Zhang and colleagues examine the efficacy of a replication-competent parainfluenza virus (PIV)-based vector for airway gene transfer applications. Using an in vitro model of rhesus airway epithelium, the authors demonstrate that PIV mediates efficient gene transfer in rhesus epithelium. In vivo experiments revealed that intranasal administration of a PIV vector expressing rhesus macaque α-fetoprotein (rhAFP) results in the transient secretion of rhAFP in both mucosal and serosal compartments.
Abstract
Over the last two decades, enormous effort has been focused on developing virus-based gene delivery vectors to target the respiratory airway epithelium as a potential treatment for cystic fibrosis (CF) lung disease. However, amongst other problems, the efficiency of gene delivery to the differentiated airway epithelial cells of the lung has been too low for clinical benefit. Although not a target for CF therapy, the nasal epithelium exhibits cellular morphology and composition similar to that of the lower airways, thus representing an accessible and relevant tissue target for evaluating novel and improved gene delivery vectors. We previously reported that replication-competent human parainfluenza virus (PIV)-based vectors efficiently deliver the cystic fibrosis transmembrane conductance regulator gene to sufficient numbers of cultured CF airway epithelial cells to completely correct the bioelectric function of CF cells to normal levels, resulting in restoration of mucus transport. Here, using an in vitro model of rhesus airway epithelium, we demonstrate that PIV mediates efficient gene transfer in rhesus epithelium as in the human counterpart. Naive rhesus macaques were inoculated intranasally with a PIV vector expressing rhesus macaque α-fetoprotein (rhAFP), and expression was monitored longitudinally. rhAFP was detected in nasal lavage fluid and in serum samples, indicating that PIV-mediated gene transfer was effective and that rhAFP was secreted into both mucosal and serosal compartments. Although expression was transient, lasting up to 10 days, it paralleled virus replication, suggesting that as PIV was cleared, rhAFP expression was lost. No adverse reactions or signs of discomfort were noted, and only mild, transient elevations of a small number of inflammatory cytokines were measured at the peak of virus replication. In summary, rhAFP proved suitable for monitoring in vivo gene delivery over time, and PIV vectors appear to be promising airway-specific gene transfer vehicles that warrant further development.
Introduction
Therapeutic gene delivery to the human respiratory epithelium has potential applications for alleviating the consequences of inherited diseases such as cystic fibrosis (CF), α1-antitrypsin deficiency, as well as acquired diseases such as asthma, surfactant protein deficiencies, and chronic obstructive pulmonary disease (COPD). Attempts to deliver therapeutic transgenes to human ciliated airway epithelium have been challenging largely because of the inability of the currently available vectors (e.g., lentivirus, adenovirus, adeno-associated viral vectors, and liposomes) to deliver transgenes to sufficient numbers of airway epithelial cells for clinical benefit (Klink et al., 2004; Tate and Elborn, 2005). Efficient gene transfer vectors for gene delivery to the human ciliated airway epithelium remain highly desirable.
We have previously reported that a recombinant human parainfluenza virus type 3 (PIV3, Respirovirus genus, Paramyxoviridae family) infects an in vitro model of human ciliated airway epithelium (HAE) and specifically targets ciliated epithelial cells (Zhang et al., 2005), the predominant cell type in human airways (Kreda et al., 2005). Moreover, because the cystic fibrosis transmembrane conductance regulator (CFTR) is normally expressed in ciliated cells, we used HAE derived from subjects with CF (CF HAE) to perform proof-of-concept studies designed to deliver CFTR to CF ciliated cells. These studies demonstrated that PIV3-based vectors successfully delivered CFTR to sufficient numbers of CF ciliated cells to completely reverse the CF phenotype to non-CF levels, that is, transepithelial ion and fluid transport, airway surface liquid volume regulation, and mucus transport were all restored to levels measured in non-CF HAE (Zhang et al., 2009).
PIV3 is a frequent cause of acute respiratory tract illness in humans, especially in the pediatric population. The virus contains a single-stranded negative-sense RNA genome of 15.5 kb (Karron and Collins, 2006). To test whether our PIV3-based vectors were capable of delivering transgenes to the respiratory epithelium in vivo, we chose to administer PIV3 vectors to the nasal epithelium of the rhesus macaque (Macaca mulatta). We chose this animal model for our in vivo studies because (1) the nasal epithelium has been used as a relevant and well-tolerated site to test gene transfer vectors that would subsequently be tested in the lower airways and because (2) PIV3 infects and replicates in cultured well-differentiated rhesus airway epithelial cells at levels similar to those in human airway epithelial cells (present study). In addition, the adult rhesus macaque approximates the size of a human infant, which would be the most likely CF patient population targeted for CFTR gene replacement.
