Abstract
Objective
To compare the effects of standard silicone oil 5700 (SSO) and heavy silicone oil (HSO) such as Densiron® 68 on intraocular pressure (IOP).
Materials and methods
Retrospective case series including 180 eyes (105 treated with SSO and 75 with HSO). IOP was measured before surgery, 1 day after, and then at 1-, 3-, 6-, and 12-month follow-ups.
Results
In the SSO group, a significant increase in IOP occurred in 14% of the eyes (15/105) at 1 day postoperatively, and persisted in 11.4% (12/105) at 1-month follow-up. In the HSO group, a persistent elevated IOP was recorded in 20% of the eyes (15/75) at 1 day postoperatively, and in 16% (12/75) at 1-month follow-up. At 12-month follow-up, mean IOP was 16.7 ± 8.7 mmHg and 19.7 ± 3.8 mmHg, respectively, in the SSO and HSO groups. The difference between the 2 groups was always not significant.
Conclusion
Overall, the use of Densiron 68 was not associated with higher IOP values as compared with SSO.
Keywords: Densiron 68, heavy silicone oil, silicone oil, intraocular pressure, vitrectomy
Introduction
The use of silicone oil (SO) in conjunction with advanced vitreous surgical techniques can successfully treat complicated retinal detachment (RD),1–4 including proliferative vitreoretinopathy, giant retinal tears, and severe trauma cases.5,6 SO is still the best-tolerated substance and is well-accepted and biologically well-tolerated in the clinical management of complicated conditions. Because the specific gravity of standard silicone oil (SSO) (0.97 g/cm3) is lower than that of water, it has an effective tamponade effect for the treatment of retinal pathologies in the upper quadrants. The most frequent complication with SSO internal tamponade is the persistence or recurrence of inferior RD.7,8
Several attempts have been undertaken to develop a vitreous tamponade with a specific gravity greater than that of water.9,10 Recently, partially fluorinated alkanes in combination with SSO have been proposed as heavy tamponade agents for long-term tamponade. The combination of these 2 substances results in a single-bubble optically clear mixture according to the proportions used.11–13
Densiron® 68 (Fluoron Gmbh, Neu-Ulm, Germany) is a solution of SSO and perfluorohexyloctane (F6H8). By adding F6H8 to silicone, the mixture has a specific gravity of 1.06 g/cm3 and a viscosity of 1387 centistokes (cSt). It is therefore heavier than water and sufficiently viscous to have a much lower propensity for dispersion (compared with F6H8 on its own). Therefore, the advantage of long-term heavy tamponades with a specific gravity greater than that of water is to provide an effective postoperative tamponade of the inferior quadrants.
Long-term glaucoma following SSO use is uncommon in current surgery, but past studies have indicated an incidence ranging from 3% to 40% of cases.4,10,14,15 Some of these cases can develop secondary glaucoma.5,14–16 The Silicone Study Report 4 has recently recorded a prevalence of chronically elevated intraocular pressure (IOP) in 8% of patients with SSO.17
The aim of this study was to compare IOP modifications in eyes treated with SSO versus heavy silicone oil (HSO) (Densiron 68) in the management of complicated RD.
Materials and methods
Study design
We performed a retrospective case study of 180 eyes of 180 patients: 105 eyes (65 phakic, 35 pseudophakic, 5 aphakic) treated with SSO 5700 cSt (Oxane 5700®; Bausch and Lomb Inc., Toulouse, France) and 75 eyes (57 phakic, 15 pseudophakic, 3 aphakic) treated with Densiron 68 tamponade.
All cases were treated from June 2006 to January 2008 in 3 different centers in Italy by 5 different vitreoretinal surgeons. The exclusion criteria were severe systemic disease, pregnancy, and pre-existing ocular inflammatory disease.
