Abstract
Tuberoinfundibular peptide of 39 residues (TIP39) and the PTH-2 receptor (PTH2R) constitute a peptide-receptor neuromodulator system. Based on the abundance of TIP39 fibers and axonal terminals as well as PTH2R-containing neurons and their processes in the hypothalamic para- and periventricular and arcuate nuclei TIP39 has been suggested to play a role in neuroendocrine regulation. We showed previously that TIP39 expression decreased dramatically by adulthood. In the present study, using in situ hybridization histochemistry, real-time RT-PCR, and immunohistochemistry, we found that TIP39 mRNA and peptide expression levels are markedly elevated in the posterior intralaminar complex of the thalamus (PIL) of lactating dams, one of the three locations of TIP39-containing cell bodies in the brain. In addition, in mother rats, these TIP39 neurons showed Fos expression in response to pup exposure. Transection of TIP39 fibers originating in the PIL resulted in an ipsilateral disappearance of TIP39 immunoreactivity throughout the mediobasal hypothalamus of mother rats, suggesting that TIP39 fibers there arise from the PIL. To elucidate the function of TIP39 activation in dams, mothers separated from their pups for 4 h on postpartum d 9 received injection of a PTH2R antagonist into the lateral ventricle 5 min before returning the pups. Blood samples were taken seven times during the experimental period through jugular cannulae. The PTH2R antagonist administered in two different concentrations markedly inhibited suckling-induced elevation of plasma prolactin levels in a dose-dependent manner. These results suggest that TIP39 neurons in the PIL may regulate suckling-induced prolactin release in rat dams.
Posterior thalamic neurons were identified that may relay suckling information to the hypothalamus to induce prolactin release and lactation using a recently identified neuropeptide transmitter.
In lactating mothers, the pituitary hormone prolactin is primarily released to the circulation in response to suckling (1) and is essential to the production of milk in the mammary gland (2). Suckling-induced prolactin release from anterior pituitary lactotroph cells, a neuroendocrine reflex, is initiated by the stimulation of mechanoreceptors in and around the nipples (3). Stimulation, lesion, and tracer studies revealed that this reflex arch relays in the lateral cervical nucleus and traverses the lateral mesencephalic tegmentum (4,5,6,7,8,9,10). It has also been established that dopamine neurons in the arcuate nucleus are the major hypothalamic regulators of prolactin release from the pituitary (11); however, components of the pathway mediating suckling-induced prolactin release and its regulation and neurochemical characterization remain to be elucidated.
Tuberoinfundibular peptide of 39 residues (TIP39), purified from bovine hypothalamus, is the only known endogenous high-affinity agonist of the PTH-2 receptor (PTH2R) (12). Anatomical evidence supports formation of a peptide-receptor neuromodulator system by TIP39 and the PTH2R (13) because the location of TIP39 fibers present in limbic and endocrine forebrain regions is very similar to the distribution of PTH2R-containing neurons and their processes (14). In particular, both TIP39-containing fiber terminals and PTH2R-expressing cells and their fibers are abundant in the hypothalamic para- and periventricular, and arcuate nuclei (14,15). TIP39-expressing cells in the brain are restricted to the subparafascicular area of the thalamus and the medial paralemniscal nucleus of the pons (16,17). The former brain area provides TIP39 fibers to the forebrain and the latter one to the hindbrain (18). The subparafascicular area TIP39 neurons can be subdivided into the medially located group in the periventricular gray of the thalamus (PVG) and a laterally positioned group in the posterior intralaminar complex of the thalamus (PIL) as reviewed recently (13). This subdivision was based on the developmentally earlier appearance of TIP39 in the PIL (19) and the selective ejaculation-induced Fos activation in TIP39 neurons of the PIL in male rats (20). The PIL includes the posterior intralaminar thalamic nucleus, the parvicellular subparafascicular nucleus, and part of the lateral-most zona incerta, ventromedial to the medial geniculate body (13,17,21). This area is situated in the rostral end of the lateral mesencephalic tegmentum, demonstrates retrogradely labeled neurons after mediobasal hypothalamic injections (22) and Fos activation in lactating rats (23), suggesting that it may participate in the mediation of suckling-induced prolactin release.
We have reported a marked decrease in the level of TIP39 but not the PTH2R expression during the period of pubertal development (24). Furthermore, we previously found a marked increase in the expression level of the ligand of another peptide-receptor system in rat dams (25). Therefore, we hypothesized that TIP39 can also be induced to act on the available PTH2Rs. In the present study, the expression of TIP39 mRNA and peptide was investigated in mother rats. In addition, Fos activation was examined in TIP39 neurons in response to pup exposure. Subsequently, we tested whether activated TIP39 neurons project to the mediobasal hypothalamus where neurons regulating prolactin release from the pituitary are located (11). Finally, we addressed the function of TIP39 activation in suckling-induced prolactin release. Because transgenic mice lacking TIP39 are sterile due to a defect in germ cell development (26), we antagonized endogenous TIP39 in mother rats by injecting a recently developed selective peptide antagonist of the PTH2R (27) into the lateral ventricle and investigated its effect on plasma prolactin levels in response to suckling.
Materials and Methods
Animals
All animal experimentation was conducted in accordance to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments were carried out according to protocols approved by the Animal Examination Ethical Council of the Animal Protection Advisory Board at the Semmelweis University, Budapest, and meet the guidelines of the Animal Hygiene and Food Control Department, Ministry of Agriculture, Hungary. A total of 84 adult female Wistar rats (260–340 g body weight; Charles River Laboratories, Budapest, Hungary) were used. All of the animals were 90–120 d old when killed. Animals were kept in standard laboratory conditions with 12-h light, 12-h dark periods (lights on at 0600 h) and supplied with food and drinking water ad libitum. Pregnant and mother rats as well as their experimental control counterparts were individually housed. Three mother rats were excluded from the study because they delivered fewer than six pups or some of their pups died. The number of pups was adjusted to 10 within 2 d of delivery. Rats were anesthetized with an im injection of anesthetic mix containing 0.2 ml/300 g body weight ketamine (100 mg/ml) and 0.2 ml/300 g body weight xylazine (20 mg/ml) for surgery, perfusions, and dissections, which took place at 9–10 d postpartum for mothers.
Pup exposure of mother rats for Fos activation study
Rat dams (n = 6) were deprived of pups on postpartum d 8–9 at 1300 h. The following day at 0900 h, pups were returned to the cage of three mother rats, whereas three control rats remained isolated. All three mothers accepted the pups, and suckling started within 10 min. All animals were killed 22 h after removing the pups, i.e. 2 h after returning the pups to the three mothers for suckling. Animals were perfused transcardially and processed for Fos and TIP39 immunocytochemistry.
