Abstract
It has been reported that the endocannabinoid anandamide (AEA) exerts an adverse effect on human sperm motility, which has been ascribed to inhibition of mitochondrial activity. This seems to be at variance with evidence suggesting a major role of glycolysis in supplying ATP for sperm motility; furthermore, the role of AEA-binding receptors in mediating mitochondrial inhibition has not yet been explored. In this study, human sperm exposure to Met-AEA (methanandamide, nonhydrolyzable analog of AEA) in the micromolar range significantly decreased mitochondrial transmembrane potential (ΔΨm), similarly to rotenone, mitochondrial complex I inhibitor. The effect of Met-AEA (1 μm) was prevented by SR141716, CB1 cannabinoid receptor antagonist, but not by SR144528, CB2 antagonist, nor by iodoresiniferatoxin, vanilloid receptor antagonist. The effect of Met-AEA did not involve activation of caspase-9 or caspase-3 and was reverted by washing. In the presence of glucose, sperm exposure either to Met-AEA up to 1 μm or to rotenone for up to 18 h did not affect sperm motility. At higher doses Met-AEA produced a CB1-independent poisoning of spermatozoa, reducing their viability. Under glycolysis blockage, 1 μm Met-AEA, similarly to rotenone, dramatically abolished sperm motility, an effect that was prevented by SR1 and reverted by washing. In conclusion, CB1 activation induced a nonapoptotic decrease of ΔΨm, the detrimental reflection on sperm motility of which could be revealed only under glycolysis blockage, unless very high doses of Met-AEA, producing CB1-independent sperm toxicity, were used. The effects of CB1 activation reported here contribute to elucidate the relationship between energetic metabolism and human sperm motility.
CB1 activation induces a reversible, nonapoptotic decrease of mitochondrial potential, whose detrimental reflection on sperm motility is revealed under glycolysis blockage.
N-Arachidonoylethanolamine (anandamide, AEA) and 2-arachidonoylglycerol (2-AG) are the best characterized prototype members of two families of endocannabinoids, the fatty acid amides and the monoacylglycerols, respectively (1). These bioactive lipids owe their name to the ability to act primarily at cannabinoid receptors, CB1 and CB2, thus reproducing some of the biological actions of natural Cannabis sativa components (the phytocannabinoids), of which Δ9-tetrahydrocannabinol (THC) is the most prominent member. CB1 is highly expressed in the central nervous system and is also present in peripheral and extraneural sites. By contrast, CB2 is mainly present in the immune system (2,3,4) but has been detected also in neuronal cells (5,6).
Biochemical studies have revealed that the biological activity of AEA at its receptors is under a metabolic control (7). AEA is released from membrane phospholipid precursors, the N-acylphosphatidylthanolamines (NAPEs), through the activity of NAPE-hydrolyzing phospholipase D (8). Furthermore, a purported bi-directional endocannabinoid membrane transporter is responsible for the uptake of extracellular AEA (9,10,11). Once inside the cell, AEA is degraded to ethanolamine and arachidonic acid by the fatty acid amide hydrolase (12,13). Unlike 2-AG, AEA also acts at the intracellular binding site of the transient receptor potential vanilloid type 1 (TRPV1) channel, naturally activated by the pungent compound of hot chilli pepper, capsaicin, as well as by the irritant extract of the Euphorbia resinifera, resiniferatoxin (14,15).
Evidence has been accumulated that (endo)cannabinoids may affect male fertility in a number of species, both in vitro and in vivo (16,17,18). Chronic administration of THC to animals reduces the production, motility, and viability of spermatozoa (19,20). In in vitro experiments, both THC and AEA inhibit egg jelly-stimulated acrosome reaction in sea urchin spermatozoa (21,22,23). Furthermore, activation of CB1 by a metabolically stable analog of AEA, methanandamide (Met-AEA), was shown to inhibit sperm capacitation in the boar, where a fully functional endocannabinoid system has been characterized (24).
Human spermatozoa, much like boar spermatozoa, exhibit the functional biochemical machinery needed to synthesize (NAPE-hydrolyzing phospholipase D) and degrade (endocannabinoid membrane transporter and fatty acid amide hydrolase) AEA (25) and express CB1 (25,26) and CB2 (27) receptors. AEA has been detected in fluids of male and female reproductive tracts (28,29), and, in human spermatozoa, (endo)cannabinoids have been reported to negatively affect acrosome reaction (26,30) and zona pellucida binding (28). Actually, the endocannabinoid system would exert a complex role in modulating human sperm functions involved in the acquisition of sperm-fertilizing ability. In fact, in addition to expressing a functional CB1 receptor that binds (endo)cannabinoids, human spermatozoa also express a functional TRPV1 receptor, the activation of which by intracellular AEA appears to be involved in the prevention of spontaneous acrosome exocytosis during capacitation, thereby preserving sperm-fertilizing potential (25).
It has been also claimed that (endo)cannabinoids negatively affect human sperm motility (26,28,30,31) and that this effect would be mediated by CB1 receptors (26,31). Because AEA and congeners have the potential to affect isolated mitochondria physiology (32,33) and AEA reduces mitochondrial activity in human spermatozoa (26), as do exogenous cannabinoids (34), it has been suggested that the effect of these substances on sperm motility could depend on a mechanism of energy depletion (35). This interpretation would implicitly ascribe a key role to mitochondrial oxidative phosphorylation in providing energy to the axoneme. This assumption should be critically revised, in the light of some interesting experimental data, suggesting a major role of glycolysis in supplying energy for sperm motility. Elegant experiments in the mouse have clearly demonstrated that glycolysis compensates for any lack of ATP production by mitochondria in maintaining sperm motility, and that mitochondrial inhibition can depress sperm motility only in the presence of glycolysis blockage (36). An obligatory role for glycolysis is supported by the lack of progressive motility in sperm from mice in which the gene for sperm-specific glyceraldehyde-3-phosphate dehydrogenases had been knocked out (37). However, it is unclear whether this glycolysis model is suitable for other mammalian spermatozoa, and there is still debate concerning whether glycolysis or oxidative phosphorylation should be considered as the major biochemical pathway for the supply of energy that supports human sperm motility (38,39,40).
In this study we further characterized the effect of the activation of AEA-binding receptors (CB1, CB2, and TRPV1), on human sperm mitochondrial function and investigated its reflection on sperm motility, providing interesting observations on the relationship between energy metabolism and motility in human spermatozoa.
