Abstract
Although there is substantial evidence that membrane progestin receptors (mPRs) perform a critical physiological role in meiotic maturation of fish oocytes, it is unknown whether they are also intermediaries in progestin signaling in the surrounding follicular cells. Here, we show that mPRα protein is located on the plasma membranes of both granulosa and theca cells (G/T cells) isolated from Atlantic croaker ovaries and is associated with the presence of a single high affinity, limited capacity, pertussis toxin-sensitive, specific progestin [17,20β,21-trihydroxy-4-pregnen-3-one (20β-S)] membrane binding site with the characteristics of mPRα. Treatment of G/T cells with 20β-S caused rapid G protein activation and a transient, pertussis toxin-sensitive, decrease in cAMP levels, whereas the selective nuclear progesterone receptor agonist, R5020, did not cause G protein activation, consistent with previous reports on mPRα signaling. 20β-S treatment decreased serum starvation-induced cell death in both G/T cells and in seatrout mPRα-transfected MDA-MB-231 cells, whereas R5020 was ineffective. Moreover, a selective mPRα agonist, 10-ethenyl-19-norprogesterone, mimicked the protective action of 20β-S against cell death, which was lost upon knockdown of mPRα protein but not after progesterone receptor knockdown, further demonstrating an involvement of mPRα. Signaling molecules involved in inhibition of apoptosis, Erk and serine-threonine kinase, were activated in G/T cells by 20β-S, which suggests a potential mechanism for mPRα inhibition of apoptosis. This is the first study to demonstrate endogenous mPR signaling in the ovarian follicle and to suggest a novel physiological role for mPRα in mediating the antiapoptotic actions of progestins in ovarian follicle cells.
Teleost granulosa and theca cell membrane-localized mPRα initiates inhibitory G-protein signaling and is necessary and sufficient to protect cells against serum-starvation induced death.
In addition to their classical genomic actions through activation of nuclear steroid receptors, progestins can also act at the cell surface to initiate rapid, nongenomic actions. For example, it was shown over 20 yr ago that progestins initiate final oocyte maturation in fish and amphibians via a nonclassical mechanism, although the identity of the membrane receptor mediating this nongenomic action remained unclear (1,2). A membrane progestin receptor (mPR) that is the likely intermediary in progestin induction of oocyte maturation in fish was first biochemically characterized in the ovaries of spotted seatrout, Cynoscion nebulosus (3). The receptor has high affinity, limited capacity, specific binding for the endogenous progestin 17,20β,21-trihydroxy-4-pregnen-3-one (20β-S) with ligand binding kinetics and a steroid specificity profile that are very different from those of the nuclear progesterone receptor (PR) characterized in the same species (3,4,5), suggesting the presence of a novel receptor protein. A cDNA unrelated to any known hormone receptor was subsequently discovered in a spotted seatrout ovarian cDNA library with the characteristics of the receptor mediating progestin induction of oocyte maturation in this species and was named mPR (6). The recombinant seatrout mPRα (stmPRα) protein produced in a mammalian cell line specifically binds 20β-S with high affinity [dissociation constant (Kd) = 7.6 nm] and activates pertussis toxin (PTX)-sensitive inhibitory G proteins (Gi) (7). Phylogenetic analyses indicate that the multiple mPR isoforms identified in humans and other vertebrate species are members of the progestin and adiponectin Q receptor protein family (8,9). The mPRs have been identified on the oocyte plasma membranes of several teleosts, including spotted seatrout (6), Atlantic croaker (10), zebrafish (11), goldfish (12), and an amphibian, Xenopus (13), and mPR mRNAs have been detected in catfish (14), sheep (15), and human ovaries (16). Of particular interest are reports of mPRα mRNA in the corpus luteum of rat (17) and sheep (15), suggesting the presence of mPRα in ovarian endocrine cells in addition to its localization in oocytes. However, there are no reports showing that mPRs are present or functional in ovarian follicle cells, although recent reports of rapid, nongenomic actions of progestins in granulosa/luteal cells from a variety of species (18,19,20,21) suggest the presence of mPRs in these cells.
Progesterone treatment increases granulosa cell survival in quail (22), cow (23,24), rat (18,20,25), and primates (21), including humans (19), yet the mechanism of progesterone’s antiapoptotic action remains unclear. Although this progestin action has been attributed to the presence of PR in some cell models (22,23,24,25), it has also been demonstrated in cells that lack PR activity (18,19,20,21). An alternative mediator of progesterone’s antiapoptotic actions, the mPR, has not been investigated to date. Atlantic croaker, Micropogonias undulatus, is a well-characterized teleost model for studies on the hypothalamic-pituitary-gonadal reproductive axis, ovarian steroidogenesis, and oocyte maturation. Croaker oocytes are surrounded by monolayers of granulosa and theca cells (G/T cells), which can be separated and cocultured, making it an excellent model for examining the actions of progestins on teleost follicular cells independent of the oocyte (26).
The presence of mPRα in Atlantic croaker G/T cells and the signal transduction pathways activated by progestins in these cells were investigated in this study. We also examined the role of progestins in serum starvation-induced follicle cell death in croaker to determine whether this progestin action is conserved across vertebrates. Investigations of mPR-specific functions in reproductive tissues are often complicated by the presence of PRs in the same cell types (17,27,28). The recent identification of an mPR-selective agonist, 10-ethenyl-19-norprogesterone (Org OD 02-0) (29), together with the PR-selective agonist, R5020 (7,27), for the first time provides pharmacological tools to distinguish progestin actions mediated by these two receptors. These selective mPR and PR agonists as well as selective knockdown of mPR or PR with small interfering RNA (siRNA) were used in this study to identify the major PR present on the surface of croaker G/T cells and its involvement in mediating the antiapoptotic effects of progestins.
Materials and Methods
Chemicals
Chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise noted. 20β-S, 17,20β-dihydroxy-4-pregnen-3-one (17,20β-P), estradiol-17β, testosterone, and cortisol were purchased from Steraloids (Newport, RI). R5020 was purchased from PerkinElmer (Waltham, MA). Org OD 02-0 and 19a-methylprogesterone (Org OD 13-0) were gifts from MSD (Oss, The Netherlands). Radiolabeled 20β-S and 17,20β-P precursors, [1,2, 3H] 11-deoxycortisol and [1,2,6,7 3H] 17α hydroxyprogesterone, were purchased from American Radiolabeled Chemicals (St. Louis, MO) and PerkinElmer NEN Life Science Products (Waltham, MA), respectively, and converted into [1,2, 3H] 20β-S and [1,2,6,7 3H] 17,20β-P with 20β-hydroxysteroid dehydrogenase as described previously (30).
Animal care and cell culture
Adult Atlantic croaker were purchased from local bait shops near Port Aransas (TX) during the reproductive season and maintained in 11,000-liter recirculating filtered seawater tanks at 24 C with a 13-h light, 11-h dark cycle photoperiod at the University of Texas Marine Science Institute. Fish were fed a diet of commercial pellets and shrimp daily.
Croaker were humanely killed according to National Institutes of Health guidelines by procedures approved by the University of Texas at Austin Institutional Animal Care and Use Committee. Primary G/T cell cultures were obtained as described previously (26).
