Abstract
Plants often attract pollinators with floral displays composed of visual, olfactory, tactile and gustatory stimuli. Since pollinators' responses to each of these stimuli are usually studied independently, the question of why plants produce multi-component floral displays remains relatively unexplored. Here we used signal detection theory to test the hypothesis that complex displays reduce a pollinator's uncertainty about the floral signal. Specifically, we asked whether one component of the floral display, scent, improved a bee's certainty about the value of another component, color hue. We first trained two groups of bumble bees (Bombus impatiens Cresson) to discriminate between rewarding and unrewarding artificial flowers of slightly different hues in the presence vs absence of scent. In a test phase, we presented these bees with a gradient of floral hues and assessed their ability to identify the hue rewarded during training. We interpreted the extent to which bees' preferences were biased away from the unrewarding hue (‘peak shift’) as an indicator of uncertainty in color discrimination. Our data show that the presence of an olfactory signal reduces uncertainty regarding color: not only was color learning facilitated on scented flowers but also bees showed a lower amount of peak shift in the presence of scent. We explore potential mechanisms by which scent might reduce uncertainty about color, and discuss the broader significance of our results for our understanding of signal evolution.
Keywords: signal detection theory, complex signals, mimicry, signal evolution, multimodal, foraging, color learning, Bombus impatiens, peak shift, plant–pollinator interactions
INTRODUCTION
Communication often involves the exchange of complex signals. For example, males courting females, prey deterring predators and humans speaking with each other generally use some combination of acoustic, visual, tactile and chemical stimuli, as reviewed by Hebets and Papaj (Hebets and Papaj, 2005). Despite the ubiquity of these multi-component or multimodal signals across taxonomic groups and behavioral contexts, most hypotheses regarding their function remain untested (Coleman, 2009). Given the potential costs of producing a more elaborate display (Partan and Marler, 2005), why signal across multiple sensory modalities rather than just one? The question of how and why animals use complex signals has attracted the interest of researchers in areas as diverse as sexual selection (Candolin, 2003), human psychophysics (Stein and Meredith, 1993) and animal cognition (Rowe, 1999).
Signal complexity, however, is not confined to interactions among animals. Plants attract animal pollinators with floral displays composed of colors, patterns, shapes, scents, textures and even tastes. Yet we know little about the possible benefits that multi-component signals offer either participant in this well-studied interaction. A recent experiment by Kulahci and colleagues (Kulahci et al., 2008) (see also Dyer and Chittka, 2004a; Reinhard et al., 2006) provided evidence that multi-component floral signals enhance pollinator foraging: bumble bees (Bombus impatiens) visited the rewarding floral type at a higher rate when flowers differed in two aspects (both scent and shape) than when flowers differed in a single aspect (only scent or only shape). The ability to accurately identify nectar-producing flowers presumably translates to a greater rate of energy intake (Harder and Real, 1987; Waser, 1983). In addition, more accurate discrimination could reduce visits to Batesian mimics (Schiestl, 2005) and other distracting stimuli. Enhanced discrimination by pollinators could benefit plants as well, if it increases the chance that bees will transport pollen to conspecific plants on subsequent visits (Levin, 1978). For example, McEwan and Vamosi recently reported that within alpine plant communities, the colors of species that flower at similar times are more different from each other than predicted by chance (McEwan and Vamosi, 2010). This finding is consistent with a scenario wherein pollinators experience uncertainty in color signals and plants thus benefit by having flowers that are distinctly different from others in the same habitat.
Why are complex floral displays easier for bees to learn? One straightforward explanation is that a complex floral display provides pollinators with multiple independent samples of information about a signal associated with a reward. For example, perhaps bees perceive flowers that differ both in shape and in scent as more different than flowers that differ in shape only or in scent only. So long as a bee can process the greater amount of information without undue cost, then choices may be more accurate when more information is available. According to this hypothesis, which is similar to the ‘redundant signals’ hypothesis of Hebets and Papaj (Hebets and Papaj, 2005), sampling both visual and olfactory stimuli allows the bee to be more certain about the identity of a rewarding flower type. Alternatively, a complex floral display may improve signal certainty because floral components are not sampled independently, but instead interact to facilitate learning and/or recall [see ‘inter-signal interaction’ hypothesis (Hebets and Papaj, 2005)]. For example, it is possible that one component of the complex display, scent, improves a bee's certainty about another component, color.
Here we tested whether the presence of a scent reduces nectar-foraging bumble bees' (B. impatiens) uncertainty about the precise color of a flower. We used a peak shift learning discrimination assay based upon signal detection theory to investigate this potential interaction between olfactory and visual floral signals.
Uncertainty, signal detection theory and learned biases
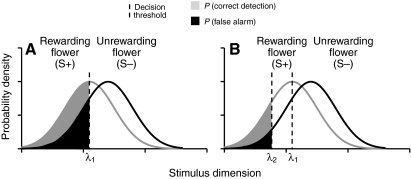
Signal detection theory (Green and Swets, 1966) provides a framework for understanding decision making in situations where perceptual or environmental noise makes discrimination between two signals difficult (Dyer et al., 2008; Wiley, 2006). For example, Fig. 1 illustrates a hypothetical signal detection problem faced by bees: flower types that differ in reward value on average differ along some perceptual dimension (such as color), but individual flowers vary in their traits. The overall distributions of trait values overlap between more and less rewarding flower types, making perfect identification of every individual impossible. This problem could apply to distinguishing rewarding flower species from rewardless mimic species (Schiestl, 2005), or, more generally, to discriminating more rewarding flower species from less rewarding ones [e.g. variation in nectar volume or concentration (Cnaani et al., 2006; Goulson, 1999)]. If bees use a threshold-based rule to decide whether to accept an individual flower, then any overlap between sensory traits of floral types generates a non-zero probability of making two types of mistake: false alarms and missed detections. If a bee accepts a flower, it may have made a correct detection (landing on a rewarding flower) or a false alarm (landing on an unrewarding flower). Bees may also reject flowers, either incorrectly (missed detection of the rewarding flower) or correctly (if the flower is unrewarding). If the cost of false alarms is higher than the cost of missed detections, the optimal decision threshold will be shifted along the perceptual axis in a direction away from the unrewarding flower, possibly even beyond the stimulus value that corresponds to the most common value of the rewarding flowers (Fig. 1).
Fig. 1.
When flowers vary in profitability but share characteristics, pollinators face a classic signal detection problem. (A) Pollinators encounter rewarding flowers (S+) that transmit stimuli (e.g. hue, wavelength, scent, size) shared by unrewarding flowers (S–). Pollinators who accept floral stimuli to the left of the decision criterion λ1 (the most common stimulus value of S+) face a certain probability (P) of correctly detecting the rewarding floral type and rejecting the unrewarding floral type (integral of S– curve from λ1 to ∞), but also risk false alarms (incorrectly accepting the unrewarding floral type) as well as missed detections (integral of S+ curve from λ1 to ∞). (B) Pollinators might adopt a more conservative decision criterion (λ2), in which more stimuli are rejected. This reduces the probability of false alarms/increases correct rejections, but also reduces correct detections/increases missed detections.
