Abstract
Repetitive correlated activation of pre- and postsynaptic neurons induced long-term potentiation (LTP) of synaptic transmission among hippocampal neurons grown on a layer of astrocytes (mixed cultures) but not among neurons cultured in glial conditioned medium. Supplement of d-serine, an agonist for the glycine-binding site of N-methyl-d-aspartate (NMDA) receptors, enhanced NMDA receptor activation and enabled LTP induction in glial conditioned medium cultures. The induction of LTP in both mixed cultures and hippocampal slices was suppressed by NMDA receptor antagonists, glycine-binding-site blockers of NMDA receptors, or an enzyme that degrades endogenous d-serine. By providing extracellular d-serine that facilitates activation of NMDA receptors, astrocytes thus play a key role in long-term synaptic plasticity.
Astrocytes, the most abundant glial cells in the brain, are found to associate closely with neuronal synapses (1, 2). Although astrocytes lack excitability in terms of action potentials, they express various neurotransmitter receptors and ion channels and can create Ca2+ waves in response to various stimuli, including neuronal activity. Furthermore, astrocytes can secrete many factors that modulate neuronal activity (3–5). Recent studies have shown that astrocytes play important roles in neurogenesis (6), synaptogenesis (7, 8), and synaptic modulation (3–5, 9–11). There is evidence that astrocytes may also contribute to high-level brain functions such as motivation, learning, and behavior (12). Given the potential role of glial factors in neuronal functions, it is of interest to know whether and how astrocytes may contribute to the induction of long-term potentiation (LTP), the most extensively studied form of synaptic plasticity that may serve as a cellular mechanism underlying learning and memory (13, 14). Deletion of glial fibrillary acidic protein (GFAP) or S-100B in the mouse (15, 16) resulted in an enhancement of LTP, but how these cytoplasmic proteins in glial cells affect synaptic plasticity remains unknown. Studies of LTP are usually carried out in slice preparations, in which glial cells are intimately associated with neurons and synapses. It is thus difficult to separate the role of glia in synaptogenesis from a direct modulatory role of glial factors in synaptic plasticity. To address this problem, we have established in the present study two types of hippocampal neuronal cultures: conventional mixed cultures, in which neurons were grown on a layer of astrocytes, and cultures in which the neurons were fed with glial conditioned medium (GCM) but grown without contacting astrocytes (see Materials and Methods). In the latter GCM cultures, apparently normal synaptogenesis occurs among hippocampal neurons. By using correlated stimulation of pre- and postsynaptic neurons to induce LTP (17), we were able to analyze directly the role of immediate astrocyte-derived factors in the induction of LTP. We found that LTP could be induced in mixed cultures, but not in GCM cultures, and that glia-derived d-serine plays a key role in LTP induction by its action on the N-methyl-d-aspartate (NMDA) subtype of the glutamate receptors. Furthermore, we showed that in hippocampal slices, the induction of LTP at Schaffer collateral–CA1 pyramidal cell synapses also critically depends on the presence of intact glial cells and extracellular d-serine. Taken together, we have identified an astrocyte-derived factor that contributes to activity-induced long-term synaptic plasticity.
Materials and Methods
Cell Cultures. Primary cultures of astrocytes were prepared as described (18, 19) with some modifications. In brief, cortices were prepared from 1-d-old rat and were dissociated by trypsin, plated on polyD-lysine-coated glass coverslips, cultured with Eagle's MEM (GIBCO) containing 10% FBS, and maintained at 37°C in a 5% CO2 incubator. Astrocytes formed a confluent layer 10–14 d after plating and were ready for mixed cultures and GCM cultures (see below). Three types of hippocampal neuronal cultures were prepared in the present study: (i) pure neuronal cultures, as described (8, 20), with some modifications. Hippocampi from embryonic day 18 rat were dissociated by trypsin treatment, plated on polyD-lysine-coated glass coverslips at a cell density of 60,000/ml and maintained in MEM containing 20% glucose, 2 mM glutamine, 1% BSA, and 10% FBS for 24 h. These cultures were then treated with cytosine arabinoside (10 μM) for 48 h to kill proliferating cells and subsequently maintained in Neurobasal medium (GIBCO) containing 20% glucose and 2% B27 supplements. (ii) GCM cultures, prepared and cultured in the same manner as pure neuronal cultures except that, after cytosine arabinoside treatment, one coverslip prepared for pure neuronal cultures and three coverslips plated confluently with astrocyte cultures (10–14 d) were cultured side-by-side in a single 35-mm dish. This allows neurons to grow in GCM, and electrophysiological studies can be performed in the absence of astrocytes and in the recording medium. (iii) Mixed culture, prepared in the same manner as pure neuronal cultures except that neurons were plated on a layer of primary astrocytes (10–14 d) rather than directly on polyD-lysine-coated glass coverslips. The absence of glial cells in GCM cultures were confirmed by immunostaining by using antibodies for neuron-specific marker MAP2 and astrocyte marker GFAP. In mixed cultures, neurons were found to grow on top of a layer of astrocytes (Fig. 1A), whereas in GCM cultures, no astrocyte was found to be associated with the neuron (Fig. 1B). Astrocytes are easily distinguished from neurons by their morphological characteristics under phase-contrast microscopy, thus GCM cultures contaminated with astrocytes were not used for electrophysiological studies. The use and care of animals in this study follow the guidelines of the Shanghai Institutes of Biological Sciences Animal Research Advisory Committee.
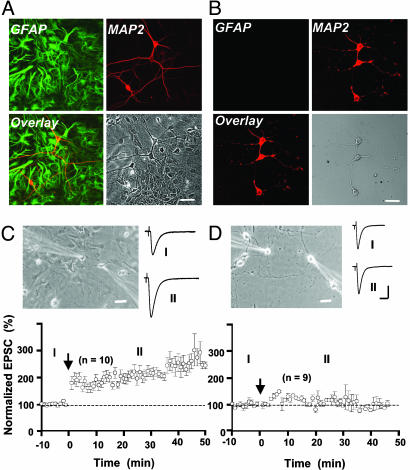
Fig. 1.