Transection of the supraoptic decussations
Mother rats (n = 3) were operated on 3 d after delivery. Holes of about 1 × 3 mm diameter were drilled into the right side of the skull above the target coordinate (antero-posterior [AP], −2.64; lateral [L], 3.0; ventral, 8.6 mm) 75° to the anteroposterior axis. Transections were performed with 2-mm-wide glass knives targeted to cut the supraoptic decussations perpendicularly. Animals were allowed to survive for 6 d after transections.
Microdissection of brain tissue samples
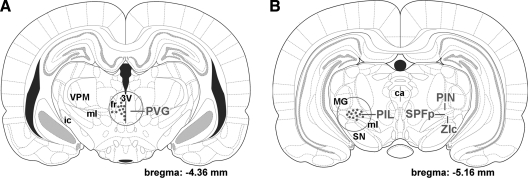
Brains of eight primiparous lactating mothers and eight primiparous mothers separated from pups immediately after parturition were removed on postpartum d 8–9 together with brains of eight age-matched nulliparous control female rats. Coronal brain sections (3 mm thick) were cut from the fresh brain around the subparafascicular area. A circular micropunch needle of 2 mm diameter was used subsequently to dissect the PVG medial to the fasciculus retroflexus (Fig. 1A) and the PIL ventromedial to the medial geniculate body (Fig. 1B). The dissected tissue samples were frozen and stored at −80 C.
Figure 1.
Localization of TIP39-expressing cell bodies in the posterior thalamus and brain regions dissected are shown in coronal drawings. The positions of TIP39-neurons are indicated by red dots, whereas the dissected brain area is shown by a red circle. A, Most TIP39 neurons in the PVG are distributed between the midline and the fasciculus retroflexus (fr). Some TIP39 neurons are located more ventrally along the third ventricle (3V) or more laterally below the fasciculus retroflexus. All of these TIP39 neurons were included in the dissection. B, The PIL is located somewhat more caudally than the PVG. TIP39 neurons are distributed in the posterior intralaminar thalamic nucleus (PIN), the parvicellular subparafascicular nucleus (SPFp), and the caudal-most part of the zona incerta (ZIc). Somewhat more rostrally, some TIP39 neurons are located medial to the major TIP39 cell group above the medial lemniscus (ml). These TIP39 neurons were also included in the dissection. ca, Cerebral aqueduct; ic, internal capsule; MG, medial geniculate body; SN, substantia nigra; VPM, ventral posteromedial thalamic nucleus.
Real-time RT-PCR
Total RNA was isolated from the microdissected PVG and PIL using TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. After diluting RNA to 2 μg/μl, it was treated with amplification-grade deoxyribonuclease I (Invitrogen), and cDNA was synthesized with a Superscript II reverse transcriptase kit (Invitrogen) according to the manufacturer’s instructions. After 10-fold dilution, 2.5 μl of the resulting cDNA was used as template in multiplex PCR using dual-fluorescence labeled TaqMan probes for TIP39 (6-FAM-CGCTAGCTGACGACGCGGCCT-TAMRA), and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH; JOE-ATGGCCTTCCGTGTTCCTACCCCC-TAMRA) as described previously (24). The primers for TIP39 (CTGCCTCAGGTGTTGCCCT and TGTAAGAGTCCAGCCAGCGG) were used at 300 nm, whereas the primers for GAPDH (CTGAACGGGAAGCTCACTGG and CGGCATGTCAGATCCACAAC) were used at 150 nm concentration. PCR were performed with iTaq DNA polymerase (Bio-Rad Laboratories, Hercules, CA). Cycle threshold values were obtained from the linear region of baseline-adjusted amplification curves. Standard curves for TIP39 and GAPDH were used to calculate the amount of cDNA in the samples.
In situ hybridization histochemistry for TIP39
Brains of three primiparous lactating mothers, three primiparous mothers separated from pups immediately after parturition, and three age-matched nulliparous control female rats were dissected and frozen on postpartum d 8–9 together with brains of eight age-matched nulliparous control female rats. The estrous stage of the control females was not analyzed because estrogen level did not have a detectable effect on TIP39 mRNA expression level (24). In situ hybridization was performed as described previously (16,17). Briefly, serial coronal sections (12 μm) were cut, mounted, dried, and stored at −80 C until use. In situ hybridization protocols are described in detail on the website http://intramural.nimh.nih.gov/lcmr/snge/Protocols/ISHH/ISHH.html. [35S]UTP-labeled riboprobes were generated using a MAXIscript transcription kit (Ambion, Austin, TX) from TIP39 cDNA subcloned into TOPO TA vectors (Invitrogen) and hybridized at 1 million decay per minute activity per slide. A region of the rat TIP39 cDNA sequence corresponding to amino acids −55 to 37, where amino acid 1 is the first residue of mature TIP39, was used to generate probes. Slides were dipped in NTB nuclear track emulsion (Eastman Kodak, Rochester, NY), stored for 3 wk at 4 C for autoradiography, developed, and fixed with Kodak Dektol developer and Kodak fixer, respectively, counterstained with Giemsa, and coverslipped.
Tissue collection for immunolabeling
Rats were deeply anesthetized and perfused transcardially with 150 ml saline followed by 300 ml ice-cold 4% paraformaldehyde prepared in phosphate buffer (PB; 0.1 m). Brains were removed and postfixed in 4% paraformaldehyde for 24 h and then transferred to PB containing 20% sucrose for 2 d. Serial coronal sections were cut at 50 μm on a sliding microtome 1.0–8.0 mm caudal to the bregma level. Sections were collected in PB and stored at 4 C.
TIP39 immunocytochemistry
Every fourth free-floating brain section of three primiparous lactating mothers and three primiparous mothers separated from pups immediately after parturition and killed 8–9 d postpartum, and three age-matched nulliparous control female rats as well as three rats with transection were immunostained for TIP39 as described previously (16,17). TIP39 was detected with an affinity-purified antiserum from a rabbit immunized with rat TIP39, which can be absorbed with synthetic TIP39 (16,17) and labels cell bodies with the same distribution as observed by in situ hybridization histochemistry (17,24). This antiserum (1:3000 for tyramide amplification and 1:700 for 3,3′-diaminobenzidine reaction) was applied for 48 h at room temperature followed by incubation of the sections in biotinylated antirabbit secondary antibody (1:800 dilution; Vector Laboratories, Burlingame, CA) and then in avidin-biotin-peroxidase complex (1:300; Vector) for 2 h. Subsequently, sections were treated with 0.06% diaminobenzidine or fluorescein isothiocyanate-tyramide (1:8000) and H2O2 in Tris-hydrochloride buffer (0.1 m, pH 8.0) for 6 min, mounted, and coverslipped.