Lynn and colleagues (Lynn et al., 2005) noted that the predicted displacement of the optimal decision threshold away from the most common (peak) value of rewarding flowers accounts for a phenomenon found in discrimination learning experiments known as peak shift (Hanson, 1959; Shettleworth, 1998). These ‘shifts’ occur when bees are trained to respond to one stimulus (‘S+’, e.g. light with a wavelength of 450 nm that provides a sucrose reward) and to withhold responses to a second, similar, stimulus (‘S–’, e.g. light with a wavelength of 470 nm that is unrewarding or punishing). In a test phase where subjects' responses are measured across a range of stimulus colors (e.g. lights with wavelengths from 400 to 500 nm), we might expect subjects' strongest (‘peak’) response to be to the S+ (450 nm), the color rewarded during training. Contrary to this expectation, however, subjects may show peak shift, responding most strongly to a novel stimulus value (e.g. 430 nm) that is biased in a direction away from the S– (470 nm, the color that was unrewarding or punishing during training). These subjects may also show a weaker form of perceptual shift known as ‘area shift’ (Cheng et al., 1997; Cheng, 2002; Lynn et al., 2005), wherein responses to test stimuli are asymmetrically distributed around the S+ (e.g. S+ is 450 nm, S– is 470 nm, and subjects show a stronger response to test stimuli between 400 and 449 nm than to stimuli between 451 and 500 nm). In experiments on bumble bees trained to discriminate between different colors of artificial flowers, Lynn and colleagues' (Lynn et al., 2005) functional, signal detection theory-based account proposes that the observed biases jointly minimize the separate probabilities of a false alarm (incorrectly landing on the S–) and a missed detection (incorrectly failing to land on the S+ when encountered).
Lynn and colleagues (Lynn et al., 2005) argued that the magnitude of peak and area shift reflect the subjects' certainty in discriminating the rewarding S+ from the punishing S–. In this view, the peak shift results from uncertainty about whether a particular stimulus value corresponds to a rewarding flower. If uncertainty about the true stimulus value is reduced, the acceptance threshold can be moved to include most of the rewarding stimulus distribution, towards the unrewarding stimulus, without an increase in false alarms (referring to Fig. 1, if the variance in the stimulus distributions is reduced, these distributions will overlap less, allowing for more accurate discrimination). Empirically, bees trained under conditions of greater uncertainty (e.g. increased variance in the color of the S+) indeed showed greater shifts in response (Lynn et al., 2005). Measuring peak shift in a discrimination task therefore provides a way to quantify bees' levels of uncertainty in discriminating the S+ from the S–.
Here we trained bees on flowers that presented either simple or complex signals. If multimodal floral signals reduce uncertainty, then bees' responses to scented color stimuli should show less peak shift than when flowers are unscented; bees should also learn to discriminate between rewarding and unrewarding floral colors faster in the presence of scent. Although we applied this methodology in the context of complex floral signals, peak shift experiments can be used to study complex signaling in any context where signal uncertainty is of interest.
MATERIALS AND METHODS
Subjects and pre-training
We used 135 worker bees as subjects, selected from three colonies of B. impatiens (Koppert Biological Systems, Romulus, MI, USA). Colonies were provided with pollen ad libitum and housed in plastic boxes (L×W×H: 22 cm×24 cm×12 cm) outside of the room-sized experimental chamber (L×W×H: 3.05 m×1.92 m×1.55 m). Mesh tubing connected the colony box to a gated buffer box (L×W×H: 35 cm×22 cm×15 cm), which led to the experimental chamber via a custom-fitted port (supplementary material Fig. S1A). The gating system permitted us to release individual foragers into the experimental chamber. All foragers were fitted on the thorax with numbered tags (E. H. Thorne Ltd, Wragby, Lincolnshire, UK) for individual recognition. All pre-training, training and testing occurred in this experimental chamber, which was illuminated by fluorescent lighting (supplementary material Fig. S2A: Sylvania Cool White 34 W, 60 Hz bulbs, no. F40CW1SS, 750 lx measured at center of array) and fitted with a screen door to permit observation. Since the flicker frequency of the ballasts powering the lights was less than B. impatiens' probable fusion frequency [∼100 Hz reported for honeybees (Srinivasan and Lehrer, 1993)], bees likely perceived a flicker.
Before experiments began, it was necessary to pre-train bees to visit the experimental chamber and forage at the floral array. We allowed the colony free access for 3 days to a vertical training array that offered three feeders, each providing 500 ml of 30% w/w sucrose. These pre-training feeders consisted of a wick on a cylindrical landing platform (L×diameter: 3.0 cm×0.5 cm) surrounded by a gray circle (diameter=4.0 cm), the same size as the artificial flowers used in training and testing. The feeders were mounted on a gray pegboard of the same color and size used in training and testing. We began experiments after this initial pre-training, but continued to provide free access to the feeders after experiments were completed each day. Daily access to the pre-training feeders ensured that newly emerged foragers learned to visit the array over the course of the experiment.
Floral array
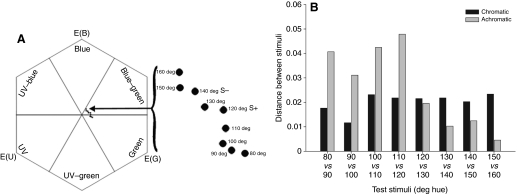
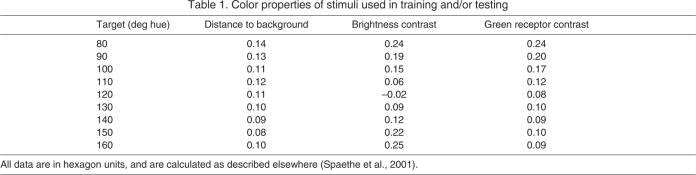
The vertical array was constructed from pegboard (L×W: 60 cm×60 cm) painted gray, with artificial flowers arranged at 10 cm intervals in a 6×6 grid (supplementary material Fig. S1B). Flowers consisted of a landing platform (cylindrical acrylic rod, L×diameter: 2.5 cm×0.5 cm) centered in a 4.0 cm diameter circle. Bees were trained on one (S+) or two (S+/S–) colors and tested on nine colors, all printed on Avery circular mailing labels (no. 8293) using a Canon Pixma MX860 inkjet printer. In HSB color-space, hue is an angular representation (measured in degrees) of a RBG color model (red: 0 deg; green: 120 deg; blue: 240 deg). Our stimuli ranged from 80 deg (human yellow–green) to 160 deg (human green–blue) in 10 deg increments (saturation: 50%, brightness: 100%). The reflectance peaks of these stimuli (supplementary material Fig. S2B) span wavelengths at which bumble bees' photoreceptor sensitivities are high (Briscoe and Chittka, 2001); additionally, Lynn and colleagues (Lynn et al., 2005) established that B. impatiens exhibit peak shift when trained and tested using these stimuli. Because HSB color-space is tuned to human vision, not bee vision, color stimuli are not likely to vary for bees in precisely regular increments. We confirmed this by plotting our nine hues in bee color space, using the color hexagon model of Chittka (Chittka, 1992) (Fig. 2A). The diagram was created using AVICOL 3.0 software (AVICOL: A program to analyse spectrometric data; free program available from the author at dodogomez@yahoo.fr), and is based upon spectral sensitivity functions (supplementary material Fig. S2C) (Briscoe and Chittka, 2001; Peitsch et al., 1992; Stavenga et al., 1993) derived from data published on B. jonellus [the closest relative for whom data are available (Cameron et al., 2002)]. The hexagon shows that the nine hues maintain their order in bee color space; Euclidean color distances between successive hue values are shown in Fig. 2B. We used this information to scale the x-axes in Figs 4 and 5.