Because no CFTR-deficient nonhuman primate model is currently available, testing the functional consequences of CFTR delivery to the rhesus nasal epithelium was not feasible. Therefore, we chose to deliver a marker gene expressed from PIV3 that enabled minimally invasive monitoring and allowed longitudinal assessment of gene expression in vivo. The marker transgene we chose to express was rhesus α-fetoprotein (rhAFP). AFP is normally produced in large quantity by the yolk sac and the liver only during fetal development (Mizejewski, 2001). Normal levels of AFP in adults are low, and AFP has no known function in healthy adults (Ball et al., 1992). The advantages of AFP as a reporter gene for in vivo studies are 4-fold: (1) the background level of AFP in adult animals is close to undetectable, enabling high sensitivity of detection; (2) expression of a species-matched AFP gene in adults is recognized as self and, thus, is nonimmunogenic; (3) AFP is readily secreted from cells, enabling quantification in bodily fluids without invasive procedures; and, (4) commercial ELISA kits for human AFP are available that cross-react with rhAFP. Species-matched AFP has previously been used to assess gene transfer in the alveolar regions of baboons after adenoviral vector administration (O'Neal et al., 2000).
Materials and Methods
Construction of recombinant PIV3 vectors expressing two transgenes simultaneously
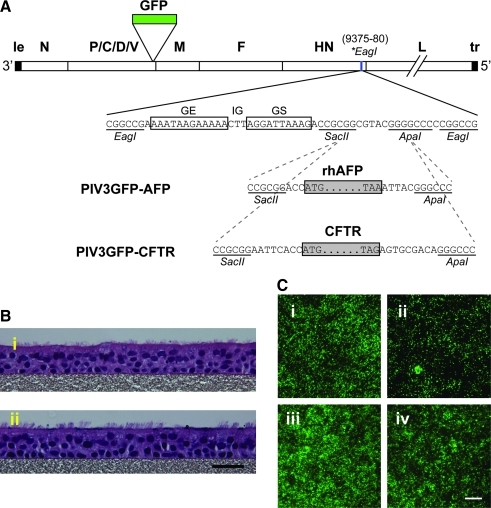
PIV3GFP virus in which the green fluorescent protein (GFP) gene was inserted between the P and M genes (Zhang et al., 2005) was used to accommodate an additional gene, either rhAFP or CFTR, between the HN and L genes (Fig. 1A). First, a unique EagI restriction site (positions 9375–9380) was generated within the downstream noncoding region of the HN gene through site-directed mutagenesis. Next, the rhesus macaque AFP-coding sequence was amplified by RT-PCR from fetal rhesus macaque liver RNA (a kind gift from A. Tarantal, University of California at Davis, Davis, CA) with the following primer set: sense (5′-CCGCGGACCATGAAGTGGGTGGAATCAA-3′) and antisense (5′-GGGCCCGTAATTTAAACTCCCAA-3′); the underlined portions represent SacII and ApaI sites, respectively. The rhesus macaque AFP, flanked by SacII and ApaI sites, was then inserted into a linker sequence containing the PIV3 gene-end (GE), intergenic (IG), and gene-start (GS) transcription signals, flanked at both ends by EagI sites. Last, this EagI cassette was inserted into the unique EagI site in PIV3GFP generated previously, to create PIV3GFP-AFP. Similarly, the full-length human CFTR-coding sequence was cloned in place of AFP to create PIV3GFP-CFTR. The viral genomes were designed to be multiples of six, which is a requirement for efficient PIV3 genome replication. The recombinant viruses were rescued and propagated in LLC-MK2 cells (American Type Culture Collection [ATCC], Manassas, VA), using reverse genetics techniques as reported previously (Durbin et al., 1997).
FIG. 1.
Construction of recombinant PIV3GFP-AFP and PIV3GFP-CFTR vectors and transduction efficiency in human and macaque ciliated airway epithelium in vitro. (A) Schematic of the genomic structures of PIV3GFP-AFP and PIV3GFP-CFTR vectors. The linear wild-type PIV3 genome is shown with virus-encoded genes (open boxes) labeled, as well as the extragenic 3′ leader (le) and 5′ trailer (tr) sequences. The GFP gene was inserted between the P and M genes. The rhesus macaque AFP or human CFTR gene (each represented as a shaded rectangle, with the ATG initiation and TAA/G termination codons indicated), together with the PIV3 gene-end (GE), intergenic (IG), and gene-start (GS) transcription signals, was inserted into a unique EagI restriction site (*EagI, positions 9375–9380, indicated by a blue vertical line) within the downstream noncoding region of the HN gene. This places the rhAFP- or CFTR-coding sequence as a separate, added gene that is expressed as a separate mRNA. Sequences are shown as positive-sense. (B) Photomicrographs of hematoxylin and eosin (H&E)-stained cross-sections of well-differentiated ciliated airway epithelial cell cultures from (panel i) human (HAE) or (panel ii) rhesus macaque (rhMAE). Note the abundance of ciliated cells facing the lumenal surface for cultures from both species. Scale bar: 30 μm. (C) Fluorescence en face photomicrographs showing GFP expression in HAE (panels i and ii) or rhMAE (panels iii and iv) 2 days after inoculation of the apical surfaces with 106 PFU (MOI, 3) of PIV3GFP-AFP (panels i and iii) or PIV3GFP-CFTR (panels ii and iv). Scale bar: 200 μm.
