Abstract
Estradiol appears to exert its anorexigenic effect by activating nuclear estrogen receptors (ERs), which are expressed widely in peripheral tissues and in the brain. Here, we used ICI-182,780 (ICI), a pure antiestrogen with limited ability to cross the blood-brain barrier, to assess the relative involvement of peripheral vs. central ERs to estradiol’s anorexigenic effect. Food intake was measured after peripheral (sc) administration of ICI or vehicle in ovariectomized rats treated with acute injections of estradiol benzoate and sesame oil over a 2-wk period. Uterine weight was assessed as a biological assay of peripheral ER activation. In a second experiment, food intake was measured after central (lateral ventricular) administration of ICI or vehicle in ovariectomized rats receiving acute injections of estradiol benzoate and oil over a period of 10 d. In order to assess the possible spread of ICI from the brain to the periphery, vaginal cytology samples were examined as a biological assay of peripheral ER activation. Peripherally administered ICI failed to attenuate estradiol’s anorexigenic effect at a dose that was sufficient to block estradiol’s uterotrophic effect. This suggests that peripheral activation of ERs is not necessary for estradiol’s anorexigenic effect. Although central infusion of 4 nm ICI blocked estradiol’s anorexigenic effect, it did not attenuate estradiol’s ability to increase the presence of cornified cells in vaginal cytology samples, suggesting that ICI did not leak into the periphery. We conclude that activation of central, but not peripheral, ERs is necessary for estradiol’s anorexigenic effect.
The ability of estradiol to reduce food intake in ovariectomized rats was blocked following central, but not peripheral, antagonism of estrogen receptors.
Many of estradiol’s behavioral and physiological actions appear to be mediated via slow, genomic effects involving activation of nuclear estrogen receptors (ERs). Two lines of evidence suggest that this is likely true of estradiol’s anorexigenic effect. First, there is a lag time of approximately 36–40 h between the initial increase in plasma estradiol levels during diestrus and the onset of decreased feeding during estrus in cycling, female rats (1). Second, ovariectomized (OVX) rats do not display a decrease in food until 24–48 h after either one or two sc injections of physiological (1–2 μg) doses of estradiol (1,2,3). Although shorter (2–14 h) latencies to reduce food intake have been reported in male and female rats receiving infusions of a water-soluble form of estradiol directly into the brain (4,5), it remains to be determined whether brain concentrations of estradiol remained within the physiological range in these studies. Consistent with estradiol’s diverse actions, nuclear ERs have been localized in multiple peripheral tissues, including the ovaries, uterus, adipose tissue, liver, and intestinal mucosa (6,7,8,9), and throughout the brain (10). Thus, there are multiple sites where estradiol could act to decrease food intake in the female rat.
Three lines of evidence suggest that estradiol’s anorexigenic effect involves peripheral targets. First, estradiol decreases fat absorption, specifically by decreasing lipoprotein lipase (LPL) activity in adipose tissue (11,12,13). This, in turn, could increase the availability of circulating metabolic fuels and, thereby, reduce appetite. Second, in vitro studies have shown that estradiol increases mRNA expression of the obese (ob) gene and secretion of leptin (the protein product of the ob gene) in adipocytes harvested from the sc, perirenal, and parametrial fat pads of OVX rats (14). This same group also reported that ovariectomy decreases ob mRNA expression in perirenal adipocytes (14). Because leptin regulates body adiposity by reducing energy intake and increasing energy expenditure (15), this peripheral action of estradiol to increase leptin signaling could promote decreased feeding. Third, estradiol has been shown to decrease gastric emptying in rats (16). Such a delay could increase stimulation of gastric mechanoreceptors, resulting in greater negative feedback via visceral afferents in the gut to brain areas that control food intake.
Much of the initial research on the involvement of central ERs in mediating estradiol’s anorexigenic effect involved site-specific administration of estradiol into the brain. In the earliest studies, central implants of concentrated, crystalline estradiol produced reliable reductions in food intake in OVX rats (17,18). However, some implants were reported to increase uterine weight, suggesting considerable spread of estradiol into the periphery (17). Other studies using more dilute concentrations of estradiol reported reductions in food intake after site-specific infusions of estradiol into the nucleus of the solitary tract (19), the arcuate nucleus of the hypothalamus (Arc) (5), the paraventricular nucleus of the hypothalamus (20,21), and the medial preoptic nucleus (4). It should be noted, however, that studies involving the latter two sites have not been replicated by other groups (20,22).
Although there is some evidence that activation of either peripheral or central ERs may be sufficient to reduce food intake, it remains less clear whether activation of peripheral and/or central ERs is necessary for estradiol’s anorexigenic effect. Progress in addressing this question is limited by the fact that many pharmacological compounds designed to target ERs (e.g. raloxifene, tamoxifen, nitromifene citrate, and methyl-piperidino-pyrazole) function as selective ER modulators (SERMs). That is, they exhibit tissue-specific, mixed agonist/antagonist effects. For example, although raloxifene exerts an antiestrogenic effect on breast tissue, it exerts estrogenic effects on uterine tissue and bone (23). These tissue-selective effects of SERMs underscore the importance of assessing the relative contributions of peripheral and central ERs in mediating estradiol’s anorexigenic effect. With respect to feeding, SERMs like raloxifene, tamoxifen, and methyl-piperidino-pyrazole have been shown to mimic, rather than antagonize, estradiol’s anorexigenic effect in female rats (24,25,26).
