Abstract
We investigated the relationship between mammary gland volume (MGV) of the breast as measured with three-dimensional chest computed tomography (CT) and breast cancer risk. Univariate analysis was used to assess the relationship between MGV and known risk factors in 427 healthy women. A case control study (97 cases and 194 controls) was conducted to assess breast cancer risk. MGV was significantly smaller for postmenopausal women than for premenopausal women, and was significantly larger for women with a family history of breast cancer than for women without. MGV, body mass index (BMI), and rate of family history of breast cancer were significantly higher among breast cancer patients than among healthy women, and number of deliveries was significantly lower among breast cancer patients. In postmenopausal women, age at menarche was significantly younger for breast cancer patients. MGV correlated well with breast cancer risk factors. The highest odds ratio was 4.9 for premenopausal women with the largest MGV. Regardless of menopausal status, the greater the MGV, the higher the odds ratio. Our results constitute the first reliable data on the relationship between MGV and breast cancer obtained through exact volume analysis.
Keywords: mammographic density, mammary gland volume, breast cancer risk factor, breast cancer risk
Introduction
The relative proportion of radio-opaque stroma, including epithelial tissues, to the entire breast as determined by mammogram is known as mammographic density (MD) or mammographic breast density. Many studies have found that MD correlates to breast cancer risk, namely, the greater the MD, the higher the risk.1–4 A significant question that remains unanswered is whether or not MD is a good indicator of the stromal volume of the breast. MD is not an absolute volume but a relative parameter that is affected by changes in fat proportion due to changes in body weight.5 In addition, the reproducibility of MD findings is problematic. Because MD does not represent the natural state of the breast, MD values can vary under different scanning conditions.6 Several studies investigating breast cancer risk have instead used the area of mammographic dense tissue as the measurement of the absolute volume,7 though these two measurements are not actually equivalent.
Since breast cancer arises in the mammary glands, its onset should theoretically depend on the mammary gland volume (MGV) of the breast.8 In this study, we aimed to investigate the relationship between MGV of the breast as measured with three-dimensional chest computed tomography (CT) and breast cancer risk.
Materials and Methods
Subjects
The initial pool from which subjects were drawn consisted of 153 patients who underwent surgery for breast cancer at our university hospital between January 2002 and December 2006. In our hospital, all breast cancer patients routinely undergo preoperative abdomen–chest CT to evaluate metastasis or to screen for other disease. Of these patients, 20 who died or whose current address was unknown and 5 who did not have preoperative CT and mammography data collected at our hospital were excluded. A written explanation of the present study was provided to the remaining 128 patients by mail or during outpatient visits to ask for their participation. Twenty-six patients did not reply and thus, a total of 102 women participated by filling out the above-mentioned self-administered questionnaire (66.7% of the total subject population).
Control subjects were members of a public school teaching union in northeastern Japan. Their cancer screening included complete physical examinations that incorporated breast cancer screening including mammography and lung cancer screening based on chest CT. Eight hundred and ninety-nine women out of 970 women who had undergone cancer screening between September 2006 and February 2007 were control subjects who had undergone both mammography and chest CT. We recruited potential study participants from among these women by distributing a written explanation of the present study. A total of 528 women participated by filling out a self-administered questionnaire. Of the questionnaire respondents, we excluded 54 women whose medical test results suggested breast cancer. We also excluded women with a history of breast cancer and women who had undergone artificial menopause due to premenopausal uterine or ovarian disease; this removed another 47 women from the participant pool. Hence, 427 women participated (47.5% of those who underwent the complete physical examination during the study period).
For the case control study, two age-matched healthy control subjects were randomly selected per patient (age difference: ±2 years). Subsequently, a total of 97 breast cancer patients and 194 healthy women were also enrolled.
The present study protocol was approved by the Local Research Ethics Committee.
