Abstract
During lactation, calcium is mobilized from the maternal skeleton to supply the breast for milk production. This results in rapid but fully reversible bone loss. Prior studies have suggested that PTHrP, secreted from the breast, and estrogen deficiency, due to suckling-induced central hypogonadism, combine to trigger bone resorption. To determine whether this combination was sufficient to explain bone loss during lactation, we raised PTHrP levels and decreased levels of estrogens in nulliparous mice. PTHrP was infused via osmotic minipumps and estrogens were decreased either by using leuprolide, a long-acting GnRH agonist, or by surgical ovariectomy (OVX). Bone mineral density declined by 23.2 ± 1.3% in the spine and 16.8 ± 1.9% in the femur over 10 d of lactation. This was accompanied by changes in trabecular architecture and an increase in both osteoblast and osteoclast numbers. OVX and PTHrP infusion both induced a modest decline in bone mineral density over 10 d, but leuprolide treatment did not. The combination of OVX and PTHrP was more effective than either treatment alone, but there was no interaction between PTHrP and leuprolide. None of the treatments reproduced the same degree of bone loss caused by lactation. However, both forms of estrogen deficiency led to an increase in osteoclasts, whereas infusion of PTHrP increased both osteoblasts and osteoclasts. Therefore, although the combination of PTHrP and estrogen deficiency contributes to bone loss, it is insufficient to reproduce the full response of the skeleton to lactation, suggesting that other factors also regulate bone metabolism during this period.
Parathyroid hormone-related protein (PTHrP) and estrogen deficiency contribute to lactational bone loss; however, infusing PTHrP and suppressing estrogens were insufficient to reproduce the effects of lactation on bone.
Lactation triggers rapid bone loss to provide calcium to the breast for milk production (1,2,3). Rodents typically lose 20–30% of their skeletal mass over 3 wk and nursing women lose 5–8% over 6 months (1,2,3). Lactation is also associated with deterioration in trabecular microarchitecture and a decrease in the mechanical integrity of bone (4,5). These changes in skeletal mass are associated with high bone turnover. Studies using biochemical markers as well as histomorphometry have demonstrated increased numbers of both osteoblasts and osteoclasts as well as increased rates of bone formation and resorption (1,4,6,7,8,9). As evidenced by the significant loss in bone mass, resorption outstrips formation during this time. Fortunately, weaning results in a rapid and full recovery of bone mass so that lactation does not result in the permanent loss of bone, and both rodents and women can reproduce multiple times without long-term damage to their skeletons (4,10,11,12,13).
Suckling induces hypogonadotropic hypogonadism, which results in low circulating levels of estrogens (14,15). The dominant effect of suckling appears to be the stimulation of afferent nerves from the breast, which relay through the hindbrain and inhibit GnRH secretion from the hypothalamus (14,15,16). In addition, suckling stimulates prolactin secretion and lactation is associated with low circulating levels of leptin, both of which reinforce the functional hypogonadism (14,15,17). Estrogen deficiency causes bone loss (18,19), and it has been suggested that withdrawal of estrogens after delivery is one of the main triggers for bone loss during lactation (1,3). This premise is supported by studies that used estrogen replacement. Mice treated with estradiol continued to lactate but lost only 50% of the expected amount of bone over a period of 12 d (9).
Elevated levels of circulating PTHrP also contribute to lactational bone loss (1,3). PTHrP is secreted into the bloodstream by the breast during lactation and circulating levels have been shown to correlate with rates of bone resorption and with bone loss in rodents and humans (1,9,20,21). When the PTHrP gene was disrupted specifically in lactating mammary epithelial cells, circulating levels of PTHrP were decreased and rates of bone loss were reduced by approximately 50% (21). Therefore, during lactation, the breast participates in mobilizing calcium from the maternal skeleton by secreting PTHrP, which then acts to increase bone resorption.
Estrogen deficiency and continuous exposure to PTH can act synergistically to increase bone turnover and accelerate bone loss (22,23,24). Because PTH and PTHrP share the use of a common G protein-coupled receptor (25), we wondered whether the combination of elevated PTHrP levels and decreased levels of estrogens observed during lactation might also synergize to drive bone loss during lactation. Furthermore, the fact that the removal of PTHrP and replacement of estrogens both inhibited bone loss by 50% (9,21) suggested to us that the combination of excess PTHrP and estrogen withdrawal might be sufficient to explain the bone loss seen during lactation. If this were the case, we reasoned that infusing PTHrP and suppressing estrogens in virgin mice might reproduce the pattern of bone loss noted during lactation. In this report, we demonstrate that although the combination of estrogen deficiency and PTHrP excess produces changes in bone architecture and bone turnover, it does not fully reproduce the effect of lactation on the skeleton.
Materials and Methods
Experimental animals and treatments
Female, 10- to 13-wk-old, CD1 virgin mice were purchased from Charles River Laboratories (Wilmington, MA). One group of mice was treated with PBS containing 100 μg of leuprolide acetate (McKesson, Northborough, MA) via sc injection, twice a day for 11 d. A second group received a continuous infusion of PTHrP (1–36) sc using Alzet miniosmotic pumps (model 2002, 14 d at 0.5 μl/h; Durect Corp., Cupertino, CA) at a rate of 10 pmol/h until the animals were killed on d 12. Lyophilized PTHrP (1–36) (a gift of Dr. Andrew Stewart, University of Pittsburgh, Pittsburgh, PA) was reconstituted with sterile water to 1.3 mm and diluted 1:60 with sterile 2% l-cysteine (pH 1.5) (Sigma-Aldrich, St. Louis, MO); loaded into the reservoir of the miniosmotic pump and preactivated in sterile saline at 37 C for several hours just before implantation beneath the interscapular skin. The leuprolide plus PTHrP group received PTHrP through the miniosmotic pump as above and was also treated with twice-daily injections of leuprolide acetate. Ovariectomy was performed by the Yale Veterinary Clinical Services. The OVX plus PTHrP mice had miniosmotic pumps delivering PTHrP placed at the time of OVX. These five groups of mice were compared with two control groups. First, placebo-treated mice received miniosmotic pumps that delivered 2% l-cysteine without PTHrP at equivalent flow rates to those delivering PTHrP and were also treated with twice-daily sc injections of sterile saline. The other control group consisted of age-matched CD1 female mice that were allowed to become pregnant, give birth, and lactate until the animals were killed on d 12. All animal experiments were reviewed and approved by the Yale institutional animal care and use committee.
Dual-energy x-ray absorptiometry (DEXA)
Bone mineral density (BMD) measurements were performed by DEXA using a Lunar PIXImus (GE Medical Systems, Lunar Division, Madison, WI) operated by the Yale Core Center for Musculoskeletal Disorders. Mice were anesthetized with 50 mg/kg ketamine (Ketaset III; Fort Dodge Animal Health, Fort Dodge, IA) and 10 mg/kg xylazine (AnaSed; Lloyd, Shenandoah, IA), by ip injection. BMD measurements included the entire thoracic and lumbar spine as well as the entire femur. The presence of the miniosmotic pumps precluded accurate measurement of total body BMD.