Patients and preoperative findings
The mean age in the first group was 64 ± 21 (15–81) years and in the second group 58 ± 15 (13–90) years. IOP was measured before surgery, 1 day after surgery, and then at 1-, 3-, 6-, and 12-month follow-ups. SSO removal was planned within 6 months (160 ± 35 days), whereas Densiron 68 removal was planned within 3 months (130 ± 15 days) from the initial surgery, according to the presence of emulsification. The reason for such a difference is that Densiron 68 contains F6H8, which might lead to a higher rate of inflammation in cases of long-term endotamponade. The following preoperative and postoperative parameters were recorded: etiology of RD, refractive status, pre-existing glaucoma, lens status, diabetes mellitus, presence of SSO in the anterior chamber, emulsification of SSO, and rubeosis iridis. Given the retrospective nature of this study, gonioscopy data were not available in all cases, and therefore such parameters have not been included in the study design. The baseline patient parameters are summarized in Table 1.
Table 1.
Baseline patient characteristics
| Group | No. eyes | Gender (M/F) | Age (years) | Lens status (Ph/Pseph/Aph) | Removal of oil |
|---|---|---|---|---|---|
| SSO 5700 | 105 | 34/73 | 64 ± 21 (15–81) | 65/35/5 | 160 ± 35 days |
| Densiron 68 | 75 | 45/30 | 58 ± 15 (13–90) | 57/15/3 | 130 ± 15 days |
Abbreviations: M, male; F, female; Ph, phakic; Pseph, pseudophakic; Aph, aphakic; SSO, standard silicone oil.
Surgical techniques
The indication for surgery and for use of oil as endotamponade was mainly RD. Moreover, 11 cases (4 in the SSO group and 7 in the HSO group) underwent surgery for stage 4 macular hole or reopening macular hole.
Surgery was performed with monitored anesthesia care and a retrobulbar block. In both groups the surgical procedure included a standard 3-port pars plana vitrectomy using the ACCURUS® System (Alcon Laboratories, Inc., Hünenberg, Switzerland) or Associate® System (DORC, Zuidland, the Netherlands) for vitrectomy. During vitrectomy, the vitreous base was thoroughly removed. Epiretinal membrane dissection and relaxing retinotomies were performed, when necessary. The retinal periphery was inspected for retinal breaks, and any break found was treated with cryocoagulation or endolaser photocoagulation. A fluid–air exchange procedure was then performed with humidified air.
At the end of the surgical procedure, SSO or HSO (Densiron 68) was injected by an automatic device. HSO with an interfacial tension against water at 25°C of 40.82 mN/m, a specific gravity of 1.06 g/cm3, and a viscosity of 1387 cSt was used. The SSO used was of 5700 cSt viscosity at 25°C, with a specific gravity of 0.965 g/cm3 and interfacial tension against water at 25°C of 35.5 mN/m. The iridotomies were performed in aphakic eyes: inferior iridotomy in the SSO group and superior iridotomy in the HSO group. At the end of surgery the eye appeared clinically completely filled by the substance. Suture of the sclerotomies followed. In all cases the surgery was not combined with scleral buckle placement.
Examinations
IOP was recorded using the Goldmann applanation tonometer. The presence of ocular hypertension (OH) was defined as postoperatively elevated IOP greater than 25 mmHg, or with a value greater than 10 mmHg above the preoperative level.
As this work was performed as an audit, our hospital did not require the study to have a specific ethical approval.
Results
We report functional and anatomical results, with particular attention given to differences in IOP and to the presence of SSO in the anterior chamber (AC). Moreover, we investigated the depth of the AC and the retinal status at the follow-up checks. Statistical analysis was performed using SPSS software version 10 (SPSS Inc., Chicago, IL, USA).