Comparing synaptic potentiation induced by correlated pre- and postsynaptic activities in two different culture preparations. (A and B) Immunostaining with anti-MAP2 (neuronal marker, red) and anti-GFAP (astrocyte marker, green) showing images of mixed (A) and GCM (B) cultures, respectively. (C and D) Correlated pre- and postsynaptic activation induced LTP in mixed cultures (C) but not in GCM cultures (D). (Upper) Phase-contrast images showing dual patch recordings from neurons in mixed (Left) and GCM (Right) cultures. The amplitude of EPSCs was normalized by the mean amplitude observed during the control period (t = -10 to 0 min). Data represent averages from all experiments (n = 10 and 9 for C and D, respectively). Arrows mark the time of repetitive correlated activation (1 Hz, 80 s), with presynaptic stimulation (1 ms, +100 mV) preceded postsynaptic stimulation (1 ms, 2 nA) by 5 ms. Traces depict samples of averaged EPSCs from one experiment during the control period (I) and at 20–30 min after correlated stimulation (II). (Scales, 20 μm for photo images; 200 pA and 20 ms for recording traces.)
Immunostaining. Cell cultures prepared as described above were fixed in 4% paraformaldehyde for 10 min before treated with blocking solution containing 10% BSA and 0.1% Triton X-100 for 1 h at room temperature. Cultures were then stained with anti-GFAP antibody (1:1,000, Chemicon) and anti-MAP2 antibody (1:100, Santa Cruz Biotechnology) overnight at 4°C. After washing to remove excess primary antibodies, the cultures were incubated for 1 h at room temperature with fluorescence-conjugated secondary antibodies (Jackson ImmunoResearch): anti-goat-IgG-Cy5 for the MAP2 antibody, and anti-rabbit-IgG-FITC for the GFAP antibody. Excess antibody was removed, and cells were imaged by using a Zeiss LSM 510 confocal microscope.
Glial Plasmalemmal Vesicle (GPV) and Synaptosome Preparation and d-Serine Measurements. Glial and nerve ending fractions were acutely isolated by discontinuous Percoll gradient centrifugation as described (21, 22) with minor modifications. d-serine was measured by a chemiluminescent assay as described (23). For detailed description, see Supporting Methods, which is published as supporting information on the PNAS web site.
Slice Preparation. Acute hippocampal slices were prepared as described (25). In brief, postnatal rats (16 ∼ 18 d) were anaesthetized with sodium pentobarbital. After decapitation, hippocampal formation was dissected rapidly and placed in ice-cold oxygenated (95% O2 and 5% CO2) solution containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, and 11 glucose, pH 7.3. Transverse slices (400 μm thick) were cut with a vibratome (Campden Instruments, Loughborough, U.K.) and maintained in an incubation chamber for at least 2 h at 26°C before recording. During experiments, an individual slice was transferred to a submersion-recording chamber and was continuously perfused with the above oxygenated solution (3.0 ml/min) at 26 ∼ 28°C. Slices were visualized with infrared optics by using an Olympus (Tokyo) microscope (BX50WI) equipped with differential interference contrast microscopy optics.
Electrophysiology. For cultured neurons, whole-cell perforated patch recordings from two hippocampal neurons were performed simultaneously, by using amphotericin B (Sigma) for perforation (17). The patch electrodes were made from borosilicate glass capillaries (Sutter Instruments, Novato, CA), with a resistance in the range of 3 ∼ 5 MΩ. The pipettes were tip-filled with internal solution and then back-filled with internal solution containing 150 ng/ml amphotericin B. The internal solution contained (in mM): 136.5 K-gluconate, 17.5 KCl, 9 NaCl, 1 MgCl2, 10 Hepes, and 0.2 EGTA (pH 7.2). The recording medium was a Hepes-buffered saline containing (in mM): 145 NaCl, 3 KCl, 10 Hepes, 3 CaCl2, 8 glucose, and 2 MgCl2 (pH 7.3). The bath was constantly perfused with fresh recording medium at a slow rate (≈1 ml/min) throughout the recording. All experiments were done at room temperature (20 ∼ 24°C). Neurons were visualized with a phase-contrast inverted microscope (IX50, Olympus). Recordings were made with two patch-clamp amplifiers (Axopatch 200B; Axon Instruments, Foster City, CA). For assaying synaptic connectivity, each neuron was stimulated at a low frequency (0.0167 Hz) by 1-ms-step depolarization from -70 to + 30 mV in voltage-clamp mode, and the responses of the other neuron as well as autaptic responses in the stimulated neuron itself were recorded. The inhibitory postsynaptic currents had distinctly longer decay times and more negative reversal potentials than excitatory postsynaptic currents (EPSCs) and were sensitive to blockage by bicuculline. Only glutamatergic synapses on postsynaptic glutamatergic neurons were studied for LTP induction. LTP was induced by repetitive correlated pre- and postsynaptic activation (1 Hz, 80 sec), with presynaptic stimulation (1 ms, +100 mV) preceded postsynaptic stimulation (1 ms, 2 nA) by 5 ms (17, 26). During correlated stimulation, presynaptic neuron was voltage-clamped at -70 mV, whereas the postsynaptic neuron was current-clamped at -70 mV to allow generation of back-propagating spikes. In experiments when the ratio NMDA receptor (NMDAR)/α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) was calculated, EPSCs or miniature EPSCs (mEPSCs) with both NMDAR and AMPAR components were recorded in Mg2+-free extracellular medium. The NMDAR and AMPAR components were separated temporally by their distinct kinetics (27, 28). The amplitude of NMDAR–mediated current was determined in a window between 15 and 25 ms after the peak of AMPAR-mediated component, which has a fast rising time <1 ms (27, 28).