In contrast to the SERMs discussed above, the steroidal compound ICI-182,780 (ICI) has been classified as a pure antiestrogen (27,28) with a binding affinity that is similar to estradiol in ER competition assays involving homogenized brain tissue (11). ICI inhibits ER activation by impairing ER dimerization (29), disrupting ER nuclear localization (30,31), and increasing ER degradation (32). Findings from several studies involving in vivo administration of this antiestrogenic compound provide evidence that ICI does not cross the blood-brain barrier. First, peripheral ICI treatment blocked in vivo uptake of tritiated estradiol in peripheral tissues (uterus, adipose tissue, and pituitary gland) but not in forebrain tissue obtained from OVX rats and hamsters (11,33). Second, peripheral ICI treatment in cycling rats did not mimic the increase in body weight or plasma levels of gonadotropins that follows ovariectomy surgery and that would have been expected if ICI had gained access to central ERs (27,28). Third, intracerebroventricular (icv) administration of ICI did not attenuate the estradiol-dependent increase in uterine weight in OVX rats (34). It should be noted, however, that others have found some evidence that ICI may gain access to the brain after peripheral administration. In one study, systemic dosing (three daily injections of 1 mg/kg ICI) resulted in detectable levels of ICI in the brains of OVX rats (35). In addition, peripheral administration of ICI either chronically or at high doses (1–3 mg/kg) attenuated estradiol-induced changes in body temperature and white adipose tissue mass in OVX rats (11,35) and induced hot flushes in premenopausal women (36). Because these physiological actions of estradiol are thought to be mediated entirely by activation of central ERs, these studies suggest that peripheral administration of ICI can gain access to central ERs under certain test conditions. At present, we are unaware of any reports that central administration of ICI either gains access to peripheral tissues or attenuates actions of estradiol thought to be mediated in the periphery.
Relative to other compounds that selectively target ERs, ICI’s unique features have made it a useful tool in distinguishing between peripheral and central sites of ER action. For example, Wade et al. (11,33) have shown that peripherally administered ICI failed to attenuate the anorexia associated with 4 wk of daily estradiol injections in OVX rats and hamsters. Although this suggests that the anorexia associated with chronically elevated concentrations of estradiol is mediated by activation of central, rather than peripheral, ERs, a direct test of this hypothesis has yet to be conducted. It is also unknown whether activation of peripheral or central ERs is required for the anorexia associated with a more acute, physiologically relevant, regimen of estradiol treatment. We addressed this last question in the present study by monitoring food intake after peripheral and central administration of ICI in OVX rats receiving an acute regimen of estradiol treatment that mimics the fluctuations in endogenous estradiol secretion observed in cycling rats.
Materials and Methods
Animals and housing conditions
Female, Long-Evans rats (Charles River Breeding Laboratories, Raleigh, NC) were housed individually in custom-designed shoebox cages located in a temperature-controlled testing room with a reverse light cycle (dark onset at 1300 h). The cages were equipped with spill-resistant food cups that facilitated accurate measurement of food intake (±0.1 g). Rats gained access to the food cups by traversing stainless-steel, wire-mesh bridges that were designed to minimize cage bedding in and around the food cups. Throughout the study, rats were given free access to powdered chow (Purina 5001) and tap water. At study onset, rats weighed 292 ± 11 g. All animal procedures were approved by The Florida State University Institutional Animal Care and Use Committee.
Surgery
At the beginning of experiments 1 and 2, rats were anesthetized with ip injections of a mixture of 50 mg/ml ketamine (Ketaset; Fort Dodge, Fort Dodge, IA) and 4.5 mg/ml xylazine (Rompun; Mobay, Shawnee, KS). Rats were then bilaterally OVX using an intraabdominal approach (e.g. see Ref. 37). Immediately after surgery, rats received single, ip injections of butorphanol (0.5 mg/kg; Fort Dodge) to minimize postsurgical pain. After 9 d of postsurgical recovery, OVX rats used in experiment 2 were anesthetized with a mixture of ketamine/xylazine (as described for OVX surgery) and then fitted with stainless-steel, osmotic pump, single connector, guide cannulas (22 G needle cut 5 mm below pedestal; Plastics One, Inc., Roanoke, VA) aimed at the right lateral ventricle. The stereotaxic coordinates, based on the rat brain atlas of Paxinos and Watson (38), were: caudal, 0.6 mm; lateral, 1.7 mm; and ventral, 3.5 mm relative to bregma. The guide cannulas were attached to osmotic minipumps (Alzet Model 1002) designed to deliver their contents across a 14-d period (flow rate, 0.25 μl/h; reservoir volume, 100 μl). After surgery, rats received ip injections of 0.5 mg/kg butorphanol and gentamicin (10 mg/ml; ProLabs Ltd., St. Joseph, MO) to minimize pain and the risk of infection, respectively. Food intake and body weight were monitored daily in each experiment to ensure that rats had fully recovered from the anesthetic and all surgical procedures before data collection.
