Abstract
Previously, we demonstrated that progesterone (P4) promoted adult rat neural progenitor cell (rNPC) proliferation with concomitant regulation of cell-cycle gene expression via the P4 receptor membrane component/ERK pathway. Here, we report the efficacy of seven clinically relevant progestins alone or in combination with 17β-estradiol (E2) on adult rNPC proliferation and hippocampal cell viability in vitro and in vivo. In vitro analyses indicated that P4, norgestimate, Nestorone, norethynodrel, norethindrone, and levonorgestrel (LNG) significantly increased in rNPC proliferation, whereas norethindrone acetate was without effect, and medroxyprogesterone acetate (MPA) inhibited rNPC proliferation. Proliferative progestins in vitro were also neuroprotective. Acute in vivo exposure to P4 and Nestorone significantly increased proliferating cell nuclear antigen and cell division cycle 2 expression and total number of hippocampal 5-bromo-2-deoxyuridine (BrdU)-positive cells, whereas LNG and MPA were without effect. Mechanistically, neurogenic progestins required activation of MAPK to promote proliferation. P4, Nestorone, and LNG significantly increased ATP synthase subunit α (complex V, subunit α) expression, whereas MPA was without effect. In combination with E2, P4, Nestorone, LNG, and MPA significantly increased BrdU incorporation. However, BrdU incorporation induced by E2 plus LNG or MPA was paralleled by a significant increase in apoptosis. A rise in Bax/Bcl-2 ratio paralleled apoptosis induced by LNG and MPA. With the exception of P4, clinical progestins antagonized E2-induced rise in complex V, subunit α. These preclinical translational findings indicate that the neurogenic response to clinical progestins varies dramatically. Progestin impact on the regenerative capacity of the brain has clinical implications for contraceptive and hormone therapy formulations prescribed for pre- and postmenopausal women.
Clinical progestins vary dramatically in their impact on the regenerative capacity of the brain and thus are likely to have clinical implications for long-term neurological function.
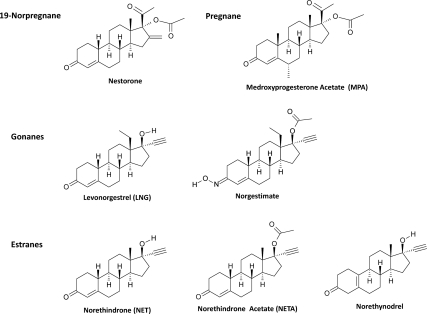
It is increasingly clear that gonadal hormone function extends well beyond reproduction (1,2,3). In brain, progesterone (P4) exerts multiple nonreproductive functions including regulating mood, inflammation, bioenergetics, neural plasticity, cognition, and recovery from traumatic brain injury (1,2,4,5,6). Clinically, P4 and related synthetic progestins have been widely used as therapeutics for fertility, contraception, and hormone replacement therapy (1,2,7,8,9,10). The majority of progestins are derived from three tetracyclic structures: pregnane, estrane, and gonane and have varying degrees of specificity for P4 receptors (PRs) (Fig. 1) (9,10). Recently, a newer class of progestins, the 19-norprogesterone molecules, has been developed with improved progestational activity with little to no androgenic activity and used for contraception and hormone therapy (11).
Figure 1.
Chemical structures of clinically relevant progestins. Progestins are derived from four tetracyclic structures: 19-norprogesterone, pregnane, estrane, and gonane. Although all three structures bear four rings, identified as A, B, C, and D, the estrane structure differs from the pregnane structure by lacking the C-19 angular methyl radical between rings A and B. Gonane structure lacks both the C-18 and C-19 angular methyl radicals and bears an ethyl group between rings C and D at C-13. Removal of C-19 methyl radical and addition of C-13 ethyl radical significantly enhances the progestational activities. The seven representative progestins were the 19-norprogesterone, Nestorone; the pregnane, MPA; the gonanes, leveonorgestrel and NGM; and the estranes, NET, NETA, and norethynodrel.
Although the effect of progestins on the reproductive system has been extensively studied, their impact on central nervous system function, particularly adult neurogenesis and cell viability, remains largely unexplored (1). The generation of new neurons or neurogenesis in the two proliferative zones of the brain, the subgranular zone of the hippocampus and the subventricular zone of the cerebral ventricles, is the principle regenerative strategy of the adult brain. In parallel, cellular viability can be a predictive indicator of the health of newly generated and existing cells. In this study, we selected seven clinically relevant progestins as representative of three different classes of progestins to determine their effects on neural progenitor proliferation and neuronal viability (Fig. 1): one with pregnane structure [medroxyprogesterone acetate (MPA)], one with 19-norprogesterone structure (Nestorone), three with estrane structure [norethindrone (NET), NET acetate (NETA), and norethynodrel], and two with gonane structure [levonorgestrel (LNG) and norgestimate (NGM)]. Notably, NETA, norethynodrel, and NGM are prodrugs; the first two require in vivo conversion to NET to exert their progestational effects, whereas the latter requires conversion to LNG for activity (8,12).
Analyses reported here were conducted to determine the impact of clinically relevant progestins on the regenerative capacity of brain when administered alone or in combination with 17β-estradiol (E2). Results of these studies indicate that acute exposure to clinically relevant progestins and E2/progestin combinations differentially regulated neurogenic and neuroprotective responses in brain. The data indicate that clinical progestins vary dramatically in their impact, which can range from promoting the regenerative function of the brain to increasing cell death.
Materials and Methods
Chemicals
P4, NET, NETA, ORG 31710, norethynodrel, LNG, MPA, and NGM were purchased from Steraloids (Newport, RI). Nestorone was provided by Sitruk-Ware (Rockefeller University and Population Council, New York, NY).
PR, androgen receptor (AR), and glucocorticoid receptor (GR) competitive binding
The binding affinities of tested progestins to PR, AR, and GR were determined by fluorescent polarization competitive binding assays using purified baculovirus-expressed human PR/AR/GR and fluorescent P4/androgen/glucocorticoid ligand PL Red (Invitrogen, Grand Island, NY) as described before (7,13), respectively. Polarization values were determined using GENios Pro microplate reader (Tecan, San Jose, CA) and plotted against the logarithm of the concentrations of test compounds. IC50 values were determined by nonlinear least-squares analysis.
Culture of rat neural progenitor cells (rNPCs)
Rat NPCs derived from adult rat dentate gyrus (gift from Fred Gage; Laboratory of Genetics, The Salk Institute, CA) were provided as cryopreserved cells. They were cultured as described previously (7,13,14) in DMEM/Hams F-12 medium (1:1; Omega Scientific, Tarzana, CA) with 1% penicillin/streptomycin/amphotericin B (Invitrogen), supplemented with N2 (1%; Invitrogen) and basic fibroblast growth factor (bFGF) (20 ng/ml; Invitrogen) in a humidified incubator (37 C and 5% CO2). Cells were plated at a density of 2 × e6 cells/flask or 7.5–15 × e4 cells/well · 96-well plates.
5-Bromo-2-deoxyuridine (BrdU) incorporation
Cell proliferation was determined by S phase incorporation of BrdU. After 4–6 h of starvation (medium without supplements), rNPCs were loaded with 10 μm BrdU in the presence or absence of bFGF and varying concentrations of P4 or test progestins in unsupplemented maintenance medium for 1d. The rNPCs were then processed as described previously (7,14). After subtracting the value of the blank (without BrdU loading), data were analyzed using a one-way ANOVA, followed by a Neuman-Keuls post hoc test.
