Abstract
The expression of basal cytokeratin markers CK5/6 in breast carcinomas has been associated with high histological grade and poor clinical outcome. A previous study has shown that CK5/6 can be detected in up to 17% of invasive lobular carcinomas (ILC). Here we study the expression of three basal cytokeratin markers (CK5/6, CK14, and CK17) in 53 ILC cases diagnosed by histology and lack of E-cadherin expression. Among them, 42 were classic lobular carcinomas, 6 were tubular-lobular carcinoma, and 5 were pleomorphic lobular carcinomas. There was no significant difference among these three groups in patients’ age, tumor size, uni- and multi-focality, expression of ER and PR, lymphovascular invasion, perineural invasion and lymph node metastasis. The only statistically different factor was HER2 over-expression, which was observed only in pleomorphic ILC (P = 0.0073). None of the 53 cases expressed CK5/6, CK14 or CK17; and 51/53 cases expressed luminal markers CK8 and CK18, and the two negative cases were both classic lobular carcinoma, with positivity for ER and PR. In conclusion, all 53 cases of ILC failed to show expression by any of the three basal CK markers, suggesting that very few ILC will demonstrate a basal phenotype when assessed by immunohistochemistry (IHC). More studies are needed to investigate molecular classification in lobular carcinoma of the breast.
Keywords: lobular carcinoma of the breast, CK5, CK14 and CK17
Introduction
Infiltrating lobular carcinoma (ILC) is the second most common histologic type of breast cancer and comprises 5%–15% of newly diagnosed invasive tumors.1,2 Its incidence has been increasing over the last 20 years, mainly in women over 50 years of age. Recent studies showed that ILC carried distinct biologic and prognostic factors when compared with infiltrating ductal carcinoma (IDC). With a large database of over 50,000 patients and a median follow-up of 87 months, Arpino et al2 found that ILC is significantly more likely to occur in older patients, more likely to be larger in size, and to be ER and PR positive and HER2 negative when compared to IDC. Contralateral involvement is more common with ILC; however, the 5 year disease-free survival and overall survival are comparable with IDC. More recently, a multi-institutional study with over 2000 patients and a median follow-up of 13 years has confirmed the above findings, and pointed out that ILC had a significantly better (P < 0.01) disease-free survival and overall survival early in the clinical course compared with IDC. However, this better prognosis was time dependent with a significant trend toward late recurrence with ILC compared to IDC (P < 0.01).3 Rakha et al4 showed that ILC has an indolent but progressive clinical course with nearly linear survival curves which cross those of IDC after approximately 10 years of follow up, thus eventually exhibiting a worse long-term outcome. Interestingly, Viale et al recently5 reported a single institution study with matched “classic” ILC and IDC for year of surgery, age, menopausal status, tumor size, nodal involvement, hormone receptor status and histological grade. In this study, there was no difference between these two groups in disease-free or over-all survival, or in locoregional relapse to time of distant metastasis. A study with over 500 cases by Orvieto et al6 have shown that tumor size, lymph node metastasis and hormone status are the most significant prognostic markers for ILC; and “classical” ILC was associated with lower axillary node metastasis and breast-related events, and better disease-free survival and overall survival compared to its variants including alveolar, solid, pleomorphic subtypes, etc. ILC cases show a distinct pattern for metastatic dissemination to peculiar anatomic sites, such as the gastrointestinal tract and serosal surface.2 ILC is associated with an increased incidence of bone metastasis but a decrease in regional and lung metastasis.3 ILC patients show a better response to adjuvant hormonal therapy with improvement in survival when compared with matched patients having IDC,4 but they are less likely to have a complete pathologic response to neoadjuvant chemotherapy.7,8
The hallmark of the molecular features of lobular carcinoma is the loss or down-regulation of E-cadherin compared to ductal lesions.9–11 E-cadherin is a calcium-dependent transmembrane protein that plays a functional role in cellular adhesion and binds to the actin cytoskeleton through interactions with β and α- catenin.12–14 Genetic studies have demonstrated that ILC and IDC will show distinctive molecular features,15,16 with different levels of expression of many genes involved in cell adhesion, motility, apoptosis, protein folding, extracellular matrix and protein phosphorylation.17 Weigelt B et al18 recently showed that 5.8% of the transcriptionally regulated genes are significantly differentially expressed in ILC compared to grade- and molecular subtype-matched IDC; while only 0.1% of genes show differential expression between classic ILC and pleomorphic ILC, supporting again that ILC and IDC are genetically distinct entities.