Experiment 1, blockade of peripheral ERs
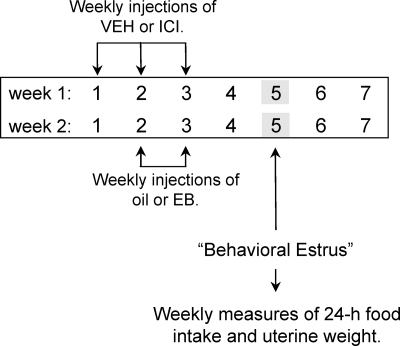
The effect of peripheral ER blockade was examined in hormone-treated OVX rats over a period of 2 wk as depicted in Fig. 1. Intrascapular, sc injections of either 2 μg 17β-estradiol benzoate (EB) (Sigma-Aldrich, Boston, MA) dissolved in 0.1 ml sesame oil or sesame oil alone were administered 1 h before dark onset on d 2 and 3 of wk 1. The reverse treatment was administered during wk 2. This acute regimen of EB treatment (injections on d 2 and 3) was chosen for two reasons. First, it induces a transient increase in plasma estradiol levels that is comparable with that seen in the proestrous rat (2). Second, it produces a reliable, anorexigenic effect on d 5, the day that models behavioral estrus (2). On d 1–3 of these same 2 wk, oil- and EB-treated OVX rats also received sc injections of either 0.5 mg/kg ICI [7α,17β-[9[(4,4,5,5,5-pentafluoropentyl) sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17-diol; Tocris Bioscience, Bristol, UK] delivered in 1 ml/kg sesame oil vehicle (VEH) or VEH alone (n = 8 per group). These injections were administered 3 h before dark onset. The combined effects of our drug (ICI or VEH) and hormone (oil and EB) treatments were assessed on d 5 of both weeks (i.e. the days that modeled estrus). Immediately after food intake was recorded each week, uterine horns were removed unilaterally under ketamine/xylazine anesthesia (as described for OVX surgery). Assessment of uterine weight was used as a biological assay of peripheral ER activation. In wk 1, the left uterine horn was excised from each rat, trimmed of adipose tissue, lightly blotted, cut to 10 mm, and weighed (±1 mg). In wk 2, the same procedure was used to remove the right uterine horn. Daily records of food intake and body weight revealed that all rats had fully recovered from the anesthetic and uterine surgery before additional data collection during wk 2. After each uterine-horn removal, rats were given ip injections of 0.5 mg/kg butorphanol to minimize any postsurgical pain.
Figure 1.
Experimental design used to examine the effect of peripheral ER blockade on EB’s anorexigenic effect. After bilateral ovariectomy, rats in the control group (VEH; n = 8) received a total of six sc injections of VEH on d 1–3 of wk 1 and 2. Rats in the experimental group (ICI; n = 8) received sc injections of 0.5 mg/kg ICI on the same days. Within each of these groups, rats received injections of either oil or 2 μg EB during wk 1. During wk 2, these hormone treatments were reversed. Each week, food intake and unilateral uterine weight were assessed on d 5, the day that models behavioral estrus using this acute regimen of EB replacement. EB, Estradiol benzoate; ICI, ICI-182,780.
Experiment 2, blockade of central ERs
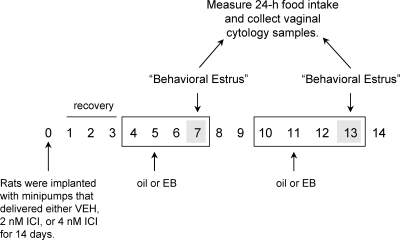
The effect of central ER blockade was examined in hormone-treated OVX rats over a period of 2 wk as depicted in Fig. 2. Three groups of OVX rats were anesthetized and implanted with icv cannulas as described above. On d 0, the cannulas were attached to osmotic minipumps filled with 1 of 3 drug treatments that were released over a course of 14 d. The control group received pumps filled with a mixture of 1% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) in physiological saline (VEH). The two experimental groups received pumps filled with either 2 or 4 nm ICI dissolved in VEH. This yielded three treatment groups (n = 6–8 per group). Rats were allowed 3 d of postsurgical recovery before two cycles of acute hormone treatment were administered. Using a within-subjects design, half the rats received sc injections of 1 μg EB, and the other half received sc injections of oil 3 h before dark onset on d 5. Treatments were then reversed on d 11. A single injection of EB was administered in this experiment, because there were only 10 test days available after the 3 d of postsurgical recovery, and we wanted to minimize the possibility of any carry-over effects of EB treatment from hormone treatment cycle 1 to hormone treatment cycle 2. Like the double EB injection model used in experiment 1, the single EB injection model produces changes in plasma estradiol levels that are similar in magnitude and duration to that observed in cycling rats (3). Specifically, this single EB injection model produces a rise in plasma estradiol concentration of approximately 270 pmol/liter, which is very similar to the proestrous peak in plasma estradiol (∼260 pmol/liter) reported in cycling rats (39). Importantly, both the single and double EB injection models produce similar decreases in food intake on the day that models estrus (1,2,3). Thus, both EB replacement protocols provide excellent models of the changes in plasma estradiol concentration reported in cycling rats as well as the estrous-related decrease in food intake observed in cycling rats. Food intake (±0.1 g) and vaginal cytology were assessed 2 d after hormone treatment on the days that modeled behavioral estrus (i.e. d 7 and 13). The appearance of vaginal cytology samples was used as a biological assay of peripheral ER activation. This replaced the assessment of uterine weights (used in experiment 1), because there was not enough time within the protocol to anesthetize rats for surgical removal of uterine horns and to wait for postsurgical recovery. The method used for obtaining and assessing vaginal cytology was the same as described previously (40,41).
Figure 2.
Experimental design used to examine the effect of central ER blockade on EB’s anorexigenic effect. Rats were OVX and equipped with osmotic minipumps that were connected to guide cannulas targeting the lateral ventricle. The minipumps delivered either VEH, 2 nm ICI, or 4 nm ICI at a rate of 0.25 μl/h over a period of 14 d. In addition to these central infusions, rats received sc injections of either oil or 1 μg EB on d 5 and 11. Food intake and vaginal cytology were monitored on d 7 and 13, the days that model behavioral estrus using this acute regimen of EB replacement. EB, Estradiol benzoate; ICI, ICI-182,780.
At the end of the 14-d infusion period, rats were anesthetized with ip injections of Nembutal (65 mg/kg; sodium pentobarbital; Henry Schein, Mellville, NY) and then perfused with 4% paraformaldehyde. Fixed brain tissue was sectioned at 40-μm intervals at the site of the guide cannula track [coordinates: caudal, 0.6 mm; lateral, 1.7 mm; and ventral, 3.5 mm relative to bregma using a cryostat (Leica, Myer Instruments, Houston, TX)]. Tissue was stained with cresyl violet, mounted on microscope slides, and coverslipped. Images of forebrain sections were captured using an Olympus AX70 microscope and Image-Pro Plus software (version 3.0; MediaCybernetics, Gaithersburg, MD). The locations of guide cannulas were then verified within the imaged tissue sections.