For recordings from hippocampal slices, all experiments were carried out in the presence of GABAA antagonist picrotoxin (50 μM). Whole-cell patch recordings were made from pyramidal neurons in the cell body layer of CA1. Recording pipettes were routinely filled with a solution containing (in mM): 125 K-gluconate, 15 KCl, 10 Hepes, 8 NaCl, 4 Mg-ATP, 0.3 Na-GTP, 10 Na2-phosphocreatine, 2 EGTA (pH 7.30). EPSPs with amplitudes in the range of 5 ∼ 7 mV were induced by constant current pulses (1 ∼ 5 μA, 200 μs, 0.033 Hz) through extracellular bipolar electrodes (MCE-100, RMI, Woodland Hill, CA). The membrane potential was maintained at a level of -70 mV by injection of a constant current (<60 pA). LTP was induced by θ-burst stimulation (TBS) protocol as described (29), with some modifications. A train of subthreshold synaptic stimulation consisted of five bursts of synaptic stimuli at 5 Hz given through the extracellular electrode. Each burst was composed of five pulses (0.2 ms, 1–5 μA) at 100 Hz, and the train was repeated twice with a 20-s interval. Each burst was paired with three postsynaptic spikes induced by intracellular current injection (2 nA, 2 ms) at 50 Hz, starting 5 ms after the presynaptic burst. For recording of cultured neurons or slices, data were accepted for analysis only when postsynaptic currents or voltages did not vary beyond 15% of the average values during the control period, and the input resistance (100–300 MΩ) remained constant throughout the experiment. Signals filtered with amplifier circuitry were sampled at 10 kHz and analyzed by using CLAMPEX 8.0 (Axon). Analysis of mEPSCs was performed with MINI ANALYSIS PROGRAM, Ver. 5.2.5 (Synaptosoft, Decatur, GA). Data were presented as means ± SEM, and statistical differences were determined by Student's t test. Reagents were obtained from Sigma–Aldrich, except where noted.
Results
Correlated Activity Induced LTP in Mixed Cultures but Not in GCM Cultures. Dual perforated whole-cell recording was made from synaptically connected pairs of hippocampal neurons in mixed or GCM cultures (Fig. 1 C and D), and repetitive correlated stimulation was applied to the pre- and postsynaptic neurons to induce LTP (17). Neurons in mixed cultures were in contact with a layer of astrocytes, whereas those in GCM cultures were not in contact with any glial cells (see Materials and Methods). Normal recording solution was used to replace culture medium during the experiment. As shown in Fig. 1C, the amplitude of EPSCs in mixed cultures was significantly elevated after a brief period of correlated activity, during which the presynaptic neuron was stimulated repetitively 5 ms before each postsynaptic spike was initiated (1 Hz, 80 s). In contrast, when the same protocol was applied to neurons in GCM cultures, we observed no significant increase in the amplitude of EPSCs (Fig. 1D). Because no astrocytes were present in GCM cultures and no glial factors were present in the recording medium, this result suggests that astrocytes provide some immediate and essential factor(s) for the induction of LTP.
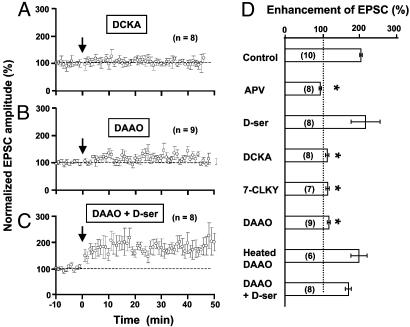
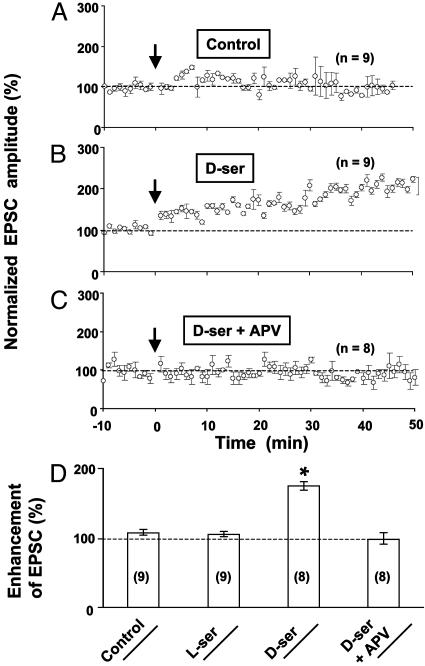
Endogenous d-Serine Is Critical for LTP Induction in Cultured Neurons. Consistent with previous reports (17, 30), we found that activation of NMDARs is required for the induction of LTP by correlated pre- and postsynaptic activity, because bath application of the NMDAR antagonist 2-amino-5-phosphonovalerate (APV, 25 μM) completely abolished LTP (Fig. 2D). Furthermore, application of 5,7-dichlorokynurenic acid (DCKA) and 7-chlorokynurenic acid, the antagonists for the glycine-binding site of NMDARs (31), also blocked LTP induction in mixed cultures (Fig. 2 A and D), indicating that LTP induction requires coactivation of the glycine-binding site of NMDARs (32). Because d-serine, an agonist for the glycine-binding site of NMDARs (31), is known to be synthesized mainly in astrocytes (23, 33–37), we tested whether the failure of LTP induction in GCM cultures (Fig. 3A) was due to the lack of d-serine that is normally supplied by astrocytes. As shown in Fig. 3 B and D, addition of d-serine (10 μM) to the culture indeed enabled the induction of LTP in GCM cultures, whereas l-serine had no effect. Furthermore, the effect of d-serine can be blocked by NMDAR antagonist APV (Fig. 3 C and D) The LTP induced in GCM cultures supplemented with d-serine appeared to be less robust than that induced in mixed cultures (comparing Figs. 1C and 3B), suggesting either a reduced capacity for LTP in neurons grown in these GCM cultures or the need of other astrocyte factors for LTP induction in addition to d-serine. The requirement of endogenous d-serine for the induction of LTP in mixed cultures was further confirmed by the effect of d-serine-degrading enzyme DAAO (34, 38). Incubation of mixed cultures with DAAO (0.1 units/ml) for 30 min significantly diminished the induction of LTP in these neurons, whereas heat-inactivated DAAO had no effect (Fig. 2 B and D). Finally, application of exogenous d-serine (10μM) during the induction period restored the capacity for LTP induction in DAAO-treated mixed cultures (Fig. 2 C and D), indicating that the enzyme DAAO did not produce nonspecific deleterious effects on the neuron that prevented LTP induction, and that excess d-serine can override the action of DAAO on the available d-serine for LTP induction.