Reverse transcription-polymerase chain reaction
G/T cells were harvested directly from culture in Tri-Reagent, and RNA was isolated following the manufacturer’s protocols and deoxyribonuclease treated (Zymo Research, Orange, CA). Reverse transcriptase (RT) was performed on 1 μg total RNA using Platinum Superscript II Reverse Transcriptase (Invitrogen, Carlsbad, CA) and oligo(dT)s. PCR was performed using Platinum PCR SuperMix High Fidelity (Invitrogen). Primers were designed against croaker mPRα (GenBank accession no. EU095257; sense, 5′GCTGGCGCTACTACTTTCTCA 3′ and antisense, 5′GGCAGCAGAAGAAATAGGCG 3′), croaker PR (Patiño, R., personal communication; sense, 5′GGCTCCTTTTCGTCTTTGATG 3′ and antisense, 5′CCTGATTGAAGCTGGGTACAGT 3′), zebrafish progesterone receptor membrane component 1 (PGRMC1) (GenBank accession no. BC085558; sense, 5′CGCTGCCCAAACTCAAGA 3′ and antisense, 5′GTTTGGGTCCGCTCTAATC 3′), and croaker 18S (sense, 5′GTTAATTCCGATAACGAACGAGACTC 3′ and antisense, 5′ACAGACCTGTTATTGCTCAATCTCGTG 3′) following the manufacturer’s instructions.
Western blot analyses
G/T cells were isolated and plated and harvested the day after in ice-cold PBS. Cells were sonicated (setting 2.5, 550 Sonic Dismembrator; Fisher Scientific, Hampton, NH) on ice in the presence of aprotinin. Lysates were centrifuged at 500 × g for 7 min at 4 C. The resulting supernatant was centrifuged at 20,000 × g for 20 min, the pellet containing the plasma membrane fraction was resuspended in reducing loading buffer (Pierce, Rockford, IL) and boiled. Membrane protein (15 μg) was run on a SDS-PAGE and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). Membranes were blotted for mPRα using an IgG-purified spotted stmPRα antibody against the N-terminal peptide sequence YRQPDQSWRYYFLTL, which is identical in seatrout and croaker (GenBank accession nos. AF262028 and EU095257, respectively). Antibody specificity was evaluated by preincubation with the antigen (3 μg peptide/μl antibody) before use. Membranes were incubated overnight at 4 C with antibody or preblocked antibody (1:500). Protein was visualized after incubation with horseradish peroxidase-linked goat antirabbit antibody (Abcam, Cambridge, MA) using SuperSignal West Pico chemiluminescent substrate (Pierce) on enhanced chemiluminescence hyperfilm (Amersham, Piscataway, NJ). Membranes were stripped and probed for pan-cadherin (1:1000) (Cell Signaling, Beverly, MA).
Erk and serine-threonine kinase (Akt) activation assay
G/T cells were cultured overnight then serum starved for 24–48 h before steroid treatment. Cells were harvested directly into 1× reducing loading dye. Samples were boiled, run on a SDS-PAGE, and transferred to polyvinylidene fluoride membranes (Bio-Rad). Membranes were probed using antibodies directed against total p42/44, phospho-p42/44 (Cell Signaling), total Akt, or phospho-Akt (Cell Signaling). ImageJ (NIH) was used to quantify band density.
Immunocytochemistry
G/T cells were grown on glass cover slides. All steps were conducted at 4 C. Cells were fixed in 2% (wt/vol) paraformaldehyde, blocked in 3% BSA in PBS for 1 h, and incubated for 1 h with mPRα or pan-cadherin primary antibody (1:5000) followed by incubation with Alexa Fluor 488 or Alexa Fluor 647-linked goat antirabbit secondary antibody (Molecular Probes, Eugene, OR) [1:5000 in PBS with 3% (wt/vol) BSA] for 1 h and stained with 300 nm 6-diamidino-2-phenylindole for 5 min. Slides were mounted using ProLong Gold Antifade Reagent (Molecular Probes) and visualized using Nikon Eclipse E600 fluorescent or Nikon C1 confocal microscopes (Nikon, Melville, NY). Granulosa cells, identified previously by 3βHSD staining (26), were distinguished from theca cells by their morphology, which is more rounded than that of theca cells, which are elongated.
[35S] GTPγS binding to G/T cell membranes
G/T cells were isolated from croaker ovaries and used immediately for the [35 S] GTPγS binding assay as previously described (7).
PR binding assays
The mPR binding assay was conducted following procedures described previously (3). The influence of PTX (List Biological, Campbell, CA) on [3H] 20β-S binding was determined by preincubating G/T cells with 6 μg/ml media activated PTX or heat-inactivated PTX (hiPTX) (31) at 18 C for 20 min before membrane isolation. Competitive binding to PR was conducted as described previously (5).
Coimmunoprecipitation of G protein αi-subunit with mPRα
Cultured G/T cells were harvested in ice-cold buffer (25 mm HEPES; 10 mm NaCl; 1 mm dithioerythritol; 1 mm EDTA, pH 7.6) and briefly sonicated. Plasma membranes were isolated as previously described, resuspended (2 mg/ml protein) in binding buffer with 100 μm GTP and 10 μm GDP and incubated with or without 20β-S for 45 min at 4 C. Membranes were pelleted by centrifugation and resuspended in 500 μl solubilization buffer [150 mm NaCl, 5 mm EDTA, 0.1% (wt/vol) SDS, and 1% (vol/vol) Triton X-100 (pH 7.4)] and incubated at 4 C. Samples were centrifuged and supernatents incubated with Gαi,o,t,z antibody (dilution of 1:200; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for 2 h. Protein A/G coated beads (Santa Cruz Biotechnology, Inc.) were added and incubated overnight. Beads were washed with PBS and resuspended in 1× reducing Western loading dye (Pierce) and boiled for 10 min. Samples were Western blotted for mPRα.
Cyclic AMP assay
Cells were cultured overnight then serum starved for 36 h. Cells were washed twice with PBS, pretreated with serum-free medium containing 10 μm 3-isobutyl-1-methylxanthine for 20–30 min, followed by the addition of 100 nm 20β-S or vehicle. Samples were harvested according to the manufacturer’s instructions, and cAMP levels were measured using an EIA kit (Cayman Chemical, Ann Arbor, MI). For PTX experiments, cells were preincubated for 30 min with 2 μm forskolin in the presence or absence of 0.5 μg/ml activated PTX or hiPTX (List Biological) (31); 100 nm 20β-S or ethanol was added and incubated for an additional 20 min. Cell extracts were processed for cAMP measurement as described previously.
Serum starvation-induced cell death
Cell death assay protocols were adapted from those described for breast cancer cells (32). G/T cells were cultured at confluence overnight. The cells were treated with serum-free DMEM with ethanol, or various steroids (1–100 nm) and incubated for 5–8 d without media change with additional steroid added every 2 d. At the end of the experiment, the combined media and adherent cells were pelleted. The pellet was resuspended in Hanks’ saline. A total of 500 cells from each sample was scored for viability using trypan blue exclusion (33). This experiment was performed three times with similar results. In other experiments, cells were stained for DNA fragmentation [terminal deoxynucleotidyl transferase biotin- 2′-deoxyuridine 5′-triphosphate nick end labeling (TUNEL)[ using the ApoAlert DNA Fragmentation Assay kit (CLONTECH, Mountain View, CA) per the manufacturer’s instructions. TUNEL-labeled cells were counted for five random fields of view on a Nikon Eclipse E600 fluorescent microscope. The experiment was repeated three times with similar results.