Fig. 2.
(A) The nine color stimuli (hues of 80–160 deg) shown in bee color space using the hexagon model of Chittka (Chittka, 1992). (B) Variation in Euclidian distance between successive hues.
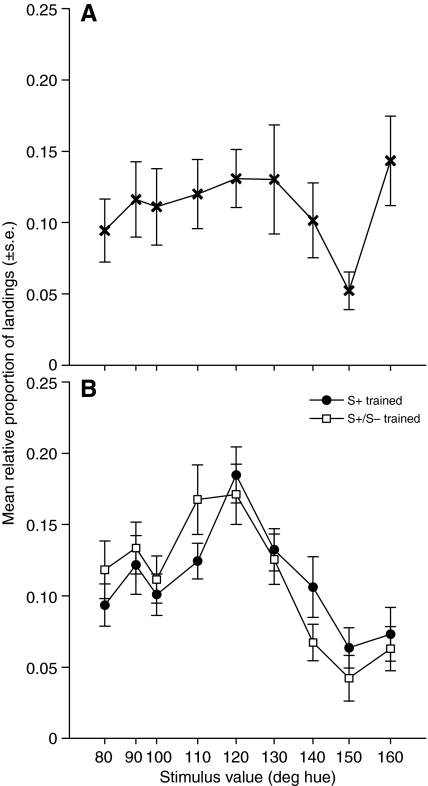
Fig. 4.
Responses of bees to nine unscented test stimuli, measured during an extinction trial. (A) Naïve bees. (B) Bees trained with unscented S+ (120 deg) only (filled circles) or unscented S+ (120 deg) vs S– (140 deg) (open squares).
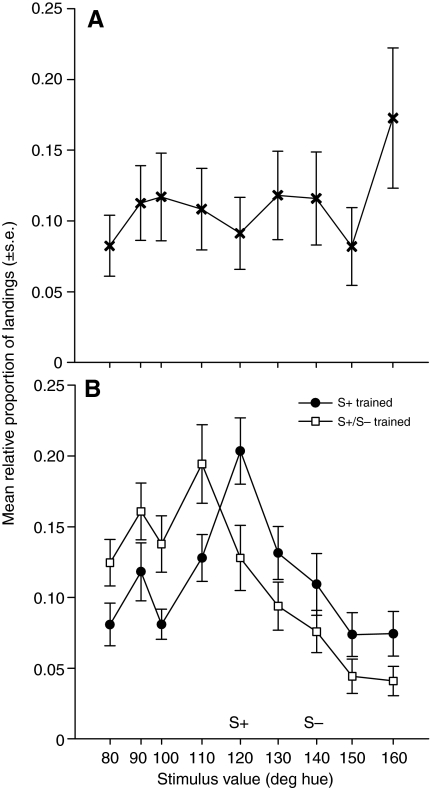
Fig. 5.
Responses of bees to nine scented test stimuli, measured during an extinction trial. (A) Naïve bees. (B) Bees trained with scented S+ (120 deg) only (filled circles) or scented S+ (120 deg) vs S– (140 deg) (open squares).
Depending on treatment, we provided 4 μl of deionized water (=unrewarding), 30% sucrose solution (=reward) or 3% NaCl solution (=punishment) on the landing platform beneath each colored circle during training and testing. We defined a landing as a stop of any duration on the landing platform; once on the platform, bees were allowed to drink whatever reinforcer was available. In trials with scented flowers, we presented 2 μl of clove or peppermint oil (Aura Cacia, Frontier Natural Products, Norway, IA, USA, 1:100 in mineral oil) in a small reservoir behind each colored circle. Each colored circle had a 2 mm hole directly above the reservoir to permit scent transmission. After each trip by an individual bee (i.e. when the bee returned to the nest after a training session), we cleaned each landing platform with 30% ethanol to remove any chemical cues deposited by foragers.
Training
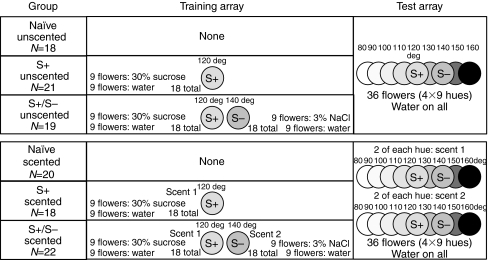
Bees were randomly assigned to one of six experimental groups (Fig. 3), three of which visited an unscented array in training and testing, and three of which visited a scented array. Bees in the ‘naïve unscented’ (N=18) and ‘naïve scented’ (N=20) groups received only the pre-training before being tested on the nine-color array. Bees in ‘S+ unscented’ (N=21), ‘S+ scented’ (N=18), ‘S+/S– unscented’ (N=19) and ‘S+/S– scented’ (N=22) groups received the following additional training. Flowers were randomly placed on the training array, and their position was changed between training sessions. Individual bees underwent multiple training sessions that lasted for a maximum of 10 min each, but were stopped when the bee left the arena and returned to the buffer box or emptied all of the rewarding flowers. Any bees tested the day after the first training session received an extra training session before the test; across treatment groups, a similar proportion of subjects underwent this multi-day training (χ2=3.44 with 3 d.f., P=0.33).
Fig. 3.