Generation of the in vitro model of well-differentiated human or rhesus macaque airway epithelium
The generation of human airway epithelial (HAE) cell cultures has been described previously (Fulcher et al., 2005). For rhesus macaque airway epithelial (rhMAE) cell cultures, macaque tracheal epithelial cells were isolated from rhesus macaque tracheas (obtained from the Gene Therapy Program at the University of Pennsylvania, Philadelphia, PA), by adapting the protocol for HAE cultures (Fulcher et al., 2005). Briefly, fresh isolated cells were seeded on collagen IV-coated permeable membrane supports (Millicells, 12 mm in diameter, 0.4-μm pore size; Millipore, Bedford, MA) at a density of 200,000 cells per culture insert. On reaching confluency (2–3 days), cultures were allowed to differentiate under an air–liquid interface (ALI), and reached the mature and well-ciliated phenotype (>50% ciliated cells) in 4–6 weeks. For histological examination, HAE and rhMAE cells were fixed in 4% paraformaldehyde (PFA), paraffin embedded, sectioned (thickness, 5 μm), and stained with hematoxylin and eosin (H&E).
Intranasal inoculations of PIV3 vectors into rhesus macaque and evaluations for transgene expression
Experiments with rhesus macaques were carried out according to protocols approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. Two healthy adult rhesus macaques, weighing 9.8 and 8.3 kg, respectively, were first confirmed to be seronegative for PIV3. The animals were anesthetized with a mixture of ketamine (10–15 mg/kg) and dexmedetomidine (0.5–1.0 mg/kg) injected intramuscularly. The macaques were then intubated with an endotracheal tube to maintain a patent airway. A pediatric Foley catheter was placed in one of the nares and, once in place, the balloon was inflated with air and the catheter pulled back until resistance was achieved. The naris opening was also blocked at the proximal side with another pediatric Foley catheter after inflation of the balloon. Approximately 2 ml of viral solution (diluted with phosphate-buffered saline [PBS]) was infused into the nasal cavity via a catheter and allowed to remain there for 5–7 min. Each macaque received PIV3GFP-AFP or PIV3GFP-CFTR at a dose of 4 ml of viral vector (1 × 106 plaque-forming units [PFU]/ml; 2 ml per naris). The macaques were observed and monitored daily for signs of rhinorrhea, sneezing, loss of appetite, and change in body temperature and weight. To detect secreted AFP after PIV3GFP-AFP vector inoculation, nasal lavage fluid (NLF) and serum samples were harvested at 2, 4, 8, 10, 14, 17, and 21 days postinoculation. To collect NLF, macaques were sedated and intubated as during viral inoculation; one pediatric Foley catheter was used to block the back end of the nasal cavity; 5 ml of PBS (per naris) was used to flush the nasal cavity, collected, aliquoted, and stored at −80°C. Viruses present in NLF were titrated by plaque assay on LLC-MK2 cells. A 4-ml blood sample per macaque was collected from the femoral vein at the same time points; and sera were aliquoted and stored at −80°C.
Results and Discussion
To construct PIV3 incorporating the rhesus macaque AFP gene (GenBank accession number XM_001103873.1), the AFP cDNA was cloned from fetal rhesus macaque liver RNA and inserted between the HN and L genes in the recombinant PIV3GFP backbone as an extra gene under the control of a set of PIV3 gene-end (GE), intergenic (IG), and gene-start (GS) regulatory sequences (Fig. 1A and Materials and Methods). This vector was designated PIV3GFP-AFP. A control PIV3 vector expressing CFTR (PIV3GFP-CFTR) was constructed in which the human CFTR gene was inserted into the viral genome in place of the rhAFP gene. Thus, both PIV3 vectors expressed two foreign genes simultaneously, that is, the 717-nucleotide GFP gene and either the 1830-nucleotide rhAFP gene or the 4443-nucleotide CFTR gene. Each foreign gene was expressed as a separate polyadenylated mRNA. The presence of these foreign genes increased the nucleotide length of the PIV3 genome from 15,426 to 18,055 nucleotides (an increase of 17%, PIV3GFP-AFP) or to 20,677 nucleotides (an increase of 34%, PIV3GPF-CFTR). Both viruses were readily rescued in LLC-MK2 cells and amplified by passage (Durbin et al., 1997). Titers generated and plaqued on LLC-MK2 cells indicated that PIV3GFP-AFP and PIV3GFP-CFTR replicated to similar levels as PIV3GFP virus (Zhang et al., 2005) but that PIV3GFP-CFTR was attenuated by 10-fold as previously reported (Zhang et al., 2009) (PIV3GFP, 4.1 × 107 PFU/ml; PIV3GFP-AFP, 1.3 × 107 PFU/ml; and PIV3GFP-CFTR, 2.12 × 106 PFU/ml).
