Abstract
Female crickets, Gryllus bimaculatus, are attracted by the male calling song and approach singing males; a behaviour known as phonotaxis. Even tethered females walking on a trackball steer towards a computer-generated male song presented from their left or right side. High-speed video analysis showed how this auditory-evoked steering was integrated with walking. Typically all the front and middle legs showed kinematic adjustments during steering, with the trajectories tilted towards the side of acoustic stimulation. Furthermore, the average speed of the tarsi contralateral to song increased relative to the ipsilateral tarsi. Kinematic changes of the hind legs were small and may be a consequence of the front and middle leg adjustments. Although phonotactic steering generally led to stereotyped adjustments there were differences in the specific combination of kinematic changes in leg trajectories. The most reliable kinematic steering response was by the contralateral front leg, such that, during its swing phase the tarsus moved towards the side of acoustic stimulation through an increased forward rotation of the femur and an increased extension of the tibia. Relating the changes in tarsal positioning of each leg to the steering velocity of the animal indicated that typically the front and middle legs contralateral to song generated the turning forces. Phonotactic steering was integrated into forward walking without changes to the walking motor cycle.
Keywords: cricket, locomotion, phonotactic steering, leg kinematics, high-speed video, trackball
INTRODUCTION
Important insight into the different steering strategies of animals has been obtained through studying the kinematics of insect walking and turning. This has been beneficial for understanding underlying neural mechanisms and for the design of biomimetic walking robots and their controllers (Pearson and Franklin, 1984; Delcomyn, 2004). Insects walk with an approximate alternating tripod gait in which a set of three legs move at any time; a front leg and hind leg on one side and a middle leg on the other (Cruse, 1976; Cruse, 1979; Matsuura et al., 2002; Delcomyn, 2004). Stepping cycles are separated into swing and stance phases. During the swing phase a leg moves from the posterior extreme position (PEP) to the anterior extreme position (AEP), which defines the starting point for that leg's stance movement. During stance the leg stays on the ground, forces are generated by muscles moving the leg from AEP to PEP, which pushes the animal forward, and can also turn the body.
Behaviour and neural circuits in crickets have been used to design robots for auditory-evoked orientation (Webb, 2006) and to compare the performance of these robots with the cricket's natural behaviour (Reeve and Webb, 2002). Recent research in control circuits, inspired by insect locomotion, aims to design walking robots able to adaptively generate different gaits and to coordinate walking with orienting behaviour based on sensory inputs (Steingrube et al., 2010). However, how sensory inputs can evoke steering that is integrated with ongoing walking is not yet well understood in insects. Studies of insect turning propose a number of possibilities for how steering is executed during walking. Asymmetries between the movements of the outer and inner legs of the turn must occur if the animal is to change walking direction. Different forms of asymmetric walking have been observed: stick insects increase their stepping frequency or step length on one side of the thorax relative to the other (Graham, 1972). Video recordings of walking stick insects performing visually induced turning (Dürr and Ebeling, 2005; Rosano and Webb, 2007) and of cockroaches with tactile-induced turning (Mu and Ritzmann, 2005) have all demonstrated the dominant role of the front legs in shaping walking direction.
Here we analysed the kinematics of the auditory-induced steering in female Gryllus bimaculatus, which walk towards singing males, attracted by their calling song; a behaviour known as phonotaxis (Murphey and Zaretsky, 1972; Weber et al., 1981; Pollack, 2000). Little is known about how the auditory steering responses are generated and integrated into the cricket's walking activity. In recent studies, tethered G. bimaculatus were tested when walking on a highly-sensitive trackball. Fast steering responses towards the song became evident (Hedwig and Poulet, 2004; Hedwig and Poulet, 2005). Rapid changes in front leg movements upon changes in song direction were demonstrated (Baden and Hedwig, 2008) but in those experiments only a single front tibia position could be measured.
In order to understand the phonotactic kinematic adjustments we used high-speed video to record tethered crickets walking and phonotactically steering on the trackball system. The high resolution of the video images captured the cricket's phonotactic motor response from the dorsal side, revealing the simultaneous adjustments in leg, body and antennae. From these images we extracted the tarsal trajectories of all legs in combination with the animal's forward and lateral velocity as obtained by the trackball system. In addition, we analysed how changes in tarsal trajectories were caused by altered leg and body angles to make predictions about the muscles involved in phonotactic steering.
MATERIALS AND METHODS
Animals
Female crickets, Gryllus bimaculatus (de Geer), from the colony in the Department of Zoology, University of Cambridge, were isolated as last instars and raised individually at 28°C with access to food and water.
Trackball and speakers
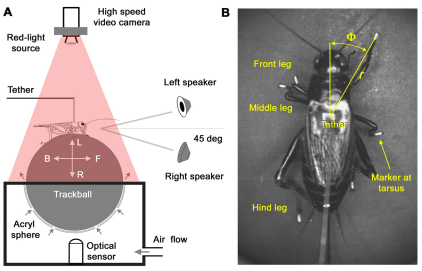
Crickets were tethered to a metal pin at the third thoracic tergite. They walked on a trackball under open-loop conditions while their body position and orientation remained the same. Trackball movements were monitored by an optical sensor and revealed the animals' forward–backward and left–right velocity components during walking. We refer to the left–right component as the animal's lateral or steering velocity. For data analysis we defined leftward steering as positive and rightward steering as negative. The speakers were positioned frontally at 45 deg to the left and right of the animal's length axis at a distance of 57 cm (Fig. 1A). The trackball system and speakers for acoustic stimulation were located inside a sound-proof chamber (Hedwig and Poulet, 2005).
Fig. 1.
(A) A tethered cricket walking on a trackball floating in an air stream. The forward–backward (F–B) and leftward–rightward (L–R) rotations of the trackball were recorded with an optical sensor. The song was presented from either of two speakers placed 45 deg to the left and right of the animal's length axis. A high-speed video camera recorded the cricket's walking movements from above. (B) Markers on the tarsi, head and abdomen were used to measure the cricket's movements. Tarsal trajectories were described by the tarsal distance (r) and tarsal angle (Φ).
Song presentation
To simulate the male calling song, six sound pulses of 21 ms duration and 21 ms pulse interval and a carrier frequency of 4.8 kHz were combined to form a chirp. Chirp duration was 252 ms and the chirp period was 500 ms (Thorson et al., 1982). The song was presented to the cricket from either the left speaker (song-left) or from the right speaker (song-right). A leg was described as ipsilateral when the song occurred from the same side as the leg considered, and contralateral when the song was presented from the opposite side. A control condition (no-song) allowed comparison of non-phonotactic and phonotactic walking.