The six experimental groups differed in the floral stimuli available during training and testing. Bees in three ‘unscented’ groups were tested on unscented flowers that differed in color. Bees in three ‘scented’ groups were tested on flowers that differed in both color and scent. Before an extinction trial on the full nine-color array, naïve groups had no training; S+-only trained groups were trained with a rewarding floral stimulus; S+/S– groups were trained with both a rewarding and a punishing floral stimulus. In the scented S+-only trained group, 11 bees had peppermint as scent 1 and clove as scent 2; seven bees had clove as scent 1/peppermint as scent 2. In the scented S+/S– trained group, 15 bees had peppermint as scent 1/clove as scent 2; seven bees had clove as scent 1/peppermint as scent 2.
In the S+ unscented and scented groups, we trained bees on the S+ (120 deg) flowers only. For bees in the S+ scented group, training flowers were scented with peppermint (N=11) or clove (N=7). Although unequal numbers of bees were assigned peppermint vs clove as their S+ scent, naïve bees showed no preference for landing on peppermint- vs clove-scented flowers (see Results). The training array held 18 of these S+ flowers, nine of which provided 30% sucrose and nine of which provided water. Having half of the training flowers provide water was an attempt to ensure that bees would not expect every single ‘rewarding flower’ to actually contain sugar, such that they would still make multiple visits to flowers during the test (an extinction assay in which all flowers provided water),
In the S+/S– unscented and scented groups, we trained bees on an array of 18 S+ (120 deg) and 18 S– (140 deg) flowers. Nine of the S+ flowers provided 30% sucrose and nine provided water, as above. Of the S– flowers, nine provided 3% NaCl and nine provided water. Bees perceive salt solution as aversive (e.g. Chandra and Smith, 1998). For bees in the S+/S– scented group, S+ flowers were scented with peppermint (N=15) or clove (N=7), and S– flowers were scented with the alternative scent. Bees in the S+/S– groups were trained until 8 of the last 10 of their landings during their last (2nd, 3rd or 4th) training session were to the S+ flower. We did not find a statistically significant difference between bees that experienced peppermint- or clove-scented S+ stimuli in the mean number of landings to reach the 80% criterion (see Results). Bees in the two S+-only trained groups were given a number of training sessions similar to those for bees in the two S+/S– trained groups (minimum 2, maximum 4; mean: S+ unscented 2.6±0.5; S+ scented 2.5±0.5; S+/S– unscented 2.5±0.6; S+/S– scented 2.5±0.5).
Testing
During testing we presented bees with 36 artificial flowers (nine hues: 80 deg, 90 deg, 100 deg, 110 deg, 120 deg, 130 deg, 140 deg, 150 deg and 160 deg, four flowers of each) arranged in a 6×6 randomized grid on a gray pegboard background. For naïve unscented, S+ unscented and S+/S– unscented groups these flowers were unscented. For naïve scented, S+ scented and S+/S– scented groups, two flowers of each hue were scented with clove and two were scented with peppermint. Scent by itself thus gave no information that would allow bees to distinguish between flower colors. For all groups, all flowers provided 4 μl of deionized water. Tests lasted for 10 min, but were stopped if the bee was away from the array for more than 3 min.
To compare bees' color certainty in the absence vs presence of scent, we considered four independent measures of response to test stimuli. First, we simply asked whether peak shift occurred under both conditions: following discrimination (S+ vs S–) training, was the most frequent (peak) response to the S+ (120 deg) test stimulus, or was it shifted away from the S+ in the direction opposite to the S– (140 deg)? Second, we compared the mean proportion of landings on the S+ (120 deg) test stimulus. Any decline in landings on the S+ potentially represents a cost to both plant (i.e. lost pollination services) and pollinator (i.e. lost reward). As a measure of how discrimination training affected overall color preference, we also compared the mean hue value bees landed upon. Finally, in perhaps the most direct assessment of a bee's uncertainty about hue, we measured the extent of aggregate bias away from the S– (‘area shift’): the mean proportion of landings on the four test stimuli of lower hues than the S+ (80 deg+90 deg+100 deg+110 deg). We used an arcsine transformation to normalize data where necessary (Zar, 1999); all means are reported ±s.e.
Scent learning experiment
To establish how well bees could learn to distinguish the scents we used (clove vs peppermint) on our floral array, we ran 17 bees through training sessions similar to those described above, on arrays in which rewarding and unrewarding flowers differed only in scent: both S+ and S– were 120 deg in hue (seven bees had clove as the S+/peppermint as the S–; 10 bees had peppermint as the S+/clove as the S–). Bees were trained until 8/10 landings were to the S+, and then were given an extinction trial (all flowers 120 deg in hue; 18 scented with clove and 18 scented with peppermint; all provided water).
RESULTS
Scent enhanced learning during training phase
If floral scent reduces bees' uncertainty in discriminating between unrewarding and rewarding flowers, then we expected that during training, bees should reach our accuracy criteria fastest when flowers were scented. Comparing the two groups of bees who underwent discrimination (S+ vs S–) training, bees whose training flowers were scented indeed reached 80% accuracy (8 out of 10 consecutive landings on S+) significantly sooner in the last training session than did bees whose training flowers were unscented (mean total number of visits to criterion for S+/S– unscented group: 47.4±4.08; for S+/S– scented group: 37.6±2.02; 2-tailed t-test t25.8=2.168, P=0.04). Although there was a trend for bees to reach the 80% accuracy criterion sooner when peppermint was the S+, this difference was not statistically significant (mean number of landings to criterion for peppermint group: 35.3±1.80; for clove group: 42.6±4.27; 2-tailed t-test t20=–1.867, P=0.077). Thus, since similar proportions of bees within training groups had peppermint vs clove as the S+ scent, and we found no effect of scent type on learning speed and no naïve preferences (see below), our first analyses below pool bees within a treatment group regardless of whether their S+ scent was peppermint or clove.
Scented flowers decreased peak shift
To establish whether floral scent reduces bees' uncertainty in color learning, we compared the magnitude of shifts in color responses observed during the test phase between S+ and S+/S– trained bees in the absence (Fig. 4) vs presence (Fig. 5) of scent. In order to establish that differences result from training, rather than innate color biases, we also report the responses of naïve bees to our nine test stimuli in Fig. 4A and Fig. 5A. Regardless of whether flowers were scented, naïve bees responded similarly to the nine color stimuli, with a peak response to the bluest stimulus, 160 deg (mean relative proportion of landings on 160 deg: unscented: 0.14±0.032, scented: 0.17±0.038). Bees' innate preference for blue stimuli is well established (Gumbert, 2000); the 160 deg stimulus was also brightest against the background (Table 1), which may have contributed to its attractiveness. Naïve bees responded least to the 150 deg stimulus, for unknown reasons; we did note that the 150 deg stimulus presented the least chromatic contrast against the grey background (Table 1). Naïve bees landed significantly more often on flowers when they were scented than when they were unscented (mean number of landings on scented: 14.9; unscented: 9.7; 2-tailed t-test t28.5=2.81, P<0.01), but did not show a bias for landing on flowers scented with peppermint vs clove (mean relative proportion of landings: peppermint: 0.48±0.031, clove: 0.52±0.031; paired t-test t19=–0.49, P=0.63).