To determine whether PIV3 vectors infect human and rhesus differentiated airway epithelial cells similarly, we inoculated in vitro cultures of HAE and rhesus macaque airway epithelial cells (rhMAE) in parallel and quantified infection efficiency and the secretion of rhAFP in both the apical (mucosal) and basolateral (serosal) compartments. Rhesus macaque airway epithelial (rhMAE) cell cultures were generated according to protocols similar to those for HAE (see Materials and Methods). Histological examination showed that the rhMAE cultures exhibited a pseudostratified mucociliary epithelium (Fig. 1B, panel ii) that was indistinguishable from HAE (Fig. 1B, panel i). Ciliated cells accounted for ∼60–80% of the lumenal surface epithelial cells in rhMAE and exhibited mucus production and transport on the lumenal surface as previously reported for HAE (Zhang et al., 2009).
To assess the extent of PIV-mediated gene transfer, the apical surface of HAE and rhMAE cultures was inoculated with PIV3GFP-AFP or PIV3GFP-CFTR at equal titer (1 × 106 PFU per 12-mm diameter culture insert; multiplicity of infection [MOI], ≈3). GFP-positive cells were visualized with an inverted fluorescence microscope (DMIRB; Leica Microsystems, Wetzlar, Germany). Representative en face photomicrographs of GFP-positive cells 2 days postinoculation are shown in Fig. 1C, demonstrating that both PIV3 vectors efficiently transduced HAE and rhMAE. Ciliated cells were the target cell type for PIV3 in both HAE and rhMAE infections (data not shown). The percentage of GFP-positive cells in HAE and rhMAE over time was quantified with ImageJ software (National Institutes of Health [NIH], Bethesda, MD) (Fig. 2A and B, respectively). Unexpectedly, this revealed a difference between HAE and rhMAE in the kinetics of GFP transduction after PIV3 inoculation. For HAE (Fig. 2A), the number of GFP-positive cells after inoculation with PIV3GFP-AFP or PIV3GFP-CFTR peaked at 2 days postinoculation, and then gradually decreased to a low, steady level 10 days postinoculation. This was the expected result given our previous demonstration that PIV3-infected ciliated cells are lost from the epithelium by a process of accelerated ciliated cell shedding (Zhang et al., 2009). At all time points, PIV3GFP-AFP infected more ciliated cells compared with PIV3GFP-CFTR; a finding likely due to the increased rate of replication for PIV3GFP-AFP because of its smaller transgene insert. In contrast to the HAE data, the extent of ciliated cell infection (GFP-positive cells) in rhMAE was more gradual and infection continued to increase for the duration of the experiment (17 days) (Fig. 2B). The prolonged infection of rhMAE by PIV3 vectors was observed with cultures derived from multiple individual animals (data not shown).
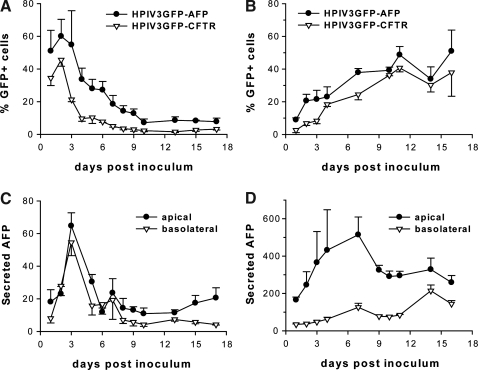
FIG. 2.
Quantification of PIV3 vector-mediated transduction in vitro. The apical surface of (A) HAE and (B) rhMAE was inoculated with PIV3GFP-AFP or PIV3GFP-CFTR as described in Fig. 1C; and the percentages of GFP-positive cells were quantified with ImageJ software (NIH). Both apical and basolateral supernatants from PIV3GFP-AFP-transduced (C) HAE and (D) rhMAE cultures were harvested over time and AFP concentrations (ng/cm2) are shown. Error bars represent the standard deviation (SD) (n = 6).
Infection by PIV3GFP-AFP was more efficient than by PIV3GFP-CFTR (compare Fig. 1C, panel i vs. ii and panel iii vs. iv), even though the cultures were inoculated with equal titers. Furthermore, the viral titers measured on the lumenal surface of HAE at 48 hr postinoculation with PIV3GFP-AFP (7.65 log10 PFU/ml) were significantly higher than those for PIV3GFP-CFTR (6.39 log10 PFU/ml), suggesting that PIV3GFP-AFP replicated 10-fold more efficiently than PIV3GFP-CFTR. These observations suggest that the smaller size of the AFP transgene insert, that is, 1.8 kb for the AFP gene (17% of PIV3 genome length) versus 4.4 kb for the CFTR gene (35% of PIV genome length), accounted for this difference in replication kinetics. The finding that larger insertions into the PIV3 genome attenuate viral growth in vivo is consistent with previous reports (Skiadopoulos et al., 2000, 2002; Zhang et al., 2009) and may be further exploited to generate more attenuated vectors for future applications.
