Abstract
Purpose
A new carboxymethylcellulose (CMC)-containing ophthalmic formulation of 0.45% ketorolac, pH 6.8 (Acuvail®) was recently developed for treatment of inflammation and pain after cataract surgery. This study compared pharmacokinetics of the new formulation with that of a prior formulation, 0.4% ketorolac, pH 7.4 (Acular LS®).
Methods
Ketorolac formulations were administered bilaterally (35 μL) to female New Zealand White rabbits. Samples from aqueous humor and iris-ciliary body were collected at multiple time points, and ketorolac was quantified using liquid chromatography-tandem mass spectrometry.
Results
In aqueous humor, the peak concentration (Cmax) and area under the concentration-time curve (AUC0–τ) of ketorolac were, respectively, 389 ng/mL and 939 ng·h/mL following administration of the CMC-containing 0.45% ketorolac, pH 6.8, and 211 ng/mL and 465 ng·hr/mL following administration of the 0.4% ketorolac, pH 7.4. In iris-ciliary body, Cmax and AUC0–τ of ketorolac were, respectively 450 ng/g and 2040 ng·h/g after administration of the CMC-containing 0.45% ketorolac, pH 6.8, and 216 ng/g and 699 ng·h/g after administration of the 0.4% ketorolac, pH 7.4. PK simulations predicted an AUC0–τ of 2910 ng·h/g for twice daily, CMC-containing 0.45% ketorolac, pH 6.8, compared to 725 ng·h/g for 4 times daily, 0.4% ketorolac, pH 7.4.
Conclusions
The CMC-containing formulation of 0.45% ketorolac, pH 6.8, increased ketorolac bioavailability by 2-fold in aqueous humor and by 3-fold in iris-ciliary body in comparison to the 0.4% ketorolac, pH 7.4, allowing a reduced dosing schedule from 4 times daily to twice daily.
Keywords: Acuvail, Acular LS, inflammation, ketorolac, ocular pharmacokinetics, twice-daily dosing
Introduction
Ketorolac is a nonsteroidal anti-inflammatory drug with analgesic and anti- inflammatory properties.1 Currently available ophthalmic formulations of ketorolac include a 0.5% solution (Acular®; Allergan Inc., Irvine, CA), indicated for treatments of inflammation following cataract extraction and ocular itching due to seasonal allergic conjunctivitis, and a 0.4% solution, (Acular LS®; Allergan Inc.), indicated for treatment of pain and burning/stinging after corneal refractive surgery.2,3 Both of these ketorolac formulations are indicated for instillation 4 times daily.
Several clinical studies have demonstrated the safety and efficacy of the 0.5% and 0.4% ketorolac formulations for the alleviation of ocular inflammation and pain, prevention and treatment of cystoid macular edema, and/or prevention of intraoperative miosis in ocular surgery patients.4–10 With 20% less active ingredient, the 0.4% ketorolac was demonstrated to be equivalent in potency to the 0.5% ketorolac in animal and human studies.8,11 Both the 0.4% and 0.5% ketorolac solutions, however, contain the preservative benzalkonium chloride (BAK), the surfactant octoxynol-40, and the metal-chelating agent sodium edetate and are associated with a high incidence of burning and stinging upon instillation.2,3,9
The formulation of ophthalmic solutions can profoundly affect the safety, bioavailability, and tissue distribution of the active ingredients. To enhance the ocular bioavailability and tolerability of ketorolac, various modifications were made to the 0.4% ketorolac formulation, which included increasing the concentration of active ingredient, lowering formulation pH, adding carboxymethylcellulose (CMC), and excluding preservative, surfactant, and metal chelating agents. The purpose of this study was to evaluate the impact of these modifications on the ocular pharmacokinetics (PK) of ketorolac.
Methods
Ketorolac formulations
The ophthalmic solution of 0.4% ketorolac (Acular LS) contains 0.006% BAK, sodium chloride, 0.015% edetate disodium, octoxynol-40, purified water, and hydrochloric acid and/or sodium hydroxide to adjust the pH to approximately 7.4.2 For the formulations tested here: a) the preservative BAK, the metal-chelating agent edetate disodium, and the surfactant octoxynol-40 were omitted; b) CMC was added as a viscosity agent; and c) the concentration of ketorolac was increased to 0.45%. The pH was adjusted by the addition of hydrochloric acid or sodium hydroxide.