Table 1.
Color properties of stimuli used in training and/or testing
On unscented flowers (Fig. 4B), S+/S– trained bees showed a distinct peak shift: their most frequent response was to the 110 deg stimulus (mean proportion of landings on 110 deg: 0.19±0.027), a shift away from the color they were actually trained to (S+ was 120 deg) in a direction opposite to the previously punishing stimulus (S– was 140 deg). S+ trained bees, in contrast, showed a peak response to the training stimulus S+ (mean proportion of landings on 120 deg: 0.20±0.024). S+/S– trained bees were significantly less likely to land on the S+ than bees whose training was to S+ only (2-tailed t-test: t38=2.538, P=0.015). Comparing the mean hue landed upon by S+ vs S+/S– trained bees also showed a significant difference (mean hue landed upon: S+-only trained bees: 119.0±1.87 deg, S+/S– trained bees: 110.9±1.72 deg; 2-tailed t-test t38=3.128, P=0.003). Relative to S+ trained bees, S+/S– trained bees showed area shift: they landed more frequently on test stimuli whose hue was lower than 120 deg (that of the S+ stimulus) (mean proportion of landings on stimuli between 80 deg and 110 deg: S+ trained bees: 0.41±0.033, S+/S– trained bees: 0.62±0.045; 2-tailed t-test t38=–3.748, P<0.001).
In contrast, on scented flowers (Fig. 5B), S+/S– trained bees did not show a significant degree of peak shift: their strongest response spanned 120 deg (S+) and 110 deg (mean relative proportion of landings: 120 deg: 0.17±0.0023; 110 deg: 0.17±0.023). Once again, S+ trained bees showed a peak response to the S+ (120 deg) stimulus (mean proportion of landings: 0.19±0.025). On scented flowers, in contrast to unscented ones, S+/S– trained bees' mean proportion of landings on the S+ was not significantly lower than that of S+ trained bees (2-tailed t-test t38=0.631, P=0.533). Nor was there a significant difference between the mean hue that S+ vs S+/S– trained bees landed upon (S+ only: 117.6±1.90 deg; S+/S– trained: 113.7±1.79 deg; 2-tailed t-test t38=1.477, P=0.148). Likewise, there was no significant area shift on scented flowers (mean proportion of landings on 80–110 deg: S+ trained bees: 0.44±0.032, S+/S– trained bees: 0.53±0.034; 2-tailed t-test t38=–1.879, P=0.064).
Comparing the proportion of landings on the S+ between bees on unscented vs scented flowers in an ANOVA revealed a non-significant interaction between training type (S+ vs S+/S–) and presence of scent (training type: F1,79=5.544, P=0.021; presence of scent: F1,79=0.863, P=0.356; training type×presence of scent: F1,79=2.501, P=0.118). A direct comparison of the mean hue landed upon showed similar results (training type: F1,79=10.535, P=0.002; presence of scent: F1,79=0.154, P=0.696; training type×presence of scent: F1,79=1.300, P=0.258). However, comparing the difference between mean proportions of landings on test stimuli between 80 deg and 110 deg on unscented vs scented flowers showed that area shift was significantly reduced on scented flowers (ANCOVA with hue as covariate: F2,5=9.102, P=0.022; hue: F1,5=3.753, P=0.110; presence of scent: F1,5=14.45, P=0.013).
In summary, as hypothesized, there is more peak shift, and thus more uncertainty on the part of the bee, if there is only a single modality available when bees have to discriminate between rewarding vs unrewarding flowers. Bees trained and tested on scented flowers behave as though they are better able to identify the S+ and the S–: in signal detection theory terms, S+/S– trained bees' stable strong response to the S+ when flowers are scented suggests that they perceive minimal overlap between the stimuli, in spite of environmental and perceptual noise. From the plant's perspective, the increased fidelity of pollinators likely contributes to reproductive success.
Scent: indicator of floral identity, or facilitator of color learning?
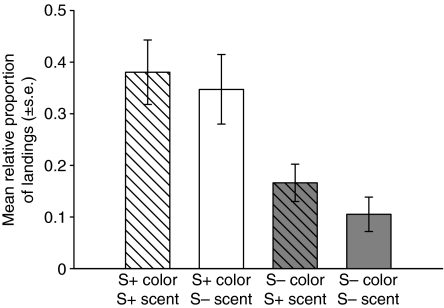
Was the bees' apparently enhanced certainty about the rewarding color associated with learning and recall of the rewarding scent? Our results suggest the answer is ‘no’: across all test stimuli, S+/S– bees showed no significant scent learning, i.e. in the unrewarded test they landed on the correct scent equally as often as on the incorrect one (mean relative proportion of landings: S+ scent: 0.55±0.0063; S– scent: 0.45±0.063; paired t-test t21=1.83, P=0.082). Fig. 6 shows the mean proportion of landings of S+/S– trained bees on four color+scent combinations of test stimuli: 120 deg (S+ color) paired with the S+ scent, 120 deg (S+) paired with the S– scent, 140 deg (S– color) paired with the S+ scent, and 140 deg (S–) paired with the S– scent. Bees were as likely to land on the S+ color regardless of which scent it was associated with (mean proportion of landings: 120 deg and S+ scent: 0.38±0.061, 120 deg and S– scent: 0.35±0.068) and were less likely to land on the S– color regardless of whether it transmitted the S+ or the S– scent (mean proportion of landings: 140 deg and S+ scent: 0.17±0.036; 140 deg and S– scent: 0.11±0.034). A repeated measures ANOVA on these test choices shows a statistically significant effect of training color (F1,21=18.14, P<0.01), but no effect of training scent (F1,21=0.37, P=0.55), and no significant interaction between color and scent (F1,21=0.40, P=0.53). The absence of an interaction, coupled with the non-significant scent main effect, suggests that, under the S+ vs S– training regime, bees either did not learn flower scents or did not use them in landing decisions on the test array.
Fig. 6.
Responses of bees in scented S+ vs S– trained group to the four test stimuli that transmitted the S+ color (120 deg) or S– color (140 deg) paired with the S+ scent or S– scent. Bees landed more often on the S+ color (ANOVA, P<0.01) but not on the S+ odor (P=0.55).