Fig. 2.
Dependence of LTP induction in mixed cultures on NMDAR activation, the glycine-binding site, and d-serine. (A–C) LTP induction in mixed cultures in the presence of glycine-binding site antagonist DCKA (10 μM, A), d-serine-degrading enzyme DAAO (0.1 units/ml, B), and d-serine (10 μM) in addition to DAAO (0.1 units/ml, C). Data were normalized in the same manner as that shown in Fig. 1C. (D) Summary of results from all experiments, including data shown in A–C. Other experiments include treatments with NMDAR antagonist APV (25 μM), glycine-binding site antagonist 7-CLKY (10 μM), glycine-binding site agonist d-serine (10 μM), and heat-inactivated DAAO (0.1 units/ml; heated at 90°C for 5 min). The enhancement of EPSCs was measured by the mean amplitude of EPSCs at 20–30 min after correlated stimulation, normalized in each neuron by the mean EPSC amplitude observed during the control period (-10 to 0 min). The number associated with each column refers to the number of neurons tested in each condition. Asterisks indicate significant difference from the control group (not treated with any drug, P < 0.001, t test).
Fig. 3.
d-serine enabled the induction of LTP in GCM cultures. (A) No LTP induction was induced in GCM cultures under control conditions. (B) Supplement of d-serine (10μM) enabled the induction of LTP in GCM cultures. (C) The enabling effect of d-serine was abolished by NMDAR antagonist APV. (D) Summary of results from all experiments, including data shown in A–C and experiments in which l-serine (10 μM) was used instead of d-serine. Asterisks indicate significant difference from the control group (P < 0.001, t test).
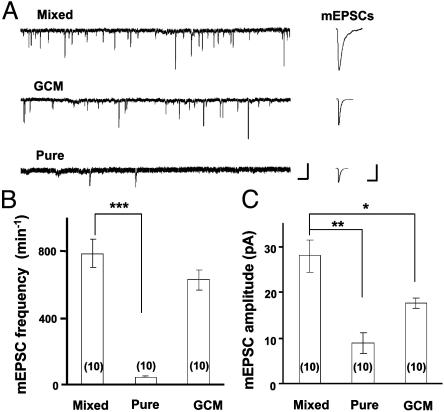
Endogenous d-Serine Is Required for NMDAR Activation in Cultured Neurons. Glia-derived factors are known to be important for synaptogenesis (7, 8). In the present study, we found that the frequency of mEPSCs in the presence of tetrodotoxin (1 μM) and bicuculline (10 μM) and in the absence of Mg2+ was not significantly different between mixed cultures and GCM cultures, whereas very few mEPSCs were recorded in neurons cultured without astrocytes or GCM (“pure” neuronal cultures; see Materials and Methods; Fig. 4 A and B). These findings suggest that synaptogenesis was similar in mixed and GCM cultures but was defective in pure neuronal cultures. We noted that the mean amplitude of mEPSCs in GCM cultures was lower than that recorded in mixed cultures (Fig. 4C). This reduced amplitude of mEPSCs may be attributed to the reduced NMDAR activation in the absence of endogenous agonist for the glycine-binding site, as suggested by the following study of the effects of d-serine on NMDA component of EPSCs in these cultures.
Fig. 4.
Comparison of miniature synaptic currents in three different culture preparations. (A) Continuous traces depict membrane currents and spontaneous mEPSCs recorded from postsynaptic neurons in three types of cultures (see Materials and Methods). Extracellular Mg2+ was omitted in the recording medium. (Scales, 25 pA and 1 s.) Traces (Right) depict samples of mEPSCs (average of 10 events) observed in the experiment depicted (Left). (Scales, 10 pA and 20 ms.) (B and C) Summary of the average frequency and amplitude of mEPSCs observed in three different cultures (n = 10 for each group). Asterisks indicate significant difference from the data of mixed cultures (*, P < 0.05; **, P < 0.01; ***, P < 0.001, t test.)