Primary neuronal culture
Hippocampal neuronal cultures were prepared as previously described (13).
Glutamate exposure and neuronal viability
Hippocampal neuronal cultures grown on 96-well culture plates for 7d in vitro were pretreated with vehicle alone or test compounds, followed by exposure to 200 μm glutamate as previously described (13). After glutamate exposure, cultures were washed with N-2-hydroxyethylpiperazine-N′-2-ethane sulfonic acid-buffered saline solution and replaced with fresh NBM containing the test compounds or combinations. Cultures were returned to the incubator and incubated for 24 h before analysis of neuronal viability using colorimetric lactate dehydrogenase (LDH) release in the media.
Animals
Use of animals was approved by the Institutional Animal Care and Use Committee at the University of Southern California (Protocol No. 10911). Embryonic d-18 fetuses derived from time-pregnant Sprague Dawley rats (Harlan, Indianapolis, IN) were used to obtain primary neuronal cultures for in vitro experiments. Ovariectomized (OVX) 3-month-old adult female Sprague Dawley rats (Harlan) were used for in vivo experiments. For progestin-only treatments (n = 5 per group), rats were sc injected 1 wk after ovariectomy with vehicle alone or P4 [30 μg/kg body weight (BW)], Nestorone (30 μg/kg BW), LNG (30 μg/kg BW), and MPA (30 μg/kg BW), respectively, and 1 h later with 100 mg/kg BW BrdU injection. For E2 and progestin combined treatments (n = 5 per group), rats were sc injected 1 wk after ovariectomy with either E2 alone (30 μg/kg BW), or E2 combined with P4/Nestorone/LNG/MPA (30 μg/kg BW), or equivalent volume of sesame oil as vehicle control, respectively, and 1 h later with 100 mg/kg BW BrdU injection. Doses of progestins were based on our previous in vivo studies, where the same P4 treatment produced P4 levels in rats of 28 pg/g in brain tissue and 20 pg/ml in serum (5). Animals were killed 24 h after injection, and one hemisphere was fixed in 4% paraformaldehyde and processed for fluorescence-activated cell sorting (FACS) analysis, and whole hippocampus (both posterior and anterior hippocampus but not alveus or entorhinal/perirhinal cortex) from the other hemispheres were manually dissected for Western blot analysis.
Western blot analyses
Hippocampal samples from progestin-treated rats were assessed for protein expression of proliferating cell nuclear antigen (PCNA) (1:500; Zymed Laboratories, San Francisco, CA) and cell division cycle 2 (CDC2) or cyclin dependent kinase 1 (Cdk1) (1:500; Novus Biologicals, Littleton, CO), mitochondria complex V, subunit α (CVα) (1:1000; MitoSciences, Inc., Eugene, OR), Bax (1:1000; Cell Signaling Technology, Danvers, MA), and Bcl-2 (50E3) (1:1000; Cell Signaling Technology) by Western blotting as previously described (7,14). Relative amounts of protein expression were quantified by optical density analysis using UN-SCAN-IT gel automated digitizing system (Silk Scientific, Inc., Orem, UT).
Nuclei extraction and flow cytometry counting
Hippocampi were dissected from the fixed hemispheres using anatomical landmarks as described (15). Extracted hippocampi were homogenized and nuclei sample collected into a 1.5 ml microcentrifuge tube, washed four times using 200 μl of PBS, and then centrifuged for 10 min at 10,000 rpm. The pellet was then resuspended in 600 μl of PBS plus 0.5% Triton X-100, heated for 1 h at 75 C for epitope retrieval, and incubated for 24 h at 4 C with primary mouse monoclonal anti-BrdU antibody (1:100, Ab12219; Abcam, Cambridge, MA) for BrdU+ cell count or with Clontech ApoAlert DNA Fragmentation Assay kit for DNA fragmentation [terminal deoxynucleotidyltransferase-mediated 2′-deoxyuridine 5′-triphosphate nick end labeling (TUNEL)] detection. The number of nuclei was estimated by counting the propidium iodide, and the number of BrdU or TUNEL-labeled cells was detected using BD LSR II flow cytometer and BD FACSDiva software (BD Biosciences, San Jose, CA).
Statistical analyses
Data are presented as mean ± sem and statistically significant differences determined by a one-way ANOVA followed by a Student-Newman-Keuls post hoc analysis.
Results
PR/GR/AR binding profiles of clinical progestins
To determine the binding profiles of tested progestins (Fig. 1) to nuclear PR (nPR), fluorescence polarization-based competitive binding assay was conducted (7,13). Competitive binding assays were also conducted to determine their binding affinities to AR and GR, respectively. Data derived from the binding experiments are summarized in Table 1. The binding IC50 values of P4, 9.01 nm for PR, 119.3 nm for AR, and 6.51 nm for GR were consistent with those reported previously (2). Among these progestins, maximal binding affinity to nPR was exhibited by Nestorone (IC50 = 5.07 nm), followed by LNG (IC50 = 5.46 nm), MPA (IC50 = 6.20 nm), NET (IC50 = 14.32 nm), NETA (IC50 = 38.92 nm), norethynodrel (IC50 = 67.76 nm), and NGM (IC50 = 482.80 nm). The low binding affinities of NETA and NGM were consistent with their prodrug properties, because they require conversion to NET and LNG, respectively, to exert progestational effects (11). With respect to AR binding affinity, LNG exhibited maximal binding affinity to AR (IC50 = 2.90 nm), followed by MPA (IC50 = 4.53 nm), NGM (IC50 = 5.02 nm), NET (IC50 = 18.02 nm), norethynodrel (IC50 = 196.70 nm), and NETA (IC50 = 876.90 nm). Nestorone did not bind to AR, consistent with previous reports indicating that Nestorone has little androgenic activity (16). Lastly, progestin binding to GR was as follows: MPA (IC50 = 1.42 nm), Nestorone (IC50 = 8.71 nm), LNG (IC50 = 16.73 nm), NET (IC50 = 97.84 nm), NETA (IC50 = 202.70 nm), NGM (IC50 = 287.10 nm), and norethynodrel (IC50 = 1306.00 nm). Overall, results from these binding analysis confirmed previous reports and provided a direct comparison of binding affinities of tested progestins with PR, AR, and GR. Further, results indicated that clinical progestins have a complex binding profile and have multiple targets in the biological systems that are likely to impact outcomes in brain.
Table 1.