X-ray microcomputed tomographic (CT) imaging
Trabecular morphometry within the distal femur and the third lumbar vertebra (L3) was quantified using x-ray micro-CT (μCT35; Scanco Medical AG, Bruttisellen, Switzerland). Specimens were scanned in 70% ethyl alcohol at 55 kV (145 μA), using 1000 cone beam projections per revolution and an integration time of 300 msec within a 12.3-mm-diameter field of view. Three-dimensional images were reconstructed at a 6-μm isometric voxel size. Trabecular morphometry, with a density threshold of 255 mg/cm3, was characterized by measuring the bone volume fraction, trabecular thickness, trabecular number, trabecular spacing, connectivity density, tissue mineral density, and structure model index (SMI), a numerical measure of trabecular geometry representative of increasingly rod-like (higher SMI) or plate-like architecture (lower SMI). Cortical bone morphometry was averaged from 232 serial cross-sectional images (1.4 mm) centered at the longitudinal midpoint of the femur, applying a density threshold of 375 mg/cm3.
Bone histology and histomorphometry
Routine bone histology was performed on 4-μm, toluidine blue-stained, methylmethacrylate-embedded, nondecalcified sections of vertebra as previously described (4,9,21). Static and dynamic histomorphometric analysis of the lumbar vertebra was performed using the Osteomeasure system (OsteoMetrics, Inc., Decatur, GA).
Biochemical measurements
Serum calcium and estradiol concentrations were measured on blood collected from the retroorbital venous plexus into capillary tubes 10 or 11 d after the initiation of leuprolide treatment or PTHrP infusion. PTH and PTHrP plasma levels were measured on blood obtained by cardiac puncture in the presence of protease inhibitors (9,21) at the time the animals were killed on the 11th day of treatment. Urinary calcium was measured on urine collected at the time the animals were killed. All calcium concentrations were determined using a commercial assay (Quantichrom; BioAssay Systems, Hayward, CA) or in the case of the cohorts of mice receiving 5,10, or 20 pmol PTHrP per hour, by atomic absorptiometer (Yale New Haven Hospital Clinical Laboratories). Urinary calcium was corrected for creatinine determined by the picric acid method (QuantiChrom creatine assay; BioAssay Systems). PTH levels were measured using a commercial, two-site immunoradiometric assay for rat PTH (Immunotopics International, San Clemente, CA) as per the manufacturer’s instructions.
PTHrP (1-36) levels were measured in a two-site immunoradiometric assay similar to the assay previously described (26). We used the same capture antibody, an affinity-purified, polyclonal rabbit 6 antiserum raised against PTHrP (1-36), and an affinity purified sheep antibody that recognizes PTHrP (1-36) as the radiolabeled signal. The sensitivity (defined as 2 sd over plasma zero) was 2.5–3.0 pm in human plasma and 5 pm in mouse plasma.
Estradiol levels were measured in a commercial RIA from Siemens Health Diagnostics Inc. (formerly known as Diagnostic Products Corp., Los Angeles, CA), following the manufacturer’s instructions. The quoted sensitivity of this assay is 1.4 pg/ml, but in our hands, the sensitivity (as defined by 88% binding or 12% displacement) was 3–5 pg/ml. Standard curves for this assay were generated using standards and a human blood-based diluent (zero calibrator) provided by the manufacturer. Our samples were diluted 1:1 with this diluent because we observed no effect of 50% mouse serum on the assay standard curve. Any value less than 5 pg/ml in the assay was set equal to 5 pg/ml for the purposes of calculations.
Statistics
Values are reported as the mean ± sem, and the error bars represent sem. To calculate statistical significance, we used the unpaired, two-tailed Student’s t test. Statistical analyses were performed using GraphPad Prism 4.00 for Windows (GraphPad Software, San Diego, CA).
Results
Raising PTHrP levels and lowering estrogens in virgin mice
Our goal was to manipulate PTHrP and estrogens in virgin mice in an attempt to reproduce the rapid bone loss of lactation. Our first task was to determine how best to mimic lactational levels of these hormones. We had previously noted that circulating PTHrP levels during lactation were approximately 1.5–2 pm, whereas circulating PTH levels during this time were approximately 3–4 pm (9,21). Therefore, the combined circulating ligand concentration to which the type 1 PTH/PTHrP receptor (PTHR1) is exposed is approximately 4.5–6 pm. We reasoned that administering PTHrP to virgin mice would raise calcium levels, which, in turn, would suppress PTH secretion. Therefore, to ensure that the combined PTHrP plus PTH levels in our experiments were similar to those measured during lactation, we infused PTHrP (1-36) with a goal of achieving circulating PTHrP levels of approximately 6 pm. We used miniosmotic infusion pumps to deliver PTHrP (1-36) sc at 5, 10, or 20 pmol/h. Calcium and PTHrP levels were measured 11 d after insertion of the pumps. As shown in Supplemental Fig. 1, published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org, both the 5- and 10-pmol/h doses produced circulating PTHrP levels of around 1800 h with a minimal degree of hypercalcemia. In contrast, the 20-pmol/h dose produced frankly elevated levels of both PTHrP and calcium. Based on these data, we chose the 10-pmol/h dose for all subsequent experiments.
Suckling stimulates afferent nerves that project to the hindbrain and hypothalamus and suppress GnRH secretion, causing hypogonadotropic hypogonadism and low levels of circulating estrogens (14,15). To induce hypogonadotropic hypogonadism in virgin mice, we injected them with the long-acting GnRH analog, leuprolide acetate (27). Supplemental Fig. 2 shows circulating estradiol levels after 10 d of leuprolide given sc twice a day. As expected, lactating mice had lower circulating levels of estradiol than did randomly cycling virgin mice. The administration of leuprolide lowered estradiol to a level similar to that in lactating mice. We also measured estradiol concentrations in mice that had undergone ovariectomy (OVX). Compared with virgins, leuprolide-treated, OVX, and lactating mice all had reductions in circulating estradiol concentrations.
Elevated PTHrP levels combined with estrogen deficiency cause bone loss but do not reproduce the effects of lactation on BMD
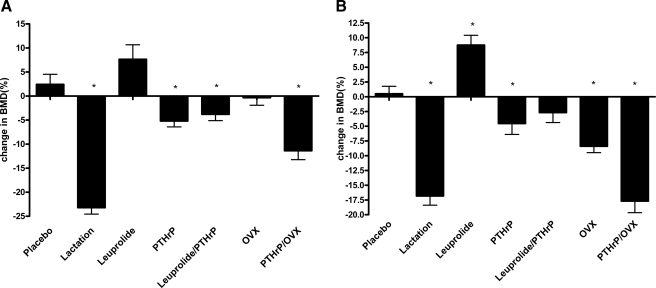
We next measured BMD by PIXIMUS (GE Medical Systems) before and after 10 d of leuprolide treatment alone, PTHrP infusion alone, and the combination of both PTHrP infusion and leuprolide treatment. Although estrogen deficiency during lactation is the result of central hypogonadism, we also examined the effects of OVX alone and OVX combined with PTHrP infusion as another model of estrogen withdrawal. Finally, we examined bone loss in age-matched lactating mice over the first 10 d postpartum. Figure 1A shows the results for measurements at the spine, and Fig. 1B shows the results for the measurements at the total femur. We could not examine total body bone density because the miniosmotic pumps interfered with these measurements. BMD declined 23.2 ± 1.3% in the spine and 16.8 ± 1.9% in the femur over 10 d of lactation. Infusion of PTHrP alone caused a modest degree of bone loss both at the spine (5.2 ± 1.2%) and at the femur (4.5 ± 1.8%). Surprisingly, leuprolide treatment was associated with a slight increase in BMD at the spine and OVX produced no change in spine BMD. At the femur, leuprolide was again associated with an increase in BMD, whereas OVX produced a significant decline in BMD. Combining leuprolide with PTHrP infusion did not magnify the bone loss associated with PTHrP infusion alone, but OVX did amplify the degree of bone loss caused by PTHrP infusion alone. None of the above maneuvers reproduced the decline in spine BMD that occurred over 10 d of lactation. At the femur, the combination of PTHrP infusion plus OVX produced a degree of bone loss equivalent to that occurring over the first 10 d of lactation, although the combination of PTHrP infusion and leuprolide did not.