Materials and Methods
Chemicals
R(+)-Methanandamide (Met-AEA), rotenone, 2-deoxy-d-glucose (DOG), and 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenimidazolyl carbocyanine iodide (JC-1) were purchased from Sigma Chemical Co. (St. Louis, MO). Iodoresiniferatoxin (IRTX) was obtain from Tocris Cookson (Bristol, UK). SR141716 (SR1) and SR144528 (SR2) were kind gifts of Sanofi-Aventis Recherche (Montpellier, France). CaspGLOW Fluorescein Active Kits for caspase-9 and caspase-3 were obtained from BioVision (Mountain View, CA). Met-AEA is supplied as a 5 mg/ml solution in absolute ethanol; thus the solvent was changed by evaporating ethanol under a gentle stream of nitrogen before the addition of dimethyl sulfoxide (DMSO), purged with inert gas to prevent oxidation of Met-AEA. Stock solutions of Met-AEA, SR1, SR2, IRTX, rotenone, and JC-1 in DMSO were diluted in Biggers, Whitten, and Wittingham (BWW) medium to obtain the working concentrations just before use.
Medium
BWW medium consisted of 5.6 mm d-glucose, 44 mm sodium lactate, 0.27 mm sodium pyruvate, 95 mm NaCl, 25 mm NaHCO3, 4.6 mm KCl, 1.7 mm CaCl2, 1.2 mm KH2PO4, 1.2 mm MgSO4, 5 U/ml penicillin, 5 mg/ml streptomycin, pH 7.4. BSA was omitted, because it is known to bind endocannabinoids (9,41), thus impairing biochemical and functional assays. BWW minus glucose (BWW−Gluc) consisted of normal BWW lacking d-glucose. In BWW−Gluc containing the glucose analog DOG, 5.6 mm DOG replaced d-glucose.
Semen samples and sperm processing
Semen samples were collected according to the World Health Organization (WHO)-recommended procedure (42) by masturbation, from healthy normozoospermic postgraduate medical students.
The study was approved by the local Institutional Review Board, and all subjects signed an informed consent statement.
All samples were produced into sterile containers and left for at least 30 min to liquefy before processing. Motile sperm suspensions were obtained by swim up procedure. Briefly, spermatozoa were washed twice (700 × g for 7 min) in BWW medium. After the second centrifugation, supernatants were removed by aspiration, leaving 0.5 ml on the pellet, and after 30 min of incubation time, supernatants containing highly concentrated motile sperm were carefully aspirated. Sperm concentration was adjusted as needed for subsequent analysis.
Flow cytometric evaluation of mitochondrial membrane potential (Δψm)
The fluorescent lipophilic cationic dye JC-1 was used to evaluate transmembrane potential of sperm mitochondria. JC-1 possesses the unique ability to differentially label mitochondria with low and high Δψm. In mitochondria with high Δψm, JC-1 forms multimeric aggregates that emit orange light (wavelength of 590 nm) when excited at 488 nm. In mitochondria with low Δψm, JC-1 forms monomers that emit green light (525–530 nm) when excited at 488 nm. Sperm suspensions (1 ml) containing 5 × 106 spermatozoa were stained with 0.5 μl of JC-1 stock solution (3 mm in DMSO). Samples were incubated at 37 C in the dark for 60 min and then analyzed using a flow cytometer (Beckman-Coulter Epics XL-4; Beckman Coulter, Inc., Fullerton, CA) equipped with a 15 mW argon-ion laser for excitation. For each sample 10,000 events were recorded at a flow rate of 200–300 cells/sec. Based on the light scatter characteristics of swim up selected spermatozoa, debris and aggregates were gated out by establishing a region around the population of interest in the forward scatter/side scatter dot plot on a log scale.
Compensation between FL1 and FL2 was carefully adjusted according to the manufacturer’s instructions. Green fluorescence (480–530 nm) was measured in the FL-1 channel and orange-red fluorescence (580–630 nm) was measured in the FL-2 channel. The percentage of positive cells was evaluated on a 1023-channel scale, using the flow cytometer System II Version 3.0 software (Beckman Coulter, Inc.).
Flow cytometric assessment of activated caspases
Activation of caspase-9 and caspase-3 was evaluated by using ApoptosisCaspGLOW Fluorescein Active Caspase-3 and 9 Staining Kits (Biovision, Inc.), according to the manufacturer’s instructions. These assays use the caspase-9 or caspase-3 inhibitors, LEHD-FMK or DEVD-FMK, respectively, conjugated to fluorescein isothiocyanate (FITC), as fluorescent markers. These cell-permeable, nontoxic peptides covalently bind to activated caspases, serving as in situ markers for apoptosis (43,44,45). Briefly, in 300 μl of sperm suspension containing 0.3 × 106 spermatozoa, 1 μl of FITC-LEHD-FMK or FITC-DEVD-FMK was added before incubation for 1 h at 37 C in an atmosphere of 5% CO2. After three centrifugations (1000 × g for 4 min), spermatozoa were resuspended in 1 ml of PBS before addition of 1 μl of propidium iodide (PI), a red nucleic acid dye that is excluded from viable cells. Flow cytometry was performed within 10 min. With this technique, four sperm subpopulations were detected. Events in the lower-left quadrant represented live (no apoptotic) spermatozoa (FITC−/PI−); events in the lower-right quadrant represented live spermatozoa with active caspases (FITC+/PI−), events in the upper-right quadrant represented dead spermatozoa showing caspase activation (FITC+/PI+), and events in the upper-left quadrant represented dead spermatozoa with no caspase activation (FITC−/PI+).
Evaluation of sperm motility and viability
Sperm motility was evaluated by Computer-Aided Semen Analysis using ATS20 (JCD, Gauville, France). Ten microliters of each sperm sample were placed into a prewarmed (37 C) Makler counting chamber (Sefi Medical Instruments, Haifa, Israel). At least 200 spermatozoa were evaluated for each sample. Setting parameters were: analysis duration of 1 sec (30 frames); minimum contrast, 80; minimum size, 3; low size gate, 0.7; high size gate, 2.6; low intensity gate, 0.34; light intensity gate, 1.40. Spermatozoa exhibiting an average pathway velocity >5 μm/sec were categorized by the software as motile spermatozoa.
Sperm viability was evaluated under light microscope by the eosin technique, according to WHO (42).
Statistical analysis
Statistical analysis was performed using the SAS statistical software (version 9.1, 2003; SAS Institute, Inc., Cary, NC). Data were analyzed by ANOVA and post hoc comparisons between pairs of groups were performed by the Tukey’s studentized range-honestly significant difference (HSD) test. Statistical significance was accepted when P ≤ 0.05. Data were expressed as mean ± sem.