Binding profile of clinical progestins to progesterone, glucocorticoid, and androgen receptors
| Progestins | PR
|
GR IC50 (nm) | AR IC50 (nm) | Selectivity (PR/GR) | Selectivity (PR/AR) | Selectivity (AR/GR) | |
|---|---|---|---|---|---|---|---|
| IC50 (nm) | RBA (%) | ||||||
| P4 | 9.01 | 100 | 6.51 | 119.30 | 4.78 | 87.72 | 0.05 |
| MPA | 6.20 | 145.30 | 1.42 | 4.53 | 0.23 | 0.73 | 0.31 |
| Nestorone | 5.07 | 177.70 | 8.71 | NB | 1.72 | NA (+∞) | NA (−∞) |
| NET | 14.32 | 62.90 | 97.84 | 18.02 | 6.83 | 1.26 | 5.43 |
| NETA | 38.92 | 23.20 | 202.70 | 876.90 | 5.21 | 22.53 | 0.23 |
| Norethynodrel | 67.76 | 13.30 | 1306.00 | 196.70 | 19.27 | 2.90 | 6.64 |
| LNG | 5.46 | 165.20 | 16.73 | 2.90 | 3.07 | 0.53 | 5.77 |
| Norgestimate | 482.80 | 1.87 | 287.10 | 5021.00 | 0.59 | 10.40 | 0.06 |
Fluorescence polarization-based competitive binding assays were conducted to determine the binding profile of test progestins to PR, GR, and AR. IC50 refers to the concentration of test progestins resulting in a half-maximal shift in polarization value, which was determined from the binding curve by a nonlinear least-squares analysis. The relative binding affinity (RBA) of test progestins is expressed as percent of the binding affinity of progesterone (RBA = 100%). Selectivity was calculated as the ratio between the binding IC50. NA, Not applicable; NB, no binding.
Clinical progestin regulation of rNPC proliferation in vitro
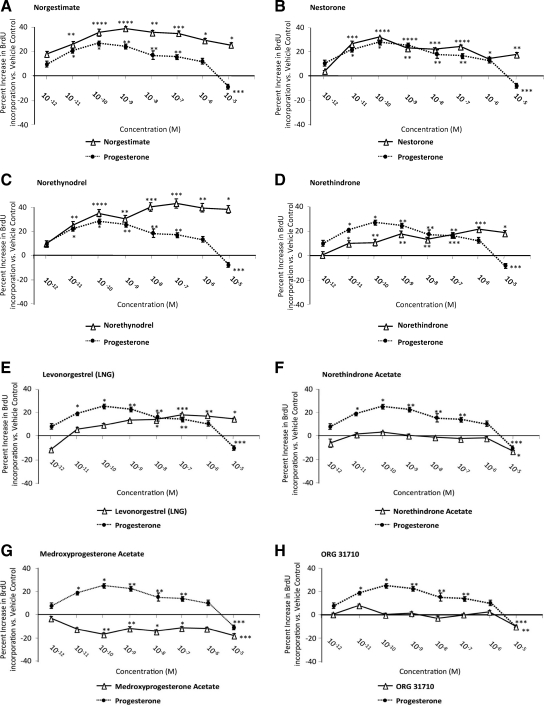
Our previous study demonstrated that P4 increased rNPC proliferation in a dose-dependent manner (7). To determine whether progestins exhibited comparable efficacy on rNPC proliferation, BrdU chemiluminescence ELISA was conducted and compared with the proliferative efficacy of P4 at the same concentration (Fig. 2). Results of these analyses indicated that at 24 h, NGM was more potent in promoting rNPC proliferation than P4 at all concentrations, with minimal effective dose of 10−11 m and a maximal effect of 40.4 ± 1.5% increase at 10−9 m (P < 0.001). Nestorone and P4 were equally efficacious at their EC100 concentrations, with both EC100 values being 10−10 m. Norethynodrel induced a comparable effect with P4 within the picomolar to low nanomolar range and was significantly (P < 0.005) more efficacious than P4 at high nanomolar to micromolar range, with minimal effective dose at 10−11 m and maximal effective effect (43.5 ± 3.7% increase) at 10−7 m. NET induced a moderate effect in promoting rNPC proliferation but was less effective than P4 with a minimally effective dose of 10−11 m and a maximal effect was 21.8 ± 1.7% increase at 10−6 m. Similarly, LNG exhibited a minimally effective dose of 10−8 m and a maximal effect of 17.7 ± 0.8% at 10−7 m. NETA and ORG 31710 exerted no effect on rNPC proliferation at all concentrations except 10−5 m, a concentration at which rNPC proliferation was significantly inhibited (P < 0.05 and P < 0.01, respectively). Lastly, MPA significantly inhibited rNPC proliferation at multiple concentrations with maximal inhibition at 10−10 m to 10−5 m with rNPC proliferation reduced by 17.0 ± 1.3% (P < 0.05) and 17.8 ± 1.2% (P < 0.05), respectively.
Figure 2.
Progestin regulation of NPC proliferation. Rat NPCs were starved for 4 h before exposure to clinical progestins (10 × e−12 to 10 × e−5 m) for 24 h. Cell proliferation was evaluated by BrdU chemiluminescence ELISA measuring BrdU incorporation. Ethanol was used as vehicle control (1 × e−6), and bFGF was used as positive control. At each concentration, the proliferative efficacies of progestins (solid lines) were compared against that of P4 (dotted line). A–C, Effects of progestins that had greater proliferative efficacy relative to P4. D and E, Progestins with lower proliferative efficacy relative to P4. F–H, Progestins with no or antagonizing effects on cell proliferation. Data are derived from three independent assays, analyzed using one-way ANOVA, followed by Neuman-Keuls post hoc test, and plotted as percentage increase vs. vehicle control (mean ± sem). *, P < 0.05; **, P < 0.01; ***, P < 0.005; and ****, P < 0.001 vs. vehicle control.
Progestin-induced cell proliferation is dependent on ERK signaling pathway and independent of nPR
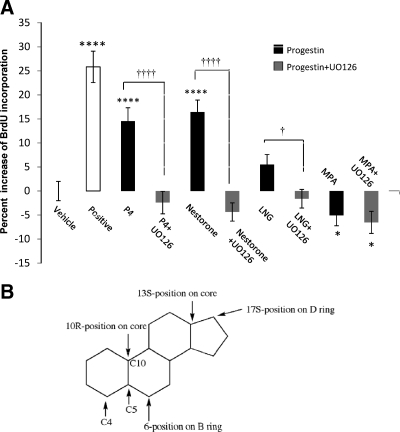
Previously we demonstrated that P4-induced cell proliferation is an ERK signaling pathway-dependent process (7). To determine whether ERK activation was required for progestin-induced cell proliferation, BrdU chemiluminescence ELISA was conducted in the presence or absence of the MEK kinase inhibitor UO126 (Fig. 3A). Consistent with results in Fig. 2, at 10−8 m, both P4 and Nestorone significantly increased BrdU incorporation by 14.6 and 16.4% compared with vehicle control, respectively (P < 0.0001 and P < 0.0001, respectively). At 10−8 m, LNG had no statistically significant effect, although a positive trend was observed, whereas MPA significantly inhibited BrdU incorporation (P < 0.05). Inhibition of ERK/MAPK signaling pathway by UO126 abolished P4-induced cell proliferation (P < 0.0001), which replicated our previous findings (7), and completely inhibited the proliferative effects of Nestorone and LNG (P < 0.0001 and P < 0.05, respectively) with no effect observed on MPA-induced inhibition of cell proliferation. These data indicate that like P4, Nestorone- induced, and LNG-induced cell proliferation require the ERK signaling pathway, whereas the inhibitory effect of MPA is independent of the ERK/MAPK signaling pathway.
Figure 3.