MDA-MB-231 cells stably transfected with vector or stmPRα, described previously (6,7), were cultured in phenol red-free DMEM with 10% fetal bovine serum and G418 (genetica to 100% confluence). The cells were washed twice with PBS and incubated with serum-free media with G418 and 10 nm 20β-S, 10 nm R5020, or ethanol control for 48 h. The cells were harvested in Hanks’ saline, and trypan blue staining was used to determine the proportion of live and dead cells. The experiment was repeated three times with similar results.
siRNA transfection and cell death
Cells were isolated and plated as previously described. One day after isolation, the cells were transfected with 100 nm siRNA (directed against croaker mPRα, croaker PR, or a control pool; Dharmacon, Lafayette, CO) using Lipofectamine 2000 (Invitrogen). Transfection was repeated the day after, and cells were treated with either starvation media or starvation media supplemented with steroid. After a 3- to 5-d incubation period, the cells were harvested as previously described and scored for cell survival.
Statistical analyses
One-way ANOVA with either Dunnett’s multiple comparison or Bonferroni’s multiple comparison was used to determine statistical differences between control and experimental treatments using GraphPad Prism (GraphPad Software, San Diego, CA). Square root or log transformations of the data were used as indicated to remove significant differences in variance. Saturation curves of [3H] progestin binding were analyzed by nonlinear regression using the GraphPad Prism software. Affinity (Kd) and saturation (Bmax) of [3H] progestin binding were calculated from nonlinear curve fitting.
Results
PR expression and localization in G/T cell cocultures
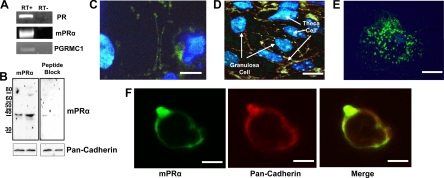
The presence of mPRα, PR, and PGRMC1 mRNA in croaker G/T cell cocultures was confirmed by RT-PCR, which amplified products of the predicted sizes (Fig. 1A). Western blot analysis of G/T cell membranes using an IgG-purified antibody directed against the N terminus of the seatrout and croaker mPRα resulted in the detection of a major immunoreactive band slightly larger than 40 kDa, as well as a minor band at 80 kDa, probably representing an mPR dimer (Fig. 1B, left panel). Peptide blocking the mPRα antibody resulted in a decrease in mPRα detection, indicating the specificity of the antibody (Fig. 1B, right panel). Immunocytochemistry using the antibody showed specific mPRα localization on the plasma membranes (Fig. 1C) where it colocalized with pan-cadherin (Fig. 1F) of both G/T cells, which can be distinguished by their morphological differences (Fig. 1D). Intracellular, perinuclear mPRα staining was also seen in some cells and is likely mPRα in the endoplasmic reticulum. Visualizing the cells under confocal microscopy revealed punctate staining of mPRα on the surface of the follicular cells (Fig. 1E). Treatment of cells with secondary antibody alone confirmed the specificity of the immunoreaction (data not shown).
Figure 1.
Presence of PRs and localization of mPRα in granulosa and theca cocultures. A, Detection of PR, mPRα, and PGRMC1 mRNA in isolated granulosa/theca cells using RT-PCR. RT plus and RT minus shown to confirm lack of DNA contamination. B, Western blotting of mPRα (top panels) in two G/T cell plasma membrane preparations using an IgG-purified antibody directed against an N-terminal mPRα peptide (left panel) and blocked with the peptide antigen (right panel). Bottom panels, Pan-cadherin loading control. C–E, Immunocytochemistry of G/T cell cultures using the mPRα antibody and 6-diamidino-2-phenylindole nuclear staining. Scale bar, 2.5 μm (C) and 10 μm (D). Confocal microscopy shows punctate mPRα staining on the cell surface. Scale bar, 5 μm (E). F, mPRα colocalizes with pan-cadherin on G/T cell membranes. Scale bar, 5 μm.
[3H]20β-S binding to G/T cell membranes
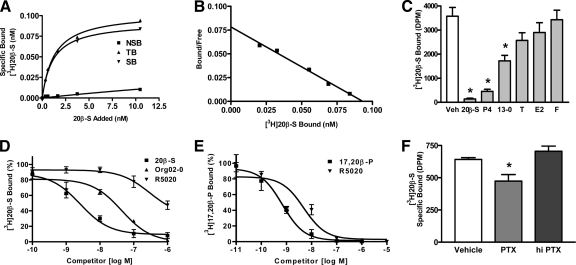
The progestin hormone in croaker, 20β-S, bound to the plasma membranes of croaker G/T cell cocultures with characteristics typical of steroid membrane receptors. Saturation binding analysis and Scatchard plotting revealed a single, high affinity (Kd = 1.7 nm 20β-S), limited capacity (Bmax = 0.0975 nm or 0.39 fmol/mg protein) progestin binding site on follicular plasma membranes (Fig. 2, A and B). Single-point competitive binding studies showed that the binding is specific for progestins; 100 nm 20β-S, progesterone, and the synthetic progestin Org OD 13-0 caused significant displacement of [3H] 20β-S from the binding site, whereas 100 nm testosterone displayed low affinity for the receptor, and estradiol-17β and cortisol were ineffective as competitors (Fig. 2C). Full-scale competition studies were performed with the selective PR agonist R5020, which has low binding affinity for seatrout and human mPR (hmPR)αs (7), to determine the likely identity of the G/T mPR, and with a selective hmPRα agonist Org OD 02-0, which has no PR agonist activity (29), to determine whether it binds to the G/T cells and can be used to distinguish progestin actions initiated by the mPR from those mediated by the PR. Competition for progestin binding sites on croaker G/T membranes showed that R5020 (EC50 = 297 nm) bound to the mPR with an affinity two orders of magnitude lower than 20β-S (EC50 = 2.6 nm) (Fig. 2D), whereas it showed a binding affinity for the G/T cytosolic receptor (EC50 = 6.3 nm) within one order of magnitude of that of the endogenous progestin 17,20β-P (EC50 = 0.73 nm) (Fig. 2E). These differing binding affinities for R5020 suggest that the PR on the surface of G/T cells is different from that of the PR in the cytosolic compartment and is likely mPRα. Therefore, low concentrations of R5020 (10–20 nm) should act as a selective agonist of PR-mediated progestin actions. The finding that Org OD 02-0 binds with relatively high affinity to the mPR (EC50 ∼ 40 nm) suggests that it can be used to investigate progestin agonist actions in croaker G/T cells mediated by mPRα. Preliminary results showing that Org OD 02-0 has mPRα agonist activity on progestin induction of oocyte maturation in another teleost model, zebrafish (34), support this hypothesis. Possible coupling of the mPR in G/T cells to Gi was investigated by examining specific binding of [3H] 20β-S to follicle cell membranes after uncoupling the G proteins by pretreatment with PTX. Preincubation of cells with activated PTX significantly decreased specific [3H] 20β-S binding by 30% compared with control and hiPTX groups (Fig. 2F), suggesting that the PR on G/T cell membranes is coupled to Gi.
Figure 2.