PK sampling
A 35 μL aliquot of test formulations or 0.4% ketorolac was topically administered to each eye of female New Zealand White rabbits (Covance Research Products Inc., Denver, PA). Samples from aqueous humor (n = 6 eyes of 3 rabbits/timepoint) were collected at 1, 2, and 4 hours postdose. Samples from iris-ciliary body (n = 4 eyes of 2 rabbits/timepoint) were collected at 1, 2, 4, and 8 hours postdose. Animals were euthanized with an intravenous injection of sodium pentobarbital. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee, and the study conformed to the principles of animal use set forth by the Association for Research in Vision and Ophthalmology Statement for Use of Animals in Ophthalmic and Vision Research.
Ketorolac extraction and quantification
Ketorolac concentrations in aqueous humor and irisciliary body were determined after liquid-liquid extraction by a liquid chromatography-tandem mass spectrometry (LC-MS/MS). Iris-ciliary body samples were prepared for extraction by homogenization in 1 mL of 50% methanol. Finasteride (500 ng/mL; 10 μL) was then added to 50 μL aliquots of aqueous humor and to 300 μL aliquots of the homogenized iris-ciliary body samples. Since stable-isotope labeled ketorolac is not available, finasteride, which has an approximately similar retention time on liquid chromatography, was used as an internal standard. Ketorolac was extracted with methyl tert-butyl ether. The organic extracts were evaporated to dryness, and the precipitates were reconstituted with 40% acetonitrile for injection onto the LC-MS/MS system utilizing an atmospheric-pressure chemical ionization heated nebulizer source. Ketorolac quantification was conducted in the positive ion multiple reaction–monitoring mode.
Data analysis
Thermo Electron Watson™ (Thermo Electron Corporation, Waltham, MA) software was used for PK calculations. All samples were analyzed for peak concentrations (Cmax), time to Cmax (Tmax), and area under the concentration-time curve from time 0 to the sampling time (AUC0–τ) using the random method for nonsequential sampling. Relative bioavailability (relative %F) was expressed as the percentage of the AUC0–τ of the 0.4% ketorolac following a single ocular instillation (35 μL). PK simulations were performed using WinNonlin Enterprise (Pharsight, Mountain View, CA).
Statistical analysis
Between-group differences in iris-ciliary body Cmax were evaluated by one-way analysis of variance (ANOVA) and Dunett’s multiple comparison test. P < 0.05 was considered a statistically significant difference.
Results
Effects of CMC and pH on ketorolac PK in aqueous humor
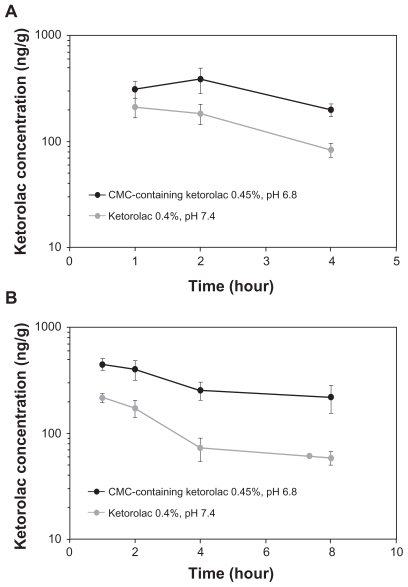
The PK of the CMC-containing test formulations at different pH were compared to that of the 0.4% ketorolac (Acular LS) (Table 1). At pH = 7.4, the inclusion of CMC as well as a 12.5% increase in ketorolac concentration enhanced ketorolac bioavailability in aqueous humor by 35% compared to ketorolac 0.4% (AUC0–4 [SEM] of 627 ± 51 ng·h/mL versus 465 ± 65 ng·h/mL, respectively). Decreasing the pH from 7.4 to 6.8 in combination with the addition of CMC enhanced ketorolac bioavailability in aqueous humor by 2-fold compared to ketorolac exposure following the 0.4% ketorolac administration (AUC0–4 [SEM] of 939 ± 163 ng·h/mL versus 465 ± 65 ng·h/mL, respectively) (Table 1). Temporal kinetics of aqueous ketorolac concentrations following instillation of the CMC-containing 0.45% ketorolac, pH 6.8, or the 0.4% ketorolac, pH 7.4, are presented in Figure 1a.
Table 1.
