Abstract
Although it is known that bisphosphonates prevent medial vascular calcification in vivo, their mechanism of action remains unknown and, in particular, whether they act directly on the blood vessels or indirectly through inhibition of bone resorption. To determine this, we studied the effects of two bisphosphonates on calcification of rat aortas in vitro and on in vivo aortic calcification and bone metabolism in rats with renal failure. We produced vascular calcification in rats with adenine-induced renal failure fed a high-phosphate diet. Daily treatment with either etidronate or pamidronate prevented aortic calcification, with the latter being 100-fold more potent. Both aortic calcification and bone formation were reduced in parallel; however, bone resorption was not significantly affected. In all uremic rats, aortic calcium content correlated with bone formation but not with bone resorption. Bisphosphonates also inhibited calcification of rat aortas in culture and arrested further calcification of precalcified vessels but did not reverse their calcification. Expression of osteogenic factors or calcification inhibitors was not altered by etidronate in vitro. Hence, these studies show that bisphosphonates can directly inhibit uremic vascular calcification independent of bone resorption. The correlation between inhibition of aortic calcification and bone mineralization is consistent with a common mechanism such as the prevention of hydroxyapatite formation and suggests that bisphosphonates may not be able to prevent vascular calcification without inhibiting bone formation in uremic rats.
Keywords: bone formation, bone histomorphometry, bone resorption, pyrophosphate, rat aorta, uremic osteodystrophy
Calcification of the medial layer of arteries is common in renal failure and is thought to contribute to the increased incidence of cardiovascular disease in this population. Recent studies have shown that medial calcification is a far more complex process than simple precipitation of calcium phosphate. It is clear that several endogenous inhibitors of hydroxyapatite formation are responsible for preventing vascular calcification under normal conditions1 and must be deficient in pathologic calcification. Vascular calcification is also associated with osteogenic changes in smooth muscle2 and with altered bone metabolism,3 suggesting additional pathophysiologic mechanisms. We have shown that pyrophosphate (PPi), a potent inhibitor of hydroyapatite formation,4–6 is produced by smooth muscle in quantities sufficient to prevent medial calcification in vitro.7 Plasma PPi levels are reduced in hemodialysis patients8 and PPi levels in the vascular wall may be reduced in uremia by virtue of increased alkaline phosphatase activity.9 Humans and mice with low levels of PPi due to the absence of PC-1, a membrane-bound ecto-nucleotide pyrophosphatase/phosphodiesterase that produces PPi,10–12, develop severe, fatal arterial calcification, indicating that PPi deficiency could contribute to vascular calcification in renal failure.
Systemic administration of PPi to vitamin D-toxic rats prevents vascular calcification13 but this approach is limited by the susceptibility of PPi to hydrolysis. Bisphosphonates are nonhydrolyzable analogs of pyrophosphate that were originally developed to treat ectopic calcification. Although early compounds were successful at preventing vascular calcification in rats,6,14,15, inhibition of bone formation limited this approach in humans.15 It was assumed that the prevention of vascular calcification as well as the inhibition of bone formation were due to a physicochemical effect of the bisphosphonates related to their PPi-like structure. Subsequent chemical modifications imparted an additional biological action that yielded newer bisphosphonates that are far more potent at inhibiting bone resorption than bone formation. Recent studies with these compounds have shown that medial vascular calcification produced by vitamin D, warfarin, or renal failure in rats can be prevented at relative potencies that match those for inhibition of bone resorption rather than bone formation.16–18
Based on these results, it has been assumed that the prevention of vascular calcification is related to the antiresorptive activity of bisphosphonates in bone, thus supporting the putative link between bone resorption and vascular calcification. However, effects on bone were not examined in these studies and bisphosphonates can inhibit calcium deposition in vascular smooth muscle cells in culture.19 Thus, the possibility that bisphosphonates directly inhibit vascular calcification in renal failure independent of effects on bone cannot be excluded. Whether calcification can be prevented without reducing bone mineralization is also unknown. Using two bisphosphonates with different relative potencies for inhibition of bone resorption and formation, we compared vascular calcification and bone metabolism in vivo in rats with renal failure. An in vitro model of vascular calcification in cultured rat aortas was used to examine direct effects of bisphosphonates independent of bone.
