Xenotropic murine leukemia virus (MLV)-related virus (XMRV) is a recently identified human retrovirus. XMRV targets XPR1 cell surface receptor, which is expressed in a broad range of human tissues including hematopoietic stem cells. In this study, Sakuma and colleagues pseudotyped HIV- and murine leukemia virus (MLV)-based vectors with XMRV envelope glycoprotein, and characterized the biological properties of the newly generated recombinant vectors.
Abstract
Retroviral and lentiviral vectors are effective gene delivery vehicles that are being evaluated in clinical trials. Variations in the viral envelope (Env) glycoproteins, which are used to pseudotype retroviral or lentiviral vectors, can alter vector performance, including stability, titers, host range, and tissue tropism. Xenotropic murine leukemia virus (MLV)-related virus (XMRV) is a novel human retrovirus identified in patients with prostate cancer. XMRV targets XPR1 cell surface receptor, which is expressed in a broad range of human tissues including hematopoietic stem cells. Pseudotyping with XMRV Env would allow targeting of XPR1-expressing tissues. Here, we characterized XMRV Env-pseudotyped retroviral and lentiviral vectors. Although HIV and MLV vectors were poorly pseudotyped with wild-type XMRV Env, replacement of the C-terminal 11 amino acid residues in the transmembrane domain of XMRV Env with the corresponding 6 amino acid residues of amphotropic MLV Env (XMRV/Rampho) significantly increased XMRV Env-pseudotyped HIV and MLV vector titers. The transduction efficiency in human CD34+ cells when using the XMRV/Rampho-pseudotyped HIV vector (10–20%) was comparable to that achieved when using the same infectious units of vesicular stomatitis virus G glycoprotein-pseudotyped vector (25%); thus the modified XMRV Env offers an alternative pseudotyping strategy for XPR1-mediated gene delivery.
Introduction
Lentiviral vectors, such as human immunodeficiency virus (HIV)-based vectors, are promising gene delivery vehicles, which allow efficient transduction of nondividing cells and sustain long-term transgene expression through integration into the host genome (Kafri et al., 1997; Poeschla et al., 1998). In addition to mRNA coding transgenes, lentiviral vectors have been used to generate transgenic animals (Hofmann et al., 2003; McGrew et al., 2004), and to transfer complex genetic structures, such as intron-containing sequences. With advances in vector design for increased biosafety, lentiviral vectors have become safer and more effective gene delivery systems, and are being evaluated in clinical trials for infectious and genetic diseases (D'Costa et al., 2009).
Variations in the viral envelope (Env) glycoproteins, used to pseudotype retroviral and lentiviral vector cores, can determine the characteristics of the vectors, such as stability, titers, host range, and tissue tropism. HIV-based vectors are often pseudotyped with vesicular stomatitis virus G glycoprotein (VSV-G). Because VSV-G uses a ubiquitous cellular factor as its receptor and enters cells through endocytosis, VSV-G-pseudotyped vectors can efficiently infect a broad range of cells (Burns et al., 1993). In addition, because of its remarkable stability, pseudotyping with VSV-G allows vector concentration by ultracentrifugation (Strang et al., 2004). The limitations of VSV-G-pseudotyped vectors include toxicity at high concentrations (Burns et al., 1993; Liu et al., 1996), sensitivity to complement-mediated neutralization (DePolo et al., 2000), and induction of innate immune response on systemic administration (Pichlmair et al., 2007). In addition, when VSV-G is stably expressed, the fusogenicity of VSV-G induces strong toxicity, which prevents its use in continuous HIV vector-producing cells (Ikeda et al., 2003). Several gammaretroviral Env glycoproteins have been used to pseudotype lentiviral vectors. Those include amphotropic murine leukemia virus (MLV-A) Env, which uses a sodium-dependent phosphate symporter (Pit2) (Leverett et al., 1998); feline endogenous virus RD114 Env, which targets a neutral amino acid transporter expressed on many human tissues (Rasko et al., 1999; Tailor et al., 1999; Green et al., 2004); and gibbon ape leukemia virus (GALV) Env, which uses a sodium-dependent phosphate symporter (Pit1) (Kavanaugh et al., 1994; Olah et al., 1994). These pseudotypes transduce human cells in a receptor-specific manner (Battini et al., 1999). Unlike VSV-G, gammaretroviral Env glycoproteins can be stably expressed in continuous HIV vector-packaging cell lines (Ikeda et al., 2003).
In MLV, the cytoplasmic tail of the transmembrane (TM) protein (p15E) is further processed by the viral protease after viral particle assembly, which produces the mature TM protein (p12E) and releases a short amino acid fragment designated the R peptide (Green et al., 1981). Cleavage of the MLV R peptide is critical for activation of the cell fusion activity of the Env protein (Ragheb and Anderson, 1994; Rein et al., 1994). Although lentiviral vectors are efficiently pseudotyped with Env glycoproteins of several different subtypes of MLV, such as MLV-A, ecotropic MLV (Schambach et al., 2006), and MLV 10A1 (Stitz et al., 2000), wild-type Env glycoproteins from GALV or RD114 do not form functional pseudotypes with lentiviral vectors because of their inefficient incorporation into lentiviral vectors and/or insufficient R peptide cleavage (Stitz et al., 2000; Christodoulopoulos and Cannon, 2001; Sandrin et al., 2002). In the case of GALV Env, sequences in the cytoplasmic tail contain the specificity determinant that prevents its incorporation into lentiviral vectors (Christodoulopoulos and Cannon, 2001). Intriguingly, coexpression of HIV vector components strongly inhibits or destabilizes wild-type GALV Env expression in producer cells (Christodoulopoulos and Cannon, 2001). Modifications in the C-terminal Env amino acid sequences, such as replacement of all or part of the cytoplasmic tail with the corresponding MLV sequence (Stitz et al., 2000; Christodoulopoulos and Cannon, 2001; Sandrin et al., 2002), removal of the R peptides (Christodoulopoulos and Cannon, 2001), or mutations in the R peptide cleavage sites (Christodoulopoulos and Cannon, 2001; Ikeda et al., 2003), can increase the pseudotyping efficiency of lentiviral vectors with GALV and RD114 Envs.