IOP
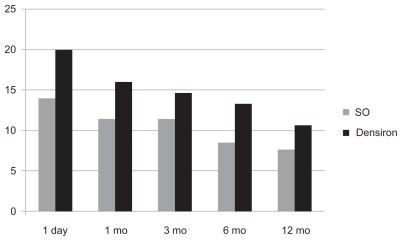
OH occurred in 14% of the eyes (15 out of 105) at 1 day postoperatively, and persisted in 11.4% of the eyes (12 out of 105) at 1-month follow-up in the presence of SSO. In the HSO group, OH occurred in 20% of the eyes (15 out of 75) at 1 day postoperatively, and persisted in 16% of the eyes (12 out of 75) at 1-month follow-up. The differences between the 2 groups were always not significant (P = 0.18, chi-square test). In the SSO group at 3-month follow-up, IOP was controlled in 12 of 15 eyes (81%), 45% with topical treatment (beta-blockers, dorzolamide, and brinzolamide), 30% with oral treatment (acetazomide), and 6% with other surgical procedures; in the HSO group at 3-month follow-up, IOP was controlled in 11 of 15 eyes (73%), 40% with topical treatment, 20% with oral treatment, and 13% with other surgical procedures. In the SSO group, mean postoperative IOP values at 1-day and 1-, 3-, and 6-month follow-up were 22.8 ± 8.9, 22.3 ± 7.4, 20.5 ± 9.5, and 18.6 ± 8.4 mmHg, respectively. In the HSO group, mean postoperative IOP values at 1-day and 1-, 3-, and 6-month follow-up were 23.4 ± 5.7, 21.8 ± 6.4, 22.3 ± 9.2, and 18.9 ± 8.7, respectively. At 12-month follow-up, mean IOP was 16.7 ± 8.7 mmHg and 19.7 ± 3.8 mmHg in the SSO and HSO groups, respectively (Figure 1). The difference in IOP between the 2 groups was not statistically significant (P = 0.21, chi-square test). At 12-month follow-up, 7.6% of patients (8 out of 105) in the SSO group and 10.6% of patients (8 out of 75) in the HSO group were still on topical treatment for glaucoma (in most cases with a combination of topical dorzolamide–timolol).
Figure 1.
Percentage of OH in SSO and Densiron 68 group at each follow-up visit.a
Note: aOH is defined as elevated IOP greater than 25 mmHg, or greater than 10 mmHg above the preoperative level.
Abbreviations: OH, ocular hypertension; SSO, standard silicone oil; mo, month; IOP, intraocular pressure.
One case (0.95%) in the SSO group and 2 cases (2.6%) in the HSO group needed surgical treatment with trabeculectomy to lower IOP.
The trabeculectomy was performed after the removal of oil in both groups. The patients did not develop neovascular glaucoma but showed a persistent elevated IOP, probably due to the emulsified SSO, which may induce changes in the trabecular meshwork with a reduction in the outflow of aqueous humor.
The oil was removed when necessary in relation to AC inflammation, emulsification, and the presence of a well-formed chorioretinal scar. Therefore, the mean time for removal of HSO was shorter than for SSO. This could influence the IOP results at the 3-month follow-up but not the final 12-month follow-up.
The clinical data of patients are summarized in Table 2 and Table 3.
Table 2.
Clinical data of patient series over the follow-up period
| SSO group |
Densiron 68 group |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IOP baseline | OH% 1 day postoperative | OH% 1 month postoperative | OH% 3 month postoperative | OH% 6 month postoperative | OH% 12 month postoperative | IOP baseline | OH% 1 day postoperative | OH% 1 month postoperative | OH% 3 month postoperative | OH% 6 month postoperative | OH% 12 month postoperative |
| 14.5 ± 6 | 14 (15/105) | 11.4 (12/105) | 11.4 (12/105) | 8.5 (9/105) | 7.6 (8/105)b | 15.2 ± 4 | 20 (15/75) | 16 (12/75) | 14.6 (11/75) | 13.3 (10/75) | 10.6 (8/75)b |
| 15/0* | 11/1a | 8/0a | 15/0a | 9/2a | 8/0a | ||||||
Notes: % patients on treatment with topical IOP-lowering drops/surgical treatment;
No statistically significant difference between the two groups.