RESULTS
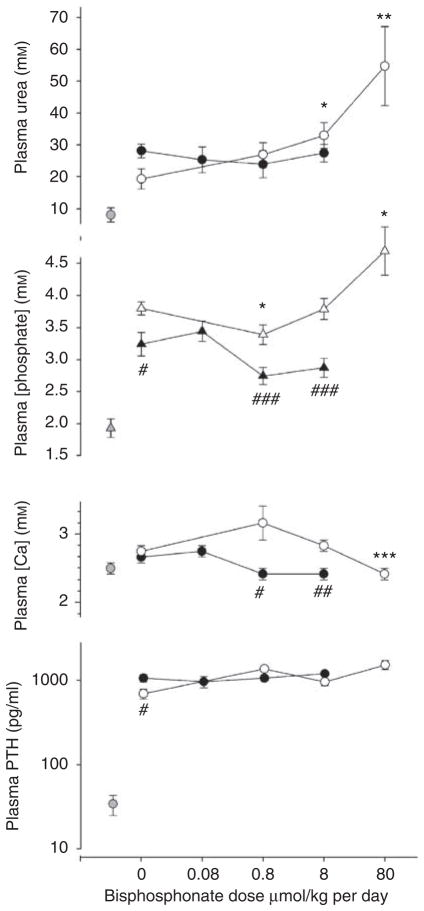
Rats were fed with adenine for 28 days together with a high-phosphate diet to induce renal failure and vascular calcification. One rat treated with the highest dose of etidronate was killed on day 22 due to distress. One rat receiving the lowest dose of pamidronate died on day 27 and was not included in the results. The resulting plasma concentrations of urea, calcium, and phosphate in rats receiving etidronate, pamidronate, or vehicle are shown in Figure 1. Plasma urea increased 2.5- to 4-fold in vehicle-treated, adenine-fed rats compared with pair-fed control rats. Pamidronate did not alter the plasma urea concentration at any dose but it was significantly increased at the two highest etidronate doses. Plasma urea did not differ between rats treated with etidronate or pamidronate at similar doses. The plasma phosphate concentration was substantially elevated in uremic rats and was slightly decreased and substantially increased at the highest and lowest doses of etidronate respectively. Plasma phosphate was not significantly altered by pamidronate although it was lower than in etidronate-treated rats. Plasma calcium was not altered by uremia or pamidronate but was significantly reduced at the highest etidronate dose. At similar doses, the plasma calcium concentration was lower in the pamidronate-treated rats. Plasma parathyroid hormone concentration was markedly elevated in uremic rats but not altered by bisphosphonates.
Figure 1. Plasma chemistries in bisphosphonate-treated rats.
Open symbols, etidronate-treated rats; solid symbols, pamidronate-treated rats; shaded symbols: nonuremic rats. There are separate untreated uremic controls for pamidronate and etidronate-treated rats. *P<0.05, **P<0.02, ***P<0.005 versus untreated adenine-fed rats; #P<0.05, ##P<0.02, ###P<0.01 versus etidronate-treated rats.