Characteristics of progestin binding in G/T cells. A and B, Representative saturation analysis (A) and Scatchard plot (B) of [3H] 20β-S binding to granulosa and theca coculture plasma membranes. TB, Total binding; NSB, nonspecific binding; SB, specific binding. Kd = 1.7 nm 20β-S, Bmax = 0.0975 nm or 0.39 fmol/mg protein. C, Single point competition with 100 nm competitors for [3H] 20β-S binding to plasma membranes of G/T cell cocultures. Data represent means disintegrations per minute/50 μg protein ± sem, n = 3; *, P < 0.001 compared with vehicle (Veh) control by one-way ANOVA and Dunnett’s multiple comparison test. P4, Progesterone; 13-0, Org OD 13-0; T, testosterone; E2, estradiol-17β; F, cortisol. D, Competition of 20β-S, Org OD 02-0, and R5020 for [3H] 20β-S binding to G/T plasma membranes expressed as a percentage of maximum specific 20β-S binding. Competition binding curves were generated from the average of three experiments. Org 02-0, Org OD 02-0. E, Competition with [3H] 17,20β-P binding to an ovarian cytosolic fraction by 17,20β-P and R5020 expressed as a percentage of maximum specific 17,20β-P binding. Competition binding curves were generated from the average of three experiments. F, Effect of 1 μg/ml PTX or hiPTX on [3H] 20β-S binding to G/T cell plasma membranes. Representative results from multiple experiments are shown. Data represent means disintegrations per minute/50 μg protein ± sem; *, P < 0.05 compared with vehicle control by one-way ANOVA and Dunnett’s multiple comparison test, n = 3.
G protein activation in response to 20β-S exposure
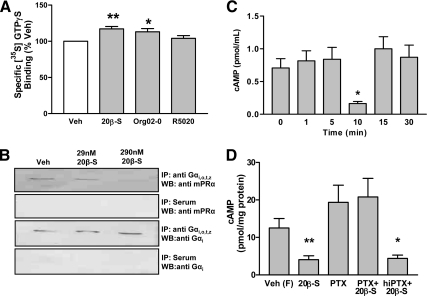
[35S] GTPγ-S binding to G/T cell membranes increased significantly after treatment with 100 nm 20β-S and 100 nm Org OD 02-0 above that measured in the vehicle-treated controls but was not altered in response to treatment with 100 nm R5020 (Fig. 3A). The results indicate that Org OD 02-0 mimics the action of 20β-S to activate G proteins, whereas R5020 does not activate a PR/G protein complex in the G/T cells.
Figure 3.
G protein activation and cAMP signaling in response to 20β-S treatment. A, Specific binding of [35S] GTPγS to plasma membranes of Atlantic croaker G/T cells in response to 100 nm progestin. Data are expressed relative to control values (100%) and represent means ± sem; **, P < 0.001, n = 7; *, P < 0.05, n = 3 compared with vehicle (Veh) control by one-way ANOVA and Dunnett’s multiple comparison test. Org 02-0, Org OD 02-0. B, Representative blot of coimmunoprecipitation of mPRα by anti-Gαi,o,t,z and effects of pretreatment with 20β-S. C and D, Effects of treatments with 100 nm 20β-S on whole-cell cAMP levels. C, Time course of changes in cAMP after 20β-S treatment. Data represent means ± sem; *, P < 0.01, compared with 0 time point by one-way ANOVA and Bonferroni’s multiple comparison test, n = 13. D, Effects of pretreatment with PTX and hiPTX on cAMP levels in response to 20β-S treatment in forskolin-treated cells. Data represent means ± sem of log transformed data to remove variance inequality. *, P < 0.05; **, P < 0.001, compared with vehicle control treated with forskolin alone (Veh F) by one-way ANOVA and Dunnett’s multiple comparison test, n = 6. IP, Immunoprecipitation; WB, Western blot.
Coimmunoprecipitation of mPRα with an Gi
mPRα coimmunoprecipitated with antibodies directed against Gαi,o,t,z (Fig. 3B). As predicted, the amount of mPRα protein coimmunoprecipitated with Gi decreased after pretreatment with a high concentration of 20β-S (290 nm) (Fig. 3B, lane 3), because hormonal activation and dissociation of the receptor/G protein complex depletes the pool of the mPRα/Gi protein complex available for coimmunoprecipitation (27). In contrast, the amounts of Gi immunoprecipitated after treatment with 29 nm 20β-S were similar to the no treatment controls (Fig. 3B, lane 2). Preimmune serum did not immunoprecipitate either mPRα (row 2) or Gi (row 4), confirming the specificity of the antibody precipitation reactions.
Cyclic AMP response to 20β-S exposure
20β-S caused a significant transient 5-fold decrease in cAMP levels in G/T cells at 10 min, which was observed in five separate experiments (Fig. 3C). Preincubation with PTX, but not hiPTX, blocked the inhibitory effect of 20β-S treatment on cAMP production by forskolin-treated G/T cells (Fig. 3D), which is consistent with progestin down-regulation of adenylyl cyclase activity through activation of Gi.
Alteration of G/T cell death by progestins
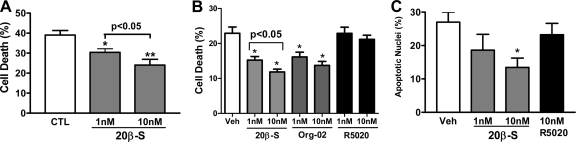
A concentration-dependent significant decrease in the incidence of follicular cell death, assessed by trypan blue exclusion, was observed after 5–8 d of treatment with 1 and 10 nm 20β-S in G/T cultures obtained from ovaries containing a high percentage of fully grown oocytes (Fig. 4A). Serum starvation-induced cell death was consistently attenuated after 7 d of treatment with 20β-S at these concentrations, whereas treatment of the cells with 1 and 10 nm R5020, which should activate PR but not mPR, did not affect the incidence of cell death (Fig. 4B). In contrast, the selective hmPRα agonist, Org OD 0-02, mimicked the actions of 20β-S, significantly decreasing the incidence of cell death over the concentration range of 1–10 nm (Fig. 4B). A higher concentration of 20β-S (100 nm) was also effective in decreasing the incidence of cell death (data not shown). TUNEL staining of G/T cells indicated that the number of cells that had undergone DNA cleavage was significantly reduced by treatment with 20β-S but not by treatment with R5020 (Fig. 4C).
Figure 4.
Effects of various progestins on serum starvation-induced cell death and apoptosis in G/T cell cultures. All data represent means ± sem. A, Changes in G/T cell serum starvation-induced death measured by trypan blue exclusion after 8 d of treatment with 1 and 10 nm 20β-S. *, P < 0.001; **, P < 0.0001 compared with vehicle control (CTL) by ANOVA and Dunnett’s multiple comparison test, n = 9. B, Changes in cell death after 7 d of treatment with various concentrations of 20β-S, Org OD 02-0, or R5020. *, P < 0.05, compared with vehicle control (Veh) by ANOVA and Dunnett’s multiple comparison test, n = 21. Org 02, Org OD 02-0. C, Percent TUNEL-stained nuclei after 7 d of treatment with various concentrations of 20β-S and R5020. *, P < 0.001, compared with vehicle control by ANOVA and Dunnett’s multiple comparison test, n = 20.