Fos immunocytochemistry
Every fourth free-floating section of three brains per group (mothers on postpartum d 8–9 deprived of pups for 22 h and mothers deprived of pups for 20 h followed by pup exposure) was immunolabeled as described for TIP39 immunostaining except that a rabbit anti-Fos primary antiserum [1:20,000; c-Fos (4) sc-52; Santa Cruz Biotechnology, Santa Cruz, CA] was used.
Double immunolabeling of TIP39 and Fos
Every fourth free-floating section of the six rat dams used for single-labeling Fos were immunolabeled at first for TIP39, as described above. Sections were then placed in rabbit anti-Fos primary antiserum (1:10,000) for 48 h at room temperature. The sections were then incubated in Alexa 594 donkey antirabbit secondary antibody (1:500; Molecular Probes, Eugene, OR) for 2 h, washed in PB overnight, mounted, and coverslipped.
Analysis of TIP39 and Fos double immunolabeling
A section containing the PIL was randomly selected from each of the six animals double labeled for TIP39 and Fos. The total number of TIP39-immunoreactive (TIP39-ir) neurons with an identifiable cell nucleus as well as the number of double-labeled cells was counted using a ×20 objective of an Olympus BX60 light microscope equipped with fluorescent epi-illumination and a filter that allowed us to see both green and red colors. Subsequently, the number of single-labeled Fos-ir cells was also calculated in the area where TIP39 neurons were distributed.
Measuring the effect of [His4,Tyr5,Try6,His7] TIP39 (HYWY-TIP39) on suckling-induced prolactin release
Mother rats (n = 24) were implanted with intracerebroventricular cannulae 3–4 d postpartum and jugular catheters 8–9 d postpartum and received the antagonist challenge and had blood collected 1 d later, on d 9–10 postpartum.
Implantation of intracerebroventricular cannulae
Rats were fixed in a stereotaxic apparatus (Stoelting, Wood Dale, IL). The skin was cut over the skull and holes of about 2 mm diameter were drilled into the left side of the skull above the lateral ventricle positioned at the following coordinates: AP, −0.5; L, 1.4; ventral, 3.6 mm. Guide cannulae (Plastics One, Roanoke, VA) were inserted into the lateral ventricle and fixed to the skull with cranioplastic cement (Plastics One). Threaded dummy cannulae were placed and screwed into the guide cannulae.
Implantation of jugular cannulae
Dams received 25-mm-long sterile polyethylene jugular cannulae (Plastics One). A ventral cervical skin incision was made right of the midline with its caudal terminus at the level of the clavicle. The right common jugular vein was mobilized, and cannulae were inserted into the vessel and secured in place with suture. Incisions were made on the skin at the midline between the scapulae, and jugular cannulae were pulled through the scapular incisions. The cannulae were filled with heparinized saline and sealed with metal pins.
Antagonist injection and blood sampling before and during suckling
The effect of the PTH2R antagonist HYWY-TIP39 (27) was examined on postpartum d 9–10. Dams were separated from their pups between 0800 and 0900 h for 4 h. The first blood sample was taken before separation (−240 min). Five minutes before the end of the separation period (−5 min), a second blood sample was taken after which the animals received the intracerebroventricular injection. The three groups received 0.375, 0.075, or 0 mg (control) HYWY-TIP39 (corresponding to 75, 15, and 0 nmol HYWY-TIP39, respectively) dissolved in 6 μl sterile saline injected over 5 min. At the end of the injection, another blood sample was taken, and the pups were returned to the dams, which time was defined as 0 min. Additional blood samples were taken 5, 15, 30, and 60 min after reunion. At each time point, 300 μl blood was obtained, and the same amount of heparinized saline was injected back into the circulation. Blood was stored on ice temporarily followed by the separation of plasma, which was stored at −20 C until assayed for prolactin.
Prolactin assay
Prolactin was measured with RIA kits kindly provided by National Hormone and Pituitary Program, National Institute of Diabetes and Digestive and Kidney Diseases, and Dr. A. F. Parlow. The procedure was a slight modification of the instructions supplied with the kit as described previously (28). The chloramine-T method was used for iodination and protein A (BactASorb; Human Rt, Godollo, Hungary) for separation of bound and free hormone. Data collection and calculations for curve fitting were made using LKB Clinigamma software. The within- and between-assay variances were 10 and 14%, respectively. The sensitivity of the prolactin assay was 0.5 ng/ml rat plasma (or 25 pg prolactin). All samples were analyzed in duplicates. We used 50 μl plasma for each measurement.
Statistical analysis
Statistical analyses were performed using Prism 5 for Windows (GraphPad Software, Inc., La Jolla, CA). For RT-PCR measurements, mRNA levels of the three groups (control female, lactating mother, and mothers deprived of pups) were compared using one-way ANOVA followed by Bonferroni post-tests for post hoc comparisons.
For the suckling experiment, plasma prolactin levels of the three groups (injection of saline and lower and higher doses of the PTH2R antagonist) were compared at each of the seven time points using two-way repeated-measures ANOVA to evaluate whether the antagonist injection had an effect on the prolactin level. To determine at which time points the antagonist injection was effective, the three groups (injection of saline and lower and higher doses of the PTH2R antagonist) were compared using one-way ANOVA followed by Bonferroni post-tests for post hoc comparisons.
Histological analysis
Sections were examined using an Olympus BX60 light microscope equipped with fluorescent epi-illumination and dark-field condenser. Images were captured at 2048 × 2048-pixel resolution with a SPOT Xplorer digital CCD camera (Diagnostic Instruments, Sterling Heights, MI). Confocal images were acquired with a Nikon Eclipse E800 confocal microscope equipped with a Bio-Rad Radiance 2100 Laser Scanning System using a ×20–60 objectives at an optical thickness of 1–3 μm.
Contrast and sharpness of the images were adjusted using the levels and sharpness commands in Adobe Photoshop CS version 8.0. Full resolution of the images was maintained until the final versions were adjusted to a resolution of 300 dpi.
Results
Induction of TIP39 mRNA in the posterior thalamus by in situ hybridization
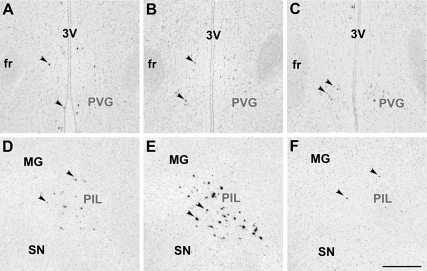
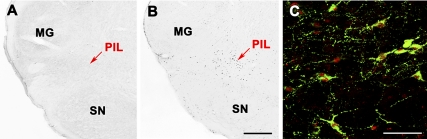
In the forebrain of lactating mother rats, TIP39 neurons were found in the ventral part of the posterior thalamus concentrated in the PVG medial to the fasciculus retroflexus (Fig. 2, A–C) and the PIL, an area ventromedial to the medial geniculate body (Fig. 2, D–F). TIP39 mRNA-containing neurons in the PIL were situated in the posterior intralaminar thalamic nucleus, the parvicellular (lateral) subparafascicular nucleus, and the caudal portion of the zona incerta. Apart from these posterior thalamic locations, we detected no signal for TIP39 mRNA in the examined parts of the brain in lactating mothers. This location of TIP39 neurons was the same as that described earlier in young adult male and female rats (17). However, the intensity of the autoradiography signal was higher in the PIL of mother rats (Fig. 2E). Neurons with high-intensity signal were evenly distributed (Fig. 2E). In contrast, the vast majority of TIP39 neurons in the PVG showed a low-intensity signal in mothers (Fig. 2B).
