Abstract
The IGF-I pathway and renin-angiotensin-aldosterone axis are both involved in the pathogenesis of hypertension and atherosclerosis, but no information is available about IGF-I and aldosterone interaction or their potential synergistic effects in vascular smooth muscle cells (VSMCs). The aims of this study were to investigate whether aldosterone influences IGF-I signaling and to determine the mechanism(s) by which aldosterone affects IGF-I function. Aldosterone resulted in significant increases in the Akt (1.87 ± 0.24, P < 0.001), MAPK (1.78 ± 0.13, P < 0.001), p70S6kinase (1.92 ± 0.15, P < 0.001), IGF-I receptor (1.69 ± 0.05, P < 0.01), and insulin receptor substrate-1 (1.7 ± 0.04, P < 0.01) (fold increase, mean ± SEM, n = 3) phosphorylation responses to IGF-I compared with IGF-I treatment alone. There were also significant increases in VSMC proliferation, migration, and protein synthesis (1.63 ± 0.03-, 1.56 ± 0.08-, and 1.51 ± 0.04-fold increases compared with IGF-I alone, respectively, n = 3, P < 0.001). Aldosterone induced osteopontin (OPN) mRNA expression and activation of αVβ3-integrin as well as an increase in the synthesis of IGF-I receptor. The enhancing effects of aldosterone were inhibited by eplerenone (10 μmol/liter), actinomycin-D (20 nmol/liter), and an anti-αVβ3-integrin antibody that blocks OPN binding. The antioxidant N-acetylcysteine (2 mmol/liter) completely inhibited the ability of aldosterone to induce any of these changes. In conclusion, our results show that aldosterone enhances IGF-I signaling and biological actions in VSMCs through induction of OPN followed by its subsequent activation of the αVβ3-integrin and by increasing IGF-I receptor. These changes are mediated in part through increased oxidative stress. The findings suggest a new mechanism by which aldosterone could accelerate the development of atherosclerosis.
Aldosterone enhancement of IGF-I actions in vascular smooth muscle cells is mediated through an increase in osteopontin and IGF-I receptor synthesis.
The proliferative phase of atherosclerotic lesion development involves vascular smooth muscle cell (VSMC) proliferation and migration, accumulation of extracellular matrix, and fibrous cap formation. IGF-I is a potent stimulator of VSMC proliferation and migration (1). IGF-I expression is increased in blood vessels after injury and in the aorta of hypertensive animals (2). We have previously reported that the ability of IGF-I to enhance VSMC proliferation requires ligand occupancy of the αVβ3 integrin (3). In vivo and in vitro studies show that blocking ligand occupancy of αVβ3 inhibits IGF-I signaling and atherosclerotic lesion progression (3,4,5). One of the ligands of αVβ3 is the extracellular matrix protein osteopontin (OPN). In response to injury OPN concentrations increase in several tissues and hypoxia, hyperglycemia, and mechanical injury to blood vessels all result in enhanced OPN expression (6,7,8). The increase in OPN induced by hyperglycemia enhances VSMC proliferation in response to IGF-I (9). These increases may be significant for blood vessel growth because overexpression of OPN has been shown to accelerate intimal thickening (10). In hypertensive patients, aldosterone levels have been reported to be independently associated with plasma OPN levels (11). However, there are no studies that have evaluated the effects of aldosterone on OPN-induced activation of αVβ3 integrin or its role on IGF-I-mediated signaling in VSMCs.
Aldosterone has a well-established pathophysiological role in hypertension and cardiovascular disease (6,12,13). In addition to its primary function in regulating blood pressure and electrolytic balance, aldosterone directly promotes vascular remodeling and profibrotic changes in blood vessels (14,15). The cardiovascular actions of aldosterone are thought to be mediated, at least in part, by increased reactive oxygen species (ROS) generation. The consequences of ROS generation are complex, but they have been shown to activate Src kinase (16) and stress glucocorticoid kinase (17) and to enhance cellular responsiveness to insulin (18). Aldosterone has also been shown to stimulate the expression of and to transactivate the epidermal growth factor receptor in different cell types, including vascular cells (19,20,21). One study reported that aldosterone could directly transactivate the IGF-I receptor (IGF-IR) in a renal cell line (22). Although both the IGF-I signaling pathway and the renin-angiotensin-aldosterone axis have been proposed to be involved in the pathogenesis of hypertension and atherosclerosis, the potential interaction between these two signaling pathways in VSMCs has not been evaluated. The present study was carried out to investigate whether aldosterone influences IGF-I signaling and biological function in VSMCs and define the roles of oxidative stress and αVβ3 integrin activation in determining the results of this interaction.
Materials and Methods
Aldosterone, actinomycin D (Act-D), N-acetyl-l-cysteine (NAC), and catalase were purchased from Sigma-Aldrich (St. Louis, MO). Human IGF-I was a gift from Genentech (South San Francisco, CA). platelet-derived growth factor (PDGF) was purchased from USB (Cleveland, OH). Eplerenone was supplied from Pfizer Inc. (Groton, CT). Phospho-Akt (Ser473), total Akt, dual-phosphorylated (active) form of p42/p44 MAPK, total p42/p44 MAPK protein, and β-actin antibodies were obtained from Cell Signaling Technology (Beverly, MA). The antiphosphotyrosine (PY99) and IGF-IR β-chain polyclonal antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The polyclonal OPN antibody was raised in rabbits against human OPN (23). Human OPN was purified using conditioned media obtained from Chinese hamster ovary cell line that had been transfected with human OPN cDNA (23). The anti-β3-polyclonal antibody was prepared by injecting rabbits with porcine β3 (4). An additional anti-αVβ3 antibody that was used for inhibiting binding of OPN to αVβ3 was prepared using the amino acid sequence (177CYDMKTTC184) of human β3 as an immunogen (25). Control IgG was prepared by protein G affinity chromatography using non immune rabbit serum. The horseradish peroxidase-conjugated mouse antirabbit, goat antimouse, and mouse antirabbit light-chain-specific antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). 2′,7′-Dichlorofluorescein diacetate (DCFDA) was purchased from Invitrogen Molecular Probes (Carlsbad, CA). Twenty-four-well glass-bottomed plates were from E&K Scientific (Santa Clara, CA). Polyvinyl difluoride membranes (Immobilon P) and the centrifugal filter device, Ultrafree-0.5, were purchased from Millipore Corp. (Bedford, MA). Autoradiographic film was obtained from Pierce (Pierce/ThermoFisher, Rockford, IL). DMEM containing 900 mg glucose/liter (5 mm), referred to as normal glucose (NG) and Hanks’ balanced salt solution were purchased from Gibco (Grand Island, NY). Streptomycin and penicillin were purchased from Invitrogen (Carlsbad, CA).
Cell culture, treatments, and cell lysis
VSMCs were isolated from the pig thoracic aorta (26) and maintained in DMEM containing 5.0 mm NG supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fetal bovine serum (FBS) in 10 cm tissue culture plates (Falcon Laboratory, Franklin Lakes NJ). VSMCs were fed every 3 d, passaged every 7 d, and used between passages 4 and 12. The cells were plated at a density of 3 × 105 in 10-cm dishes (Falcon) and then grown to 90% confluency. Confluent monolayers were washed three times in serum-free medium (SFM) and incubated overnight (18–20 h) in SFM DMEM with or without aldosterone (10−9 mol/liter, 18 h) before IGF-I (50 ng/ml, 5–20 min) stimulation. Exposure to aldosterone (10−9 mol/liter, 18 h) did not change the differentiation state of VSMCs (Supplemental Fig. 1). Where indicated, VSMCs were preincubated with Eplerenone (10−6 mol/liter), Act-D (20 nmol/liter), or NAC (2 mmol/liter) for 1 h before the addition of aldosterone. Eplerenone was dissolved in ethanol, which when added alone had no effect on aldosterone or IGF-I signaling. VSMCs were lysed in ice-cold lysis buffer (4). After centrifugation at 14,000 × g for 10 min, solubilized proteins were quantified by the Bradford method (Pierce Chemical Co., Rockford, IL). Equal amounts of lysates were separated by SDS-PAGE and the proteins visualized by Western immunoblotting. To detect OPN, SFM was collected and concentrated 20-fold using an Ultrafree 0.5-μl centrifugal filter device, and then 30 μl was resuspended in an equal volume of 2× reducing Laemmli buffer and the proteins separated by SDS-PAGE.