Data analysis
In experiment 1, two-factor, mixed-design ANOVAs were used to examine the effects of the between-subjects variable, drug treatment (VEH or ICI), and the within-subjects variable, hormone treatment (oil and EB), on 24-h food intake and uterine weight. Two rats (receiving VEH/oil and VEH/EB treatment) were not included in the uterine weight analysis because they were not sufficiently unresponsive after receiving anesthesia on d 5. Because the missing uterine weight data could not be expected to influence food intake, these two rats were included in the analysis of food intake. In experiment 2, two-factor, mixed-design ANOVAs were used to examine the effects of the between-subjects variable, drug treatment (VEH, 2 nm ICI or 4 nm ICI), and the within-subjects variable, hormone treatment (oil and EB), on 24-h food intake. For both experiments, Newman-Keuls post hoc tests were used to examine differences between means after significant (P < 0.05) main or interactive ANOVA effects.
Results
Experiment 1, blockade of peripheral ERs
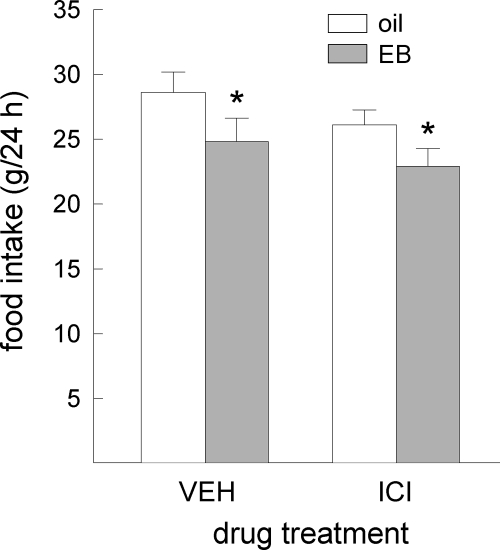
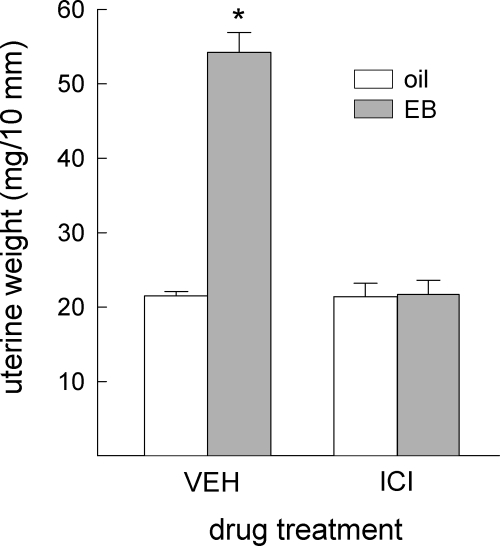
Acute administration of EB reduced food intake in all rats, F(1,14) = 15.08, P < 0.005 (Fig. 3). This anorexigenic effect of EB was similar in ICI- and VEH-treated rats. No main or interactive effects of ICI treatment on food intake were detected, F(1,14) = 1.25 and 0.09, respectively, not significant. In contrast, EB’s ability to increase uterine weight was differentially affected by ICI/VEH treatment, F(1,10) = 136.31, P < 0.0001 (Fig. 4). The increase in uterine weight observed in VEH-treated rats (P < 0.05) was blocked in ICI-treated rats.
Figure 3.
Peripheral blockade of ERs failed to attenuate EB’s anorexigenic effect in OVX rats. Data are means ± sems. EB produced similar decreases in food intake in VEH- and ICI-treated rats. An asterisk indicates EB treatment less than oil treatment (Ps < 0.05). EB, Estradiol benzoate; ICI, ICI-182,780.
Figure 4.
Peripheral blockade of ERs prevented EB’s uterotrophic effect in OVX rats. Data are means ± sems. EB treatment increased uterine weight in rats that received peripheral VEH treatment. This action of EB was blocked by peripheral ICI treatment. An asterisk indicates greater than all other groups (Ps < 0.05). EB, Estradiol benzoate; ICI, ICI-182,780.
Experiment 2, blockade of central ERs
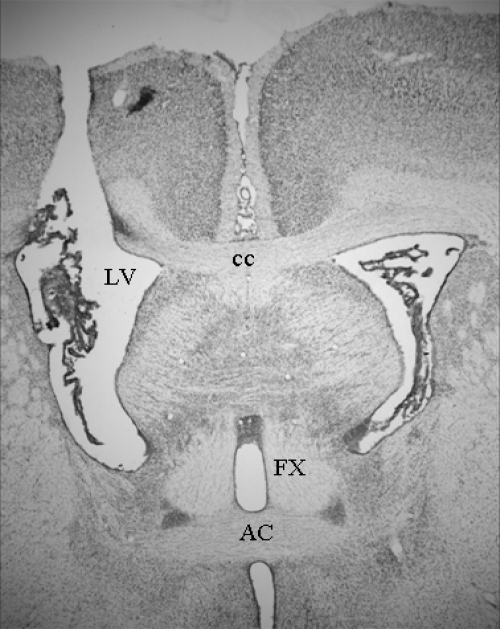
Histological examination of forebrain sections revealed that four of 22 rats had misplaced cannulas that failed to target the lateral ventricle (VEH, n = 1; 2 nm ICI, n = 1; 4 nm ICI, n = 2). The data from these rats were not analyzed. Correct cannula placement was verified in the remaining 18 rats. A representative photomicrograph image showing correct cannula placement is depicted in Fig. 5.