Figure 2.
Bright-field photomicrographs of in situ hybridization histochemical sections demonstrate the expression of TIP39 mRNA in the PVG (A–C) and the PIL (D–F). Arrowheads indicate some examples of the autoradiography signal above TIP39-expressing neurons. In the PVG, most TIP39 neurons have a weak autoradiography signal in control female (A) and lactating mother (B) as well as in a pup-deprived mother (C). In contrast, the low level of TIP39 mRNA in the control female (D) is increased in all parts of the PIL in lactating mother (E), whereas removing the pups reduced the level of TIP39 mRNA as indicated by the low density of TIP39-expressing cells and their weak labeling (F) similar to the image in D. fr, Fasciculus retroflexus; MG, medial geniculate body; SN, substantia nigra; 3V, third ventricle. Scale bar, 500 μm.
RT-PCR measurement of TIP39 mRNA levels in the PIL and PVG
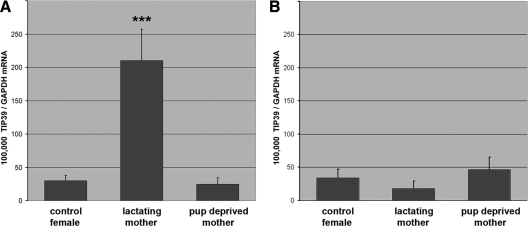
In the PIL, lactating mother rats had a 6.9 times higher level of TIP39 mRNA than age-matched nulliparous control female rats, whereas the TIP39 mRNA level decreased to the level of control females when the pups were taken away from mothers (Fig. 3A). The mRNA level of TIP39 (expressed as 100,000 × mRNA level of TIP39/mRNA level of GAPDH) was 30 ± 7 in control female rats, 210 ± 47 in lactating mother rats, and 25 ± 9 for mothers deprived of pups. These values represent a significant increase in the lactating rats with pups, based on one-way ANOVA. The change is in TIP39 mRNA because there was no difference in the level of GAPDH mRNA in the PIL among the three groups (4944 ± 1871 fg/μl in control females, 6018 ± 1726 fg/μl in rat dams, and 7894 ± 2064 fg/μl in mother rats deprived of pups). In contrast to changes in the PIL, there was no significant difference in the TIP39 mRNA level in the PVG (Fig. 3B) between the three groups as determined by one-way ANOVA. The measured values were the following (expressed as 100,000 × mRNA level of TIP39/mRNA level of GAPDH): 34 ± 13 in control female rats, 18 ± 11 in lactating mother rats, and 46 ± 19 for mothers deprived of pups. There was also no significant difference in the level of GAPDH mRNA among the three groups in the PVG (6277 ± 2323 fg/μl in control females, 3310 ± 919 fg/μl in rat dams, and 4712 ± 1099 fg/μl in mother rats deprived of pups).
Figure 3.
TIP39 is selectively induced in the PIL of rat dams as demonstrated by quantitative real-time RT-PCR. A, In the PIL, the level of TIP39 mRNA is significantly higher (***, P < 0.001) in lactating mothers than in age-matched nulliparous control female rats and mother rats deprived of pups immediately after delivery (n = 8 in each group) as revealed by using one-way ANOVA (F = 15.03). Bonferroni post-tests for post hoc comparisons further demonstrated that TIP39 mRNA level in the lactating mother was significantly (P < 0.001) higher than that in control female rats (t = 4.43) and mothers deprived of their pups (t = 5.03), whereas the TIP39 mRNA levels did not differ in the latter two groups (t = 0.08). B, In the PVG, there was a tendency for a reduced level of TIP39 mRNA in lactating mothers. However, there were no significant differences between rat dams and controls in the level of TIP39 mRNA in the PVG as determined by one-way ANOVA (F = 0.82). Data are expressed in the ratio of TIP39 to GAPDH mRNA.
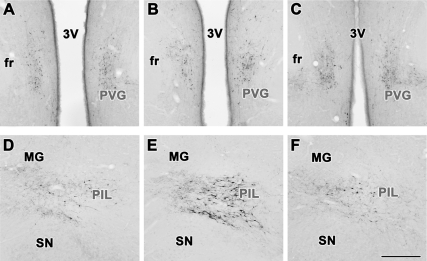
Induction of TIP39 immunoreactivity in the brain of rat dams
TIP39-ir cell bodies were present in the PVG (Fig. 4, A–C) as well as in the PIL (Fig. 4, D–F). In the PVG, we observed similarly weak TIP39 immunolabeling in control female rats (Fig. 4A), lactating mothers (Fig. 4B), and mothers separated from their pups immediately after delivery (Fig. 4C). In contrast, in the PIL, the intensity of immunolabeling was increased in lactating mother rats (Fig. 4E). Thus, a large number (more than 40 per section) of TIP39-ir cell bodies were observed in the PIL of rat dams (Fig. 4E), whereas only a few (fewer than 10 per section) TIP39-ir cell bodies were detected in the PIL of control female rats (Fig. 4D) and mothers separated from their pups immediately after delivery (Fig. 4F). In rat dams, these immunolabeled neuronal perikarya were located in the posterior intralaminar thalamic nucleus, the parvicellular (lateral) subparafascicular nucleus, and the caudal zona incerta (Fig. 4E). Thus, the distribution of TIP39-ir neurons in the PIL of lactating mother rats was the same as that of TIP39 mRNA-expressing neurons.
Figure 4.
TIP39-ir neurons in the PVG and the PIL. In the PVG, immunocytochemistry reveals TIP39-positive neuronal cell bodies in control females (A) and lactating mothers (B) as well as in pup-deprived mothers (C). The distribution of the relatively weakly stained cell bodies is similar in the three groups and also similar to that of TIP39 mRNA-containing neurons. In contrast, the PIL contains only a low density of weakly immunolabeled neuronal cell bodies the control female rats (D); however, intensely immunolabeled TIP39 cell bodies are distributed in all parts of the PIL in lactating mother (E). After removal of the pups, the intensity of the immunolabeling was reduced (F) to the level of the control female shown in D. fr, Fasciculus retroflexus; MG, medial geniculate body; SN, substantia nigra; 3V, third ventricle. Scale bar, 500 μm.