Figure 1.
Changes in bone density. A, Changes in bone density at the spine in response to the different treatments noted on the graph. As expected, lactation led to a large decline in BMD at the spine. PTHrP infusion, PTHrP infusion plus leuprolide treatment, and PTHrP infusion plus OVX all caused a modest degree of bone loss but not to the same extent as lactation. B, Changes in bone density at the femur in response to the different treatments noted on the graph. As with the spine, lactation was associated with a large decline in BMD. Again, PTHrP infusion, PTHrP infusion plus leuprolide treatment, and PTHrP infusion plus OVX all caused a significant degree of bone loss. However, unlike the spine, the combination of PTHrP and OVX caused a decline in BMD equal to that seen during lactation. Each group contained the following numbers of mice: placebo, 10; lactation, 10; leuprolide, 10; PTHrP, 10; PTHrP/leuprolide, 12; OVX, 11; PTHrP/OVX, nine. The presence of an asterisk denotes a P < 0.05 compared with placebo-treated mice.
We also examined bone mass and architecture using micro-CT. As we had observed previously, in trabecular bone at both the spine and femur, lactation was associated with significant decreases in bone mass, trabecular thickness, and tissue density as well as significant increases in the SMI and bone surface to volume ratios (Tables 1 and 2) (4). Despite the changes in BMD measured by DEXA, none of the manipulations in virgin mice reproduced the pattern of changes in trabecular bone mass and architecture that occurred during lactation. In vertebral trabecular bone, PTHrP, alone or in combination with leuprolide treatment or OVX, resulted in a decline in tissue density similar to that seen in lactation. However, individually, leuprolide or OVX had no effect on this parameter, suggesting that the decline in tissue density may be caused by PTHrP. However, in femoral trabecular bone, the combination of leuprolide with PTHrP lowered tissue density, but neither PTHrP alone nor OVX plus PTHrP exerted the same effect. Although there were some isolated significant differences in other parameters with one or more treatments, no other obvious patterns emerged from these data. Thus, with the possible exception of an effect of PTHrP on tissue density, neither short-term withdrawal of estrogens nor short-term PTHrP infusion, alone or in combination, reproduced the effects of lactation on micro-CT parameters of trabecular bone mass or structure.
Table 1.
Micro-CT measurements of vertebral trabecular bone
| BV/TV (%) | Trabecular number (1/mm) | Trabecular thickness (μm) | Connective density (1/mm3) | Tissue density (mg/ccmHA) | SMI | BS/BV (1/mm) | |
|---|---|---|---|---|---|---|---|
| Placebo (n = 9) | 21.2 ± 1.37 | 4.23 ± 0.16 | 49.64 ± 1.36 | 199.05 ± 14.43 | 1083.17 ± 8.60 | 0.96 ± 0.17 | 45.50 ± 1.54 |
| Lactation (n = 10) | 14.7 ± 1.24 | 4.4 ± 0.29 | 40.38 ± 0.80 | 253.33 ± 35.66 | 1045.81 ± 6.22 | 1.64 ± 0.11 | 60.52 ± 1.63 |
| Leuprolide (n = 10) | 22.7 ± 1.94 | 4.59 ± 0.15 | 51.05 ± 1.41 | 195.58 ± 12.91 | 1089.12 ± 2.54 | 0.86 ± 0.20 | 44.38 ± 1.78 |
| PTHrP (n = 10) | 19.6 ± 1.23 | 4.32 ± 0.22 | 46.0 ± 0.96 | 283.0 ± 42.34 | 1036.0 ± 11.24 | 1.1 ± 0.09 | 50.2 ± 1.31 |
| PTHrP/leuprolide (n = 11) | 21.1 ± 1.2 | 4.16 ± 0.23 | 48.68 ± 1.04 | 218.66 ± 11.67 | 1053.31 ± 6.23 | 0.78 ± 0.13 | 46.35 ± 1.54 |
| OVX (n = 10) | 20.1 ± 1.07 | 3.87 ± 0.17 | 51.04 ± 1.39 | 149.12 ± 10.08 | 1098.26 ± 12.32 | 0.86 ± 0.12 | 44.17 ± 1.47 |
| PTHrP/OVX (n = 9) | 21.9 ± 1.96 | 4.66 ± 0.24 | 48.90 ± 2.17 | 260.28 ± 33.75 | 1042.40 ± 16.22 | 0.98 ± 0.21 | 47.98 ± 2.57 |
The chart contains the mean ± sem of trabecular parameters measured on the third lumbar vertebra on mice from the various treatment groups. All measurements were compared against the same parameter assessed on placebo-treated mice. Statistically significant differences (P < 0.05) are denoted in bold type. The number of mice in each group is noted on the chart. ccmHA, Cubic centimeters of hydroxyapatite.
Table 2.
Micro-CT measurements of femur trabecular bone
| BV/TV (%) | Trabecular number (1/mm) | Trabecular thickness (1/mm) | Connective density (1/mm3) | Tissue density (mg/ccmHA) | SMI | BS/BV (1/mm) | |
|---|---|---|---|---|---|---|---|
| Placebo (n = 5) | 0.16 ± 0.017 | 4.79 ± 0.18 | 0.045 ± 0.002 | 263.75 ± 26.28 | 818.95 ± 5.62 | 1.57 ± 0.13 | 59.48 ± 2.81 |
| Lactation (n = 5) | 0.09 ± 0.012 | 4.08 ± 0.58 | 0.038 ± 0.001 | 221.12 ± 47.14 | 781.50 ± 12.67 | 2.09 ± 0.07 | 74.02 ± 1.76 |
| Leuprolide (n = 5) | 0.17 ± 0.006 | 4.83 ± 0.11 | 0.049 ± 0.001 | 242.59 ± 18.59 | 826.23 ± 4.97 | 1.49 ± 0.07 | 55.04 ± 1.19 |
| PTHrP (n = 5) | 0.12 ± 0.013 | 3.63 ± 0.21 | 0.050 ± 0.003 | 173.03 ± 24.51 | 803.37 ± 9.71 | 1.65 ± 0.15 | 57.28 ± 3.34 |
| PTHrP/leuprolide (n = 5) | 0.15 ± 0.033 | 4.47 ± 0.61 | 0.044 ± 0.003 | 274.01 ± 54.48 | 765.84 ± 19.48 | 1.65 ± 0.28 | 62.99 ± 5.31 |
| OVX (n = 5) | 0.15 ± 0.008 | 3.87 ± 0.21 | 0.047 ± 0.0004 | 377.24 ± 55.17 | 870.63 ± 6.89 | 1.19 ± 0.08 | 56.08 ± 0.75 |
| PTHrP/OVX (n = 5) | 0.16 ± 0.032 | 4.56 ± 0.51 | 0.040 ± 0.002 | 550.28 ± 100.64 | 815.42 ± 9.19 | 1.44 ± 0.27 | 68.79 ± 4.97 |
The chart contains the mean ± sem of trabecular parameters measured in the distal femoral metaphasis on mice from the various treatment groups. All measurements were compared against the same parameter assessed on placebo-treated mice. Statistically significant differences (P < 0.05) are denoted in bold type. The number of mice in each group is noted on the chart. ccmHA, Cubic centimeters of hydroxyapatite.