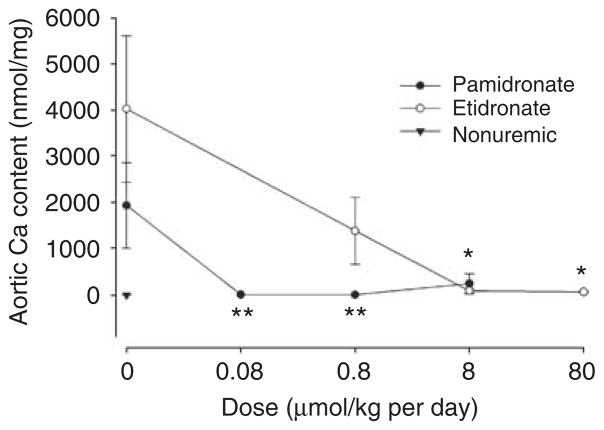
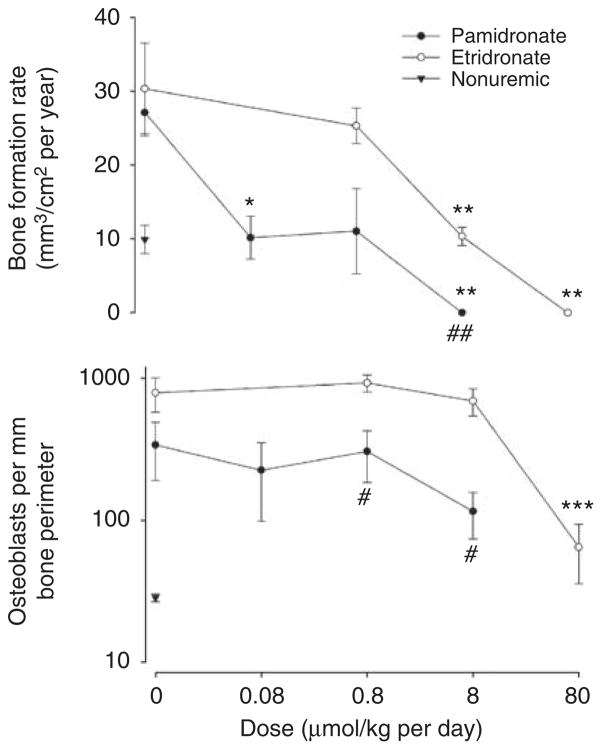
Extensive aortic calcification developed in half of the uremic rats treated with vehicle alone. As shown in Figure 2, both bisphosphonates reduced aortic calcification. Complete inhibition was obtained at 0.08 μmol/kg per day of pamidronate and at 8 μmol/kg per day of etidronate. Samples of bone were examined from the same rats. Bone volume, osteoid volume, and cortical thickness were not altered in uremic rats and were unaffected by bisphosphonate (Table 1). Parameters of bone resorption are shown in Figure 3. Erosion depth and osteoclast number both increased (but not significantly) in adenine-fed rats compared with pair-fed nonuremic rats and tended to be higher in etidronate-treated rats than in pamidronate-treated rats. Although there was a decreasing trend with increasing doses of bisphosphonate, none of the changes were significant. Parameters of bone formation are presented in Figure 4. Both bone formation rate (2.7-fold; P = 0.0009) and osteoblast number (20-fold; P = 0.0013) were increased in the adenine-fed rats. Bisphosphonates completely eliminated bone formation, with pamidronate being at least 10-fold more potent, but osteoblast number was reduced only at the highest dose of etidronate. Mineralizing surface (1.5-fold; P = 0.05) was also increased in the adenine-fed rats and substantially reduced with bisphosphonates, again with pamidronate being at least 10-fold more potent (Table 1).
Figure 2. Effect of bisphosphonates on aortic calcification in renal failure.
Solid symbols, pamidronate-treated rats; **P<0.05 versus untreated uremic rats by Mann–Whitney U-test. Open symbols, etidronate-treated rats; *P<0.02 versus untreated uremic rats by Mann–Whitney U-test.
Table 1.