Immunoprecipitation and immunoblotting
Equal amounts of cell lysate were incubated overnight at 4 C with the following antibody dilutions of anti-IGF-IR (1:200), anti-αVβ3 (1:300), antiinsulin receptor substrate (IRS)-1 (1:100). The immune complexes were precipitated by adding protein A Sepharose and incubating for 2 h at 4 C and then centrifuging at 14,000 × g for 10 min. The pellets were washed four times with lysis buffer and resuspended in 40 μl of 2× reducing Laemmli buffer (0.2 m final concentration dithiothreitol), boiled for 5 min, and separated by SDS-PAGE (9% gel). After transfer to Immobilon-P membranes and blocking (4), the blots were incubated overnight at 4 C with the indicated antibodies (1:1000 for anti p-Tyr, anti-αVβ3, anti-IGF-IR, anti-IRS-1; 1:2000 for anti-OPN). For direct immunoblotting, 15 μg of cell lysate was mixed with an equal volume of 2× Laemmli sample buffer and then the proteins separated by SDS-PAGE (11% gel). The blots were incubated with a (1:1000 dilution) of anti-pAkt, anti-Akt, anti-pMAPK, anti-MAPK, anti-β-Actin, or anti-phospho-p70S6K. The immune complexes were visualized using a peroxidase-labeled secondary antibody and enhanced chemiluminescence following the manufacturer’s instructions (Pierce). The labeled immune complexes were detected by autoradiography.
Real-time quantitative RT-PCR
OPN mRNA expression was measured by quantitative RT-PCR. Total RNA was isolated from VSMCs by adding 1 ml of Trizol (Invitrogen Life Technologies, Carlsbad, CA) to 10-cm plates. Two hundred microliters of chloroform were added to each sample, and then the samples were centrifuged at 8000 × g and the total cellular RNA purified using an RNeasy kit (QIAGEN, Valencia, CA). Deoxyribonuclease-treated, total RNA (1 μg) was reverse transcribed using high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). PCR amplification of cDNA was performed with the ABI-PRISM 7700 system (total volume 2 μl), using a TaqMan PCR core reagent kit. Each sample was analyzed in triplicate and normalized to the nonaldosterone treatment sample. FAM-labeled primers and probes sets for porcine OPN (Applied Biosystems catalog no. Ss03391324_m1; GenBank sequence NM_214023.1) were used.
Proliferation assay
VSMCs grown in NG were plated at 3 × 104 cells/well in each well of a 24-well plate (catalog no. 3047; BD Falcon) in SFM-NG plus 2% FBS. After attachment the medium was replaced with SFM-NG for 24 h and then replaced with SFM containing 0.2% platelet-derived poor plasma and the various treatments for 48 h. Cell number was determined after trypsinization, trypan blue staining, and counting. Each treatment was analyzed in triplicate, and the results represent mean values of three independent experiments.
Migration assay
VSMCs were seeded into six-well plates (catalog no. 3046; BD Falcon) and fed with NG media 3 d after seeding. Four days later the monolayers were wounded with a razor blade and the areas to be counted were selected (23,26). After wounding, VSMCs were incubated with SFM-NG plus 0.2% FBS in the presence or absence of the indicated treatments at 37 C for 48 h. The wounded monolayers were fixed and stained (Diff Quick; Dade Behring, Inc., Newark, DE), and the number of cells migrating at least 5 μm into the wound area was determined. At least five previously selected 1-mm areas at the edge of the wound were analyzed per well (total two wells) for each data point, and the results represent mean value of three independent experiments.
Protein synthesis assay
VSMCs were grown to confluence in 24-well culture plates. The cultures were rinsed once with serum-free DMEM and incubated with 0.5 ml of low (10−7 mol/liter) methionine MEM for 18 h in the presence of 50 μCi per well of [35S]methionine (specific activity 1206 Ci/mmol) and each of the treatments. After 18 h the amount of [35S]methionine that had been incorporated into total protein was determined (27). In each experiment there were two determinations for each test condition and the results represent mean value of three independent experiments.
Measurement of ROS
ROS were measured using the fluorogenic substrate DCFDA, a nonfluorescent cell-permeable dye. The acetate groups are removed by intracellular esterases and 2′,7′-dichlorofluorescein (DCF) is oxidized by H2O2. Cells were plated at a density of 4 × 104 in 24-well glass bottom plates (E&K Scientific) and grown to 90% confluency. After 24 that they were in SFM DMEM, the cells were washed twice with Hanks’ balanced salt solution containing 2.5 mm CaCl2 and 1 mm MgSO4 and incubated for 1 h at 37 C under darkness with 10 μm DCFDA with or without aldosterone treatment (10−9 to 10−8 mol/liter) for 60 min. Relative fluorescence was measured using a fluorescence plate reader (CytoFlor; PerSeptive Biosystems, Foster City, CA) using excitation and emission wavelenghts of 485 and 530 nm, respectively. Subsequently the cells were lysed and the total cellular protein quantified. The H2O2 level is expressed in fluorescence units per milligram of cellular protein.
Data analysis
For the biological assays, statistical significance was evaluated by one-way ANOVA followed by Tukey’s multiple comparison test when P < 0.05 (GraphPad Prism, La Jolla, CA). For biochemical analyses, the Student’s t test was used. The results that are shown are representative of at least three separate experiments and P < 0.05 was considered statistically significant.
Results
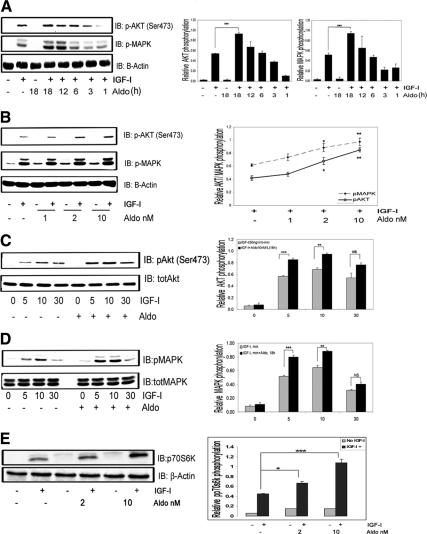
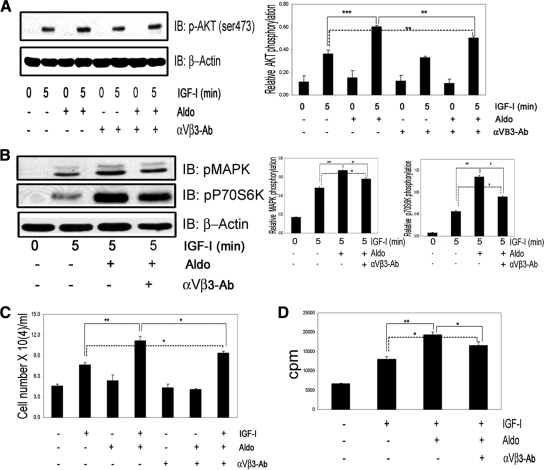
Aldosterone enhances IGF-I-stimulated Akt, MAPK, and p70S6K phosphorylation in VSMCs
In VSMCs maintained in 5 mm glucose, IGF-I (50 ng/ml) alone stimulated a significant increase in Akt (ser473) phosphorylation, but prior exposure to aldosterone (10−9 mol/liter, 18 h) resulted in a significant, additional increase (1.87 ± 0.24-fold, mean ± sem, n = 5, P < 0.001) (Fig. 1A). Incubation with aldosterone alone had no effect (Fig. 1A). IGF-I-stimulated MAPK activation was increased significantly after aldosterone pretreatment (1.78 ± 0.13-fold increase over IGF-I alone, mean ± sem, n = 5, P < 0.001), but aldosterone alone had no effect (Fig. 1A). Exposure to aldosterone for shorter time periods (3–12 h) had no enhancing effect on IGF-I signaling. When VSMCs were exposed to increasing amounts of aldosterone (1–10 nmol/liter) (Fig. 1B), the lowest effective concentration that significantly enhanced IGF-I stimulated Akt and MAPK phosphorylation was 2.0 nmol/liter (1.61 ± 0.09- and 1.43 ± 0.07-fold increases, respectively, for pAkt and pMAPK, compared with IGF-I treatment alone, mean ± sem, n = 3, P < 0.05). Concentrations of aldosterone greater than 10 nmol/liter were not tested because these are not representative of concentrations in vivo. Aldosterone exposure did not alter the time course of the response to IGF-I, but it significantly increased Akt (Fig. 1C) and MAPK (Fig. 1D) phosphorylation after 5 and 10 min of IGF-I stimulation [1.41 ± 0.03- and 1.38 ± 0.02-fold increase in pAkt and pMAPK, respectively (mean ± sem, n = 3, P < 0.01)]. Aldosterone also enhanced the p70S6K (Thr389) phosphorylation response to IGF-I [1.5 ± 0.03- (P < 0.05) and 1.92 ± 0.15-fold (P < 0.001) increases with 2 or 10 nmol/liter aldosterone, respectively, vs. IGF-I stimulation alone, mean ± sem, n = 3] (Fig. 1E). To determine whether the enhancing effect of aldosterone on downstream signaling was specific, we stimulated VSMCs with 10 ng/ml of PDGF for 5, 15, and 30 min after exposure to aldosterone. There was no significant difference in the Akt phosphorylation response when cells that had been pretreated with aldosterone were compared with PDGF alone (Supplemental Fig. 2).
