loss was removed from the study and euthanized. At least 3 wk after cannulation, the animals were divided into five groups. The first group (n = 14) had free access to food and was injected icv with 6 μl aCSF containing 0.1% BSA and 100 μl saline ip every 6 h for the duration of the experiment. The second (n = 9), third (n = 10), fourth (n = 6), and fifth (n = 16) groups were fasted for 36 h beginning at 2000 h on the first day and ending between 0800 and 0900 h on the third day, injected icv with α-MSH in 2 μl aCSF, with ip leptin 1 mg/kg, with icv 10 nmol HS014 in 2 μl aCSF, or with 2 μl aCSF respectively, every 6 h.
In previous studies (9,15), these doses were shown to prevent fasting-induced inhibition of TRH mRNA in the PVN. All icv injections were made in freely moving animals through a 32-gauge needle that extended 1 mm below the guide cannula, connected by polyethylene tubing to a 10-μl Hamilton syringe and infused over 2 min by hand. At completion of the experiment, the animals were anesthetized with isoflurane and decapitated for truncal blood, pituitary, and brain collection. The brains were removed and frozen by immersion in dry ice. Cannula placement was confirmed by light microscopic examination, and animals with cannulas outside the lateral ventricle were excluded from further analysis. Fasted animals lost approximately 17% body weight during the experiment, whereas the fed mice gained 3–5% body weight. Fasted animals receiving α-MSH by icv infusion also showed significant weight reduction (18.6 ± 1.1%) that was not significantly different from the weight loss of the fasted control animals.
Total T4 measurements
RIA technique was used to measure serum total T4 levels of baseline and posttreatment mouse serum by using the Coat-A-Count Total T4 kit as recommended by the manufacturer (TKT41; Siemens Medical Solution Diagnostics, Berkeley, CA).
Supplementary Material
Acknowledgments
We thank Dr. Amanda Vanhoose for critically reading the manuscript and John Murphy and Meghan Rowland for technical assistance.
Footnotes
Our work is supported by NIH RO1 DK070332 (to R.D.C.), NIH RO1 DK078090 (A.N.H.), and Canadian Institutes of Health Research Fellowship Award (129207 to M.G.-L.).
Disclosure Summary: The authors of this manuscript have nothing to declare.
First Published Online October 13, 2010
Abbreviations: aCSF, Artificial cerebrospinal fluid; AgRP, agouti-related peptide; ARC, arcuate nucleus of the hypothalamus; FG, fluorogold; GABA(A), γ-aminobutyric acid(A); GFP, green fluorescent protein; HPT, hypothalamic-pituitary-thyroid; icv, intracerebroventricularl; IHC, immunohistochemistry; ISH, in situ hybridization; KYN, kynurenic acid; LTS, low threshold potentials; MC4-R, melanocortin-4 receptor; ME, median eminence; NPH, neurophysin; NPY, neuropeptide Y; PIC, picrotoxin; POMC, proopiomelanocortin; PP, posterior pituitary; pSTAT3, phospho-signal transducer and activator of transcription 3; PVN, paraventricular nucleus; TH, thyroid hormone; TTX, tetrodotoxin.
References
- Lechan RM, Fekete C 2006 The TRH neuron: a hypothalamic integrator of energy metabolism. Prog Brain Res 153:209–235 [DOI] [PubMed] [Google Scholar]
- Segerson TP, Kauer J, Wolfe HC, Mobtaker H, Wu P, Jackson IM, Lechan RM 1987 Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science 238:78–80 [DOI] [PubMed] [Google Scholar]
- Kakucska I, Rand W, Lechan RM 1992 Thyrotropin-releasing hormone gene expression in the hypothalamic paraventricular nucleus is dependent on feedback regulation by both triiodothyronine and thyroxine. Endocrinology 130:2845–2850 [DOI] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Arancibia S, Astier H 1985 Evidence for α1-adrenergic stimulatory control of in vitro release of immunoreactive thyrotropin-releasing hormone from rat median eminence: in vivo corroboration. Endocrinology 116:2314–2319 [DOI] [PubMed] [Google Scholar]
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjøorbaek C, Elmquist JK, Flier JS, Hollenberg AN 2001 Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 107:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Mihály E, Luo LG, Kelly J, Clausen JT, Mao Q, Rand WM, Moss LG, Kuhar M, Emerson CH, Jackson IM, Lechan RM 2000 Association of cocaine- and amphetamine-regulated transcript-immunoreactive elements with thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and its role in the regulation of the hypothalamic-pituitary-thyroid axis during fasting. J Neurosci 20:9224–9234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perello M, Stuart RC, Nillni EA 2006 The role of intracerebroventricular administration of leptin in the stimulation of prothyrotropin releasing hormone neurons in the hypothalamic paraventricular nucleus. Endocrinology 147:3296–3306 [DOI] [PubMed] [Google Scholar]