Figure 5.
A representative photomicrograph of a cannula track depicting correct cannula placement in the lateral ventricle. Image was taken at a magnification of ×2. AC, Anterior commissure; cc, corpus callosum; FX, fornix; LV, lateral ventricle.
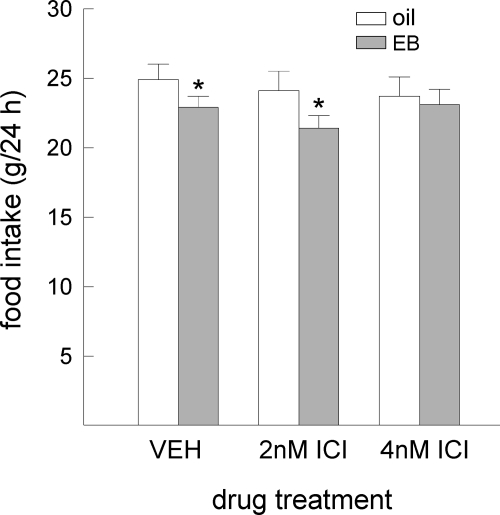
EB’s anorexigenic effect was influenced by central ICI treatment, F(2,15) = 3.61, P < 0.05 (Fig. 6). Although EB treatment decreased food intake in rats that received central infusions of VEH or 2 nm ICI (Ps < 0.05), this action of EB was blocked in rats treated with 4 nm ICI. All of the vaginal samples obtained from EB-treated rats, regardless of ICI or VEH treatment, contained nonnucleated, cornified cells indicative of estrus. In comparison, nonnucleated, cornified cells were not apparent in any of the vaginal samples obtained from oil-treated rats. The predominant cell types observed in these samples included small, nucleated epithelial cells and leukocytes, a pattern associated with the diestrous stage of the cycle.
Figure 6.
Central blockade of ERs is capable of blocking EB’s anorexigenic effect in OVX rats. Data are means ± sems. EB treatment decreased food intake in rats that received icv infusions of VEH and 2 nm ICI. The anorexigenic action of EB was, however, blocked in rats that received icv infusions of 4 nm ICI. An asterisk indicates EB treatment less than oil treatment (Ps < 0.05). EB, Estradiol benzoate; ICI, ICI-182,780.
Discussion
The relative necessity of peripheral and central ERs in mediating estradiol’s anorexigenic effect is poorly understood. Progress in addressing this question has been hampered by the fact that most compounds designed to selectively target ERs cross the blood-brain barrier and exert tissue-specific, mixed estrogenic/antiestrogenic effects. Here, however, we were able to investigate the relative necessity of peripheral vs. central ERs in mediating estradiol’s anorexigenic effect. Food intake was monitored in EB-treated OVX rats after either peripheral or central pretreatment with ICI, a pure antiestrogenic compound that appears to cross the blood-brain barrier under very limited conditions. The anorexigenic effect of our acute regimen of EB treatment, which was designed to model the proestrous increase in estradiol secretion (2,3), was blocked by central, but not peripheral, administration of ICI. Thus, our findings provide the first evidence that activation of central, but not peripheral, ERs is necessary for estradiol’s anorexigenic effect.
Peripheral administration of ICI failed to alter EB’s anorexigenic effect. That is, the reduction in food intake after an acute regimen of EB treatment was identical in rats that received peripheral injections of VEH or ICI. This finding is consistent with previous reports that peripherally administered ICI failed to attenuate the more prolonged anorexia associated with chronic (4 wk) estradiol treatment in OVX rats and hamsters (11,33). Our finding extends these reports by showing that peripherally administered ICI fails to attenuate the anorexigenic effect of a more physiological regimen of acute EB replacement, which was chosen to mimic the changes in endogenous estradiol secretion across the estrous cycle in intact rats (2,3). It is unlikely that this null finding was the result of inadequate blockade of peripheral ERs, because the same dose of ICI blocked EB’s ability to increase uterine weight, a well-established peripheral action of estradiol. Because our regimen of ICI treatment blocked ERs in the uterus, a peripheral tissue with high ER expression, it was likely sufficient to provide widespread blockade of peripheral ERs. There is also no reason to believe that ICI was rapidly metabolized, because peripheral ICI treatment increased plasma ICI levels for at least 24 h in OVX rats (35) and acute im injection of ICI increased plasma ICI levels for up to 7 d in humans (42).
Previous studies have provided some evidence that activation of peripheral ERs may be sufficient to reduce food intake in OVX rats. For example, estradiol decreases LPL activity (11,12,13), decreases gastrointestinal emptying (16), and increases ob mRNA expression and leptin secretion in adipocytes (14). Each of these peripheral actions of estradiol could function to reduce food intake. It should be noted, however, that the temporal relationship between estradiol’s effects on LPL activity and food intake are not tightly correlated. For example, in one study, estradiol decreased food intake for 2–7 d, whereas it decreased LPL activity for 2–14 d, after hormone treatment (13). A similar time-course study has not been conducted to determine whether estradiol’s effects on gastrointestinal emptying and food intake are temporally correlated. It also remains to be determined whether estradiol’s ability to increase leptin expression/secretion (14) would promote the types of transient changes modeled here by acute estradiol treatment. Rather, it is possible that estradiol’s ability to regulate leptin expression/secretion from adipocytes plays a more critical role in the maintenance and deposition of body fat than the short-term control of food intake. Although it remains plausible that a peripheral action of estradiol on adipocytes and/or the gut is sufficient to decrease food intake, the current findings provide compelling evidence that activation of peripheral ERs is not necessary for estradiol’s acute, anorexigenic effect.