Results
Effect of CB1 activation on sperm mitochondrial transmembrane potential (Δψm)
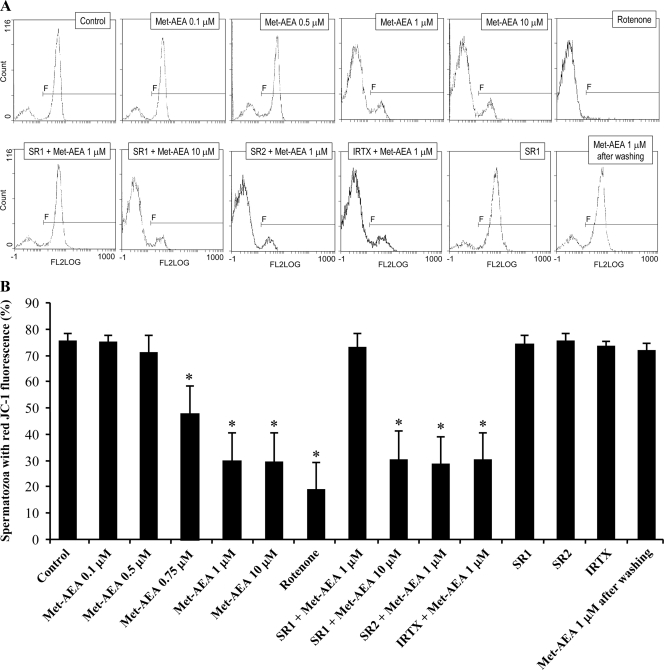
Aliquots of the same motile sperm suspensions were exposed to increasing concentrations of Met-AEA (0.1, 0.5, 1, and 10 μm). As shown in Fig. 1, the exposure to 1 and 10 μm Met-AEA for 1 h produced a significant decrease in sperm mitochondrial transmembrane potential (Δψm), as indicated by a decrease on the percentage of spermatozoa exhibiting red-orange fluorescence, with respect to controls exposed to a proper dilution of DMSO. Conversely, sperm Δψm was not affected by Met-AEA at lower concentrations. As expected, Δψm was suppressed by the mitochondrial respiratory chain complex I inhibitor rotenone (1 μm), used as a positive control.
Figure 1.
A, Typical histograms of fluorescence from flow cytometric analysis of sperm mitochondrial transmembrane potential (Δψm), evaluated by JC-1 staining. In each setting, a negative control of high quality, with high Δψm (Control), was used to set a region (F) including all spermatozoa exhibiting red-orange fluorescence for JC-1 (high Δψm). FL2LOG, fluorescence detector 2 log; SR1, SR141716 (0.1 μm); SR2, SR144528 (0.1 μm); IRTX (1 μm). B, Percentage of spermatozoa exhibiting red-orange fluorescence for JC-1 (high Δψm) under different experimental conditions. Means ± sem of four independent experiments with different donors.
To explore the possible involvement of AEA-binding receptors in mediating the Met-AEA effect on sperm Δψm, cell suspensions were separately exposed to 0.1 μm SR1, 0.1 μm SR2, or 1 μm IRTX, selective antagonists of CB1, CB2 or TRPV1 respectively, for 15 min before addition of Met-AEA. Each antagonist was used at a concentration previously shown to block the corresponding target in sperm (24,25). SR1 prevented the inhibitory effect of 1 μm Met-AEA, whereas SR2 or IRTX were ineffective. On the other hand, SR1 did not block inhibition by 10 μm Met-AEA, and none of the antagonists affected sperm Δψm when used alone (Fig. 1). Because CB1 was the only AEA-binding receptor engaged in Δψm modulation, the involvement of CB2 and TRPV1 was not investigated in the subsequent experiments.
To further characterize the mechanism of sperm Δψm inhibition by Met-AEA, we checked if the effect of this substance could be reverted by a simple washing step. To this end, sperm suspensions exposed for 1 h to 1 μm Met-AEA were divided into two aliquots: one of them was directly stained with JC-1 for Δψm evaluation; the other one was washed twice in BWW (1000 × g, × 4 min) and, after 1 h incubation, was exposed to JC-1. Simple sperm washing completely abolished the inhibitory effect of 1 μm Met-AEA on Δψm (Fig. 1).
Effect of Met-AEA on caspase activation
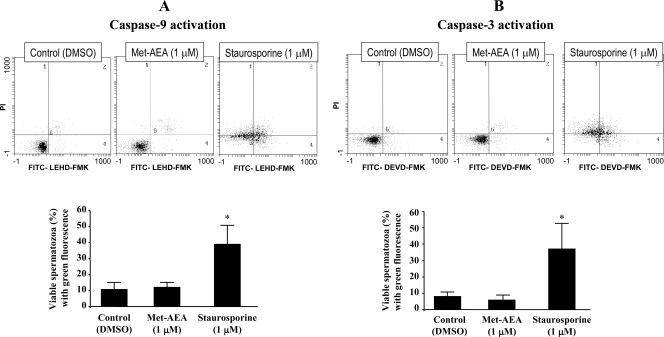
Because the loss of Δψm could reflect an early apoptotic stage, preceding phosphatidylserine externalization and coinciding with caspase activation (46,47,48), and endocannabinoids and plant-derived cannabinoids have emerged as key promoters of CB receptor-dependent apoptosis in central and peripheral cells (49,50,51,52,53,54), we checked the activation of caspase-9 (induced by mitochondrial apoptotic pathway) and caspase-3 (the downstream effector caspase) upon exposure to 1 μm Met-AEA. As shown in Fig. 2, in samples exposed to 1 μm Met-AEA for 1 h, the percentage of viable spermatozoa exhibiting activated caspase-9 or caspase-3 was similar to that observed in controls exposed to proper dilutions of DMSO. Staurosporine, a protein kinase inhibitor, has been characterized as a strong inducer of caspase activity and apoptosis in different cell types (55,56,57). Thus, this substance was used as a positive control for caspase activation. As shown in Fig. 2, the exposure to staurosporine (1 μm) for 1 h negatively affected sperm viability while producing a significant increase in the proportion of viable spermatozoa with activated caspase-9 and caspase-3.
Figure 2.
Effect of Met-AEA on caspase-9 (A) and caspase-3 (B) activation. Top, Flow cytometric dot plots of double staining with PI and FITC label that allows detection of activated caspase-9 (FITC-LEHD-FMK) and activated caspase-3 (FITC-DEVD-FMK). Staurosporine was used as positive control for caspase activation. Bottom, Percentage of viable (PI−) spermatozoa exhibiting green fluorescence (FITC+) of caspase activation. Means ± sem of three independent experiments with different donors.
Effect of Met-AEA on sperm motility
Because CB1 activation inhibited mitochondrial function, in the second part of the study we explored its reflection on sperm motility.