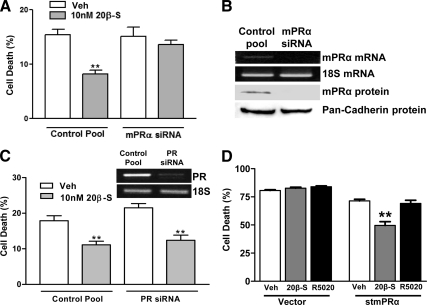
Reduction of mPRα mRNA and protein expression by siRNA directed against mPRα (Fig. 5B) abrogated progestin’s ability to inhibit serum starvation-induced death (Fig. 5A), whereas control siRNA did not alter the progestin response. In contrast, siRNA directed against croaker PR did not alter the progestin-induced decrease in cell death (Fig. 5C). Treatment of serum-starved MDA-MB-231 cells stably transfected with the stmPRα with 20β-S for 48 h caused a significant decrease in cell death compared with the vehicle-treated controls, whereas R5020 was ineffective (Fig. 5D). In contrast, 20β-S treatment did not alter cell death in cells transfected with the empty vector. Taken together, these data suggest that progestin inhibition of cell death occurs via a progestin-induced decrease in the incidence of DNA fragmentation mediated by mPRα activation.
Figure 5.
Effects of stmPRα and PR levels on progestin inhibition of serum starvation-induced cell death. All data represent means ± sem. A, G/T cell serum starvation-induced death in response to 20β-S after transfection with siRNA directed against croaker mPRα or control pool after 6 d of treatment with 10 nm 20β-S. **, P < 0.05 compared with vehicle (Veh) control by one-way ANOVA with Dunnett’s multiple comparison, n = 9. B, Croaker mPRα mRNA and protein levels after treatment with siRNA directed against mPRα or control pool siRNA. C, G/T cell serum starvation-induced death in response to 20β-S after transfection with siRNA directed against croaker PR or control pool after 8 d of treatment with 10 nm 20β-S. Inset shows PCR demonstrating reduced levels of PR mRNA. **, P < 0.05 compared with vehicle control by one-way ANOVA with Dunnett’s multiple comparison, n = 9. D, Changes in the rate of serum starvation-induced cell death of MDA-MB-231 cells stably transfected with stmPRα or vector alone in response to a 48-h exposure to 10 nm 20β-S or R5020. **, P < 0.001 compared with vehicle control by one way ANOVA with Dunnett’s multiple comparison, n = 6.
Erk and Akt activation by 20β-S exposure
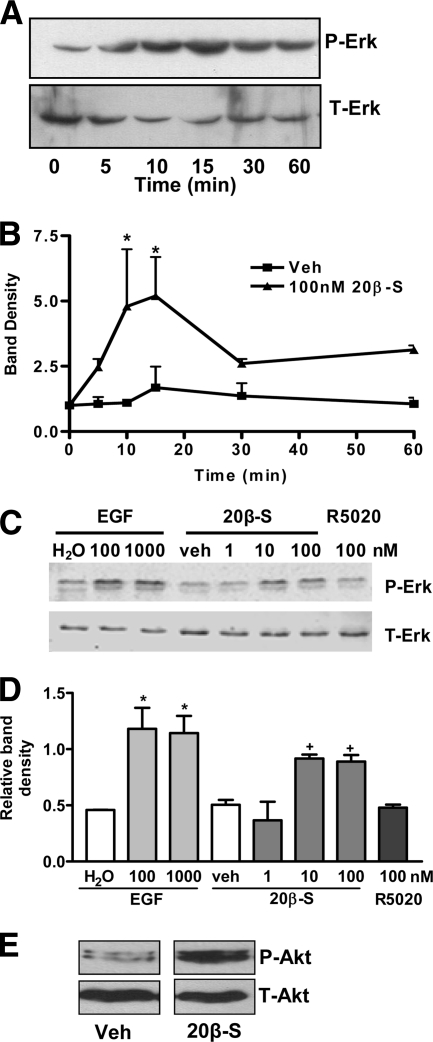
Inhibition of apoptosis in mammalian cells may involve activation of Erk and Akt (35). Exposure of follicular cells to 100 nm 20β-S resulted in increased Erk phosphorylation beginning at 10 min (Fig. 6A). Mean Erk activation was increased 5-fold over initial (time 0) levels after 10- and 15-min treatments with 100 nm 20β-S (Fig. 6B). Treatment with 20β-S for 10 min, but not with R5020, resulted in an increase in phospho-Erk compared with the vehicle-treated control (Fig. 6, C and D). Mammalian epidermal growth factor was used as a positive control. Western blotting for phospho-Akt and total Akt showed that 20β-S treatment increased Akt phosphorylation compared with that observed with the vehicle-treated controls (Fig. 6E).
Figure 6.
Effects of various progestin treatments on activation of Erk and Akt in granulosa and theca cocultures. P-Erk, Phosphrylated Erk; T-Erk, total Erk; P-Akt, phosphorylated Akt; T-Akt, total Akt. A, Representative blot showing Erk phosphorylation after 100 nm 20β-S treatment for various periods. B, Band density shown as percent time 0 adjusted for total Erk protein of treated and control cell extracts. Data represents mean percent band density to time 0 adjusted to total p42/44 ± sem; *, P < 0.05 compared with time 0 by one-way ANOVA and Dunnett’s multiple comparison test on square root transformed data, n = 3. C, Representative blot showing Erk activation in G/T cells after 15 min of treatment with 1, 10, or 100 nm 20β-S, 100 nm R5020, 100 or 1000 nm epidermal growth factor (EGF), or vehicles (Veh). D, Band density of P-Erk divided by band density of T-Erk. Average of three experiments. * and +, P < 0.05 compared with respective vehicle and water controls. E, Representative blot showing Akt phosphorylation in response to a 60-min exposure to 50 nm 20β-S.
Discussion
The results of this study provide the first evidence in vertebrates that mPRα is present on the surface of ovarian follicle cells and functions as an intermediary for the antiapoptotic actions of progestins in G/T cells. The PR detected on G/T cells has the binding characteristics of mPRα, including low binding affinity for the PR agonist, R5020 (7,27,36), and high binding affinity for the selective hmPR agonist, Org OD 02-0 (29). Org OD 02-0 was also effective in mimicking the protective effects of 20β-S on serum-starved cell death. Moreover, these protective progestin effects were abrogated in G/T cells when mPRα protein levels were decreased by mPRα siRNA. A role for mPRs in mediating progestin’s protective effects on cell death has not previously been described in any vertebrate cells. PR was also detected in G/T cells, but the results of experiments using the PR-selective agonist R5020, and siRNA directed against PR, indicate that it is not involved in this antiapoptotic action of progestins.
Immunocytochemical studies clearly show that mPRα protein localizes at the periphery of both G/T cells, and Western blot analysis confirms the presence of the receptor in the plasma membrane fraction. The punctuate pattern of mPRα labeling on the surface of croaker follicular cells is typical of that observed with 7-transmembrane (7-TM) receptors associated with clathrin-coated pits and is consistent with recent evidence for an involvement of a clathrin-dependent mechanism in mPRα internalization in human immortalized myometrial (M11) cells (37). Endocytosis of 7-TM receptors, including mPRα in M11 cells, results in their accumulation intracellularly (37). 7-TM receptors are trafficked to the plasma membrane via the endoplasmic reticulum, which often retains the majority of the receptor protein (38). Therefore, it is not surprising that mPRα was also detected intracellularly in the perinuclear region of croaker G/T cells, where the endoplasmic reticulum is located. The presence of mPRα in both G/T cells suggests that the receptor has important biological functions in both follicular cell types. Identification of progesterone binding sites on plasma membranes of bovine theca and granulosa cells (39) raises the possibility that mPRα is also present in mammalian follicular cells. In support of this, we have identified mPRα in spontaneously immortalized rat granulosa cells (our unpublished data).
