Abstract
The Kiss1 gene and its product kisspeptin are important regulators of reproduction. In rodents, Kiss1 is expressed in the hypothalamic arcuate (ARC) and anteroventral periventricular (AVPV)/rostral periventricular (PeN) nuclei. In the AVPV/PeN, females have more Kiss1 and tyrosine hydroxylase (TH) neurons than males. We explored the ontogeny of the Kiss1 sex difference, and the role of cell death in establishing Kiss1 and TH cell number. We also determined whether Kiss1 cells in AVPV/PeN coexpress TH. AVPV/PeN Kiss1 neurons were first detected in both sexes on postnatal d 10, but the Kiss1 sex difference did not emerge until postnatal d 12. The role of BAX-mediated apoptosis in generating this sex difference was tested in adult Bax knockout (KO) and wild-type mice. Deletion of Bax did not diminish the sex difference in Kiss1 expression in the AVPV/PeN. TH expression was sexually dimorphic in the AVPV of both wild-type and Bax KO mice but, unlike Kiss1, was not sexually dimorphic in the PeN of either genotype. Double-label analysis determined that most Kiss1 neurons coexpress TH mRNA, but many TH neurons do not coexpress Kiss1, especially in the PeN. These findings suggest that several subpopulations of TH cells reside within the AVPV/PeN, only one of which coexpresses Kiss1. In the ARC, Kiss1 cell number was markedly increased in Bax KO mice of both sexes, indicating that although BAX-dependent apoptosis does not generate the sex difference in either Kiss1 or TH expression in AVPV/PeN, BAX does importantly regulate Kiss1 cell number in the ARC.
Kiss1 neurons in the hypothalamus co-express tyrosine hydroxylase and are developmentally regulated by BAX-mediated apoptosis, but the sex difference in Kiss1 neurons is independent of BAX signaling.
The neuroendocrine reproductive axis varies between males and females, with sex differences observed in the onset of puberty and the ability to generate estrogen-induced preovulatory LH surges (1,2,3,4,5). Additionally, sex differences exist in several reproductive health disorders, such as idiopathic hypogonadotropic hypogonadism, constitutional delayed puberty, and precocious puberty (4,6,7,8,9). Many of these sex differences likely reflect underlying differences in the nervous system, although in most cases, the specific neural populations involved are unknown. The final common pathway by which the brain controls reproduction is via GnRH neurons, which are themselves not sexually dimorphic. Numerous other brain populations, however, are sexually differentiated, such as the amygdala, bed nucleus of the stria terminalis (BNST), and several hypothalamic nuclei, including the anteroventral periventricular nucleus (AVPV) (9,10,11,12,13,14,15). In rodents, the AVPV is larger in overall volume and cell number in females than in males. Females also possess more γ-aminobutyric acid(GABA)/glutamate-expressing neurons and more dopaminergic neurons, identified by tyrosine hydroxylase (TH) expression, in the AVPV than do males (16,17,18,19). However, evidence implicating these AVPV subpopulations in sexually dimorphic functions has remained equivocal. In particular, despite the fact that AVPV TH neurons are a well-characterized sexually differentiated population, their physiological function remains poorly understood.
The Kiss1 gene, and its protein product kisspeptin, has recently been shown to be critical for puberty onset and adult fertility (20,21). Kisspeptin directly activates GnRH neurons (22,23,24,25,26) and, in rodents, is expressed in two discrete regions of the hypothalamus: the anatomical continuum comprising the AVPV and neighboring rostral periventricular nucleus (PeN) (AVPV/PeN) and, more caudally, the arcuate nucleus (ARC) (22,25,27). ARC Kiss1 neurons may mediate negative feedback effects of sex steroids on basal pulsatile GnRH secretion in both sexes, whereas AVPV/PeN Kiss1 neurons in females are thought to mediate estradiol’s (E2) positive feedback induction of the preovulatory LH surge (22,25,27). In rodents, the sex-specific ability of females to display an LH surge may reflect underlying sex differences in the AVPV/PeN Kiss1 population. The number of Kiss1-expressing neurons in the rat and mouse AVPV/PeN, as well as the amount of Kiss1 mRNA per cell, is much greater in females than males (28,29,30). These sex differences can be reversed by castrating newborn males or treating neonatal females with gonadal steroids (28,31,32,33), indicating that perinatal sex steroids organize the AVPV/PeN Kiss1 sex difference.
Although sexual differentiation of the AVPV/PeN Kiss1 system is clearly governed by postnatal sex steroids, it is unknown exactly how and when the Kiss1 sex difference arises. Several developmental mechanisms, such as neurogenesis, cell migration, and programmed cell death (apoptosis), could contribute (13,14,34), although thus far, differential apoptosis in males and females is the best established mechanism for generating sex differences in neuron number (15,16,35,36,37,38). Bcl-2 (B-cell lymphoma 2)-associated protein X (BAX) is a proapoptotic protein of the Bcl-2 family that is required for developmental apoptosis in many neural regions, including sexually dimorphic nuclei (39). In the developing rat AVPV, males have higher BAX protein and mRNA levels than females (15,40), and the sex difference in the total number of AVPV cells is eliminated in Bax knockout (KO) mice (16). By contrast, Bax KO mice still exhibit normal sexual differentiation of AVPV TH-immunoreactive neurons (16). It is currently unknown if the AVPV Kiss1 sex difference is caused by BAX-mediated apoptosis (like overall AVPV cell number) or is independent of BAX (like AVPV TH immunoreactivity).
Here, we examined the emergence of Kiss1 expression in the AVPV/PeN, focusing on when Kiss1 expression first occurs, whether this happens at the same age in both sexes, and whether the sex difference in Kiss1 neurons emerges concurrently or later than age of first expression. Next, we determined whether Kiss1 neurons are developmentally regulated by BAX-mediated apoptosis by analyzing Kiss1 cell number and Kiss1 mRNA per cell in the AVPV and PeN of adult male and female Bax KO mice and their wild-type (WT) littermates. Moreover, given observed similarities between Kiss1 and TH in neuroanatomical distribution, sexual differentiation, and degree of regulation by BAX, we also assessed whether these two cell types are in fact the same population by determining the degree of coexpression of Kiss1 and TH within the AVPV/PeN. Finally, because Bax is expressed in the rodent ARC and apoptosis occurs in this region during development (41,42), we also tested whether ARC Kiss1 neurons might be developmentally regulated by BAX-mediated apoptosis.
Materials and Methods
Animals
Experiment 1 used C57BL6 mice housed at the University of California, San Diego. Experiments 2–4 used mice with a targeted deletion of the Bax gene (Bax KO) on a C57BL/6J background (The Jackson Laboratory, Bar Harbor, ME) and their WT littermates. Offspring of Bax heterozygous breeding pairs were genotyped as described previously (16) and housed at the University of Massachusetts, Amherst. Animals in all experiments were housed on a 12-h light, 12-h dark cycle with food and water available ad libitum. All experiments were conducted in accordance with the National Institutes of Health Animal Care and Use Guidelines and with approval of the local Animal Care and Use Committees of the University of California, San Diego, and the University of Massachusetts, Amherst.