A novel human retrovirus, xenotropic murine leukemia virus-related virus (XMRV), was discovered in patients with prostate cancer (Urisman et al., 2006) and chronic fatigue syndrome (Lombardi et al., 2009). Genetic analysis has revealed XMRV to be a gammaretrovirus, closely related to xenotropic MLV (MLV-X). Similar to MLV-X, XMRV also uses a phosphate transporter, xenotropic and polytropic retrovirus receptor 1 (XPR1), for viral entry (Hong et al., 2009; Lombardi et al., 2009). Because various human tissues including pancreas, kidney, placenta, and heart as well as hematopoietic tissues express XPR1 (Battini et al., 1999; Tailor et al., 1999), pseudotyping of lentiviral vectors with Env of XMRV or MLV-X is an attractive strategy for XPR1-targeted gene delivery. However, previous studies using xenotropic MLV Env-pseudotyped retroviral vectors showed relatively low viral titers (Forestell et al., 1997).
In this paper, we pseudotyped HIV- and MLV-based vectors with XMRV Env, and characterized their biological properties. Although HIV and MLV vectors were poorly pseudotyped with the wild-type XMRV Env, replacement of the C-terminal 11 amino acid residues of XMRV Env with the corresponding 6 amino acid residues of amphotropic MLV Env increased the XMRV Env-pseudotyped HIV and MLV vector titers approximately 10-fold. HIV vector pseudotyped with the modified XMRV Env (XMRV/Rampho) could transduce a broad range of human cells, but inefficiently infected XMRV-producing cells. When human hematopoietic stem cells were transduced with the HIV vectors pseudotyped with XMRV/Rampho at a multiplicity of infection (MOI) of 0.5 on 293T cells, 10 to 20% of the cells were transduced with XMRV/Rampho. Our data demonstrate successful XPR1-targeted gene delivery by an HIV vector pseudotyped with a modified XMRV Env, thus offering an important alternative option for pseudotyping of HIV vectors. Further optimization or modification is likely to further increase pseudotyping efficiency.
Materials and Methods
Cells
293T and HeLa cells were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin, and streptomycin. Jurkat, LNCaP, and 22Rv1 cells were maintained in RPMI containing 10% FBS, penicillin, and streptomycin. All the cells were placed in a 37°C incubator with 5% CO2.
Titration of retroviral and lentiviral vectors
HIV- and MLV-based vectors were generated as reported previously (Strang et al., 2004, 2005). Briefly, a packaging plasmid (0.25 μg), a green fluorescent protein (GFP) vector plasmid (0.5 μg), and an Env-encoding plasmid (0.25 μg) were cotransfected into 293T cells, using FuGENE-6 (Roche, Indianapolis, IN). Three days after transfection, supernatants were filtered through 0.45-μm (pore size) filters (Millipore, Bedford, MA) and 293T cells (5 × 104) were infected in the presence of Polybrene (8 μg/ml). Supernatants from 293T cells (100, 20, and 2 μl) were used for the infection. At 3 days posttransduction cells were fixed with paraformaldehyde, GFP-positive cell populations were determined by flow cytometry (FACScan; BD Biosciences, San Jose, CA), and viral titers were calculated as infective units per milliliter (IU/ml), using the following equation: amount of cells per well × percent GFP-positive cells from flow cytometry analysis × 1/100 × 1000/amount of supernatant from 293T cells. GFP-carrying XMRV was generated by transfecting 293T cells with VP62/pcDNA3.1(–) (Dong et al., 2007) and a GFP-carrying retroviral vector genome construct as reported previously (Sakuma et al., 2010).
XMRV wild-type and XMRV chimera construction
XMRV Env was amplified by PCR using primers 5′-CAG TGTGGTGGAATTCGCCACCATGGAAAGTCCAGCGTTC TCAAAAC-3′ and 5′-GATGACCGGTACGCGTTTATTCA CGTGATTCCACTTCTTCTGG-3′. Underlined sequences indicate EcoRV and MluI sites. The resulting PCR fragment was cloned with an In-Fusion Advantage PCR cloning kit (Clontech, Palo Alto, CA), and the sequenced PCR-amplified XMRV was identical to the wild type. In order to construct the XMRV-MLV-A Env chimera (Fig. 1B), PCR was performed using XMRV Env as a template, and primers 5′-CGTCTCGAGGCCACCATGGAAAGTCCAGCGTTCTC-3′ (XhoI restriction site is underlined) and 5′-ACGTACGCGTTCATGGCTCGTACTCTATGGGTTTGAGTTGGTGATACTGTTGGGTCAG-3′ (MluI restriction site is underlined). The insert was cloned into pUB6 (Invitrogen, Carlsbad, CA). The sequence was analyzed to confirm that there were no unexpected mutations.
FIG. 1.
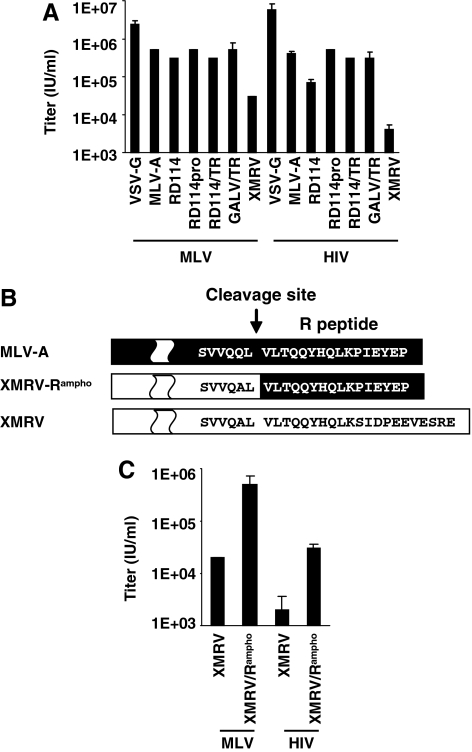
Transduction efficiency of XMRV Env-pseudotyped vectors. (A) XMRV Env-pseudotyped HIV and MLV vectors were compared with vectors pseudotyped with other viral glycoproteins. Shown are average values, with error bars, based on two experiments. (B) Schematic diagram of the C-terminal cytoplasmic tail is shown with amino acid sequence alignment of MLV-A, XMRV/MLV-A chimeric Env (XMRV/Rampho), and XMRV. The R-peptide region (six amino acids) of XMRV/Rampho was changed to that of MLV-A. (C) Infectious titers of wild-type XMRV Env- and XMRV/Rampho-pseudotyped vectors were determined in 293T cells in the presence of Polybrene (8 μg/ml).