Methods
Measurements and analysis of data from control subjects
CT was performed on all subjects by a single radiologist who is skilled at diagnostic imaging and computer-assisted measurement. MGV, including fibrous and vascular components of the breast, was measured using chest CT data obtained during lung cancer screening. CT was performed using an Aquillion16 system (Toshiba Medical Systems Corporation, Tokyo, Japan) with a tube voltage of 120 kV and a current of 30 mA. The area from the supraclavicular fossa to below the diaphragm was imaged through 16-row helical scanning. The pixel size of the resulting images was 0.78 mm × 0.78 mm × 0.5 mm; images were reconstructed with a slice thickness of 5 mm and a slice interval of 5 mm without any gaps. Images were displayed with a field of view (FOV) of 40 cm and a matrix size of 512 × 512. All images were saved as Digital Imaging and Communications in Medicine (DICOM) files. Images were displayed using a 1280 × 1024 monitor, and processed using Image J software.9
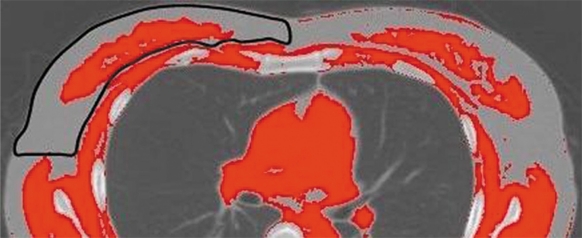
The maximum CT value of a region of interest (ROI) with a diameter of 6 to 20 mm set at the axillary fat in each patient was used as a reference value. The breast was manually identified excluding the skin, and the mammary gland was defined as all areas with CT values higher than the reference CT value (Fig. 1). This process was repeated for the entire breast, and the total number of pixels identified as mammary gland tissue was multiplied by the slice thickness (5 mm) to calculate MGV. Under these scanning conditions, each pixel was equivalent to 0.003 cm3.
Figure 1.
Estimation of mammary gland volume (MGV).
Note: The breast is manually identified on a binary CT image (outlined in black). Within the breast, areas with high CT values are considered to represent mammary gland tissue.
The CT scans of 30 of the 427 subjects were randomly selected. The concordance rate of left and right MGV was investigated to confirm the reliability of measuring MGV through CT.10
Body mass index (BMI) was calculated based on height and body weight measured during examinations. The self-administered questionnaire included items on age at menarche, parity (number of childbirths), age at first live delivery, duration of infant breastfeeding, age at menopause, and family history of breast cancer.
MGV was assessed in relation to family history and menopausal status. Patients were asked to report family history of breast cancer within two generations (grandmother, mother, sister, daughter, and granddaughter). Relationships between MGV and other breast cancer risk factors such as age, BMI, age at menarche and menopause, and reproductive history were investigated in pre- and postmenopausal women separately. Subjects were divided according to age into five-year groups (<45 years, 45–49 years, 50–54 years, 55–59 years, and ≥60 years); according to BMI into four groups (<20 kg/m2, 20–<25 kg/m2, 25–<30 kg/m2, and ≥30 kg/m2); according to age at menarche into four groups (<12 years, 12 years, 13 years, and ≥14 years); according to number of live deliveries; and, in the case of parous women, according to age at first live delivery into three groups (<25 years, 25–29 years, and ≥30 years); according to total duration of breastfeeding (ie, the sum of the durations for which each child was breastfed) into five groups (never breastfed, 0–6 months, 7–12 months, 13–24 months, and ≥25 months); and, finally, according to age at onset of menopause into four groups (<49 years, 49–50 years, 51–52 years, and ≥53 years).
A nonparametric test was conducted to ascertain the relationship between MGV and family history as well as that between MGV and menopausal status. Rank-sum correlation coefficients, ρ, were calculated to evaluate the correlation between MGV and breast cancer risk factors.
Case control study
For breast cancer patients, height and body weight were measured before breast cancer surgery, and the same questionnaire given to control subjects was administered. Preoperative chest CT data gathered for therapeutic planning were used. CT was performed using Asteion (Toshiba Medical Systems Corporation) with a tube voltage of 120 kV; current was set automatically. Images were collected and analyzed using the same protocols that were used for control subjects. MGV was measured on the unaffected side in breast cancer patients, and on the same side in each patient’s age-matched cases. The data from each patient’s two age-matched cases were used as control data.
In both breast cancer patients and controls, MGV, BMI, and other risk factors were examined through univariate analysis in premenopausal and postmenopausal women separately. A nonparametric test was used to assess statistical significance. Conditional logistic regression analysis was performed to examine the relationship between MGV and breast cancer. The subjects were divided into quartiles according to MGV, and the odds ratio for the subgroup with the lowest MGV to the other subgroups was determined (p for trend). In this analysis, factors used as risk factors in this report (BMI, age at menarche, number of deliveries, age at first delivery, breastfeeding duration, family history, and age at menopause in postmenopausal women) were used as adjusted factors.