High-speed video recordings of stepping cycles
For video recordings the tarsi of all legs were marked with droplets of white titanium (IV) dioxide (TiO2; Sigma T8341, St Louis, MO, USA) mixed with Roti-Histokitt (C. Roth, Karlsruhe, Germany). Additionally markers on the head and abdomen indicated the body-length axis as a reference for data analysis (Fig. 1B).
A computer-controlled high-speed video camera with a resolution of 512×512 pixels (Photron 512 PCI, Photron Ltd, Marlow, Bucks., UK), mounted above the cricket, recorded the movements of the body and its appendages. A ring light with a red filter (Ilford Dark Room Safety Filter 915; Ilford Imaging, Knutsford, UK) provided shadow-free illumination without a disturbing effect on the animal's behaviour (Fig. 1A). Experiments took place at 24–30°C.
High-speed video recordings on each trial were taken for 8.192 s at 500 frames s–1 and a shutter speed of 1/2000 s. Recordings were started when a cricket was walking continuously. A TTL pulse synchronised with the timing of video frames was recorded with the song pattern and trackball data. Four females walked in 25 trials; 16 song-left trials; 6 song-right trials and 2 trials in the no-song condition. Approximately 500 steps of walking during song presentation and 90 steps of walking in the absence of song were recorded and analysed.
Data acquisition
The trackball data, the TTL and video frame signals and the song stimuli were sampled online at 10 kHz per channel using an A/D board (PCI-Mio 16-E-4; National Instruments, Newbury, UK) controlled by LabView 5.01. AVI video files were imported into SIMI motion software (SIMI Reality Motion Systems, Unterschleissheim, Germany) to automatically track the trajectories of the marked tarsi, head and abdomen, as x,y positions on the video image. Trajectories were imported into MATLAB (The Mathworks, Natick, MA, USA) and aligned with velocity signals from the trackball, song stimuli and video frame time data using custom-written software (Hedwig and Knepper, 1992). Trajectory data were low-pass filtered with a fifth order, zero phase lag, Butterworth filter with a 25 Hz cut-off frequency.
Kinematic analysis
Positions of tarsal trajectories during walking and steering Each tarsal position was defined in polar coordinates (r, Φ) providing a cricket-based reference. The origin was the attachment point of the tether at the third thoracic tergite with the direction of 0 deg pointing forward (Fig. 1B). The distance of the tarsus from the origin was referred to as ‘tarsal distance’ (r):
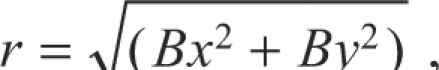 |
(1) |
and the angle formed by the animal's length axis, the origin and the tarsus was referred to as ‘tarsal angle’ (Φ):
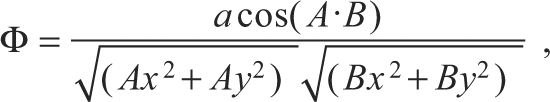 |
(2) |
where A=head(x,y)–tether(x,y) and B=tarsal(x,y)–tether (x,y).
Similar to previous methods (Jindrich and Full, 1999) for the cricket's right legs the anterior extreme position of a stepping cycle (AEP) was defined by the position (r, Φ) where Φ was minimal, and the posterior extreme position (PEP) was defined by the position (r, Φ) where Φ was maximal. For the cricket's left legs the opposite criterion was used, because of the polar coordinate system. This reliably enabled the separation of stepping cycles in all legs. Owing to the spatial relations of the different legs to the origin of the coordinate system, the magnitudes of alterations of the Φ and distance r scaled differently, e.g. changes in the tarsal angle of the front legs during a step cycle were larger than the changes in the hind legs. The mean and peak tarsal speeds for all legs were calculated from the changes in leg positions over each stepping cycle duration (AEP–PEP–AEP). For all legs the swing/stance ratio was calculated by dividing the duration of the swing phase by the duration of the stance phase.
The tilt of the tarsal trajectories, defined as the angle between the connecting line of PEP and AEP, and the animal's length axis was used to describe the change in the alignment of tarsal trajectories towards the direction of song.
Leg trajectories and steering velocities
The possible contribution of the legs in generating steering forces was examined by plotting tarsal angles, Φ, of each leg and the concurrent steering velocity over successive stepping cycles. Steering can only occur during the stance phase, so examining how increases in steering velocity related to the step cycle provided insight into which tripod was responsible for turning. The step cycle duration for each leg was calculated as the time between consecutive tarsal AEPs. This analysis was performed on a trial-by-trial basis, with an average of 25 stepping cycles available for each walking trial. Before averaging over the steps, the time function of the tarsal angle and the steering velocity of each step cycle was normalised using the MATLAB resampling function to 300 ms, a value well within the range reported for cricket stepping cycles (Laurent and Richard, 1986; Baden and Hedwig, 2008).
For comparison of tarsal trajectories and steering velocities across conditions, the stepping cycle of a middle leg was used as reference: the duration of the tarsal trajectory and the steering velocity for each leg was based on consecutive AEPs of the reference leg. The time functions of r and Φ for each leg and the steering velocity were averaged after normalising by resampling to the same duration.
Leg adjustments during steering
To provide an understanding of which leg and body adjustments caused the observed auditory-related changes in tarsal trajectories, three different angles were measured in six representative steps of each stimulus condition (see Fig. 8A). First, the angle of the femur to the thorax was determined, which indicated movement of the femur away from the thorax (abduction) or towards the thorax (adduction). Second, the angle of the femur–tibia joint was analysed, which indicated flexion and extension movements of the tibia. The femur is attached to the thorax via the trochanter and coxal joints, which are obscured by the thorax in our videos. We therefore measured the femur–thorax angle to gain understanding of how adjustments in these joints may influence tarsal trajectories. The angle of the femur–tibia joint can be accurately measured with the single camera when the femur axis is parallel to the plane of the image. When the tip of the femur points upwards, the extension of the joint would be overestimated, and when the tip of the femur points downwards, flexion would be underestimated. Only the front legs were considered for this analysis. In the middle legs the tibia position was occluded by the femur and it was not possible to measure the femur–tibia angle. Third, the bending of the head and prothoracic segment was analysed by measuring the deflection angle of the head from the y-axis of the video image coordinates. Before averaging, each set of measurements was normalised to the phase of the right middle leg step cycle and fitted with a spline.
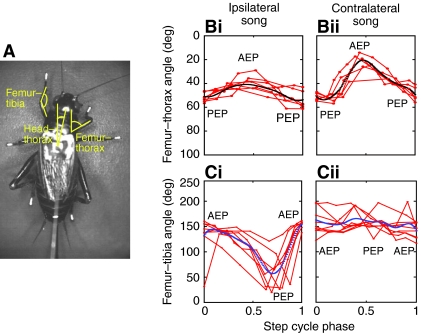
Fig. 8.