The major finding of the current study was that EB’s anorexigenic effect was blocked by icv infusion of 4 nm ICI. That a smaller (2 nm) dose of ICI failed to produce a similar effect suggests that the threshold dose of ICI for blocking the relevant central ERs that mediate estradiol’s anorexigenic effect must lie between 2 and 4 nm. The present findings provide the first demonstration that activation of central ERs is necessary for estradiol’s anorexigenic effect. Our use of a physiologically relevant dose of EB, designed to model the fluctuations in estradiol secretion in cycling rats (2,3), suggests that the estrous-related decrease in food intake in cycling rats is also dependent upon activation of central, rather than peripheral, ERs. As expected, our physiological regimen of EB treatment induced the appearance of cornified cells (indicative of estrus) in vaginal cytology samples obtained from OVX rats receiving central infusion of VEH. The fact that this peripheral action of estradiol was identical in rats receiving central infusion of either ICI or VEH suggests that none (or a negligible amount) of the ICI leaked from the brain to the periphery. This is consistent with an earlier report that ventricular, crystalline implants of ICI failed to attenuate estradiol-induced uterine hypertrophy in OVX rats (34). Unlike the results obtained after central ICI treatment, our regimen of peripheral ICI treatment failed to attenuate EB’s ability to reduce food intake in OVX rats, an effect that would have been expected if peripherally administered ICI had gained access to central ERs. Thus, under our experimental conditions, we found no evidence that ICI is capable of crossing the blood-brain barrier.
Although this is consistent with some studies (11,27,28,33,34), it conflicts with other reports that peripherally administered ICI can reach the brain and attenuate certain central actions of estradiol (11,35,36). It is likely that methodological differences across studies account for the discrepant findings. For example, in the single report that ICI was detectable in the brains of OVX rats after three daily sc injections of ICI (35), the daily dose of ICI was at least twice that used in the current study. Similarly, the reports that ICI attenuates estradiol-induced changes in thermoregulatory behaviors and white adipose tissue in OVX rats (11,35), and induces hot flushes in premenopausal women (36), involved chronic application of ICI rather than the more acute dosing regimen used here. Thus, although this and other studies (11,27,28,33,34) suggest that ICI does not cross the blood-brain barrier, it appears as if test conditions do exist that may promote leakage of ICI from the periphery to the brain. Nevertheless, the current study, which is the first to combine both peripheral and central administration of ICI, strengthens earlier reports (11,33) of its potential use in discerning central vs. peripheral actions of estradiol.
This study does not disclose where the critical, central ERs are located. Previously, site-specific administration of dilute estradiol into the nucleus of the solitary tract, Arc, paraventricular nucleus of the hypothalamus, and medial preoptic nucleus was sufficient to decrease food intake in OVX rats in some (4,5,19,20,21), but not all, studies (see Refs. 20,22). In addition, chronic, peripheral estradiol implants increased the number of glutamatergic, excitatory inputs to anorexigenic, proopiomelanocortin neurons (5), further implicating the Arc as a possible central target. Additional research involving site-specific interference of ERs within these and other brain areas could be helpful in determining the brain areas critical for mediating estradiol’s anorexigenic effect. To this end, one group used site-specific administration of an adeno-associated viral vector designed to silence ERα in the ventromedial hypothalamus of OVX mice. These mice were, however, equally responsive to the anorexigenic effect of chronic estradiol treatment as control mice (43). This finding extends previous research demonstrating that lesions of the ventromedial hypothalamus do not disrupt estradiol-induced reductions in food intake in OVX rats (44,45,46). Future studies involving site-specific administration of ICI should prove very useful in determining which brain areas are necessary for estradiol’s anorexigenic effect.
ICI binds to both nuclear ER subtypes, ERα and ERβ, with equal affinity (47). As a result, the present findings do not disclose the relative roles of the centrally located ER subtypes in mediating estradiol’s anorexigenic effect. Previous studies reported decreased feeding in rats after peripheral administration of an ERα-selective, but not an ERβ-selective, agonist (48,49,50). Although these studies suggest a role for ERα, other studies involving disruptions in either ERα or ERβ signaling provide equivocal evidence regarding the necessity of each ER subtype in mediating estradiol’s anorexigenic effect. In one study, ERα knockout (αERKO) mice were not responsive to the anorexigenic effects of estradiol (51). In another study, αERKO mice displayed a limited response to the feeding-inhibitory effects of chronic estradiol treatment (52), suggesting some involvement of ERβ. Caution is warranted, however, in interpreting the results of studies involving null mutations of ERα due to the possibility of developmental compensation and the fact that αERKO mice display a 10-fold increase in estradiol levels that could lead to changes in signaling through ERβ (52). In a study that avoided both of these issues, the anorexigenic effect of estradiol was blocked after ventricular administration of ERβ-targeted, but not ERα-targeted, antisense oligonucleotides in adult OVX rats (53). Taken together, there is little consensus regarding the relative contribution of ERα and ERβ to estradiol’s anorexigenic effect.
In summary, the present findings provide the first demonstration that activation of central ERs is necessary for estradiol’s anorexigenic effect. Additional studies are needed to determine the exact neural substrates, the specific ER subtypes, and the intracellular signaling pathways underlying estradiol’s ability to reduce food intake in the female rat. A greater understanding of the mechanism underlying estradiol’s role in the normal control of food intake has obvious translational importance for the development of effective therapies for the dysregulated ingestive behavior associated with eating disorders and obesity, both of which are more prevalent in women than in men.
Acknowledgments
We thank Dr. David Knight and Dr. Michelina Messina for providing technical instruction related to the central implantation of osmotic minipumps and Gretha Boersma for assisting in the collection of pilot data used to design the current study.