Immunoblotting analysis of XMRV Env incorporation into vector particles
XMRV wild-type Env or XMRV/Rampho Env expression plasmids were cotransfected with retro- or lentiviral expression plasmids in 293T cells (2 × 105) using FuGENE-6 (Roche). Culture supernatants were harvested and filtered through 0.45-μm (pore size) filters. Vector particles were purified by centrifugation through a 20% sucrose cushion at 13,000 rpm at 4°C for 1 hr (5417R; Eppendorf, Hamburg, Germany). Pellets were resuspended in PBS and centrifuged at 13,000 rpm at 4°C for 1 hr. Purified vector pellets were resuspended in sample buffer and subjected to Western blotting analysis. Producer cells were lysed in radioimmunoprecipitation assay (RIPA) buffer and subjected to Western blotting analysis. XMRV Env protein and MLV Gag proteins were detected with goat polyclonal anti-MLV gp70/p30 antibody (diluted 1:3000). HIV-1 Gag proteins were detected with a mixture of mouse anti-p24 antibody 183-H12-5C (diluted 1:1000) and mouse anti-Ag3.0 (diluted 1:500) (AIDS Research and Reference Reagent Program, National Institutes of Health, Bethesda, MD). To quantify the bands obtained from Western blots, ImageJ software-based analysis (http://rsb.info.nih.gov/ij/) was applied. Areas under the curve of the specific signals (pixel density) were compared.
Characterization of XMRV/Rampho-pseudotyped vectors
Vector samples were placed at −80°C and 37°C three times to determine heat stability. To determine half-life time of vector pseudotypes, vector samples were incubated at 37°C for 6 hr. After 15, 30, 60, 120, 180, and 360 min of incubation, 293T cells (5 × 104) were infected with vectors in the presence of Polybrene (8 μg/ml). Seventy-two hours posttransduction, GFP-positive cells were counted by flow cytometry. All the experiments were repeated twice and average titers obtained from the experiments were used for data analysis.
Self-inhibitory effects of vectors with different retroviral Env glycoproteins
Inhibitory effects of viral vectors were examined after vectors were concentrated by ultracentrifugation (Beckman Coulter, Fullerton, CA) at 15,000 rpm for 4 hr at 4°C. 293 T cells (1 × 104) were plated on 96-well plates 1 day before infection. Two-fold serial dilutions of vector sets were incubated at 37°C for 72 hr, and then cells were fixed with paraformaldehyde and analyzed by flow cytometry.
Transduction efficiency in human CD34+ cells
Human CD34+ cells were isolated from granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood of two healthy donors (institutional regulatory board-approved NIH protocol 94-I-0073). The cells were prestimulated in X-VIVO 10 (supplemented with 1% human serum albumin, stem cell factor [SCF, 50 ng/ml], thrombopoietin [TPO, 50 ng/ml], FLT3 ligand [FLT3-L, 50 ng/ml], and interleukin-3 [IL-3, 5 ng/ml]) for 24 hr before exposure to vector by spinoculation for 30 min at 32°C, in the presence of protamine sulfate (6 μg/ml) on RetroNectin-coated plates. Cells were infected with a multiplicity of infection (MOI) of 0.5 of each concentrated vector pseudotyped with VSV-G, MLV-A, or XMRV/Rampho, that is, 2 × 107, 3.5 × 106, or 1.7 × 106 IU/ml, respectively.
Results
HIV vectors pseudotyped with XMRV Env
To determine the pseudotyping efficiency of XMRV Env with HIV and MLV particles, a plasmid expressing wild-type XMRV Env was cotransfected with an HIV-packaging plasmid and a GFP-expressing transfer vector plasmid, or with an MLV-packaging plasmid and a GFP-expressing vector plasmid, in 293T cells. For direct comparison, we also generated HIV and MLV vectors pseudotyped with VSV-G, MLV-A Env, wild-type RD114 Env, RD114pro (RD114 Env with an HIV protease cleavage site), RD114/TR, and GALV/TR by transient transfection in 293T cells as described previously (Ikeda et al., 2003; Strang et al., 2004). Infectious vector titers were determined by infecting 293T cells with serially diluted vector supernatants. As shown in Fig. 1A, HIV-based vector pseudotyped with XMRV Env showed the lowest titer (4 × 103 IU/ml) compared with HIV vector pseudotyped with other Envs (i.e., VSV-G, MLV-A, RD114, RD114pro, RD114/TR, GALV/TR). Although the XMRV Env-pseudotyped MLV vector titer (3 × 104 IU/ml) was higher than the XMRV Env-pseudotyped HIV vector titer, it was still lower than those of the other retroviral Env-pseudotyped MLV vectors. As we and others have reported previously (Sandrin et al., 2002; Strang et al., 2004), modifications in the RD114 cytoplasmic tail region markedly increased pseudotyped HIV vector titers.
To rule out the possibility that the poor titers of XMRV Env pseudotype were due to low XPR1 receptor expression in 293T target cells, we examined the susceptibility of 293T cells to wild-type XMRV. To monitor XMRV infectivity, we generated a GFP-expressing XMRV (XMRV-GFP) by cross-packaging the same GFP-encoding MLV vector genome with full-length XMRV clone VP62. The infectivity of XMRV-GFP in 293T cells reached as high as 6 × 106 IU/ml, suggesting that 293T cells express sufficient XPR1 receptor for viral entry. We therefore concluded that the poor titers with XMRV Env-pseudotyped HIV and MLV vectors were due to inefficient pseudotyping.