Bone parameters in control and bisphosphonate-treated uremic rats
| Treatment | Dose μmol/kg per day | Bone volume % | Cortical thickness μm | Osteoid thickness μm | Mineralization surface % |
|---|---|---|---|---|---|
| No adenine | 12.5±1.6 | 494±34 | 3.64±0.50 | 13.0±1.4 | |
| Adenine+ etidronate | 0 | 14.5±2.1 | 594±31 | 3.82±0.58 | 21.4±3.8 |
| 0.8 | 10.9±1.1 | 637±28 | 5.04±1.0 | 21.7±2.6 | |
| 8 | 14.6±1.1 | 649±20 | 12.2±3.0** | 9.2±1.5** | |
| 80 | 15.5±1.3 | 621±27 | 6.43±1.5 | 3.3±0.9*** | |
| Adenine+ pamidronate | 0 | 13.8±2.3 | 478±31 | 4.36±0.45 | 19.7±2.8 |
| 0.08 | 10.4±0.6 | 540±29 | 6.23±2.2 | 10.3±1.6** | |
| 0.8 | 10.3±1.7 | 525±23 | 3.27±0.61 | 9.6±2.1* | |
| 8 | 13.9±2.1 | 466±18 | 5.75±1.7 | 1.4±0.2*** |
P<0.05,
P<0.02,
P<0.005 vs untreated uremic.
Figure 3. Parameters of bone resorption in bisphosphonate-treated rats.
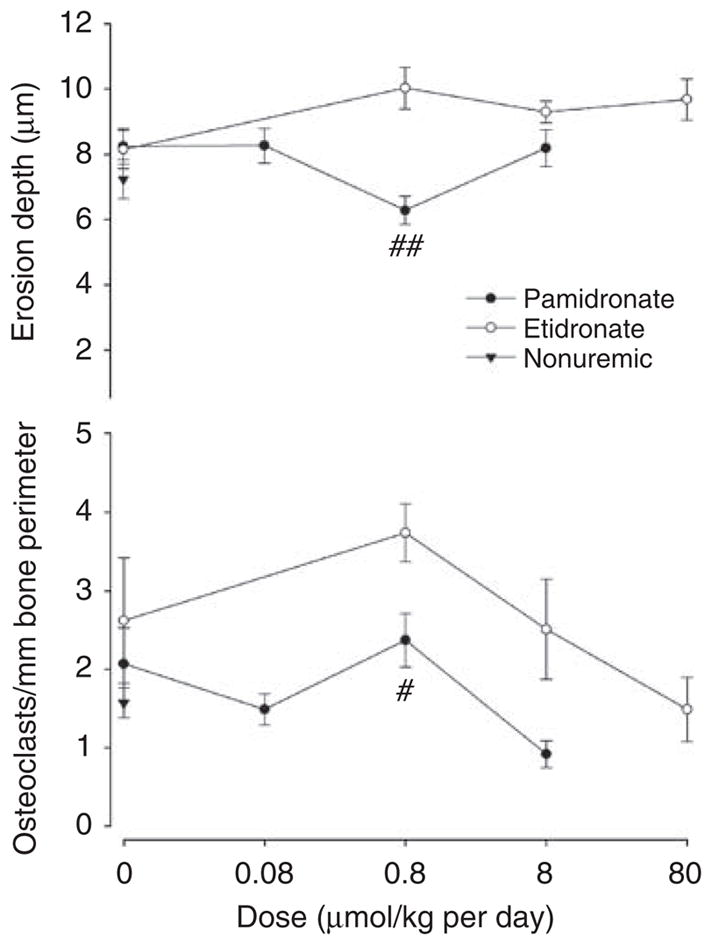
Top: erosion depth. Bottom: osteoclast number. Solid symbols, pamidronate-treated; open symbols, etidronate-treated rats; triangles: control, nonuremic rats. There are separate untreated uremic controls for pamidronate and etidronate-treated rats. #P<0.02, ##P<0.005 versus etidronate-treated rats.
Figure 4. Parameters of bone formation in bisphosphonate-treated rats.
Top: Bone formation rate per bone surface. Bottom: Number of osteoblasts per mm bone perimeter. There are separate untreated uremic controls for pamidronate and etidronate-treated rats. *P<0.01, **P<0.005, ***P<0.002 versus, control uremic rats; #P<0.01, ##P<0.005 versus etidronate-treated rats.