Aqueous Humor pharmacokinetics of ketorolac following administration of the 0.4% ketorolac, pH 7.4 (Acular LS), and the CMC-containing 0.45% ketorolac, pH 6.8 (Acuvail), formulations at varying pH
| Ketorolac (%w/v) | CMC | pH | Tmax (hour) | Cmax (ng/mL [SD]) | AUC0–4 (ng·h/mL [SEM]) | Relative %F |
|---|---|---|---|---|---|---|
| 0.40 | − | 7.4 | 1.0 | 211 (106) | 465 (65) | 100 |
| 0.45 | + | 7.4 | 2.0 | 265 (71) | 627 (51) | 135 |
| 0.45 | + | 7.2 | 1.0 | 240 (84) | 619 (50) | 133 |
| 0.45 | + | 7.0 | 1.0 | 268 (125) | 658 (73) | 142 |
| 0.45 | + | 6.8 | 2.0 | 389 (258) | 939 (163) | 202 |
Abbreviations: AUC0–4, area under the concentration-time curve from time 0 to 4 hours; CMC, carboxymethylcellulose; Cmax, peak concentration; relative %F, percentage of the AUC0–4 value compared to that of the 0.4% ketorolac; SEM, standard error of the mean; SD, standard deviation; Tmax, time to Cmax.
Figure 1.
Temporal kinetics of ketorolac concentrations (±SE M) following a single topical administration of the CMC-containing 0.45% ketorolac, pH 6.8 (Acuvail), and the 0.4% ketorolac, pH 7.4 (Acular LS), in aqueous humor (a) and iris-ciliary body (b).
Abbreviation: CMC, carboxymethylcellulose.
Effects of CMC and pH on ketorolac PK in iris-ciliary body
In iris-ciliary body, the Cmax (SD) and AUC0–8 (SEM) of ketorolac were, respectively, 450 ± 117 ng/g and 2040 ± 240 ng·h/g following administration of the CMC-containing 0.45% ketorolac, pH 6.8, and 216 ± 43 ng/g and 699 ± 74 ng·h/g after administration of the 0.4% ketorolac (Table 2). The between-group difference in ketorolac Cmax was statistically significant (P < 0.05). Overall, ketorolac bioavailability increased by 3-fold in iris ciliary body following administration of the CMC-containing 0.45% ketorolac, pH 6.8, compared to the 0.4% ketorolac, pH 7.4. Temporal kinetics of ketorolac concentrations in iris-ciliary body following instillation of the CMC-containing 0.45% ketorolac, pH 6.8, or the 0.4% ketorolac, pH 7.4, are presented in Figure 1b.
Table 2.
Pharmacokinetics of ketorolac in iris-ciliary body following administrations of the CMC-containing 0.45% ketorolac, pH 6.8 (Acuvail), and the 0.4% ketorolac, pH 7.4 (Acular LS)
| Tmax (hour) | Cmax (ng/g [SD]) | AUC0–τ (ng h/g [SEM]) | Relative %F | |
|---|---|---|---|---|
| CMC-containing 0.45% ketorolac, pH 6.8 | 1.0 | 450a (117) | 2040 (240) | 292 |
| 0.4% ketorolac, pH 7.4 | 1.0 | 216 (43) | 699 (74) | 100 |
Note: P < 0.05 compared to the 0.4% ketorolac, pH 7.4 (Acular LS).
Abbreviations: AUC0–4, area under the concentration-time curve from time 0 to 4 hours; CMC, carboxymethylcellulose; Cmax, peak concentration; relative %F, percentage of the AUC0–4 value compared to that of the 0.4% ketorolac; SEM, standard error of the mean; SD, standard deviation; Tmax, time to Cmax.
PK simulation of ketorolac concentrations following multiple dose administration over a 24-hour period
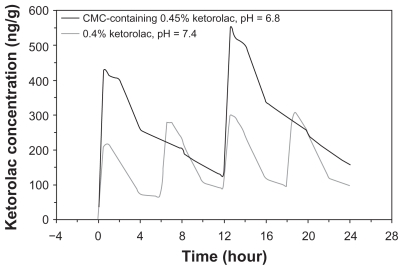
PK simulations of multiple-dose regimens derived from data collected following a single dose predicted an AUC0–τ of 2910 ng·h/g for twice daily, CMC-containing 0.45% ketorolac, pH 6.8, compared to 725 ng·h/g for 4 times daily 0.4% ketorolac, pH 7.4 (Figure 2).
Figure 2.