Surgical treatments and tissue collection
In adulthood, sex steroids up-regulate Kiss1 gene expression in the AVPV/PeN and down-regulate Kiss1 in the ARC (28,30,43,44), and AVPV TH expression may also be hormonally regulated (45). Thus, circulating sex steroid levels were equalized among groups in experiments 2–4. Adult WT and Bax KO mice (8–12 wk old) of both sexes were anesthetized and bilaterally gonadectomized (GDX). At the time of gonadectomy, one cohort received a sc E2-filled SILASTIC (Dow Corning Corp., Midland, MI) implant, constructed as previously described (39,42) and shown to produce physiological blood levels of E2 and to significantly regulate Kiss1 expression in the brains of adult mice (43,46). The remaining GDX mice, used in experiment 4, received no hormone implant. All animals were killed 9–10 d after surgery. Brains were collected, immediately frozen on dry ice, and stored at −80 C. Five series of 20-μm sections were cut and thaw-mounted onto Superfrost plus slides. Slides were stored at −80 C until processing via single or double-label in situ hybridization (ISH).
Single-label ISH
Single-label ISH for Kiss1 or TH mRNA was performed as previously described (28,46,47,48), using a validated Kiss1 riboprobe (47) or a cloned TH antisense riboprobe designed to bind bases 383–900 of the mouse TH mRNA sequence (GenBank accession no. NM_009377). Briefly, slide-mounted sections encompassing the AVPV/PeN or ARC were fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× sodium citrate, sodium chloride (SSC), delipidated in chloroform, dehydrated in ethanols, and air dried. Radiolabeled (P33) antisense riboprobe (0.05 pmol/ml) was combined with 1/20 volume yeast tRNA (Roche Biochemicals, Indianapolis, IN), heat denatured, added to hybridization buffer, and applied to each slide (100 μl/slide). Slides were coverslipped and placed in a humidity chamber at 55 C for 18 h. After hybridization, the slides were washed in 4× SSC and then placed into RNAse [37 mg/ml RNAse A in 0.15 m sodium chloride, 10 mm Tris, 1 mm EDTA (pH 8.0)] for 30 min at 37 C, then in RNAse buffer without RNAse at 37 C for 30 min. After a wash in 2× SSC at room temperature, slides were washed in 0.1× SSC at 62 C, dehydrated in ethanols, and air dried. Slides were then dipped in Kodak NTB emulsion, air dried, and stored at 4 C for 8–12 d (depending on the assay). Slides were then developed, dehydrated in ethanols, cleared in Citrasolv (Fisher Scientific, Pittsburgh, PA), and coverslipped with Permaslip (Sigma, St. Louis, MO). No signal was detected after application of sense probes.
Double-label ISH
Slides were treated similarly to single-label ISH with the following modifications. Digoxigenin (DIG)-labeled antisense Kiss1 cRNA was synthesized with DIG labeling mix (Roche Biochemicals) according to the manufacturer’s protocol. Radio-labeled (P33) antisense TH (0.05 pmol/ml) and DIG-labeled Kiss1 (1:400) riboprobes were denatured by boiling with tRNA, dissolved together in hybridization buffer, applied to slides (100 μl/slide), and hybridized for 18 h. After the 62 C washes on d 2, slides were incubated in 2× SSC with 0.05% Triton X-100 containing 2% sheep serum for 1 h at room temperature. The slides were then washed in buffer 1 [100 mm Tris-HCl (pH 7.5) and 150 mm NaCl] and incubated overnight at room temperature with anti-DIG antibody conjugated to alkaline phosphatase (diluted 1:200 in buffer 1 containing 1% sheep serum and 0.3% Triton X-100; Roche Biochemicals). The next day, slides were washed with buffer 1 and incubated with Vector Red alkaline phosphatase substrate (Vector Laboratories, Burlingame, CA) for 2 h at room temperature. The slides were then air dried, dipped in emulsion, stored at 4 C, and developed and coverslipped 10 d later.
Quantification and analysis of ISH data
ISH slides were analyzed with an automated image processing system by a person unaware of the treatment group of each slide (49). The system consists of a computer running custom grain-counting software (Don Clifton, University of Washington, Seattle, WA). For single-label experiments, the software counted the number of silver grain clusters representing cells, as well as the number of silver grains over each cell (a semiquantitative index of mRNA content per cell) (25,26,30,40). Cells were considered Kiss1 positive when the number of silver grains in a cluster exceeded that of background by 3-fold. For double-label assays, DIG-containing cells (Kiss1 cells) were identified under fluorescence microscopy, and the grain-counting software was used to quantify silver grains (representing TH mRNA) overlying each cell. Signal-to-background ratios for individual cells were calculated, and a cell was considered double-labeled if its signal-to-background ratio was more than 3.
Experiment 1, development of Kiss1 gene expression and the Kiss1 sex difference in the AVPV/PeN
Experiment 1 examined the ontogeny of postnatal Kiss1 mRNA expression in the AVPV/PeN to determine 1) when Kiss1 expression is first evident in the AVPV/PeN of each sex, and 2) when the Kiss1 sex difference first emerges. Intact male and female C57BL/6J mice aged 1 (day of birth), 3, 6, 8, 10, 12 14, and 16 d (five to seven animals/group) were killed and brains collected. Single-label ISH was used to compare the onset of Kiss1 mRNA expression in the AVPV/PeN of each sex.
Experiment 2A, Kiss1 expression in the AVPV/PeN of adult Bax KO and WT mice
This experiment tested the hypothesis that the number of Kiss1 cells in the AVPV/PeN is regulated by BAX-dependent apoptosis. E2 up-regulates Kiss1 levels in the AVPV/PeN of both sexes (28,43,46). Therefore, to maximize the detection of AVPV/PeN Kiss1 cells, we examined adult Bax KO and WT mice (seven to ten animals/group) that were GDX and treated with constant E2 for 10 d. The number of Kiss1-expressing cells in the AVPV and PeN, as well as the relative amount of Kiss1 mRNA per cell, was determined via single-label ISH. The stereotaxic coordinates for the AVPV and PeN correspond to plates 26–31 and 31–33, respectively, in the Franklin and Paxinos 2008 Mouse Brain Atlas.
Experiment 2B, TH gene expression in AVPV/PeN of adult Bax KO and WT mice
The role of BAX-dependent cell death in the sexual differentiation of dopaminergic neuron number in the AVPV (but not the PeN) has previously been explored by examining TH protein immunoreactivity in adult, gonadally intact Bax KO and WT mice (16). The results of experiment 2A led us to revisit this question using detection of TH mRNA in sex steroid-clamped adult WT and Bax KO animals. One series of AVPV/PeN sections from GDX+E2 males and females in experiment 2A was processed for single label TH ISH. The number of TH-expressing cells, as well as the relative amount of TH mRNA per cell, was determined for the AVPV and PeN of each animal, as above (n = 7–10 animals/group).