- Toni R, Jackson IM, Lechan RM 1990 Neuropeptide-Y-immunoreactive innervation of thyrotropin-releasing hormone-synthesizing neurons in the rat hypothalamic paraventricular nucleus. Endocrinology 126:2444–2453 [DOI] [PubMed] [Google Scholar]
- Fekete C, Légrádi G, Mihály E, Huang QH, Tatro JB, Rand WM, Emerson CH, Lechan RM 2000 α-Melanocyte-stimulating hormone is contained in nerve terminals innervating thyrotropin-releasing hormone-synthesizing neurons in the hypothalamic paraventricular nucleus and prevents fasting-induced suppression of prothyrotropin-releasing hormone gene expression. J Neurosci 20:1550–1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioda S, Nakai Y, Sato A, Sunayama S, Shimoda Y 1986 Electron-microscopic cytochemistry of the catecholaminergic innervation of TRH neurons in the rat hypothalamus. Cell Tissue Res 245:247–252 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW, Grzanna R, Howe PR, Bloom SR, Polak JM 1985 Colocalization of neuropeptide Y immunoreactivity in brainstem catecholaminergic neurons that project to the paraventricular nucleus of the hypothalamus. J Comp Neurol 241:138–153 [DOI] [PubMed] [Google Scholar]
- ter Horst GJ, Luiten PG 1986 The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res Bull 16:231–248 [DOI] [PubMed] [Google Scholar]
- Keys A, Brozek J, Henschel J 1950 The biology of human starvation. Minneapolis: University of Minnesota Press [Google Scholar]
- Bray GA, Fisler J, York DA 1990 Neuroendocrine control of the development of obesity: understanding gained from studies of experimental animal models. Front Neuroendocrinol 11:128–181 [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS 1996 Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252 [DOI] [PubMed] [Google Scholar]
- Légrádi G, Emerson CH, Ahima RS, Flier JS, Lechan RM 1997 Leptin prevents fasting induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 138:2569–2576 [DOI] [PubMed] [Google Scholar]
- Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, Karalis A, Mantzoros CS 2004 Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 351:987–997 [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL 2005 Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. The J Clin Invest 115:3579–3586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete C, Marks DL, Sarkar S, Emerson CH, Rand WM, Cone RD, Lechan RM 2004 Effect of agouti-related protein in regulation of the hypothalamic-pituitary-thyroid axis in the melanocortin 4 receptor knockout mouse. Endocrinology 145:4816–4821 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB 1998 Distribution of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol 395:535–547 [PubMed] [Google Scholar]
- Bates SH, Dundon TA, Seifert M, Carlson M, Maratos-Flier E, Myers Jr MG 2004 LRb-STAT3 signaling is required for the neuroendocrine regulation of energy expenditure by leptin. Diabetes 53:3067–3073 [DOI] [PubMed] [Google Scholar]
- Hübschle T, Thom E, Watson A, Roth J, Klaus S, Meyerhof W 2001 Leptin-induced nuclear translocation of STAT3 immunoreactivity in hypothalamic nuclei involved in body weight regulation. J Neurosci 21:2413–2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi G, Emerson CH, Ahima RS, Rand WM, Flier JS, Lechan RM 1998 Arcuate nucleus ablation prevents fasting-induced suppression of proTRH mRNA in the hypothalamic paraventricular nucleus. Neuroendocrinology 68:89–97 [DOI] [PubMed] [Google Scholar]
- Sarkar S, Légrádi G, Lechan RM 2002 Intracerebroventricular administration of α-melanocyte stimulating hormone increases phosphorylation of CREB in TRH- and CRH-producing neurons of the hypothalamic paraventricular nucleus. Brain Res 945:50–59 [DOI] [PubMed] [Google Scholar]
- Fekete C, Kelly J, Mihaly E, Sarkar S, Rand WM, Légrádi G, Emerson CH, Lechan RM 2001 Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology 142:2606–2613 [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, Cheetham T, O'Rahilly S 2000 Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest 106:271–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A 1998 Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet 19:155–157 [DOI] [PubMed] [Google Scholar]
- Erickson JC, Ahima RS, Hollopeter G, Flier JS, Palmiter RD 1997 Endocrine function of neuropeptide Y knockout mice. Regul Pept 70:199–202 [DOI] [PubMed] [Google Scholar]