Scent learning experiment
Bees given S+/S– training on flowers that differed only in scent readily learned to distinguish peppermint from clove, landing significantly more often on the scent rewarded in training during an extinction test (paired t-test t15=7.73 P<0.001). However, it took bees longer during training to reach the same level of accuracy (8/10 consecutive visits to the rewarding flower) when flowers differed only in scent vs when flowers differed only in color (scent learning: 81.8±8.48 landings vs color learning by bees in the unscented S+/S– training group: 47.4±4.08 landings; Mann–Whitney U-test: Ncolor only=19, Nscent only=17, U=49.5, P<0.001).
DISCUSSION
Why do flowers commonly signal in both visual and olfactory modalities? Our results suggest that a more complex signal reduces pollinators' uncertainty in identifying rewarding flowers in a given modality. Although previous studies have shown that bees' discrimination improves when artificial flowers differ in multiple aspects (Dyer and Chittka, 2004a; Kulahci et al., 2008), our results provide the first evidence that scent can improve the perceived reliability of visual stimuli. Using a discrimination learning design adapted from the classic psychophysical ‘peak shift’ experiments (Hanson, 1959; Lynn et al., 2005), we established that bumble bees behave as though more certain about the colors of unrewarding and rewarding flower types when they learn and make this discrimination in the presence of floral scent. Without floral scent, bees show peak shift: rather than landing on the color rewarded during training (S+, 120 deg) they prefer to land on a novel color (110 deg) that is more different from the color previously associated with punishment (S–, 140 deg). They also show area shift, a heightened response to all stimuli with hues less than the S+ (80–110 deg). A signal detection theory-based interpretation of these perceptual biases is that, on unscented flowers, bees have difficulty in distinguishing the S+ from the S–. They thus become conservative in their responses such that they decrease the risk of so-called ‘false alarm’ errors, i.e. landing on unrewarding or punishing flowers, even at the cost of missing some rewarding flowers (Lynn et al., 2005). It is worth noting that other studies indicate that bees show improved discrimination between similar colors following differential (S+ vs S–) rather than absolute (S+ only) conditioning (Dyer and Chittka, 2004b; Giurfa, 2004). Although these experiments did not measure the response of bees across a wide range of stimuli, their findings are consistent with the peak shift observed in our experiment.
Interestingly, this reduction in uncertainty occurred even though bees did not discriminate between scents themselves in our test. Taken as a whole, these results point towards an interaction between olfactory and visual components of a floral display, whereby scent facilitates learning about color even when not providing additional information about floral identity.
Multimodality and the acquisition of more vs better information
Like most chemical signals (Bradbury and Vehrencamp, 1998), floral scents may travel long distances (Dobson, 1994), be regulated to reflect the state of their producer (Dudareva et al., 2004), and serve as unique identifiers (Raguso, 2008). Bees even deposit their own scents on flowers when visual discrimination is difficult (Giurfa et al., 1994). Our experiment reveals a new role for scent in plant–pollinator communication: even if scent does not provide additional information about floral identity or quality, in its presence pollinators more effectively acquire visual information. Certainly, one implication of our results is that experiments which use unscented stimuli are likely to underestimate bees' potential performance on visual learning tasks.
How might scent allow pollinators to better acquire visual information? One explanation may be attentional: in humans, for example, the presence of an olfactory stimulus can influence visual attention (Ho and Spence, 2005; Michael et al., 2003; Zhou et al., 2010). In an analogous manner, scent may alert or affect attention to other floral stimuli: because pollinators can only process a limited subset of all stimuli currently available (Dukas, 2002), scent may draw pollinators' visual attention to floral stimuli and away from non-floral stimuli. A direct test of this hypothesis might involve comparing bees' detection of other visual stimuli (e.g. response to a predator model) when foraging on flowers that are scented vs unscented, or assessing whether scent extends the in-flight period during which visual signals are thought to be learned (Menzel, 1983). A field-based study by Reinhard and colleagues (Reinhard et al., 2006) suggests that scent can serve an alerting function: honeybees trained to feeders differing in both color and scent later selectively visited the correct, unscented, feeder when its previously corresponding scent was released in their colony. While quantifying the attention-altering or alerting function of scent was beyond the scope of our experiment, van Swinderen and Greenspan (van Swinderen and Greenspan, 2003) have shown that in Drosophila melanogaster, attention-like increases in brain activity (local field potentials of 20–30 Hz in the medial protocerebrum) occur when flies are presented with a conditioned visual stimulus; of direct relevance to the current experiment, adding scent to the visual stimulus increased levels of this characteristic 20–30 Hz signature (van Swinderen and Greenspan, 2003).
From a functional perspective, the hypothesis that one signal in a complex display draws attention to a second signal has so far largely been explored in the context of sexual selection (Elias et al., 2003; Grafe and Wagner, 2007) (but see VanderSal and Hebets, 2007). The possibility that floral scent might draw pollinators' attention towards color was first suggested by Kunze and Gumbert's finding that bumble bees (B. terrestris) learn to discriminate between two floral colors faster when they transmit the same scent vs when they are both unscented (Kunze and Gumbert, 2001). In that study, as here, evidence that scent focuses attention is indirect; for example, we have noted that bees seem to spend more time on scented training flowers, an effect which might lengthen the sampling of visual information.
Secondly, rather than directing bees' attention towards color, scent could simply provide a context for learning visual signals, identifying flowers as potential nectar sources. Pollinators encounter and learn visual stimuli in contexts other than foraging, such as host plant selection (Weiss and Papaj, 2003) and colony location (Worden et al., 2005). In any one of these contexts, they learn not only the association between stimuli and reinforcement but also ‘background’ stimuli (for a review, see Shettleworth, 1998). As a contextual cue, scent might facilitate learning of visual stimuli by reducing confusion between different tasks (e.g. foraging vs nest location), or by eliciting an innate foraging response: as most flowers are scented, it may have been more difficult for bees to learn that unscented artificial flowers were food sources. Indeed, we found that naïve bees landed less often on unscented flowers; Giurfa and colleagues similarly reported that naïve honeybees would not land on unscented targets (Giurfa et al., 1995). A test of this hypothesis might involve training bees to distinguish two colors in the presence of a shared scent, then determining whether a change or removal of the scent disrupts performance.
Why did our subjects not show evidence of having learned the identity of training scents in the peak shift assay? Bees are famously adept at olfactory learning, and even show peak shift in the olfactory modality following discrimination training with scent blends (Wright et al., 2009). While our free-flight assay allowed us to observe floral learning in a semi-natural context, it is possible that blending between scent types on the vertical array made discriminating clove vs peppermint difficult. Our scent learning experiment showed that bees can learn to discriminate between stimuli that differ only in scent; however, it took many more landings for them to reach 80% accuracy than for bees learning color differences (120 deg vs 140 deg). Presented with a noisy olfactory signal and a clearer color signal, bees may have simply ignored scent as a potential source of information about floral identity.