Recent studies on molecular classification of the breast carcinomas have shown that basal subtype has a worse prognosis when compared to luminal subtype, and one of the IHC markers for basal subtype is CK5/6. One previous study has shown that the basal marker CK5/6 can be detected in up to 17% of ILC.19 Here we study the expression of three basal cytokeratin markers in 53 cases of histologically and E-cadherin confirmed ILC.
Methods
Fifty-three cases of ILC between 2000 and 2005 were identified from the files of the Department of Pathology and confirmed by two pathologists (NK, PT). The expression of E-cadherin was also analyzed by immunohistochemistry (IHC) and showed that none of the cases in this study expressed it, including both the tubular and lobular component of the tubular-lobular carcinoma. Clinical and pathological information including the patients’ age, tumor size, multifocality, ER, PR and HER2 status, lymphovascular invasion, perineural invasion, and status of lymph nodes were reviewed and recorded. One representative section from each case was also stained with antibodies to basal markers CK5/6 (clone D5/16B4, Dako), CK14 (clone LL002 Noracastra) and CK17 (clone E3, Dako), and luminal markers CK8 (clone 35bH11, Dako) and CK18 (clone DC10, Dako). ER (clone ID5, Dako) and PR (clone PgR636, Dako) were scored using the Allred scoring system with less than or equal to 2 as negative, and a score of 3 or greater as positive.20 HER2 (Herceptest, Dako) was scored according to the new CAP/ASCO guidelines.21 CK5/6, CK14, CK17, CK8 and CK18 were scored as positive with any strong cytoplasmic/membrane staining. An antibody panel for breast cancer classification based on IHC analysis of four markers described by Nelson et al22 was used in this study. Briefly, Liminal A subtype was defined as ER and PR positive, HER2 negative, CK5 and EGFR positive or negative; Luminal B subtype as ER and PR positive, HER2 positive, CK5 and EGFR positive or negative; HER2 over-expression subtype as ER and PR negative, HER2 positive, CK5 and EGFR positive or negative; and Basal subtype as ER and PR negative, HER2 negative, CK5 and/or EGFR positive.
For the statistical analysis, the means of age and size of tumors of specific types of ILC were given, and the differences between types of ILC were tested by using t-test. Software SAS 9.1.3 was used to perform Fisher’s exact test to detect the difference between classic ILC, tubular-lobular carcinoma and pleomorphic ILC.
Results
Among the 53 cases of ILC, 42 were classic lobular carcinomas, 6 were tubular-lobular carcinomas, and 5 were pleomorphic lobular carcinomas. There was no significant difference among these three groups in patients’ age, tumor size, uni- or multifocality, expression of ER and PR, lymphovascular invasion, perineural invasion and lymph node metastasis. The only statistically different factor was HER2 over-expression, which was only observed in the pleomorphic invasive lobular carcinomas (P = 0.0073) (Table 1; Figure 1). None of the 53 cases was positive for basal cytokeratin markers CK5/6, CK14 or CK17. All but two cases expressed CK8 and CK18, and the two negative cases were both classic lobular carcinomas and positive for both ER and PR (Table 2). Interestingly, both luminal cytokeratin markers (CK8 and CK18) were uniformly positive or negative in every case. Using the antibody panel for breast cancer classification, ILC in our study could be classified as Luminal A subtype for 100% of the classic lobular and tubular-lobular carcinomas and 60% of the pleomorphic lobular carcinomas; the other 40% were classified as Luminal B subtype due to the over-expression of HER2 (Table 3).
Table 1.