Fos activation of TIP39 neurons in rat dams in response to pup exposure
Suckling started within 10 min of returning the pups to mothers for each dam. Fos-ir neurons appeared in response to pup exposure in a number of brain regions including, for example, the medial preoptic nucleus, the anterior periventricular hypothalamic nucleus, the periaqueductal gray, and the lateral septal nucleus. Similar to these brain regions, very few Fos-ir neurons (fewer than 10 per section) were detected in the PIL of rat dams 22 h after separating them from their pups (Fig. 5A). However, when the dams were exposed to their pups for 2 h after 20 h of separation, Fos-ir nuclei (over 50 per section) appeared in the PIL (Fig. 5B). We observed that Fos-ir nuclei were evenly distributed within the PIL, whereas adjacent brain areas including the medial geniculate body and the substantia nigra remained devoid of Fos-ir cells. The number of TIP39-ir neurons with an identifiable nucleus in the PIL was 40 ± 5 in randomly selected sections (one section from each of three animals) containing the PIL. Double labeling revealed that 93% of TIP39-ir neurons in the PIL were Fos positive (Fig. 5C) because the number of Fos-positive TIP39 neurons in the counted PIL sections was 37 ± 5. The few TIP39-ir neurons whose cell bodies lacked Fos immunoreactivity did not seem to form a separate cell group but rather were present in all parts of the PIL. Interestingly, however, 27 ± 4 Fos-positive but TIP39-negative neurons were also observed in the PIL. In the region of the PVG where TIP39 neurons are distributed, only 10 ± 4 Fos-ir nuclei per section appeared in response to pup exposure. Furthermore, we did not find Fos-positive TIP39 neurons in this area (not shown).
Figure 5.
Fos activation in response to pup exposure. A, A very low density of Fos-immunopositive neurons are present in the PIL of mother rats deprived of pups for 22 h. B, The number of Fos-ir neurons (black dot-like structures) rises markedly in all parts of the PIL 2 h after the pups are given back to their mothers. C, Most TIP39 cells (green cell bodies) contain Fos (red-stained nuclei) in the PIL after pup exposure as demonstrated by double-fluorescent immunolabeling. Scale bar, 1 mm (B) and 100 μm (C).
Hypothalamic projection of TIP39 neurons from the PIL in rat dams
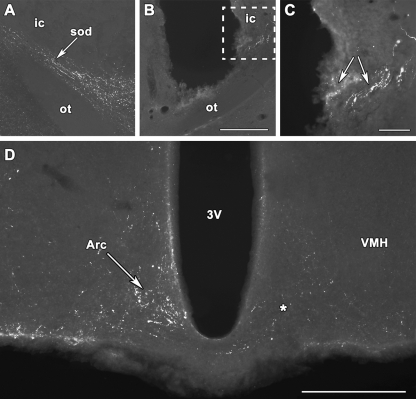
The arcuate, paraventricular, and periventricular nuclei of the hypothalamus contained a high density of TIP39-ir fibers in lactating mother rats. The distribution of TIP39 fibers and fiber terminals was similar in lactating and control female rats, although the observed intensity of immunolabeling was higher in mothers. TIP39 could be followed from the PIL toward the supraoptic decussations where a high density of TIP39 fibers was observed to project in a ventromedial direction (Fig. 6A). Transections reaching the optic tract ventrally at AP −2.64, L 3.0 mm from the bragma level resulted in the accumulation of TIP39 immunoreactivity immediately caudal to the transection within the fibers of the supraoptic decussations (Fig. 6, B and C). In these animals, a marked reduction was found in the density of TIP39-containing fibers and terminals in the arcuate (Fig. 6D), paraventricular, and periventricular nuclei, ipsilateral to the transection.
Figure 6.
Projection of TIP39 neurons is demonstrated to the arcuate nucleus in rat dams. A, TIP39 fibers project toward the hypothalamus in the supraoptic decussation. B, A transection perpendicular to the supraoptic decussations is shown in a coronal section. The disappearance of TIP39 fibers is observed medial to the transaction. C, A high-magnification photomicrograph showing the framed area in B demonstrates the accumulation of TIP39 immunoreactivity (arrows) in the supraoptic decussations immediately lateral to the transection. D, TIP39 fibers are abundant in the arcuate nucleus of rat dams (white arrow) contralaterally but disappeared from the arcuate nucleus on the side of transaction indicated by an asterisk. Arc, Arcuate nucleus; ic, internal capsule; ot, optic tract; sod, supraoptic decussations; VMH, ventromedial hypothalamic nucleus; 3V, third ventricle. Scale bar, 400 (B), 100 (C), and 500 (D) μm.
Antagonism of the PTH2R suppresses suckling-induced prolactin release in mother rats
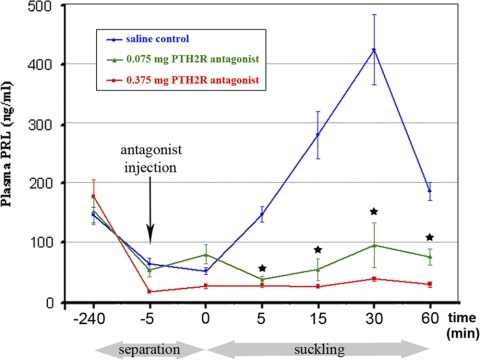
The plasma prolactin concentration was at a basal level by the end of the separation period (44 ± 10 ng/ml) and remained low immediately after the injection (0 min) of saline or the lower (0.075 mg) or higher (0.375 mg) concentration of HYWY-TIP39. At 0 min, the pups were returned to the mothers, which led to pup attachment and suckling. The plasma prolactin levels rose after suckling in the control group until 423 ± 118 ng/ml 30 min after reunion, whereas the antagonist injection significantly (P < 0.001) reduced the elevation in plasma prolactin level as determined by using two-way repeated-measures ANOVA (Fig. 7). Injection of 0.075 mg PTH2R antagonist into the lateral ventricle significantly (P < 0.05) reduced the suckling-induced elevation of the plasma prolactin level 5, 15, 30, and 60 min after giving back the pups as determined by using one-way ANOVA followed by Bonferroni post-tests for post hoc comparisons. Injection of 0.375 mg PTH2R antagonist completely prevented the prolactin release.
Figure 7.