(A) Video image showing the femur–tibia angle, femur–thorax angle and head–thorax angle that were analysed on six selected steps for song-left and song-right. Measures were taken for at least eight points with reference to the step cycle of the left front leg. (B) Change in femur–thorax angle during ipsilateral (Bi, left) and contralateral (Bii, right) acoustic stimulation. (C) Change in femur–tibia angle during ipsilateral (Ci, left) and contralateral (Cii, right) acoustic stimulation. Individual steps are indicated in red and the averages in black (top) or blue (bottom). AEP, anterior extreme position of the tarsus; PEP, posterior extreme position of the tarsus.
Differences in measures dependent on the direction of song were assessed for statistical significance using the Wilcoxon signed rank test with a significance level of P<0.05. Unless otherwise stated, parameters were averaged over a walking trial, and then this mean averaged across all walking trials. Means are expressed ± the standard deviation.
RESULTS
Steering and kinematic changes in a stepping sequence
The tarsal angles of all legs (Fig. 1B, Φ) and forward and steering velocities from the trackball, were aligned and compared across all stimulus conditions by plotting their time course (Fig. 2A–C). The rhythmic alterations of the tarsal angle represent the swing and stance phase of each leg, which were most pronounced for the front and middle legs. As expected, the movements of the legs of one tripod were in phase and consequently they were out of phase for each pair of legs. The forward velocity for all test conditions showed rhythmic oscillations in its amplitude caused by the combined effect of the force generated during the stance phases of all legs. Occasional pauses during walking trials were not further analysed.
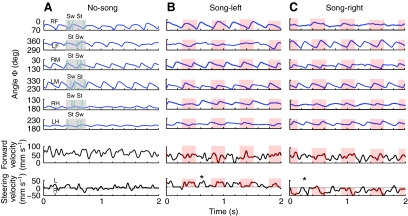
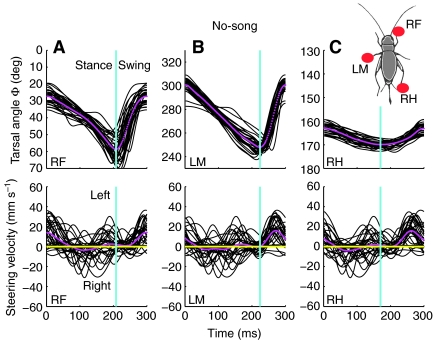
Fig. 2.
Changes in tarsal angle for all six legs and concurrent walking and steering velocity. Three 2 s periods of walking in the no-song (A), song-left (B) and song-right (C) conditions. Red shading on the traces represents the occurrence of a chirp. (A) No-song, steering velocity fluctuated around zero. (B,C) When song was present, differences in tarsal angle were most obvious in the front legs. The steering velocity increased in the direction of the song (L, leftwards and R, rightwards). RF and LF, right and left front; RM and LM, right and left middle; RH and LH, left and right hind tarsi; Sw, swing; St, stance. *Legs maintaining their movement pattern between chirps.
Walking without acoustic stimulation: no-song
In the no-song condition (Fig. 2A) the difference in tarsal angle, Φ, between AEP and PEP was similar for the right and left legs on each step. For example, for the steps in Fig. 2 the tarsal angle of the left front leg changed by 49 deg between 350 deg at AEP to 301 deg at PEP, compared with a change of 50 deg between 18 deg at AEP and 68 deg at PEP for the right front leg. Although individual steps could show smaller changes in tarsal angle, overall there were only minor differences between the left and the right. We therefore present data pooled from each pair of legs.
Averages of all walking steps during no-song showed the front legs moved through a tarsal angle of 23.8±4.0 deg, the middle legs moved through a tarsal angle of 45.7±4.2 deg and the hind legs moved through 6.0±0.3 deg (supplementary material Table S2).
Stepping cycle durations were spread over a range of 128–738 ms. There were no differences in stepping cycle duration between the legs in the no-song condition. The average step cycle duration was 244.7±24.6 ms for the front legs, 248.9±48.9 ms for the middle and 242.3±38.5 ms for the hind legs (supplementary material Table S1). The swing phase of the middle leg occupied 25–30% of the stepping cycle, whereas for the front and hind legs it occupied between 30–45%.
The mean forward velocity was 51.8±4.9 mm s–1 and could reach transient peaks of 100 mm s–1. Characteristically, lateral velocity averaged around 0 mm s–1, indicating straight walking. Fluctuations in steering velocity occurred both to the left and the right. These fluctuations were predominantly small in amplitude reaching 5–10 mm s–1, with occasional peaks of 36 mm s–1; and they were transient, lasting between 50 and 100 ms (Fig. 2A, lower panel). During this walking trial, there were slightly more fluctuations in steering to the left than to the right.
Walking during song-left and song-right
Since the auditory-evoked steering responses to song-left and song-right were correspondingly similar, we combined the description of the responses of the ipsilateral and contralateral legs. In both conditions there were characteristic asymmetries in the changes of the tarsal angle across each pair of legs. Typically, tarsal angle range between AEP and PEP was greater for the contralateral front leg. For example, during song-left, the maximum range of tarsal angle for the right front leg was 71 deg measured between AEP at 0.1 deg and PEP at 71 deg. By contrast, the maximum range of the left front leg was 44 deg, measured between 348 deg at AEP and 304 deg at PEP. However, over most steps the front legs showed considerably smaller changes in tarsal angle (Fig. 2B,C). Averaged over all the phonotactic trials (Fig. 4A,D, upper panels), the change in the contralateral front leg was 26.6±6.2 deg, and was significantly greater than the tarsal angle of the ipsilateral front leg that changed by 13.3±2.2 deg over a step (P<0.001). Alterations in tarsal angle for the middle legs were similar and were 39.6±8.2 deg for the contralateral and 43.2±7.4 deg for the ipsilateral leg (non-significant). Additionally, although the change of tarsal angle of the hind legs was small, there was a significantly larger average change of 9.4±1.9 deg in the contralateral hind tarsus compared with 5.4±2.2 deg in the ipsilateral hind tarsus (P<0.001; supplementary material Table S2).
Fig. 4.
The tarsal angle (Φ) and steering velocity for steps during the song-left condition. Top diagrams (A–C) show the tripod comprising the right front (RF), left middle (LM) and the right hind (RH) tarsi. Lower diagrams (D–F) show tarsal angle and steering velocity for the other tripod comprising the left front (LF), right middle (RM) and the left hind(LH) tarsi. Red markers on the schematic cricket (right) indicate the tripod considered. Individual traces are shown in black, average traces in magenta, average time of the stance–swing transition is indicated by a vertical line (cyan).
There were no differences in average stepping cycle duration between the legs and between bilateral pairs of legs (Fig. 2A–C). For example, the average step cycle duration of the contralateral middle leg was 274.4±18.7 ms and the step cycle duration of the ipsilateral middle leg was 275.6±63.2 ms (supplementary material Table S1).