In contrast to trabecular bone, the combination of leuprolide treatment and PTHrP infusion as well as the combination of OVX and PTHrP infusion decreased cortical bone mass and cortical thickness in the femur (Table 3). Whereas the circumference of the periosteal surface did not change, the endosteal circumference increased in response to both manipulations. These changes were also reflected in a significant increase in the bone surface to volume ratio for both treatments. None of these parameters changed with PTHrP infusion, leuprolide treatment, or OVX alone, suggesting they resulted from an interaction between low estrogens and excess PTHrP. Given the lack of any changes in trabecular bone, these changes in the cortex may explain the significant decrease in femur BMD noted for the combination of OVX and PTHrP infusion. Interestingly, although past studies have shown that lactation is associated with an increase in endosteal bone resorption and decreases in cortical bone mass and thickness, in the present study, we did not find significant changes in the micro-CT measurements of cortical bone in the femurs from lactating mice.
Table 3.
Micro-CT measurements of femur cortical bone
| BV/TV (%) | Tissue density (mg/ccmHA) | BS/BV (1/mm) | Cortical thickness (mm) | Outer circumference (mm) | Inner circumference (mm) | |
|---|---|---|---|---|---|---|
| Placebo (n = 5) | 0.91 ± 0.0057 | 976.22 ± 13.23 | 10.70 ± 0.32 | 0.19 ± 0.005 | 5.26 ± 0.10 | 4.09 ± 0.12 |
| Lactation (n = 5) | 0.89 ± 0.008 | 1023.11 ± 12.82 | 11.31 ± 0.46 | 0.18 ± 0.007 | 5.23 ± 0.16 | 4.12 ± 0.16 |
| Leuprolide (n = 5) | 0.92 ± 0.005 | 996.27 ± 19.59 | 10.54 ± 0.42 | 0.19 ± 0.007 | 5.34 ± 0.06 | 4.15 ± 0.80 |
| PTHrP (n = 5) | 0.91 ± 0.004 | 1039.94 ± 6.12 | 10.85 ± 0.27 | 0.18 ± 0.005 | 5.39 ± 0.09 | 4.23 ± 0.11 |
| PTHrP/leuprolide (n = 5) | 0.88 ± 0.012 | 964.37 ± 10.33 | 14.36 ± 1.22 | 0.14 ± 0.012 | 5.52 ± 0.09 | 4.62 ± 0.05 |
| OVX (n = 5) | 0.92 ± 0.003 | 1048.85 ± 14.17 | 9.96 ± 0.16 | 0.20 ± 0.003 | 5.17 ± 0.12 | 3.91 ± 0.14 |
| PTHrP/OVX (n = 5) | 0.89 ± 0.007 | 1014.61 ± 31.57 | 12.75 ± 0.62 | 0.16 ± 0.008 | 5.50 ± 0.16 | 4.51 ± 0.18 |
The chart contains the mean ± sem of cortical parameters measured in the femoral shaft on mice from the various treatment groups. All measurements were compared against the same parameter assessed on placebo-treated mice. Statistically significant differences (P < 0.05) are denoted in bold type. The number of mice in each group is noted on the chart. ccmHA, Cubic centimeters of hydroxyapatite.
Low levels of estrogens and PTHrP infusion alter bone turnover
Table 4 displays the results for histomorphometry performed on vertebrae harvested from mice after 10 d of the various treatments. Consistent with the previous DEXA and micro-CT data, only lactation produced a significant decrease in both bone volume/tissue volume (BV/TV) and trabecular thickness as measured histologically. Lactation was associated with increases both in osteoblast and osteoclast numbers, results consistent with multiple previous studies characterizing this period as one of elevated bone turnover (1,4,7,8,9,13,21). Despite the lack of change in bone mass, treatment with leuprolide, PTHrP, OVX, and the combinations of leuprolide plus PTHrP and OVX plus PTHrP all resulted in significant changes in the numbers of osteoblasts and/or osteoclasts. Compared with placebo, leuprolide treatment and OVX both increased osteoclast numbers. PTHrP infusion increased both osteoblasts and osteoclasts, as did the combination of leuprolide treatment with PTHrP infusion. In contrast, the combination of OVX and PTHrP infusion was associated with an increase in osteoclast numbers but not osteoblast numbers.
Table 4.
Static histomorphometry of vertebral bone
| BV/TV (%) | TbTh (μm) | Nob/TAR (1/mm2) | Nob/Bpm (1/mm) | Noc/TAR (1/mm2) Noc/Bpm (1/mm) | Placebo (n = 7) | |
|---|---|---|---|---|---|---|
| Lactation (n = 8) | 25.39 ± 1.41 | 28.46 ± 1.48 | 345.89 ± 43.58 | 24.74 ± 3.11 | 25.46 ± 1.17 | 1.84 ± 0.13 |
| Leuprolide (n = 8) | 32.20 ± 1.85 | 34.84 ± 1.14 | 184.31 ± 38.61 | 12.92 ± 2.57 | 30.76 ± 2.30 | 2.11 ± 0.10 |
| PTHrP (n = 8) | 32.52 ± 0.75 | 34.39 ± 0.96 | 470.67 ± 53.64 | 31.35 ± 3.02 | 30.18 ± 3.33 | 2.01 ± 0.19 |
| PTHrP/leuprolide (n = 8) | 32.54 ± 0.95 | 32.69 ± 1.07 | 369.71 ± 62.25 | 23.24 ± 3.70 | 33.81 ± 2.30 | 2.16 ± 0.13 |
| OVX (n = 8) | 30.37 ± 1.93 | 35.18 ± 1.52 | 156.25 ± 23.85 | 11.58 ± 1.72 | 27.46 ± 3.49 | 2.03 ± 0.24 |
| PTHrP/OVX (n = 7) | 27.66 ± 2.10 | 30.72 ± 0.71 | 186.85 ± 23.84 | 13.46 ± 1.90 | 37.79 ± 6.34 | 2.60 ± 0.34 |
The chart contains the mean ± sem of histomorphometric measurements of bone volume and osteoclast and osteoblast numbers in the first and second lumbar vertebrae of mice from the various groups listed in the chart. All measurements were compared against the same parameter assessed on placebo-treated mice. Statistically significant differences (P < 0.05) are denoted in bold type. The number of mice in each group is noted on the chart. TbTh, Trabecular thickness; Nob/TAR, number of ostéoblasts per tissue area; Nob/Bpm, number of osteoblasts per bone perimeter; Noc/TAR, number of osteoclasts per tissue area; Noc/Bpm, number of osteoclasts per bone perimeter.
PTHrP infusion reduces urinary calcium excretion
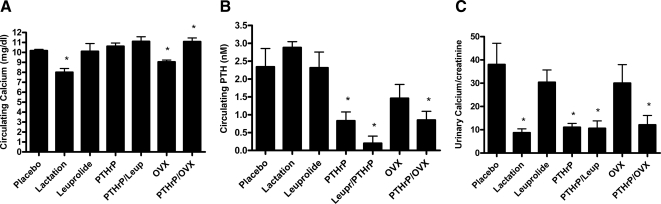
Figure 2 shows the results of biochemical measurements performed on mice from the different experimental groups. Lactation was associated with a slight decrease in the circulating level of calcium as well as a decrease in the urinary calcium to creatinine ratio (Ca/Cr). After 10 d of treatment, PTHrP infusion at these doses led to minimal elevations in serum calcium but was associated with significant reductions in circulating PTH levels. Likewise, PTHrP infusion led to significant reductions in urinary Ca/Cr, equal to those seen in lactating mice. Neither leuprolide nor OVX alone reduced urinary calcium excretion, and neither method of suppressing estrogens blunted the effects of PTHrP on urinary Ca/Cr.