Clinical and Pathological features in each histologic subtype of invasive lobular carcinomas.
|
Types of ILC |
Pvalue | |||
|---|---|---|---|---|
| Classic ILC | Tubular-lobular | PLC | ||
| Case number | 42 | 6 | 5 | |
| Clinical-pathological | ||||
| Age (mean, years) | 58.4 | 58.8 | 55 | 0.8386 |
| Size (cm) | 2.56 | 3.43 | 7.8 | 0.8067 |
| Multifocal | 6 (14%) | 2 (33%) | 1 (20%) | 0.3325 |
| ER+ | 41 (98%) | 6 (100%) | 5 (100%) | 1.0000 |
| PR+ | 35 (83%) | 5 (83%) | 5 (100%) | 1.0000 |
| HER2+ | 0 (0%) | 0 (0%) | 2 (40%) | 0.0073 |
| LVI | 7 (17%) | 2 (33%) | 1 (20%) | 0.5669 |
| PNI | 2 (5%) | 1 (17%) | 0 (0%) | 0.5099 |
| LN | 9/33 (27%) | 2/6 (33%) | 3/4 (75%) | 0.2333 |
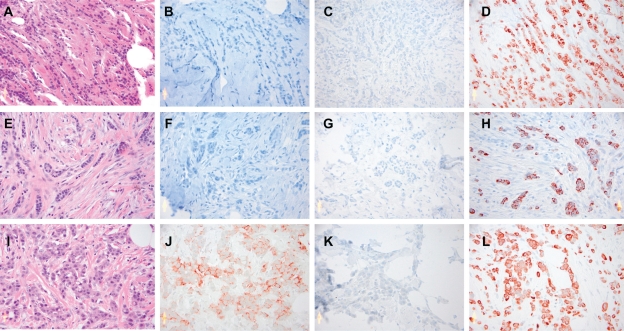
Figure 1.
Examples of the staining patterns of each subtype of ILC. A–D) Classic ILC for HE, HER2, CK5/6 and CK8; E–H) Tubular-lobular ILC for HE, HER2, CK5/6 and CK8; I–L) Pleomorphic ILC for HE, HER2, CK5/6 and CK8.
Table 2.
Expression of CK5, CK8, CK18, CK14 and CK17 in each histologic subtype of invasive lobular carcinomas.
|
Types of ILC |
Pvalue | |||
|---|---|---|---|---|
| Classic ILC | Tubular-lobular | PLC | ||
| Case number | 42 | 6 | 5 | |
| CK expression | 1.0000 | |||
| CK5 | 0% | 0% | 0% | |
| CK8 | 95% | 100% | 100% | |
| CK18 | 95% | 100% | 100% | |
| CK14 | 0% | 0% | 0% | |
| CK17 | 0% | 0% | 0% | |
Table 3.
Molecular classification for each histologic subtype of invasive lobular carcinomas.
|
Types of ILC |
|||
|---|---|---|---|
| Classic ILC | Tubular-lobular | PLC | |
| Case number | 42 | 6 | 5 |
| Luminal A | 100% | 100% | 60% |
| Luminal B | 0% | 0% | 40% |
| HER2 | 0% | 0% | 0% |
| Basal | 0% | 0% | 0% |
Discussion
Invasive ‘breast cancer’ represents a heterogeneous group of distinct entities that vary widely in terms of their morphologic spectrum, tumor biology, clinical presentation and behavior. Roughly 25% of invasive breast tumors can be recognized as ‘special histologic types’ based on distinctive cytologic features and growth patterns. While these ‘special type’ tumors have demonstrated considerable prognostic significance in clinical studies, little attention has been paid to the molecular genetic basis for these histologic entities in recent attempts at molecular classification of breast cancer, which have been derived primarily from the study of invasive ductal carcinoma of no specific type (IDC NOS). The integration of the histologic special types of breast cancer into current molecular classification schemes may have important prognostic and predictive implications for clinical management. It is also unclear at present whether prognostic gene sets, including the 70-gene prognosis profile23 and 21-gene recurrence score24 have similar prognostic power when applied to the special types of breast cancer. Weigelt et al18,25 demonstrated that classic ILC and tubular carcinomas showed similarities at the level of gene expression and immunohistochemical profiles, falling into a luminal subtype, with low levels of e-cadherin expression distinguishing ILC. Such tumors would be expected to demonstrate expression of ER and to have a more indolent clinical course of disease.