Several lines of evidence indicate that the progestin binding moiety on G/T cell membranes is mPRα. The G/T cell receptor has a single binding site with high affinity for 20β-S (Kd = 1.7 nm), similar to that of stmPRα in ovarian membrane preparations (Kd = 1.5 nm) (3) and mammalian expression systems (Kd = 7.58 nm) (7). Association of [3H] 20β-S with the receptor is rapid, like that with the recombinant stmPRα (7), and reaches equilibrium within 30 min, whereas the rate of association of progestin binding to the seatrout PR is much slower, and a 6-h incubation is required to reach equilibrium (5). The steroid specificity for the G/T PR is similar to that previously observed with recombinant stmPRα (6,7). In addition, two synthetic progestins, Org OD 02-0 and Org OD 0-13, which have recently been identified as mPRα-selective agonists with high binding affinities for recombinant hmPRα (29), are agonists on mPRα-mediated induction of oocyte maturation in zebrafish (34) and are also effective competitors for 20β-S binding to G/T cells. In contrast, the steroid binding characteristics of the G/T cell membranes differ from those of PR and PGRMC1, which were also identified by RT-PCR in croaker ovarian follicle cells. The PR agonist, R5020, displays a much higher affinity for the cytosolic PR in G/T cells than it does for the PR on the cell membranes of these cells. R5020 has low binding affinity for recombinant stmPRα and hmPRα (7). Recombinant mammalian PGRMC1 and partially purified PGRMC1 display 30–40 times lower affinity binding to progestins than that observed to the G/T membranes, with Kds in the 200 to 300 nm range (20,40,41), and also bind cortisol with high affinity (42). Collectively, the results provide strong evidence that the PR on croaker G/T cell membranes is mPRα.
The signaling characteristics of the mPR on G/T cells, involving activation of Gi and decreases in cAMP production, are characteristic of mPRα (43). A higher concentration of progestins (100 nm) was used in this study to demonstrate activation of G proteins and second messengers than those used (1–10 nm) to investigate physiological responses, because our previous studies have shown that 100 nm progestin treatments are required to significantly increase G protein activation above the high constitutive levels of activity (7,27,29,36). However, this higher progestin concentration is likely to be within the physiological range of hormone levels in croaker ovaries, because they synthesize large amounts of these steroids. The data show that mPR agonists increase GDP/GTP exchange (a hallmark of G protein activation) and mPRα is immunoprecipitated using antibodies directed against Gi. These results are consistent with previous findings demonstrating that both endogenous stmPRα in oocytes and recombinant stmPRα in mammalian cells, as well as endogenous hmPRα present in myometrial cells and overexpressed in breast cancer cells, activate G proteins and are coimmunoprecipitated with Gi (7,27,43). Blockade of progestin-induced cAMP reduction by activated PTX provides additional evidence that mPRα in G/T cells is coupled to Gi. These current data corroborate previous findings with recombinant stmPRα and hmPRα in mammalian cells (7), and with hmPRα and hmPRβ in myometrial cells (27). The decrease in [3H] 20β-S binding to G/T cell membranes after PTX treatment is additional evidence for a direct coupling between mPR and Gi, because PTX ribosylates the ADP on inhibitory α G protein subunits resulting in Gi inactivation, its uncoupling from the receptor, and a decrease in receptor ligand binding affinity (44). PTX inhibition of progestin binding has previously been demonstrated in seatrout ovarian membranes (45), and with recombinant stmPRα and hmPRα (7).
Low physiological levels of progestins (10 nm) clearly inhibited teleost G/T cell death in a manner and magnitude similar to previous reports in tetrapods (18,19,20,21,22,23,24,25), suggesting that this is a conserved action of progestins in vertebrates. The protective effects of progestins observed in other species around the periovulatory period may also occur in teleost ovarian follicles when a high percentage of oocytes are fully grown and ready to undergo meiotic maturation and release. Croaker are batch spawners, and we hypothesize that fully grown ovarian follicles are protected from apoptosis in croaker by the presence of 20β-S just before spawning, allowing for the synchronization of oocyte maturation and enabling the fish to spawn the greatest number of fertilizable eggs. Interestingly, apoptosis of the ovarian follicle is critical in the reabsorption of the postovulatory follicle (46), as well as in atresia of excess vertebrate oocytes (47), and thus, the absence or decline in progestins in croaker after spawning may increase apoptosis of the follicle cells and reabsorption of the oocytes. However, in vivo studies will be required to test these hypotheses.
Experiments aimed toward determining the identity of the PR mediating progestin’s protective effects indicate that mPRα is necessary and sufficient to inhibit apoptosis under serum starvation conditions. Repression of mPRα protein synthesis resulted in a corresponding loss in progestin protection from serum starvation-induced death, indicating that mPRα is necessary for progestin’s protective actions. In contrast, siRNA directed against croaker PR had no effect on progestin’s protective characteristics. Experiments demonstrating that human cells showed decreased cell death in response to 20β-S after they had been transfected with stmPRα provide further support for a role for mPRα in mediating progestin inhibition of apoptosis. A functional teleost mPRα is present in these transfected human breast cancer cells as indicated by the presence of high amounts of specific [3H] 20β-S binding and 20β-S activation of intracellular signaling pathways (7). It is concluded from these studies that mPRα mediates 20β-S’s protective properties.
A potential mechanism for mPR inhibition of apoptosis in G/T cells is via activation of Erk and Akt. Previous studies demonstrated that progestins activate Akt and Erk in croaker oocytes (48) and mPR activates Erk in human cell lines stably expressing stmPRα (6) or zebrafish mPRα and mPRβ (11). Both Erk and Akt activation directly and indirectly inhibit apoptosis in mammals (35). Activated Erk up-regulates the expression of antiapoptotic members (49) and inactivates the proapoptotic members (50) of the Bcl-2 family of proteins. Akt directly inhibits apoptosis through inhibition of the proapoptotic Bcl-2 family member BAD (BCL2 antagonist of cell death) (51) and caspase-9 (52), an upstream mediator of apoptosis.
Alternate mechanisms and outcomes of progesterone action on cell survival have been reported in reproductive tissues. High micromolar concentrations of progestins cocultured with 10% fetal calf serum caused apoptosis of human PR-negative uterine carcinoma cells (53). The high concentrations of progestins, the presence of growth factor signaling, and the transformed nature of the cells may explain the different responses observed in carcinoma cells compared with those seen in nontransformed croaker G/T cells. Interestingly, PR has been implicated in mediating the antiapoptotic actions of progesterone on granulosa/luteal cells isolated from rat ovaries after the LH surge (54,55). It is possible that the receptor mechanism mediating this progesterone action of changes after the LH surge because it initiates extensive changes in granulosa cells, including the up-regulation of PR.