Experiment 3, Kiss1 and TH coexpression in the AVPV and PeN of WT mice
We previously determined that colocalization of TH and Kiss1 is low in the AVPV of female rats (28), suggesting two largely separate populations of cells in this region. However, in mice, TH and Kiss1 show strikingly similar neuroanatomical and sexually dimorphic patterns of gene expression in the AVPV (higher expression in females), and both genes also display a similar independence of developmental BAX signaling (experiment 2). These observed similarities led us to determine whether the Kiss1 and TH populations in the AVPV, and perhaps PeN, are in fact the same population of cells in mice or, as in rats, are two separate populations. One series of brain sections from WT males and females in experiment 2 was processed via double-label ISH for Kiss1 and TH expression in the AVPV and PeN. The extent of colabeling was determined for each region in both sexes (six to ten animals/group).
Experiment 4, effect of BAX-mediated apoptosis on Kiss1 neuron development in the ARC
Bax is expressed in the rodent ARC and apoptosis occurs in the region during development (41,42). Thus, this experiment determined whether Kiss1 neurons in the ARC are developmentally regulated by BAX-mediated apoptosis. In the adult ARC, Kiss1 expression is decreased by circulating sex steroids and is enhanced by low/absent sex steroids (30). To maximize detection of Kiss1-expressing neurons in the ARC, adult male and female Bax KO and WT mice were GDX and received no hormone replacement before killing 9 d later. ARC tissue was processed for single-label ISH for Kiss1 expression, as in earlier experiments (seven to nine animals/group).
Statistical analysis
All data are expressed as the mean ± sem for each group. In experiment 1, differences in group means were assessed via overall ANOVA, with post hoc analysis determined by Fisher’s least significant difference test. In experiments 2–4, differences were analyzed by two-way ANOVA, followed by post hoc comparisons via Fisher’s least significant difference. For all comparisons, statistical significance was set at P < 0.05. All analyses were performed with Statview 5.0.1 (SAS Institute, Cary, NC).
Results
Experiment 1, development of Kiss1 gene expression and the Kiss1 sex difference in the AVPV/PeN
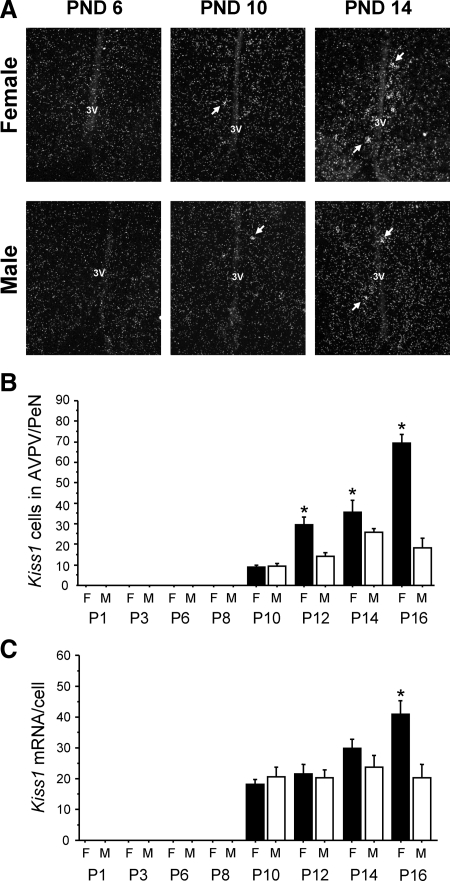
The development profile of postnatal Kiss1 mRNA expression in the AVPV/PeN was assessed in each sex to determine when Kiss1 expression begins and when the Kiss1 sex difference is first evident. No Kiss1-expressing neurons were detected in the AVPV/PeN in either sex on postnatal d (PND) 1, 3, 6, or 8. In both males and females, a small number of AVPV/PeN Kiss1 neurons was first detectable on PND 10, but there was no sex difference in Kiss1 expression at this age (Fig. 1). At PND 12, a sex difference in the AVPV/PeN was first evident, with females having approximately twice as many Kiss1 cells as males (P < 0.05) (Fig. 1). The sex difference in relative amount of Kiss1 mRNA per cell was not observed until PND 16 (P < 0.05) (Fig. 1). Thus, AVPV/PeN Kiss1 neurons are first detectable on PND 10 in both male and female mice, but the Kiss1 sex difference does not arise until several days later. This pattern contrasts with the ARC, where Kiss1 is present in both sexes on PND1 and at all PNDs examined (Poling, M. C., and A. S. Kauffman, unpublished data).
Figure 1.
Development of the Kiss1 sex difference in the AVPV/PeN of mice. A, Representative photomicrographs of ISH for Kiss1 mRNA in the AVPV/PeN of male (M) and female (F) mice on PND 6, 10, and 14. B, Mean number of Kiss1 neurons in the AVPV/PeN of females and males over the course of early postnatal development. Kiss1 cells were first detected in each sex on PND 10. The number of Kiss1 neurons was significantly higher in females than males on PND 12 and later. C, Mean level of Kiss1 mRNA/cell in the AVPV/PeN of females and males over the course of early postnatal development. This measure was not significantly higher in females than males until PND 16. *, Significantly different from males of same age.
Experiment 2A, Kiss1 expression in the AVPV/PeN of adult Bax KO and WT mice
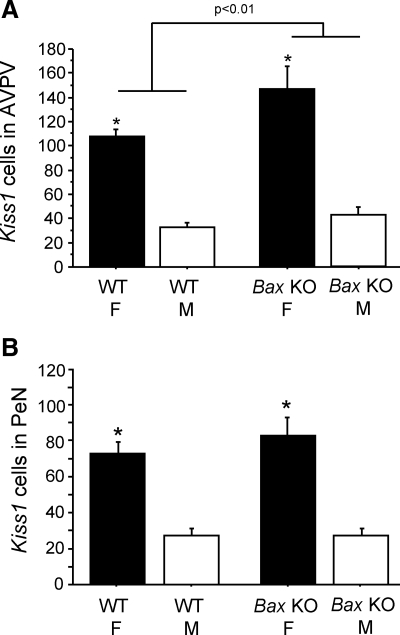
This experiment used Bax KO mice and their WT littermates to assess whether absolute Kiss1 cell number in the AVPV/PeN, or sexual differentiation of Kiss1 expression, is regulated by BAX-mediated apoptosis. Because BAX regulates cell death throughout the developing brain (39), we predicted that Bax KO mice of both sexes might have more Kiss1-expressing cells. Moreover, if BAX-dependent apoptosis is an important organizer of the Kiss1 sex difference, then this sex difference might be eliminated in mice lacking BAX. As expected, WT females had significantly more Kiss1 neurons in both the AVPV and PeN than did WT males (P < 0.01 for both regions). These sex differences persisted in Bax KO animals (P < 0.01) (Figs. 2 and 3), indicating that the Kiss1 sex differences in the AVPV and PeN are not dependent on BAX-mediated cell death. In the AVPV, there was a modest, but significant, main effect of genotype on Kiss1 cell number, such that Bax KO mice had more Kiss1 cells than WT mice (P < 0.01) (Fig. 3). In contrast, no effect of Bax genotype was seen in the PeN (Fig. 3). In both the AVPV and PeN, there was a main effect of sex on relative Kiss1 mRNA content per cell, with significantly greater (approximately three times higher) levels in females than males (P < 0.01), and this measure was not affected by genotype (data not shown).