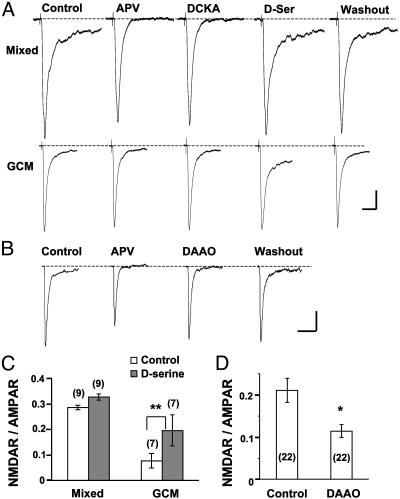
Previous studies have shown that glycine or d-serine is necessary for NMDAR activation (31). A slow current component of EPSCs, with characteristics of the current mediated by NMDARs and sensitive to APV or DCKA, was frequently recorded in mixed cultures but not in GCM cultures (Fig. 5A). Treatment with DCKA significantly reduced the amplitude of EPSCs in mixed cultures to 75.0 ± 11.3% (SEM, n = 12) but did not affect the amplitude of EPSCs in GCM cultures (96.6 ± 7.5%, n = 11). The NMDAR- and non-NMDA- or AMPAR-mediated EPSCs can be separated temporally according to their kinetics. The current amplitude ratio of NMDAR and AMPAR components was then determined (see Materials and Methods; ref. 28). We found that the ratio in mixed cultures (0.29 ± 0.01, n = 9) was >3-fold higher than that in GCM cultures (0.08 ± 0.03, n = 7, Fig. 5C). These results indicate that in GCM cultures, NMDARs were not significantly activated during synaptic transmission. As shown in Fig. 5 A and C, a supplement with d-serine (10 μM) significantly increased the slow component of EPSCs in GCM cultures but had much less effect on EPSCs recorded in mixed cultures. Thus, the lack of NMDAR activation during synaptic transmission in GCM cultures was due to a deficiency in the agonist for the glycine-binding site rather than in NMDAR expression. To test whether endogenous d-serine is responsible for the activation of NMDARs in mixed cultures, we recorded mEPSCs in mixed cultures with or without a 15-min pretreatment of DAAO (0.1 units/ml). We found that the ratio NMDAR/AMPAR for mEPSCs was decreased significantly after DAAO pretreatment (Fig. 5 B and D).
Fig. 5.
Analysis of NMDAR-mediated EPSCs and mEPSCs in mixed and GCM cultures. (A) Samples of EPSCs (average of 5 ∼ 10 events) recorded in the mixed and GCM cultures before (control) and during the perfusion with APV (25 μM) or DCKA (10 μM) or d-serine (10 μM), and after washout of d-serine. Note the significant increase in the NMDAR component (APV-sensitive slow component) in the GCM culture after perfusion with d-serine. (Scales, 100 pA and 50 ms.) (B) Samples of mEPSCs (average of 22 events) recorded in the mixed cultures before (control) during the perfusion with APV or DAAO and after washout of DAAO. (Scales, 5 pA and 20 ms.) (C) Ratio of the NMDAR and AMPAR components of EPSCs obtained from data similar to those shown in A, before and after perfusion with d-serine. (D) Ratio of the NMDAR and AMPAR components of mEPSCs obtained from experiments in mixed cultures, similar to those shown in B, before and after treatment with DAAO. Asterisks indicate significant differences between the two groups (*, P < 0.01; **, P < 0.001, t test).
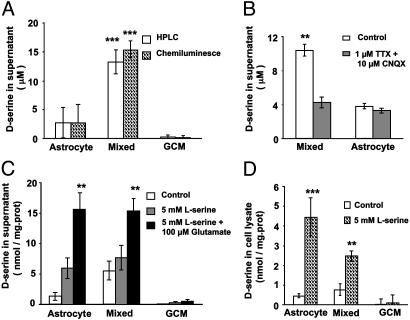
d-Serine Release from Cultured Astrocytes. To examine directly whether astrocytes are the main source of d-serine in our culture system, we measured the d-serine level in the culture supernatant and cell lysates by using chemiluminescence and/or HPLC. The results obtained with the chemiluminescence assay were similar to those obtained with HPLC (Fig. 6A). We detected a significant level of d-serine in the culture supernatant and cell lysates from pure astrocyte cultures and mixed cultures, but not from GCM cultures (Fig. 6). Furthermore, a supplement of l-serine to the culture medium significantly increased d-serine concentration in pure astrocyte or mixed cultures but not in GCM cultures (Fig. 6 C and D), consistent with the reports that astrocytes, rather than neurons, express serine racemase (23). We found that medium from mixed cultures contained significantly higher d-serine than that from pure astrocyte cultures (Fig. 6), possibly due to an elevated d-serine release from astrocytes in response to spontaneous neuronal activity in mixed cultures. This idea is supported by the finding that treatment of cultures with Tetrodotoxin (1 μM) and CNQX (10 μM) to block neuronal activity significantly decreased the concentration of d-serine in the supernatant of mixed cultures to a level comparable to that in the supernatant of pure astrocyte cultures, whereas in the later cultures, the same treatment had no effect on d-serine level (Fig. 6B). Furthermore, bath application of glutamate to mimic neuronal activity resulted in a significant increase in the d-serine release in pure astrocyte cultures or mixed cultures but not in GCM cultures (Fig. 6C).
Fig. 6.
d-serine synthesis and release in different cultures. (A) Free d-serine level in the supernatant of three different cultures was assayed by chemiluminescence and HPLC methods. There is significant difference between astrocyte culture and GCM culture (P < 0.01), between astrocyte culture and mixed culture (P < 0.001), and between mixed culture and GCM culture (P < 0.001). (B) Treatment of cultures with tetrodotoxin and CNQX significantly decreased d-serine in the supernatant of mixed cultures but not in the supernatant of pure astrocyte cultures. (C) d-serine in the supernatant from different cultures under control, pretreatment of l-serine for 48 h, or 48-h pretreatment of l-serine plus 10-min exposure to glutamate. Data were normalized with cell content (protein level) of cultures. (D) d-Serine measured from cell lysates from different cultures with or without 48-h pretreatment with l-serine. d-serine was measured by chemiluminescence assay in B–D. Results were from four independent experiments in each group for A–D. Asterisks in B–D indicate significant differences from other groups of the same culture preparation (**, P < 0.01; ***, P < 0.001, t test).