To measure PIV3-mediated rhAFP gene transfer longitudinally in airway cultures, we collected apical washes (volume, 0.3 ml) and basolateral medium (1 ml) from PIV3GFP-AFP-infected HAE and rhMAE every 24 hr for 17 days. The concentration of rhAFP in these samples was quantified with a commercial human AFP ELISA kit (Quantikine DAFP00; R&D Systems, Minneapolis, MN), and total secreted rhAFP was calculated by adjusting to the respective volume (apical, 0.3 ml; basolateral, 1 ml) and expressed as nanograms per square centimeter of epithelium (Fig. 2C and D). Although rhAFP was readily detected in the apical and basolateral compartments of both HAE and rhMAE, significantly more rhAFP was secreted from rhMAE (Fig. 2D) than from HAE (Fig. 2C) both in terms of the total amount and the amount secreted into the apical compartment. Secretion of rhAFP into the basolateral compartment of rhMAE cultures approximated the levels of rhAFP secretion into the basolateral compartment of HAE despite fewer ciliated cells infected. However, for rhMAE but not HAE, rhAFP was secreted at 5-fold higher levels into the apical compared with the basolateral compartment. PIV3GFP-CFTR failed to increase rhAFP above the limit of detection in apical or basolateral compartments of either HAE or rhMAE (data not shown).
The duration of rhAFP secretion in HAE and rhMAE was consistent with the quantification of GFP-positive cells in each of these culture types, with HAE ciliated cell infection and rhAFP secretion being transient and peaking at 2 or 3 days postinoculation whereas ciliated cell infection and rhAFP secretion from rhMAE remained high throughout the experiment (Fig. 2B). At present, we have no explanation for the slower and prolonged PIV3 infection in rhMAE compared with HAE. It is possible that, in this in vitro model, appropriate innate antiviral responses of macaque epithelial cells to human PIV3 infection are less effective. These in vitro data suggest that PIV3GFP-AFP transduction in vivo would result in rhAFP secretion into both mucosal and serosal compartments, thus enabling rhAFP measurements in both airway lumenal lavage and serum, respectively.
To investigate PIV3 vector-mediated gene transfer to rhesus macaque airways in vivo, we delivered our vectors to the nasal epithelium of adult rhesus macaques. This was done for two reasons: (1) the nasal epithelium is an easily accessible site for vector administration and the harvesting of samples, using noninvasive procedures; and (2) the human nasal epithelium of patients with CF has proven to be a robust model for feasibility studies to deliver CFTR to the CF airway epithelium (Knowles et al., 1981; Boucher et al., 1994; Flotte et al., 2003; Roxo-Rosa et al., 2006).
Two adult rhesus macaques (weight, 9.8 and 8.3 kg) were confirmed to be seronegative for PIV3 before the onset of the experiment. One animal was inoculated intranasally with 4 ml of PIV3GFP-AFP (106 PFU/ml) and the other received the same dose of control vector, PIV3GFP-CFTR. Both nares from an individual animal were inoculated with one virus type (2 ml/naris). This viral dose (4 × 106 PFU/macaque) is relatively low in comparison with doses used in previous work involving Sendai virus inoculation of mouse nasal epithelium (1–7 × 107 PFU in a 100-μl volume) (Ban et al., 2007; Ferrari et al., 2007). The inoculate was administered to the animals under sedation; two pediatric Foley balloon catheters were used to plug the proximal and distal ends of the nasal passages, enabling retention of vector in defined regions of the respiratory epithelium for 5 min. We have previously shown that as little as a 5-min exposure time of PIV3 to the apical surface of HAE results in approximately 80% of the maximal transduction achieved after a 2-hr incubation time (Zhang et al., 2009). To harvest samples for evaluation of vector replication rates and transgene expression, 5 ml of nasal lavage fluid (NLF in PBS) and 4 ml of serum per macaque were collected at 2, 4, 8, 10, 14, 17, and 21 days postinoculation. During this time period, with either PIV3 vector, there were no clinical indications of disease including onset of fever, weight loss, or abnormal nasal discharge.
Viral titers in NLF were determined by a standard plaque assay (Durbin et al., 1997). Evaluation of viral titers in sera was not performed as PIV3 does not shed into the basolateral compartment of HAE or into the sera of humans infected with PIV3 (Zhang et al., 2005; Karron and Collins, 2006). PIV3 was detected in NLF from both animals between 2 and 10 days postinoculation (Fig. 3A and B). Viral titers represented production of progeny virus, not residual inocula, because little or no virus was detected before 2 days postinoculation (Fig. 3A and B). The growth kinetics for both vectors showed similar peak titers (∼104 PFU/ml) at 4–8 days, with undetectable viral titers by 14 days postinoculation. However, some differences between virus growth kinetics were noted. PIV3GFP-CFTR reached peak titers later than PIV3GFP-AFP (8 vs. 4 days postinoculation, respectively), suggesting that the PIV3GFP-CFTR vector was attenuated compared with PIV3GFP-AFP in vivo. These data are consistent with the in vitro results in HAE and rhMAE (Fig. 2A and B). Furthermore, titers of PIV3GFP-CFTR remained high at 10 days postinoculation whereas those for PIV3GFP-AFP were almost undetectable on day 10 postinoculation. We propose that the longer period of virus production with PIV3GFP-CFTR is related to attenuated shedding of infected ciliated cells. We have previously shown that human ciliated cells are shed less rapidly when infected with PIV3CFTR versus PIV3GFP, presumably because of the 10-fold reduction in virus replication seen with the larger insert (Zhang et al., 2009).