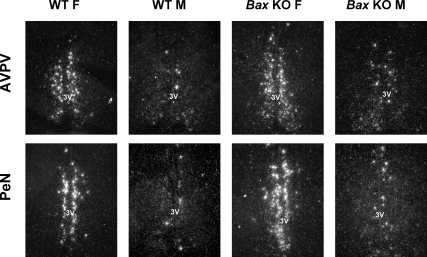
Figure 2.
Representative photomicrographs of Kiss1 labeling in the AVPV and PeN of adult female (F) and male (M) WT and Bax KO animals. All animals were GDX and treated with E2 for 10 d before brain collection. 3V, Third ventricle.
Figure 3.
Mean number of Kiss1 cells in the AVPV and PeN of adult WT and Bax KO females (F) and males (M) that were GDX and treated with E2 before killing. Kiss1 gene expression in both the AVPV (A) and PeN (B) was significantly higher in females than males in both genotypes. There were significantly more Kiss1 cells in Bax KO animals in the AVPV but not in the PeN. *, Significantly different from males of same genotype (P < 0.05).
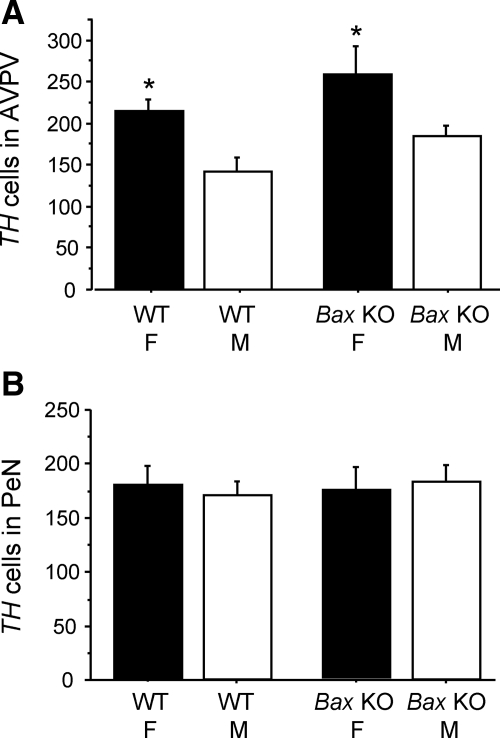
Experiment 2B, TH gene expression in AVPV and PeN of adult Bax KO and WT mice
We previously reported that sex differences in AVPV dopaminergic cell number, as measured by TH immunoreactivity, was independent of BAX (16). However, that study used gonadally intact mice and did not control for gender or genotypic differences in circulating sex steroid levels. Moreover, TH mRNA levels were not examined, nor was the PeN examined. This experiment assessed whether sexual differentiation of TH mRNA expression in the AVPV or PeN involves BAX-mediated apoptosis in E2-clamped GDX animals. Two-way ANOVA revealed a main effect of sex on AVPV TH cell number (P < 0.01), with no significant effect of genotype or sex-by-genotype interaction. We confirm that WT female mice have significantly more TH neurons in the AVPV than do WT males (P < 0.05) (Figs. 4 and 5). This AVPV TH sex difference does not depend on BAX-mediated apoptosis, because it was not eliminated in Bax KO mice (P < 0.05) (Figs. 4 and 5). Bax KO mice tended to have higher TH cell numbers in the AVPV relative to WT mice (Fig. 5), but this did not reach statistical significance (P = 0.09). Likewise, there was a tendency for higher TH mRNA content per cell in the AVPV of females relative to males that fell short of significance (P = 0.08) (data not shown).
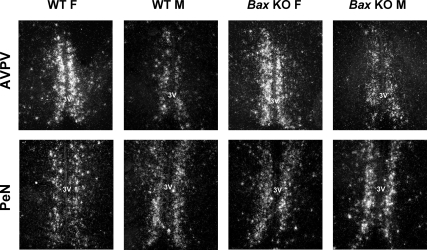
Figure 4.
Representative photomicrographs of TH expression in the AVPV and PeN of adult female (F) and male (M) WT and Bax KO animals. 3V, Third ventricle.
Figure 5.
Mean number of TH cells in the AVPV (A) and PeN (B) of adult WT and Bax KO females (F) and males (M). TH cell number in the AVPV was significantly greater in females than males in both genotypes. In contrast, there was no significant sex difference in the PeN. *, Significantly different from males of same genotype (P < 0.05).
Interestingly, in the PeN, TH neuron number was not significantly different between males and females of either genotype (Figs. 4 and 5), unlike PeN Kiss1 levels in experiment 2A. There also was no sex or genotype effect on relative TH mRNA content per cell in the PeN (data not shown).
Experiment 3, Kiss1 and TH coexpression in the AVPV and PeN
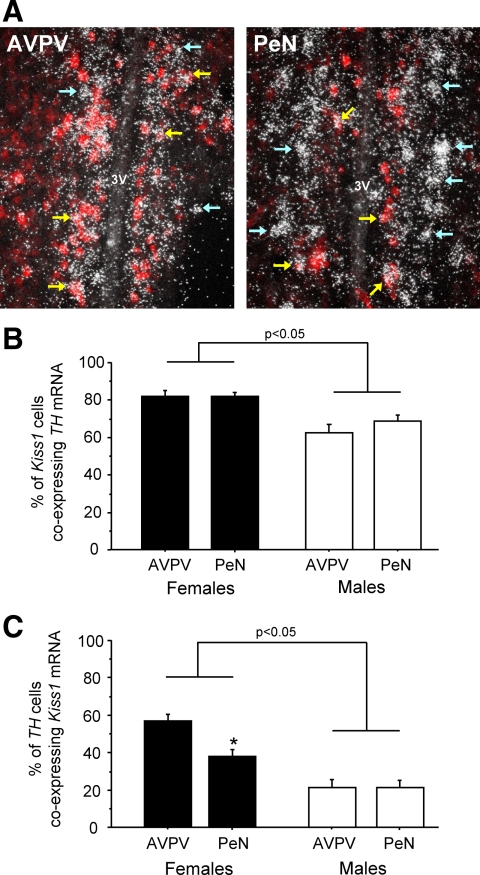
Given the similar anatomical expression patterns of the Kiss1 and TH genes in the AVPV/PeN, the fact that both cell types are more numerous in females, and that neither population is sexually differentiated via BAX-mediated apoptosis, it is possible that Kiss1 and TH cells are in fact the same neuronal populations. We tested this idea by measuring coexpression of Kiss1 and TH in the AVPV and PeN of WT male and female mice. In both regions, the majority of Kiss1 neurons coexpress TH mRNA (Fig. 6): approximately 80% of Kiss1 neurons in AVPV and PeN of females also express TH, with slightly lower coexpression in males (P < 0.05) (Fig. 6).