Uncertainty reduction and the complex signal
Pollinators searching for floral rewards, females evaluating potential mates, and, to some extent, predators selecting palatable prey must often locate a resource and distinguish it from alternative, unprofitable, types that share many characteristics (e.g. mimics, heterospecific males, unpalatable prey). To complicate matters, although the signaler may broadcast their location, identity or quality, this message is inevitably obscured by environmental and sensory noise. Signal detection and classification are thus major challenges for receivers (reviewed in Wiley, 2006). When incorrect responses are costly relative to missed detections, receivers may cope with this uncertainty by adopting a conservative decision strategy: shifting their responses to signal values displaced away from those shared with the lower-quality resource. In many cases, these perceptual shifts will not benefit the signaler. In any circumstance where signalers benefit from increased receiver confidence, we should therefore expect to find display characteristics that serve this purpose. An individual plant, for example, will likely experience a decline in successful pollen transfer if pollinators show a reduced response to the most common value of its floral signal. Thus, a plant potentially benefits from transmitting scent if this reduces pollinators' uncertainty regarding color. While facilitation of color learning is clearly not the only function of floral scent (Raguso, 2008), it may be central to understanding the evolution of floral signal complexity.
Integrating uncertainty reduction into the study of signal evolution provides a functional counterpoint to recent research on the neural correlates of uncertainty (e.g. Kepecs et al., 2008; Kiani and Shadlen, 2009). Given that decision-makers' uncertainty can now be quantified physiologically and behaviorally, how have signalers evolved to manage this aspect of receiver psychology? Adding a second component to a display probably involves production costs, and may even increase the risk of attracting predators or parasites; for example, production of floral scent can attract herbivores in addition to pollinators (Theis, 2006). It has also been argued that more complex displays may require more time and energy for receivers to assess (Partan and Marler, 2005). We propose that a complex (multicomponent or multimodal) display's ability to reduce uncertainty, relative to simpler displays, may outweigh these costs and thus explain their ubiquity in the natural world.
Supplementary Material
Acknowledgments
We thank R. Kaczorowski for comments on the manuscript, B. Jones and T. Comi for help with data collection and P. Marek for assistance with spectrophotometry.
Supplementary material available online at http://jeb.biologists.org/cgi/content/full/214/1/113/DC1
This work was supported by NSF Grant no. IOS-0921280 and by the University of Arizona Center for Insect Science through NIH Training Grant no. 1K12 GM000708. Deposited in PMC for release after 12 months.
REFERENCES
- Bradbury J. W., Vehrencamp S. L. (1998). Principles of Animal Communication. Sunderland, MA: Sinauer Associates; [Google Scholar]
- Briscoe A. D., Chittka L. (2001). The evolution of color vision in insects. Annu. Rev. Entomol. 46, 471-510 [DOI] [PubMed] [Google Scholar]
- Cameron S. A., Hines H. M., Williams P. H. (2002). A comprehensive phylogeny of the bumble bees (Bombus). Biol. J. Linn. Soc. 91, 161-188 [Google Scholar]
- Candolin U. (2003). The use of multiple cues in mate choice. Biol. Rev. 78, 575-595 [DOI] [PubMed] [Google Scholar]
- Chandra S., Smith B. (1998). An analysis of synthetic processing of odor mixtures in the honey bee (Apis mellifera). J. Exp. Biol. 201, 3113-3121 [DOI] [PubMed] [Google Scholar]
- Cheng K. (2002). Generalization: mechanistic and functional explanations. Anim. Cogn. 5, 33-40 [DOI] [PubMed] [Google Scholar]
- Cheng K., Spetch M. L., Johnson M. (1997). Spatial peak shift and generalization in pigeons. J. Exp. Psychol. Anim. Behav. Process. 23, 469-481 [Google Scholar]
- Chittka L. (1992). The colour hexagon: a chromaticity diagram based on photoreceptor excitations as a generalized representation of colour opponency. J. Comp. Physiol. A 170, 533-543 [Google Scholar]
- Cnaani J., Thomson J. D., Papaj D. R. (2006). Flower choice and learning in foraging bumblebees: effect of variation in nectar volume and concentration. Ethology 112, 278-285 [Google Scholar]
- Coleman S. W. (2009). Taxonomic and sensory biases in the mate-choice literature: there are far too few studies of chemical and multimodal communication. Acta Ethol. 12, 45-48 [Google Scholar]
- Dobson H. E. M. (1994). Floral volatiles in insect biology. In Insect–Plant Interactions, Vol. 5 (ed. Bernays E.), pp. 47-82 Boca Raton, FL: CRC; [Google Scholar]
- Dudareva N., Pichersky E., Gershenzon J. (2004). Biochemistry of plant volatiles. Plant Physiol. 135, 1893-1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dukas R. D. (2002). Behavioural and ecological consequences of limited attention. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 357, 1539-1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer A. G., Chittka L. (2004a). Biological significance of distinguishing between similar colours in spectrally variable illumination: bumblebees (Bombus terrestris) as a case study. J. Comp. Physiol. A 190, 105-114 [DOI] [PubMed] [Google Scholar]
- Dyer A. G., Chittka L. (2004b). Fine colour discrimination requires differential conditioning. Naturwissenschaften 91, 224-227 [DOI] [PubMed] [Google Scholar]
- Dyer A. G., Rosa M. G., Reser D. H. (2008). Honeybees can recognise images of complex natural scenes for use as potential landmarks. J. Exp. Biol. 211, 1180-1186 [DOI] [PubMed] [Google Scholar]
- Elias D. O., Mason A. C., Maddison W. P., Hoy R. R. (2003). Seismic signals in a courting male jumping spider (Araneae: Salticidae). J. Exp. Biol. 206, 4029-4039 [DOI] [PubMed] [Google Scholar]
- Giurfa M. (2004). Conditioning procedure and color discrimination in the honeybee Apis mellifera. Naturwissenschaften 91, 228-231 [DOI] [PubMed] [Google Scholar]
- Giurfa M., Núñez J., Backhaus W. (1994). Odour and colour information in the foraging choice behavior of the honeybee. J. Comp. Physiol. A 175, 773-779 [Google Scholar]
- Giurfa M., Núñez J., Chittka L., Menzel R. (1995). Color preferences of flower-naïve honeybees. J. Comp. Physiol. A. 177, 247-259 [Google Scholar]