Figure 1.
Aldosterone enhances IGF-I signaling in VSMCs. A, Aldosterone (10 nmol/liter) was added for the indicated times then IGF-I (50 ng/ml) for 5 min. Cell lysates were immunoblotted for pAKT, pMAPK, and β-actin. IB, Immunoblot; Aldo; aldosterone. ***, P < 0.001 when IGF-I plus aldosterone was compared with IGF-I alone. B, VSMCs were treated as in A except increasing concentrations of aldosterone (1–10 nmol/liter) were added for 18 h. *, P < 0.05, **, P < 0.01 aldosterone (2 or 10 nmol/liter) plus IGF-I compared with IGF-I alone. C, VSMCs were treated as in A, and then IGF-I (50 ng/ml) was added for the indicated times. Cell lysates were immunoblotted for pAkt and then reprobed using anti-Akt antibody. ***, P < 0.001, **,P < 0.01 IGF-I for 5 or 10 min plus aldosterone compared with IGF-I alone. D, The same experimental conditions as in C were used to detect MAPK phosphorylation. E, VSMCs were treated as in A. Cell lysates were immunoblotted for phos-p70S6K or with anti-β-actin. *, P < 0.05 (2 nmol/liter), ***, P < 0.001 (10 nmol/liter) aldosterone plus IGF-I compared with IGF-I alone. The results represent the mean ± sem scanning densitometry units from three experiments and are expressed as the ratio of the phosphorylated form divided by the total protein.
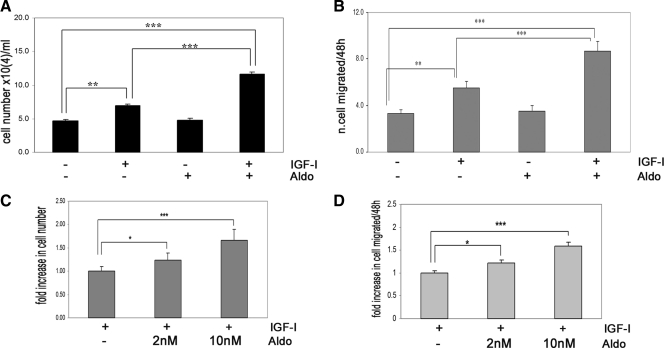
Aldosterone enhances proliferation and migration of VSMCs in response to IGF-I
In VSMCs IGF-I treatment alone resulted in a 1.4 ± 0.09-fold increase in cell number (mean ± sem; n = 3; P < 0.01), whereas aldosterone treatment alone had no effect (Fig. 2A). When both aldosterone and IGF-I were added for 48 h, VSMC proliferation was significantly increased (2.3 ± 0.09-fold increase, mean ± sem; n = 3; P < 0.001). This change was significantly greater than the response to IGF-I alone (1.63 ± 0.03-fold increase, mean ± sem; n = 3; P < 0.001). Similarly, IGF-I stimulated a 1.67 ± 0.12-fold increase in cell migration (mean ± sem; n = 3; P < 0.001), whereas aldosterone and IGF-I together induced an additional 1.56 ± 0.08-fold increase (mean ± sem; n = 3; P < 0.001 compared with the IGF-I treatment alone) (Fig. 2B). Aldosterone alone had no effect. The addition of 2 nmol/liter aldosterone with IGF-I stimulated VSMC proliferation and migration significantly (Fig 2, C and D).
Figure 2.
Aldosterone enhances IGF-I biological function in VSMCs. A, Cell proliferation response to aldosterone (10 nmol/liter) alone or in combination with IGF-I (50 ng/ml). Aldo, Aldosterone. **, P < 0.01 and ***, P < 0.001 when the response to IGF-I is compared with nonexposed cells or to aldosterone and IGF-I; ***, P < 0.001 when the response to IGF-I plus aldosterone is compared with nontreated cells. The results are the mean ± sem. B, Cell migration. The results shown are the mean ± sem of three independent experiments for aldosterone (10 nmol/liter) alone or in combination with IGF-I (50 μg/ml). **, P < 0.01 and ***, P < 0.001 when the response to IGF-I is compared with control cells or cells exposed to aldosterone and IGF-I; ***, P < 0.001 when the number of cells migrating in response to IGF-I plus aldosterone is compared with the control cells. C and D, Dose response. *, P < 0.05, ***, P < 0.001 when the number of proliferating (C) or migrating (D) cells in response to 2 nmol/liter and 10 nmol/liter aldosterone plus IGF-I is compared with IGF-I alone.
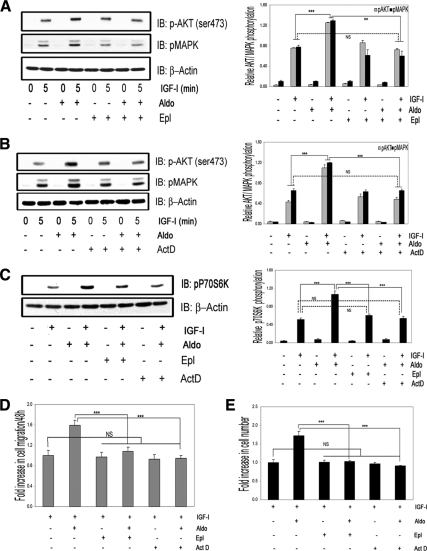
The aldosterone-induced enhancement in IGF-I signaling and biological actions requires aldosterone binding to the mineralocorticoid receptor (MR) and activation of gene transcription
The enhancing effects of aldosterone on IGF-I induced Akt and MAPK phosphorylation were completely inhibited by the MR antagonist eplerone (Epl) (Fig. 3A) (1.74 ± 0.04- and 1.83 ± 0.05-fold greater responses when IGF-I plus aldosterone treatment was compared with IGF-I plus Epl plus aldosterone, mean ± sem, n = 3, P < 0.01). Epl had no effect on the response to IGF-I alone. Pretreatment with Act-D blocked the effect of aldosterone on both IGF-I-stimulated Akt and MAPK phosphorylation (1.9 ± 0.01- and 1.8 ± 0.02-fold greater responses when IGF-I plus aldosterone treatment was compared with IGF-I plus Act-D plus aldosterone, mean ± sem, n = 3, P < 0.001), but it had no effect on the response to IGF-I alone (Fig. 3B). The p70S6K phosphorylation response to IGF-I plus aldosterone was also inhibited by Act-D (1.8 ± 0.02-fold greater response when IGF-I plus aldosterone treatment was compared with IGF-I plus Act-D plus aldosterone, mean ± sem, n = 3, P < 0.001) and by Epl (1.7 ± 0.01-fold greater response when IGF-I plus aldosterone was compared with IGF-I plus Epl plus aldosterone, mean ± sem, n = 3, P < 0.001) (Fig. 3C).
Figure 3.
The enhancing actions of aldosterone on IGF-I signaling and biological function require aldosterone binding to MR and gene transcription. A, VSMCs were incubated with eplerenone (10 μmol/liter) for 1 h, and then 10 nmol/liter of aldosterone for 18 h, or with eplerenone alone, and then IGF-I (50 ng/ml) for 5 min. IB, Immunoblot; Aldo, aldosterone. B, VSMCs were incubated with Act-D (20 nmol/liter) for 1 h followed by aldosterone (10 nmol/liter) for 18 h or Act-D alone, and then IGF-I (50 ng/ml) was added for 5 min. C, VSMCs were incubated with Epl (10 μmol/liter) or Act-D (20 nmol/liter) for 1 h and then 10 nmol/liter of aldosterone for 18 h or with Act-D alone for 18 h and then IGF-I (50 ng/ml) for 5 min. A–C, The levels of phosphorylated Akt, MAPK, and p70S6K were determined by direct immunoblotting and normalized to β-actin. The bar graphs show the mean ± sem (n = 3). ***, P < 0.001; **, P < 0.01. D, Cell migration. Aldosterone (10 nmol/liter), Epl (10 μmol/liter), and Act-D (20 nmol/liter) were added in combination with IGF-I or IGF-I plus aldosterone. ***, P < 0.001, when the response to aldosterone plus IGF-I is compared with IGF-I plus aldosterone plus Epl or to IGF-I plus aldosterone plus Act-D. NS, Not significant when the response to Epl or Act-D plus IGF-I is compared with the response to IGF-I alone. The results are the mean ± sem. E, Cell proliferation. Aldosterone (10 nmol/liter), Epl (10 μmol/liter), and Act-D (20 nmol/liter) were added in combination with IGF-I. ***, P < 0.001 when aldosterone plus IGF-I is compared with the response of cells to aldosterone plus IGF-I plus Epl or to IGF-I plus aldosterone plus Act-D. NS, Not significant when Epl or Act-D plus IGF-I is compared with the response of cells to IGF-I alone.