Statistical analyses were conducted using SPSS software (SPSS Japan Inc., Tokyo, Japan), with the level of significance set at P < 0.05 (two-sided).
Results
Measurements and analysis of data from control subjects
The distribution of breast cancer risk factors in the 427 healthy women is presented in Table 1. The sum correlation coefficient of concordance between left and right MGV as measured through CT was 0.91. The median MGV was 45.9 cm3 (interquartile range: 22.2–78.2 cm3). MGV was significantly smaller in postmenopausal women than in premenopausal women. The median MGV was 60.6 cm3 (interquartile range: 5.4–90.6 cm3) for women with a family history of breast cancer and 42.6 cm3 (19.5–71.6) for women without. MGV was statistically significantly larger for women with a family history of breast cancer than for women without.
Table 1.
Distribution of breast cancer risk factors and MGV (healthy women).
| Factors | Number of subjects n = 427 | Median (range) |
|---|---|---|
| Age (years) | 427 | 50 (34–62) |
| BMI (kg/m2) | 427 | 22.9 (15.2–40.2) |
| Age at menarche (years) | 427 | 12 (10–15) |
| Number of deliveries (live births) | 367 (85.9%) | 2 (1–3) |
| Age at first delivery (years) | 367 (85.9%) | 28 (21–37) |
| Duration of breastfeeding (months) | 341 (80.0%) | 7 (0–60) |
| Positive family historya | 48 (11.2%) | |
| Age at menopause (years) | 163 (38.2%) | 50 (41–56) |
Note:
Family history of breast cancer among first- and second-degree relatives.
The relationships between MGV and breast cancer risk factors in the pre- and postmenopausal groups are listed in Table 2. For both pre- and postmenopausal women, MGV was significantly smaller when the number of live births was high or the duration of breastfeeding was long. For postmenopausal women, MGV was significantly smaller when age at menarche was later or current age was older. For both pre- and postmenopausal women, no significant correlation was observed between BMI and MGV.
Table 2.
Relationships between MGV and breast cancer risk factors (healthy women).
| Factors |
Premenopausal |
Postmenopausal |
||
|---|---|---|---|---|
| Number of subjects | Mammary gland volume (cm3) Median (IQR) | Number of subjects | Mammary gland volume (cm3) Median (IQR) | |
| Overall | 264 | 57.9 (28.1–88.2) | 163 | 33.6** (13.9–51.7) |
| Age (years) | 264 | 163 | ||
| <45 | 86 | 60.7 (28.1–91.3) | 0 | |
| 45–49 | 102 | 55.9 (23.8–85.0) | 3 | 33.6 (13.2–50.9) |
| 50–54 | 71 | 57.3 (33.5–87.1) | 26 | 35.9 (9.9–54.1) |
| 55–59 | 5 | 93.5 (84.7–115.6) | 107 | 37.2 (16.3–63.0) |
| ≥60 | 0 | 27 | 19.1 (9.0–27.0) | |
| ρ | 0.01 | −0.16* | ||
| BMI | 264 | 163 | ||
| <20.0 | 37 | 57.8 (27.6–87.5) | 3 | 41.3 (3.8–96.2) |
| 20.0 to <25.0 | 149 | 57.3 (28.7–88.9) | 98 | 30.2 (13.2–46.6) |
| 25.0 to <30.0 | 74 | 58.5 (28.2–84.0) | 48 | 40.2 (16.3–72.0) |
| ≥30.0 | 4 | 71.6 (60.8–72.3) | 14 | 38.9 (27.6–50.9) |
| ρ | −0.02 | 0.14 | ||
| Age at menarche (years) | 264 | 163 | ||
| <12 | 72 | 49.8 (31.1–87.1) | 34 | 45.7 (33.6–51.7) |
| 12 | 87 | 59.5 (30.7–79.1) | 27 | 37.9 (14.2–45.2) |
| 13 | 45 | 63.6 (19.5–114.2) | 54 | 22.2 (13.8–46.6) |
| ≥14 | 60 | 57.8 (23.8–90.0) | 48 | 30.4 (8.9–66.9) |
| ρ | −0.01 | −0.18* | ||