Figure 2.
Biochemical changes. A, Systemic calcium levels after 10 d of the various treatments noted on the graph. Compared with placebo, lactation and OVX were associated with slightly decreased calcium level, and the combination of PTHrP infusion with OVX was associated with a slightly increased calcium level. Each group contained the following numbers of mice: placebo, 10; lactation, 10; leuprolide, nine; PTHrP, 10; PTHrP/leuprolide, 11; OVX, 11; PTHrP/OVX, nine. B, Systemic PTH levels after 11 d of the various treatments noted on the graph. Compared with placebo, PTHrP infusion, the combination of PTHrP infusion with leuprolide, and the combination of PTHrP infusion with OVX were associated with a significant reduction in PTH levels. Each group contained the following numbers of mice: placebo, five; lactation, five; leuprolide, seven; PTHrP, seven; PTHrP/leuprolide, four; OVX, five; PTHrP/OVX, six. C, Urinary Ca/Cr after 11 d of the various treatments noted on the graph. Compared with placebo, lactation, PTHrP infusion, the combination of PTHrP infusion with leuprolide treatment, and the combination of PTHrP infusion with OVX all resulted in lower calcium excretion. Each group contained the following numbers of mice: placebo, six; lactation, seven; leuprolide, seven; PTHrP, seven; PTHrP/leuprolide, seven; OVX, eight; PTHrP/OVX, six. For each panel, an asterisk denotes a P < 0.05 compared with placebo-treated mice.
Discussion
Previous studies have suggested that the combination of low estrogens due to central hypogonadism and increased PTHrP secretion from the mammary gland trigger rapid bone loss during lactation (1,3,9,20,21). To test this hypothesis, we attempted to reproduce the same rate of bone loss in nulliparous mice by raising PTHrP levels and/or by lowering levels of estrogens. Although these manipulations altered bone turnover, they were unable to generate bone loss equivalent to that caused by lactation. Bone mass, as measured by DEXA, declined by 23% at the spine and 17% at the femur over 10 d of lactation. Likewise, we found significant decreases in bone mass, trabecular thickness, and tissue density as well as significant increases in the SMI and bone surface to bone volume (BS/BV) in lactating mice as assessed by micro-CT. In contrast, 10 d of leuprolide treatment actually increased bone density slightly by DEXA and had no effect on trabecular or cortical bone mass or microarchitecture as assessed by micro-CT. OVX resulted in a modest decline in femur BMD by DEXA but was not associated with significant changes in bone mass or microarchitecture on micro-CT measurements of either the vertebrae or femur. Short-term infusion of PTHrP also was associated with modest bone loss at both the spine and femur. On micro-CT, PTHrP infusion lowered trabecular BV/TV in the distal femur, although the difference did not quite reach significance (P = 0.09). PTHrP infusion also was associated with a reduction in trabecular number and connective density. Bone loss was no greater with the combination of PTHrP infusion and leuprolide treatment, but the combination of PTHrP infusion and OVX was associated with enhanced bone loss, especially at the femur. In fact, this combination caused femoral BMD to fall to an equivalent level as lactation. However, the bone loss caused by the combination of OVX and PTHrP was predominantly from cortical sites because there was a decrease in cortical BV/TV and cortical thickness accompanied by trend toward an increase in the endosteal circumference of the cortex (P = 0.096 compared with placebo). Thus, over the course of 10 d, simultaneously decreasing estrogens and increasing PTHrP did not reproduce the specific pattern of rapid bone loss characteristic of lactation.
Both leuprolide treatment and OVX increased the numbers of osteoclasts but not the numbers of osteoblasts. This is likely to be related to estrogen deficiency because estrogens are known to inhibit the formation of osteoclasts and to induce osteoclast apoptosis (18,28,29). Furthermore, it occurred in both the setting of primary and central hypogonadism, so it is unlikely to have been caused by other pituitary or ovarian hormones. In contrast, the infusion of PTHrP increased the numbers of both osteoclasts and osteoblasts. This was expected because prior studies have demonstrated that activation of the PTHR1 in bone cells leads to an overall increase in bone turnover with either a net gain in bone in the setting of intermittent administration of PTH or a net loss of bone in the setting of continuous exposure to PTH (30). Interestingly, although both the numbers of osteoclasts and osteoblasts were elevated by the combination of leuprolide and PTHrP, only the number of osteoclasts was significantly elevated by the combination of OVX and PTHrP. This relative imbalance may explain the greater degree of bone loss seen with the latter combination, even though the combination of leuprolide and PTHrP more closely reproduced the effects of lactation on the numbers of osteoclasts and osteoblasts. These observations underscore two points. First, the experimental maneuvers did alter bone turnover, even though they were relatively ineffective in causing net bone loss over the short time period of our experiment. Second, PTHrP appears to increase both osteoclast and osteoblast numbers, which might be expected to limit bone loss. Thus, lactation must be associated with the regulation of relative osteoclast vs. osteoblast activity over and above the alterations in cell numbers to generate such rapid bone loss.
It is interesting that OVX appeared to be more efficacious than leuprolide at triggering bone loss in our experiments. There are several possible explanations for this observation. First, it is possible that leuprolide may have stimulated the production of estrogens before they were suppressed as a result of the expected initial increase in gonadotrophins (27). In contrast, OVX would be expected to lower estrogens immediately. Because our experiments were very short in duration, this may be an important difference. In fact, in long-term experiments, leuprolide has been shown to cause significant bone loss in rats over 16 wk of treatment, and a 1-yr comparison of leuprolide with OVX demonstrated equivalent degrees of bone loss in rats (31,32,33). It is worth noting, however, that mice undergo a postpartum estrous cycle before gonadotrophin secretion is inhibited by suckling (34). Therefore, leuprolide treatment may reproduce the normal lactational pattern of circulating estrogens more closely than OVX. Second, it is likely that estradiol levels were absolutely lower in OVX mice than in leuprolide-treated mice because more OVX values were below the detection limit of the assay. Third, it has been suggested that elevated FSH levels may contribute to OVX-associated bone loss in mice (35). Because leuprolide would lead to a suppression of FSH levels, the lack of FSH action on osteoclasts may also have contributed to the lack of short-term bone loss in the leuprolide-treated groups. Finally, previous studies examining leuprolide treatment have used rats, and many studies examining OVX in mice have been performed in C57BL/6 or 129P3 mice (36). We used CD1 mice, and thus, our findings may reflect species and/or strain differences.
During lactation, renal calcium reabsorption is enhanced and urinary calcium excretion is very low (1,3). Both estrogens and PTHrP are known to affect renal calcium handling, although most data suggest that they act in opposite directions. Estrogen deficiency in postmenopausal women has been shown to be associated with higher rates of urinary calcium excretion, and replacement of estrogens in this setting usually results in a reduction in urinary calcium (37,38,39). PTH and PTHrP are known to act on the PTHR1 in the distal tubule to reduce fractional calcium excretion (40,41). Our data suggest that elevated levels of PTHrP may be responsible for the reduction in urinary calcium excretion seen in lactation. The infusion of PTHrP into nulliparous mice reduced urinary calcium excretion to levels equivalent to that observed during lactation. This also agrees with the results of PTHrP infusions in human subjects (40,41). Neither leuprolide treatment nor OVX alone affected renal calcium excretion, and neither blunted the effects of PTHrP on lowering urinary calcium levels. These data suggest that the secretion of PTHrP from the breast plays a dominant role in altering renal calcium handling during lactation.