To improve the pseudotyping efficiency with XMRV Env, we employed the C-terminal tail replacement strategy, which was successfully used to increase pseudotyping efficiency of RD114 and GALV Env glycoproteins with HIV vectors (Stitz et al., 2000; Christodoulopoulos and Cannon, 2001; Sandrin et al., 2002). We constructed a plasmid encoding a modified XMRV Env with chimeric transmembrane (TM) protein originated from XMRV and MLV-A (Fig. 1B). The six-amino acid (R-peptide) modification of the C-terminal tail in wild-type XMRV Env, designated as XMRV/Rampho, increased the HIV vector titer approximately 10-fold (3 × 104 IU/ml), whereas XMRV/Rampho-pseudotyped MLV vector showed a 25-fold higher titer (5 × 105 IU/ml) than wild-type XMRV Env pseudotypes (2 × 104 IU/ml) (Fig. 1C). Because of the improved pseudotyping efficiency, we used HIV and MLV vectors with XMRV/Rampho for further characterization.
XMRV Env incorporation into vector particles
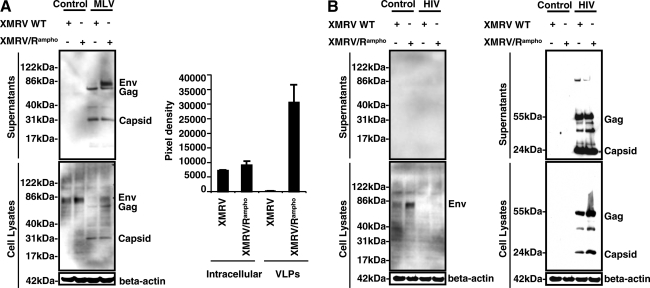
Increased pseudotyping efficiency can be due to increased XMRV Env expression in producer cells and/or improved XMRV Env incorporation into vector particles. To better understand the titer improvement with XMRV/Rampho pseudotypes, Western blotting was performed (Fig. 2). We also measured the density of the XMRV Env signals (Fig. 2A). A 2-fold increase in the levels of XMRV Env was observed in cells expressing XMRV/Rampho Env. The levels of MLV vector particle-associated XMRV Env were remarkably higher in XMRV/Rampho-pseudotyped MLV particles (Fig. 2A), indicating that the C-terminal tail modification slightly increased XMRV Env stability and strongly improved XMRV Env incorporation into the MLV vector. In contrast, when HIV vector components were cotransfected with XMRV Env-expressing plasmid, the levels of XMRV Env in producer cells or on HIV vector particles were severely reduced (Fig. 2B). There was no difference between the wild-type and modified XMRV Env, suggesting the inhibition or destabilization of XMRV Env glycoproteins by HIV vector components. Although the XMRV/Rampho-pseudotyped vector showed an infectious titer of 3 × 104 IU/ml, we failed to detect XMRV Env incorporated into the HIV particles. These results indicate that the relatively low titers of XMRV Env-pseudotyped HIV vectors were due to poor incorporation of XMRV Env.
FIG. 2.
Incorporation of XMRV wild-type (WT) or XMRV/Rampho Env protein into MLV and HIV vectors. Plasmid expressing XMRV WT or XMRV/Rampho Env glycoprotein was transfected into 293T cells in the presence or absence of MLV components (A) for 3 days. Western blot analysis with goat polyclonal anti-MLV gp70/30 antibody is shown (left) and the signal density of XMRV Env (right) was analyzed with ImageJ software as described in Materials and Methods and demonstrated as the average of two independent studies experiments. For the HIV pseudotypes (B), plasmid expressing XMRV WT or XMRV/Rampho Env glycoprotein was transfected into 293T cells in the presence or absence of HIV components for 3 days. Cell lysates were then extracted after 3 days of transduction. Western blot analysis with goat polyclonal anti-MLV gp70/30 antibody (left) and a mixture of mouse anti-p24 antibody 183-H12-5C and mouse anti-Ag3.0 antibody (right) is shown.
Stability of XMRV Env-pseudotyped vectors
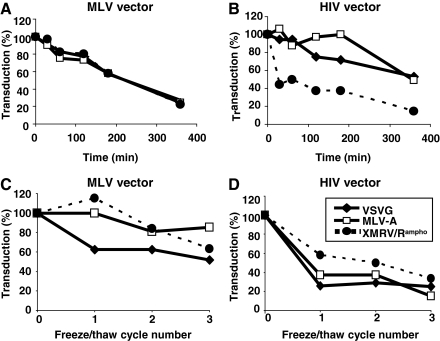
Storage conditions change vector transduction efficiencies (Strang et al., 2004). To characterize vectors pseudotyped with XMRV/Rampho, vector stability at 37°C and after repeated freeze–thaw cycles was examined (Fig. 3). Data from MLV and HIV vectors pseudotyped with VSV-G or MLV-A Env were also included to compare stability. As shown in Fig. 3A, the stability of XMRV/Rampho-pseudotyped MLV vector at 37°C was similar to that of VSV-G- and MLV-A Env-pseudotyped MLV vectors. The half-life of XMRV/Rampho-pseudotyped MLV vector was estimated as approximately 3 hr (Fig. 3A). The stability of MLV-based vectors may be more dependent on the viral particle itself than on the Env glycoproteins used for pseudotyping. In contrast, the various Env glycoproteins on HIV-based vectors strongly affected vector stability (Fig. 3B). Our results showed that the half-life of VSV-G- and MLV-A-pseudotyped vectors was 6 hr, whereas XMRV/Rampho-pseudotyped HIV vector showed a short half-life, less than 15 min (Fig. 3B), indicating the unstable nature of XMRV/Rampho Env on HIV vector at 37°C.
FIG. 3.
Characterization of XMRV/Rampho pseudotypes. Transduction efficiencies of MLV vectors (A) and HIV vectors (B) pseudotyped with VSV-G, MLV-A, or XMRV/Rampho glycoprotein were determined after incubation at 37°C. (C and D) Freeze–thaw resistance was tested by subjecting samples to 37°C and −80°C three times. Results from MLV-based vector pseudotypes (C) and HIV-based vector pseudotypes (D) are shown. Transduction efficiency before vector freezing was set to 100% and compared with transduction efficiency after various numbers of freezing cycles. All the experiments were repeated twice and average values are shown.