| Number of deliveries (live births) | 264 | 163 | ||
| 0 | 45 | 72.3 (58.2–99.0) | 15 | 48.3 (33.6–100.3) |
| 1 | 39 | 74.9 (26.6–135.0) | 27 | 30.4 (13.9–60.4) |
| 2 | 102 | 53.5 (23.5–81.0) | 77 | 34.5 (14.2–51.7) |
| 3 | 78 | 47.3 (27.6–78.1) | 44 | 25.2 (5.8–45.2) |
| ρ | −0.25** | −0.19** | ||
| Age at first delivery (years) | 219 | 148 | ||
| <25 | 24 | 54.3 (27.6–74.7) | 22 | 35.9 (14.2–44.6) |
| 25–29 | 119 | 49.4 (26.3–84.4) | 109 | 27.2 (10.6–47.0) |
| ≥30 | 76 | 58.5 (25.5–110.4) | 17 | 51.7 (30.4–75.0) |
| ρ | 0.05 | 0.16 | ||
| Breastfeeding duration (months) | 264 | 163 | ||
| never breastfed | 54 | 74.8 (59.1–99.0) | 32 | 44.6 (19.1–80.6) |
| 0–6 | 96 | 69.0 (39.9–111.6) | 73 | 34.5 (13.9–51.7) |
| 7–12 | 36 | 55.9 (24.6–96.4) | 21 | 34.6 (13.8–53.7) |
| 13–24 | 49 | 29.8 (17.0–51.7) | 15 | 23.8 (9.7–75.0) |
| ≥25 | 29 | 29.9 (22.3–54.2) | 22 | 22.2 (6.7–40.2) |
| ρ | −0.42** | −0.16* | ||
| Age at menopause (years) | 163 | |||
| <49 | 33 | 33.6 (14.2–42.4) | ||
| 49–50 | 55 | 29.4 (13.9–51.6) | ||
| 51–52 | 40 | 30.4 (13.2–56.1) | ||
| ≥53 | 35 | 40.3 (21.1–75.0) | ||
| ρ | 0.15 | |||
Nonparametric test:
P < 0.05;
P < 0.01.
ρ = rank-sum correlation coefficient.
Abbreviation: IQR, interquartile range.
Case control study
The MGV and other breast cancer risk factor data for the 97 breast cancer patients and 194 healthy women are summarized in Table 3. Median MGVs for premenopausal and postmenopausal women were 108.0 and 53.0 cm3, respectively. Regardless of menopausal status, MGV was significantly higher in breast cancer patients than in healthy women. Breast cancer patients, both premenopausal and postmenopausal, had significantly low numbers of deliveries and significantly high BMIs and rates of family history of breast cancer. Postmenopausal breast cancer patients reported significantly younger ages at menarche than their age-matched controls.
Table 3.
MGV and breast cancer risk factors in premenopausal and postmenopausal women.
| Cases Median (range) | Controls Median (range) | ||
|---|---|---|---|
| Premenopausal | Factors | n = 34 | n = 68 |
| Mammary gland volume (cm3) (IQR) | 108.0 (67.0–162.1)** | 68.7 (40.6–110.9) | |
| Age (years) | 49 (34–54) | 49 (34–56) | |
| BMI | 22.7 (16.0–39.8)** | 19.6 (15.2–38.0) | |
| Age at menarche (years) | 12 (11–13) | 12 (10–15) | |
| Number of deliveries (live births) | 1 (0–2)** | 2 (0–3) | |
| Age at first delivery (years) | 27 (23–36) | 28 (22–36) | |
| Breastfeeding duration (months) | 2 (0–12) | 1 (0–60) | |
| Positive family history (%)a§ | 14.7** | 1.5 | |
| Postmenopausal | Factors | n = 63 | n = 126 |
| Mammary gland volume (cm3) (IQR) | 53.0 (30.4–108.0)** | 34.2 (13.9–53.8) | |
| Age (years) | 58 (55–64) | 59 (55–52) | |
| BMI | 24.6 (16.6–40.7)** | 23.0 (19.3–35.6) | |
| Age at menarche (years) | 12 (10–14)** | 13 (10–15) | |
| Number of deliveries (live births) | 2 (0–3)** | 2 (0–3) | |
| Age at first delivery (years) | 25 (23–37) | 26 (23–33) | |
| Breastfeeding duration (months) | 3 (0–24) | 6 (0–40) | |
| Positive family history (%)a§ | 9.5* | 2.4 | |
| Age at menopause (years) | 50 (42–54) | 50 (45–56) |
Nonparametric test:
P < 0.05;
P < 0.01;
§, chi-square test.