Neither Act-D nor Epl had an effect on IGF-I-stimulated VSMC proliferation in response to IGF-I, but they both significantly inhibited the ability of aldosterone to stimulate a further increase in VSMC proliferation (1.1 ± 0.02- and 1.0 ± 0.01-fold greater responses when IGF-I plus aldosterone plus Epl or Act-D treatment was compared with IGF-I alone, mean ± sem; n = 3, P = NS) (Fig. 3D). Similar results were obtained for VSMC migration (1.02 ± 0.03- and 0.93 ± 0.06-fold greater responses when IGF-I plus aldosterone plus Epl or Act-D treatment was respectively compared with IGF-I alone (mean ± sem; n = 3, P = NS) (Fig. 3E).
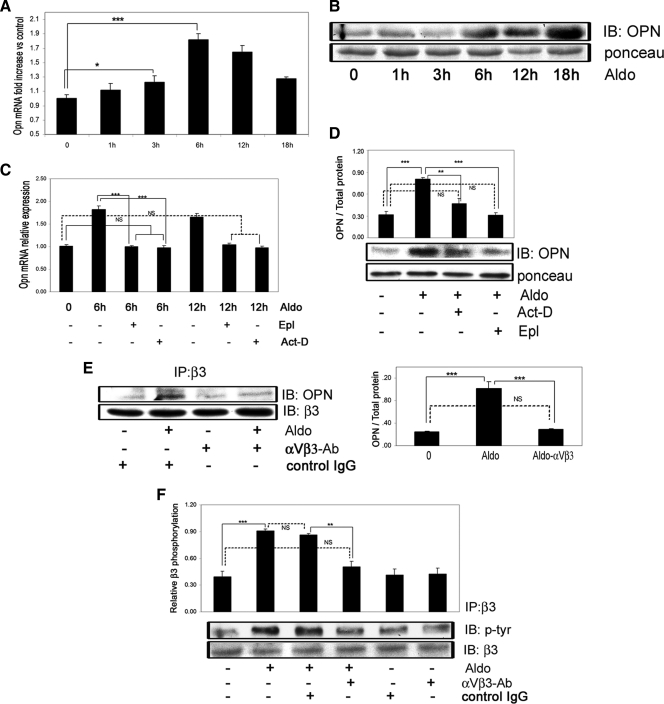
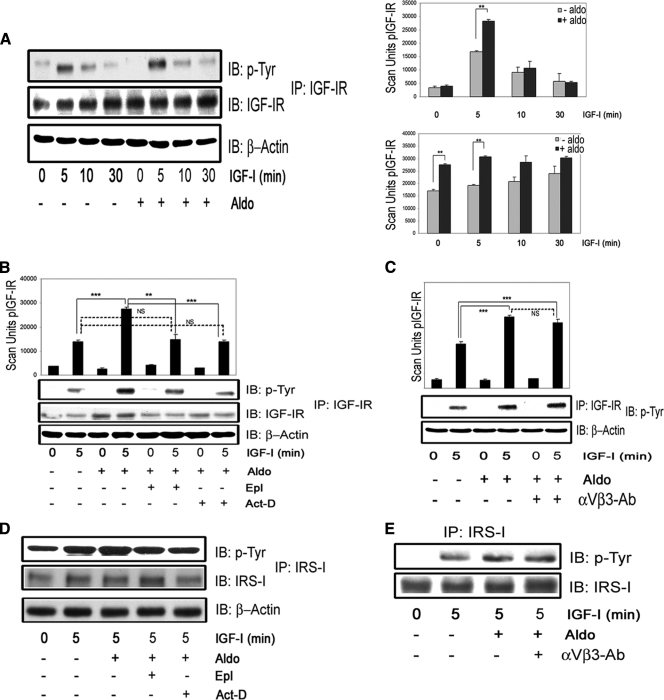
The enhancing action of aldosterone on IGF-I-mediated signaling is due in part to OPN activation of the αVβ3 integrin
We have previously reported (23) that in VSMCs maintained in 5 mm glucose, the attainment of an optimal response to IGF-I requires αVβ3 occupancy; therefore, we determined whether the effect of aldosterone was mediated by an increase in the αVβ3 ligand, OPN. Aldosterone significantly increased OPN mRNA levels in a time-dependent manner. A significant increase was observed at 3 h (1.24 ± 0.08-fold, P < 0.05, n = 3) with peak expression observed at 6 h (1.82 ± 0.09-fold, P < 0.001, n = 3) (Fig 4A). When conditioned media were analyzed, OPN was significantly increased after 6 h (3.3 ± 0.1-fold increase, mean ± sem, P < 0.001, n = 3), and it continued to increase up to 18 h (5.6 ± 0.2-fold increase, mean ± sem, P < 0.001, n = 3) (Fig. 4B). Both Act-D and Epl inhibited the induction of OPN mRNA expression by aldosterone (comparing aldosterone to aldosterone plus Epl or to aldosterone plus Act-D, mean ± sd, P < 0.001, n = 3) (Fig. 4C). They also significantly inhibited OPN secretion (Fig. 4D). To determine whether the increase in OPN that was induced by aldosterone resulted in increased OPN binding to αVβ3, we immunoprecipitated αVβ3 and immunoblotted for OPN. An increase in OPN association with αVβ3 was detected in aldosterone-treated cells (Fig. 4E), and this increase was inhibited by exposure to a purified anti-αVβ3 antibody (Fig. 4E), which inhibits OPN binding to αVβ3 (3). The αVβ3 tyrosine phosphorylation is a marker of αVβ3 activation and is required for optimal IGF-I signaling responses in VSMCs (28). Aldosterone induced αVβ3 phosphorylation and this effect was significantly inhibited by exposure to the αVβ3 antibody that inhibited OPN binding (Fig. 4F).
Figure 4.
Aldosterone stimulates OPN mRNA and protein expression in VSMCs and induces OPN binding to the integrin αVβ3. A, VSMCs were exposed to aldosterone (10 nmol/liter) for the indicated times. OPN mRNA expression is shown as fold increase over nontreated cells (mean ± sd of three independent experiments). B, VSMCs were exposed to aldosterone for the indicated times, and OPN protein levels in condition media were determined. IB, Immunoblot; Aldo, aldosterone. C, After pretreatment with or without Epl (10 μmol/liter) or Act-D (20 nmol/liter) for 1 h, VSMCs were exposed to aldosterone (10 nmol/liter) for 6 or 12 h, and OPN mRNA expression was measured (n = 3). D, VSMCs were exposed to Epl (10 μmol/liter) or Act-D (20 nmol/liter) for 1 h before aldosterone (10 nmol/liter) for 18 h and media OPN protein levels were determined. E, Quiescent cells were incubated for 1 h with or without the purified anti-αVβ3 IgG (1 μg/ml) before being exposed to aldosterone for 18 h. Cell lysates were immunoprecipitated with a different anti-αVβ3 antibody and then immunoblotted for OPN. To control for loading, αVβ3 was quantified. F, The cells were exposed to the same treatments as in E. Cell lysates were immunoprecipitated with anti-αVβ3 and immunoblotted with an antiphosphotyrosine antibody. The blots were stripped and reprobed with the anti-αVβ3 antibody. The results are expressed as the ratio of the phosphorylated to total protein levels from three independent experiments. IP, Immunoprecipitation. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To determine whether the aldosterone-mediated increase in αVβ3 phosphorylation was responsible for the change in IGF-I signaling, we added an anti-αVβ3 antibody, which inhibits αVβ3 activation (3), and determined its effect on Akt (Fig. 5A), MAPK, and p70S6K phosphorylation after IGF-I and aldosterone stimulation (Fig. 5B). The antibody significantly reduced the enhancing effect of aldosterone (P < 0.05 for p70S6K and P < 0.01 for pAkt and pMAPK). In contrast, there was no significant effect on the response to IGF-I treatment alone (Fig. 5A). Aldosterone enhancement of IGF-I stimulated cell proliferation was significantly reduced by the αVβ3 antibody (1.2 ± 0.02-fold difference in cell number when aldosterone-IGF-I and aldosterone-IGF-I-αVβ3 antibody treatments were compared, P < 0.05, n = 3) (Fig. 5C). Similar results were also obtained when protein synthesis was quantified (1.3 ± 0.03-fold difference in 35S-methionine incorporation between aldosterone-IGF-I and aldosterone-IGF-I-αVβ3 antibody treatment, P < 0.05, n = 3) (Fig. 5D).