Initial evidence for molecular subtypes of breast carcinomas came from a cDNA-microarray study of gene expression, which divided tumors into basal-like, luminal A, luminal B, HER2 over-expression, and normal breast-like subgroups, each with distinct clinical outcomes.26–28 In an effort to develop a similar classification that is clinically significant, technically simple, reproducible and readily available, several IHC-based molecular classifications for breast cancer have been investigated extensively. These include: 1) Cytokeratin-based classification divides breast carcinomas into basal subtype (CK5/6, CK14, CK17 positive), and luminal subtype (CK8, CK18 positive and basal negative);29–33 2) ER, PR and HER2-based classification defines the basal subtype as an absence of expression of ER, PR and HER2;34–38 3) ER, HER2, EGFR and CK5/6-based classification22,39 defines the basal subtype as ER and HER2 negative, and CK5/6 and/or EGFR positive, with 76% sensitivity and 100% specificity, respectively, compared to basal subtype defined by gene expression profiling. Although these IHC-based molecular classifications all show basal subtype has the worse prognosis, they are not interchangeable.40 In addition to various definitions with similar terminology for molecular classification, other limitations for IHC-based molecular classifications include differences in patient cohorts, tumor grades, antibody methodology, and definition of positive staining for each marker.
Fadare et al19 used one basal marker (CK5/6) to study 82 cases of invasive lobular carcinoma, and observed that 17% of the cases expressed CK5/6. In contrast, we did not identify any expression in any of our cases by any of the three basal cytokeratin markers (CK5/6, CK14 and CK17). In Fadare et al’s study, CK5/6 was considered as immunologically reactive if there was cytoplasmic staining unequivocally above the background, similar to our definition of positive staining for any CK marker. 8/14 of their cases showed strong diffuse and intense stain, while the other 6 remaining cases showed patchy and intense stain. Our study used the same monoclonal antibody for CK5/6 as was used by Fadare et al purchased from the same vendor. Our experience of IHC analysis on CK5/6, CK14 and CK17 positive cases of IDC has been that most cases present with strong patchy stains while only a few cases present with strong and diffuse stain. The reason for the different observation could be due to the number of pre-analytical variables between our two laboratories. Another less likely possibility would be due to differences in the patient population included in these two studies.
About 20 years ago, Eusebi et al41 showed that PLC is a more aggressive tumor with apocrine differentiation. Since then many studies have focused on this more aggressive subtype of ILC. Buchanan et al42 have found that pleomorphic lobular carcinomas are larger tumors, have more positive nodes, more frequently develop metastatic disease, and more often require mastectomies. By gene expression profiling, classic ILC falls into the luminal subtype,25 while PLC may be of luminal, HER2 or molecular apocrine subtype by expression profiling, although PLC seems to share a common molecular genetic pathway with classic lobular carcinomas.43–45 Although histologic subtype was not- mentioned in the report by Fadare et al19 they did mention the correlation between CK5/6 expression and ER negativity, high histologic grade, and high mitotic index, suggesting some of their cases were likely to be pleomorphic ILC. There are 5 pleomorphic lobular carcinomas in our study, and none of them expressed any of the three basal CK markers. Pleomorphic ILC consists of 9.4% of all lobular carcinomas in our study, comparable with a rate of 10.8% observed by Buchanan et al42 in a much larger study; 2 of them (40%) over-express HER2, compatible with a prior study by Frolit et al46 who found 53% of PLC over-expressed HER2.
In summary, although one prior study suggested that a significant portion of invasive lobular carcinomas express basal cytokeratin markers,19 our study with three commonly used basal cytokeratin markers failed to confirm their findings. It is very possible that we have not studied enough cases to make a conclusion, but it is unlikely that the level of basal CK marker expression would reach 17%. More studies are needed to investigate the molecular classification in lobular lesions.
Footnotes
Disclosures
This manuscript has been read and approved by all authors. This paper is unique and is not under consideration by any other publication and has not been published elsewhere. The authors and peer reviewers of this paper report no conflicts of interest. The authors confirm that they have permission to reproduce any copyrighted material.