Footnotes
This work was supported by National Institutes of Health Grants DK-073936 and MH-063932 (to L.A.E.) and T32 DC-00044 (to H.M.R.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 3, 2010
Abbreviations: Arc, Arcuate nucleus of the hypothalamus; EB, estradiol benzoate; ER, estrogen receptor; αERKO, ERα knockout; ICI, ICI-182,780; icv, intracerebroventricular; LPL, lipoprotein lipase; OVX, ovariectomized; SERM, selective ER modulator; VEH, vehicle.
References
- Eckel LA 2004 Estradiol: a rhythmic, inhibitory, indirect control of meal size. Physiol Behav 82:35–41 [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L 1999 Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav 67:141–147 [DOI] [PubMed] [Google Scholar]
- Asarian L, Geary N 2002 Cyclic estradiol treatment normalizes body weight and restores physiological patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav 42:461–471 [DOI] [PubMed] [Google Scholar]
- Dagnault A, Richard D 1997 Involvement of the medial preoptic area in the anorectic action of estrogens. Am J Physiol Regul Integr Comp Physiol 272:R311–R317 [DOI] [PubMed] [Google Scholar]
- Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao X-B, Mobbs C, Shulman GI, Diano S, Horvath TL 2007 Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 13:89–94 [DOI] [PubMed] [Google Scholar]
- Gray JM, Dudley SD, Wade GN 1981 In vivo cell nuclear binding of 17β-[3H]estradiol in rat adipose tissues. Am J Physiol Endocrinol Metab 240:E43–E46 [DOI] [PubMed] [Google Scholar]
- Eisenfeld AJ, Aten R, Weinberger M, Haselbacher G, Halpern K, Krakoff L 1976 Estrogen receptor in the mammalian liver. Science 191:862–865 [DOI] [PubMed] [Google Scholar]
- Thomas ML, Xu X, Norfleet AM, Watson CS 1993 The presence of functional estrogen receptors in intestinal epithelial cells. Endocrinology 132:426–430 [DOI] [PubMed] [Google Scholar]
- Pelletier G, Labrie C, Labrie F 2000 Localization of oestrogen receptor α, oestrogen receptor β and androgen receptors in the rat reproductive organs. J Endocrinol 165:359–370 [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I 1997 Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol 388:507–525 [DOI] [PubMed] [Google Scholar]
- Wade GN, Blaustein JD, Gray JM, Meredith JM 1993 ICI 182,780: a pure antiestrogen that affects behaviors and energy balance in rats without acting in the brain. Am J Physiol Regul Integr Comp Physiol 265:R1392–R1398 [DOI] [PubMed] [Google Scholar]
- Ramirez I 1981 Estradiol-induced changes in lipoprotein lipase, eating, and body weight in rats. Am J Physiol Endocrinol Metab 240:E533–E538 [DOI] [PubMed] [Google Scholar]
- Gray JM, Greenwood MR 1982 Time course of effects of ovarian hormones on food intake and metabolism. Am J Physiol Endocrinol Metab 243:E407–E412 [DOI] [PubMed] [Google Scholar]
- Machinal F, Dieudonne M-N, Leneveu M-C, Pecquery R, Giudicelli Y 1999 In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology 140:1567–1574 [DOI] [PubMed] [Google Scholar]
- Hamann A, Matthaei S 1996 Regulation of energy balance by leptin. Exp Clin Endocrinol Diabetes 104:293–300 [DOI] [PubMed] [Google Scholar]
- Chen TS, Doong ML, Chang FY, Lee SD, Wang PS 1995 Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol Gastrointest Liver Physiol 268:G171–G176 [DOI] [PubMed] [Google Scholar]
- Palmer K, Gray JM 1986 Central vs. peripheral effects of estrogen on food intake and lipoprotein lipase activity in ovariectomized rats. Physiol Behav 37:187–189 [DOI] [PubMed] [Google Scholar]
- Wade GN, Zucker I 1970 Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J Comp Physiol Psychol 72:328–336 [DOI] [PubMed] [Google Scholar]
- Thammacharoen S, Lutz TA, Geary N, Asarian L 2008 Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-α-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology 149:1609–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butera PC, Beikirch RJ 1989 Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res 491:266–273 [DOI] [PubMed] [Google Scholar]
- Butera PC, Xiong M, Davis RJ, Platania SP 1996 Central implants of dilute estradiol enhance the satiety effect of CCK-8. Behav Neurosci 110:823–830 [DOI] [PubMed] [Google Scholar]
- Hrupka BJ, Smith GP, Geary N 2002 Hypothalamic implants of dilute estradiol fail to reduce feeding in ovariectomized rats. Physiol Behav 77:233–241 [DOI] [PubMed] [Google Scholar]
- Dey M, Lyttle CR, Pickar JH 2000 Recent insights into the varying activity of estrogens. Maturitas 34:S25–S33 [DOI] [PubMed] [Google Scholar]
- Wade GN, Heller HW 1993 Tamoxifen mimics the effects of estradiol on food intake, body weight, and body composition in rats. Am J Physiol Regul Integr Comp Physiol 264:R1219–R1223 [DOI] [PubMed] [Google Scholar]
- Santollo J, Eckel LA 2009 Effect of a putative ERα antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiol Behav 97:193–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge MA, Kavaliers M, Baird JP, Ossenkopp KP 2009 Tamoxifen and raloxifene produce conditioned taste avoidance in female rats: a microstructural analysis of licking patterns. Life Sci 84:282–289 [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Bowler J 1992 ICI 182,780, a new antioestrogen with clinical potential. J Steroid Biochem Mol Biol 43:173–177 [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J 1991 A potent specific pure antiestrogen with clinical potential. Cancer Res 51:3867–3873 [PubMed] [Google Scholar]