During the song the crickets walked with an average forward velocity of 49±4.8 mm s–1, reaching a maximum of 88 mm s–1. This was similar to the forward velocity recorded in the no-song condition. However, the characteristic changes of lateral velocity were very different from the no-song condition. Typically there were only large modulations in steering velocity towards the song source that reached 50 mm s–1 and generally lasted approximately 200 ms, before returning to a value close to 0 mm s–1 (Fig. 2B,C, lower panels).
Changes in steering velocity were not tightly coupled to the occurrence of chirps, and steering towards the song source also occurred during silent 250-ms intervals between the chirps, with the legs maintaining their movement pattern (marked with asterisks in Fig. 2B,C, lower panel).
Steering during walking
Legs can only apply forces to the trackball when they contact its surface during stance. Owing to mechanical coupling of the legs via the trackball it is difficult to separate the contributions of individual legs in stance to steering. To elucidate how each leg may contribute to steering, the tarsal angle Φ with the corresponding steering velocity was plotted over time for steps of different stimulus conditions. This revealed the legs that were in stance phase and hence could apply forces to the trackball.
In the no-song condition, the tripod involving the left middle leg (RF, LM, RH) was considered. All three legs showed changes of the tarsal angle, reflecting the stance and swing phases of the stepping cycle (Fig. 3A–C). Stepping trajectories were very regular and the tarsal angles covered by each leg were consistent over successive steps (Fig. 3A–C, upper panel). As the tripod gait of insects is never perfect over a series of steps, the onset and offset of stepping cycles within the same tripod occurred at slightly different times. Consequently, during the stance phase of the tripod comprising RF, LM and RH the step-wise steering velocity profiles were similar for all three legs, however, they were not identical.
Fig. 3.
Tarsal angle (Φ) and steering velocity were superimposed and averaged over 30 steps of a typical walking trial during the no-song condition. They are shown in A–C for the tripod comprising the right front (RF), left middle (LF) and right hind (RH) tarsi. The red markers on the schematic cricket (right) indicate the tripod considered. Individual traces of angles are shown in black, average traces in magenta, and average time of the stance–swing transition is indicated by a vertical line (cyan).
On average, only minor deviations in steering velocity to the left and right occurred (Fig. 3A–C, lower panel, magenta trace). However, when the legs of this tripod were in swing phase, steering was transiently to the left. This was due to forces generated by the other tripod (not shown) and indicated that the animal had a slight leftward bias in its walking direction.
Also during song-left, the changes in tarsal angle, Φ, indicated the stance and swing phases of the legs (Fig. 4A–C, upper panel). When the tripod involving of the ipsilateral middle leg (RF, LM, RH) was considered, average steering was always to the left. Peaks in the average steering velocity occurred at the beginning of the stance phase and during the swing phase (Fig. 4A–C). It is possible that steering during the stance phase could result from forces generated by all legs of this tripod because of mechanical coupling via the trackball. However, we suggest a dominant involvement of the contralateral front leg (RF), as this produced the most salient kinematic change during phonotaxis (Fig. 2, see also Figs 5, 6, 7). Steering velocity also increased during swing but this effect must have been generated by the other tripod.
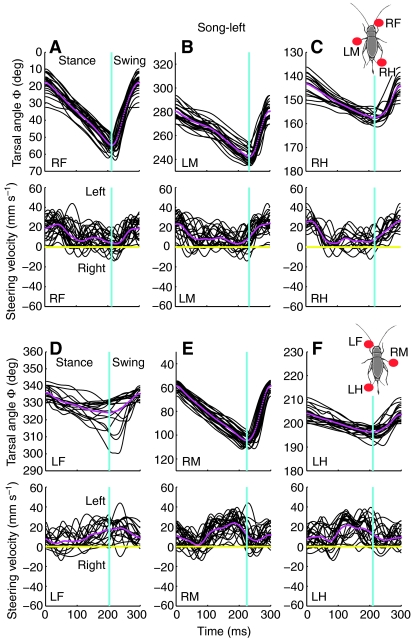
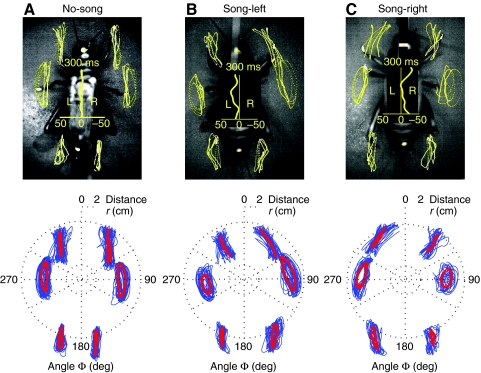
Fig. 5.
Video images and tarsal trajectories of female crickets walking on a trackball during (A) no-song, (B) song-left and (C) song-right conditions. Lower panels, tarsal trajectories and average steering velocity for a 2-s walking period. The extracted tarsal trajectories (blue) were plotted in polar coordinates based on tarsal distance (r) and tarsal angle (Φ). Mean tarsal trajectories were calculated by averaging and are superimposed in red.
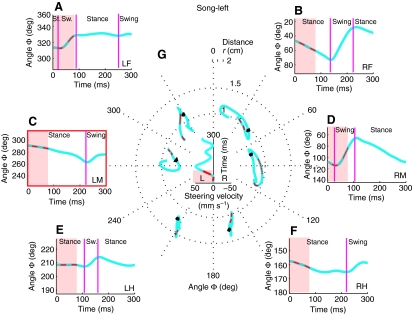
Fig. 6.
Tarsal trajectories of each leg for a single step during song-left. The left middle leg was used as reference for the stepping cycle (indicated in red). Outer diagrams (A–F) show the tarsal angle (Φ) for each leg. Pink shading indicates the presentation of the song and red dots indicate the occurrence of syllables. The transition between swing and stance phases is marked with vertical magenta lines. In the centre (G) the polar plot shows the steering velocity over the step, together with the tarsal trajectories of all legs. Black arrowheads indicate the movement direction of the tarsi and red dots indicate the timing of syllables.
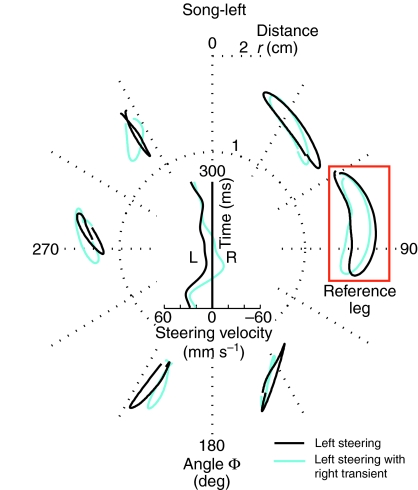
Fig. 7.