Abbrevations: SSO, standard silicone oil; IOP, intraocular pressure; OH, ocular hypertension is defined as elevated IOP greater than 25 mmHg or greater than 10 mmHg above the preoperative level.
Table 3.
Anatomical and functional outcomes at different follow-up intervals
| Variable | Baseline | 1 day | 1 month | 3 month | 6 month | 12 month |
|---|---|---|---|---|---|---|
| SSO groupa | ||||||
| IOP | 15.2 ± 3.1 | 22.8 ± 8.9 | 22.3 ± 7.4 | 20.5 ± 9.5 | 18.6 ± 8.4 | 16.7 ± 8.7 |
| Anatomical success rate (%) | 105 (100) | 98 (93) | 94 (89) | 92 (88) | 103 (98) | |
| BCVA logMar | 1.21 ± 0.94 | 1.01 ± 0.76 | 0.86 ± 0.35 | 0.94 ± 0.75 | 0.60 ± 0.54 | 0.56 ± 0.51 |
| Presence of SO (in/out) | 105/0 | 105/0 | 105/0 | 77/28 | 10/95 | 1/104 |
| Densiron 68 groupb | ||||||
| IOP | 14.8 ± 2.8 | 23.4 ± 5.7 | 21.8 ± 6.4 | 22.3 ± 9.2 | 18.9 ± 8.7 | 19.7 ± 3.8 |
| Anatomical success rate (%) | 75 (100) | 72 (96) | 68 (91) | 64 (85) | 74 (99) | |
| BCVA logMar | 1.74 ± 0.83 | 1.1 ± 0.79 | 0.78 ± 0.65 | 0.9 ± 0.84 | 0.68 ± 0.63 | 0.48 ± 0.81 |
| Presence of Densiron 68 (in/out) | 75/0 | 75/0 | 75/0 | 8/67 | 0/75 | 0/75 |
Notes: Mean time of SO removal was 160 ± 35 days;
Mean time of SO removal was 130 ± 15 days.
Abbreviations: SSO, standard silicone oil; IOP, intraocular pressure; BCVA, best corrected visual acuity.
Anatomical results
Anatomical success was defined as complete retinal reattachment without the presence of any endotamponade. Because of its specific gravity, SSO does not provide adequate support to the inferior retina, which HSO provides for the superior retina.
One month after the removal of oil, anatomical success was achieved in 92 out of 105 eyes (87.6%) in the SSO group. In this group we also noted an RD not involving the posterior pole in 2 out of 105 eyes (1.9%) in the superior quadrant and in 6 out of 105 eyes (5.7%) in the inferior quadrant. Thirteen eyes required further surgery (Table 3).
In the HSO group, anatomical success was achieved in 64 out of 75 eyes (85.3%). An RD not involving the posterior pole was noted in 3 out of 75 eyes (4%) in the superior quadrant, and in 4 out of 75 eyes (5.3%) in the inferior quadrant. Eleven eyes required further surgery. In all reattachments SSO was used as endotamponade (Table 3).
The best corrected visual acuity improved from 1.21 ± 0.94 to 0.56 ± 0.51 logMar in the SSO group, and from 1.74 ± 0.83 to 0.48 ± 0.81 logMar in the HSO group.
Anterior chamber findings
At 1-day follow-up, in the SSO group, a shallow AC was found in 2 eyes out of 105 (1.9%), which were both pseudophakic. No oil was found in the AC of these patients.
In the HSO group, a shallow AC was found in 4 out of 75 eyes (5.3%): 3 pseudophakic and 1 aphakic. The presence of oil in the AC was recorded in 10 eyes (13.3%), 9 of which were pseudophakic and 1 aphakic.