Note:
Family history of breast cancer among first- and second-degree relatives.
Abbreviation: IQR, interquartile range.
The results of our conditional logistic regression analysis for the relationship between MGV and breast cancer are presented in Table 4. Compared to the subgroup with the smallest MGV, the highest odds ratio was observed in the subgroup with the largest MGV for both premenopausal and postmenopausal women. The highest odds ratio for premenopausal women was 4.9 (95% confidence interval: 1.2–20.4) for women with MGVs greater than 110.9; that for postmenopausal women was 3.6 (95% confidence interval: 1.4–9.1) for women with MGVs greater than 53.8. Regardless of menopausal status, the greater the MGV, the higher the odds ratio (P for trend among premenopausal women = 0.030, and P for trend among postmenopausal women = 0.007).
Table 4.
Odds ratio of each subgroup in relation to the subgroup with the lowest MGV (case control study).
| Mammary gland volume (cm3) | Cases | Controls | Adjusted odds ratio (95% confidence interval) | Pfor trend | |
|---|---|---|---|---|---|
| Premenopausal | n = 34 | n = 68 | |||
| <40.6 | 3 | 17 | 1 | ||
| 40.6–68.7 | 8 | 17 | 2.4 (0.5–10.7) | ||
| 68.7–110.9 | 9 | 17 | 3.0 (0.7–13.1) | ||
| >110.9 | 14 | 17 | 4.9 (1.2–20.4)** | 0.030 | |
| Postmenopausal | n = 63 | n = 126 | |||
| <13.9 | 8 | 31 | 1 | ||
| 13.9–34.2 | 10 | 32 | 1.1 (0.4–3.2) | ||
| 34.2–53.8 | 17 | 32 | 1.8 (0.7–5.0) | ||
| >53.8 | 28 | 31 | 3.6 (1.4–9.1)** | 0.007 |
Conditional logistic regression analysis:
P < 0.05.
Notes: Postmenopausal adjusted factors: BMI, age at menarche, number of deliveries, age at first delivery, duration of breastfeeding, family history; Postmenopausal adjusted factors: BMI, age at menarche, number of deliveries, age at first delivery, duration of breastfeeding, family history, age at menopause.
Discussion
Breast cancer has been theoretically assumed to occur more often in individuals with a greater volume of mammary gland tissue in the breast. A previous study reported that involution in breast was associated with breast cancer reduction.11 MGV reflects the amount of fibroglandular tissue in the breast. The risk of suffering a malignant transformation should thus be in proportion to the number of cells. The present study investigated the relationship between exact gland volume and breast cancer risk, though our MGV measurement included not only mammary gland tissue but also fibrous and vascular components. Among premenopausal and postmenopausal women alike, breast cancer patients exhibited statistically significant differences from healthy women in terms of MGV, BMI, number of deliveries, and incidence of positive family history of breast cancer. Regardless of menopausal status, the odds ratio increased with increasing MGV. We thus confirmed that high MGV, as measured using our technique for exact volume estimation, is a risk factor for breast cancer.
We estimated the exact gland volume as MGV using chest CT data. The volume of mammary gland tissue can be evaluated more precisely by chest CT than by mammogram because recent advances in CT technology have enabled three-dimensional estimates. Nevertheless, no previous report has studied gland volume using measurements obtained through CT. This may be due to a desire to avoid exposing study subjects to unnecessary radiation. The present study, however, did not require participants to receive any unnecessary radiation exposure because we used CT data that had been obtained previously for another purpose. The rate of concordance between left and right MGV was very high, indicating the reliability of MGV measurements obtained through CT.