Simulation of ketorolac concentrations in iris-ciliary body following multiple topical administrations of the CMC-containing 0.45% ketorolac, pH 6.8 (Acuvail), and the 0.4% ketorolac, pH 7.4 (Acular LS).
Abbreviation: CMC, carboxymethylcellulose.
Discussion
Ketorolac ophthalmic formulations have been used successfully since 1997 for the control of inflammation after cataract surgery and the reduction of pain after refractive surgery. However, both the 0.4% ketorolac solution (Acular LS) and the 0.5% ketorolac solution (Acular) were associated with a high incidence of burning and stinging upon instillation as reported in their package inserts (“20%–40%” and “up to 40%”, respectively).2,3 The high rate of burning and stinging may negatively impact patient acceptance and compliance.
The most important finding of the current study was that the CMC-containing 0.45% ketorolac, pH 6.8 (Acuvail®; Allergan Inc., Irvine, CA), delivered higher ketorolac concentrations to aqueous humor and iris-ciliary body than did the 0.4% ketorolac, pH 7.4. The new formulation also provided greater aqueous humor exposure to ketorolac in comparison to historical PK data from the 0.5% ketorolac formulation, pH 7.4 (AUC0–4 = 939 ng·h/mL versus AUC0–4 = 583 ng·h/mL, respectively).12 The 0.5% ketorolac formulation, pH 7.4, achieves <4% absolute ocular bioavailability in the anterior chamber.12
Low ocular bioavailability following topical administration is a well recognized challenge in ophthalmic drug delivery, which is attributed to the drainage of the majority of drug through the nasolacrimal duct. In comparison to the anterior chamber, it is even more difficult for topical NSAIDs to penetrate into and maintain therapeutic levels in the posterior chamber. Previous studies demonstrated that the 0.4% ketorolac penetrated into vitreous and significantly lowered vitreous prostaglandin E2 levels compared to control. 13 Further studies are warranted to evaluate whether the new 0.45% formulation can deliver higher concentrations of ketorolac into the posterior chamber compared to the 0.4% ketorolac.
In this study, factors that contributed to the improvement of ketorolac delivery into ocular tissues were a small increase in ketorolac concentration, the addition of CMC, and a decrease in formulation pH. CMC is a cellulose derivative that is soluble in aqueous solutions and is used to provide viscosity without gelling. CMC increases the ocular residence time of topical ophthalmic drugs and thereby enhances ocular absorption.14–16 In our study, the addition of CMC to a formulation of 0.45% ketorolac at pH = 7.4 substantially increased ketorolac absorption into aqueous humor in comparison to the CMC-free 0.4% ketorolac, pH 7.4. An additional benefit of the use of CMC in ophthalmic solutions is its ability to promote corneal epithelial wound healing.17 This feature is important for ophthalmic treatments following cataract surgery.
The un-ionized form of a drug is more readily available for ocular absorption than the ionized form. The primary factor affecting ionization is pH.18 With a pKa of 3.5, ketorolac is mostly ionized in the pH range of 6.8 to 7.4, according to the Henderson–Hasselbalch equation, pH = pKa + log[A−]/[HA], where [A−] and [HA] represent the concentration of ionized and un-ionized ketorolac, respectively.18 As the pH is decreased from 7.4 to 6.8, relatively more ketorolac becomes un-ionized and, as a corollary, bioavailable. For the CMC-containing 0.45% ketorolac formulation, a shift from pH 7.4 to pH 6.8 results in 4-fold higher percentage of un-ionized drug that is available for absorption (0.0125% versus 0.05%, respectively).
Other formulation modifications included the omission of BAK, edetate disodium, and octoxynol–40. BAK has been shown to promote absorption of some ophthalmic drugs.19 The impact of BAK on ocular absorption of ketorolac is, however, controversial. In vivo, BAK did not improve the bioavailability of ketorolac in rabbits with intact corneas and decreased ocular absorption of ketorolac in rabbits with de-epithelialized corneas.20 In vitro, however, BAK improved the rate of ocular absorption of ketorolac.21 Nevertheless, reformulation of ketorolac without BAK, edetate disodium, and octoxynol-40 may improve tolerability and ocular comfort.