Figure 5.
The enhancing action of aldosterone on IGF-I mediated signaling and biological actions is mediated in part by OPN binding to αVβ3 integrin. A and B, Quiescent VSMCs were incubated for 1 h with or without purified anti-αVβ3 IgG (1 μg/ml), and then aldosterone (10 nmol/liter) was added for 18 h followed by IGF-I (50 ng/ml) for 5 min and the levels of phosphorylated Akt, MAPK, and p70S6K were determined. The graph shows the ratio of phospho/total protein (mean ± sem, n = 3). IB, Immunoblot; Aldo, aldosterone. C, Cell proliferation. Cells were treated with or without purified anti-αVβ3 IgG (1 μg/ml), aldosterone (10 nmol/liter), and/or IGF-I (50 ng/ml) for 48 h, as indicated. Each value represents the mean ± sem of number of cells (×104) of three replicate measurements from three independent experiments. D, Protein synthesis. VSMCs were incubated for 18 h with or without purified anti-αVβ3 IgG (1 μg/ml), aldosterone (10 nmol/liter), and/or IGF-I (50 ng/ml), as indicated. Each data point is the mean ± sd cpm of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Aldosterone up-regulates IGF-IR protein and enhances IGF-I-mediated IGF-IR and IRS-1 tyrosine phosphorylation
Because the enhancing effects of aldosterone on IGF-I signaling and biological actions were not completely explained by the activation of the αVβ3 integrin, we determined whether aldosterone induced other changes that could alter IGF-I signaling. Aldosterone alone had no ability to transactivate IGF-IR (Fig. 6A). However, aldosterone did induce an increase in IGF-IR protein (1.60 ± 0.09-fold increase compared with IGF-I alone, mean ± sem, n = 4, P < 0.01), and it induced an increase in IGF-IR phosphorylation after IGF-I stimulation (1.69 ± 0.05-fold increase compared with IGF-I alone, mean ± sem, n = 4, P < 0.01) (Fig. 6A). The increase in IGF-IR total protein and IGF-IR phosphorylation was of a similar magnitude (Fig 6A). In the presence of Act-D or Epl, the effects of aldosterone were completely inhibited (Fig. 6B), indicating that the aldosterone-mediated increase in IGF-IR protein and phosphorylation require gene transcription and aldosterone binding to MR. On the other hand, there was no effect on IGF-IR phosphorylation after aldosterone and IGF-I exposure in the presence of the anti-αVβ3 antibody (Fig. 6C). These results support previous data from our group showing that αVβ3 integrin activation does not alter the IGF-IR phosphorylation response to IGF-I (3). To determine whether the activation of IGF-IR was leading to a change in downstream signaling, we evaluated the effect of aldosterone on IGF-I induced IRS-1 tyrosine phosphorylation. Aldosterone induced a significant increase in IRS-1 tyrosine phosphorylation after IGF-I stimulation, compared with IGF-I treatment alone (1.7 ± 0.04-fold increase, mean ± sem, n = 3, P < 0.01) (Fig. 6D), which was inhibited by Epl and Act-D. However, it was not altered by anti-αVβ3 IgG (1.67 ± 0.3-fold increase compared with IGF-I alone, mean ± sem, n = 3, P = NS compared with cultures receiving the same treatment in the absence of anti-αVβ3 IgG) (Fig. 6E). We also determined whether aldosterone treatment altered IRS-1 abundance by direct immunoblotting. There was no change in total IRS-1 when cells exposed to aldosterone were compared with control (Supplemental Fig. 3). Epl and the anti-αVβ3 antibody had no effect on the IGF-IR or IRS-1 responses to IGF-I alone. However, as would be expected, 18 h exposure to the transcriptional inhibitor Act-D had a small inhibitory effect on IGF-IR protein levels (Supplemental Fig. 4).
Figure 6.
Aldosterone increases synthesis of IGF-IR and enhances IGF-I-mediated IGF-IR and IRS-1 phosphorylation. Aldosterone (10 nmol/liter) was added for 18 h and then IGF-I (50 ng/ml) for the indicated times. A, IGF-IR phosphorylation was determined as described in Materials and Methods. The results from three experiments are expressed as arbitrary scan units of the phosphorylated IGF-IR or as the ratio of phospho/total IGF-IR and are presented as mean ± sem. IB, Immunoblot; Aldo, aldosterone. B, VSMCs were incubated with eplerenone (10 μmol/liter) or Act-D (20 nmol/liter) for 1 h and then aldosterone (10 nmol/liter) for 18 h. The same procedures as in A were used. C, Cells were incubated for 1 h with or without anti-αVβ3 IgG (1 μg/ml) and aldosterone (10 nmol/liter) for 18 h and then IGF-I (50 ng/ml) for 5 min. IGF-IR phosphorylation was determined and expressed as in A. D, The same experimental conditions as in B were used. Cell lysates were immunoprecipitated with an anti-IRS-1 and immunoblotted with anti p-Tyr. The membranes were stripped and reprobed with an anti-IRS-1. E, The same experimental conditions as in C were used. IRS-1 phosphorylation was determined as above. **, P < 0.01; ***, P < 0.001. NS, Not significant.
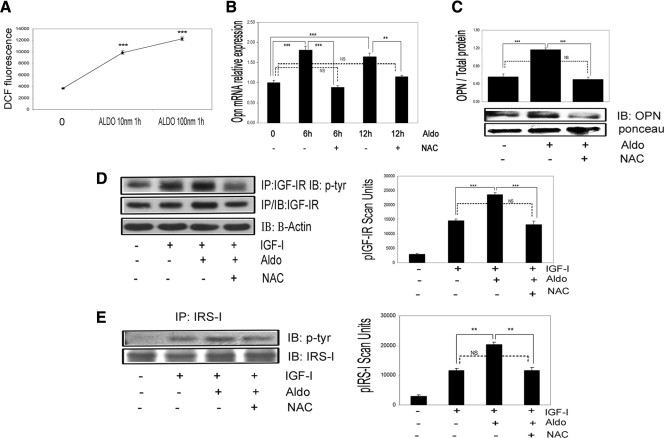
Increased oxidative stress leads to aldosterone enhancement of IGF-I-stimulated signaling and biological actions in VSMCs
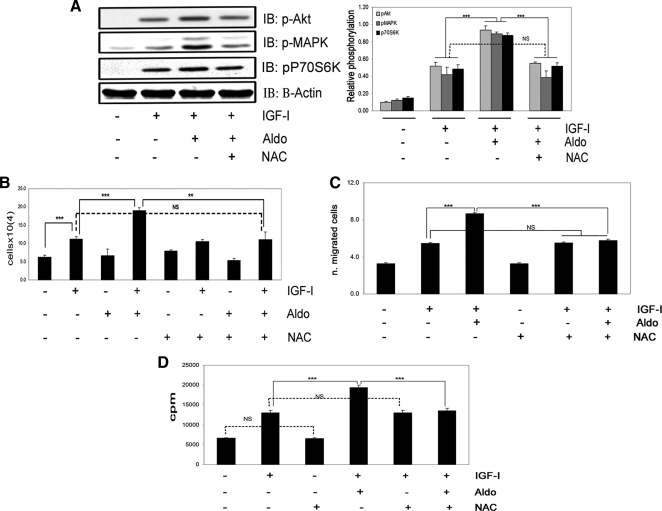
Our prior studies have determined that hyperglycemia induces an increase in OPN synthesis, which activates αVβ3 in VSMCs (23). Because both hyperglycemia (29,30) and aldosterone (16,17,18) stimulate ROS production, we analyzed the effect of ROS induction on OPN synthesis. DCF fluorescence was significantly increased after 60 min of aldosterone treatment (Fig. 7A). Aldosterone dose dependently increased ROS production in VSMCs (2.7 ± 0.08-fold increase with 10 nmol/liter, 3.3 ± 0.07-fold increase with 100 nmol/liter, mean ± sem, n = 3, P < 0.001). Exposure to the antioxidant NAC also completely inhibited the aldosterone-induced increases in OPN mRNA expression (Fig. 7B) and protein secretion (Fig. 7C). Exposure of VSMCs to NAC significantly reduced the aldosterone-induced increase in IGF-IR and the IGF-IR phosphorylation response to aldosterone plus IGF-I (Fig. 7D). Additionally the incremental increase in IRS-1 phosphorylation induced by aldosterone in response to IGF-I was inhibited by NAC (Fig. 7E). NAC had no effect on the responses to IGF-I alone (Supplemental Fig. 5). Treatment with NAC completely abolished the enhancing effect of aldosterone on IGF-I-stimulated Akt, MAPK, and p70S6K phosphorylation (n = 3, P < 0.001 when IGF-I plus aldosterone treatment is compared with IGF-I plus aldosterone plus NAC) (Fig. 8A). NAC had no effect on the response to IGF-I alone (Supplemental Fig. 6). These results were confirmed using another antioxidant, catalase (Supplemental Fig. 7). NAC also completely inhibited the ability of aldosterone to enhance IGF-I-stimulated cell growth (Fig. 8B), cell migration (Fig. 8C), and protein synthesis (Fig. 8D).