Effect on sperm motility in the presence of glucose
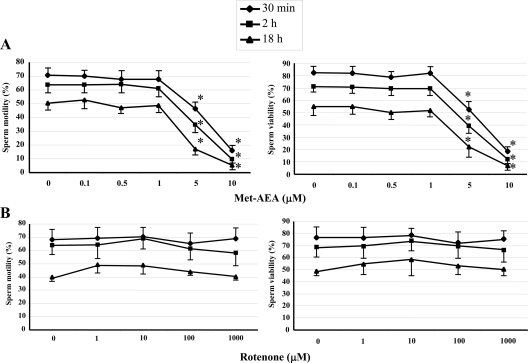
When a complete glucose-containing BWW medium was used, the exposure to scalar doses of Met-AEA up to 1 μm and for up to 18 h did not produce any change either in the percentage of motile spermatozoa (Fig. 3A) or in the quality of motility (Table 1) with respect to controls. On the contrary, sperm motility was significantly affected by higher doses of Met-AEA (5 and 10 μm), due to cell poisoning that reduced sperm viability (Fig. 3A). In a subsequent set of experiments, the detrimental effect of 10 μm Met-AEA was shown to be CB1 independent, because it was not prevented by SR1 after 30 min of treatment (16.2 ± 4.0% vs. 15.8 ± 4.0%).
Figure 3.
Effect of Met-AEA (A) and rotenone (B) on motility (left) and viability (right) of human spermatozoa in the presence of glucose (complete BWW medium). Number of replicates = 6, with different donors. Overall significance for treatment variations: P < 0.001 with ANOVA; *, P < 0.05 vs. ≤1 μm Met-AEA (Tukey’s HSD test).
Table 1.
Effect of Met-AEA on the quality of sperm motility, evaluated by Computer-Aided Semen Analysis (CASA), in the presence of glucose
| Met-AEA (μm) | VCL | VSL | VAP | |
|---|---|---|---|---|
| 30 min | 0 | 82.7 ± 0.5 | 66.9 ± 2.4 | 70.0 ± 1.6 |
| 0.1 | 79.1 ± 2.4 | 65.0 ± 1.9 | 67.1 ± 1.5 | |
| 0.5 | 82.6 ± 2.5 | 60.0 ± 2.0 | 65.4 ± 1.9 | |
| 1 | 79.3 ± 3 | 61.7 ± 1.6 | 67.0 ± 2.1 | |
| 5 | 46.7 ± 3.5a | 33.4 ± 3.1a | 29.0 ± 3.0a | |
| 10 | 0.1 ± 0.1a | 0.7 ± 0.2a | 0.1 ± 0.1a | |
| 2 h | 0 | 61.3 ± 2.5 | 48.0 ± 2.9 | 59.1 ± 3.4 |
| 0.1 | 62.9 ± 2.2 | 48.3 ± 2.5 | 52.8 ± 2.8 | |
| 0.5 | 63.3 ± 4.6 | 49.7 ± 2.9 | 54.0 ± 2.7 | |
| 1 | 60.7 ± 3.7 | 45.7 ± 2.2 | 51.3 ± 2.5 | |
| 5 | 35.6 ± 2.8a | 22.7 ± 0.8a | 22.0 ± 1.4a | |
| 10 | 0.4 ± 0.2a | 0.0 ± 0.0a | 0.3 ± 0.2a | |
| 18 h | 0 | 41.0 ± 3.8 | 29.3 ± 2.8 | 32.3 ± 2.3 |
| 0.1 | 40.0 ± 4.1 | 28.0 ± 2.1 | 31.0 ± 1.0 | |
| 0.5 | 39.7 ± 4.4 | 22.7 ± 1.5 | 28.0 ± 4.1 | |
| 1 | 39.0 ± 3.5 | 20.3 ± 0.3 | 30.0 ± 2.5 | |
| 5 | 19.3 ± 3.4a | 10.0 ± 1.0a | 15.3 ± 3.2a | |
| 10 | 0.3 ± 0.3a | 0.0 ± 0.0a | 0.0 ± 0.0a |
Number of replicates = 6, with different donors. Overall significance for treatment variations: P < 0.001 with ANOVA;
P < 0.05 vs. ≤1 μm Met-AEA (Tukey’s HSD test). VCL, Curvilinear velocity (μm/sec); VSL, straight line velocity (μm/sec); VAP, average pathway velocity (μm/sec).
Similarly, sperm exposure to scalar concentrations (1, 10, 100, and 1000 μm) of rotenone for up to 18 h did not affect sperm motility (Fig. 3B).
Effect on sperm motility under glycolysis blockage
Because mitochondrial inhibition did not suppress sperm motility in the presence of glucose (Fig. 3), we checked experimental conditions under which the reflection of mitochondrial inhibition on sperm motility could be revealed.
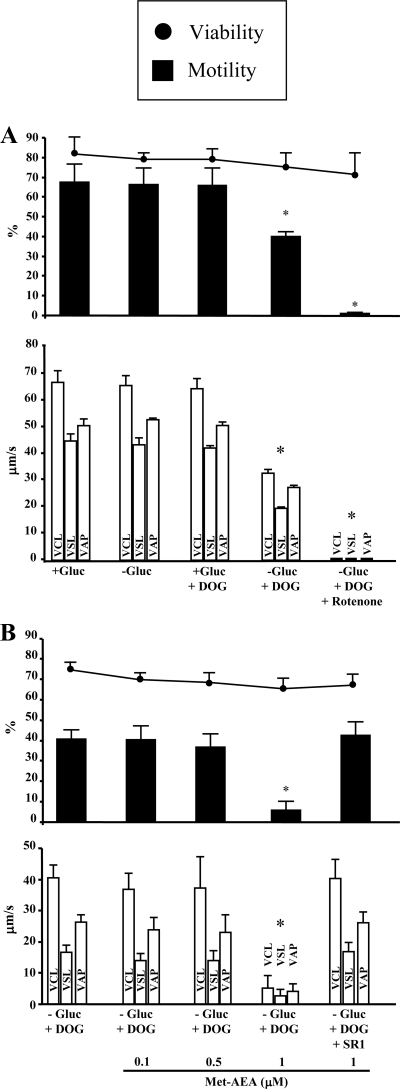
As shown in Fig. 4A, motility remained unchanged in spermatozoa suspended for 1 h in BWW either depleted of glucose (−Gluc) or complete and containing 5.6 mm 2-deoxy-d-glucose (DOG), an inhibitor of glycolysis. It decreased significantly when spermatozoa were exposed to DOG in glucose-depleted BWW medium (−Gluc+DOG), and was completely abolished only when sperm suspensions were exposed also to rotenone (1 μm), in addition to glycolysis blockage (BWW-Gluc+DOG). Therefore, the latter experimental condition was singled out for revealing a possible negative effect of Met-AEA on sperm motility, reflecting its inhibition of mitochondrial activity. In BWW-Gluc+DOG medium, 1 μm Met-AEA dramatically suppressed sperm motility, without affecting sperm viability, when compared with control under the same experimental conditions (Fig. 4B). In keeping with the effects on mitochondrial activity (Fig. 1), sperm motility was not significantly affected by lower concentrations (0.1 μm and 0.5 μm) of Met-AEA (Fig. 4B). Furthermore, the effect of 1 μm Met-AEA was completely prevented by 0.1 μm SR1 (Fig. 4B). In another set of experiments, the inhibition of sperm motility by 1 μm Met-AEA was found to be completely reversed by the washing step described above: under glycolysis blockage (BWW-Gluc+DOG), the percentage of motile spermatozoa dropped from 42.0 ± 3.0% to 4.0 ± 1.0% in samples exposed to 1 μm Met-AEA (P < 0.05) and was restored (35.0 ± 5.0%) after washing in BWW-Gluc + DOG (P < 0.05).