With a longer retention time and higher bioavailability on the ocular surface, the CMC-containing 0.45% ketorolac, pH 6.8, exhibits the potential for use at longer intervals. PK simulations indicated that twice daily instillation of the new formulation may provide greater drug exposure than does 4 times daily instillation of 0.4% ketorolac, pH 7.4, in iris-ciliary body over a 24-hour period. Considering the omission of the preservative, surfactant, and metal-chelating agents, this finding suggests that the CMC-containing 0.45% ketorolac, pH 6.8, is likely to provide a similar efficacy to those of the 0.4% and 0.5% formulations in reducing inflammation and pain but with improved ocular comfort at a reduced dosing frequency. Pivotal phase 3 trials have recently demonstrated that twice daily instillation of the CMC-containing 0.45% ketorolac, pH 6.8, was well tolerated and effectively treated both inflammation and pain in cataract surgery patients.22 Based on these findings, the Food and Drug Administration approved twice-daily CMC-containing 0.45% ketorolac for treatment of ocular inflammation and pain following cataract surgery.23 In addition to convenience, a less frequent dosing regimen may increase patient compliance and adherence to the recommended dosing schedule.24,25 Together these data demonstrate that by targeting formulation modifications to the small fraction of drug available for absorption, substantial effects on increasing ocular penetration can be observed. Further studies are warranted to compare the PK and efficacy of the new ketorolac formulation to those of other ophthalmic NSAIDs for the treatment of prostaglandin-mediated ocular inflammatory conditions.
Acknowledgments
Thanks to Irwin Loh for excellent technical assistance with utilization of the bioanalytical method for sample analysis. The authors also thank Julia R Gage, PhD, for assistance with writing the manuscript.
Footnotes
Disclosure
Allergan, Inc., Irvine, CA, USA provided funding for the research. Authors MA, RS, LB, QF, and DW are employees of Allergan, Inc.
References
- 1.Kim SJ, Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Surv Ophthalmol. 2010;55(2):108–133. doi: 10.1016/j.survophthal.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Acular® LS [package insert] Irvine, CA: Allergan, Inc; 2008. [Google Scholar]
- 3.Acular LS® [package insert] Irvine, CA: Allergan, Inc; 1997. [Google Scholar]
- 4.Donnenfeld ED, Perry HD, Wittpenn JR, Solomon R, Nattis A, Chou T. Preoperative ketorolac tromethamine 0.4% in phacoemulsification outcomes: pharmacokinetic-response curve. J Cataract Refract Surg. 2006;32(9):1474–1482. doi: 10.1016/j.jcrs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Perry HD, Donnenfeld ED. An update on the use of ophthalmic ketorolac tromethamine 0.4% Expert Opin Pharmacother. 2006;7(1):99–107. doi: 10.1517/14656566.7.1.99. [DOI] [PubMed] [Google Scholar]
- 6.Price FW, Jr, Price MO, Zeh W, Dobbins K. Pain reduction after laser in situ keratomileusis with ketorolac tromethamine ophthalmic solution 0.5%: a randomized, double-masked, placebo-controlled trial. J Refract Surg. 2002;18(2):140–144. doi: 10.3928/1081-597X-20020301-07. [DOI] [PubMed] [Google Scholar]
- 7.Price MO, Price FW. Efficacy of topical ketorolac tromethamine 0.4% for control of pain or discomfort associated with cataract surgery. Curr Med Res Opin. 2004;20(12):2015–2019. doi: 10.1185/030079904x16759. [DOI] [PubMed] [Google Scholar]
- 8.Sandoval HP, Férnandez de Castro LE, Vroman DT, Solomon KD. Evaluation of 0.4% ketorolac tromethamine ophthalmic solution versus 0.5% ketorolac tromethamine ophthalmic solution after phacoemulsification and intraocular lens implantation. J Ocul Pharmacol Ther. 2006;22(4):251–257. doi: 10.1089/jop.2006.22.251. [DOI] [PubMed] [Google Scholar]