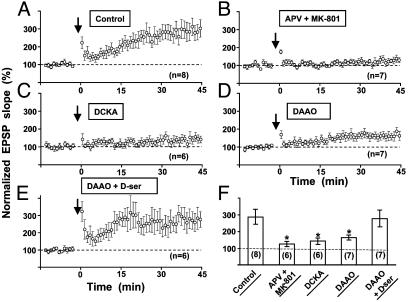
LTP Induction in Hippocampal Slices Requires Endogenous d-Serine. To extend the above findings in cell cultures to a more intact preparation, we further examined whether d-serine is also critical for LTP induction in rat hippocampal slices. Extracellular stimulation was made in the s. radiatum at a distance of about 100 ∼ 200 μm from the CA1 cell body layer to stimulate Schaffer collaterals. Whole-cell recording was made in CA1 pyramidal neurons, and LTP was induced by TBS paired with postsynaptic spikes (see Materials and Methods). Picrotoxin (50 μM) was added to the media to block GABAA receptor-mediated inhibitory postsynaptic currents. As reported (29), the induction of LTP by TBS depends on the activation of NMDARs. Treatment of the slices with NMDAR antagonists APV (100 μM) and MK-801 (20 μM) abolished LTP (Fig. 7 B and F). Similar to results from mixed cultures (Fig. 2 A and D), DCKA significantly reduced the magnitude of LTP induced by TBS (Fig. 7 C and F). Furthermore, preincubation of slices with DAAO (0.1 units/ml) for 45 min to degrade endogenous d-serine also greatly reduced LTP (Fig. 7 D and F). Finally, addition of d-serine (100 μM) to the slices 10 min before the onset of the experiment largely reversed the effect of DAAO preincubation on LTP (Fig. 7 E and F). Taken together, these results show that endogenous d-serine is also critical for the induction of LTP in hippocampal slices.
Fig. 7.
The involvement of endogenous d-serine in the induction of LTP in hippocampal slices. (A) Summary of all experiments in control slices showing the induction of LTP in CA1 pyramidal neurons by TBS (see Materials and Methods). (B–E) Under the same stimulation conditions as in A, LTP induction was significantly inhibited by perfusing the slice with NMDA receptor antagonists, APV and MK801 (B), antagonist of glycine-binding site DCKA (C), and d-serine degrading enzyme DAAO (D). The blockade of LTP induction by DAAO was rescued by perfusing d-serine (100 μM) in addition to DAAO (E). Data shown are averaged values of EPSP slope normalized by the mean EPSP slope observed during the control period (-10 to 0 min) in each experiment. The arrow marks the time of the TBS. (F) Summary of results from all experiments shown in A–E. Data represent the averaged slope of EPSP at 35–45 min after TBS, normalized in each neuron by its mean EPSP slope observed during the control period. Numbers associated with each column refer to the number of neurons tested in each condition. Asterisks indicate data significantly different from the data obtained in control slices (*, P < 0.001, t test).
DAAO Suppressed NMDAR EPSCs in Hippocampal Slices. To further test the idea that DAAO inhibits LTP induction in hippocampal slices by suppressing NMDAR activation through the depletion of endogenous d-serine, we directly examined the effect of DAAO on the NMDAR-mediated component of EPSCs. This was done by omitting Mg2+ and adding DNQX (25 μM) in the recording solution. After perfusion of 0.1 units/ml DAAO for >20 min, we found that the NMDAR-EPSCs were significantly suppressed, and this suppression was reversible when DAAO was washed out (Fig. 8, which is published as supporting information on the PNAS web site). In contrast, the same concentration of DAAO did not affect AMPAR-EPSCs that were evoked in the presence of 100 μMAPV and 2 mM Mg2+, indicating specific action of DAAO on NMDARs.
d-Serine Is Released from Glial Terminals but Not from Neuronal Terminals. To address the question whether endogenous d-serine releases from glial cells or neurons in acute brain tissue, we prepared highly purified neuronal terminal synaptosomes and glial terminal GPV (21, 22). We found that treatment of GPV with 0.2 mM glutamate for 5 min increased d-serine in the supernatants of GPV from 3.65 ± 0.77 (control group, n = 4) to 5.05 ± 0.82 nmol/mg protein (n = 4, P < 0.01, compared with control group). In contrast, d-serine in the supernatant of synaptosomes was undetectable, even after being treated with glutamate (0.2 mM, 5 min) or high K+ (120 mM, 5 min).
Discussion
In the present study, we found that correlated pre- and postsynaptic spiking induced LTP in mixed cultures in which neurons were in contact with astrocytes, but not in GCM cultures in which neurons were fed with glial-conditioned media in the absence of astrocyte contact. Astrocytes have been reported to play an important role in synaptogenesis between retinal ganglion cells (7, 8). The failure in LTP induction in GCM cultures we observed is unlikely to be caused by a deficiency in synapse formation, because apparent normal spontaneous and evoked synaptic transmission was found in GCM cultures (Figs. 1 and 4; see also refs. 7 and 8).
Induction of LTP by correlated pre- and postsynaptic activity in both hippocampal slice and cultures depends on Ca2+ influx through NMDARs (17, 39). Full activation of the NMDARs requires the presence of coagonists for its glycine-binding site, e.g., glycine or d-serine (31). We found that antagonists of either NMDARs or their glycine-binding sites blocked LTP induction in hippocampal neurons in mixed cultures and freshly isolated slices (Figs. 2 and 7), consistent with previous reports (17, 32, 39). Furthermore, supplement of d-serine in GCM cultures enabled LTP induction in these neurons (Fig. 3 B and D), suggesting that the failure in LTP induction in these neurons is due to the deficiency of this ligand for the glycine-binding site of NMDARs. This notion is supported by the finding that in GCM cultures, the NMDAR/AMPAR ratio of EPSCs was significantly reduced, and supplement of d-serine resulted in a much more pronounced increase in the ratio, as compared with that in mixed cultures (Fig. 5 A and C).