Vector stabilities before and after freezing are important parameters to be examined. To test the stability of vector pseudotypes, we repeated freezing at −80°C and thawing at 37°C three times and the infectivity of each vector in 293T cells was determined (Fig. 3C and D). Transduction efficiency of MLV-based vector pseudotyped with VSV-G (52%), MLV-A (82%), and XMRV/Rampho (62%) was more resistant compared with HIV-based vector pseudotyped with VSV-G (15%), MLV-A (25%), and XMRV/Rampho (33%). The results also showed that HIV vector pseudotyped with XMRV/Rampho had higher transduction efficiency than that with either VSV-G, or MLV-A Env. Thus, XMRV/Rampho pseudotypes are comparable to or better than the other two pseudotypes tested in this study, although vector difference (MLV vs. HIV) strongly affects its stability; MLV vector is more resistant compared with HIV vector pseudotypes.
No evidence of inhibitory effects by XMRV Env pseudotypes
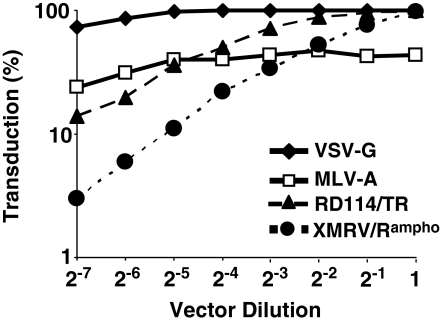
Previously, we have shown that MLV-A pseudotypes contain large amounts of free MLV-A Env (Strang et al., 2004). Excess soluble Env results in inhibition of vector transduction by competing with vector particles for their cellular receptor (Arai et al., 1998; Slingsby et al., 2000; Strang et al., 2004). Because of the free MLV-A Env in vector preparations, MLV-A pseudotypes do not show typical dose-dependent infection kinetics and the transduction efficiency of MLV-A pseudotypes can be decreased at high doses (Strang et al., 2004). In contrast, VSV-G uses a ubiquitous cellular factor as its receptor, facilitating efficient transduction by VSV-G pseudotypes without self-inhibitory effects. VSV-G pseudotypes readily achieve 100% infection at high doses (Strang et al., 2004). To assess whether XMRV/Rampho pseudotypes show such inhibitory effects, we examined their dose-dependent transduction kinetics. 293T cells were infected with 2-fold serially diluted concentrated MLV-A, VSV-G, and XMRV/Rampho vectors (Fig. 4). As reported previously (Slingsby et al., 2000; Strang et al., 2004), MLV-A-pseudotyped vector failed to achieve 100% transduction at high doses. The highest percentage of transduction was about 44%, whereas the highest concentration of both VSV-G- and RD114/TR-pseudotyped vectors achieved 99% transduction efficiency (Fig. 4). Similarly, XMRV/Rampho Env-pseudotyped vector showed linear dose-dependent transduction kinetics at low doses, and achieved 97% transduction of 293T cells at the highest concentration, indicating no evidence of inhibitory effects of XMRV/Rampho Env pseudotypes.
FIG. 4.
Inhibitory effects of XMRV Env-pseudotyped HIV vectors. Concentrated vector stocks were serially diluted 2-fold and titrated on 293T cells in the presence of Polybrene (8 μg/ml). In this experiment the initial MOI used was 100.
Transduction efficiency of XMRV Env pseudotypes in different human cells
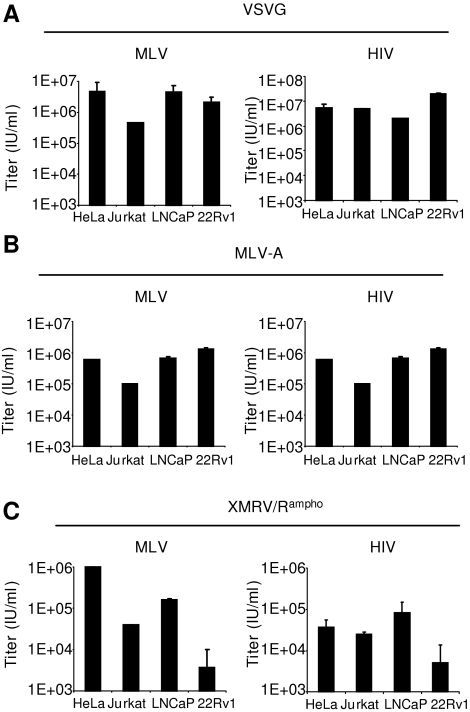
Next, we examined the infectivity of XMRV/Rampho-pseudotyped MLV and HIV vectors on four cell lines, including HeLa, Jurkat, LNCaP, and 22Rv1 cells. 22Rv1 cells, which constitutively produce XMRV (Knouf et al., 2009), were used as an XPR1-blind control. We also included VSV-G and MLV-A pseudotypes. Viral titers from MLV-based vectors pseudotyped with VSV-G were more than 106 IU/ml, except for Jurkat cells, which had 10-fold lower titers (105 IU/ml) than other cells (Fig. 5A). HIV-based vectors pseudotyped with VSV-G showed high titers, ranging between 2 × 106 and 2 × 107 IU/ml (Fig. 5A). Similarly, viral titers for MLV- and HIV-based vectors with MLV-A Env were more than 105 IU/ml for most of the cells (Fig. 5B). Although XMRV was originally isolated from prostate tissues, the titers of XMRV/Rampho-pseudotyped MLV and HIV vectors in prostate cancer-derived LNCaP cells (2 × 105 and 8 × 104 IU/ml for MLV- and HIV-based vectors, respectively) were similar to those in 293T or HeLa cells (Fig. 5C). Another prostate cancer line, 22Rv1, which stably produces XMRV, showed strong resistance to XMRV/Rampho-pseudotyped vectors (Fig. 5C). Similarly, XMRV-infected LNCaP cells were resistant to XMRV/Rampho-pseudotyped vectors, suggesting XPR1 receptor interference by the endogenously produced XMRV.
FIG. 5.
Transduction efficiency of XMRV Env-pseudotyped HIV and MLV vectors in various types of cells. Titers of MLV or HIV vector pseudotyped with VSV-G (A), MLV-A (B), or XMRV/Rampho (C) were determined in HeLa, Jurkat, LNCaP, and 22Rv1 cells in the presence of Polybrene (8 μg/ml). All experiments were repeated twice and average values from flow cytometric analyses were used for data analysis.