- Chen D, Washbrook E, Sarwar N, Bates GJ, Pace PE, Thirunuvakkarasu V, Taylor J, Epstein RJ, Fuller-Pace FV, Egly J-M, Coombes RC, Ali S 2002 Phosphorylation of human estrogen receptor α at serine 118 by two distinct signal transduction pathways revealed by phosphorylation-specific antisera. Oncogene 21:4921–4931 [DOI] [PubMed] [Google Scholar]
- Htun H, Holth LT, Walker D, Davie JR, Hager GL 1999 Direct visualization of the human estrogen receptor α reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell 10:471–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvois S, White R, Parker MG 1993 The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci 106:1377–1388 [DOI] [PubMed] [Google Scholar]
- Long X, Nephew KP 2006 Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-α. J Biol Chem 281:9607–9615 [DOI] [PubMed] [Google Scholar]
- Wade GN, Powers JB, Blaustein JD, Green DE 1993 ICI 182,780 antagonizes the effects of estradiol on estrous behavior and energy balance in Syrian hamsters. Am J Physiol Regul Integr Comp Physiol 265:R1399–R1403 [DOI] [PubMed] [Google Scholar]
- Steyn FJ, Anderson GM, Grattan DR 2006 Differential effects of centrally-administered oestrogen antagonist ICI-182,780 on oestrogen-sensitive functions in the hypothalamus. J Neuroendocrinol 19:26–33 [DOI] [PubMed] [Google Scholar]
- Alfinito PD, Chen X, Atherton J, Cosmi S, Deecher DC 2008 ICI 182,780 penetrates brain and hypothalamic tissue and has functional effects in the brain after systemic dosing. Endocrinology 149:5219–5226 [DOI] [PubMed] [Google Scholar]
- Young OE, Renshaw L, Macaskill EJ, White S, Faratian D, Thomas JS, Dixon JM 2008 Effects of fulvestrant 750 mg in premenopausal women with oestrogen-receptor-positive primary breast cancer. Eur J Cancer 44:391–399 [DOI] [PubMed] [Google Scholar]
- Rivera HM, Eckel LA 2005 The anorectic effect of fenfluramine is increased by estradiol treatment in ovariectomized rats. Physiol Behav 86:331–337 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1998 The rat brain in stereotaxic coordinates. 4th ed. San Diego, CA: Academic Press [Google Scholar]
- Smith MS, Freeman ME, Neill JD 1975 The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 96:219–226 [DOI] [PubMed] [Google Scholar]
- Eckel LA, Houpt TA, Geary N 2000 Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav 70:397–405 [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E 2005 Strategies and methods for research on sex differences in brain and behavior. Endocrinology 146:1650–1673 [DOI] [PubMed] [Google Scholar]
- Robertson JF, Erikstein B, Osborne KC, Pippen J, Come SE, Parker LM, Gertler S, Harrison MP, Clarke DA 2004 Pharmacokinetic profile of intramuscular fulvestrant in advanced breast cancer. Clin Pharmacokinet 43:529–538 [DOI] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang X-J, Clegg DJ, Kaplitt MG, Ogawa S 2007 Silencing of estrogen receptor α in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA 104:2501–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitz JW, Goy RW, Keesey RE 1977 Effects of gonadectomy on hypothalamic obesity in male and female rats. Int J Obes 1:259–270 [PubMed] [Google Scholar]
- King JM, Cox VC 1973 The effects of estrogens on food intake and body weight following ventromedial hypothalamic lesions. Physiol Psychol 1:261–264 [Google Scholar]
- Thompson ME, Cox VC 1979 The effects of estradiol on body weight and food intake in normal weight VMH-lesioned rats. Physiol Behav 22:627–629 [DOI] [PubMed] [Google Scholar]
- Sun J, Huang YR, Harrington WR, Sheng S, Katzenellenbogen JA, Katzenellenbogen BS 2002 Antagonists selective for estrogen receptor α. Endocrinology 143:941–947 [DOI] [PubMed] [Google Scholar]
- Roesch DM 2006 Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav 87:39–44 [DOI] [PubMed] [Google Scholar]
- Santollo J, Wiley MD, Eckel LA 2007 Acute activation of ERα decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol 293:R2194–R2201 [DOI] [PubMed] [Google Scholar]
- Thammacharoen S, Geary N, Lutz TA, Ogawa S, Asarian L 2009 Divergent effects of estradiol and the estrogen receptor-α agonist PPT on eating and activation of PVN CRH neurons in ovariectomized rats and mice. Brain Res 1268:88–96 [DOI] [PubMed] [Google Scholar]
- Geary N, Asarian L, Korach KS, Pfaff DW, Ogawa S 2001 Deficits in E2-dependent control of feeding, weight gain, and cholecystokinin satiation in ER-α null mice. Endocrinology 142:4751–4757 [DOI] [PubMed] [Google Scholar]
- Naaz A, Zakroczymski M, Heine P, Taylor J, Saunders P, Lubahn D, Cooke PS 2002 Effect of ovariectomy on adipose tissue of mice in the absence of estrogen receptor α (ERα): a potential role for estrogen receptor β (ERβ). Horm Metab Res 34:758–763 [DOI] [PubMed] [Google Scholar]
- Liang Y-Q, Akishita M, Kim S, Ako J, Hashimoto M, Iijima K, Ohike Y, Watanabe T, Sudoh N, Toba K, Yoshizumi M, Ouchi Y 2002 Estrogen receptor β is involved in the anorectic action of estrogen. Int J Obes 26:1103–1109 [DOI] [PubMed] [Google Scholar]