Figure 6.
A, Representative photomicrographs of Kiss1-TH coexpression in the AVPV and PeN of adult female mice. Red DIG staining, Kiss1 mRNA; white silver grains, TH mRNA. Yellow arrows denote example double-labeled cells; light blue arrows denote single-labeled TH cells. B, Mean percentage of Kiss1 neurons coexpressing TH mRNA in the AVPV and PeN of adult male and female mice. C, Mean percentage of TH cells coexpressing Kiss1 mRNA in the AVPV and PeN of adult males and females. *, Significantly different from AVPV in same sex.
Despite this high level of overlap between the two genes, we also noticed that many TH neurons in the AVPV and PeN did not coexpress Kiss1 (Fig. 6). Specifically, 56% of TH cells expressed Kiss1 in the AVPV in females, with even fewer (36%) TH cells coexpressing Kiss1 in the PeN. In males, only 20–25% of TH neurons colabeled with Kiss1 mRNA in either the AVPV or PeN (P < 0.05 vs. females in each region) (Fig. 6, B and C). Thus, in both sexes there appear to be many more TH neurons than Kiss1 neurons in both the AVPV and PeN, with many TH cells not coexpressing Kiss1. Moreover, whereas Kiss1 neurons closely apposed the third ventricle, many TH cells in both the caudal AVPV and PeN were found slightly more laterally (see Fig. 6A). These findings suggest that there are at least two subpopulations of TH cells in the AVPV/PeN region: one immediately adjacent to the third ventricle, which coexpresses Kiss1, and another that lies slightly more lateral and does not express Kiss1.
Experiment 4, effect of BAX-mediated apoptosis on Kiss1 neuron development in the ARC
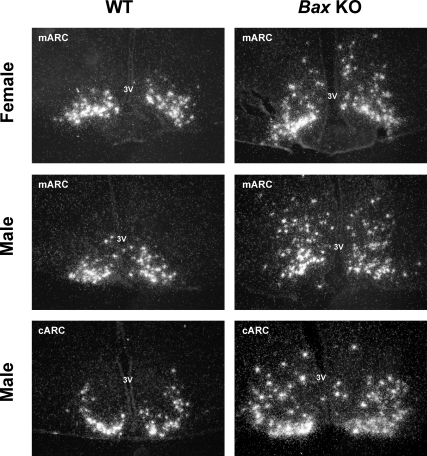
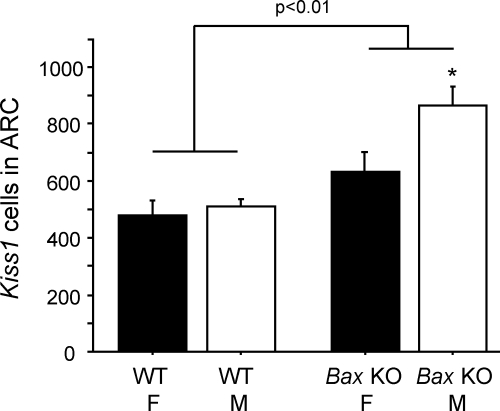
This experiment used GDX Bax KO and WT adult mice of both sexes to determine whether Kiss1 mRNA expression in the ARC is dependent on BAX-mediated apoptosis. As expected, there was no sex difference in Kiss1 cell number in the ARC. However, a striking main effect of genotype was observed, with Bax KO mice having more ARC Kiss1 cells than WT mice (P < 0.01) (Figs. 7 and 8). Many of the “extra” ARC Kiss1 cells of Bax KO animals appeared to be located dorsally, particularly evident in the middle and caudal regions of the ARC (Fig. 7), although we did not attempt to quantify this. Quantitatively, Bax KO mice of both sexes had significantly more Kiss1 neurons than WT mice of the same sex (P < 0.05) (Fig. 8). Interestingly, Bax KO males had more ARC Kiss1 cells than Bax KO females (P < 0.05) (Fig. 8). Unlike cell number, Kiss1 mRNA per cell did not significantly differ between Bax KO and WT mice (data not shown).
Figure 7.
Representative photomicrographs of Kiss1 gene expression in the ARC of GDX adult WT and Bax KO mice. 3V, Third ventricle; cARC, caudal ARC; mARC, medial ARC.
Figure 8.
A, Mean number of Kiss1 cells in the ARC of female (F) and male (M) Bax KO and WT animals that were GDX for 9 d before sacrifice. Kiss1 gene expression in the ARC was significantly higher in Bax KO mice than WT mice (P < 0.01), and this was especially true in males. *, Significantly different from female mice of same genotype.
Discussion
Sexual differentiation of Kiss1 neurons in the AVPV/PeN is well established and may be a neural mechanism underlying sexually dimorphic reproductive phenotypes, such as the ability of females to generate an LH surge or earlier puberty onset in females. Here, we provide new evidence on the ontogeny of this sex difference and the involvement of BAX-mediated apoptosis in shaping Kiss1 cell number in the AVPV/PeN and ARC. Bax gene KO caused a slight increase in Kiss1 cell number in the AVPV, but unlike a number of other sexually dimorphic traits in the brain, the sex difference in AVPV/PeN Kiss1 neurons is not dependent on BAX-mediated apoptosis. In contrast to the modest effect in the AVPV/PeN, Bax gene deletion caused a robust increase in Kiss1 cells in ARC. Thus, absolute Kiss1 cell number in ARC, but not the Kiss1 sex difference in AVPV/PeN, is regulated by BAX-dependent cell death. We also demonstrate that TH cells in the AVPV, but not PeN, are sexually dimorphic and that a majority of Kiss1 neurons in both the AVPV and PeN express TH mRNA. Many TH neurons do not coexpress Kiss1, however, arguing for the presence of multiple TH cell populations within the AVPV/PeN continuum.
We find that Kiss1 neurons in the AVPV/PeN are first detectable in both sexes on PND 10. This is the earliest age at which Kiss1 has been reported in the AVPV/PeN of developing rodents. A recent developmental study of kisspeptin protein expression in the mouse (50) found no kisspeptin-immunoreactive cells in the female AVPV/PeN at PND 10 and a small number of kisspeptin cells at PND 15 (the next age examined). Males were not included in that study. Whether the absence of significant kisspeptin-immunoreactive cells previously reported at PND 10 reflects differences in the sensitivities of the techniques used (immunocytochemistry vs. ISH) or a developmental delay between mRNA transcription and protein translation is unknown. Intriguingly, we found no sex difference in AVPV/PeN Kiss1 neurons at PND 10. This well-established sex difference was first evident, however, on PND 12, and became even more robust on PND 14 and 16. Thus, AVPV/PeN Kiss1 expression first arises at the same time in both sexes, but the sex difference takes several more days to develop.