Progestin-induced cell proliferation is dependent on MAPK and independent of cPR. A, BrdU chemiluminescence ELISA was carried out to determine the signaling cascade of progestin-induced cell proliferation. Rat NPCs were starved for 4 h before exposure to 100 pm progestin alone or progestin combined with MAPK inhibitor UO126 (100 nm) for 24 h, and cell proliferation was evaluated by BrdU chemiluminescence ELISA measuring BrdU incorporation. Ethanol + DMSO (1 × e−6) was used as vehicle control, and bFGF was used as positive control. Data were collected from four independent assays, analyzed using one-way ANOVA, followed by Neuman-Keuls post hoc test, and plotted as percentage increase vs. vehicle control (mean ± sem). *, P < 0.05; **, P < 0.01; ***, P < 0.005; and ****, P < 0.0001 vs. vehicle control; †, P < 0.05; ††††, P < 0.001 vs. progestin-alone group. B, Structure-activity analysis of progestin-induced proliferative effects. Critical structural features of progestins required for proliferative effects from SAR analysis.
Analysis of the structure-activity relationship (SAR) was conducted to determine structural molecular features required for proliferative efficacy of clinical progestins. The presence of an acetyl or hydroxyl group at the 17S-position of the D ring was favorable for rNPC proliferation, whereas a methyl group at the 6-position, whether it was in the R or S configuration, negatively impacted cell proliferation (Fig. 3B). A modification at the 10R-position or at the 13S-position had no effect on the proliferative capabilities of the molecule nor did the position of the double bond between C-4 and C-5 or between C-5 and C-10. Interestingly, addition of a hydroxyl group at the C-17-position is known to eliminate the progestational effect of progestins, whereas acetylation of the C-17-OH can reverse this effect to render the progestins orally active. Addition of the 13-C ethyl group is positively associated with the highest progestational activity, consistent with findings that helix 12 Met909 is the key residue for PR activation by both testosterone- derived and P4-derived progestins with a 13-methyl or a 13-ethyl substitutions (17). Surprisingly, modifications favoring the proliferative effect of the progestins are negatively correlated with their binding affinity to nPR. Thus, it is likely that the proliferative effects of progestins are not mediated by nPR, which is consistent with our previous finding that nPR is not expressed in rNPCs, that the proliferative effect of P4 in rNPCs is not mediated by nPR but is instead mediated by the membrane PR membrane component 1 (PGRMC-1) (7).
Clinical progestin regulation of neuroprotection in vitro
Previous analyses indicated that P4 exerted significant neuroprotection against neurodegenerative insults (5,18). We therefore determined whether clinical progestins exerted comparable efficacy in protecting primary hippocampal neurons against degeneration induced by excitoxic glutamate. LDH release in the culture medium served as an indicator of neuronal membrane integrity, a minimum requirement for neuroprotection (13). Dose-response analysis was conducted for each progestin (Fig. 4) with neuroprotective efficiency (NE) calculated as follows:
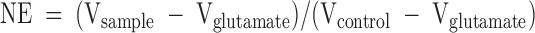 |
Figure 4.
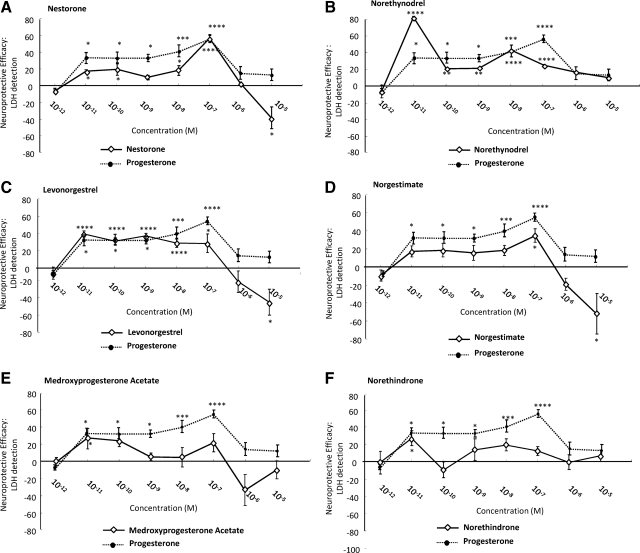
Neuroprotective efficacy of clinical progestins. Rat primary hippocampal neurons were exposed to neurotoxic glutamate 200 μm and the protective effects of progestins determined by LDH release. The neuroprotective effect was defined as NE and calculated as follows: NE = (Vsample − Vglutamate)/(Vcontrol − Vglutamate). Data are presented as the percentage of values produced by vehicle-treated control cultures, and the proliferative efficacy of each progestin (solid line) was compared with that of P4 (dotted line). A–C, Progestins with neuroprotective efficacy comparable with P4. D–F, Clinical progestins with modest neuroprotective efficacy. *, P < 0.05; **, P < 0.01; ***, P < 0.005; and ****, P < 0.001 compared with glutamate-treated cultures.
Consistent with previous findings (5,18), P4 induced a significant (P < 0.05) protective effect with a minimally effective concentration of 10−11 m and maximal effect of 55.7 ± 13.3% at 10−7 m. Nestorone induced comparable neuroprotective efficacy with that of P4, with a minimally effective concentration of 10−11 m and a maximal effect of 57.0 ± 4.4% at 10−7 m. Norethynodrel was more potent than P4, and its minimal effective concentration was 10−11 m, a concentration at which it also exhibited maximal neuroprotection of 80.8 ± 1.8% (P < 0.001). LNG induced comparable efficacy with P4, with a minimally effective concentration 10−11 m, a concentration at which LNG also induced maximal neuroprotection of 39.6 ± 3.2% (P < 0.001). In contrast, NGM was much less potent with neuroprotection at 10−7 m, whereas both MPA and NET showed modest neuroprotection at 10−11 m but were without effect at other concentrations. Similar to the results of clinical progestin regulation of rNPCs proliferation, no association was found between the neuroprotective efficacy of the progestins and their binding affinities to PR, AR, or GR.