References
- 1.Martinez V, Azzopardi JG. Invasive lobular carcinoma of the breast: incidence and variants. Histopathology. 1979;3:467–88. doi: 10.1111/j.1365-2559.1979.tb03029.x. [DOI] [PubMed] [Google Scholar]
- 2.Arpino G, Bardou VJ, Clark GM, et al. Infiltrating lobular carinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;4:R149–56. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pestalozz1 BC, Zahrieh D, Mallon E, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 international breast cancer study group clinical trails. J Clin Oncol. 2008;26:3006–14. doi: 10.1200/JCO.2007.14.9336. [DOI] [PubMed] [Google Scholar]
- 4.Rakha EA, El-Sayed ME, Powe DG, et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcome. Eur J Cancer. 2008;44:73–83. doi: 10.1016/j.ejca.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 5.Viale G, Rotmensz N, Maisonneuve P, et al. Lack of prognostic significance of “classic” lobular breast carcinoma: a matched, single institution series. Breast Cancer Res Treat. 2009;117:211–4. doi: 10.1007/s10549-008-0112-4. [DOI] [PubMed] [Google Scholar]
- 6.Orvieta E, Maiorano E, Bottiglieri L, et al. Clinicopathologic characteristics of invasive lobular carcinoma of the breast. Cancer. 2008;113:1511–20. doi: 10.1002/cncr.23811. [DOI] [PubMed] [Google Scholar]
- 7.Cristofanilli M, Gonzalez-Angulo A, Sneige N, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23:41–8. doi: 10.1200/JCO.2005.03.111. [DOI] [PubMed] [Google Scholar]
- 8.Tubiana-Hulin M, Stevens D, Lasry S, et al. Response to neoadjuvant chemotherapy in lobular and ductal carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17:1228–33. doi: 10.1093/annonc/mdl114. [DOI] [PubMed] [Google Scholar]
- 9.Moll R, Mitze M, Frixen UH, Birchmeier W. Differential loss of E-cadherin expression in infiltrating ductal and lobular breast carcinomas. Am J Pathol. 1993;143:1731–42. [PMC free article] [PubMed] [Google Scholar]
- 10.Berx G, Cleton-Jansen AM, Strumane K, et al. E-cadherin is inactivated in majority of invasive human lobular breast cancers by truncation mutations throughout its extracellular domain. Oncogene. 1996;13:1919–25. [PubMed] [Google Scholar]
- 11.Leuw WJF, Berx G, Vos CBJ, et al. Simultaneous loss of E-cadherin and catenins in invasive lobular breast cancer and lobular carcinoma in situ. J Pathol. 1997;183:404–11. doi: 10.1002/(SICI)1096-9896(199712)183:4<404::AID-PATH1148>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Berx G, van Roy F. The –E-cadherin/catenin complex: an important gatekeeper in breast cancer tumorigenesis and malignant progression. Breast Cancer Res. 2001;3:289–93. doi: 10.1186/bcr309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baranwal S, Alahari SK. Molecular mechanism controlling E-cadherin expression in breast cancer. Biochem Biophy Res Commun. 2009;384:6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakha EA, Ellis IO. Lobular breast carcinoma and its variants. Semin Diagn Pathol. 2010;27:49–41. doi: 10.1053/j.semdp.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 15.Korkola JE, DeVries S, Firdlyand J, et al. Differentiation of lobular versus ductal breast carcinomas by expression microarray analysis. Cancer Res. 2003;63:7167–75. [PubMed] [Google Scholar]
- 16.Zhao H, Langerod A, Ji Y, et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15:2523–36. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertucci E, Orsetti B, Negre V, et al. Lobular and ductal carcinomas of the breast have distinct genomic and expression profiles. Oncogene. 2008;27:5359–72. doi: 10.1038/onc.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weigelt B, Geyer FC, Natrajan R, et al. The molecular underpinning of lobular histological growth pattern: a genome-wide transcriptomic analysis of invasive lobular carcinomas and grade- and molecular subtype-matched invasive ductal carcinomas of no special type. J Pathol. 2010;220:45–75. doi: 10.1002/path.2629. [DOI] [PubMed] [Google Scholar]
- 19.Fadare O, Wang SA, Hileeto D. The expression of cytokeratin 5/6 in lobular carcinoma of the breast: evidence of a basal-like subset? Hum Pathol. 2008;39:331–6. doi: 10.1016/j.humpath.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Harvey JM, Clark GM, Osborne K, et al. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Schartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 22.Nielsen TO, Hsu FD, Jensen K, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–74. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 23.van’t Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–6. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 24.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–26. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 25.Weigelt B, Horlings HM, Kreike B, et al. Refinement of breast cancer classification by molecular characterization of histological special types. J Pathol. 2008;216:141–50. doi: 10.1002/path.2407. [DOI] [PubMed] [Google Scholar]