It is possible that the presumptive Kiss1 neurons are not yet present in the AVPV/PeN before PND 10, but we consider this unlikely. The neurons in this region are generated well before birth (51,52). Moreover, perinatal gonadal steroid manipulations sexually differentiate the AVPV/PeN Kiss1 system (28,31,32), despite the fact that such treatments occur more than 1 wk before the first sign of Kiss1 expression. Thus, the AVPV/PeN Kiss1 neurons are likely present, and perhaps even sexually differentiated, before PND 10, but the Kiss1 gene is not transcriptionally active until PND 10 and later. The emergence of AVPV/PeN Kiss1 expression around PND 10 coincides with the reported onset of gonadal steroidogenesis in rodents (53,54,55,56). Whether the increased sex steroid production triggers the start of AVPV/PeN Kiss1 transcription, or whether the sex steroidogenesis is instead a downstream result of emerging AVPV/PeN Kiss1 signaling, remains to be determined. In adulthood, E2 stimulates AVPV/PeN Kiss1 expression via ERα (43) through an estrogen response element pathway (57); whether this also applies to earliest Kiss1 expression in development is unknown.
If Kiss1 cells are present but not yet transcriptionally active in the AVPV/PeN before PND 10, how does the sex difference in Kiss1 cell number develop? We examined the possibility that programmed cell death underlies the AVPV/PeN Kiss1 sex difference. Cell death in many brain regions, including the AVPV, depends on BAX (16,39), and we have observed a near absence of apoptotic cells in the AVPV region of Bax KO mice during the first postnatal week (Forger, N. G., unpublished data). However, Bax KO mice still exhibit a robust sex difference in Kiss1 cell number, with many more Kiss1 cells in Bax KO females than males in both the AVPV and PeN. This indicates that BAX-dependent cell death is not the mechanism responsible for the Kiss1 sex difference. This is somewhat unusual, as several other sex differences in neuron number, including overall cell number in AVPV, require BAX-dependent apoptosis (13,16,35). The Kiss1 data reported here, however, mirror our previous report that neither absolute TH cell number nor the sex difference in TH immunoreactivity in the AVPV is altered by Bax deletion (16). Here, we confirmed and extended this finding by analyzing TH mRNA expression in Bax KO and WT mice in which gonadal steroids were equalized across groups. Thus, sex differences in both Kiss1 and TH gene expression persist in the AVPV in the absence of BAX signaling.
Although BAX-mediated apoptosis is the primary route for programmed cell death in neurons, our data do not rule out the possibility that some other apoptosis pathways may be involved in producing the AVPV Kiss1 or TH sex difference (40). E2-induced changes in TH cell number in rat AVPV explants can be blocked with a caspase inhibitor, suggesting a cell death mechanism (37). Moreover, an alternate apoptotic pathway, along with changes in Bax expression, has recently been implicated in the differentiation of γ-aminobutyric acidergic neurons in the rat AVPV (40). Alternatively, it is possible that the Kiss1 and TH sex differences are not produced via apoptosis at all but are induced by other regulatory mechanisms. Recent findings point to a role for hormone-dependent epigenetic modifications in sexual differentiation of the brain (34,53). We suggest that developmental modifications in the epigenetic silencing/activating of the Kiss1 and TH genes may generate sex differences in their expression in adulthood, a possibility our labs are now pursuing.
Interestingly, unlike the AVPV, we find that TH expression is not sexually dimorphic in the rostral PeN in either Bax KO or WT mice. Thus, within the AVPV/PeN continuum, TH sexual dimorphism is restricted to the AVPV. This is in striking contrast to the Kiss1 system, which we found to be robustly sexually dimorphic in both the AVPV and PeN. As far as we know, this is the first study to analyze sexual differentiation of the TH population separately within these anatomical regions, and highlights an important distinction between the AVPV and PeN TH neurons. This finding suggests the possibility of two different TH populations along the AVPV/PeN continuum. Whether these TH populations serve similar or different functions remains to be determined.
Based on double-label ISH, we found a high degree of coexpression of Kiss1 and TH in both the AVPV and PeN, with approximately 80% of female Kiss1 neurons coexpressing TH mRNA, respectively. By contrast, in female rats, only about 20% of AVPV Kiss1 cells coexpressed TH under the same hormonal condition (28). Whether this is a functionally important species difference is not clear, but there are several notable differences in the neuroendocrine reproductive circuits of rats and mice (58). Our present findings of high colocalization of TH in Kiss1 neurons in mice, along with the fact that kisspeptin cells in AVPV/PeN project directly to GnRH neurons (29,59), suggest that the two molecules may be coreleased on GnRH neurons and that studies of kisspeptin/dopamine interactions might be fruitful. At present, the function of dopaminergic neurons in AVPV/PeN is completely unknown. Although some studies indicate an inhibitory effect of dopamine on LH secretion, others have not confirmed this (60,61,62,63,64,65).
Although we report that most Kiss1 cells in the mouse coexpress TH, the reverse is not true, especially in the PeN. We found that, in females, only 56% of TH neurons in the AVPV and 36% of TH cells in the PeN coexpress Kiss1. Thus, although most Kiss1 cells in the PeN coexpress TH, a majority of TH cells in this region do not coexpress Kiss1. This is not likely to be due to differences in the labeling efficiencies of our two probes, because, notably, most of the non-Kiss1-expressing TH cells were found lateral to the double-labeled Kiss1-TH cells, especially in the caudal AVPV and PeN. These observations again suggest several subpopulations of TH neurons in AVPV/PeN. Whether these different TH subpopulations have similar or different functions remains to be determined.
In contrast to the AVPV/PeN, Kiss1 neurons in the ARC are present at birth and all ages afterwards, are down-regulated by sex steroids, and are not sexually dimorphic in adulthood (28,30). However, the factors regulating the development of ARC Kiss1 neurons have not been studied. Bax is expressed in the rodent ARC, and apoptosis occurs in the region during development (41,42). Moreover, rodents treated with high doses of sex steroids or steroid agonists during early postnatal life exhibit varying degrees of decreased Kiss1 levels in the ARC in adulthood (28,66,67), raising the possibility that ARC Kiss1 neurons may be controlled during development by sex steroid-mediated apoptosis. Indeed, we find that both Bax KO males and females had significantly more Kiss1-expressing neurons in the ARC than WT animals. In many cases, Bax KO mice tended to have increased Kiss1 cells present dorsally, and sometimes laterally, in the ARC, suggesting that programmed cell death might developmentally regulate the final location of Kiss1 cells in the ARC. Intriguingly, Bax deletion increased Kiss1 cell number more in males than in females, despite the fact that there is not normally an adult sex difference in Kiss1 cell number in this region. This raises the possibility that early in development, males may possess more ARC Kiss1 neurons than females, but that this is subsequently offset by a higher rate of perinatal apoptosis in males. In this model, lack of a sex difference in adult ARC Kiss1 neurons is actively regulated during early development, a process which De Vries et al. (68) have recently discussed in terms of compensatory mechanisms serving to eliminate adult sex differences in the brain.