Clinical progestins regulate neurogenic activity and cell viability in vivo
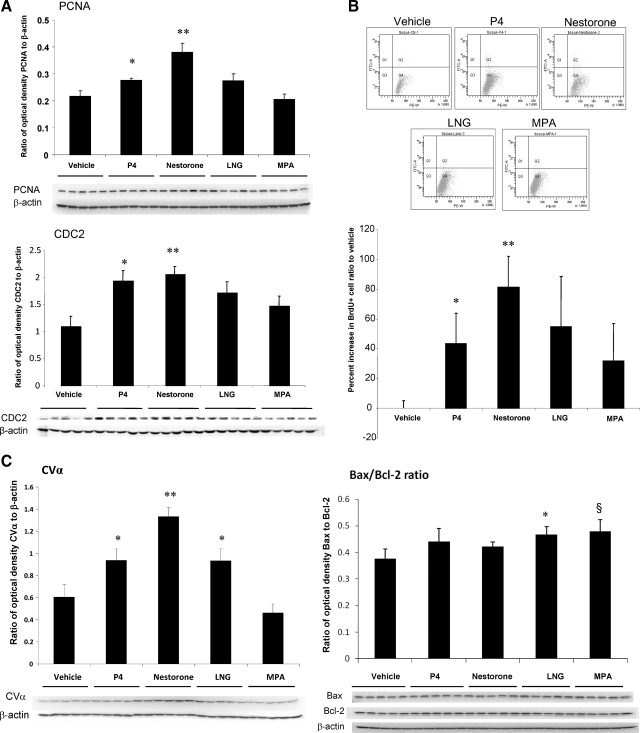
To determine the generalizability of in vitro findings to the in vivo condition, analyses of rNPC proliferation and cell viability were conducted in 3-month-old Sprague Dawley OVX female rats treated with selected progestins. Selection of progestins for in vivo testing was based on the requirement that the progestin exert both a significant increase in rNPC proliferation and neuroprotection. Among these progestins, Nestorone, norethynodrel, and LNG fulfilled these criteria. However, norethynodrel was not advanced to in vivo testing because of its aromatization in liver to form the potent estrogen ethinyl-estradiol (19) and thus potential involvement of ER in proliferative action in vivo could not be excluded. Although MPA exerted a decrease in both rNPC proliferation and neuroprotection, it was included for in vivo analysis based on its continued clinical use and its controversial effects in brain (5,20,21). In addition to FACS determination of total number of BrdU+ hippocampal cells, two cell cycle protein markers, PCNA, which is associated with transition through S phase, and CDC2 (CDK1), which is associated with transition through the mitotic M phase, were assessed (7,14). Results of cell cycle protein expression as determined by Western blotting indicated that both P4 and Nestorone significantly increased PCNA expression at the protein level (P < 0.05 and P < 0.01, respectively) (Fig. 5A), whereas LNG and MPA had no significant effect upon PCNA protein expression (P = 0.15 and P = 0.08, respectively) (Fig. 5A, top). CDC2 protein expression was significantly increased by P4 and Nestorone (P < 0.05 and P < 0.01, respectively), whereas LNG and MPA had no significant effect on CDC2 protein expression (P = 0.07 and P = 0.23, respectively) (Fig. 5A, bottom).
Figure 5.
Progestin regulation of neurogenic and neuroprotective activity in the hippocampus of OVX adult female rat. P4, Nestorone, LNG, and MPA were selected for in vivo efficacy assessment for their regulation of neurogenic activity; 30 μg/kg BW progestins were injected to 3-month-old OVX female rat followed by BrdU injection 1 h later. A, Effect of tested progestins on cell proliferation marker PCNA and CDK1 protein level in the hippocampus. B, Progestin regulation of BrdU+ cell count in the hippocampus of OVX adult female rat. *, P < 0.05 and **, P < 0.01 compared with vehicle control. C, left, Effects of progestins on CVα expression in rat hippocampus at protein level. Right, Effects of progestins on Bax/Bcl-2 expression ratio at protein level. P < 0.05 and P = 0.08, respectively.
To assess the total number of BrdU+ cells, the contralateral hippocampal hemisphere used for protein analysis was fixed and processed for FACS analysis. Total number of BrdU+ cells per each hippocampus was determined and normalized to that of vehicle control. Results were plotted as the percent increase in BrdU+ cell ratio compared with vehicle control (Fig. 5B). Results derived from FACS analysis indicated that P4 and Nestorone significantly increased BrdU+ cell number (P < 0.05 and P < 0.01, respectively), whereas LNG induced a comparable increase in BrdU incorporation with P4 but was not statistically significant (P = 0.054). Further, MPA, which inhibited rNPC proliferation in vitro, had no significant effect on rNPC proliferation in vivo. Results of FACS analyses are consistent with data derived from Western blot analyses.
Previous analyses demonstrated that P4 increased cell viability by promoting mitochondrial function and reducing oxidative damage (4). As a marker of cell viability, expression of the α-subunit of ATP synthase-complex V (CVα) of the mitochondrial oxidative phosphorylation pathway was assessed by Western blot analysis. Consistent with previous results (4), P4 significantly increased CVα expression in vivo by 1- to 2-fold (P < 0.05) (Fig. 5C). Nestorone and LNG also significantly increased CVα expression in vivo (P < 0.01 and P < 0.05 compared with vehicle control, respectively) (Fig. 5C), whereas MPA exerted no significant effect on CVα expression level, although a trend toward a decrease was observed (P = 0.18).
One of the closely associated events of increased cell viability is decreased apoptosis. To determine the effects of progestins on apoptosis, Western blot analyses were conducted to determine the expression level of Bax, a mediator of apoptosis by translocating to the mitochondria to release apoptotic factors such as cytochrome c (22), and Bcl-2, an antiapoptotic protein that is a key component of the neuroprotective effect of E2 (22). Ratio of Bax to Bcl-2 was used as the indicator of in vivo apoptotic activity, because it positively correlates with apoptosis. P4 and Nestorone had no effect on the ratio of Bax/Bcl-2 expression, whereas LNG (P < 0.05) and MPA (P = 0.05) significantly increased Bax/Bcl-2 ratio consistent with its proapoptotic effect in uterus reported by other groups (23,24,25). Together with previous results on CVα expression, the in vitro neuroprotective efficacy of clinical progestins paralleled their efficacy in vivo to induce markers of cell viability.
Impact of clinical progestins in combination with E2 on neurogenesis and cell viability in vivo
When used clinically for hormone replacement therapy/contraceptive therapy, progestins are administered in conjunction with an estrogen. To determine the impact of the combination of E2 and clinical progestins on neurogenesis and cell viability in vivo, young adult OVX female Sprague Dawley rats were divided into six groups and received sc injection with either E2 alone (30 μg/kg BW) or E2 combined with either P4, Nestorone, LNG, MPA (30 μg/kg BW), or equivalent volume of sesame oil as vehicle control. Hippocampi were isolated 24 h later for Western blot analysis and flow cytometry to determine the impact of treatment on cell viability and neurogenesis, respectively.
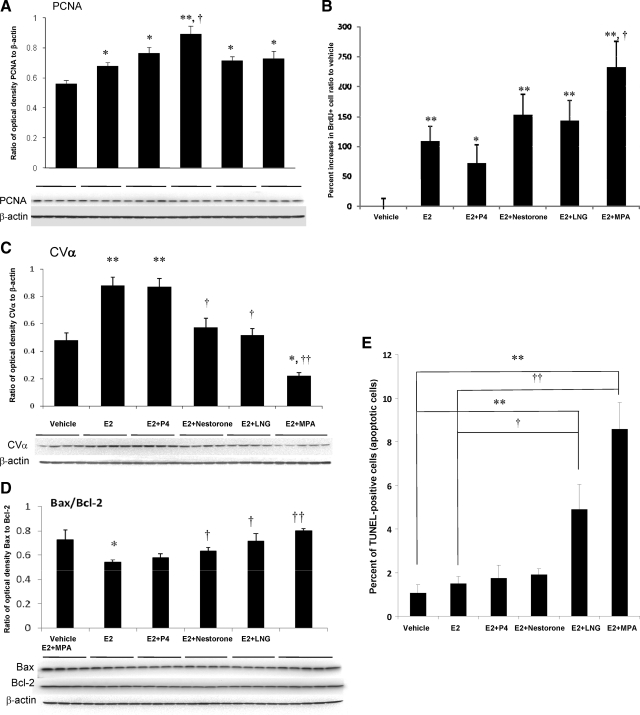
Expression level of PCNA, which is associated with transition through S phase, was assessed to determine the impact of treatment groups on the entry into the cell cycle required for neurogenesis. Results of these analyses indicated that E2 significantly increased PCNA expression at the protein level, and combination of E2 and progestins were progestin dependent (Fig. 6A): E2+Nestorone induced the greatest magnitude of PCNA expression (P < 0.01), whereas E2+P4, E2+LNG and E2+MPA exhibited comparable increase in PCNA level (P < 0.05). Further, E2+Nestorone-induced PCNA expression was significantly greater when compared with E2 alone (P < 0.05). Entry into the cell cycle was confirmed by FACS analysis, which detected BrdU incorporation in the contralateral hippocampal hemisphere (Fig. 6B). Consistent with PCNA expression, E2 and its combination with progestins significantly increased the BrdU+ cell population. In contrast to the effect of MPA in vitro, where MPA inhibited rNPC proliferation, and in vivo, where it had no effect on PCNA expression or BrdU incorporation as detected by FACS, the E2+MPA group exhibited the greatest increase in the number of BrdU+ cells indicated by a significant 4-fold increase compared with vehicle control (P < 0.01) and a 1-fold increase when compared with that of E2-alone group (P < 0.05). Because neither LNG nor MPA induced a rise in PCNA, CDC2, or BrdU incorporation when administered in the absence of E2, we determined whether the rise in BrdU induced by these progestins in the presence of E2 was associated with an increase in apoptosis.