Tarsal trajectories of each leg were separated depending on steering velocity. Black traces indicate steering to the left and cyan traces indicate overall steering towards the left yet with a rightward transient. Salient changes in the trajectories of the ipsilateral front leg indicate the role of this leg in increasing the average steering velocity towards the song. The right middle leg was used as reference for the stepping cycle (red box).
When the tripod with the contralateral middle leg (LF, RM, LH) was considered (Fig. 4D–F), the most salient feature was that the tarsal angles of the ipsilateral front leg (LF) were very variable (see also Fig. 7). There was no obvious coupling of leftward steering to the stance phase of this leg. However, the change in tarsal angle of the contralateral middle leg (RM) was very consistent. A clear peak in the leftward steering velocity occurred during the stance phase highlighting the possible importance of the leg for steering. The angular changes over the stepping cycles of the ipsilateral hind leg (LH) were similarly consistent. Owing to coupling of the legs in the tripod the lateral velocity also had a peak during the stance phase of the hind leg, although less pronounced than in the contralateral middle leg.
During song-right the opposite pattern of changes in tarsal angle and peak steering velocity occurred in the tripods (graphs not shown). Again the data indicated that the contralateral front and middle leg played a major role in changing steering velocity over a step.
High-speed images and changes in leg positioning during phonotactic steering
High-speed images with overlaid tarsal trajectories provided a more comprehensive description of the kinematic adjustments (Fig. 5, upper). Intrinsic biomechanical constraints for each pair of legs led to differing characteristic trajectories. Describing the no-song condition first allowed us to identify auditory-induced alterations to the characteristic trajectories.
Leg trajectories during walking without acoustic stimulation
During normal walking, the tarsi of the front legs were directed forward. Movements of the front and middle tarsi are known to occur through tibial flexion and extension together with rotation of the femur (Laurent and Richard, 1986). This led to front tarsal trajectories (defined in Fig. 1B) which were in parallel to the animal's length axis in swing and stance phase (Fig. 5A). The middle femura pointed at right angles to the length axis, with femur rotations dominating the movement of the tarsus between AEP and PEP. During the swing phase, tibial extension led to a slight outward displacement of the tarsi, whereas the stance trajectory was in parallel with the length axis. The hind legs were positioned almost in parallel with the animal's length axis, and moved through flexion and extension of the tibia, rather than by rotation at the coxa (Laurent and Richard, 1986). Similar to the front legs, their tarsal trajectories remained close to parallel with the animal's length axis. Thus, during no-song, the tarsal trajectories of all legs were noticeably straight between AEP and PEP. On average they were tilted by less than 5 deg from being parallel to the animal's length axis. Left and right trajectories were close to symmetrical (Fig. 5A).
Consistent with straight walking, average tarsal stepping speed during the no-song condition was similar for each pair of legs (mean: LF, 6.3±1.1cm s–1; RF, 6.7±2.7 cm s–1; LM, 7.5±1.9 cm s–1; RM, 7.0±2.1 cm s–1; LH, 5.5±0.4 cm s–1; RH, 5.6±1.4 cm s–1). These small differences between the stepping speed of the left and right tarsi reflected a small leftward walking deviation in this animal (Fig. 5A, centre).
Leg trajectories during phonotactic steering
Alterations in tarsal trajectories underlying phonotactic steering typically involved a combination of changes in angular positioning, Φ, and distance of the tarsus from the thorax, r (Fig. 5B,C upper).
First, front and middle leg tarsal trajectories were tilted in the direction of the song. This was particularly clear for the front legs; for example, in song-right, the rightward tilt was an average of 29.5±4.9 deg for the right front leg; and 32.6±15.4 deg for the left front leg. Tilting of the middle legs could reach similar magnitudes but it did not occur consistently on all steps. Tilting of the hind leg trajectories was less than 10 deg and opposite to the direction of the song.
Second, the distance covered by the trajectory of the contralateral front leg was significantly greater than that of the ipsilateral legs. The parameter r gave the distance between the tether and the tarsus. It showed that the difference in path length was due to positioning of the contralateral front leg at AEP, but not at PEP, meaning that these legs were moved further to the front during steering. During song-left, the right front leg r at AEP was an average 1.7±0.01 cm. It was significantly greater than r at AEP of the left front leg that was 1.5±0.05 cm (P<0.05). The average path length differed between the left and right front legs. The average path length between AEP and PEP was 0.35±0.1 cm for the ipsilateral front leg compared with 0.57±0.2 cm for the contralateral front leg (P<0.01). There were no significant differences in path length between AEP and PEP of the middle legs (0.52±0.2 cm for contralateral and 0.54±0.2 cm for ipsilateral) although the path length of the ipsilateral middle legs was substantially reduced on some steps (Fig. 7). The contralateral hind leg showed an increased path relative to the ipsilateral hind leg (P<0.01; supplementary material Table S2).
Third, consistent with the generally larger steps of the contralateral legs, the mean tarsal stepping speed was also higher. The mean speed for the contralateral front leg was 7.2±1.1 cm s–1 compared with a speed 5.7±0.9 cm s–1 for the ipsilateral front leg. The mean speed for the contralateral middle leg was 7.4±1.0 cm s–1 compared with 6.7±1.0 cm s–1 for the ipsilateral middle leg. There was a significant difference in the tarsal stepping speed of the hind legs, with a mean of 5.6±0.7cm s–1 for the contralateral hind leg as compared with 5.0±0.6 cm s–1 for the ipsilateral hind leg (supplementary material Table S2).
These three types of changes, i.e. tilting of trajectories, changes in path length and stepping speed generally occurred together but they also could occur in different combinations.
An individual step when the song was presented from the left, illustrates these three features of phonotactic steering (Fig. 6). Steering velocity increased from close to 0 mm s–1 at the beginning of the step to a maximum of about 50 mm s–1 during the step (Fig. 6G). The tarsal trajectories of the front and middle legs tilted in the direction of the song. In detail, steering-related changes in trajectories in the right front and left middle leg encompassed a stance phase tilted to the left, followed by a swing phase, also strongly directed to the left. The hind legs tilted slightly to the right (Fig. 6G). Corresponding to the sequence of steps illustrated in Fig. 2, the angle Φ covered by the contralateral front tarsus (45.1 deg) and middle tarsus (48.5 deg) were greater than the angle covered by the ipsilateral front tarsus (18.7 deg) and middle tarsus (28.2 deg) (Fig. 6A–D). Changes in Φ of the hind legs were considerably smaller (Figs 6E,F). Distance covered during swing phase by the right hind legs was larger as compared with the distance covered during the swing phase of the left hind legs.