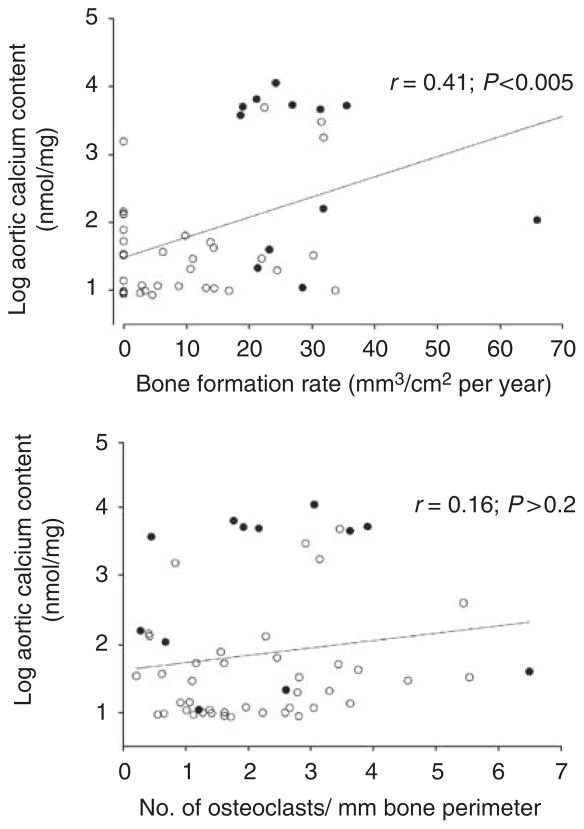
Parameters of bone formation and resorption were compared with aortic calcium content by linear regression in the entire cohort of adenine-fed rats (Figure 5). Owing to the large range of values for aortic calcium, regression was performed using the logarithm of calcium content to minimize skewing of the data. Of all the parameters of bone formation examined, bone formation rate correlated best with aortic calcium content (Figure 5, top). The correlation between aortic calcium content and bone formation rate was 0.41 (0.48 when the outlying value was omitted). Aortic calcium also correlated significantly with osteoblast surface (r = 0.31), osteoblast number (r = 0.35), and mineralizing surface (r = 0.34). In contrast, there was no significant correlation between aortic calcium content and osteoclast number (Figure 5, bottom). Correlation with other parameters of bone resorption was even poorer (not shown).
Figure 5. Correlation between aortic calcium content and bone histomorphometry in uremic rats treated with or without bisphosphonates.
Top: bone formation rate per bone surface; bottom: number of osteoclasts per bone perimeter. Open symbols: rats treated with etidronate or pamidronate. Solid symbols: rats treated with vehicle alone. Lines are linear regressions.
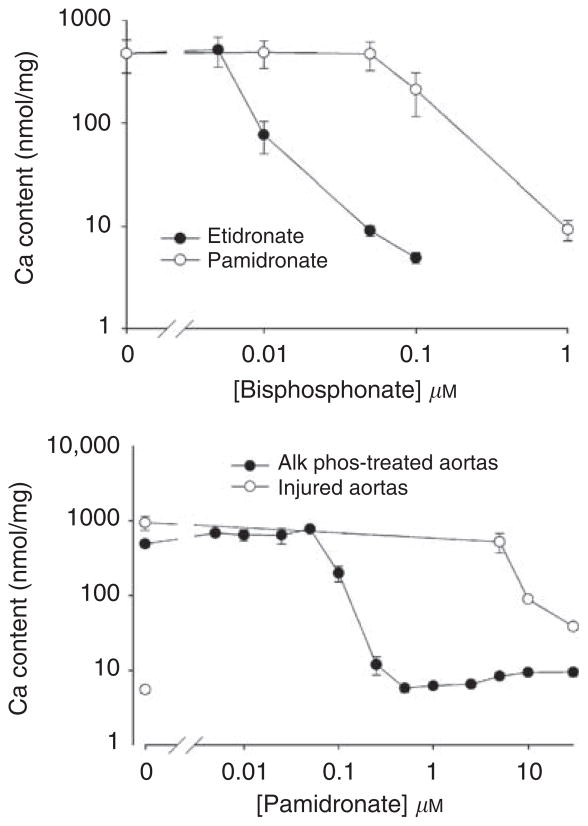
To determine whether bisphosphonates can affect vascular calcification directly, independent of effects on bone, studies were repeated in normal rat aortas in culture. Culture of rat aortas with alkaline phosphatase results in medial calcification after 9 days by virtue of removing pyrophosphate.7 Warfarin, which increases calcification in vitro by reducing levels of matrix Gla protein (KA Lomashvili and WC O’Neill, unpublished data), was added to maximize calcification and provide a more precise comparison between the two agents. As shown in Figure 6a, calcification was prevented by increasing concentrations of etidronate or pamidronate, with complete inhibition at 0.1 and 1 μM, respectively. Inhibition of calcification was also observed in the absence of MGP (not shown). Medial calcification of aortas can also be produced in culture without alkaline phosphatase when the vessels are injured prior to culture by rubbing with a cotton swab. As shown in Figure 6b, substantially greater concentrations of pamidronate were required to inhibit calcification in injured aortas than in uninjured aortas cultured with alkaline phosphatase. A similar decrease in potency in injured aortas was observed with etidronate (not shown).