Figure 6.
Combined effect of E2 and progestin on neurogenic activity and apoptosis in vivo. Vehicle, 30 μg/kg BW E2, and 30 μg/kg BW progestins combined with 30 μg/kg BW E2 were injected to 3-month-old OVX female rat followed by BrdU injection 1 h later. A, Effects of E2 and E2 + progestins on PCNA expression level in hippocampus. B, Combined effect of E2 + progestin in regulating BrdU+ cell count in the hippocampus of OVX adult female rat. *, P < 0.05 and **, P < 0.01 compared with vehicle control; †, P < 0.05 compared with E2-alone group. C, Effects of E2 and E2 + progestins on CVα expression at protein level. Western blot analysis was conducted on protein isolated from hippocampus. D, Effects of E2 and E2 + progestins on Bax/Bcl-2 expression at protein level. E, Combined effect of E2 + progestin in regulating TUNEL+ cell count in the hippocampus of OVX adult female rat. *, P < 0.05 and **, P < 0.01 compared with vehicle control; †, P < 0.05 and ††, P < 0.01 compared with E2 group.
To address this issue, analysis of cell viability and apoptosis were conducted. Indicators of cell viability were expression of mitochondrial ATP synthase subunit CVα expression and Bax/Bcl-2 ratio (Fig. 6, C and D). Consistent with previous analyses, E2 increased cell viability by promoting mitochondrial function and reducing oxidative damage (26), E2 significantly increased CVα protein expression relative to vehicle control (Fig. 6C). E2+P4 significantly increased CVα expression comparable with that of E2-alone group, whereas E2+Nestorone and E2+LNG significantly decreased CVα protein expression relative to E2 alone. Remarkably, E2+MPA significantly reduced CVα expression below that expressed in response to either vehicle or E2 (P < 0.05 and P < 0.01, respectively) (Fig. 6C). In parallel, E2 significantly decreased the apoptosis indicator Bax/Bcl-2 ratio, whereas E2+Nestorone, or LNG or MPA, increased the Bax/Bcl-2 ratio relative to E2 alone, although the increase did not exceed the vehicle (Fig. 6D). These results are consistent with previous reports from our group and others indicating a lack of synergy in the neuroprotective effects when P4 and E2 were administered in combination (18,27) and that MPA could inhibit E2-induced neuroprotective effect by antagonizing E2-induced attenuation of intracellular calcium concentration (5,18,21).
The rise in the Bax/Bcl2 ratio relative to E2 suggested the potential of an apoptotic effect. To determine whether a rise in apoptosis paralleled the rise in Bax/Bcl2 ratio, TUNEL labeling was performed to identify apoptotic nuclei in response to different treatments of E2 and progestins (Fig. 6E), and TUNEL+ cell numbers were detected via flow cytometry analysis. Results of the FACS analysis indicated that treatment with either E2 alone, E2+P4, or E2+Nestorone had no effect on TUNEL+ cell number relative to vehicle-treated animals. In contrast, E2+LNG induced a 4- and a 2-fold increase in the number of TUNEL+ cells relative to vehicle and E2-alone group, respectively (P < 0.01 and P < 0.05). The magnitude of TUNEL+ cells was maximal in the hippocampi derived from E2+MPA-treated animals. E2+MPA-treated animals exhibited an 8- and a 4-fold increase in TUNEL+ cell count relative to vehicle and E2-alone group, respectively (P < 0.01 and P < 0.01). This is consistent with the proapoptotic effect of LNG and MPA in the uterus (23,24,25). Collectively, these data indicate that clinical progestins can exert significantly different outcomes on the survival of proliferating NPCs in vivo.
Discussion
In the present study, we conducted comparative analyses of seven clinically relevant progestins to determine their neurogenic and neuroprotective efficacy both in vitro and in vivo, as well as their efficacy in regulating neurogenesis and cell viability in vivo when coadministered with E2. Results of these analyses indicated that clinical progestins exert a range of effects that span promotion of neurogenesis and neuroprotection to inducing apoptosis and reduced cell viability. P4, the endogenous regulator of P4 action promoted neurogenesis and neuroprotection served as the comparator positive control. Four of the seven clinical progestins exerted an increase of rNPC proliferation in vitro and were also neuroprotective. In vivo analyses indicated that when administered alone, P4 and the clinical progestin Nestorone significantly increased rNPC proliferation; and Nestorone and LNG increased CVα expression with an efficacy comparable with or greater than P4. MPA was without effect in both neurogenesis and cell viability measurements. When coadministered with E2, P4, Nestorone, LNG, and MPA increased entry into cell cycle, whereas LNG and MPA increased apoptosis in parallel. The induction of apoptosis by LNG and MPA is consistent with their antagonism of E2-induced increase in CVα expression and decrease in Bax/Bcl-2 ratio.
As an initial comparative approach for the in vivo experiments, we used comparable doses of all progestins based on an effective dose of P4 to induce rNPC proliferation in vivo. The dose of P4 used in this study translates into a concentration of 20 pg/ml in serum (21), comparable with that of P4 in woman’s brain during follicle phase but much lower than luteal phase (10–30 ng/ml) or pregnancy (50 ng/ml). Thus the effects of progestins at the tested concentration are within clinically relevant range. However, uterine bioassays of the progestational activity of these progestins in uterus indicate substantially different dose response profiles (8,12). Further analyses are required to investigate the impact of comparatively equivalent doses of the progestins based on their progestational potency in human uterus.
Progestin regulation of neurogenic activity and cell viability in vitro and in vivo
A major finding of our analysis was that similar to P4, progestins can regulate rNPC proliferation in a dose-dependent manner. Among the seven progestins tested, NGM, Nestorone, norethynodrel, NET, and LNG increased BrdU incorporation comparable with that of P4, NETA exhibited no effect on rNPC proliferation, whereas MPA significantly inhibited rNPC proliferation. Further, the prodrugs norethynodrel and NGM were significantly more potent than their active metabolites, NET and LNG, respectively, indicating that conversion to active metabolites and binding to nPR were not required for their in vitro proliferative action.