Figure 4.
Effect of rotenone (A) and Met-AEA (B) on sperm viability and motility, as evaluated with Computer-Aided Semen Analysis, during 1 h incubation under conditions of glycolysis blockage. Number of replicates = 4, with different donors. Overall significance for treatment variations: P < 0.001 with ANOVA; *. P < 0.05 vs. all the others (Tukey’s HSD test). −Gluc, glucose-free BWW (containing pyruvate and lactate); +Gluc, complete BWW (containing glucose); DOG, (5.6 mm); SR1, SR141716 (0.1 μm); VCL, curvilinear velocity (μm/sec); VSL, straight line velocity (μm/sec); VAP, average pathway velocity (μm/sec).
Discussion
The inhibitory effects of cannabinoids on mitochondrial respiration has been known since the 1960s–1970s, when it was demonstrated that THC, the main active phytocannabinoid compound contained in marijuana, inhibited oxygen consumption in rat brain homogenates and in liver mitochondria (58,59,60). Similar findings were more recently reported in a number of cell types, where mitochondrial inhibition appeared to be involved in biological actions of phytocannabinoids (34,61,62), as well as of their endogenous counterparts (32,33). Mechanisms mediating this inhibition are as yet unknown (35).
According to previous results obtained by using AEA (26), in the present study 1 μm Met-AEA significantly inhibited Δψm of human spermatozoa. The first novel and interesting observation arising from this study is that the effect of Met-AEA on sperm Δψm occurred via activation of CB1, because it was prevented by the CB1-selective antagonist SR1. Instead, the two other AEA-binding receptors CB2 and TRPV1 were not involved, as demonstrated by using the selective antagonists SR2 and IRTX, respectively. Incidentally, it should be recalled that, whereas TRPV1 clearly impacts human sperm function (25), CB2 is expressed in these cells (27) but does not seem to be functional (25). Some evidence of a mitochondrial localization of CB1 receptors in human spermatozoa has been recently reported (63), emphasizing the need to examine their possible involvement in mediating the effect of cannabinoids on mitochondria.
Recently, evidence has been collected for the ability of (endo)cannabinoids to trigger apoptotic pathways through CB1 or CB2 (49,50,51,52,53,54). Although a decrease in Δψm, evaluated by JC-1 staining, could represent an early marker of apoptosis (46,47,48), here the possible involvement of apoptotic pathways in the effect of Met-AEA on sperm mitochondrial activity was ruled out by caspase activation assays. Activation of these cytosolic cysteine proteases is one of the earliest and most common features of programmed cell death (64). In particular, along the apoptotic cascade of events mitochondrial membrane damage/permeabilization leads to the release of cytochrome c, which activates caspase-9 and ultimately caspase-3, the downstream executioner of apoptosis (65). Treatment with 1 μm Met-AEA inhibited sperm Δψm (Fig. 1), yet it did not produce any detectable activation of caspase-9 or of caspase-3 (Fig. 2). Furthermore, consistent with the lack of apoptosis, a simple sperm washing completely abolished the inhibitory effect of 1 μm Met-AEA on Δψm. At higher concentrations (10 μm), this substance became toxic, and reduced sperm viability in a CB1-independent manner.
Another major outcome of this investigation is that, in the presence of extracellular glucose (i.e. standard BWW medium), exposure to Met-AEA at concentrations up to 1 μm and for up to 18 h did not affect sperm motility. Similarly, sperm motility was not affected by exposure for up to 18 h to rotenone, a well-known inhibitor of the respiratory chain complex I. Recently, short-term (4 h) exposure to rotenone failed to inhibit human sperm motility but significantly reduced it when the incubation period was extended to 24 h (66). This effect was a possible consequence of late lipoperoxidative damage induced by mitochondrial generation of reactive oxygen species (66). The observation that in the presence of glucose sperm motility was not affected by mitochondrial inhibition, caused by either Met-AEA or rotenone, indicates that ATP generated by glycolysis (rather than by mitochondrial respiration) fully supports flagellar movement, thus extending previous data from mouse models (36,37). Consistently, the inhibitory effect of Met-AEA and rotenone on sperm motility could be revealed only in the presence of glycolysis blockage. In a medium lacking glycolysable sugars, substrates for mitochondrial respiration present in BWW medium (e.g. pyruvate and lactate) could support high-motility levels, by at least two mechanisms: 1) ATP is synthesized in mitochondria and then is supplied to the entire flagellum by diffusion; 2) energy produced by mitochondrial respiration is used to drive gluconeogenesis and thus to provide glucose for glycolytic ATP production in the flagellum. The first hypothesis could be ruled out, because the addition of DOG significantly decreased motility supported by pyruvate and lactate in the absence of glucose. It should be recalled that, once inside the cell, DOG is phosphorylated by sperm hexokinase but is not metabolized any further (67); additionally, DOG inhibits glycolysis by competing with glucose for key enzymes, the affinity for glucose of which is much higher than that for DOG itself (36). This fact could explain the lack of effects of DOG on sperm motility in the presence of extracellular glucose (Fig. 4A). Furthermore, in the presence of pyruvate and lactate (BWW-Gluc), sperm motility was strongly impaired but not completely abolished by DOG, suggesting that the amount of glucose produced by mitochondrial gluconeogenesis could still compete to some extent with DOG, due to the higher affinity of glycolytic enzymes for glucose. This hypothesis has been challenged (38), because phosphorylation of DOC would consume most of phosphate in the sperm, making it unavailable for oxidative phosphorylation; consequently, sperm motility would be affected. As a matter of fact, Williams and Ford (68) reported that, in human spermatozoa incubated in BWW-Gluc+DOG, ATP content was significantly lower than in those incubated in sugar-free BWW. As postulated by Ford (38), the reduced availability of phosphate for oxidative phosphorylation should increase the mitochondrial membrane potential by preventing the discharge of the proton motive force (38). We evaluated the effect of DOG in glucose-free BWW on Δψm and on sperm motility at 1 h. In four experiments, the mean intensity of JC-1 red fluorescence in spermatozoa incubated in BWW-Gluc+DOG showed a nonsignificant approximately 5% increase compared with those kept in glucose-free BWW (data not shown). At any rate, whether or not DOG could affect mitochondrial respiration does not seem to represent a major issue in the context of the present study; in fact, a decreased mitochondrial ATP production could affect gluconeogenesis, and subsequently the glucose availability for glycolysis, rather than affecting motility through a reduced availability of mitochondrial ATP along the flagellum. Direct evidence for the occurrence of gluconeogenesis, glycogen storage, and subsequent decrease of glycogen levels has been provided in dog spermatozoa incubated in a sugar-free medium (69).