Figure 7.
Inhibition of the aldosterone-induced increase in ROS inhibits OPN expression and IGF-I-stimulated signaling. A, VSMCs were exposed to 10 and 100 nmol/liter aldosterone for 60 min. ***, P < 0.001 when DCF fluorescence after 10 and 100 nmol/liter of aldosterone was compared with no treatment. B, VSMCs were pretreated with or without NAC (2 mmol/liter) for 60 min, and then aldosterone (10 nmol/liter) was added for 6 or 12 h; NAC was not removed. OPN mRNA expression is expressed as fold increase over nontreated cultures and presented as mean ± sd of three experiments. IB, Immunoblot. C, VSMCs were exposed to NAC (2 mmol/liter) for 60 min and then aldosterone (10 nmol/liter) for 18 h and media OPN levels determined. Aldo, Aldosterone. D and E, Cells were incubated with aldosterone and NAC as described in C and then IGF-I (50 ng/ml) for 5 min. IP, Immunoprecipitation. D, IGF-IR phosphorylation. E, IRS-1 phosphorylation. In D and E, the results are from three independent experiments and are mean ± sem of phospho-IGF-IR or IRS-1. **, P < 0.01; ***, P < 0.001. NS, Not significant.
Figure 8.
The enhancing action of aldosterone on IGF-I-mediated signaling and biological function is completely inhibited by NAC. A, VSMCs were incubated for 60 min with or without NAC (2 mmol/liter) and aldosterone (10 nmol/liter) for 18 h before IGF-I (50 ng/ml) was added for 5 min. Levels of Akt, MAPK, and p70S6K phosphorylation were assessed by immunoblotting. The blots were stripped and reprobed with anti-β-actin. The results from three similar experiments are expressed as the ratio between the phosphorylated protein and β-actin. IB, Immunoblot; Aldo, aldosterone. B, Cell proliferation. Cells were treated with or without NAC (2 mmol/liter), aldosterone (10 nmol/liter), and/or IGF-I (50 ng/ml) for 48 h, as indicated. Each data point represents the mean ± sem number of cells (×104) of three replicate measurements from three independent experiments. C, Cell migration. NAC (2 mmol/liter), aldosterone (10 nmol/liter), and/or IGF-I (50 ng/ml) were added for 48 h, as indicated. ***, P < 0.001 when aldosterone plus IGF-I is compared with the response of cells to IGF-I alone or IGF-I plus aldosterone compared with IGF-I plus aldosterone plus NAC. NS, Not significant when aldosterone and NAC plus IGF-I is compared with the response of cells to IGF-I or NAC alone. D, Protein synthesis. VSMCs were incubated for 18 h with or without NAC (2 mmol/liter), aldosterone (10 nmol/liter), and/or IGF-I (50 ng/ml), as indicated. Each data point represents the mean ± sd cpm of two replicate measurements from three independent experiments. ***, P < 0.001. NS, Not significant.
Discussion
The findings in this study demonstrate that aldosterone enhances IGF-I mediated, IRS-1, Akt, MAPK, and p70S6K phosphorylation. Aldosterone also enhances IGF-I-induced VSMC proliferation, migration, and protein synthesis. The mechanisms mediating these changes were determined to be: 1) induction of OPN production followed by its activation of the αVβ3 integrin; and 2) an increase in IGF-IR protein that was accompanied by a similar in increase IGF-IR activation in response to IGF-I. Both of these actions of aldosterone require gene transcription and aldosterone binding to MR. The findings also show that an aldosterone-induced increase in oxidative stress plays a key role in mediating both the changes in OPN secretion and IGF-IR abundance.
The interaction between aldosterone exposure and IGF-I signaling has been studied in the heart (31) and the kidney (22), but no studies evaluating their interaction in the vasculature have been reported. Holzman et al. (22) demonstrated that in an immortalized renal epithelial cell line aldosterone (1.5 μm per 10 min) transactivates IGF-IR, and this was accompanied by IRS-1 and Akt phosphorylation. This effect was dependent on aldosterone binding to MR but did not require gene transcription. Bunda et al. (31) studied the interaction between aldosterone and IGF-I in human cardiac fibroblasts and its relevance to remodeling of cardiac extracellular matrix. They reported that aldosterone induces elastin fiber deposition and that this effect was dependent on activation of the IGF-IR. Elastin transcription has been reported to be increased by IGF-I (32), and this effect was enhanced in response to aldosterone. They reported that aldosterone (50 nm per 10 min) in the presence of 10% FBS induced IGF-IR phosphorylation but that in SFM it had no effect. They also reported that aldosterone enhanced the effect of IGF-I on tyrosine phosphorylation of IGF-IR, but in contrast to our results, this effect of aldosterone was not inhibited by spironolactone (31).
In the present study, we evaluated the effect of lower concentrations (i.e. 2–10 nmol/liter) of aldosterone because these are the concentrations that occur in hypertensive subjects and in other states of aldosterone excess (33). This is significantly lower than those used by Bunda et al. (31). In contrast to prior studies (22,31), we focused on the effect of long-term aldosterone exposure (18 h) in the absence of serum. Aldosterone (18 h per 10 nmol/liter) had no direct effect on IGF-IR phosphorylation, but it increased IGF-IR protein levels, leading to enhanced IGF-IR activation by IGF-I and downstream signaling (e.g. IRS-1, Akt, MAPK, and p70S6K phosphorylation). These effects required both aldosterone binding to MR and aldosterone-stimulated gene transcription. The observation that an 18 h exposure to aldosterone was required to demonstrate these changes is consistent with the requirement for the de novo protein synthesis.
The increased IGF-IR activation in response to aldosterone was accounted for by an increase in IGF-IR protein. Other growth factor signaling systems have been shown to be responsive to MR stimulation. Aldosterone stimulates epidermal growth factor receptor synthesis in VSMCs, and this effect requires MR binding (20,21). Importantly, angiotensin II stimulates IGF-IR transcription in VSMCs (34), and this response is mediated in part through changes in ROS. Because the variables that regulate IGF-IR expression are major determinants of the cellular response to IGF-I, these findings support the conclusion that these components of the renin-angiotensin-aldosterone system axis have the potential to significantly alter the VSMC response to IGF-I stimulation in vivo (17).
The increase in IGF-IR phosphorylation was accompanied by increased IRS-1 phosphorylation in response to IGF-IR activation. Others (35) reported that aldosterone induced IRS-1 degradation in rat smooth muscle cells. However, that study was conducted in 25 mm glucose, which is a major stimulant of ROS generation and has been shown to induce IRS-1 degradation in multiple cell types (36,37,38). Our studies were conducted in 5 mm glucose; therefore, this difference in experimental conditions could account for the different results in the response of IRS-1 to aldosterone.
In addition to inducing IGF-IR, our results clearly demonstrate that aldosterone stimulates OPN synthesis and secretion, and this results in activation of αVβ3. Our previous studies have demonstrated that OPN-induced αVβ3 activation enhances IGF-I stimulated VSMC responses including MAPK pathway activation and cellular proliferation (23). This conclusion is supported by our observation that a monoclonal antibody that inhibits αVβ3 activation inhibited aldosterone enhancement of IGF-I-stimulated MAPK activation and cellular proliferation. That this effect was independent of aldosterone enhancement of IGF-IR activation was shown by demonstrating that exposure to the αVβ3 antibody had no effect on the ability of aldosterone to enhance IGF-I stimulated IGF-IR tyrosine phosphorylation. This observation also supports the conclusion that the effect of aldosterone on IGF-IR and IRS-1 phosphorylation is independent of its ability to stimulate OPN synthesis and OPN activation of αVβ3.