Effect of the PTH2R antagonist HYWY-TIP39 on the suckling-induced prolactin release. Plasma prolactin levels are expressed in nanograms per milliliter. Each data point represents six to eight animals. The x-axis shows time (minutes) in a nonlinear fashion. Below the x-axis, the periods of pup separation and suckling are indicated in gray. The time points of significant changes are indicated by black stars. During the initial three time points (−240, −5, and 0 min), plasma prolactin levels in the three groups of animals did not differ significantly as determined by one-way ANOVA at each time point (F = 0.16, 2.58, and 2.51, respectively). Plasma prolactin concentration was measured again immediately before injection of saline (blue curve), 0.075 mg HYWY-TIP39 (green curve), or 0.375 mg HYWY-TIP39 (red curve) dissolved in saline. The plasma prolactin levels rose after suckling in the control group, whereas the antagonist injection significantly (P < 0.001) reduced the elevation in plasma prolactin level as demonstrated by using two-way repeated-measures ANOVA (F = 11.68). Plasma prolactin concentrations at 5, 15, 30, and 60 min after returning the pups were as follows (ng/ml): 145 ± 26, 280 ± 79, 423 ± 118, and 185 ± 31 after saline injection; 37 ± 13, 53 ± 36, 94 ± 76, and 75 ± 28 after the injection of 0.075 mg HYWY-TIP39; and 27 ± 8, 25 ± 7, 38 ± 8, and 28 ± 10 after the injection of 0.375 mg HYWY-TIP39, respectively. The suckling-induced prolactin release was significantly (P < 0.01) reduced by the injection of the antagonist at 5, 15, 30, and 60 min after returning the pups determined at each time point independently by one-way ANOVA (F = 12.36, 8.75, 5.55, and 11.01, respectively). Bonferroni post-tests after ANOVA for post hoc comparisons revealed that both 0.075 mg (P < 0.05) and 0.375 mg HYWY-TIP39 (P < 0.01) significantly reduced plasma prolactin levels at 5, 15, 30, and 60 min after returning the pups (for 0.075 mg HYWY-TIP39, t = 3.54, 3.05, 2.31, and 2.78; for 0.375 mg HYWY-TIP39, t = 4.51, 3.98, 3.11, and 4.56, respectively).
Discussion
The role of TIP39 in lactating mother rats was studied by several different approaches in the present study. Increased levels and expression of TIP39 in the PIL of mother rats were demonstrated by in situ hybridization histochemistry, real-time RT-PCR, and immunohistochemistry indicating that TIP39 is available to act on the PTH2R present in the mediobasal hypothalamus during lactation. The disappearance of TIP39 immunoreactivity from the mediobasal hypothalamus after lesions of TIP39 fibers arising from the PIL suggests that TIP39 neurons in the PIL project to this brain area, which is involved in the regulation of lactation. The finding that TIP39 neurons in the PIL are activated by pup exposure suggests that suckling is involved in the activation of these neurons. Finally, the remarkable inhibitory effect of the PTH2R antagonist on the prolactin release evoked by pup exposure also supports the conclusion that TIP39 may be involved in the physiological regulation of suckling-induced prolactin release.
Activation of TIP39 neurons
Induction of TIP39 in the PIL of lactating mother rats was suggested on the basis of in situ hybridization histochemistry and confirmed by the independent technique of RT-PCR. The distribution of TIP39 neurons in the PIL of lactating mother rats was similar to that described previously in young rats (17,24), suggesting that TIP39 reappears in the same neurons, which expressed it during earlier stages of ontogenic development. Furthermore, the increased number of immunolabeled neurons observed in the PIL of lactating mothers suggests that the elevated level of mRNA expression of TIP39 is also reflected in an increased synthesis of TIP39 peptide.
Fos, an immediate early gene expressed in activated cells (29), appears in the nuclei of TIP39 neurons of the PIL in response to suckling indicating an elevated activity of TIP39 neurons in lactating rat dams in this area. This finding confirmed previously reported expression of Fos in the PIL area of lactating rats (23). Furthermore, the finding that Fos appears in TIP39 neurons also suggests that TIP39 is induced in the PIL of rat dams by the suckling stimulus. However, our data do not provide information on the time course of TIP39 induction. It is possible that TIP39 is induced in response to mating, during pregnancy, or in the peripartum period in response to hormonal alterations, and pup exposure only maintains its elevated level. Furthermore, the type of pup-related stimulus that maintains elevated TIP39 levels remains undetermined. Apart from the suckling reflex, visual, auditory, or olfactory exteroceptive stimuli or hormonal changes associated with the presence of pups could also activate TIP39 neurons in response to pup exposure and could induce prolactin release (30,31).
It is particularly striking that TIP39 expression is induced only in the PIL, whereas the medial subparafascicular TIP39 cell group in the PVG continues to express a low level of TIP39 in lactating dams. Although TIP39 disappears from the PIL earlier than from the PVG during ontogeny (19), the adult levels are markedly reduced in both regions. Furthermore, brain areas that receive TIP39 axons exclusively from the PVG, including the lateral septal nucleus and the medial prefrontal cortex (18,32), also continue to possess a high PTH2R level (24), suggesting that this TIP39 cell group may also be activated in response to some physiological stimuli.
Neuronal connections of TIP39 neurons in the PIL
Neuronal inputs to TIP39 neurons in the PIL are largely unknown. However, afferent connections of the parvicellular subparafascicular nucleus, which is part of the PIL (13), have been studied by tracer injections in male rats (33). An area that expresses Fos in response to ejaculation was described to receive spinothalamic projection conveying genitosensory information (33,34). Because TIP39 neurons express Fos in response to ejaculation in male rats (20), spinal input to TIP39 neurons is expected.
Using electrical microstimulations and lesions, the pathway of the suckling reflex was described to traverse from the mesencephalic lateral tegmentum toward the hypothalamus in a position ventromedial to the medial geniculate body (4,35) where TIP39 neurons in the PIL are located. Destroying relay neurons in this area by local injections of ibotenic acid inhibited male ejaculation as well as the milk-ejection reflex (8). These data are consistent with the possibility that TIP39 neurons receive input related to the suckling stimulus.
Lesioning the PIL resulted in the loss of TIP39 immunoreactivity in the hypothalamus, whereas septal and limbic cortical TIP39 fibers disappeared only after lesions of the medial subparafascicular area in the PVG (18). Hypothalamic projections of an area partially overlapping with the PIL have also been established in a tracer study (36). TIP39 fibers were abundant in the supraoptic decussations (17) and could be followed to their origin in the PIL in the developing brain (19). Unilateral transection of this pathway in mother rats in the present study resulted in the disappearance of TIP39 fibers from the arcuate and periventricular nuclei, suggesting that their TIP39 fibers and fiber terminals originate in TIP39 neurons in the PIL. Hypothalamic transections probably including ascending TIP39 fibers have been reported to inhibit suckling-induced prolactin release (37).