Collectively, these findings advance our understanding of the development and regulation of hypothalamic Kiss1 and TH neurons. We provide new evidence on the developmental time course of both Kiss1 expression and the emergence of the AVPV/PeN Kiss1 sex difference, as well as the role of BAX-mediated apoptosis in governing the development of Kiss1 cells in the AVPV/PeN and ARC. We also provide novel observations on the TH-expressing cells in the AVPV and PeN and show that most Kiss1 cells in the AVPV/PeN are highly colabeled with TH mRNA, but a large population of AVPV/PeN TH neurons does not coexpress Kiss1.
Acknowledgments
We thank Megan Varnum and Jill McCutcheon for technical support on this project.
Footnotes
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Grants R00 HD056157 (to A.S.K.) and T32 HD07203 (to S.J.S.) and National Institutes of Health (NIH) Grant R01 MH068482 (to N.G.F.). Additional support to A.S.K. was provided by a NIH funded DERC pilot grant as well as the NICHD through cooperative agreement U54 HD012303, as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 6, 2010
Abbreviations: ARC, Arcuate nucleus; AVPV, anteroventral periventricular nucleus; BAX, B-cell lymphoma 2-associated protein X; BNST, bed nucleus of the stria terminalis; DIG, digoxigenin; E2, estradiol; GDX, gonadectomized; ISH, in situ hybridization; KO, knockout; PeN, rostral periventricular nucleus; PND, postnatal day; SSC, sodium citrate; sodium chloride; TH, tyrosine hydroxylase; WT, wild type.
References
- Corbier P, Roffi J, Rhoda J 1983 Female sexual behavior in male rats: effect of hour of castration at birth. Physiol Behav 30:613–616 [DOI] [PubMed] [Google Scholar]
- Grumbach MM 2002 The neuroendocrinology of human puberty revisited. Horm Res 57(Suppl 2):2–14 [DOI] [PubMed] [Google Scholar]
- Corbier P 1985 Sexual differentiation of positive feedback: effect of hour of castration at birth on estradiol-induced luteinizing hormone secretion in immature male rats. Endocrinology 116:142–147 [DOI] [PubMed] [Google Scholar]
- Fechner PY 2002 Gender differences in puberty. J Adolesc Health 30:44–48 [DOI] [PubMed] [Google Scholar]
- Handa RJ, Corbier P, Shryne JE, Schoonmaker JN, Gorski RA 1985 Differential effects of the perinatal steroid environment on three sexually dimorphic parameters of the rat brain. Biol Reprod 32:855–864 [DOI] [PubMed] [Google Scholar]
- Cesario SK, Hughes LA 2007 Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs 36:263–274 [DOI] [PubMed] [Google Scholar]
- Fechner A, Fong S, McGovern P 2008 A review of Kallmann syndrome: genetics, pathophysiology, and clinical management. Obstet Gynecol Surv 63:189–194 [DOI] [PubMed] [Google Scholar]
- Bianco SD, Kaiser UB 2009 The genetic and molecular basis of idiopathic hypogonadotropic hypogonadism. Nat Rev Endocrinol 5:569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semaan SJ, Kauffman AS 2010 Sexual differentiation and development of forebrain reproductive circuits. Curr Opin Neurobiol 20:424–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM 2004 Sexual differentiation of the vertebrate nervous system. Nat Neurosci 7:1034–1039 [DOI] [PubMed] [Google Scholar]
- Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM 1998 Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol 19:323–362 [DOI] [PubMed] [Google Scholar]
- Simerly RB 2002 Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci 25:507–536 [DOI] [PubMed] [Google Scholar]
- Forger NG 2009 Control of cell number in the sexually dimorphic brain and spinal cord. J Neuroendocrinol 21:393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Schwarz JM, Wright CL, Dean SL 2008 Mechanisms mediating oestradiol modulation of the developing brain. J Neuroendocrinol 20:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S 2009 Sex differences and the roles of sex steroids in apoptosis of sexually dimorphic nuclei of the preoptic area in postnatal rats. J Neuroendocrinol 21:370–376 [DOI] [PubMed] [Google Scholar]
- Forger NG, Rosen GJ, Waters EM, Jacob D, Simerly RB, de Vries GJ 2004 Deletion of Bax eliminates sex differences in the mouse forebrain. Proc Natl Acad Sci USA 101:13666–13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Zee MC, Pendleton JW, Lubahn DB, Korach KS 1997 Estrogen receptor-dependent sexual differentiation of dopaminergic neurons in the preoptic region of the mouse. Proc Natl Acad Sci USA 94:14077–14082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW 1987 The distribution of neurotransmitter-specific cells and fibers in the anteroventral periventricular nucleus: implications for the control of gonadotropin secretion in the rat. Brain Res 400:11–34 [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW, Handa RJ, Gorski RA 1985 Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology 40:501–510 [DOI] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E 2003 Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100:10972–10976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno Jr JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley Jr WF, Aparicio SA, Colledge WH 2003 The GPR54 gene as a regulator of puberty. N Engl J Med 349:1614–1627 [DOI] [PubMed] [Google Scholar]
- Kauffman AS 2010 Coming of age in the Kisspeptin era: sex differences, development, and puberty. Mol Cell Endocrinol 324:51–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge WH 2009 Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides 30:34–41 [DOI] [PubMed] [Google Scholar]
- Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M 2008 New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol 29:48–69 [DOI] [PubMed] [Google Scholar]
- Smith JT 2009 Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides 30:94–102 [DOI] [PubMed] [Google Scholar]
- Plant TM, Ramaswamy S 2009 Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta). Peptides 30:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley AE, Clifton DK, Steiner RA 2009 Kisspeptin signaling in the brain. Endocr Rev 30:713–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, Hoffman GE, Steiner RA, Tena-Sempere M 2007 Sexual differentiation of Kiss1 gene expression in the brain of the rat. Endocrinology 148:1774–1783 [DOI] [PubMed] [Google Scholar]
- Clarkson J, Herbison AE 2006 Postnatal development of kisspeptin neurons in mouse hypothalamus; sexual dimorphism and projections to gonadotropin-releasing hormone neurons. Endocrinology 147:5817–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA 2009 Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab 297:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma T, Sakakibara M, Yamada S, Kinoshita M, Iwata K, Tomikawa J, Kanazawa T, Matsui H, Takatsu Y, Ohtaki T, Matsumoto H, Uenoyama Y, Maeda K, Tsukamura H 2009 Significance of neonatal testicular sex steroids to defeminize anteroventral periventricular Kisspeptin neurons and the GnRH/LH surge system in male rats. Biol Reprod 81:1216–1225 [DOI] [PubMed] [Google Scholar]