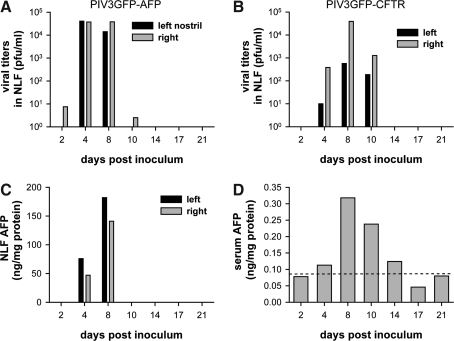
FIG. 3.
PIV3 vector-mediated gene expression in macaque nasal airways is efficient and transient. Shown are viral titers in nasal lavage fluid (NLF) obtained from a rhesus macaque inoculated intranasally with 4 × 106 PFU of either PIV3GFP-AFP (A) or PIV3GFP-CFTR (B). Samples were harvested at intervals from 2 to 21 days postinoculation. AFP concentrations present in NLF (C) or sera (D) were quantified by ELISA, and normalized by the amount of total protein present in each sample. Dashed line in (D) represents the baseline AFP level detected in serum obtained from the animal inoculated with PIV3GFP-CFTR.
The concentrations of rhAFP in the NLF and serum were quantified by ELISA. Although PIV3GFP-CFTR inoculation did not increase rhAFP in the NLF or sera beyond the low baseline levels (data not shown), rhAFP was detected in the NLF and serum from the PIV3GFP-AFP-inoculated macaque (Fig. 3C and D). Significant rhAFP was detected in NLF obtained from both nostrils 4 and 8 days postinoculation (Fig. 3C). Considering the brief period of nasal flushing during NLF collection, the detection of rhAFP in NLF samples indicated significant PIV-mediated gene delivery to the nasal epithelium. Serum rhAFP, a result of rhAFP being secreted into the basolateral compartments of the nasal epithelium and subsequent systemic distribution, was detected 4 to 14 days postinoculation, with a peak in expression at 8 days postinoculation. The lower levels of rhAFP in the serum compared with those in the NLF are likely due to the significant dilution of rhAFP into the systemic circulation.
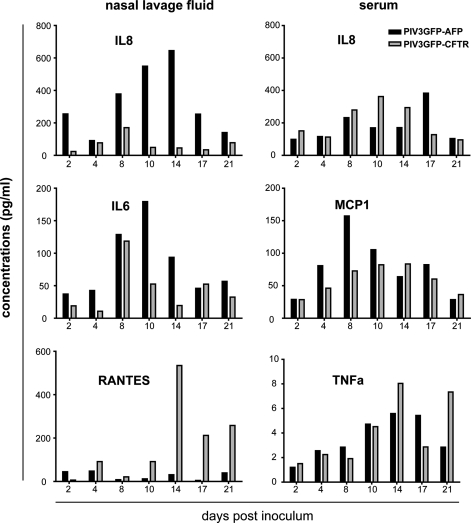
Interestingly, the transient expression of rhAFP in the NLF and serum mirrored the rhAFP quantification in vitro (Fig. 2B vs. D). Although it is tempting to speculate that similar host defenses are responsible for the transient nature of PIV3-mediated gene delivery in vitro and in vivo, that is, ciliated cell shedding, it is likely that other host factors in vivo (e.g., infiltrating inflammatory cells) also result in clearance of the virus and thus termination of rhAFP gene expression. To determine the extent of inflammatory mediator production induced by PIV3 vector transduction, we assayed for select cytokines/chemokines in the NLF and serum samples over time, using Luminex multiplex bead-based technology (Upstate flex kits; Millipore). Eleven macaque-specific analytes were quantified including interleukin (IL)-1β, IL-6, IL-8, monocyte chemotactic protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, tumor necrosis factor (TNF)-α, RANTES (regulated on activation, normal T-cell expressed and secreted), inflammatory protein (IP)-10, IL-13, interferon (IFN)-γ, and IL-2. Only IL-6, IL-8, and RANTES were found to be modestly elevated in NLF (Fig. 4, left), whereas other analytes did not increase above baseline levels (data not shown). Interestingly, IL-8 levels in the NLF of the PIV3GFP-AFP-inoculated macaque were higher than for the PIV3GFP-CFTR-inoculated macaque, whereas more RANTES was detected in the macaque inoculated with the PIV3GFP-CFTR vector compared with the PIV3GFP-AFP vector. In serum samples, only IL-8, MCP-1, and TNF-α were elevated, with other analytes not increased above baseline (Fig. 4, right). Thus, intranasal inoculation of macaques with PIV3 vectors was mildly inflammatory and the kinetics of inflammatory mediator production mirrored that of viral titers.