Discussion
The cause of raised IOP following the use of endotamponade in the surgical treatment of complicated RD may be multifactorial, including inflammation, previous vitreoretinal procedures, and overfilling.16 Clinically significant increased IOP could represent a complication following vitreoretinal procedures, which can lead to the development of secondary glaucoma.18,19 Previous studies have reported effective control of IOP in this type of OH with medical and surgical procedures, with possible use of antimetabolites or filtration tubes.20,21
Henderer et al reported that 21% (80 out of 383 eyes) of patients treated with SSO for complex RD had an elevated IOP (greater than 25 mm Hg) at 12-month follow-up.22 These authors pointed out that risk factors for an elevated postoperative IOP were a history of glaucoma, diabetes mellitus, and a high IOP on the first postoperative day.22
Wong et al reported a case-control study on 128 patients to compare the postoperative IOP in patients treated with HSO with those treated with SSO.23 At the first day and at 2 weeks postoperatively, mean IOP was higher in patients treated with HSO (P = 0.05 and 0.01, respectively). By the fourth week, the IOP difference between the 2 groups was insignificant (P = 0.17).23 On day 1, 12.7% eyes (9 out of 71) in the HSO group and 3.5% eyes (2 out of 57) in the SSO group had an IOP greater than 30 mmHg. At 4 weeks, an IOP of more than 30 mmHg was still seen in 12.7% (9 out of 71) of the HSO-treated group and in 1.8% (9 out of 57) in the SSO group.23 In our series we did not find any significant IOP regression after removal of SSO or HSO.
In a series of 70 eyes of 70 patients, Tranos et al showed that although medical treatment is successful in lowering IOP in most patients, there was a mild progression of the mean vertical cup/disc ratio from 0.6 (SD 0.2) to 0.7 (SD 0.2) in the majority of patients during the follow-up period.24 Moreover, the visual outcome of eyes with final IOP greater than 21 mmHg was significantly worse than that exhibited by eyes with a normal (6–21 mmHg) IOP range.25
There are several important limitations to this study, including the retrospective nature, diurnal variation of IOP, and variety of surgeons, techniques, and instruments used. The analysis of SSO emulsification is complex due to the multifactorial etiology. The presence of red blood cell membranes, plasma lipoproteins, purified high-density lipoprotein apolipoproteins, an encircling band, and even the oil/aqueous movement generated by high-speed vitrectomy handpieces result in shearing forces and support the SSO emulsification.22–26
To the best of our knowledge, there are no reports on long-term changes in IOP after the use of HSO.
In our series, there was no significant difference between the 2 groups, but there appeared to be a trend for somewhat higher values in the HSO group, both in the early postoperative period and at longer follow-up times. Our findings could be explained by the different tendency for emulsification of the 2 tamponades.26 HSO and SSO are known to remain stable for 3 and 6 months, respectively.27,28 At the time of oil removal, some droplets may be involuntarily left in the vitreous cavity. Over time, these small amounts of tamponade may emulsify and then may induce a chronic reaction. The macrophages react toward the tamponade emulsion as if it were a foreign body, as already reported by Hiscott et al28 for F6H8, and thus they are able to promote, theoretically, an IOP increase. This effect seems to be higher in the HSO group of patients.
In our series we did not report any case of pupillary block associated with SSO or HSO caused by overfilling of the globe at the end of surgery. We believe that the long-term increased IOP depends on an open-angle mechanism rather than a closed-angle mechanism. We can speculate that the persistence of a raised pressure could be related to the presence of emulsificated oil in the AC and in the trabecular meshwork, which persists after the removal of oil.
A high IOP value on the first postoperative day represents a prognostic negative risk factor for the development of glaucoma in long-term follow-up. In most cases, topical treatment is sufficient to keep the pressure under control. Prophylactic treatment should be considered in high-risk eyes.
Footnotes
Disclosure
The authors declare no conflicts of interest.