Membrane PR-mediated cell survival of somatic cells in female vertebrates has potentially broad and far-reaching implications. To date, mPRα has been detected in all the human cell types or human-derived cell lines examined (our unpublished data). mPR expression is particularly relevant in women, where circulating progesterone levels would regularly activate the mPR. This has significance in ovarian steroid-driven cancers of the breast. Indeed, mPRα is present in human breast cancer cell lines irrespective of nuclear receptor expression (28). Progesterone-mediated cell survival of breast cancer cells via mPR activation, particularly where the PR is not detected, could have implications for current breast cancer treatment protocols and warrants further study.
In conclusion, this study demonstrates the presence of mPR and its membrane localization and signaling in the ovarian follicle of a vertebrate species. The results show that progestins also inhibit apoptosis of ovarian follicle cells in a teleost fish species, similar to their actions in mammals and birds, suggesting that this is a fundamental, evolutionarily conserved function of progestins in vertebrates. Additionally, and perhaps most importantly, this work is the first to suggest a novel antiapoptotic role for the mPR in the ovarian follicle and to link the mPR to apoptotic processes. Additional studies are needed to elucidate the signaling mechanisms leading from progestin activation of mPR to inhibition of DNA cleavage and apoptosis and to examine the potential interactions of the mPR, PR, and PGRMC1 in ovarian follicular apoptosis.
Acknowledgments
We thank the assistance of Susan Lawson with fish care.
Footnotes
This work was supported by the National Institutes of Health Grant ESO 12961 (to P.T.). This work was also supported by a Lund Fellowship (G.E.D.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 20, 2010
Abbreviations: Akt, Serine-threonine kinase; Bmax, saturation; Gi, inhibitory G protein; G/T cell, granulosa and theca cell; hiPTX, heat-inactivated PTX; hmPRα, human mPRα; Kd, dissociation constant; mPR, membrane progestin receptor; Org OD 02-2, 10-ethenyl-19-norprogesterone; Org OD 13-0, 19a-methylprogesterone; 17,20β-P, 17,20β-dihydroxy-4-pregnen-3-one; PGRMC1, progesterone receptor membrane component 1; PR, progesterone receptor; PTX, pertussis toxin; 20β-S, 17,20β,21-trihydroxy-4-pregnen-3-one; RT, reverse transcriptase; siRNA, small interfering RNA; stmPRα, seatrout mPRα; 7-TM, 7-transmembrane; TUNEL, terminal deoxynucleotidyl transferase biotin- 2′-deoxyuridine 5′-triphosphate nick end labeling.
References
- Masui Y, Markert CL 1971 Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool 177:129–145 [DOI] [PubMed] [Google Scholar]
- Thomas P, Zhu Y, Pace M 2002 Progestin membrane receptors involved in the meiotic maturation of teleost oocytes: a review with some new findings. Steroids 67:511–517 [DOI] [PubMed] [Google Scholar]
- Patiño R, Thomas P 1990 Characterization of membrane receptor activity for 17α,20β,21-tryhydroxy-4-pregnen-3-one in ovaries of spotted seatrout (Cynoscion nebulosus). Gen Comp Endocrinol 78:204–217 [DOI] [PubMed] [Google Scholar]
- Pinter J, Thomas P 1997 The ovarian progestogen receptor in the spotted seatrout, Cynoscion nebulosus, demonstrates steroid specificity different from the progesterone receptors in other vertebrates. J Steroid Biochem Mol Biol 60:113–119 [DOI] [PubMed] [Google Scholar]
- Pinter J, Thomas P 1995 Characterization of a progestogen receptor in the ovary of the spotted sea-trout, Cynoscion Nebulosus. Biol Reprod 52:667–675 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P 2003 Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA 100:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Dong J, Groenen P, Kelder J, de Vlieg J, Zhu Y, Tubbs C 2007 Steroid and G protein binding characteristics of the seatrout and human progestin membrane receptor α subtypes and their evolutionary origins. Endocrinology 148:705–718 [DOI] [PubMed] [Google Scholar]
- Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD 2005 PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol 61:372–380 [DOI] [PubMed] [Google Scholar]
- Lyons TJ, Villa NY, Regalla LM, Kupchak BR, Vagstad A, Eide DJ 2004 Metalloregulation of yeast membrane steroid receptor homologs. Proc Natl Acad Sci USA 101:5506–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubbs C, Pace M, Thomas P 2010 Expression and gonadotropin regulation of membrane progestin receptor α in Atlantic croaker (Micropogonias undulatus) gonads: role in gamete maturation. Gen Comp Endocrinol 165:144–154 [DOI] [PubMed] [Google Scholar]
- Hanna R, Pang Y, Thomas P, Zhu Y 2006 Cell-surface expression, progestin binding, and rapid nongenomic signaling of zebrafish membrane progestin receptors α and β in transfected cells. J Endocrinol 190:247–260 [DOI] [PubMed] [Google Scholar]
- Tokumoto M, Nagahama Y, Thomas P, Tokumoto T 2006 Cloning and identification of a membrane progestin receptor in goldfish ovaries and evidence it is an intermediary in oocyte meiotic maturation. Gen Comp Endocrinol 145:101–108 [DOI] [PubMed] [Google Scholar]
- Josefsberg Ben-Yehoshua L, Lewellyn AL, Thomas P, Maller Jl 2007 The role of Xenopus membrane progesterone receptor β in mediating the effect of progesterone on oocyte maturation. Mol Endocrinol 21:664–673 [DOI] [PubMed] [Google Scholar]
- Kazeto Y, Goto-Kazeto R, Trant JM 2005 Membrane-bound progestin receptors in channel catfish and zebrafish ovary: changes in gene expression associated with the reproductive cycles and hormonal reagents. Gen Comp Endocrinol 142:204–211 [DOI] [PubMed] [Google Scholar]
- Ashley RL, Clay CM, Farmerie TA, Niswender GD, Nett TM 2006 Cloning and characterization of an ovine intracellular seven transmembrane receptor for progesterone that mediates calcium mobilization. Endocrinology 147:4151–4159 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bond J, Thomas P 2003 Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Stocco CO 2005 Expression and regulation of progestin membrane receptors in the rat corpus luteum. Endocrinology 146:5522–5532 [DOI] [PubMed] [Google Scholar]
- Telleria CM, Stocco CO, Stati AO, Deis RP 1999 Progesterone receptor is not required for progesterone action in the rat corpus luteum of pregnancy. Steroids 64:760–766 [DOI] [PubMed] [Google Scholar]
- Engmann L, Losel R, Wehling M, Peluso JJ 2006 Progesterone regulation of human granulosa/luteal cell viability by an RU486-independent mechanism. J Clin Endocrinol Metab 91:4962–4968 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Pappalardo A, Losel R, Wehling M 2006 Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology 147:3133–3140 [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL 2000 Role of gonadotrophins and progesterone in the regulation of morphological remodelling and atresia in the monkey peri-ovulatory follicle. Hum Reprod 15:2489–2495 [DOI] [PubMed] [Google Scholar]