Both glycine and d-serine are effective ligands for the glycine-binding site of NMDARs (31, 40). It has been suggested that astrocytes may play a key role in NMDAR function by controlling the extracellular concentration of glycine or d-serine (33, 34, 37, 41, 42). Although cerebrospinal fluid contains relatively high concentration of glycine (31), the synaptically available glycine for the activation of NMDARs may not exist at saturating levels (43, 44), possibly due to the presence of the glycine transporters expressed on astrocytes (42–45). Recent studies suggest that in many brain regions, including hippocampus, d-serine rather than glycine may serve as the endogenous ligand for the glycine-binding site (23, 33–37). In the present study, a significant d-serine level was found in the culture supernatant and in the cell lysates from pure astrocyte cultures or mixed cultures, but not from GCM cultures (Fig. 6). Pretreatment of l-serine to promote synthesis of d-serine or acute application of glutamate to mimic neuronal excitation increased d-serine level in the pure astrocyte cultures and mixed cultures but not in GCM cultures (Fig. 6 C and D). We also found that d-serine was detectable in the supernatants of glial terminal GPV, but not in that of neuronal terminal synaptosomes acutely isolated from hippocampus. Furthermore, treatment with glutamate increased d-serine in the supernatants of GPV but not in that of synaptosomes. All of these results are consistent with previous reports that astrocytes, rather than neurons, are the main source of d-serine in the brain (23, 33–37). The finding that the basal d-serine level in the supernatant of mixed cultures is higher than that in astrocyte cultures (Fig. 6) is consistent with the expectation that glutamate release associated with spontaneous neuronal activities in these cultures induced d-serine release from astrocytes. Our finding that pretreatment with d-serine-degrading enzyme DAAO inhibited LTP induction in both mixed cultures (Fig. 2) and hippocampal slices (Fig. 7) suggests that endogenous d-serine rather than glycine is critical for LTP induction. This notion is further supported by the finding that DAAO reversibly reduced the NMDAR component of EPSCs in both cultured neurons and hippocampal slices (Figs. 5 and 8; ref. 34). The inhibitory effect of DAAO on NMDAR-EPSCs was found to be more pronounced in mixed cultures than in slices (compare Fig. 5 B and D to Fig. 8). This may be attributed to a more severe diffusion barrier for DAAO in the slice preparation (34). Although DAAO caused only ≈30% suppression in the amplitude of NMDAREPSCs in CA1 pyramidal neurons (Fig. 8), it significantly diminished the magnitude of LTP (Fig. 7F), suggesting that high-level activation of NMDARs is normally required for LTP induction.
The potential involvement of glial cells in neuronal plasticity and higher brain functions has long been suggested (10, 12, 15, 16, 46). The present study provides evidence for the involvement of astrocytes in LTP induction and the underlying mechanism. Astrocyte-derived d-serine, by modulating the level of NMDA receptor activation, may regulate the extent of LTP induced by correlated synaptic activity. Because the glutamate released due to synaptic activity may evoke d-serine release through activation of non-NMDA receptors in astrocytes (36), and release from a large population of astrocytes may be triggered through propagated Ca2+ waves (3–5, 18), glia-dependent modulation of synaptic plasticity may serve for long-range cross-talk between activated synapses. The prevalence and the physiological implications of such glia-mediated synaptic cross-talk in the processing and storage of information in the neural network remain to be further explored.
Supplementary Material
Acknowledgments
We thank Dr. Qian Hu for confocal technical help and Xiao-Yuan Zhu for preparing cell cultures. This work was supported by a grant from the Major State Basic Research Program of China (G200077800) and by Shanghai Science and Technology Development Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LTP, long-term potentiation; GCM, glial conditioned medium; NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor; GFAP, glial fibrillary acidic protein; GPV, glial plasmalemmal vesicle; DAAO, d-amino acid oxidase; EPSC, excitatory postsynaptic current; mEPSC, miniature EPSC; AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; APV, 2-amino-5-phosphonovalerate; DCKA, 5,7-dichlorokynurenic acid; TBS, θ-burst stimulation.
References
- 1.Grosche, J., Matyash, V., Moller, T., Verkhratsky, A., Reichenbach, A. & Kettenmann, H. (1999) Nat. Neurosci. 2, 139-143. [DOI] [PubMed] [Google Scholar]
- 2.Ventura, R. & Harris, K. M. (1999) J. Neurosci. 19, 6897-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araque, A., Carmignoto, G. & Haydon, P. G. (2001) Annu. Rev. Physiol. 63, 795-813. [DOI] [PubMed] [Google Scholar]
- 4.Bezzi, P. & Volterra, A. (2001) Curr. Opin. Neurobiol. 11, 387-394. [DOI] [PubMed] [Google Scholar]
- 5.Haydon, P. G. (2001) Nat. Rev. Neurosci. 2, 185-193. [DOI] [PubMed] [Google Scholar]
- 6.Song, H., Stevens, C. F. & Gage, F. H. (2002) Nature 417, 39-44. [DOI] [PubMed] [Google Scholar]
- 7.Mauch, D. H., Nagler, K., Schumacher, S., Goritz, C., Muller, E. C., Otto, A. & Pfrieger, F. W. (2001) Science 294, 1354-1357. [DOI] [PubMed] [Google Scholar]
- 8.Ullian, E. M., Sapperstein, S. K., Christopherson, K. S. & Barres, B. A. (2001) Science 291, 657-661. [DOI] [PubMed] [Google Scholar]
- 9.Beattie, E. C., Stellwagen, D., Morishita, W., Bresnahan, J. C., Ha, B. K., Von Zastrow, M., Beattie, M. S. & Malenka, R. C. (2002) Science 295, 2282-2285. [DOI] [PubMed] [Google Scholar]
- 10.Oliet, S. H., Piet, R. & Poulain, D. A. (2001) Science 292, 923-926. [DOI] [PubMed] [Google Scholar]
- 11.Smit, A. B., Syed, N. I., Schaap, D., van Minnen, J., Klumperman, J., Kits, K. S., Lodder, H., van der Schors, R. C., van Elk, R., Sorgedrager, B., et al. (2001) Nature 411, 261-268. [DOI] [PubMed] [Google Scholar]
- 12.Laming, P. R., Kimelberg, H., Robinson, S., Salm, A., Hawrylak, N., Muller, C., Roots, B. & Ng, K. (2000) Biobehav. Rev. 24, 295-340. [DOI] [PubMed] [Google Scholar]
- 13.Bliss, T. V. & Collingridge, G. L. (1993) Nature 361, 31-39. [DOI] [PubMed] [Google Scholar]
- 14.Martin, S. J., Grimwood, P. D. & Morris, R. G. (2000) Annu. Rev. Neurosci. 23, 649-711. [DOI] [PubMed] [Google Scholar]
- 15.McCall, M. A., Gregg, R. G., Behringer, R. R., Brenner, M., Delaney, C. L., Galbreath, E. J., Zhang, C. L., Pearce, R. A., Chiu, S. Y. & Messing, A. (1996) Proc. Natl. Acad. Sci. USA 93, 6361-6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishiyama, H., Knopfel, T., Endo, S. & Itohara, S. (2002) Proc. Natl. Acad. Sci. USA 99, 4037-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bi, G. Q. & Poo, M. M. (1998) J. Neurosci. 18, 10464-10472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duan, S., Anderson, C. M., Keung, E. C., Chen, Y. M., Chen, Y. R. & Swanson, R. A. (2003) J. Neurosci. 23, 1320-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duan, S., Anderson, C. M., Stein, B. A. & Swanson, R. A. (1999) J. Neurosci. 19, 10193-10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosilin, K., Asmussen, H. & Banker, G. (1998) in Culturing Nerve Cells, eds. Banker, G. & Gosilin, K. (MIT Press, Cambridge, MA), pp. 339-370.