Potential mechanisms of the inhibition of the suckling-induced prolactin release by HYWY-TIP39
The experimental design of suckling-induced prolactin release is known to elevate plasma prolactin levels within minutes of pups’ return to the mothers deprived of pups for 4 h, and plasma prolactin concentrations peak 30 min after the beginning of suckling (38). Saline injection into the lateral ventricle did not influence the prolactin level elicited by suckling, which matched the expected curve. Injection of the PTH2R antagonist HYWY-TIP39, however, dose-dependently blocked the elevation of plasma prolactin levels. HYWY-TIP39 binds selectively to the PTH2R (27), suggesting that HYWY-TIP39 injected into the lateral ventricle exerted its inhibitory action on the suckling-induced prolactin release via the PTH2R. PTH2R-expressing neurons are abundant in the periventricular and arcuate nuclei of the hypothalamus (14,15). PTH2Rs in these neurons are possible targets mediating the effect of HYWY-TIP39 on prolactin release. However, a direct effect of HYWY-TIP39 on dopaminergic neurons is not likely because the PTH2R was not double labeled with tyrosine hydroxylase (39,40), and because close appositions between tyrosine hydroxylase neurons and fiber terminals projecting to the mediobasal hypothalamus from the PIL were not detected (22). Therefore, HYWY-TIP39 might influence dopaminergic neurons in the mediobasal hypothalamus via interneurons expressing the PTH2R. Dynorphin-containing neurons in the arcuate nucleus are one of the candidates because they are innervated by axon terminals derived from the PIL (22), innervate tuberoinfundibular dopaminergic neurons (41), and may be responsible for the effects of opioid peptides on suckling-induced prolactin release by inhibiting tuberoinfundibular dopaminergic neurons (42,43,44). It is also a possibility that TIP39 evokes prolactin release by directly or indirectly stimulating prolactin-releasing substance-containing neurons (11,45).
The role of TIP39 in suckling-induced prolactin release
TIP39 neurons in the PIL are ideally positioned to receive sensory input from the nipples and transfer this information to the mediobasal hypothalamus. Fos expression by these neurons in response to pup exposure suggests that they are indeed activated by suckling. The presence of the TIP39 in these neurons supports the role of this neuropeptide in the regulation of maternal functions. The finding that TIP39 levels are markedly elevated in lactating mother rats provides additional evidence for a specific maternal function of TIP39. It is only in mothers that suckling can elicit prolactin release from the pituitary, and the blockade of this release by an antagonist of the receptor for TIP39 suggests that TIP39 plays a physiological role in the regulation of suckling-induced prolactin release. Anatomical evidence suggests that TIP39 neurons do not directly act on dopaminergic neurons in the mediobasal hypothalamus. Rather, a model is proposed in which TIP39 may excite interneurons in the arcuate nucleus, which in turn inhibit tuberoinfundibular dopamine neurons to induce prolactin release from the pituitary.
Acknowledgments
We thank Szilvia Deák for technical assistance.
Footnotes
This work was supported by Bolyai János Fellowship Grants, NFM-OTKA NNF78219 and NKTH-OTKA K67646 for A.D., OTKA NK72929 for M.P., OTKA K81522 for G.M.N., and NIMH IRP for T.B.U.
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 22, 2010
For editorial see page 5568
Abbreviations: AP, Antero-posterior; L, lateral; PB, phosphate buffer; PIL, posterior intralaminar complex of the thalamus; PTH2R, PTH type 2 receptor; PVG, periventricular gray of the thalamus; TIP39, tuberoinfundibular peptide of 39 residues; TIP39-ir, TIP39-immunoreactive.
References
- Neill JD, Nagy GM 1994 Prolactin secretion and its control. In: Knobil E, Neill JD, eds. Physiology of reproduction. New York: Raven Press; 1833–1860 [Google Scholar]
- Neville MC 2006 Lactation and its hormonal control. In: Neill JD, ed. Physiology of reproduction. Amsterdam: Academic Press; 2993–3054 [Google Scholar]
- Voogt JL, Lee Y, Yang S, Arbogast L 2001 Regulation of prolactin secretion during pregnancy and lactation. Prog Brain Res 133:173–185 [DOI] [PubMed] [Google Scholar]
- Tindal JS, Knaggs GS 1977 Pathways in the forebrain of the rat concerned with the release of prolactin. Brain Res 119:211–221 [DOI] [PubMed] [Google Scholar]
- Tindal JS, Knaggs GS, Turvey A 1969 The afferent path of the milk-ejection reflex in the brain of the rabbit. J Endocrinol 43:663–671 [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Armstrong WE, Tribollet E, Dreifuss JJ 1985 Somatosensory systems and the milk-ejection reflex in the rat. I. Lesions of the mesencephalic lateral tegmentum disrupt the reflex and damage mesencephalic somatosensory connections. Neuroscience 15:1111–1129 [DOI] [PubMed] [Google Scholar]
- Factor EM, Mayer AD, Rosenblatt JS 1993 Peripeduncular nucleus lesions in the rat: I. Effects on maternal aggression, lactation, and maternal behavior during pre- and postpartum periods. Behav Neurosci 107:166–185 [DOI] [PubMed] [Google Scholar]
- Hansen S, Köhler C 1984 The importance of the peripeduncular nucleus in the neuroendocrine control of sexual behavior and milk ejection in the rat. Neuroendocrinology 39:563–572 [DOI] [PubMed] [Google Scholar]
- Dubois-Dauphin M, Armstrong WE, Tribollet E, Dreifuss JJ 1985 Somatosensory systems and the milk-ejection reflex in the rat. II. The effects of lesions in the ventroposterior thalamic complex, dorsal columns and lateral cervical nucleus-dorsolateral funiculus. Neuroscience 15:1131–1140 [DOI] [PubMed] [Google Scholar]
- Tasker JG, Theodosis DT, Poulain DA 1986 Afferent projections from the mammary glands to the spinal cord in the lactating rat. I. A neuroanatomical study using the transganglionic transport of horseradish peroxidase-wheatgerm agglutinin. Neuroscience 19:495–509 [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy GM 2000 Prolactin: structure, function, and regulation of secretion. Physiol Rev 80:1523–1631 [DOI] [PubMed] [Google Scholar]