Under conditions that blocked glycolysis, inhibition of mitochondrial activity by Met-AEA, as well as by rotenone, led to sperm immobilization without affecting sperm viability (Fig. 4). Also this effect of Met-AEA, much like that on sperm Δψm, was mediated by CB1 receptors, although the underlying signal transduction remains to be clarified. At any rate, these effects mediated by CB1 receptors were exerted by Met-AEA doses higher than the physiological levels of AEA in the male and female reproductive tracts (38,39). It will be interesting to explore whether they could occur in the case of increased levels of endogenous cannabinoids (e.g. in obesity), or of exposure to exogenous cannabinoids. It will be also interesting to explore whether these effects could be exerted by physiological levels of 2-AG, which in a recent report (70) appeared to be involved in the suppression of progressive motility of mouse spermatozoa in the caput of epididymis, through CB1 activation.
In conclusion, the present study provides unprecedented evidence that in human sperm CB1 activation induces an apoptosis-independent decrease of mitochondrial membrane potential. This inhibition does not produce detectable effects on sperm motility in vitro, when standard glucose-containing media are used, because glycolysis can compensate for any loss of ATP produced by mitochondria. An adverse effect of Met-AEA on sperm motility can be revealed only in the presence of glycolysis blockage, or at poisoning doses of this compound. How and to what extend these in vitro findings could be extended to in vivo sperm exposure to (endo)cannabinoids is hard to predict. Both glycolysis and mitochondrial respiration participate in ATP production and depend on each other to control the functional state of sperm in environments with different energetic substrates (39). Notably, in fluids of the female genital tract of a number of species, the levels of lactate are higher than those of glucose and other glycolysable substrates (71,72,73,74), suggesting a possible major contribution of mitochondrial respiration to support sperm motility. This hypothesis could explain why a sperm cell that, during its differentiation, dramatically reduces its volume by removing any unnecessary structure must retain its mitochondria. In line with this, in the female genital tract sperm exposure to exogenous cannabinoids like THC, by impairing mitochondrial activity, could adversely affect sperm motility. Taken together, the effects of CB1 activation reported here represent a further improvement in understanding the relationship between energetic metabolism and motility in human sperm physiology, with a potential therapeutic exploitation for the treatment of human infertility.
Acknowledgments
We thank all staff members of the S. Liberatore Hospital of Atri: their generous hospitality after the 2009 earthquake of L’Aquila allowed us to complete this study.
Footnotes
This work was supported by a grant from Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR).
Disclosure Summary: The authors have nothing to disclose.
First Published Online October 20, 2010
Abbreviations: AEA, Anandamide; 2-AG, 2-arachidonoylglycerol; BWW, Biggers, Whitten, and Wittingham; DMSO, dimethyl sulfoxide; DOG, 2-deoxy-d-glucose; FITC, fluorescein isothiocyanate; HSD, honestly significant difference; IRTX, iodoresiniferatoxin; JC-1,5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenimidazolyl carbocyanine iodide; Met-AEA, methanandamide, nonhydrolyzable analog of AEA; NAPE, N-acylphosphatidylthanolamine; PI, propidium iodide; THC, Δ9-tetrahydrocannabinol; TRPV1, transient receptor potential vanilloid type 1.
References
- Hanus LO, Mechoulam R 2010 Novel natural and synthetic ligands of the endocannabinoid system. Curr Med Chem 17:1341–1359 [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI 1990 Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561–564 [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M 1993 Molecular characterization of a peripheral receptor for cannabinoids. Nature 365:61–65 [DOI] [PubMed] [Google Scholar]
- Pertwee RG 1997 Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther 74:129–180 [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA 2005 Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310:329–332 [DOI] [PubMed] [Google Scholar]
- Viscomi MT, Oddi S, Latini L, Pasquariello N, Florenzano F, Bernardi G, Molinari M, Maccarrone M 2009 Selective CB2 receptor agonism protects central neurons from remote axotomy-induced apoptosis through the PI3K/Akt pathway. J Neurosci 29:4564–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari M, Battista N, Fezza F, Gasperi V, Maccarrone M 2006 New insights into endocannabinoid degradation and its therapeutic potential. Mini Rev Med Chem 6:257–268 [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N 2004 Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem 279:5298–5305 [DOI] [PubMed] [Google Scholar]
- Hillard CJ, Jarrahian A 2003 Cellular accumulation of anandamide: consensus and controversy. Br J Pharmacol 140:802–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser ST, Kaczocha M, Deutsch DG 2005 Anandamide transport: a critical review. Life Sci 77:1584–1604 [DOI] [PubMed] [Google Scholar]
- Yates ML, Barker EL 2009 Organized trafficking of anandamide and related lipids. Vitam Horm 81:25–53 [DOI] [PubMed] [Google Scholar]
- McKinney MK, Cravatt BF 2005 Structure and function of fatty acid amide hydrolase. Annu Rev Biochem 74:411–432 [DOI] [PubMed] [Google Scholar]
- Fezza F, De Simone C, Amadio D, Maccarrone M 2008 Fatty acid amide hydrolase: a gate-keeper of the endocannabinoid system. Subcell Biochem 49:101–132 [DOI] [PubMed] [Google Scholar]
- Acs G, Palkovits M, Blumberg PM 1994 Comparison of [3H]resiniferatoxin binding by the vanilloid (capsaicin) receptor in dorsal root ganglia, spinal cord, dorsal vagal complex, sciatic and vagal nerve and urinary bladder of the rat. Life Sci 55:1017–1026 [DOI] [PubMed] [Google Scholar]