References
- 1.Cibis PA, Becker B, Okun E, et al. The use of liquid silicone in retinal detachment surgery. Arch Ophthalmol. 1962;68:590–599. doi: 10.1001/archopht.1962.00960030594005. [DOI] [PubMed] [Google Scholar]
- 2.Lucke KH, Foerster MH, Laqua H. Long-term results of vitrectomy and silicone oil in 500 cases of complicated retinal detachments. Am J Ophthalmol. 1987;104:624–633. doi: 10.1016/0002-9394(87)90176-0. [DOI] [PubMed] [Google Scholar]
- 3.Cox MS, Trese TM, Murphy LP. Silicone oil for advanced proliferative vitreoretinopathy. Ophthalmology. 1986;93:646–650. doi: 10.1016/s0161-6420(86)33686-8. [DOI] [PubMed] [Google Scholar]
- 4.Riedel KG, Gabel VP, Neubauer L, et al. Intravitreal silicone oil injection: complications and treatment of 415 consecutive patients. Graefes Arch Clin Exp Ophthalmol. 1990;228:19–23. doi: 10.1007/BF02764284. [DOI] [PubMed] [Google Scholar]
- 5.Azen SP, Scott IU, Flynn HW, Jr, et al. Silicone oil in the repair of complex retinal detachments. A prospective observational multicenter study. Ophthalmology. 1998;105:1587–1597. doi: 10.1016/S0161-6420(98)99023-6. [DOI] [PubMed] [Google Scholar]
- 6.Yeo JH, Glaser BM, Michels RG. Silicone oil in the treatment of complicated retinal detachments. Ophthalmology. 1987;94:1109–1113. doi: 10.1016/s0161-6420(87)33328-7. [DOI] [PubMed] [Google Scholar]
- 7.Sharma T, Gopal L, Shanmugam MP, et al. Management of recurrent retinal detachment in silicone oil-filled eyes. Retina. 2002;22:153–157. doi: 10.1097/00006982-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Abrams GW, Azen SP, McCuen BW, II, et al. Vitrectomy with silicone oil or long-acting gas in eyes with severe proliferative vitreoretinopathy: results of additional and long-term follow-up. Silicone Study report 11. Arch Ophthalmol. 1997;115:335–344. doi: 10.1001/archopht.1997.01100150337005. [DOI] [PubMed] [Google Scholar]
- 9.Hammer ME, Rinder DF, Hicks L, et al. In: PVR Tolerance of Perfluorocarbons, Fluorosilicone, and Silicone Liquids in the Vitreous. Freeman HM, Tolentino FI, editors. Springer; 1988. pp. 156–161. [Google Scholar]
- 10.Yamamoto S, Takeuchi S. Silicone oil and fluorosilicone. Semin Ophthalmol. 2000;15:15–24. doi: 10.3109/08820530009037847. [DOI] [PubMed] [Google Scholar]
- 11.Meinert H, Roy T. Semifluorinated alkanes – a new class of compounds with outstanding properties for use in ophthalmology. Eur J Ophthalmol. 2000;10:189–197. [PubMed] [Google Scholar]
- 12.Hoerauf H, Kobuch K, Dresp J, Menz DH. Combined use of partially fluorinated alkanes, perfluorocarbon liquids and silicone oil: an experimental study. Graefes Arch Clin Exp Ophthalmol. 2001;239:373–381. doi: 10.1007/s004170100264. [DOI] [PubMed] [Google Scholar]
- 13.Herbert E, Stappler T, Wetterqvist C, Wong D. Tamponade properties of double-filling with perfuorohexyloctane and silicone oil in a model eye chamber. Graefes Arch Clin Exp Ophthalmol. 2004;242:250–254. doi: 10.1007/s00417-003-0830-6. [DOI] [PubMed] [Google Scholar]
- 14.Honavar SG, Goyal M, Majji AB, Sen PK, et al. Glaucoma after pars plana vitrectomy and silicone oil injection for complicated retinal detachments. Ophthalmology. 1999;106:169–176. doi: 10.1016/S0161-6420(99)90017-9. [DOI] [PubMed] [Google Scholar]