When calcium is mobilized from the skeleton by suckling, it is transported into milk and, as a result, we found that circulating calcium levels are slightly lower than in the placebo-treated controls (1,2). In contrast, infusion of PTHrP into virgin mice activated bone resorption in the absence of milk production, resulting in mild hypercalcemia. Although this effect was minimal at the dose of PTHrP that we used, circulating calcium levels in the various treatment groups (with the exception of OVX) were higher than the calcium levels in lactating mice. This may be a limitation of our model because calcium has been shown to inhibit bone resorption both directly and indirectly. Osteoclasts and osteoclast precursors have been reported to express the extracellular calcium-sensing receptor, and studies have suggested that signaling from this receptor can induce osteoclast apoptosis (42,43). In addition, elevated levels of circulating calcium can stimulate calcitonin release from thyroid C cells (44,45). Calcitonin is known to inhibit osteoclast function and the laboratory of Kovacs and colleagues (46) has demonstrated that endogenous calcitonin normally acts to restrain excessive bone loss during lactation. Finally, because PTHrP also increased the numbers of osteoblasts, the released calcium may simply have been recycled back into bone. Therefore, a lack of milk production resulting in hypercalcemia may have limited our attempts to reproduce the full degree of lactational bone loss in nulliparous mice. However, the combination of OVX and PTHrP infusion produced the greatest degree of bone loss and the highest calcium levels. As a result, we think that the mild (not statistically significant) elevations in calcium in our experiments are unlikely to be a significant factor explaining the failure to reproduce the full effects of lactation on bone mass. Instead, we believe that the minimal elevations of calcium reflect the inability of these treatments to trigger sufficient net bone resorption.
Another possible limitation of our studies was the fact that we infused amino-terminal PTHrP continuously. It is possible that full-length PTHrP or other fragments of PTHrP secreted from the mammary gland might exert additional effects on bone metabolism during lactation. In addition, it has been shown that suckling-induced spikes of prolactin increase PTHrP mRNA levels in the lactating breast, suggesting that PTHrP secretion during lactation may be pulsatile (47). Despite this suggestion, there are no data examining the 24-h profile of circulating levels of PTHrP during normal lactation in mice. The half-life of PTHrP mRNA in the breast after suckling has stopped is about 1 h, whereas PTHrP levels in milk declined only 20–30%, 4 h after pup withdrawal (47,48). However studies examining normal lactating behavior in mice have shown that pups suckle approximately 30–50% of a 24-h period with an average suckling period of 1 h and an average suckling-free interval of 30 min (maximum off time of 2 h) (49,50,51). Therefore, although there may be some modulation of PTHrP levels during the day, we think that it is likely that circulating PTHrP is continuously elevated during lactation.
If high PTHrP levels and low estrogen levels are not sufficient to reproduce lactation-associated bone loss, then something else must contribute. One possibility is that an antecedent pregnancy is required for PTHrP and estrogen deficiency to trigger bone loss. In a preliminary study, we infused PTHrP in combination with leuprolide treatment in immediately post-partum mice that were not allowed to nurse. These mice lost no more bone by DEXA than equivalently treated nulliparous mice, suggesting that the absence of a prior pregnancy does not explain the inability to mimic bone loss during lactation (Ardeshirpour, L., P. Dann, and J. J. Wysolmerski, unpublished data). Several studies have shown that the amount of bone lost during lactation is proportional to suckling intensity (1,3,5,52). Suckling triggers a series of complex neuroendocrine events that modulate maternal energy homeostasis, maternal behavior, and reproductive physiology (15). In addition to suppressing GnRH secretion, suckling stimulates the secretion of oxytocin and prolactin (14,15). Furthermore, circulating levels of inhibin have been shown to be decreased during lactation in both humans and rodents (53,54,55). All three of these hormones have been shown to affect bone turnover (56). Both leuprolide treatment and OVX would be expected to lower circulating inhibin levels, making it unlikely to be the missing factor in our experiments. In contrast, our experimental manipulations would not be expected to increase prolactin or oxytocin levels. Oxytocin has been shown to increase bone formation and increase bone mass, so it is also an unlikely candidate to contribute to bone catabolism (57,58). However, prolactin has been reported to inhibit osteoblast function and to increase receptor activator of nuclear factor-κB ligand production, thus indirectly stimulating osteoclast function (59,60). Thus, hyperprolactinemia may be an additional factor required for bone loss during lactation. Finally, it has become increasingly clear that bone cell behavior can be regulated by direct neural input from the hypothalamus (61,62,63). In this regard, it is interesting to note that suckling stimulates the production of neuropeptide Y (NPY) in the hypothalamus and that NPY contributes to alterations in feeding behavior and GnRH secretion during lactation (15,64,65). Hypothalamic NPY can also act via central Y2 receptors (which are increased during lactation) to inhibit osteoblast activity (66,67,68). Therefore, direct central effects of suckling may be important in altering the balance between osteoclast and osteoblast activity that is required for rapid bone loss.
In summary, although elevated PTHrP and decreased estrogens in the circulation clearly contribute to bone loss during lactation, they are not, by themselves, sufficient to reproduce the full effect of suckling on skeletal metabolism. Lactation induces a complex series of physiological adaptations in maternal metabolism. It is likely that other factors associated with these changes also contribute to skeletal catabolism. In particular, we hypothesize that suckling-induced prolactin secretion and/or central alterations in hypothalamic function also affect bone cell function during lactation. Further studies will be required to fully elucidate the integrated control of bone metabolism during lactation.
Supplementary Material
Acknowledgments
We thank Dr. Andrew Stewart for the gift of PTHrP (1-36) for our infusion studies. We also thank Dr. Doug Adams for assistance with micro-CT measurements. We thank Nancy Troiano and Tracy Nelson in the Yale Core Center for Musculoskeletal Disorders for expert technical assistance. Finally, we thank Drs. Karl Insogna and Arthur Broadus for helpful conversations.
Footnotes
This work was supported by National Institutes of Health Grants DK077565, AR46032, and DK081731.
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 3, 2010
Abbreviations: BMD, Bone mineral density; BS/BV, bone surface to bone volume; BV/TV, bone volume/tissue volume; Ca/Cr, calcium to creatinine ratio; CT, computed tomography; DEXA, dual-energy x-ray absorptiometry; NPY, neuropeptide Y; OVX, ovariectomy; PTHR1, type 1 PTH/PTHrP receptor; SMI, structure model index.