- Usdin TB, Hoare SR, Wang T, Mezey E, Kowalak JA 1999 TIP39: a new neuropeptide and PTH2-receptor agonist from hypothalamus. Nat Neurosci 2:941–943 [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB 2010 The TIP39-PTH2 receptor system: unique peptidergic cell groups in the brainstem and their interactions with central regulatory mechanisms. Prog Neurobiol 90:29–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber CA, Dobolyi A, Sleeman M, Usdin TB 2007 Distribution of tuberoinfundibular peptide of 39 residues and its receptor, parathyroid hormone 2 receptor, in the mouse brain. J Comp Neurol 502:563–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Palkovits M, Rusnak M, Mezey E, Usdin TB 2000 Distribution of parathyroid hormone-2 receptor-like immunoreactivity and messenger RNA in the rat nervous system. Neuroscience 100:629–649 [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Ueda H, Uchida H, Palkovits M, Usdin TB 2002 Anatomical and physiological evidence for involvement of tuberoinfundibular peptide of 39 residues in nociception. Proc Natl Acad Sci USA 99:1651–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Usdin TB 2003 Expression and distribution of tuberoinfundibular peptide of 39 residues in the rat central nervous system. J Comp Neurol 455:547–566 [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Palkovits M, Bodnár I, Usdin TB 2003 Neurons containing tuberoinfundibular peptide of 39 residues project to limbic, endocrine, auditory and spinal areas in rat. Neuroscience 122:1093–1105 [DOI] [PubMed] [Google Scholar]
- Brenner D, Bagó AG, Gallatz K, Palkovits M, Usdin TB, Dobolyi A 2008 Tuberoinfundibular peptide of 39 residues in the embryonic and early postnatal rat brain. J Chem Neuroanat 36:59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Coolen LM, Brown JL, Usdin TB 2006 Neurons containing tuberoinfundibular peptide of 39 residues are activated following male sexual behavior. Neuropeptides 40:403–408 [DOI] [PubMed] [Google Scholar]
- Ledoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ 1987 Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol 264:123–146 [DOI] [PubMed] [Google Scholar]
- Szabo FK, Snyder N, Usdin TB, Hoffman GE 2010 A direct neuronal connection between the subparafascicular and ventrolateral arcuate nuclei in non-lactating female rats. Could this pathway play a role in the suckling-induced prolactin release? Endocrine 37:62–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SH, Miyata S, Matsunaga W, Kawarabayashi T, Nakashima T, Kiyohara T 1998 Metabolic mapping of the brain in pregnant, parturient and lactating rats using fos immunohistochemistry. Brain Res 787:226–236 [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Wang J, Irwin S, Usdin TB 2006 Postnatal development and gender-dependent expression of TIP39 in the rat brain. J Comp Neurol 498:375–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobolyi A 2009 Central amylin expression and its induction in rat dams. J Neurochem 111:1490–1500 [DOI] [PubMed] [Google Scholar]
- Usdin TB, Paciga M, Riordan T, Kuo J, Parmelee A, petukova G, Camerini-Otero RD, Mezey E 2008 Tuberoinfundibular peptide of 39 residues is required for germ cell development. Endocrinology 149:4292–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J, Usdin TB 2007 Development of a rat parathyroid hormone 2 receptor antagonist. Peptides 28:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnár I, Bánky Z, Nagy GM, Halász B 2005 Non-NMDA glutamate receptor antagonist injected into the hypothalamic paraventricular nucleus blocks the suckling stimulus-induced release of prolactin. Brain Res Bull 65:163–168 [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T 1991 Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci 14:421–451 [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Saito TR, Furudate S, Takahashi KW 2001 Prolactin levels and maternal behavior induced by ultrasonic vocalizations of the rat pup. Exp Anim 50:307–312 [DOI] [PubMed] [Google Scholar]
- Terkel J, Damassa DA, Sawyer CH 1979 Ultrasonic cries from infant rats stimulate prolactin release in lactating mothers. Horm Behav 12:95–102 [DOI] [PubMed] [Google Scholar]
- Wang J, Palkovits M, Usdin TB, Dobolyi A 2006 Forebrain projections of tuberoinfundibular peptide of 39 residues (TIP39)-containing subparafascicular neurons. Neuroscience 138:1245–1263 [DOI] [PubMed] [Google Scholar]
- Coolen LM, Veening JG, Wells AB, Shipley MT 2003 Afferent connections of the parvocellular subparafascicular thalamic nucleus in the rat: evidence for functional subdivisions. J Comp Neurol 463:132–156 [DOI] [PubMed] [Google Scholar]
- Coolen LM, Allard J, Truitt WA, McKenna KE 2004 Central regulation of ejaculation. Physiol Behav 83:203–215 [DOI] [PubMed] [Google Scholar]
- Tindal JS, Knaggs GS 1971 Determination of the detailed hypothalamic route of the milk-ejection reflex in the guinea-pig. J Endocrinol 50:135–152 [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson Jr SJ 2000 Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo-pituitary-adrenocortical axis in response to audiogenic stress in rats: an anterograde and retrograde tract tracing study combined with Fos expression. J Comp Neurol 423:474–491. [PubMed] [Google Scholar]
- Bodnár I, Bánky ZS, Tóth BE, Nagy GM, Halász B 2002 Brain structures mediating the suckling stimulus-induced release of prolactin. J Neuroendocrinol 14:384–396 [DOI] [PubMed] [Google Scholar]
- Bodnár I, Mravec B, Kubovcakova L, Tóth EB, Fülöp F, Fekete MI, Kvetnansky R, Nagy GM 2004 Stress- as well as suckling-induced prolactin release is blocked by a structural analogue of the putative hypophysiotrophic prolactin-releasing factor, salsolinol. J Neuroendocrinol 16:208–213 [DOI] [PubMed] [Google Scholar]
- Dobolyi A, Irwin S, Wang J, Usdin TB 2006 The distribution and neurochemistry of the parathyroid hormone 2 receptor in the rat hypothalamus. Neurochem Res 31:227–236 [DOI] [PubMed] [Google Scholar]
- Usdin TB, Dobolyi A, Ueda H, Palkovits M 2003 Emerging functions for tuberoinfundibular peptide of 39 residues. Trends Endocrinol Metab 14:14–19 [DOI] [PubMed] [Google Scholar]
- Fitzsimmons MD, Olschowka JA, Wiegand SJ, Hoffman GE 1992 Interaction of opioid peptide-containing terminals with dopaminergic perikarya in the rat hypothalamus. Brain Res 581:10–18 [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL 1998 Endogenous opioid peptides contribute to suckling-induced prolactin release by suppressing tyrosine hydroxylase activity and messenger ribonucleic acid levels in tuberoinfundibular dopaminergic neurons. Endocrinology 139:2857–2862 [DOI] [PubMed] [Google Scholar]
- Callahan P, Klosterman S, Prunty D, Tompkins J, Janik J 2000 Immunoneutralization of endogenous opioid peptides prevents the suckling-induced prolactin increase and the inhibition of tuberoinfundibular dopaminergic neurons. Neuroendocrinology 71:268–276 [DOI] [PubMed] [Google Scholar]
- Selmanoff M, Gregerson KA 1986 Suckling-induced prolactin release is suppressed by naloxone and simulated by β-endorphin. Neuroendocrinology 42:255–259 [DOI] [PubMed] [Google Scholar]
- Andrews ZB 2005 Neuroendocrine regulation of prolactin secretion during late pregnancy: easing the transition into lactation. J Neuroendocrinol 17:466–473 [DOI] [PubMed] [Google Scholar]