Transduction efficiency of XMRV/Rampho pseudotypes in human CD34+ cells
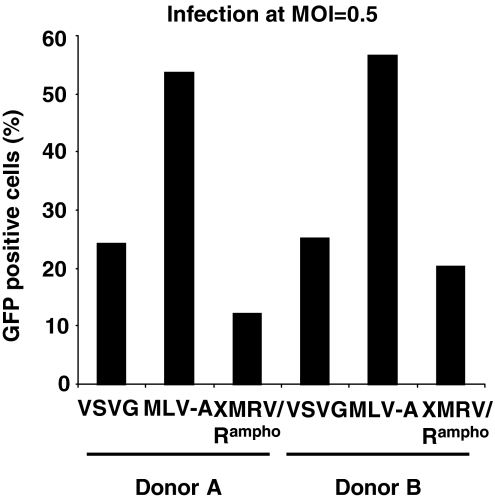
Retroviral and lentiviral vectors have been used for gene therapy of hematopoietic diseases, as the vectors allow sustained transgene expression in hematopoietic cells and hematopoietic cell progenies through integration into the host genome (Bank, 2003; Hacein-Bey-Abina et al., 2008; Cartier et al., 2009). Because high levels of XPR1 are expressed in human hematopoietic cells (Tailor et al., 1999), we next examined the transduction efficiency of the XMRV/Rampho vector in human CD34+ cells. CD34+ cells from two donors (donors A and B) were expanded and infected with HIV vectors pseudotyped with VSV-G, MLV-A, or XMRV/Rampho Env in the presence of RetroNectin. Cells were transduced with equivalent titers of vectors (MOI, 0.5). XMRV/Rampho-pseudotyped HIV vector transduced approximately 20% of human CD34+ cells at an MOI of 0.5, which was comparable to the transduction efficiency with VSV-G-pseudotyped vector (25%) (Fig. 6). At an MOI of 0.5, the highest transduction of human CD34+ cells was achieved with vector pseudotyped with MLV-A Env (Fig. 6). Although further modification is required to increase pseudotyped vector titers, these results indicate the feasibility of XMRV Env-pseudotyped lentiviral vectors in hematopoietic cell transduction.
FIG. 6.
Transduction of human CD34+ cells with XMRV Env-pseudotyped HIV vector. Human CD34+ cells isolated from two donors (donors A and B) were transduced with HIV vectors pseudotyped with various Env glycoproteins (VSV-G, MLV-A, or XMRV/Rampho) at an MOI of 0.5. GFP-positive cells were analyzed by flow cytometry.
Discussion
In this study, we generated HIV and MLV vectors pseudotyped with XMRV Env, and characterized their biological properties. Similar to GALV or RD114 Env-pseudotyped HIV vectors, the pseudotyping efficiency with XMRV Env was improved by replacement of the C-terminal 11 amino acid residues of XMRV Env with the corresponding 6 amino acid residues of MLV-A Env. Unlike MLV-A Env pseudotypes, the XMRV/Rampho-pseudotyped HIV vector showed no self-inhibitory effect. Given XPR1 expression in many clinically relevant cell types including human hematopoietic stem cells, the modified XMRV Env will provide a useful alternative to pseudotype HIV- or MLV-based vectors.
Wild-type GALV and RD114 Envs can efficiently pseudotype MLV but not HIV vectors. The modifications in the C-terminal tails of GALV and RD114 Envs result in improved pseudotyping with HIV vectors (Stitz et al., 2000; Christodoulopoulos and Cannon, 2001; Sandrin et al., 2002; Ikeda et al., 2003; Strang et al., 2004). The specificity determinant in the C-terminal region of GALV Env, which has been identified, prevents GALV Env incorporation into HIV particles (Christodoulopoulos and Cannon, 2001). XMRV is closely related to xenotropic MLV. Unexpectedly, the titer of wild-type XMRV Env-pseudotyped MLV vector was relatively low, which was improved by C-terminal modification of the XMRV Env glycoprotein. The poor titers of XMRV Env-pseudotyped vector are unlikely due to insufficient cleavage of the XMRV R peptide by MLV protease, as the amino acid sequences adjacent to the cleavage sites were identical between XMRV and MLV-A Env glycoproteins (Fig. 1B). Indeed, we found that the C-terminal modification markedly increased the levels of XMRV Env in the virus-like particles (VLPs). These observations suggest the existence of an incorporation specificity determinant in the XMRV R peptide region, which prevents its incorporation into Moloney MLV particles. Previously, relatively low vector titers were observed with MLV-X-pseudotyped retroviral vectors (Forestell et al., 1997). This is likely due to inefficient MLV-X Env incorporation, as the R peptide regions of XMRV and MLV-X NZB-9-1 Env glycoproteins are highly conserved except for a single amino acid substitution (D637E). Further studies will be required to elucidate the mechanism by which MLV-X or closely related XMRV Env glycoproteins minimize cross-packaging of other MLV particles.
Another unexpected finding was the suppression of XMRV Env expression or degradation of XMRV protein in the presence of HIV vector components (HIV-1 Gag, Pol, Rev, and Tat). The precise mechanism remains to be determined. It is, however, notable that wild-type GALV Env expression is suppressed in the presence of HIV vector components (Christodoulopoulos and Cannon, 2001). Given the suppression of XMRV Env expression, HIV protein(s) may inhibit heterologous retroviral Env expression as a viral strategy to avoid unnecessary pseudotyping with other retroviral proteins. However, we do not know whether the inhibition is specific to Env glycoproteins versus other membrane glycoproteins and whether there may be an advantage to degrading membrane glycoproteins.
Although we demonstrated improved pseudotyping efficiency by modifying the C-terminal end of XMRV Env, the improved titers of HIV and MLV vectors were 3 × 104 and 5 × 105 IU/ml, respectively, which were not comparable to the titers (6 × 106 IU/ml) seen with wild-type XMRV in the same target 293T cells. This suggests that the relatively low titers seen with XMRV/Rampho-pseudotyped vectors were not due to limited XPR1 expression on these cells. It is therefore plausible that the XMRV Env-pseudotyped vector titers can be further increased by improvement of the efficiency of vector pseudotyping with XMRV Env. Alternative strategies may be used to improve pseudotyping efficiency, including removal of the R peptides (Christodoulopoulos and Cannon, 2001) and mutations in the R peptide cleavage sites (Christodoulopoulos and Cannon, 2001; Ikeda et al., 2003) for XMRV Env.