- Di Marzo V, De Petrocellis L 2010 Endocannabinoids as regulators of transient receptor potential (TRP) channels: a further opportunity to develop new endocannabinoid-based therapeutic drugs. Curr Med Chem 17:1430–1449 [DOI] [PubMed] [Google Scholar]
- Grimaldi P, Orlando P, Di Siena S, Lolicato F, Petrosino S, Bisogno T, Geremia R, De Petrocellis L, Di Marzo V 2009 The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proc Natl Acad Sci USA 106:11131–11136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierantoni R, Cobellis G, Meccariello R, Cacciola G, Chianese R, Chioccarelli T, Fasano S 2009 CB1 activity in male reproduction: mammalian and nonmammalian animal models. Vitam Horm 81:367–387 [DOI] [PubMed] [Google Scholar]
- Maccarrone M 2009 Endocannabinoids: friends and foes of reproduction. Prog Lipid Res 48:344–354 [DOI] [PubMed] [Google Scholar]
- Hall W, Solowij N 1998 Adverse effects of cannabis. Lancet 352:1611–1616 [DOI] [PubMed] [Google Scholar]
- Wenger T, Ledent C, Csernus V, Gerendai I 2001 The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochem Biophys Res Commun 284:363–368 [DOI] [PubMed] [Google Scholar]
- Schuel H, Schuel R, Zimmerman AM, Zimmerman S 1987 Cannabinoids reduce fertility of sea urchin sperm. Biochem Cell Biol 65:130–136 [DOI] [PubMed] [Google Scholar]
- Chang MC, Berkery D, Schuel R, Laychock SG, Zimmerman AM, Zimmerman S, Schuel H 1993 Evidence for a cannabinoid receptor in sea urchin sperm and its role in blockade of the acrosome reaction. Mol Reprod Dev 36:507–516 [DOI] [PubMed] [Google Scholar]
- Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S 1994 Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proc Natl Acad Sci USA 91:7678–7682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccarrone M, Barboni B, Paradisi A, Bernabò N, Gasperi V, Pistilli MG, Fezza F, Lucidi P, Mattioli M 2005 Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J Cell Sci 118:4393–4404 [DOI] [PubMed] [Google Scholar]
- Francavilla F, Battista N, Barbonetti A, Vassallo MR, Rapino C, Antonangelo C, Pasquariello N, Catanzaro G, Barboni B, Maccarrone M 2009 Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology 150:4692–4700 [DOI] [PubMed] [Google Scholar]
- Rossato M, Ion Popa F, Ferigo M, Clari G, Foresta C 2005 Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. J Clin Endocrinol Metab 90:984–991 [DOI] [PubMed] [Google Scholar]
- Agirregoitia E, Carracedo A, Subirán N, Valdivia A, Agirregoitia N, Peralta L, Velasco G, Irazusta J 2010 The CB(2) cannabinoid receptor regulates human sperm cell motility. Fertil Steril 93:1378–1387 [DOI] [PubMed] [Google Scholar]
- Schuel H, Burkman LJ, Lippes J, Crickard K, Mahony MC, Giuffrida A, Picone RP, Makriyannis A 2002 Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Mol Reprod Dev 63:376–387 [DOI] [PubMed] [Google Scholar]
- Schuel H, Burkman LJ, Lippes J, Crickard K, Forester E, Piomelli D, Giuffrida A 2002 N-Acylethanolamines in human reproductive fluids. Chem Phys Lipids 121:211–227 [DOI] [PubMed] [Google Scholar]
- Whan LB, West MC, McClure N, Lewis SE 2006 Effects of delta-9-tetrahydrocannabinol, the primary psychoactive cannabinoid in marijuana, on human sperm function in vitro. Fertil Steril 85:653–660 [DOI] [PubMed] [Google Scholar]
- Aquila S, Guido C, Laezza C, Santoro A, Pezzi V, Panza S, Andò S, Bifulco M 2009 A new role of anandamide in human sperm: focus on metabolism. J Cell Physiol 221:147–153 [DOI] [PubMed] [Google Scholar]
- Catanzaro G, Rapino C, Oddi S, Maccarrone M 2009 Anandamide increases swelling and reduces calcium sensitivity of mitochondria. Biochem Biophys Res Commun 388:439–442 [DOI] [PubMed] [Google Scholar]
- Zaccagnino P, Saltarella M, D'Oria S, Corcelli A, Saponetti MS, Lorusso M 2009 N-Arachidonylglycine causes ROS production and cytochrome c release in liver mitochondria. Free Radic Biol Med 47:585–592 [DOI] [PubMed] [Google Scholar]
- Badawy ZS, Chohan KR, Whyte DA, Penefsky HS, Brown OM, Souid AK 2009 Cannabinoids inhibit the respiration of human sperm. Fertil Steril 91:2471–2476 [DOI] [PubMed] [Google Scholar]
- Rossato M 2008 Endocannabinoids, sperm functions and energy metabolism. Mol Cell Endocrinol 286:S31–S35 [DOI] [PubMed] [Google Scholar]
- Mukai C, Okuno M 2004 Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod 71:540–547 [DOI] [PubMed] [Google Scholar]
- Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA 2004 Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA 101:16501–16506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford WC 2006 Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round? Hum Reprod Update 12:269–274 [DOI] [PubMed] [Google Scholar]
- Miki K 2007 Energy metabolism and sperm function. Soc Reprod Fertil Suppl 65:309–325 [PubMed] [Google Scholar]
- Ruiz-Pesini E, Díez-Sánchez C, López-Pérez MJ, Enríquez JA 2007 The role of the mitochondrion in sperm function: is there a place for oxidative phosphorylation or is this a purely glycolytic process? Curr Top Dev Biol 77:3–19 [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V 2001 The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem 276:12856–12863 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) 1999 WHO laboratory manual for the examination of human semen and semen-cervical mucus interaction. 4th ed. London: Cambridge University Press; 4–33 [Google Scholar]
- Tzeng SJ, Bolland S, Inabe K, Kurosaki T, Pierce SK 2005 The B cell inhibitory Fc receptor triggers apoptosis by a novel c-Abl family kinase-dependent pathway. J Biol Chem 280:35247–35254 [DOI] [PubMed] [Google Scholar]
- Jamadar-Shroff V, Papich MG, Suter SE 2009 Soy-derived isoflavones inhibit the growth of canine lymphoid cell lines. Clin Cancer Res 15:1269–1276 [DOI] [PubMed] [Google Scholar]
- Zaheen A, Boulianne B, Parsa JY, Ramachandran S, Gommerman JL, Martin A 2009 AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood 114:547–554 [DOI] [PubMed] [Google Scholar]
- Salvioli S, Ardizzoni A, Franceschi C, Cossarizza A 1997 JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess δ ψ changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett 411:77–82 [DOI] [PubMed] [Google Scholar]