- Challis BG, Coll AP, Yeo GS, Pinnock SB, Dickson SL, Thresher RR, Dixon J, Zahn D, Rochford JJ, White A, Oliver RL, Millington G, Aparicio SA, Colledge WH, Russ AP, Carlton MB, O'Rahilly S 2004 Mice lacking pro-opiomelanocortin are sensitive to high-fat feeding but respond normally to the acute anorectic effects of peptide-YY(3-36). Proc Natl Acad Sci USA 101:4695–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin NM, Small CJ, Sajedi A, Liao XH, Weiss RE, Gardiner JV, Ghatei MA, Bloom SR 2004 Abnormalities of the hypothalamo-pituitary-thyroid axis in the pro-opiomelanocortin deficient mouse. Regul Pept 122:169–172 [DOI] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Yasenchak J, Murphy A, Valenzuela D, Diano S, Yancopoulos GD, Wiegand SJ, Sleeman MW 2005 Agouti-related protein-deficient mice display an age-related lean phenotype. Cell Metab 2:421–427 [DOI] [PubMed] [Google Scholar]
- Nillni EA, Vaslet C, Harris M, Hollenberg A, Bjørbak C, Flier JS 2000 Leptin regulates prothyrotropin-releasing hormone biosynthesis. J Biol Chem 275:36124–36133 [DOI] [PubMed] [Google Scholar]
- Guo F, Bakal K, Minokoshi Y, Hollenberg AN 2004 Leptin signaling targets the thyrotropin-releasing hormone gene promoter in vivo. Endocrinology 145:2221–2227 [DOI] [PubMed] [Google Scholar]
- Sugrue ML, Vella KR, Morales C, Lopez ME, Hollenberg AN 2010 The thyrotropin-releasing hormone gene is regulated by thyroid hormone at the level of transcription in vivo. Endocrinology 151:793–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Kuypers HG 1980 The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol 194:555–570 [DOI] [PubMed] [Google Scholar]
- Lechan RM, Jackson IMD 1982 Immunohistochemical localization of thyrotropin-releasing hormone in the rat hypothalamus and pituitary. Endocrinology 111:55–65 [DOI] [PubMed] [Google Scholar]
- Bern H, Knowles F 1966 Neurosecretion. In: Martini L, Ganong W, eds. Neuroendocrinology. New York: Academic; 139–186 [Google Scholar]
- Merchenthaler I 1991 Neurons with access to the general circulation in the central nervous system of the rat: a retrograde tracing study with fluoro-gold. Neuroscience 44:655–662 [DOI] [PubMed] [Google Scholar]
- Luther JA, Daftary SS, Boudaba C, Gould GC, Halmos KC, Tasker JG 2002 Neurosecretory and non-neurosecretory parvocellular neurones of the hypothalamic paraventricular nucleus express distinct electrophysiological properties. J Neuroendocrinol 14:929–932 [DOI] [PubMed] [Google Scholar]
- Stern JE 2001 Electrophysiological and morphological properties of pre-autonomic neurones in the rat hypothalamic paraventricular nucleus. J Physiol 537:161–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasker JG, Dudek FE 1991 Electrophysiological properties of neurones in the region of the paraventricular nucleus in slices of rat hypothalamus. J Physiol 434:271–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther JA, Tasker JG 2000 Voltage-gated currents distinguish parvocellular from magnocellular neurones in the rat hypothalamic paraventricular nucleus. J Physiol 523:193–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK 2003 Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci 23:7143–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Hisadome K, Al-Qassab H, Heffron H, Withers DJ, Ashford ML 2007 Melanocortins and agouti-related protein modulate the excitability of two arcuate nucleus neuron populations by alteration of resting potassium conductances. J Physiol 578(Pt 2):425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong TM, Van der Ploeg LHT 2000 A melanocortin agonist reduces neuronal firing rate in rat hypothalamic slices. Neurosci Lett 283:5–8 [DOI] [PubMed] [Google Scholar]
- Schiöth HB, Mutulis F, Muceniece R, Prusis P, Wikberg JE 1998 Discovery of novel melanocortin4 receptor selective MSH analogues. Br J Pharmacol 124:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD 1999 Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: Evidence of a cellular basis for the adipostat. Neuron 24:155–163 [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- Powis JE, Bains JS, Ferguson AV 1998 Leptin depolarizes rat hypothalamic paraventricular nucleus neurons. Am J Physiol Regul Integr Comp Physiol 274:R1468–R1472 [DOI] [PubMed] [Google Scholar]
- Ben-Barak Y, Russell JT, Whitnall MH, Ozato K, Gainer H 1985 Neurophysin in the hypothalamo-neurohypophysial system. I. Production and characterization of monoclonal antibodies. J Neurosci 5:81–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T, Yamada M, Monden T, Iizuka M, Mori M 1992 Cloning of the mouse hypothalamic preprothyrotropin-releasing hormone (TRH) cDNA and tissue distribution of its mRNA. Brain Res Mol Brain Res 14:131–135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.