Variation of tarsal trajectories during phonotactic steering
For one cricket, walking during song-left, although the overall steering was always to the left, we observed two distinctly different kinematic patterns of leg movements coupled to differences in steering velocity. Based on the trackball data we separated steps that showed constant leftward steering towards the song source (black traces) from steps that also contained a transient rightward steering component (cyan traces; Fig. 7). To examine whether any kinematic differences could account for these differences in steering velocity, the tarsal trajectories of both types of steps were separately averaged. Steps containing the rightward steering transient were found to occur when the ipsilateral front leg was not tilted in the direction of song.
Tilting of the ipsilateral front leg trajectories towards the song source occurred less consistently than tilting the trajectories of the contralateral front leg. When it occurred, however, it contributed to the strength of phonotactic steering. Furthermore, there were steps where the ipsilateral and not the contralateral front tarsus was rapidly moved in the direction of song, indicating that the ipsilateral front leg could be dominant for steering on an individual step. In this example (Fig. 7) there is also a clear difference in the path length of the middle legs, highlighting the asymmetrical trajectories of the middle legs. However, in subsequent walking of this same animal, the trajectories of the middle legs were similar.
Changes in femur–thorax angle and in femur–tibia angle
The front leg femur–tibia joint angle, the femur–thorax angle and the head–body angle were measured to gain a more precise understanding of how the changes in tarsal trajectories were produced by the animal (Fig. 8A).
During phonotactic steps the femur–thorax angle covered by the contralateral front leg (33 deg) was always greater than in the ipsilateral front leg (14 deg; Fig. 8Bi,ii). The angle at PEP was similar on both sides (54 deg and 51 deg); however, at AEP the femur–thorax angle of the contralateral front leg was an average of 20 deg compared with an average of 41 deg in the ipsilateral front leg. This smaller femur–thorax angle of the contralateral leg at AEP reflected increased leg adduction, which allowed its tibia and tarsus to move further forward. By contrast, the ipsilateral femur–thorax angle indicated leg abduction that kept the tarsus towards the direction of the song.
Changes of the femur–tibia angle of the front leg further indicated how the animals adjusted their stepping trajectories (Fig. 8Ci,ii). During phonotactic steering, the femur–tibia angle of the contralateral front leg only moved between a minimum of 142 deg at PEP and a maximum of 166 deg at AEP, revealing that the tibia remained extended for the whole step. Thus for the contralateral leg, the extension of the femur–tibia joint in combination with the adduction of the femur, moved the AEP in the direction of song and increased the path length. In the ipsilateral leg the femur–tibia angle changed from a maximum of 154 deg at AEP to a minimum of 57 deg at PEP, indicating that the tibia was flexed during the stance phase. In this way the path length of the ipsilateral front leg was decreased. Taken together, these two asymmetric adjustments in front leg angles contributed to shaping the tarsal trajectories underlying steering, and supported the movement of the animal towards the song source.
Global effects
Changes occurred in the head–thorax angle as the animals steered towards the calling song (Fig. 5). During no-song the average head–thorax angle was just 0.2±0.4 deg. During phonotaxis the head–thorax angle rhythmically altered by a mean of 3.9±1.2 deg towards the side of acoustic stimulation. Steering was accompanied by movements of the antennae towards the song source.
DISCUSSION
In earlier video studies of cricket phonotaxis (Murphey and Zaretsky, 1972; Bailey and Thomson, 1977) the temporal resolution of the recordings was too low and insufficient to reliably extract leg trajectories during walking. Combining high-speed video sequences with recordings of the animals' walking and steering velocity has allowed us to analyse functional relationships between leg movements and steering. In order to steer a cricket must show some form of asymmetry in its leg movements, as has been described in other studies of insect turning (Graham, 1972; Franklin et al., 1981; Frantsevich and Mokrushov, 1980).
Coordinative strategies and adjustment in leg positioning
We found no evidence that crickets changed stepping frequency of legs on one side of the thorax in order to steer (Fig. 2). Therefore asymmetries in stepping cycle duration or the duration of swing and stance were not the means by which crickets turned during phonotaxis. Consistent with earlier work (Baden and Hedwig, 2008) there was no evidence for any coupling between the chirp pattern and the onset of the stepping cycles. Instead, distinct adjusted positioning of the legs occurred, suggesting that sound-evoked steering movements of the legs were integrated into ongoing motor activity. Three auditory-evoked responses i.e. tilting of trajectories, changes in path length and stepping speed contributed to steering but could occur in different combinations. The general pattern of changes showed that the trajectories of the front and middle legs tilted towards song although there was some individual variability. During phonotactic steering the step length and speed of the contralateral front and, also often of the middle leg, were larger than that of the ipsilateral front and middle leg. For any walking animal, tilting the trajectories in the direction of the stimulus and moving the outer legs of the turn with greater speed over a greater distance will be sufficient for turning.
Similar adjustments to leg movements during walking, with directed positioning and asymmetric step lengths of the front and middle legs have been shown during turning in stick insects (Dürr and Ebeling, 2005).
Role of the front legs
We examined the role of each individual leg in phonotactic turning by relating its tarsal movements to the animal's steering velocity (Figs 2, 4). Since the stance phase of the contralateral front tarsus was closely coupled to increases in steering velocity, we conclude that movement and force generation of this leg contribute to steering. In agreement with earlier observations (Baden and Hedwig, 2008) in most steering steps the front leg contralateral to song extended towards song, forwarding the tarsal AEP at the end of swing so that the next stance phase moved the female in the direction of song (Fig. 6). This was the most salient and reliably occurring motor adjustment during phonotactic steering. A closer analysis showed there were at least two clear changes that enabled this altered trajectory of the contralateral front leg. First, the female decreased the femur–thorax angle (Fig. 8A,Bii). This positioned the distal tip of the femur more proximal to the thorax (adduction) and further forward during swing (protraction). Second, the femur–tibia angle (Fig. 8A,Cii) remained extended around 150 deg during both swing and stance phases. As a consequence the AEP of the contralateral front tarsus was positioned further forward and closer to the song source.
The ipsilateral front tarsus could be adjusted during phonotactic steering, with the trajectories tilted in the direction of song. Since the tarsal adjustments were variable they did not provide evidence for consistent coupling with steering velocity. When the trajectory was directed towards song (Fig. 5B,C) the femur was positioned away from the thorax (abduction) (Fig. 8Bi) and the femur–tibia angle decreased during stance due to tibial flexion (Fig. 8Ci). This contributed to a reduction in the distance covered in the step by the ipsilateral front tarsus (Fig. 6), which would contribute to turning. When the tarsal trajectory of the ipsilateral front leg did not tilt in the direction of song, phonotactic steering had lower average amplitude over those steps (Fig. 7). We also observed occasional steps where the ipsilateral and not the contralateral front tarsus rapidly moved in the direction of song (Baden and Hedwig, 2008). However, our data do not provide any evidence for the claim that the ipsilateral front leg was dominant for shaping the female's path (Murphey and Zaretsky, 1972).