In summary, our results demonstrate the feasibility of XMRV Env-pseudotyped HIV and MLV vectors for XPR1-targeted gene transfer. Further improvement of pseudotyping efficiency will enable efficient gene delivery into clinically relevant target cells, such as hematopoietic cells, for human gene therapy.
Acknowledgments
This work was supported by the National Institutes of Health (R56AI074363), by Mayo Clinic Career Development Project in Prostate SPORE grant CA91956-080013, and the Mayo Foundation (Y.I.), and by a Siebens Ph.D. Training Fellowship (S.O.).
Author Disclosure Statement
The authors declare no conflict of interest.
References
- Arai T. Matsumoto K. Saitoh K. Ui M. Ito T. Murakami M. Kanegae Y. Saito I. Cosset F.L. Takeuchi Y. Iba H. A new system for stringent, high-titer vesicular stomatitis virus G protein-pseudotyped retrovirus vector induction by introduction of Cre recombinase into stable prepackaging cell lines. J. Virol. 1998;72:1115–1121. doi: 10.1128/jvi.72.2.1115-1121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank A. Hematopoietic stem cell gene therapy: Selecting only the best. J. Clin. Invest. 2003;112:1478–1480. doi: 10.1172/JCI20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battini J.L. Rasko J.E. Miller A.D. A human cell-surface receptor for xenotropic and polytropic murine leukemia viruses: Possible role in G protein-coupled signal transduction. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1385–1390. doi: 10.1073/pnas.96.4.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J.C. Friedmann T. Driever W. Burrascano M. Yee J.K. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: Concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N. Hacein-Bey-Abina S. Bartholomae C.C. Veres G. Schmidt M. Kutschera I. Vidaud M. Abel U. Dal-Cortivo L. Caccavelli L. Mahlaoui N. Kiermer V. Mittelstaedt D. Bellesme C. Lahlou N. Lefrère F. Blanche S. Audit M. Payen E. Leboulch P. l'Homme B. Bougnères P. Von Kalle C. Fischer A. Cavazzana-Calvo M. Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326:818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- Christodoulopoulos I. Cannon P.M. Sequences in the cytoplasmic tail of the gibbon ape leukemia virus envelope protein that prevent its incorporation into lentivirus vectors. J. Virol. 2001;75:4129–4138. doi: 10.1128/JVI.75.9.4129-4138.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Costa J. Mansfield S.G. Humeau L.M. Lentiviral vectors in clinical trials: Current status. Curr. Opin. Mol. Ther. 2009;11:554–564. [PubMed] [Google Scholar]
- DePolo N.J. Reed J.D. Sheridan P.L. Townsend K. Sauter S.L. Jolly D.J. Dubensky T.W., Jr VSV-G pseudotyped lentiviral vector particles produced in human cells are inactivated by human serum. Mol. Ther. 2000;2:218–222. doi: 10.1006/mthe.2000.0116. [DOI] [PubMed] [Google Scholar]
- Dong B. Kim S. Hong S. Das Gupta J. Malathi K. Klein E.A. Ganem D. Derisi J.L. Chow S.A. Silverman R.H. An infectious retrovirus susceptible to an IFN antiviral pathway from human prostate tumors. Proc. Natl. Acad. Sci. U.S.A. 2007;104:1655–1660. doi: 10.1073/pnas.0610291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestell S.P. Dando J.S. Chen J. de Vries P. Böhnlein E. Rigg R.J. Novel retroviral packaging cell lines: Complementary tropisms and improved vector production for efficient gene transfer. Gene Ther. 1997;4:600–610. doi: 10.1038/sj.gt.3300420. [DOI] [PubMed] [Google Scholar]
- Green N. Shinnick T.M. Witte O. Ponticelli A. Sutcliffe J.G. Lerner R.A. Sequence-specific antibodies show that maturation of Moloney leukemia virus envelope polyprotein involves removal of a COOH-terminal peptide. Proc. Natl. Acad. Sci. U.S.A. 1981;78:6023–6027. doi: 10.1073/pnas.78.10.6023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green B.J. Lee C.S. Rasko J.E. Biodistribution of the RD114/mammalian type D retrovirus receptor, RDR. J. Gene Med. 2004;6:249–259. doi: 10.1002/jgm.517. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S. Garrigue A. Wang G.P. Soulier J. Lim A. Morillon E. Clappier E. Caccavelli L. Delabesse E. Beldjord K. Asnafi V. MacIntyre E. Dal Cortivo L. Radford I. Brousse N. Sigaux F. Moshous D. Hauer J. Borkhardt A. Belohradsky B.H. Wintergerst U. Velez M.C. Leiva L. Sorensen R. Wulffraat N. Blanche S. Bushman F.D. Fischer A. Cavazzana-Calvo M. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Invest. 2008;118:3132–3142. doi: 10.1172/JCI35700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann A. Kessler B. Ewerling S. Weppert M. Vogg B. Ludwig H. Stojkovic M. Boelhauve M. Brem G. Wolf E. Pfeifer A. Efficient transgenesis in farm animals by lentiviral vectors. EMBO Rep. 2003;4:1054–1060. doi: 10.1038/sj.embor.7400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. Klein E.A. Das Gupta J. Hanke K. Weight C.J. Nguyen C. Gaughan C. Kim K.A. Bannert N. Kirchhoff F. Munch J. Silverman R.H. Fibrils of prostatic acid phosphatase fragments boost infections with XMRV (xenotropic murine leukemia virus-related virus), a human retrovirus associated with prostate cancer. J. Virol. 2009;83:6995–7003. doi: 10.1128/JVI.00268-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y. Takeuchi Y. Martin F. Cosset F.L. Mitrophanous K. Collins M. Continuous high-titer HIV-1 vector production. Nat. Biotechnol. 2003;21:569–572. doi: 10.1038/nbt815. [DOI] [PubMed] [Google Scholar]