- Wadia JS, Chalmers-Redman RM, Ju WJ, Carlile GW, Phillips JL, Fraser AD, Tatton WG 1998 Mitochondrial membrane potential and nuclear changes in apoptosis caused by serum and nerve growth factor withdrawal: time course and modification by (-)-deprenyl. J Neurosci 18:932–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M, Anderson BO, Caldwell E, Sedghinasab M, Paty PB, Hockenbery DM 1997 Mitochondrial proliferation and paradoxical membrane depolarization during terminal differentiation and apoptosis in a human colon carcinoma cell line. J Cell Biol 138:449–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifulco M, Laezza C, Gazzerro P, Pentimalli F 2007 Endocannabinoids as emerging suppressors of angiogenesis and tumor invasion. Oncol Rep 17:813–816 [PubMed] [Google Scholar]
- Wang D, Wang H, Ning W, Backlund MG, Dey SK, DuBois RN 2008 Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res 68:6468–6476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer M, Hanemann CO, Zajicek J 2008 High concentrations of cannabinoids activate apoptosis in human U373MG glioma cells. J Neurosci Res 86:3212–3220 [DOI] [PubMed] [Google Scholar]
- Pasquariello N, Catanzaro G, Marzano V, Amadio D, Barcaroli D, Oddi S, Federici G, Urbani A, Finazzi Agrò A, Maccarrone M 2009 Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J Biol Chem 284:29413–29426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhough A, Patsos HA, Williams AC, Paraskeva C 2007 The cannabinoid Δ9-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int J Cancer 121:2172–2180 [DOI] [PubMed] [Google Scholar]
- Cianchi F, Papucci L, Schiavone N, Lulli M, Magnelli L, Vinci MC, Messerini L, Manera C, Ronconi E, Romagnani P, Donnini M, Perigli G, Trallori G, Tanganelli E, Capaccioli S, Masini E 2008 Cannabinoid receptor activation induces apoptosis through tumor necrosis factor α-mediated ceramide de novo synthesis in colon cancer cells. Clin Cancer Res 14:7691–7700 [DOI] [PubMed] [Google Scholar]
- Belmokhtar CA, Hillion J, Ségal-Bendirdjian E 2001 Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 20:3354–3362 [DOI] [PubMed] [Google Scholar]
- Xue LY, Chiu SM, Oleinick NL 2003 Staurosporine-induced death of MCF-7 human breast cancer cells: distinction between caspase-3-dependent steps of apoptosis and the critical lethal lesions. Exp Cell Res 283:135–145 [DOI] [PubMed] [Google Scholar]
- Cen H, Mao F, Aronchik I, Fuentes RJ, Firestone GL 2008 DEVD-NucView488: a novel class of enzyme substrates for real-time detection of caspase-3 activity in live cells. FASEB J 22:2243–2252 [DOI] [PubMed] [Google Scholar]
- Bose BC, Saifi AQ, Bhagwat AW 1963 Effect of Cannabis indica on hexobarbital sleeping time and tissue respiration of rat brain. Arch Int Pharmacodyn Ther 141:520–524 [PubMed] [Google Scholar]
- Mahoney JM, Harris RA 1972 Effect of 9-tetrahydrocannabinol on mitochondrial precesses. Biochem Pharmacol 21:1217–1226 [DOI] [PubMed] [Google Scholar]
- Bartova A, Birmingham MK 1976 Effect of Δ9-tetrahydrocannabinol on mitochondrial NADH-oxidase activity. J Biol Chem 251:5002–5006 [PubMed] [Google Scholar]
- Sarafian TA, Kouyoumjian S, Khoshaghideh F, Tashkin DP, Roth MD 2003 Δ9-Tetrahydrocannabinol disrupts mitochondrial function and cell energetics. Am J Physiol Lung Cell Mol Physiol 284:L298–L306 [DOI] [PubMed] [Google Scholar]
- Athanasiou A, Clarke AB, Turner AE, Kumaran NM, Vakilpour S, Smith PA, Bagiokou D, Bradshaw TD, Westwell AD, Fang L, Lobo DN, Constantinescu CS, Calabrese V, Loesch A, Alexander SP, Clothier RH, Kendall DA, Bates TE 2007 Cannabinoid receptor agonists are mitochondrial inhibitors: a unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem Biophys Res Commun 364:131–137 [DOI] [PubMed] [Google Scholar]
- Aquila S, Guido C, Santoro A, Perrotta I, Laezza, Bifulco M, Andò S 2010 Human sperm anatomy: ultrastructural localization of the cannabinoid 1 receptor and a potential role of anandamide in sperm survival and acrosome reaction. Anat Rec 293:298–309 [DOI] [PubMed] [Google Scholar]
- Thornberry NA, Lazebnik Y 1998 Caspase: enemies within. Science 281:1312–1316 [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Strasser A, McDunn JE, Swanson PE 2009 Cell death. N Engl J Med 361:1570–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers AJ, De Iuliis GN, Finnie JM, McLaughlin EA, Aitken RJ 2008 Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J Clin Endocrinol Metab 93:3199–3207 [DOI] [PubMed] [Google Scholar]
- Hiipakka RA, Hammerstedt RH 1978 2-Deoxyglucose transport and phosphorylation by bovine sperm. Biol Reprod 19:368–379 [DOI] [PubMed] [Google Scholar]
- Williams AC, Ford WC 2001 The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl 22:680–695 [PubMed] [Google Scholar]
- Albarracín JL, Fernández-Novell JM, Ballester J, Rauch MC, Quintero-Moreno A, Peña A, Mogas T, Rigau T, Yañez A, Guinovart JJ, Slebe JC, Concha II, Rodríguez-Gil JE 2004 Gluconeogenesis-linked glycogen metabolism is important in the achievement of in vitro capacitation of dog spermatozoa in a medium without glucose. Biol Reprod 71:1437–1445 [DOI] [PubMed] [Google Scholar]
- Cobellis G, Ricci G, Cacciola G, Orlando P, Petrosino S, Cascio GM, Bisogno T, De Petrocellis L, Choccarelli T, Altucci L, Fasano S, Meccariello R, Pierantoni R, Ledent C, Di Marzo V 2010 A gradient of 2-arachidoylglycerol regulates mouse epididymal sperm cell start-up. Biol Reprod 82:451–458 [DOI] [PubMed] [Google Scholar]
- Jones AR 1998 Chemical interference with sperm metabolic pathways. J Reprod Fertil Suppl 53:227–234 [PubMed] [Google Scholar]
- Windsor DP 1997 Mitochondrial function and ram sperm fertility. Reprod Fertil Dev 9:279–284 [DOI] [PubMed] [Google Scholar]
- Harris SE, Gopichandran N, Picton HM, Leese HJ, Orsi NM 2005 Nutrient concentrations in murine follicular fluid and the female reproductive tract. Theriogenology 64:992–1006 [DOI] [PubMed] [Google Scholar]
- Galantino-Homer HL, Florman HM, Storey BT, Dobrinski I, Kopf GS 2004 Bovine sperm capacitation: assessment of phosphodiesterase activity and intracellular alkalinization on capacitation-associated protein tyrosine phosphorylation. Mol Reprod Dev 67:487–500 [DOI] [PubMed] [Google Scholar]