Irita et al. (39) demonstrated that aldosterone dose and time dependently induced a significant increase in OPN expression in rat renal fibroblasts, and OPN-small interfering RNA completely inhibited the induction of cell proliferation and collagen synthesis in response to aldosterone. In particular, they also identified a specific cis regulatory element (activator protein-1 and nuclear factor-κB) in the OPN promoter responsive to aldosterone (39). Aldosterone also induces OPN in both endothelial (40) and mesangial cells (41). Significant relationships have also been identified between aldosterone and OPN in vivo. Zhang et al. (42) showed that aldosterone stimulated OPN-induced cardiac fibrosis in vivo. MR antagonists block OPN synthesis in blood vessels of angiotensin II transgenic mice (43), and OPN knockout mice demonstrate reduced cardiac fibrosis after aldosterone infusion (44). In humans changes in plasma OPN concentrations correlate with changes in aldosterone and patients with primary hyperaldosteronism have increased plasma levels of OPN (45). In patients with essential hypertension, plasma OPN levels are positively correlated with carotid artery intima-media thickness (11). These findings suggest that aldosterone could induce changes in OPN that lead to vascular remodeling. OPN has been proposed to be a mediator of increased neointimal formation, and it is increased in VSMC response to hyperglycemia (10,23,46). We reported that optimal IGF-I stimulation of VSMCs requires OPN binding to αVβ3 integrin (23). In this study we also show that inhibition of OPN binding to αVβ3 and its consequent activation significantly inhibited the potentiating action of aldosterone on IGF-I stimulated (Akt, MAPK, p70S6K phosphorylation) as well as cell proliferation and protein synthesis. Because we have shown previously that these responses are enhanced by OPN in the cells in response to hyperglycemia (23), we conclude that aldosterone is inducing this response by a similar mechanism that is independent of the effect of MR on IGF-IR abundance. However, the enhancing effect of aldosterone that was mediated through αVβ3 was minimal and its significance needs to be confirmed in vivo.
In this study we showed that aldosterone induced a significant increase in ROS production in VSMCs and that the antioxidants, NAC and catalase, inhibited the aldosterone-mediated changes in VSMC responsiveness that were secondary to enhanced IGF-I-stimulated IGF-IR phosphorylation as well as increased OPN synthesis and αVβ3 activation. This suggests that the effects of aldosterone on IGF-I signaling are dependent on the level of oxidative stress and that the increase in ROS is enhancing IGF-I actions through both signaling mechanisms. Further study will be required to determine the mechanism by which increased ROS production enhances both OPN and IGF-IR synthesis.
ROS are a major source of oxidative stress in the cardiovascular and renal parenchymal system and have an important role in the pathogenesis of atherosclerosis (47). ROS have been implicated in the pathophysiology of aldosterone-induced cardiovascular injury (18,47,48). Several basic and clinical studies suggest that aldosterone-induced vascular dysfunction is a consequence of a vasculopathy that results from the propensity for aldosterone to generate ROS (13,18,47,49). Aldosterone induction of cardiovascular cell hypertrophy has been reported to be secondary to activation of nicotinamide adenine dinucleotide phosphate activity and MR antagonism decreases nicotinamide adenine dinucleotide phosphate activity and superoxide anion generation (24). Given the role of IGF-I in stimulating changes in vascular function, these changes in ROS may be a critical determinant of the response of vascular cells to this growth factor.
In conclusion, this study demonstrates that IGF-I and aldosterone signaling pathways interact to enhance IGF-I stimulated VSMC proliferation, migration, and protein synthesis. Aldosterone enhances cellular responsiveness to IGF-I by both inducing OPN synthesis leading to activation of αVβ3 and increasing ROS generation, which leads to an increase in IGF-IR synthesis, activation, and downstream signaling. Taken together these results not only indicate a novel mechanism of aldosterone-induced pathophysiologic changes but also provide new insight about a possible role of aldosterone cross talk with IGF-I and integrins in the pathogensis of vascular diseases.
Supplementary Material
Acknowledgments
We are thankful to Ms. Laura Lindsey for her help in preparing the manuscript. We thank Pfizer Inc. (New York, NY) for supplying eplerenone.
Footnotes
This work was supported by Grants HL56850 and AG02331 from the National Institutes of Health.
Disclosure Summary: Eplerenone was supplied to D.R.C. from Pfizer Inc. in an amount less than $10,000. The authors have nothing else to disclose.
First Published Online September 29, 2010
Abbreviations: Act-D, Actinomycin D; DCF, 2′,7′-dichlorofluorescein; DCFDA, DCF diacetate; Epl, eplerone; FBS, fetal bovine serum; IGF-IR, IGF-I receptor; IRS, insulin receptor substrate; MR, mineralocorticoid receptor; NAC, N-acetyl-l-cysteine; NG, normal glucose; OPN, osteopontin; PDGF, platelet-derived growth factor; ROS, reactive oxygen species; SFM, serum-free medium; VSMC, vascular smooth muscle cell.
References
- Delafontaine P, Song YH, Li Y 2004 Expression, regulation, and function of IGF-1, IGF-1R, and IGF-1 binding proteins in blood vessels. Arterioscler Thromb Vasc Biol 24:435–444 [DOI] [PubMed] [Google Scholar]
- Fath KA, Alexander RW, Delafontaine P 1993 Abdominal coarctation increases insulin-like growth factor I mRNA levels in rat aorta. Circ Res 72:271–277 [DOI] [PubMed] [Google Scholar]
- Maile LA, Busby WH, Sitko K, Capps BE, Sergent T, Badley- Clarke J, Clemmons DR 2006 Insulin-like growth factor-I signaling in smooth muscle cells is regulated by ligand binding to the 177CYDMKTTC184 sequence of the β3-subunit of αVβ3. Mol Endocrinol 20:405–413 [DOI] [PubMed] [Google Scholar]
- Xi G, Maile LA, Yoo SE, Clemmons DR 2008 Expression of the human β3 integrin subunit in mouse smooth muscle cells enhances IGF-I-stimulated signaling and proliferation. J Cell Physiol 214:306–315 [DOI] [PubMed] [Google Scholar]
- Nichols TC, du Laney T, Zheng B, Bellinger DA, Nickols GA, Engleman W, Clemmons DR 1999 Reduction in atherosclerotic lesion size in pigs by αVβ3 inhibitors is associated with inhibition of insulin-like growth factor-I-mediated signaling. Circ Res 85:1040–1045 [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J 1999 The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 341:709–717 [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM 1993 Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest 92:1686–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Wen JK, Zheng B, Liu Z, Chen Y 2007 Blockade of integrin β3-FAK signaling pathway activated by osteopontin inhibits neointimal formation after balloon injury. Cardiovasc Pathol 16:283–290 [DOI] [PubMed] [Google Scholar]
- Sodhi P, Phadke SA, Battle D, Sahai A 2001 Hypoxia stimulates osteopontin expression and proliferation of cultured vascular smooth muscle cells: proliferation by high glucose. Diabetes 50:1482–1460 [DOI] [PubMed] [Google Scholar]
- Isoda K, Nishikawa K, Kamezawa Y, Yoshida M, Kusuhara M, Moroi M, Tada N, Ohsuzu F 2002 Osteopontin plays and important role in the development of medial thickening and neointimal formation. Circ Res 91:77–82 [DOI] [PubMed] [Google Scholar]
- Kurata M, Okura T, Watanabe S, Fukuoka T, Higaki J 2006 Osteopontin and carotid atherosclerosis in patients with essential hypertension. Clin Sci (Lond) 111:319–324 [DOI] [PubMed] [Google Scholar]
- Pitt B, Williams G, Remme W, Martinez F, Lopez-Sendon J, Zannad F, Neaton J, Roniker B, Hurley S, Burns D, Bittman R, Kleiman J 2001 The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther 15:79–87 [DOI] [PubMed] [Google Scholar]
- Struthers AD 2002 Aldosterone: cardiovascular assault. Am Heart J 144:S2–S7 [DOI] [PubMed] [Google Scholar]