References
- Kovacs CS, Kronenberg HM 1997 Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18:832–872 [DOI] [PubMed] [Google Scholar]
- VanHouten J 2005 Maternal calcium and bone metabolism during lactation. Curr Opin Endocrinol Diabetes 12:477–482 [Google Scholar]
- Wysolmerski JJ 2010 Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann NY Acad Sci 1192:161–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, Wysolmerski JJ 2007 Weaning triggers a decrease in receptor activator of nuclear factor-κB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology 148:3875–3886 [DOI] [PubMed] [Google Scholar]
- Peng TC, Garner SC, Kusy RP, Hirsch PF 1988 Effect of number of suckling pups and dietary calcium on bone mineral content and mechanical properties of femurs of lactating rats. Bone Miner 3:293–304 [PubMed] [Google Scholar]
- Miller SC, Bowman BM 1998 Comparison of bone loss during normal lactation with estrogen deficiency osteopenia and immobilization osteopenia in the rat. Anat Rec 251:265–274 [DOI] [PubMed] [Google Scholar]
- Miller SC, Halloran BP, DeLuca HF, Jee WS 1982 Role of vitamin D in maternal skeletal changes during pregnancy and lactation: a histomorphometric study. Calcif Tissue Int 34:245–252 [DOI] [PubMed] [Google Scholar]
- Sowers M, Eyre D, Hollis BW, Randolph JF, Shapiro B, Jannausch ML, Crutchfield M 1995 Biochemical markers of bone turnover in lactating and nonlactating postpartum women. J Clin Endocrinol Metab 80:2210–2216 [DOI] [PubMed] [Google Scholar]
- VanHouten JN, Wysolmerski JJ 2003 Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology 144:5521–5529 [DOI] [PubMed] [Google Scholar]
- Bowman BM, Siska CC, Miller SC 2002 Greatly increased cancellous bone formation with rapid improvements in bone structure in the rat maternal skeleton after lactation. J Bone Miner Res 17:1954–1960 [DOI] [PubMed] [Google Scholar]
- Karlsson C, Obrant KJ, Karlsson M 2001 Pregnancy and lactation confer reversible bone loss in humans. Osteoporos Int 12:828–834 [DOI] [PubMed] [Google Scholar]
- Laskey MA, Prentice A 1999 Bone mineral changes during and after lactation. Obstet Gynecol 94:608–615 [DOI] [PubMed] [Google Scholar]
- Miller SC, Bowman BM 2007 Rapid inactivation and apoptosis of osteoclasts in the maternal skeleton during the bone remodeling reversal at the end of lactation. Anat Rec (Hoboken) 290:65–73 [DOI] [PubMed] [Google Scholar]
- McNeilly AS, Tay CC, Glasier A 1994 Physiological mechanisms underlying lactational amenorrhea. Ann NY Acad Sci 709:145–155 [DOI] [PubMed] [Google Scholar]
- Smith MS, Grove KL 2002 Integration of the regulation of reproductive function and energy balance: lactation as a model. Front Neuroendocrinol 23:225–256 [DOI] [PubMed] [Google Scholar]
- Xu J, Kirigiti MA, Cowley MA, Grove KL, Smith MS 2009 Suppression of basal spontaneous gonadotropin-releasing hormone neuronal activity during lactation: role of inhibitory effects of neuropeptide Y. Endocrinology 150:333–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kirigiti MA, Grove KL, Smith MS 2009 Regulation of food intake and gonadotropin-releasing hormone/luteinizing hormone during lactation: role of insulin and leptin. Endocrinology 150:4231–4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL 2000 The mechanisms of estrogen regulation of bone resorption. J Clin Invest 106:1203–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EP, Specker B, Korach KS 2010 Recent experimental and clinical findings in the skeleton associated with loss of estrogen hormone or estrogen receptor activity. J Steroid Biochem Mol Biol 118:264–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney CA, Zhang D, Schork A, Crutchfield M, Stanczyk F, Russell-Aulet M 1996 Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA 276:549–554 [PubMed] [Google Scholar]
- VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ 2003 Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest 112:1429–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman F, Shen V, Xie F, Seibel M, Ratcliffe A, Lindsay R 1993 Estrogen protection against bone resorbing effects of parathyroid hormone infusion. Assessment by use of biochemical markers. Ann Intern Med 118:337–343 [DOI] [PubMed] [Google Scholar]
- Grey AB, Stapleton JP, Evans MC, Tatnell MA, Reid IR 1996 Effect of hormone replacement therapy on bone mineral density in postmenopausal women with mild primary hyperparathyroidism. A randomized, controlled trial. Ann Intern Med 125:360–368 [DOI] [PubMed] [Google Scholar]
- Masiukiewicz US, Mitnick M, Grey AB, Insogna KL 2000 Estrogen modulates parathyroid hormone-induced interleukin-6 production in vivo and in vitro. Endocrinology 141:2526–2531 [DOI] [PubMed] [Google Scholar]
- Strewler GJ 2000 The physiology of parathyroid hormone-related protein. N Engl J Med 342:177–185 [DOI] [PubMed] [Google Scholar]
- Plawner LL, Philbrick WM, Burtis WJ, Broadus AE, Stewart AF 1995 Cell type-specific secretion of parathyroid hormone-related protein via the regulated versus the constitutive secretory pathway. J Biol Chem 270:14078–14084 [DOI] [PubMed] [Google Scholar]
- Garner C 1994 Uses of GnRH agonists. J Obstet Gynecol Neonatal Nurs 23:563–570 [DOI] [PubMed] [Google Scholar]
- Frenkel B, Hong A, Baniwal SK, Coetzee GA, Ohlsson C, Khalid O, Gabet Y 2010 Regulation of adult bone turnover by sex steroids. J Cell Physiol 224:305–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S 2007 Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 130:811–823 [DOI] [PubMed] [Google Scholar]
- Datta NS, Abou-Samra AB 2009 PTH and PTHrP signaling in osteoblasts. Cell Signal 21:1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurabayashi T, Fujimaki T, Yasuda M, Yamamoto Y, Tanaka K 1993 Time-course of vertebral and femoral bone loss in rats administered gonadotrophin-releasing hormone agonist. J Endocrinol 138:115–125 [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Sassa S, Mitamura T, Kudo H, Suzuki S, Yoshimura S, Zhou Y, Kikuchi T, Shinoda H 1999 Prevention of osteopenia induced with a gonadotropin-releasing hormone agonist in rats. Calcif Tissue Int 65:152–155 [DOI] [PubMed] [Google Scholar]
- Wang Y, Yano T, Kikuchi A, Yano N, Matsumi H, Ando K, Kasai Y, Watanabe M, Okagaki R, Osuga Y, Taketani Y 2000 Comparison of the effects of add-back therapy with various natural oestrogens on bone metabolism in rats administered a long-acting gonadotrophin-releasing hormone agonist. J Endocrinol 165:467–473 [DOI] [PubMed] [Google Scholar]
- Gilbert AN 1984 Postpartum and lactational estrus: a comparative analysis in rodentia. J Comp Psychol 98:232–245 [PubMed] [Google Scholar]
- Sun L, Peng Y, Sharrow AC, Iqbal J, Zhang Z, Papachristou DJ, Zaidi S, Zhu LL, Yaroslavskiy BB, Zhou H, Zallone A, Sairam MR, Kumar TR, Bo W, Braun J, Cardoso-Landa L, Schaffler MB, Moonga BS, Blair HC, Zaidi M 2006 FSH directly regulates bone mass. Cell 125:247–260 [DOI] [PubMed] [Google Scholar]
- Iwaniec UT, Yuan D, Power RA, Wronski TJ 2006 Strain-dependent variations in the response of cancellous bone to ovariectomy in mice. J Bone Miner Res 21:1068–1074 [DOI] [PubMed] [Google Scholar]