FIG. 4.
Intranasal inoculation of PIV3 vectors in macaque caused minimal and transient inflammatory responses. The NLF and serum samples obtained on the indicated days after PIV3GFP-AFP (black bars) or PIV3GFP-CFTR (gray bars) inoculation were assayed for the presence of 11 cytokines/chemokines (IL-1β, IL-6, IL-8, MCP-1, MIP-1α, TNF-α, RANTES, IP-10, IL-13, IFN-γ, and IL-2) using macaque-specific Luminex multiplex assays (Upstate Flex kits; Millipore): NLF (left), serum (right). Only those cytokines/chemokines with values above background levels are presented. Values from NLF are the average of the left and right nares.
In summary, we show that PIV3 vectors mediate detectable delivery and expression of a marker transgene to in vitro and in vivo models of rhesus airway epithelium. Although the duration of gene expression was transient, these studies demonstrate the usefulness of our in vitro and in vivo macaque models for assessing the ability of PIV3 to deliver genes to the respiratory epithelium. Future studies to test PIV3 vectors that have been attenuated for replication or engineered to remove cytotoxic and immunogenic glycoproteins from the virus genome will determine whether such vectors can improve the duration of gene expression (Murphy and Collins, 2002; Ferrari et al., 2007). We demonstrated that the dose of PIV3 vectors used in our studies was effective at conferring measurable gene expression in vivo and did not cause any major adverse reactions in the animals. Importantly, we showed the utility of the secretory gene AFP as an ideal reporter transgene suitable for long-term in vitro and in vivo studies that require high sensitivity of transgene detection, thus eliminating the need to sacrifice treated animals for gene expression assays. This study establishes a platform for comparison of vector-mediated gene delivery using in vitro and in vivo models, and suggests that additional modifications of PIV3 vectors will be required for future gene delivery applications for the ciliated airway epithelium.
Acknowledgments
The authors thank the UNC CF Center Tissue Culture Core for supplying primary human bronchial epithelial cells and associated reagents, the UNC CF Center Histology Core for histological assistance, and the Clinical Proteomics Core Facility for performing the Luminex assays for chemokine/cytokine measurements. The authors are grateful to Rebecca L. Grant (University of Pennsylvania) for technical assistance in the monkey experiments, and to Dr. Alice Tarantal (UC Davis) for providing liver RNA from rhesus macaques. This work was supported by the National Institutes of Health (R01 HL77844, P01 HL051818, P30 DK065988, P30 DK047757) and the Cystic Fibrosis Foundation (ZHAN0310). P.L.C. was supported by the NIAID Intramural Program.
Author Disclosure Statement
J.M.W. is a consultant to ReGenX Holdings, and is a founder of, holds equity in, and receives a grant from affiliates of ReGenX Holdings; in addition, he is an inventor on patents licensed to various biopharmaceutical companies, including affiliates of ReGenX Holdings. For all other authors, no competing financial interests exist.
References
- Ball D. Rose E. Alpert E. α-Fetoprotein levels in normal adults. Am. J. Med. Sci. 1992;303:157–159. doi: 10.1097/00000441-199203000-00004. [DOI] [PubMed] [Google Scholar]
- Ban H. Inoue M. Griesenbach U. Munkonge F. Chan M. Iida A. Alton E.W. Hasegawa M. Expression and maturation of Sendai virus vector-derived CFTR protein: Functional and biochemical evidence using a GFP-CFTR fusion protein. Gene Ther. 2007;14:1688–1694. doi: 10.1038/sj.gt.3303032. [DOI] [PubMed] [Google Scholar]
- Boucher R.C. Knowles M.R. Johnson L.G. Olsen J.C. Pickles R. Wilson J.M. Engelhardt J. Yang Y. Grossman M. Gene therapy for cystic fibrosis using E1-deleted adenovirus: A phase I trial in the nasal cavity. Hum. Gene Ther. 1994;5:615–639. doi: 10.1089/hum.1994.5.5-615. [DOI] [PubMed] [Google Scholar]
- Durbin A.P. Hall S.L. Siew J.W. Whitehead S.S. Collins P.L. Murphy B.R. Recovery of infectious human parainfluenza virus type 3 from cDNA. Virology. 1997;235:323–332. doi: 10.1006/viro.1997.8697. [DOI] [PubMed] [Google Scholar]