- Mussche S, D'Herde K 2001 Contribution of progesterone, follicle stimulating hormone and glucocorticoids in survival of serum-free cultured granulosa cell explants. J Endocrinol 169:321–331 [DOI] [PubMed] [Google Scholar]
- Quirk SM, Cowan RG, Harman RM 2004 Progesterone receptor and the cell cycle modulate apoptosis in granulosa cells. Endocrinology 145:5033–5043 [DOI] [PubMed] [Google Scholar]
- Rueda BR, Hendry IR, Hendry III W, Stormshak F, Slayden OD, Davis JS 2000 Decreased progesterone levels and progesterone receptor antagonists promote apoptotic cell death in bovine luteal cells. Biol Reprod 62:269–276 [DOI] [PubMed] [Google Scholar]
- Svensson EC, Markström E, Andersson M, Billig H 2000 Progesterone receptor-mediated inhibition of apoptosis in granulosa cells isolated from rats treated with human chorionic gonadotropin. Biol Reprod 63:1457–1464 [DOI] [PubMed] [Google Scholar]
- Benninghoff AD, Thomas P 2006 Gonadotropin regulation of testosterone production by primary cultured theca and granulosa cells of Atlantic croaker: I. Novel role of CaMKs and interactions between calcium- and adenylyl cyclase-dependent pathways. Gen Comp Endocrinol 147:276–287 [DOI] [PubMed] [Google Scholar]
- Karteris E, Zervou S, Pang Y, Dong J, Hillhouse EW, Randeva HS, Thomas P 2006 Progesterone signaling in human myometrium through two novel membrane G protein coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol 20:1519–1534 [DOI] [PubMed] [Google Scholar]
- Dressing GE, Thomas P 2007 Identification of membrane progestin receptors in human breast cancer cell lines and biopsies and their potential involvement in breast cancer. Steroids 72:111–116 [DOI] [PubMed] [Google Scholar]
- Kelder J, Azevedo R, Pang Y, de Vlieg J, Dong J, Thomas P 2010 Comparison between steroid binding to the human progestin membrane receptor α subtype and to the human progestin nuclear receptor: correlations with physiochemical properties assessed by comparative molecular field analysis. Steroids 75:314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott AP, Sheldrick EL, Flint APF 1982 Measurement of 17α,20β-dihydroxy-4-pregnen-3-one in plasma of trout (Salmo gairdneri Richardson): seasonal changes and response to salmon pituitary extract. Gen Comp Endocrinol 46:444–451 [DOI] [PubMed] [Google Scholar]
- Kaslow HR, Lim LK, Moss J, Lesikar DD 1987 Structure-activity analysis of the activation of pertussis toxin. Biochemistry 26:123–127 [DOI] [PubMed] [Google Scholar]
- Moore MR, Spence JB, Kiningham KK, Dillon JL 2006 Progestin inhibition of cell death in human breast cancer cell lines. J Steroid Biochem Mol Biol 98:218–227 [DOI] [PubMed] [Google Scholar]
- Freshney RI 1994 Culture of animal cells: a manual of basic technique. 4th ed. New York: Wiley-Liss [Google Scholar]
- Thomas P, Harris C, Pang Y 2008 The roles of different types of progestin receptors in oocyte maturation of zebrafish. Biol Reprod (Sp Iss SI) 705:220 (Abstract) [Google Scholar]
- Song G, Ouyang G, Bao S 2005 The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9:59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleiter N, Pang Y, Park C, Horton TH, Dong J, Thomas P, Levine JE 2009 Progesterone receptor A (PRA) and PRB-independent effects of progesterone on gonadotropin-releasing hormone release. Endocrinology 150:3833–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster H, Reynolds A, Stenbeck G, Dong J, Thomas P, Karteris E 2009 Internalization of membrane progesterone receptor α (mPRα) after treatment with progesterone: potential involvement of a clathrin-dependent pathway. Mol Med Report 3:27–35 [DOI] [PubMed] [Google Scholar]
- Petaja-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M 2000 Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human δ opioid receptor. J Biol Chem 275:13727–13736 [DOI] [PubMed] [Google Scholar]
- Rae MT, Menzies GS, Bramley TA 1998 Bovine ovarian non-genomic progesterone binding sites: presence in follicular and luteal cell membranes. J Endocrinol 159:413–427 [DOI] [PubMed] [Google Scholar]
- Meyer C, Schmid R, Scriba PC, Wehling M 1996 Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem 239:726–731 [DOI] [PubMed] [Google Scholar]
- Peluso JJ, Fernandez G, Pappalardo A, White BA 2001 Characterization of a putative membrane receptor for progesterone in rat granulosa cells. Biol Reprod 65:94–101 [DOI] [PubMed] [Google Scholar]
- Cahill MA 2007 Progesterone receptor membrane component 1: an integrative review. J Ster Biochem Mol Biol 105:16–36 [DOI] [PubMed] [Google Scholar]
- Thomas P 2008 Characteristics of membrane progestin receptor α (mPRα) and progesterone membrane receptor component one (PGRMC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol 29:292–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West Jr RE, Moss J, Vaughn M, Lui T, Lui TY 1985 Pertussis toxin catalyzed ADP-ribosylation of transducin. Cysteine 347 is the ADP-ribose acceptor site. J Biol Chem 260:14428–14430 [PubMed] [Google Scholar]
- Pace MC, Thomas P 2005 Activation of a pertussis toxin-sensitive, inhibitory G-protein is necessary for steroid-mediated oocyte maturation in spotted seatrout. Dev Biol 285:70–79 [DOI] [PubMed] [Google Scholar]
- Drummond CD, Bazzoli N, Rizzo E, Sato Y 2000 Postovulatory follicle: a model for experimental studies of programmed cell death or apoptosis in teleosts. J Exp Zool 287:176–182 [DOI] [PubMed] [Google Scholar]
- Johnson AL 2003 Intracellular mechanisms regulating cell survival in ovarian follicles. Anim Reprod Sci 78:185–201 [DOI] [PubMed] [Google Scholar]
- Pace MC, Thomas P 2005 Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod 73:988–996 [DOI] [PubMed] [Google Scholar]
- Lin H, Chen C, Li X, Chen BD 2002 Activation of the MEK/MAPK pathway is involved in bryostatin 1-induced monocytic differentiation and up-regulation of X-linked inhibitor of apoptosis protein. Exp Cell Res 272:192–198 [DOI] [PubMed] [Google Scholar]
- She QB, Ma WY, Zhong S, Dong Z 2002 Activation of JNK1, RSK2, and MSK1 is involved in serine 112 phosphorylation of Bad by ultraviolet B radiation. J Biol Chem 277:24039–24048 [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME 1997 Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241 [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC 1998 Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318–1321 [DOI] [PubMed] [Google Scholar]
- Bertelsen EL, Endresen PC, Orbo A, Sager G 2004 Non-genomic cell growth inhibition by progesterone. cell cycle retardation and induction of cell death. Anticancer Res 24:3749–3755 [PubMed] [Google Scholar]
- Friberg PA, Larsson DG, Billig H 2009 Dominant role of nuclear progesterone receptor in the control of rat periovulatory granulosa cell apoptosis. Biol Reprod 80:1160–1167 [DOI] [PubMed] [Google Scholar]
- Friberg PA, Larsson DG, Rung E, Billig H 2007 Apoptotic effects of a progesterone receptor antagonist on rat granulosa cells are not mediated via reduced protein isoprenylation. Mol Reprod Dev 74:1317–1326 [DOI] [PubMed] [Google Scholar]