- 15.Al-Jazzaf AM, Netland PA, Charles S. Incidence and management of elevated intraocular pressure after silicone oil injection. J Glaucoma. 2005;14:40–46. doi: 10.1097/01.ijg.0000145811.62095.fa. [DOI] [PubMed] [Google Scholar]
- 16.Ichhpujani P, Jindal A, Jay Katz L. Silicone oil induced glaucoma: a review. Graefes Arch Clin Exp Ophthalmol. 2009;247:1585–1593. doi: 10.1007/s00417-009-1155-x. [DOI] [PubMed] [Google Scholar]
- 17.Henderer JD, Budenz DL, Flynn HW, et al. Elevated intraocular pressure and hypotony following silicone oil retinal tamponade for complex retinal detachment: incidence and risk factors. Arch Ophthalmol. 1999;117:189–195. doi: 10.1001/archopht.117.2.189. [DOI] [PubMed] [Google Scholar]
- 18.Weinberg RS, Peyman GA, Huamonte FU. Elevation of intraocular pressure after pars plana vitrectomy. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1976;200:157–611. doi: 10.1007/BF00414365. [DOI] [PubMed] [Google Scholar]
- 19.Aaberg TM, van Horn DL. Late complications of pars plana vitreous surgery. Ophthalmology. 1978;85:126–140. doi: 10.1016/s0161-6420(78)35683-9. [DOI] [PubMed] [Google Scholar]
- 20.Barr CC, Lai MY, Lean JS, Linton KL, Trese M, et al. Postoperative intraocular pressure abnormalities in the silicone study. Silicone study Report 4. Ophthalmology. 1993;100:1629–1635. doi: 10.1016/s0161-6420(93)31425-9. [DOI] [PubMed] [Google Scholar]
- 21.Heimann H, Stappler T, Wong D. Heavy tamponade 1: a review of indications, use, and complications. Eye. 2008;22:1342–1359. doi: 10.1038/eye.2008.61. [DOI] [PubMed] [Google Scholar]
- 22.Henderer JD, Budenz DL, Flynn HW, Jr, et al. Elevated intraocular pressure and hypotony following silicone oil retinal tamponade for complex retinal detachment: incidence and risk factors. Arch Ophthalmol. 1999;117:189–195. doi: 10.1001/archopht.117.2.189. [DOI] [PubMed] [Google Scholar]
- 23.Wong D, Kumar I, Quah SA, et al. Comparison of postoperative intraocular pressure in patients with Densiron 68 vs conventional silicone oil: a case-control study. Eye. 2009;23:190–194. doi: 10.1038/sj.eye.6703055. [DOI] [PubMed] [Google Scholar]
- 24.Tranos P, Asaria R, Aylward W, Sullivan P, Franks W. Long term outcome of secondary glaucoma following vitreoretinal surgery. Br J Ophthalmol. 2004;88:341–343. doi: 10.1136/bjo.2003.028076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonas JB, Knorr HL, Rank RM, et al. Intraocular pressure and silicone oil endotamponade. J Glaucoma. 2001;10:102–108. doi: 10.1097/00061198-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Caramoy A, Schröder S, Fauser S, Kirchhof B. In vitro emulsification assessment of new silicone oils. Br J Ophthalmol. 2010;94:509–512. doi: 10.1136/bjo.2009.170852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen QH, Lloyd MA, Heuer DK, et al. Incidence and management of glaucoma after intravitreal silicone oil injection for complicated retinal detachments. Ophthalmology. 1992;99:1520–1526. doi: 10.1016/s0161-6420(92)31771-3. [DOI] [PubMed] [Google Scholar]
- 28.Hiscott P, Magee RM, Colthurst M, Lois N, Wong D. Clinicopathological correlation of epiretinal membranes and posterior lens opacification following perfluorohexyloctane (F6H8) tamponade. Br J Ophthalmol. 2001;85:179–183. doi: 10.1136/bjo.85.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]