Role of the middle legs
Relating tarsal angles to steering velocity indicated that both middle legs contributed to phonotactic steering (Fig. 4). The close coupling between steering velocity and the tarsal angle of the contralateral middle leg suggests that this leg provided the dominant steering force for this tripod. When the opposite tripod was considered, the ipsilateral middle leg contributed to steering together with the contralateral front leg.
Often during phonotaxis, the middle legs were tilted towards song (Figs 5, 6, 7), with the AEP and PEP no longer parallel to the animal's body length axis. For the ipsilateral middle leg, the AEP tended to lie further away from the thorax, whereas the PEP was closer. For the contralateral middle leg the opposite occurred, with the AEP closer to the thorax and the PEP further away. Although on average the path length of both middle legs were similar, in steps where the animal was strongly steering towards the song source the ipsilateral middle tarsus was reduced while the path length of the contralateral middle tarsus increased (Fig. 7). These two features of the trajectories support phonotactic steering.
The active involvement of the middle legs in phonotactic steering was observed in previous studies (Murphey and Zaretsky, 1972). The contribution of the middle legs to steering was supported by females orienting towards male calling song despite immobilization of their front legs (Weber et al., 1988).
Role of the hind legs
Since cricket hind legs are adapted for jumping, their contribution to walking occurred through flexion and extension of the femur–tibia joint. This limited the movements of the hind tarsi and kinematic evidence for the active contribution of the hind leg to phonotactic steering was less obvious (Figs 5, 6, 7). The length of the tarsal trajectories of the hind leg was not different from a no-song step cycle. In many steps, the contralateral tarsal velocity was slightly higher than the ipsilateral, which may have supported steering. The trajectories generally were slightly tilted away from the song. This tilt appeared to be the consequence of the trackball rotating around the cricket's central vertical axis. As the rotations of the trackball were generated by the steering front and middle legs, they could have imposed a tilt on the hind leg trajectories in the opposite direction. However, similar tilts in hind leg trajectories were found when cockroaches turned on a slippery surface, which mechanically decoupled their legs (Mu and Ritzmann, 2005).
Tripod responsible for phonotactic steering
Reliably linking force generation to kinematics is a major challenge for walking studies in insects. Our experiments provide a first insight into which legs generate force for phonotactic steering, although it is still not known how individual legs contributed to force generation (Fig. 4). In order to turn, legs must produce directed forces during the stance phase. The kinematic data indicate that both tripods can contribute to this as females consistently used the contralateral middle and/or front legs for song-directed steering. However, besides force generation, the positioning of the tarsus at AEP as a result of the swing phase, critically contributes to steering because it determines the starting point of the stance phase.
Our data would suggest that during phonotaxis the adjustment in the female's contralateral front leg consistently set the direction for auditory steering (Figs 2, 6 and 7) and that the contralateral front and middle legs were dominant in producing the necessary force required to generate changes in steering velocity (Fig. 4).
The experiments also provide clear evidence that phonotactic steering responses were integrated into the ongoing walking activity without changes occurring in the walking motor rhythm. Interestingly, auditory-evoked motor responses related to steering encompassed not only the front and middle legs, but movements of the prothoracic segment, the head and even adjustments of the antennae, which all turn towards the sound. Thus phonotactic steering has a wide impact on the female's motor activity.
Contribution of legs to turning in other insects
Consistent with our data, adjusting leg positioning over a step has been shown to be an important means of turning by other insects. The dominance of the front leg movements in generating directional changes and adjustments has been reported in stick insects turning in response to visual stimuli (Dürr and Ebeling, 2005; Rosano and Webb, 2007) with the role of the middle legs still discussed (Rosano and Webb, 2007). There is no evidence in this species for hind legs making an active contribution to steering.
Unlike the stick insect, in which steering and walking occur more slowly, and coordinative changes in gait can be effective in supporting turning, the female cricket cannot rely on coordinative strategies as both steering and walking are executed very quickly. High-speed video has previously provided an important insight into how faster walking insects, such as the cockroach, adjust the trajectories of their front and middle legs to execute a sensory-induced turn (Mu and Ritzmann, 2005). In our study of cricket phonotaxis there is the additional advantage of understanding the kinematic adjustments made to steer towards an experimentally controlled sensory input.
Leg muscles involved in phonotactic steering
Based on the analysis of tarsal trajectories, the femur–tibia joint angle and femur–thorax angle, we can identify some muscles that must contribute to phonotactic steering. In the front and middle legs, the tibial extensor and tibial flexor muscles control the femur–tibia angle, which crucially contributes to steering (Fig. 8Ci,ii). The front leg tibial flexor and extensor motoneurons were identified (Baden and Hedwig, 2008), and electromyogram recordings indicate auditory responses in pro-thoracic fast flexor tibia and slow extensor tibia motoneurons.
In the front legs, also the coxal rotators, which promote the femur and which can adduct or abduct the femur, must contribute to the observed adjustment in the femur–thorax angle of the front leg (Fig. 8Bi,ii). The motoneurons controlling these muscles have been identified (Laurent and Richard, 1986). We predict that during phonotaxis there is auditory-evoked input to coxal motoneurons of both pro-thoracic and mesothoracic ganglia besides input to the front leg tibial motoneurons. Recording the membrane potential of the motoneurons involved in phonotactic steering will provide important information on what kind of auditory-evoked motor commands are generated within the CNS and how these are integrated with the ongoing walking motor activity.
Head movements and bending of the prothoracic segment
We also found a consistent bend of the head against the thorax (Fig. 5B,C). Since the prothoracic segment is also moveable against the mesothoracic segment this contributed to positioning the front legs during steering (Fig. 6). The bending of the prothorax would require the recruitment of neck and longitudinal muscles to tilt the prothorax and head. Recording electromyograms in flying crickets, showed auditory input to abdominal dorsal longitudinal muscles (Pollack and Hoy, 1980). Thus steering during flight and steering during walking might share common motor activity and control systems.
Conclusion
The combined use of high-speed video and trackball velocity recordings during the phonotactic walking of female crickets has enabled the analysis of a large sample of tarsal trajectories. When combined with detailed measurements of joint angle changes during phonotactic steering, predictions were possible about motoneuron activity during phonotaxis.
Supplementary Material
LIST OF SYMBOLS AND ABBREVIATIONS
- AEP
anterior extreme position of the tarsus
- LF
left front leg
- LH
left hind leg
- LM
left middle leg
- PEP
posterior extreme position of the tarsus
- r
distance from the tether to the tarsus
- RF
right front leg
- RH
right hind leg
- RM
right middle leg
- Φ
head–tether–tarsus angle
This study was supported by a Wellcome Trust Advanced Training Fellowship to Alice Witney and support to Berthold Hedwig from the BBSRC. We thank our colleagues in the Zoology Department in Cambridge for comments on an earlier version of the manuscript. Deposited in PMC for release after 6 months.