- Bateman HL, Patisaul HB 2008 Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology 29:988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Todd KL, Mickens JA, Adewale HB 2009 Impact of neonatal exposure to the ERα agonist PPT, bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology 30:350–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM, Auger AP, Bale TL, De Vries GJ, Dunn GA, Forger NG, Murray EK, Nugent BM, Schwarz JM, Wilson ME 2009 The epigenetics of sex differences in the brain. J Neurosci 29:12815–12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotsiridze T, Kang N, Jacob D, Forger NG 2007 Development of sex differences in the principal nucleus of the bed nucleus of the stria terminalis of mice: role of Bax-dependent cell death. Dev Neurobiol 67:355–362 [DOI] [PubMed] [Google Scholar]
- Tsukahara S, Inami K, Maekawa F, Kakeyama M, Yokoyama T, Yuji M, Kitagawa H, Kannan Y, Yamanouchi K 2004 Postnatal apoptosis, development, and sex difference in the lateral septum of rats. J Comp Neurol 475:177–187 [DOI] [PubMed] [Google Scholar]
- Waters EM, Simerly RB 2009 Estrogen induces caspase-dependent cell death during hypothalamic development. J Neurosci 29:9714–9718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zup SL, Carrier H, Waters EM, Tabor A, Bengston L, Rosen GJ, Simerly RB, Forger NG 2003 Overexpression of bcl-2 reduces sex differences in neuron number in the brain and spinal cord. J Neurosci 23:2357–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Keller-Peck CR, Knudson CM, Korsmeyer SJ, Snider WD 1998 Widespread elimination of naturally occurring neuronal death in Bax-deficient mice. J Neurosci 18:1428–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S, Intlekofer KA, Aggison LK, Petersen SL 2009 Central role of TRAF-interacting protein in a new model of brain sexual differentiation. Proc Natl Acad Sci USA 106:16692–16697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraes JC, Coope A, Morari J, Cintra DE, Roman EA, Pauli JR, Romanatto T, Carvalheira JB, Oliveira AL, Saad MJ, Velloso LA 2009 High-fat diet induces apoptosis of hypothalamic neurons. PLoS One 4:e5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broad KD, Curley JP, Keverne EB 2009 Increased apoptosis during neonatal brain development underlies the adult behavioral deficits seen in mice lacking a functional paternally expressed gene 3 (Peg3). Dev Neurobiol 69:314–325 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly RB 1989 Hormonal control of the development and regulation of tyrosine hydroxylase expression within a sexually dimorphic population of dopaminergic cells in the hypothalamus. Brain Res Mol Brain Res 6:297–310 [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA 2004 A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145:4073–4077 [DOI] [PubMed] [Google Scholar]
- Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS 2009 Circadian regulation of Kiss1 neurons: implications for timing the preovulatory GnRH/LH surge. Endocrinology 150:3664–3671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowen JA, Clifton DK 1991 Semiquantitative analysis of cellular somatostatin mRNA levels by in situ hybridization histochemistry. Method Neurosci 5:137–158 [Google Scholar]
- Clarkson J, Boon WC, Simpson ER, Herbison AE 2009 Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150:3214–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka M, Sumida H, Kano Y, Arai Y 1993 Formation of neurons in the sexually dimorphic anteroventral periventricular nucleus of the preoptic area of the rat: effects of prenatal treatment with testosterone propionate. J Neuroendocrinol 5:569–573 [DOI] [PubMed] [Google Scholar]
- Creps ES 1974 Time of neuron origin in preoptic and septal areas of the mouse: an autoradiographic study. J Comp Neurol 157:161–243 [DOI] [PubMed] [Google Scholar]
- Golovine K, Schwerin M, Vanselow J 2003 Three different promoters control expression of the aromatase cytochrome p450 gene (cyp19) in mouse gonads and brain. Biol Reprod 68:978–984 [DOI] [PubMed] [Google Scholar]
- Mannan MA, O'Shaughnessy PJ 1988 Ovarian steroid metabolism during post-natal development in the normal mouse and in the adult hypogonadal (hpg) mouse. J Reprod Fertil 82:727–734 [DOI] [PubMed] [Google Scholar]
- Mannan MA, O'Shaughnessy PJ 1991 Steroidogenesis during postnatal development in the mouse ovary. J Endocrinol 130:101–106 [DOI] [PubMed] [Google Scholar]
- Smeaton TC, Arcondoulis DE, Steele PA 1975 The synthesis of testosterone and estradiol-17β by the gonads of neonatal rats in vitro. Steroids 26:181–192 [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA 2009 Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci 29:9390–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthuis PJ, Cox KH, Searcy BT, Kumar P, Tobet S, Rissman EF Of mice and rats: key species variations in the sexual differentiation of brain and behavior. Front Neuroendocrinol [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne HJ, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE 2006 Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 52:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar DK, Fink G 1981 Gonadotropin-releasing hormone surge: possible modulation through postsynaptic α-adrenoreceptors and two pharmacologically distinct dopamine receptors. Endocrinology 108:862–867 [DOI] [PubMed] [Google Scholar]
- Sarkar DK, Smith GC, Fink G 1981 Effect of manipulating central catecholamines on puberty and the surge of luteinizing hormone and gonadotropin releasing hormone induced by pregnant mare serum gonadotropin in female rats. Brain Res 213:335–349 [DOI] [PubMed] [Google Scholar]
- Drouva SV, Gallo RV 1977 Further evidence for inhibition of episodic luteinizing hormone release in ovariectomized rats by stimulation of dopamine receptors. Endocrinology 100:792–798 [DOI] [PubMed] [Google Scholar]
- Gallo RV, Drouva SV 1979 Effect of intraventricular infusion of catecholamines on luteinizing hormone release in ovariectomized and ovariectomized, steroid-primed rats. Neuroendocrinology 29:149–162 [DOI] [PubMed] [Google Scholar]
- Aurich C, Gerlach T, Aurich JE, Hoppen HO, Lange J, Parvizi N 2002 Dopaminergic and opioidergic regulation of gonadotropin and prolactin release in stallions. Reprod Domest Anim 37:335–340 [DOI] [PubMed] [Google Scholar]
- Luderer U, Schwartz NB 1991 Sex differences in acute luteinizing hormone responses to gonadectomy remain after progesterone antagonist and dopamine agonist treatment. Biol Reprod 45:918–926 [DOI] [PubMed] [Google Scholar]
- Navarro VM, Sánchez-Garrido MA, Castellano JM, Roa J, García-Galiano D, Pineda R, Aguilar E, Pinilla L, Tena-Sempere M 2009 Persistent impairment of hypothalamic KiSS-1 system after exposures to estrogenic compounds at critical periods of brain sex differentiation. Endocrinology 150:2359–2367 [DOI] [PubMed] [Google Scholar]
- Yoshida K, Homma T, Inamoto Y, Tomikawa J, Uenoyama Y, Maeda K, Tsukamura H Effect of neonatal estrogen on brain kisspeptin expression in adult mice: possible role of estrogen receptor α. International Congress of Endocrinology, 14th Meeting, Kyoto, Japan, 2010 (Abstract P7-16-3) [Google Scholar]
- De Vries GJ 2004 Minireview: sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology 145:1063–1068 [DOI] [PubMed] [Google Scholar]