- 9.Solomon KD, Donnenfeld ED, Raizman M, et al. Safety and efficacy of ketorolac tromethamine 0.4% ophthalmic solution in post-photorefractive keratectomy patients. J Cataract Refract Surg. 2004;30(8):1653–1660. doi: 10.1016/j.jcrs.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Flach AJ, Jampol LM, Weinberg D, et al. Improvement in visual acuity in chronic aphakic and pseudophakic cystoid macular edema after treatment with topical 0.5% ketorolac tromethamine. Am J Ophthalmol. 1991;112(5):514–519. doi: 10.1016/s0002-9394(14)76851-5. [DOI] [PubMed] [Google Scholar]
- 11.Waterbury LD, Flach AJ. Efficacy of low concentrations of ketorolac tromethamine in animal models of ocular inflammation. J Ocul Pharmacol Ther. 2004;20(4):345–352. doi: 10.1089/1080768041725380. [DOI] [PubMed] [Google Scholar]
- 12.Ling TL, Combs DL. Ocular bioavailability and tissue distribution of [14C] ketorolac tromethamine in rabbits. J Pharm Sci. 1987;76(4):289–294. doi: 10.1002/jps.2600760405. [DOI] [PubMed] [Google Scholar]
- 13.Heier JS, Awh CC, Busbee BG, et al. Vitreous nonsteroidal antiinflammatory drug concentrations and prostaglandin E2 levels in vitrectomy patients treated with ketorolac 0.4%, bromfenac 0.09%, and nepafenac 0.1% Retina. 2009;29(9):1310–1313. doi: 10.1097/IAE.0b013e3181b094e6. [DOI] [PubMed] [Google Scholar]
- 14.Kyyronen K, Urtti A. Improved ocular: systemic absorption ratio of timolol by viscous vehicle and phenylephrine. Invest Ophthalmol Vis Sci. 1990;31(9):1827–1833. [PubMed] [Google Scholar]
- 15.Paugh JR, Chatelier RC, Huff JW. Ocular residence time of carboxymethylcellulose solutions. Adv Exp Med Biol. 1998;438:761–767. doi: 10.1007/978-1-4615-5359-5_107. [DOI] [PubMed] [Google Scholar]
- 16.Sasaki H, Yamamura K, Mukai T, et al. Pharmacokinetic prediction of the ocular absorption of an instilled drug with ophthalmic viscous vehicle. Biol Pharm Bull. 2000;23(11):1352–1356. doi: 10.1248/bpb.23.1352. [DOI] [PubMed] [Google Scholar]
- 17.Garrett Q, Simmons PA, Xu S, et al. Carboxymethylcellulose binds to human corneal epithelial cells and is a modulator of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2007;48(4):1559–1567. doi: 10.1167/iovs.06-0848. [DOI] [PubMed] [Google Scholar]
- 18.Katzung BG. Basic principles. In: Katzung BG, editor. Basic and Clinical Pharmacology. 6th ed. London, UK: Prentice Hall International; 1995. pp. 1–8. [Google Scholar]
- 19.Sasaki H, Nagano T, Yamamura K, Nishida K, Nakamura J. Ophthalmic preservatives as absorption promoters for ocular drug delivery. J Pharm Pharmacol. 1995;47(9):703–707. doi: 10.1111/j.2042-7158.1995.tb06726.x. [DOI] [PubMed] [Google Scholar]
- 20.Madhu C, Rix PJ, Shackleton MJ, Nguyen TG, Tang-Liu DD. Effect of benzalkonium chloride/EDTA on the ocular bioavailability of ketorolac tromethamine following ocular instillation to normal and de-epithelialized corneas of rabbits. J Pharm Sci. 1996;85(4):415–418. doi: 10.1021/js9504189. [DOI] [PubMed] [Google Scholar]
- 21.Malhotra M, Majumdar DK. Effect of preservative, antioxidant and viscolizing agents on in vitro transcorneal permeation of ketorolac tromethamine. Indian J Exp Biol. 2002;40(5):555–559. [PubMed] [Google Scholar]
- 22.Donnenfeld E, Nichamin LD, Hardten DR, et al. Twice-daily, preservative- free ketorolac 0.45% for treatment of inflammation and pain following cataract surgery. Am J Ophthalmol. 2010 doi: 10.1016/j.ajo.2010.09.003. in press. [DOI] [PubMed] [Google Scholar]
- 23.Acuvail® [package insert] Irvine, CA: Allergan, Inc; 2009. [Google Scholar]
- 24.Eisen SA, Miller DK, Woodward RS, Spitznagel E, Przybeck TR. The effect of prescribed daily dose frequency on patient medication compliance. Arch Intern Med. 1990;150(9):1881–1884. [PubMed] [Google Scholar]
- 25.Kruse W, Eggert-Kruse W, Rampmaier J, Runnebaum B, Weber E. Dosage frequency and drug-compliance behaviour – a comparative study on compliance with a medication to be taken twice or four times daily. Eur J Clin Pharmacol. 1991;41(6):589–592. doi: 10.1007/BF00314990. [DOI] [PubMed] [Google Scholar]