- Ferrari S. Griesenbach U. Iida A. Farley R. Wright A.M. Zhu J. Munkonge F.M. Smith S.N. You J. Ban H. Inoue M. Chan M. Singh C. Verdon B. Argent B.E. Wainwright B. Jeffery P.K. Geddes D.M. Porteous D.J. Hyde S.C. Gray M.A. Hasegawa M. Alton E.W. Sendai virus-mediated CFTR gene transfer to the airway epithelium. Gene Ther. 2007;14:1371–1379. doi: 10.1038/sj.gt.3302991. [DOI] [PubMed] [Google Scholar]
- Flotte T.R. Zeitlin P.L. Reynolds T.C. Heald A.E. Pedersen P. Beck S. Conrad C.K. Brass-Ernst L. Humphries M. Sullivan K. Wetzel R. Taylor G. Carter B.J. Guggino W.B. Phase I trial of intranasal and endobronchial administration of a recombinant adeno-associated virus serotype 2 (rAAV2)-CFTR vector in adult cystic fibrosis patients: A two-part clinical study. Hum. Gene Ther. 2003;14:1079–1088. doi: 10.1089/104303403322124792. [DOI] [PubMed] [Google Scholar]
- Fulcher M.L. Gabriel S. Burns K.A. Yankaskas J.R. Randell S.H. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- Karron R.A. Collins P.L. Parainfluenza viruses. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- Klink D. Schindelhauer D. Laner A. Tucker T. Bebok Z. Schwiebert E.M. Boyd A.C. Scholte B.J. Gene delivery systems—gene therapy vectors for cystic fibrosis. J. Cyst. Fibros. 2004;3(Suppl. 2):203–212. doi: 10.1016/j.jcf.2004.05.042. [DOI] [PubMed] [Google Scholar]
- Knowles M. Gatzy J. Boucher R. Increased bioelectric potential difference across respiratory epithelia in cystic fibrosis. N. Engl. J. Med. 1981;305:1489–1495. doi: 10.1056/NEJM198112173052502. [DOI] [PubMed] [Google Scholar]
- Kreda S.M. Mall M. Mengos A. Rochelle L. Yankaskas J. Riordan J.R. Boucher R.C. Characterization of wild-type and ΔF508 cystic fibrosis transmembrane regulator in human respiratory epithelia. Mol. Biol. Cell. 2005;16:2154–2167. doi: 10.1091/mbc.E04-11-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizejewski G.J. α-Fetoprotein structure and function: Relevance to isoforms, epitopes, and conformational variants. Exp. Biol. Med. (Maywood) 2001;226:377–408. doi: 10.1177/153537020122600503. [DOI] [PubMed] [Google Scholar]
- Murphy B.R. Collins P.L. Live-attenuated virus vaccines for respiratory syncytial and parainfluenza viruses: Applications of reverse genetics. J. Clin. Invest. 2002;110:21–27. doi: 10.1172/JCI16077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal W.K. Rose E. Zhou H. Langston C. Rice K. Carey D. Beaudet A.L. Multiple advantages of α-fetoprotein as a marker for in vivo gene transfer. Mol. Ther. 2000;2:640–648. doi: 10.1006/mthe.2000.0198. [DOI] [PubMed] [Google Scholar]
- Roxo-Rosa M. Da Costa G. Luider T.M. Scholte B.J. Coelho A.V. Amaral M.D. Penque D. Proteomic analysis of nasal cells from cystic fibrosis patients and non-cystic fibrosis control individuals: Search for novel biomarkers of cystic fibrosis lung disease. Proteomics. 2006;6:2314–2325. doi: 10.1002/pmic.200500273. [DOI] [PubMed] [Google Scholar]
- Skiadopoulos M.H. Surman S.R. Durbin A.P. Collins P.L. Murphy B.R. Long nucleotide insertions between the HN and L protein coding regions of human parainfluenza virus type 3 yield viruses with temperature-sensitive and attenuation phenotypes. Virology. 2000;272:225–234. doi: 10.1006/viro.2000.0372. [DOI] [PubMed] [Google Scholar]
- Skiadopoulos M.H. Surman S.R. Riggs J.M. Orvell C. Collins P.L. Murphy B.R. Evaluation of the replication and immunogenicity of recombinant human parainfluenza virus type 3 vectors expressing up to three foreign glycoproteins. Virology. 2002;297:136–152. doi: 10.1006/viro.2002.1415. [DOI] [PubMed] [Google Scholar]
- Tate S. Elborn S. Progress towards gene therapy for cystic fibrosis. Expert Opin. Drug Deliv. 2005;2:269–280. doi: 10.1517/17425247.2.2.269. [DOI] [PubMed] [Google Scholar]
- Zhang L. Bukreyev A. Thompson C.I. Watson B. Peeples M.E. Collins P.L. Pickles R.J. Infection of ciliated cells by human parainfluenza virus type 3 in an in vitro model of human airway epithelium. J. Virol. 2005;79:1113–1124. doi: 10.1128/JVI.79.2.1113-1124.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L. Button B. Gabriel S.E. Burkett S. Yan Y. Skiadopoulos M.H. Dang Y.L. Vogel L.N. McKay T. Mengos A. Boucher R.C. Collins P.L. Pickles R.J. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 2009;7:e1000155. doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]