- 21.Nakamura, Y., Iga, K., Shibata, T., Shudo, M. & Kataoka, K. (1993) Glia 9, 48-56. [DOI] [PubMed] [Google Scholar]
- 22.Daniels, K. K. & Vickroy, T. W. (1998) Neurochem. Res. 23, 103-113. [DOI] [PubMed] [Google Scholar]
- 23.Wolosker, H., Blackshaw, S. & Snyder, S. H. (1999) Proc. Natl. Acad. Sci. USA 96, 13409-13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto, A., Nishikawa, T., Oka, T., Takahashi, K. & Hayashi, T. (1992) J. Chromatogr. 582, 41-48. [DOI] [PubMed] [Google Scholar]
- 25.Wang, Z., Xu, N., Wu, C. P., Duan, S. & Poo, M. (2003) Neuron, 37, 463-472. [DOI] [PubMed] [Google Scholar]
- 26.Bi, G. Q. & Poo, M. M. (1999) Nature 401, 792-796. [DOI] [PubMed] [Google Scholar]
- 27.Hestrin, S., Sah, P. & Nicoll, R. A. (1990) Neuron 5, 247-253. [DOI] [PubMed] [Google Scholar]
- 28.Watt, A. J., van Rossum, M. C., MacLeod, K. M., Nelson, S. B. & Turrigiano, G. G. (2000) Neuron 26, 659-670. [DOI] [PubMed] [Google Scholar]
- 29.Magee, J. C. & Johnston, D. (1997) Science 275, 209-213. [DOI] [PubMed] [Google Scholar]
- 30.Markram, H., Lubke, J., Frotscher, M. & Sakmann, B. (1997) Science 275, 213-215. [DOI] [PubMed] [Google Scholar]
- 31.Kemp, J. A. & Leeson, P. D. (1993) Trends Pharmacol. Sci. 14, 20-25. [DOI] [PubMed] [Google Scholar]
- 32.Baron, B. M., Harrison, B. L., Miller, F. P., McDonald, I. A., Salituro, F. G., Schmidt, C. J., Sorensen, S. M., White, H. S. & Palfreyman, M. G. (1990) Mol. Pharmacol. 38, 554-561. [PubMed] [Google Scholar]
- 33.Baranano, D. E., Ferris, C. D. & Snyder, S. H. (2001) Trends Neurosci. 24, 99-106. [DOI] [PubMed] [Google Scholar]
- 34.Mothet, J. P., Parent, A. T., Wolosker, H., Brady, R. O., Jr., Linden, D. J., Ferris, C. D., Rogawski, M. A. & Snyder, S. H. (2000) Proc. Natl. Acad. Sci. USA 97, 4926-4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schell, M. J., Brady, R. O., Jr., Molliver, M. E. & Snyder, S. H. (1997) J. Neurosci. 17, 1604-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schell, M. J., Molliver, M. E. & Snyder, S. H. (1995) Proc. Natl. Acad. Sci. USA 92, 3948-3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Snyder, S. H. & Kim, P. M. (2000) Neurochem. Res. 25, 553-560. [DOI] [PubMed] [Google Scholar]
- 38.Nagata, Y. (1992) Experientia 48, 753-755. [DOI] [PubMed] [Google Scholar]
- 39.Malenka, R. C. & Nicoll, R. A. (1999) Science 285, 1870-1874. [DOI] [PubMed] [Google Scholar]
- 40.Forsythe, I. D., Westbrook, G. L. & Mayer, M. L. (1988) J. Neurosci. 8, 3733-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cull-Candy, S. (1995) Curr. Biol. 5, 841-843. [DOI] [PubMed] [Google Scholar]
- 42.Roux, M. J. & Supplisson, S. (2000) Neuron 25, 373-383. [DOI] [PubMed] [Google Scholar]
- 43.Adams, R. H., Sato, K., Shimada, S., Tohyama, M., Puschel, A. W. & Betz, H. (1995) J. Neurosci. 15, 2524-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berger, A. J., Dieudonne, S. & Ascher, P. (1998) J. Neurophysiol. 80, 3336-3340. [DOI] [PubMed] [Google Scholar]
- 45.Bergeron, R., Meyer, T. M., Coyle, J. T. & Greene, R. W. (1998) Proc. Natl. Acad. Sci. USA 95, 15730-15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muller, C. M. & Best, J. (1989) Nature 342, 427-430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.