- Gomez D. (2006). AVICOL: A program to analyse spectrometric data. Free program available from the author at dodogomez@yahoo.fr
- Goulson D. (1999). Foraging strategies of insects for gathering nectar and pollen, and implications for plant ecology and evolution. Perspect. Plant Ecol. 2, 185-209 [Google Scholar]
- Grafe T. U., Wagner T. C. (2007). Multimodal signaling in male and female foot-flagging frogs Staurois guttatus (Ranidae): an alerting function of calling. Ethology 113, 772-781 [Google Scholar]
- Green D. M., Swets J. A. (1966). Signal Detection Theory and Psychophysics. New York: Wiley and Sons; [Google Scholar]
- Gumbert A. (2000). Color choices by bumble bees (Bombus terrestris): innate prefernces and generalization after learning. Behav. Ecol. Sociobiol. 48, 36-43 [Google Scholar]
- Hanson H. M. (1959). Effets of discrimination training on stimulus generalization. J. Exp. Psychol. 58, 321-334 [DOI] [PubMed] [Google Scholar]
- Harder L. D., Real L. A. (1987). Why are bumble bees risk averse? Ecology 68, 1104-1108 [Google Scholar]
- Hebets E. A., Papaj D. R. (2005). Complex signal function: developing a framework of testable hypotheses. Behav. Ecol. Sociobiol. 57, 197-214 [Google Scholar]
- Ho C., Spence C. (2005). Olfactory facilitation of dual-task performance. Neurosci. Lett. 389, 35-40 [DOI] [PubMed] [Google Scholar]
- Kepecs A., Uchida N., Zariwala H., Mainen Z. F. (2008). Neural correlates, computation, and behavioral impact of decision confidence. Nature 455, 227-231 [DOI] [PubMed] [Google Scholar]
- Kiani R., Shadlen M. N. (2009). Representation of confidence associated with a decision by neurons in the parietal cortex. Science 374, 759-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulahci I. G., Dornhaus A., Papaj D. R. (2008). Multimodal signals enhance decision making in foraging bumblebees. Proc. R. Soc. Lond. B. Biol. Sci. 275, 797-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze J., Gumbert A. (2001). The combined effect of color and scent on flower choice behavior of bumble bees in flower mimicry systems. Behav. Ecol. 12, 447-456 [Google Scholar]
- Levin D. A. (1978). Pollinator behaviour and the breeding structure of plant populations. In The Pollination of Flowers by Insects (ed. Richards A. J.), pp. 133-150 London: Academic Press; [Google Scholar]
- Lynn S. K., Cnaai J., Papaj D. R. (2005). Peak shift discrimination learning as a mechanism of signal evolution. Evolution 59, 1300-1305 [PubMed] [Google Scholar]
- McEwan J. R., Vamosi J. C. (2010). Floral colour versus phylogeny in structuring subalpine communities. Proc. Biol. Sci. 277, 2957-2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R. (1983). Neurobiology of learning and memory: the honeybee as a model system. Naturwissenschaften 70, 504-511 [DOI] [PubMed] [Google Scholar]
- Michael G. A., Jacquot L., Millot J., Brand G. (2003). Ambient odors modulate visual attention capture. Neurosci. Lett. 352, 221-225 [DOI] [PubMed] [Google Scholar]
- Partan S., Marler P. (2005). Issues in the classification of multimodal communication signals. Am. Nat. 166, 231-245 [DOI] [PubMed] [Google Scholar]
- Peitsch D., Fietz A., Hertel H., de Souza J., Ventura D. F., Menzel R. (1992). The spectral input systems of hymenopteran insects and their receptor-based colour vision. J. Comp. Physiol. A 170, 23-40 [DOI] [PubMed] [Google Scholar]
- Raguso R. A. (2008). Wake up and smell the roses: the ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 39, 549-569 [Google Scholar]
- Reinhard J., Srinivasan M. V., Zhang S. (2006). Complex memories in honeybees: can there be more than two? J. Comp. Physiol. A 192, 409-416 [DOI] [PubMed] [Google Scholar]
- Rowe C. (1999). Receiver psychology and the evolution of multicomponent signals. Anim. Behav. 58, 921-931 [DOI] [PubMed] [Google Scholar]
- Schiestl F. P. (2005). On the success of a swindle: pollination by deception in orchids. Naturwissenschaften 92, 255-264 [DOI] [PubMed] [Google Scholar]
- Shettleworth S. J. (1998). Cognition, Evolution and Behavior. New York: Oxford University Press; [Google Scholar]
- Spaethe J., Tautz J., Chittka L. (2001). Visual constraints in foraging bumblebees: flower size and color affect search time and flight behavior. Proc. Natl. Acad. Sci. USA 98, 3898-3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasen M., Lehrer M. (1985). Temporal resolution of colour vision in the honeybee. J. Comp. Physiol. A 157, 579-586 [DOI] [PubMed] [Google Scholar]
- Stavenga D. G., Smits R. P., Hoenders B. J. (1993). Simple exponential functions describing the absorbance bands of visual pigment spectra. Vision Res. 33, 1011-1017 [DOI] [PubMed] [Google Scholar]
- Stein B. E., Meredith M. A. (1993). The Merging of the Senses. Cambridge, MA and London: The MIT Press; [Google Scholar]
- Theis N. (2006). Fragrance of Canada thistle (Cirsium arvense) attracts both floral herbivores and pollinators. J. Chem. Ecol. 32, 1573-1561 [DOI] [PubMed] [Google Scholar]
- Van Swinderen B., Greenspan R. J. (2003). Salience modulates 20–30 Hz brain activity in Drosophila. Nat. Neurosci. 6, 579-586 [DOI] [PubMed] [Google Scholar]
- VanderSal N. D., Hebets E. A. (2007). Cross-modal effects on learning: a seismic stimulus improves color discrimination learning in a jumping spider. J. Exp. Biol. 210, 3689-3695 [DOI] [PubMed] [Google Scholar]
- Waser N. M. (1983). The adaptive nature of floral traits: ideas and evidence. In Pollination Biology (ed. Real L. A.), pp. 241-285 New York: Academic Press; [Google Scholar]
- Weiss M. R., Papaj D. R. (2003). Butterfly color learning in two different behavioral contexts: how much can a butterfly keep in mind? Anim. Behav. 65, 425-434 [Google Scholar]
- Wiley R. H. (2006). Signal detection and animal communication. Adv. Stud. Behav. 36, 217-247 [Google Scholar]
- Worden B. D., Skemp A. K., Papaj D. R. (2005). Learning in two contexts: the effects of interference and body size in bumblebees. J. Exp. Biol. 208, 2045-2053 [DOI] [PubMed] [Google Scholar]
- Wright G. A., Choudhary A. F., Bentley M. A. (2009). Reward quality influences the development of learned olfactory biases in honeybees. Proc. R. Soc. Lond. B. Biol. Sci. 276, 2597-2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. H. (1999). Biostatistical Analysis, 4th edn.Upper Saddle River, NJ: Prentice Hall; [Google Scholar]
- Zhou W., Jiang Y., He S., Chen D. (2010). Olfaction modulates visual perception in binocular rivalry. Curr. Biol. 20, 1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.