Many studies have investigated the relationship between MD and breast cancer, which remains unclear. The most important factors influencing the determination of an individual’s breast cancer risk based on MD are body weight and BMI. Many previous studies have found that BMI is inversely related to MD. Boyd et al found an inverse correlation between absolute density and BMI,5 that is, between absolute density and absolute nondensity areas, indicating that, when breast cancer risk is estimated on the basis of MD, higher BMI is associated with lower estimates. This is in sharp contrast to the fact that higher BMI is associated with higher breast cancer risk; particularly among postmenopausal women, the importance of obesity as a risk factor is well established.12 The present study found no correlation between BMI and MGV in healthy women. High BMI and larger MGV were scored in breast cancer patients. Our results suggest that MGV and fat volume independently influence breast cancer risk, which is not seen in MD with fat density (body weight and BMI). These results make good sense in understanding that MD is an intermediate factor in breast cancer risk. For these reasons, we support Boyd et al’s recommendation that breast cancer risk be assessed on the basis of MGV and fat volume as separate measurements rather than on MD.
We investigated the relationship between MGV and breast cancer risk factors in control subjects. In both premenopausal and postmenopausal women, MGV was correlated with number of deliveries, breastfeeding duration, and family history of breast cancer. In postmenopausal women, it was correlated with age and with age at menarche. Our results were similar in many ways to the results of previous studies assessing breast cancer risk based on MD or mammographic dense tissue.13–15 The relationship between family history of breast cancer and high MD has been clarified many studies; it is known that family history and genetic factors can influence MD.16,17 In the present study, women with a family history of breast cancer had significantly greater MGVs than those without; this suggests that MGV is correlated to a family history of breast cancer. In fact, the rate of positive family history was significantly higher in patients with breast cancer than in healthy women in our case control study. Thus, the genetic factors that determine gland volume might also determine breast cancer risk.
While there have been several reports that have estimated fibroglandular tissue volume in breast MRI images,18,19 we could not find any previous epidemiological reports that investigated the relationship between breast cancer risk and precise three-dimensional “mammary (fibroglandular tissue) volume”. There has only been one epidemiological report based on volumetric breast density through mammography.20 Nevertheless, volumetric breast density is not equal to MGV because of the difference in the state of the breast—whether or not it is compressed. Our method is excellent in that measurements are conducted with the breast in its natural state, not compressed as in mammography. In addition, it is frequently difficult to examine the entire breast using conventional mammography due to partial deficits in dense tissue, particularly among Japanese women who tend to have small breasts. With CT, all areas of the breast can be examined and MGV can be measured. These breast imaging deficits have not previously been discussed in any published European or American studies assessing breast cancer risk.
Limitations of the present study include the small number of breast cancer patients and the fact that our measurements of the volume of nonfat tissue included fibrous and vascular components that are less likely to be related to breast cancer than glandular tissue. Nevertheless, our results constitute the first reliable data on the relationship between mammary gland volume and breast cancer obtained through exact volume analysis. Because it exposes patients to a high dose of radiation, we do not recommend that chest CT be routinely used to assess breast cancer risk.
Acknowledgments
This work was supported by a Grant-in-Aid from the Global COE Program of the Japan Society for the Promotion of Science.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Wolfe JN, Saftlas AF, Salane M. Mammographic parenchymal patterns and quantitative evaluation of mammographic densities: a case-control study. Ajr American Journal of Roentgenology. 1987;148(6):1087–92. doi: 10.2214/ajr.148.6.1087. [DOI] [PubMed] [Google Scholar]