- Park JB, Schiffrin EL 2002 Cardiac and vascular fibrosis and hypertrophy in aldosterone-infused rats: role of endothelin-1. Am J Hypertens 15:164–169 [DOI] [PubMed] [Google Scholar]
- Miyata K, Hitomi H, Guo P, Zhang GX, Kimura S, Kiyomoto H, Hosomi N, Kagami S, Kohno M, Nishiyama A 2008 Possible involvement of Rho-kinase in aldosterone-induced vascular smooth muscle cell remodeling. Hypertens Res 31:1407–1413 [DOI] [PubMed] [Google Scholar]
- Zhang A, Jia Z, Guo X, Yang T 2007 Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am J Physiol Renal Physiol 293:F723–F731 [DOI] [PubMed] [Google Scholar]
- Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique C, Stas S, Sowers JR 2007 Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol 293:H2009–H2023 [DOI] [PubMed] [Google Scholar]
- Miyata K, Rahman M, Shokoji T, Nagai Y, Zhang GX, Sun GP, Kimura S, Yukimura T, Kiyomoto H, Kohno M, Abe Y, Nishiyama A 2005 Aldosterone stimulates reactive oxygen species production through activation of NADPH oxidase in rat mesangial cells. J Am Soc Nephrol 16:2906–2912 [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Osborn HL, Grekin R, Webb RC 2001 Spironolactone reduces cerebral infarct size and EGF-receptor mRNA in stroke-prone rats. Am J Physiol Regul Integr Comp Physiol 281:R944–R950 [DOI] [PubMed] [Google Scholar]
- Grossmann C, Gekle M 2008 Nongenotropic aldosterone effects and the EGFR: interaction and biological relevance. Steroids 73:973–978 [DOI] [PubMed] [Google Scholar]
- Grossmann C, Krug AW, Freudinger R, Mildenberger S, Voelker K, Gekle M 2007 Aldosterone-induced EGFR expression: interaction between the human mineralocorticoid receptor and the human EGFR promoter. Am J Physiol Endocrinol Metab 292:E1790–E1800 [DOI] [PubMed] [Google Scholar]
- Holzman JL, Liu L, Duke BJ, Kemendy AE, Eaton DC 2007 Transactivation of the IGF-1R by aldosterone. Am J Physiol Renal Physiol 292:F1219–F1228 [DOI] [PubMed] [Google Scholar]
- Maile LA, Capps BE, Ling Y, Xi G, Clemmons DR 2007 Hyperglycemia alters the responsiveness of smooth muscle cells to insulin-like growth factor-I. Endocrinology 148:2435–2443 [DOI] [PubMed] [Google Scholar]
- Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR 2007 Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology 148:3773–3780 [DOI] [PubMed] [Google Scholar]
- Maile LA, Busby WH, Sitko K, Capps BE, Sergent T, Badley-Clarke J, Ling Y, Clemmons DR 2006 The heparin binding domain of vitronectin is the region that is required to enhance insulin-like growth factor-I signaling. Mol Endocrinol 20:881–892 [DOI] [PubMed] [Google Scholar]
- Gockerman A, Prevette T, Jones JI, Clemmons DR 1995 Insulin-like growth factor (IGF)-binding proteins inhibit the smooth muscle cell migration responses to IGF-I and IGF-II. Endocrinology 136:4168–41473 [DOI] [PubMed] [Google Scholar]
- Zheng B, Clemmons DR 1998 Blocking ligand occupancy of the αVβ3 integrin inhibits insulin-like growth factor I signaling in vascular smooth muscle cells. Proc Natl Acad Sci USA 95:11217–11222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Maile LA, Clemmons DR 2003 Tyrosine phosphorylation of the β3-subunit of the αVβ3 integrin is required for membrane association of the tyrosine phosphatase SHP-2 and its further recruitment to the insulin-like growth factor I receptor. Mol Endocrinol 17:1824–1833 [DOI] [PubMed] [Google Scholar]
- Gao L, Mann GE 2009 Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc Res 82:9–20 [DOI] [PubMed] [Google Scholar]
- Busik JV, Mohr S, Grant MB 2008 Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes 57:1952–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunda S, Liu P, Wang Y, Liu K, Hinek A 2007 Aldosterone induces elastin production in cardiac fibroblasts through activation of insulin-like growth factor-I receptors in a mineralocorticoid receptor-independent manner. Am J Pathol 171:809–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe BL, Rich CB, Goud HD, Terpstra AJ, Bashir M, Rosenbloom J, Sonenshein GE, Foster JA 1993 Insulin-like growth factor-I regulates transcription of the elastin gene. J Biol Chem 268:12418–12426 [PubMed] [Google Scholar]
- Marney AM, Brown NJ 2007 Aldosterone and end organ damage. Clin Sci 133:267–278 [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhang L, Peng T, Cheng J, Taneja S, Zhang J, Delafontaine P, Du J 2006 Angiotensin II stimulates transcription of insulin-like growth factor I receptor in vascular smooth muscle cells: role of nuclear factor-κB. Endocrinology 147:1256–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi H, Kiyomoto H, Nishiyama A, Hara T, Moriwaki K, Kaifu K, Ihara G, Fujita Y, Ugawa T, Kohno M 2007 Aldosterone suppresses insulin signaling via the downregulation of insulin receptor substrate-1 in vascular smooth muscle cells. Hypertension 50:750–755 [DOI] [PubMed] [Google Scholar]
- Renström F, Burén J, Eriksson JW 2005 Insulin receptor substrates-1 and -2 are both depleted but via different mechanisms after down-regulation of glucose transport in rat adipocytes. Endocrinology 146:3044–3051 [DOI] [PubMed] [Google Scholar]
- Kim B, Feldman EL 2009 Insulin receptor substrate (IRS)-2, not IRS-1, protects human neuroblastoma cells against apoptosis. Apoptosis 14:665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishnan Y, Busby Jr WH, Shen X, Maile LA, Clemmons DR 2010 Insulin-like growth factor-1 (IGF-I) stimulated insulin receptor substrate-1 negatively regulates Src homology 2 domain-containing protein-tyrosine phosphatase substrate-1 function in vascular smooth muscle cells. J Biol Chem 285:15682–15695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irita J, Okura T, Kurata M, Miyoshi K, Fukuoka T, Higaki J 2008 Osteopontin in rat renal fibroblasts: functional properties and transcriptional regulation by aldosterone. Hypertension 51:507–513 [DOI] [PubMed] [Google Scholar]
- Sugiyama T, Yoshimoto T, Hirono Y, Suzuki N, Sakurada M, Tsuchiya K, Minami I, Iwashima F, Sakai H, Tateno T, Sato R, Hirata Y 2005 Aldosterone increases osteopontin gene expression in rat endothelial cells. Biochem Biophys Res Commun 336:163–167 [DOI] [PubMed] [Google Scholar]
- Gauer S, Hauser IA, Obermüller N, Holzmann Y, Geiger H, Goppelt-Struebe M 2008 Synergistic induction of osteopontin by aldosterone and inflammatory cytokines in mesangial cells. J Cell Biochem 103:615–623 [DOI] [PubMed] [Google Scholar]
- Zhang YL, Zhou SX, Lei J, Yuan GY, Wang JF 2008 Blockades of angiotensin and aldosterone reduce osteopontin expression and interstitial fibrosis infiltration in rats with myocardial infarction. Chin Med J 121:2192–2196 [PubMed] [Google Scholar]
- Sakurabayashi-Kitade S, Aoka Y, Nagashima H, Kasanuki H, Hagiwara N, Kawana M 2009 Aldosterone blockade by Spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis 206:54–60 [DOI] [PubMed] [Google Scholar]
- Sam F, Xie Z, Ooi H, Kerstetter DL, Colucci WS, Singh M, Singh K 2004 Mice lacking osteopontin exhibit increased left ventricular dilation and reduced fibrosis after aldosterone infusion. Am J Hypertens 17:188–193 [DOI] [PubMed] [Google Scholar]
- Irita J, Okura T, Manabe S, Kurata M, Miyoshi K, Watanabe S, Fukuoka T, Higaki J 2006 Plasma osteopontin levels are higher in patients with primary aldosteronism than in patients with essential hypertension. Am J Hypertens 19:293–297 [DOI] [PubMed] [Google Scholar]
- Sun J, Xu Y, Dai Z, Sun Y 2009 Intermittent high glucose enhances proliferation of vascular smooth muscle cells by upregulating osteopontin. Mol Cell Endocrinol 313:64–69 [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL 2002 Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension 40:504–510 [DOI] [PubMed] [Google Scholar]
- Keidar S, Kaplan M, Pavlotzky E, Coleman R, Hayek T, Hamoud S, Aviram M 2004 Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation 109:2213–2220 [DOI] [PubMed] [Google Scholar]
- Maron BA, Zhang YY, Handy DE, Beuve A, Tang SS, Loscalzo J, Leopold JA 2009 Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem 284:7665–7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.