Consistent with our previous findings (7), proliferation induced by clinical progestins was dependent on the ERK signaling pathway and independent of nPR binding affinity as evidenced by further SAR analysis. Interestingly, MPA-induced inhibition of cell proliferation was not dependent on MAPK/ERK signaling pathway, because coadministration of UO126 had no impact on MPA-induced inhibition. This could be explained by our previous finding that MPA-induced pERK is not translocated to the nucleus (18,28), indicating that signaling pathways other than the ERK may mediate the inhibitory effect of MPA.
A likely PR candidate for mediating rNPC proliferation is the membrane-associated PGRMC-1. Our previous report demonstrated that P4 significantly increased rNPC proliferation in a PGRMC-1 and MAPK-dependent manner (7). SAR analysis highlighted several critical structures for their proliferative efficacy of clinical progestins, and those structural features revealed no clear association with other binding affinity to nPR. The lack of an effect of the pure nPR agonist ORG 31710 supports the postulate that nPR was not the mediator of the proliferative effect of progestins. It is possible, however, that the proliferative effects of the progestins were combined effects of binding to multiple receptors rather than one. Indeed, as reported herein and by others, LNG and NGM exerts androgenic effects by binding to and activating the nuclear AR. However, NGM in contrast with LNG inhibited nuclear translocation of the AR, revealing an antiandrogenic property (29). Also norethynodrel induces estrogenic activity through aromatization in vivo. Although Nestorone can bind to the nuclear GR, it showed no glucocorticoid activity in vivo (16,30,31). In addition, nPR is expressed in the dentate gyrus of hippocampus, and activation of nPR by P4 is known to inhibit cell proliferation under various conditions (1). Further, the effects of progestins on glial cell population and potential glial-neuronal cross talk remained undetermined.
An increase in neural responses induced by selected progestins was also evident in vivo. Consistent with their in vitro proliferative effects, P4 and Nestorone significantly increased cell cycle protein expression and BrdU+ cell number in FACS analyses compared with vehicle-treated animals. The profile for LNG was consistent in that LNG failed to increase PCNA/CDC2 expression or total BrdU+ cell number as determined by FACS analysis. Although the lineage of the newly generated cells remains to be determined, these data provide preclinical evidence that clinically relevant progestins could significantly impact the regenerative capability of the brain.
The in vitro and in vivo neuroprotective effect of P4 has been reported by multiple groups (2,4,5,20). Results from the current analyses extend these findings to now include up-regulation of a key marker of mitochondrial function and cell viability in vivo. Further, our results demonstrate that clinically relevant progestins can differentially regulate neuron survival and viability. In vitro, norethynodrel, Nestorone, and LNG protected rat primary hippocampal neurons against glutamate-induced toxicity, whereas NGM, NET, and MPA exerted no significant effect. The lack of neuroprotective capability of MPA is consistent with our previous report (18,32). In vivo analyses confirmed that P4, Nestorone, and LNG induced a significant rise in CVα expression, whereas MPA showed no effect, indicating the neuroprotective effects of the progestins are closely related to their ability in regulating mitochondria function. In addition, only LNG and MPA significantly increased Bax/Bcl-2 ratio, which is consistent with previous report that P4 does not directly regulate apoptosis, whereas both LNG and MPA induce a proapoptotic effect in the uterus (23,24,25).
Combined E2 and clinical progestin regulation of neurogenic activity and cell viability
It is well documented that E2 promotes neurogenesis and protection against neurodegenerative insults (33). Reported here, 30 μg/kg BW E2 significantly increased both PCNA expression level and BrdU+ cell number at a magnitude comparable with that of P4 alone. No significant differences were observed between E2-alone, E2+P4, and E2+Nestorone group. This is in contrast with previous reports from Tanapat et al. (27), where P4 administration subsequent to estradiol exposure decreased the amplitude of E2-induced increase in BrdU+ cell count. However, this could be explained by the differences in the treatment paradigm; Tanapat et al. (27) used chronic E2 exposure followed by P4 administration, whereas in our study, E2 and P4 treatment administration was acute and simultaneous. Further, there was no synergy in E2+P4-induced response compared with E2 or P4 alone. This is consistent with our previous report of a lack of synergy when P4 and E2 were administered in combination (18,28).
Both LNG and MPA increased BrdU+ cell count when administered in combination with E2. However, in parallel to the increase in BrdU+ cells, LNG and MPA significantly increased the number of TUNEL+ cells indicative of increased apoptosis, which is consistent with the proapoptotic effects of LNG and MPA in the uterus (23,24). Although the phenotype of the apoptotic cells remains unidentified at this point, it is probable that the increase of TUNEL+ cell population reflects the premature death of the newly generated cells (BrdU+ cells).
Therapeutic implications of progestin regulation of neural proliferation and viability
Clinical progestins are an integral constituent of oral contraceptive therapy, progestin-only contraception, fertility therapy, and postmenopausal hormone therapies. Progestin containing contraceptives account for an increasing proportion of modern contraceptive formulations used by women around the world. In the United Kingdom, the progestins mainly used are 19-nortestosterone derivatives (NETA, norgestrel, and LNG) (8,34). In France, micronized P4 and 19-norprogesterone (such as promegestone and nomegestrol acetate) are commonly prescribed, whereas MPA is the most prescribed progestin in the United States and the progestin used in commonly randomized controlled hormone therapy trials including the Women’s Health Initiative (35) and Women’s Health Initiative Memory Study (36). Typically, clinical progestins are chronically administered extending over many years to decades. For example, depomedroxyprogesterone acetate, a long-acting formulation of MPA, is extensively prescribed for adolescent females (37), and Norplant implant delivers constant infusion of LNG for 5–7 yr (38). Although data contained herein are derived from acute in vitro and in vivo exposures, our emergent findings suggest that chronic use could have long-term implications for neural function, regenerative capacity, and viability. Although the impact of chronic exposure to clinical progestins remains undetermined, the acute in vivo data indicate that P4 and Nestorone have potential beneficial outcomes for inducing and sustaining regenerative capacity of the brain. In contrast, LNG and MPA could have potential adverse outcomes on regenerative capacity of the brain.
Collectively, results of these preclinical translational analyses indicate that clinical progestins vary dramatically in their impact on the regenerative capacity of the brain and thus are likely to have clinical implications for long-term neurological function.
Acknowledgments
We thank Dr. Ronald Irwin, Zisu Mao, and Syeda Ahmed for their contributions.
Footnotes
This work was supported by National Institute on Aging Grant 1 PO1 AG026572, Project 3 of Progesterone in Brain Aging and Alzheimer’s Disease Program Project (to R.D.B.).
Disclosure Summary: L.L., H.S., S.C., K.M., L.Z., J.M.W., C.W., and R.D.B. have no conflicts to declare. R.S.-W. is employed by the Population Council a nonprofit nongovernmental agency that develops Nestorone for contraceptive formulations.