- 2.Saftlas AF, Hoover RN, Brinton LA, et al. Mammographic densities and risk of breast cancer. Cancer. 1991;67(11):2833–8. doi: 10.1002/1097-0142(19910601)67:11<2833::aid-cncr2820671121>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.van Gils CH, Hendriks JH, Holland R, et al. Changes in mammographic breast density and concomitant changes in breast cancer risk. European Journal of Cancer Prevention. 1999;8(6):509–15. doi: 10.1097/00008469-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Boyd NF, Byng JW, Jong RA, et al. Quantitative classification of mammographic densities and breast cancer risk: results from the Canadian National Breast Screening Study. Journal of the National Cancer Institute. 1995;87(9):670–5. doi: 10.1093/jnci/87.9.670. [DOI] [PubMed] [Google Scholar]
- 5.Boyd NF, Lockewood GA, Byng JW, et al. The relationship of anthropometric measures to radiological features of the breast in premenopausal women. British Journal of Cancer. 1998;78(9):1233–8. doi: 10.1038/bjc.1998.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harvey JA, Bovbjerg VE. Quantitative assessment of mammographic breast density: relationship with breast cancer risk. Radiology. 2004;230(1):29–41. doi: 10.1148/radiol.2301020870. [DOI] [PubMed] [Google Scholar]
- 7.Vachon CM, Brandt KR, Ghosh K, et al. Mammographic breast density as a general marker of breast cancer risk. Cancer Epidemiology, Biomarkers and Prevention. 2007;16(1):43–9. doi: 10.1158/1055-9965.EPI-06-0738. [DOI] [PubMed] [Google Scholar]
- 8.Albanes D, Winick M. Are cell number and cell proliferation risk factors for cancer? Journal of the National Cancer Institute. 1988;80(10):772–5. doi: 10.1093/jnci/80.10.772. [DOI] [PubMed] [Google Scholar]
- 9.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with Image J. Biophotonics International. 2004;11(7):36–42. [Google Scholar]
- 10.Byng JW, Boyd NF, Little L, et al. Symmetry of projection in the quantitative analysis of mammographic images. European Journal of Cancer Prevention. 1996;5(5):319–27. doi: 10.1097/00008469-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Milanese TR, Hartmann LC, Sellers TA, et al. Age-related lobular involution and risk of breast cancer. Journal of the National Cancer Institute. 2006;98(22):1600–7. doi: 10.1093/jnci/djj439. [DOI] [PubMed] [Google Scholar]
- 12.Hunter DJ, Willett WC. Diet, body size, and breast cancer. Epidemiologic Reviews. 1993;15(1):110–32. doi: 10.1093/oxfordjournals.epirev.a036096. [DOI] [PubMed] [Google Scholar]
- 13.Haars G, van Noord PA, van Gils CH, Grobbee DE, Peeters PH. Measurements of breast density: no ratio for a ratio. Cancer Epidemiology, Biomarkers and Prevention. 2005;14(11 Pt 1):2634–40. doi: 10.1158/1055-9965.EPI-05-0824. [DOI] [PubMed] [Google Scholar]
- 14.Riza E, Silva IS, de Stavola B, et al. Correlates of high-density mammographic parenchymal patterns by menopausal status in a rural population in Northern Greece. European Journal of Cancer. 2005;41(4):590–600. doi: 10.1016/j.ejca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 15.El-Bastawissi AY, White E, Mandelson MT, Taplin SH. Reproductive and hormonal factors associated with mammographic breast density by age (United States) Cancer Causes and Control. 2000;11(10):955–63. doi: 10.1023/a:1026514032085. [DOI] [PubMed] [Google Scholar]
- 16.Ziv E, Shepherd J, Smith-Bindman R, Kerlikowske K. Mammographic breast density and family history of breast cancer. Journal of the National Cancer Institute. 2003;95(7):556–8. doi: 10.1093/jnci/95.7.556. [DOI] [PubMed] [Google Scholar]
- 17.Pankow JS, Vachon CM, Kuni CC, et al. Genetic analysis of mammographic breast density in adult women: evidence of a gene effect. Journal of the National Cancer Institute. 1997;89(8):549–56. doi: 10.1093/jnci/89.8.549. [DOI] [PubMed] [Google Scholar]
- 18.Lee NA, Rusinek H, Weinreb J, et al. Fatty and fibroglandular tissue volumes in the breasts of women 20–83 years old: comparison of X-ray mammography and computer-assisted MR imaging. Ajr American Journal of Roentgenology. 1997;168(2):501–6. doi: 10.2214/ajr.168.2.9016235. [DOI] [PubMed] [Google Scholar]
- 19.Wei J, Chan HP, Helvie MA, et al. Correlation between mammographic density and volumetric fibroglandular tissue estimated on breast MR images. Medical Physics. 2004;31(4):933–42. doi: 10.1118/1.1668512. [DOI] [PubMed] [Google Scholar]
- 20.Jeffreys M, Warren R, Highnam R, Smith GD. Breast cancer risk factors and a novel measure of volumetric breast density: cross-sectional study. British Journal of Cancer. 2008;98(1):210–6. doi: 10.1038/sj.bjc.6604122. [DOI] [PMC free article] [PubMed] [Google Scholar]