- Kafri T. Blömer U. Peterson D.A. Gage F.H. Verma I.M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat. Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- Kavanaugh M.P. Miller D.G. Zhang W. Law W. Kozak S.L. Kabat D. Miller A.D. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knouf E.C. Metzger M.J. Mitchell P.S. Arroyo J.D. Chevillet J.R. Tewari M. Miller A.D. Multiple integrated copies and high-level production of the human retrovirus XMRV (xenotropic murine leukemia virus-related virus) from 22Rv1 prostate carcinoma cells. J. Virol. 2009;83:7353–7356. doi: 10.1128/JVI.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverett B.D. Farrell K.B. Eiden M.V. Wilson C.A. Entry of amphotropic murine leukemia virus is influenced by residues in the putative second extracellular domain of its receptor, Pit2. J. Virol. 1998;72:4956–4961. doi: 10.1128/jvi.72.6.4956-4961.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.L. Winther B.L. Kay M.A. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)-Moloney murine leukemia virus-derived retrovirus vectors: Comparison of VSV-G and amphotrophic vectors for hepatic gene transfer. J. Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi V.C. Ruscetti F.W. Das Gupta J. Pfost M.A. Hagen K.S. Peterson D.L. Ruscetti S.K. Bagni R.K. Petrow-Sadowski C. Gold B. Dean M. Silverman R.H. Mikovits J.A. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009;326:585–589. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- McGrew M.J. Sherman A. Ellard F.M. Lillico S.G. Gilhooley H.J. Kingsman A.J. Mitrophanous K.A. Sang H. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 2004;5:728–733. doi: 10.1038/sj.embor.7400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olah Z. Lehel C. Anderson W.B. Eiden M.V. Wilson C.A. The cellular receptor for gibbon ape leukemia virus is a novel high affinity sodium-dependent phosphate transporter. J. Biol. Chem. 1994;269:25426–25431. [PubMed] [Google Scholar]
- Pichlmair A. Diebold S.S. Gschmeissner S. Takeuchi Y. Ikeda Y. Collins M.K. Reis e Sousa C. Tubulovesicular structures within vesicular stomatitis virus G protein-pseudotyped lentiviral vector preparations carry DNA and stimulate antiviral responses via Toll-like receptor 9. J. Virol. 2007;81:539–547. doi: 10.1128/JVI.01818-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeschla E.M. Wong-Staal F. Looney D.J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat. Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- Ragheb J.A. Anderson W.F. Uncoupled expression of Moloney murine leukemia virus envelope polypeptides SU and TM: A functional analysis of the role of TM domains in viral entry. J. Virol. 1994;68:3207–3219. doi: 10.1128/jvi.68.5.3207-3219.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko J.E. Battini J.L. Gottschalk R.J. Mazo I. Miller A.D. The RD114/simian type D retrovirus receptor is a neutral amino acid transporter. Proc. Natl. Acad. Sci. U.S.A. 1999;96:2129–2134. doi: 10.1073/pnas.96.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A. Mirro J. Haynes J.G. Ernst S.M. Nagashima K. Function of the cytoplasmic domain of a retroviral transmembrane protein: p15E–p2E cleavage activates the membrane fusion capability of the murine leukemia virus Env protein. J. Virol. 1994;68:1773–1781. doi: 10.1128/jvi.68.3.1773-1781.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuma R. Sakuma T. Ohmine S. Silverman R.H. Ikeda Y. Xenotropic murine leukemia virus-related virus is susceptible to AZT. Virology. 2010;397:1–6. doi: 10.1016/j.virol.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrin V. Boson B. Salmon P. Gay W. Nègre D. Le Grand R. Trono D. Cosset F.L. Lentiviral vectors pseudotyped with a modified RD114 envelope glycoprotein show increased stability in sera and augmented transduction of primary lymphocytes and CD34+ cells derived from human and nonhuman primates. Blood. 2002;100:823–832. doi: 10.1182/blood-2001-11-0042. [DOI] [PubMed] [Google Scholar]
- Schambach A. Galla M. Modlich U. Will E. Chandra S. Reeves L. Colbert M. Williams D.A. von Kalle C. Baum C. Lentiviral vectors pseudotyped with murine ecotropic envelope: Increased biosafety and convenience in preclinical research. Exp. Hematol. 2006;34:588–592. doi: 10.1016/j.exphem.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Slingsby J.H. Baban D. Sutton J. Esapa M. Price T. Kingsman S.M. Kingsman A.J. Slade A. Analysis of 4070A envelope levels in retroviral preparations and effect on target cell transduction efficiency. Hum. Gene Ther. 2000;11:1439–1451. doi: 10.1089/10430340050057512. [DOI] [PubMed] [Google Scholar]
- Stitz J. Buchholz C.J. Engelstädter M. Uckert W. Bloemer U. Schmitt I. Cichutek K. Lentiviral vectors pseudotyped with envelope glycoproteins derived from gibbon ape leukemia virus and murine leukemia virus 10A1. Virology. 2000;273:16–20. doi: 10.1006/viro.2000.0394. [DOI] [PubMed] [Google Scholar]
- Strang B.L. Ikeda Y. Cosset F.L. Collins M.K. Takeuchi Y. Characterization of HIV-1 vectors with gammaretrovirus envelope glycoproteins produced from stable packaging cells. Gene Ther. 2004;11:591–598. doi: 10.1038/sj.gt.3302189. [DOI] [PubMed] [Google Scholar]
- Strang B.L. Takeuchi Y. Relander T. Richter J. Bailey R. Sanders D.A. Collins M.K. Ikeda Y. Human immunodeficiency virus type 1 vectors with alphavirus envelope glycoproteins produced from stable packaging cells. J. Virol. 2005;79:1765–1771. doi: 10.1128/JVI.79.3.1765-1771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tailor C.S. Nouri A. Lee C.G. Kozak C. Kabat D. Cloning and characterization of a cell surface receptor for xenotropic and polytropic murine leukemia viruses. Proc. Natl. Acad. Sci. U.S.A. 1999;96:927–932. doi: 10.1073/pnas.96.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urisman A. Molinaro R.J. Fischer N. Plummer S.J. Casey G. Klein E.A. Malathi K. Magi-Galluzzi C. Tubbs R.R. Ganem D. Silverman R.H. DeRisi J.L. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog. 2006;2:e25. doi: 10.1371/journal.ppat.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]