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/214/1/69/DC1
REFERENCES
- Baden T., Hedwig B. (2008). Front leg movements and tibial motoneurons underlying auditory steering in the cricket (Gryllus bimaculatus deGeer). J. Exp. Biol. 211, 2123-2133 [DOI] [PubMed] [Google Scholar]
- Bailey W. J., Thomson P. (1977). Acoustic orientation in the cricket Teleogryllus oceanicus (Le Guillou). J. Exp. Biol. 67, 61-75 [Google Scholar]
- Cruse H. (1976). The control of body position in the stick insect (Carausius morosus), when walking over uneven surfaces. Biol. Cybernet. 24, 25-33 [Google Scholar]
- Cruse H. (1979). The control of the anterior extreme position of the hindleg of a walking insect, Carausius morosus. Physiol. Entomol. 4, 121-124 [Google Scholar]
- Delcomyn F. (2004). Insect walking and robotics. A. Rev. Entomol. 49, 51-70 [DOI] [PubMed] [Google Scholar]
- Dürr V., Ebeling W. (2005). The behavioural transition from straight to curve walking: kinetics of leg movement parameters and the initiation of turning. J. Exp. Biol. 208, 2237-2252 [DOI] [PubMed] [Google Scholar]
- Franklin R., Bell W. J., Jander R. (1981). Rotational locomotion by the cockroach Blattella germanica. J. Insect Physiol. 27, 249-255 [Google Scholar]
- Frantsevich L. I., Mokrushov P. A. (1980). Turning and righting in Geotrupes (Coleoptera, Scarabeidae). J. Comp. Physiol. A 136, 279-289 [Google Scholar]
- Graham D. (1972). A behavioural analysis of the temporal organization of walking movements in the 1st instar and adult stick insect (Carausius morosus). J. Comp. Physiol. A 81, 23-52 [Google Scholar]
- Hedwig B., Knepper M. (1992). NEUROLAB, a comprehensive program for the analysis of neurophysiological and behavioural data. J. Neurosci. Methods 45, 135-148 [DOI] [PubMed] [Google Scholar]
- Hedwig B., Poulet J. F. A. (2004). Complex auditory behaviour emerges from simple reactive steering. Nature 430, 781-785 [DOI] [PubMed] [Google Scholar]
- Hedwig B., Poulet J. F. A. (2005). Mechanisms underlying phonotactic steering in the cricket Gryllus bimaculatus revealed with a fast trackball system. J. Exp. Biol. 208, 915-927 [DOI] [PubMed] [Google Scholar]
- Jindrich D. L., Full R. J. (1999). Many-legged maneuverability: dynamics of turning in hexapods. J. Exp. Biol. 202, 1603-1623 [DOI] [PubMed] [Google Scholar]
- Laurent G., Richard D. (1986). The organization and role during locomotion of the proximal musculature of the cricket foreleg. I. Anatomy and innervation. J. Exp. Biol. 123, 255-283 [Google Scholar]
- Mason A. C., Lee N., Oshinksky M. L. (2005). The start of phonotactic walking in the fly Ormia ochracea: a kinematic study. J. Exp. Biol. 208, 4699-4708 [DOI] [PubMed] [Google Scholar]
- Matsuura T., Kanou M., Yamaguchi T. (2002). Motor program initiation and selection in crickets, with special reference to swimming and flying behaviour. J. Comp. Physiol. A 187, 987-995 [DOI] [PubMed] [Google Scholar]
- Mu L., Ritzmann R. E. (2005). Kinematics and motor activity during tethered walking and turning in the cockroach, Blaberus discoidalis. J. Comp. Physiol. A 191, 1037-1054 [DOI] [PubMed] [Google Scholar]
- Murphey R. K., Zaretsky M. (1972). Orientation to calling song by female crickets, Scapsipedus margiatus (Gryllidae). J. Exp. Biol. 56, 335-352 [DOI] [PubMed] [Google Scholar]
- Pearson K. G., Franklin R. (1984). Characteristics of leg movements and patterns of coordination in locusts walking on rough terrain. Int. J. Robot. Res. 3, 101-112 [Google Scholar]
- Pollack G. S. (2000). Who, what, where? Recognition and localization of acoustic signals by insects. Curr. Opin. Neurobiol. 10, 763-767 [DOI] [PubMed] [Google Scholar]
- Pollack G. S., Hoy R. R. (1980). Phonotaxis in flying crickets: Neural correlates. J. Insect Physiol. 27, 41-45 [Google Scholar]
- Poulet J. F. A., Hedwig B. (2005). Auditory orientation in crickets: pattern recognition controls reactive steering. Proc. Natl. Acad. Sci. USA 102, 15665-15669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve R., Webb B. (2002). New neural circuits for robot phonotaxis. Phil. Trans. R. Soc. London, Series A 361, 2245-2266 [DOI] [PubMed] [Google Scholar]
- Rosano H., Webb B. (2007). A dynamic model of thoracic differentiation for the control of turning in the stick insect. Biol. Cybernet. 97, 229-246 [DOI] [PubMed] [Google Scholar]
- Schmitz B., Scharstein H., Wendler G. (1982). Phonotaxis in Gryllus campestris L. (Orthoptera, Gryllidae). I. Mechanisms of acoustic orientation in intact female crickets. J. Comp. Physiol. A 148, 431-444 [Google Scholar]
- Steingrube S., Timme M., Wörgötter F., Manoonpong P. (2010). Self-organized adaptation of a simple neural circuit enables complex robot behaviour. Nature Phys. doi: 10.1038/NPHYS1508 [Google Scholar]
- Thorson J., Weber T., Huber F. (1982). Auditory behaviour of the cricket. II. Simplicity of calling-song recognition in Gryllus, and anomalous phontaxis at abnormal carrier frequencies. J. Comp. Physiol. A 146, 361-378 [Google Scholar]
- Webb B. (2006). Validating biorobotic models. J. Neural Eng. 3, R25-R35 [DOI] [PubMed] [Google Scholar]
- Weber T., Thorson J., Huber F. (1981). Auditory behaviour of the cricket. I. Dynamics of compensated walking and discrimation on the Kramer treadmill. J. Comp. Physiol. A 146, 361-378 [Google Scholar]
- Weber T., Thorson J., Huber F. (1988). Cricket phonotaxis: females with forelegs fixed off the ground can track male song. Naturwissenschaften 75, 317-319 [Google Scholar]
- Wendler G., Dambach M., Schmitz B., Scharstein H. (1980). Analysis of the acoustic orientation behaviour in crickets (Gryllus campestris L.). Naturwissenschaften 67, 99-101 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.