- 26.Perou CM, Sorlie T, Eisen MB, et al. Molecular portrait of human breast tumours. Nature. 2000;406:747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 27.Sortie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boecker W, Moll R, Poremba C, et al. Common adult stem cells in the human breast give rise to glandular and myoepithelial cell lineages: A new cell biological concept. Lab Invest. 2002;82:737–45. doi: 10.1097/01.lab.0000017371.72714.c5. [DOI] [PubMed] [Google Scholar]
- 30.Korsching E, Packeisen J, Agelopoulos K, et al. Cytogenetic alterations and cytokeratin expression patterns in breast cancer: integrating a new model of breast differentiation into cytogenetic pathways of breast carcinogenesis. Lab Invest. 2002;82:1525–33. doi: 10.1097/01.lab.0000038508.86221.b3. [DOI] [PubMed] [Google Scholar]
- 31.Fulford LG, Easton DF, Reis-Filho JS, et al. Specific morphological features predictive for the basal phenotype in grade 3 invasive ductal carcinoma of breast. Histopathol. 2006;49:22–34. doi: 10.1111/j.1365-2559.2006.02453.x. [DOI] [PubMed] [Google Scholar]
- 32.Tang P, Wang X, Schiffhauer L, et al. Relationship between nuclear grade of ductal carcinoma in situ and cell origin markers. Ann Clin Lab Sci. 2006;36:16–22. [PubMed] [Google Scholar]
- 33.Steinman S, Wang J, Bourne P, Yang Q, Tang P. Expression of cytokeratin markers, ER-alpha, PR, Her-2/neu, and EGFR in pure ductal carcinoma in situ (DCIS) and DCIS with co-existing invasive ductal carcinoma (IDC) of the breast. Ann Clin Lab Sci. 2007;37:127–34. [PubMed] [Google Scholar]
- 34.Rakha EA, El-Sayed ME, Green AR, et al. Prognostic markers in triple negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 35.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype. A population-based study from the California Cancer Registry. Cancer. 2007;109:1721–8. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 36.Dent R, Trudeau M, Pritchard KI, et al. Triple negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 37.Cleator S, Heller W, Coombes RC. Triple-negative breast cancer, therapeutic options. Lancet Oncol. 2007;8:235–44. doi: 10.1016/S1470-2045(07)70074-8. [DOI] [PubMed] [Google Scholar]
- 38.Carey LA, Dees EC, Sawyer L, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–34. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 39.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA. 2006;295:2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 40.Tang P, Wang J, Bourne P. Molecular classifications of breast carcinoma with similar terminology and different definitions, are they the same? Human Pathol. 2008;39:506–13. doi: 10.1016/j.humpath.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Eusebi V, Magalhaes F, Azzopardi JD. Pleomorphic lobular carcinoma of the breast: an aggressive tumor showing apocrine differentiation. Hum Pathol. 1992;23:655–62. doi: 10.1016/0046-8177(92)90321-s. [DOI] [PubMed] [Google Scholar]
- 42.Buchanan CL, Flynn LW, Murray MP, et al. Is pleomorphic lobular carcinoma really a distinct clinical entity? J Surg Oncol. 2008;98:314–7. doi: 10.1002/jso.21121. [DOI] [PubMed] [Google Scholar]
- 43.Reis-Filho JS, Simpson P, Jones C, et al. Pleomorphic lobular carcinoma of the breast: role of comprehensive molecular pathology in characterization of an entity. J Pathol. 2005;207:1–13. doi: 10.1002/path.1806. [DOI] [PubMed] [Google Scholar]
- 44.Simpson PT, Reis-Filho JS, Lambros MBK, et al. Molecular profiling pleomorphic lobular carcinomas of the breast: evidence for a common molecular genetic pathway with classic lobular carciniomas. J Pathol. 2008;215:231–44. doi: 10.1002/path.2358. [DOI] [PubMed] [Google Scholar]
- 45.Vargas AC, Lakhhani SR, Simpson PT. Pleomorphic lobular carcinoma of the breast: molecular pathology and clinical impact. Future Oncol. 2009;5:233–43. doi: 10.2217/14796694.5.2.233. [DOI] [PubMed] [Google Scholar]
- 46.Frolik D, Caduff R, Varga Z. Pleomorphic lobular carcinoma of the breast: its cell kinetics, expression of oncogenes and tumour suppressor gene compared with invasive ductal carcinomas and classical infiltrating lobular carcinomas. Histopathology. 2001;39:503–13. doi: 10.1046/j.1365-2559.2001.01252.x. [DOI] [PubMed] [Google Scholar]