- Adami S, Gatti D, Bertoldo F, Rossini M, Fratta-Pasini A, Zamberlan N, Facci E, Lo Cascio V 1992 The effects of menopause and estrogen replacement therapy on the renal handling of calcium. Osteoporos Int 2:180–185 [DOI] [PubMed] [Google Scholar]
- Dick IM, Devine A, Beilby J, Prince RL 2005 Effects of endogenous estrogen on renal calcium and phosphate handling in elderly women. Am J Physiol Endocrinol Metab 288:E430–E435 [DOI] [PubMed] [Google Scholar]
- Oz OK, Hajibeigi A, Howard K, Cummins CL, van Abel M, Bindels RJ, Word RA, Kuro-o M, Pak CY, Zerwekh JE 2007 Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice. J Bone Miner Res 22:1893–1902 [DOI] [PubMed] [Google Scholar]
- Horwitz MJ, Tedesco MB, Sereika SM, Hollis BW, Garcia-Ocaña A, Stewart AF 2003 Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab 88:1603–1609 [DOI] [PubMed] [Google Scholar]
- Syed MA, Horwitz MJ, Tedesco MB, Garcia-Ocaña A, Wisniewski SR, Stewart AF 2001 Parathyroid hormone-related protein-(1-36) stimulates renal tubular calcium reabsorption in normal human volunteers: implications for the pathogenesis of humoral hypercalcemia of malignancy. J Clin Endocrinol Metab 86:1525–1531 [DOI] [PubMed] [Google Scholar]
- Lorget F, Kamel S, Mentaverri R, Wattel A, Naassila M, Maamer M, Brazier M 2000 High extracellular calcium concentrations directly stimulate osteoclast apoptosis. Biochem Biophys Res Commun 268:899–903 [DOI] [PubMed] [Google Scholar]
- Mentaverri R, Yano S, Chattopadhyay N, Petit L, Kifor O, Kamel S, Terwilliger EF, Brazier M, Brown EM 2006 The calcium sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J 20:2562–2564 [DOI] [PubMed] [Google Scholar]
- Fudge NJ, Kovacs CS 2004 Physiological studies in heterozygous calcium sensing receptor (CaSR) gene-ablated mice confirm that the CaSR regulates calcitonin release in vivo. BMC Physiol 4:5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, Mithal A, Gagel RF, Brown EM 1995 Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology 136:5202–5211 [DOI] [PubMed] [Google Scholar]
- Woodrow JP, Sharpe CJ, Fudge NJ, Hoff AO, Gagel RF, Kovacs CS 2006 Calcitonin plays a critical role in regulating skeletal mineral metabolism during lactation. Endocrinology 147:4010–4021 [DOI] [PubMed] [Google Scholar]
- Thiede MA 1989 The mRNA encoding a parathyroid hormone-like peptide is produced in mammary tissue in response to elevations in serum prolactin. Mol Endocrinol 3:1443–1447 [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Fisher JE, Thiede MA, Caulfield MP, Rosenblatt M, Duong LT 1992 Concentrations of parathyroid hormone-related protein in rat milk change with duration of lactation and interval from previous suckling, but not with milk calcium. Endocrinology 130:741–747 [DOI] [PubMed] [Google Scholar]
- Markov AG 1991 [The duration of feedings and the intervals between them in mice during established lactation]. Fiziol Zh SSSR Im I M Sechenova 77:142–148 [PubMed] [Google Scholar]
- Speakman JR, Gidney A, Bett J, Mitchell IP, Johnson MS 2001 Limits to sustained energy intake. IV. Effect of variation in food quality on lactating mice Mus musculus. J Exp Biol 204:1957–1965 [DOI] [PubMed] [Google Scholar]
- Zhao ZJ, Chi QS, Cao J 2010 Milk energy output during peak lactation in shaved Swiss mice. Physiol Behav 101:59–66 [DOI] [PubMed] [Google Scholar]
- Laskey MA, Prentice A, Hanratty LA, Jarjou LM, Dibba B, Beavan SR, Cole TJ 1998 Bone changes after 3 mo of lactation: influence of calcium intake, breast-milk output, and vitamin D-receptor genotype. Am J Clin Nutr 67:685–692 [DOI] [PubMed] [Google Scholar]
- Burger HG, Hee JP, Mamers P, Bangah M, Zissimos M, McCloud PI 1994 Serum inhibin during lactation: relation to the gonadotrophins and gonadal steroids. Clin Endocrinol (Oxf) 41:771–777 [DOI] [PubMed] [Google Scholar]
- Kremer JA, Schellekens LA, Segers MF, Thomas CM, Rolland R 1994 Circulating inhibin levels in lactating and nonlactating women. Fertil Steril 62:1150–1156 [DOI] [PubMed] [Google Scholar]
- Yohkaichiya T, O'Connor A, de Kretser DM 1991 Circulating immunoreactive inhibin, gonadotropin, and prolactin levels during pregnancy, lactation, and postweaning estrous cycle in the rat. Biol Reprod 44:6–12 [DOI] [PubMed] [Google Scholar]
- Nicks KM, Fowler TW, Gaddy D 2010 Reproductive hormones and bone. Curr Osteoporos Rep 8:60–67 [DOI] [PubMed] [Google Scholar]
- Elabd C, Basillais A, Beaupied H, Breuil V, Wagner N, Scheideler M, Zaragosi LE, Massiéra F, Lemichez E, Trajanoski Z, Carle G, Euller-Ziegler L, Ailhaud G, Benhamou CL, Dani C, Amri EZ 2008 Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 26:2399–2407 [DOI] [PubMed] [Google Scholar]
- Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, Colucci S, Grano M, Faccio R, Liu X, Li J, Usmani S, Bachar M, Bab I, Nishimori K, Young LJ, Buettner C, Iqbal J, Sun L, Zaidi M, Zallone A 2009 Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci USA 106:7149–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seriwatanachai D, Krishnamra N, van Leeuwen JP 2009 Evidence for direct effects of prolactin on human osteoblasts: Inhibition of cell growth and mineralization. J Cell Biochem 107:677–685 [DOI] [PubMed] [Google Scholar]
- Seriwatanachai D, Thongchote K, Charoenphandhu N, Pandaranandaka J, Tudpor K, Teerapornpuntakit J, Suthiphongchai T, Krishnamra N 2008 Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor κB ligand/osteoprotegerin ratio. Bone 42:535–546 [DOI] [PubMed] [Google Scholar]
- Takeda S, Karsenty G 2008 Molecular bases of the sympathetic regulation of bone mass. Bone 42:837–840 [DOI] [PubMed] [Google Scholar]
- Wong IP, Zengin A, Herzog H, Baldock PA 2008 Central regulation of bone mass. Semin Cell Dev Biol 19:452–458 [DOI] [PubMed] [Google Scholar]
- Zengin A, Zhang L, Herzog H, Baldock PA, Sainsbury A 2010 Neuropeptide Y and sex hormone interactions in humoral and neuronal regulation of bone and fat. Trends Endocrinol Metab 21:411–418 [DOI] [PubMed] [Google Scholar]
- Chen P, Smith MS 2003 Suckling-induced activation of neuronal input to the dorsomedial nucleus of the hypothalamus: possible candidates for mediating the activation of DMH neuropeptide Y neurons during lactation. Brain Res 984:11–20 [DOI] [PubMed] [Google Scholar]
- Crowley WR, Ramoz G, Torto R, Keefe KA, Wang JJ, Kalra SP 2007 Neuroendocrine actions and regulation of hypothalamic neuropeptide Y during lactation. Peptides 28:447–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldock PA, Lee NJ, Driessler F, Lin S, Allison S, Stehrer B, Lin EJ, Zhang L, Enriquez RF, Wong IP, McDonald MM, During M, Pierroz DD, Slack K, Shi YC, Yulyaningsih E, Aljanova A, Little DG, Ferrari SL, Sainsbury A, Eisman JA, Herzog H 2009 Neuropeptide Y knockout mice reveal a central role of NPY in the coordination of bone mass to body weight. PLoS One 4:e8415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, Herzog H 2002 Hypothalamic Y2 receptors regulate bone formation. J Clin Invest 109:915–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammie SC, Hasen NS, Awad TA, Auger AP, Jessen HM, Panksepp JB, Bronikowski AM 2005 Gene array profiling of large hypothalamic CNS regions in lactating and randomly cycling virgin mice. Brain Res Mol Brain Res 139:201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.