First Published Online October 13, 2010
Abbreviations: AR, Androgen receptor; bFGF, basic fibroblast growth factor; BrdU, 5-bromo-2-deoxyuridine; BW, body weight; CDC2, cell division cycle 2; Cdk1, cyclin dependent kinase 1; CVα, complex V, subunit α; E2, 17β-estradiol; FACS, fluorescence-activated cell sorting; GR, glucocorticoid receptor; LDH, lactate dehydrogenase; LNG, levonorgestrel; MPA, medroxyprogesterone acetate; NE, neuroprotective efficiency; NET, norethindrone; NETA, NET acetate; NGM, norgestimate; nPR, nuclear PR; OVX, ovariectomized; P4, progesterone; PCNA, proliferating cell nuclear antigen; PGRMC-1, PR membrane component 1; PR, P4 receptor; rNPC, rat neural progenitor cell; SAR, structure-activity relationship; TUNEL, terminal deoxynucleotidyltransferase-mediated 2′-deoxyuridine 5′-triphosphate nick end labeling.
References
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J 2008 Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE 2007 Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev 28:387–439 [DOI] [PubMed] [Google Scholar]
- Waters EM, Torres-Reveron A, McEwen BS, Milner TA 2008 Ultrastructural localization of extranuclear progestin receptors in the rat hippocampal formation. J Comp Neurol 511:34–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J 2008 Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology 149:3167–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD 2002 Impact of progestins on estradiol potentiation of the glutamate calcium response. Neuroreport 13:825–830 [DOI] [PubMed] [Google Scholar]
- Stein DG, Hoffman SW 2003 Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil 6:13–22 [DOI] [PubMed] [Google Scholar]
- Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD 2009 Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology 150:3186–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R 2002 Progestogens in hormonal replacement therapy: new molecules, risks, and benefits. Menopause 9:6–15 [DOI] [PubMed] [Google Scholar]
- Stanczyk FZ 2003 All progestins are not created equal. Steroids 68:879–890 [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R 2008 Pharmacological profile of progestins. Maturitas 61:151–157 [DOI] [PubMed] [Google Scholar]
- Nath A, Sitruk-Ware R 2009 Parenteral administration of progestins for hormonal replacement therapy. Eur J Contracept Reprod Health Care 14:88–96 [DOI] [PubMed] [Google Scholar]
- Sitruk-Ware R 2002 Hormonal replacement therapy. Rev Endocr Metab Disord 3:243–256 [DOI] [PubMed] [Google Scholar]
- Zhao L, Mao Z, Brinton RD 2009 A select combination of clinically relevant phytoestrogens enhances estrogen receptor β-binding selectivity and neuroprotective activities in vitro and in vivo. Endocrinology 150:770–783 [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD 2005 The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci 25:4706–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland JG, Haldon C, Goddard J, Oliver K, Murray F, Wheeldon A, Cumberbatch J, McAllister G, Munoz-Sanjuan I 2006 A rapid method for the quantification of mouse hippocampal neurogenesis in vivo by flow cytometry. Validation with conventional and enhanced immunohistochemical methods. J Neurosci Methods 157:54–63 [DOI] [PubMed] [Google Scholar]
- Kumar N, Koide SS, Tsong Y, Sundaram K 2000 Nestorone: a progestin with a unique pharmacological profile. Steroids 65:629–636 [DOI] [PubMed] [Google Scholar]
- Petit-Topin I, Turque N, Fagart J, Fay M, Ulmann A, Gainer E, Rafestin-Oblin ME 2009 Met909 plays a key role in the activation of the progesterone receptor and also in the high potency of 13-ethyl progestins. Mol Pharmacol 75:1317–1324 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD 2003 Divergent impact of progesterone and medroxyprogesterone acetate (Provera) on nuclear mitogen-activated protein kinase signaling. Proc Natl Acad Sci USA 100:10506–10511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl H, Wiegratz I 2007 Can 19-nortestosterone derivatives be aromatized in the liver of adult humans? Are there clinical implications? Climacteric 10:344–353 [DOI] [PubMed] [Google Scholar]
- Brinton RD, Nilsen J 2003 Effects of estrogen plus progestin on risk of dementia. JAMA 290:1706; author reply 1707–1708 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD 2002 Impact of progestins on estrogen-induced neuroprotection: synergy by progesterone and 19-norprogesterone and antagonism by medroxyprogesterone acetate. Endocrinology 143:205–212 [DOI] [PubMed] [Google Scholar]
- Wu TW, Wang JM, Chen S, Brinton RD 2005 17β-Estradiol induced Ca2+ influx via L-type calcium channels activates the Src/ERK/cyclic-AMP response element binding protein signal pathway and BCL-2 expression in rat hippocampal neurons: a potential initiation mechanism for estrogen-induced neuroprotection. Neuroscience 135:59–72 [DOI] [PubMed] [Google Scholar]
- Akcali KC, Khan SA, Moulton BC 1996 Effect of decidualization on the expression of bax and bcl-2 in the rat uterine endometrium. Endocrinology 137:3123–3131 [DOI] [PubMed] [Google Scholar]
- Vereide AB, Kaino T, Sager G, Ørbo A 2005 Bcl-2, BAX, and apoptosis in endometrial hyperplasia after high dose gestagen therapy: a comparison of responses in patients treated with intrauterine levonorgestrel and systemic medroxyprogesterone. Gynecol Oncol 97:740–750 [DOI] [PubMed] [Google Scholar]
- Rogers PA, Lederman F, Plunkett D, Affandi B 2000 Bcl-2, Fas and caspase 3 expression in endometrium from levonorgestrel implant users with and without breakthrough bleeding. Hum Reprod 15(Suppl 3):152–161 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Irwin RW, Gallaher TK, Brinton RD 2007 Estradiol in vivo regulation of brain mitochondrial proteome. J Neurosci 27:14069–14077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E 2005 Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol 481:252–265 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Deng J, Brinton RD 2005 Impact of clinically relevant progestins on the neural effects of estradiol and the signaling pathways involved. Drug News Perspect 18:545–553 [DOI] [PubMed] [Google Scholar]
- Paris F, Rabeolina F, Balaguer P, Bacquet A, Sultan C 2007 Antiandrogenic activity of norgestimate in a human androgen-dependent stable-transfected cell line. Gynecol Endocrinol 23:193–197 [DOI] [PubMed] [Google Scholar]
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH 2003 Classification and pharmacology of progestins. Maturitas 46(Suppl 1):S7–S16 [DOI] [PubMed] [Google Scholar]
- Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH 2008 Classification and pharmacology of progestins. Maturitas 61:171–180 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Morales A, Brinton RD 2006 Medroxyprogesterone acetate exacerbates glutamate excitotoxicity. Gynecol Endocrinol 22:355–361 [DOI] [PubMed] [Google Scholar]
- Brinton RD 2009 Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci 30:212–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lignières B, de Vathaire F, Fournier S, Urbinelli R, Allaert F, Le MG, Kuttenn F 2002 Combined hormone replacement therapy and risk of breast cancer in a French cohort study of 3175 women. Climacteric 5:332–340 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y 2003 Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol 71:3–29 [DOI] [PubMed] [Google Scholar]
- Khoiny FE 1996 Use of depo-provera in teens. J Pediatr Health Care 10:195–201 [DOI] [PubMed] [Google Scholar]
- Kovalevsky G, Barnhart K 2001 Norplant and other implantable contraceptives. Clin Obstet Gynecol 44:92–100 [DOI] [PubMed] [Google Scholar]