Figure 6. Effect of bisphosphonates on calcification of rat aortas in culture.
Top: Aortas were incubated in DMEM containing 3.8 mM phosphate, 3.75 U/ml of alkaline phosphatase, 25 μM warfarin, and the indicated concentrations of bisphosphonate for 9 days. Results are the means±s.e. of seven aortic rings. Bottom: Injured and uninjured aortas were incubated in DMEM with 3.8 mM phosphate, with or without 3.75 U/ml of alkaline phosphatase, and the indicated concentrations of pamidronate for 9 days. Results are the means±s.e. of at least three aortic rings.
As bisphosphonates have been reported to inhibit alkaline phosphatase,20 reduction of PPi hydrolysis by alkaline phosphatase could be a potential mechanism for the prevention of calcification by bisphosphonates. However, hydrolysis of PPi by rat aortas was not altered by 10 μM pamidronate (data not shown), a concentration that completely prevented calcification in cultured aortas. Studies were also performed to determine whether bisphosphonates alter the expression of calcification inhibitors or osteogenic factors in vascular smooth muscle (Table 2). Messenger RNA was measured by quantitative polymerase chain reaction and normalized to 18S RNA. Substantial quantities of mRNA for both matrix Gla protein and osteopontin were detected after 3 days in normal medium and even higher levels were observed after 9 days in high-phosphate medium with alkaline phosphatase (calcifying conditions). The levels in aortas cultured for 9 days in normal medium were similar to those after 3 days (not shown). In contrast, expression of the transcription factor runx2 (cbfa-1) was very low after 3 days in normal medium, consistent with levels in fresh aorta (not shown). The slightly higher levels in aortas cultured for 9 days under calcifying conditions were also observed in aortas cultured for 9 days in normal medium (not shown). Expression of the bone-specific transcription factor osterix was extremely low. Etidronate at 1 μM did not alter the content of any of the mRNAs at either 3 days in normal medium or 9 days under calcifying conditions.
Table 2.
Abundance of mRNA in aortas cultured with or without 1 μM etidronate
| 3 days (low phosphate without AP) |
9 days (high phosphate with AP) |
|||||
|---|---|---|---|---|---|---|
| Etidronate | Control | P | Etidronate | Control | P | |
| MGP | 776±66 | 888±76 | NS | 1760±729 | 2630±1110 | NS |
| OPN | 194±26 | 128±21 | NS | 1420±494 | 1770±634 | NS |
| Runx2 | 1.7±0.4 | 1.6±0.5 | NS | 17±3 | 68±40 | NS |
| Osterix | 0.07±0.03 | 0.038±0.024 | NS | <0.01 | <0.01 | NS |
AP, alkaline phosphatase; MGP, matrix gla protein; OPN, osteopontin.
Values are copies per 105 copies of 18S RNA.
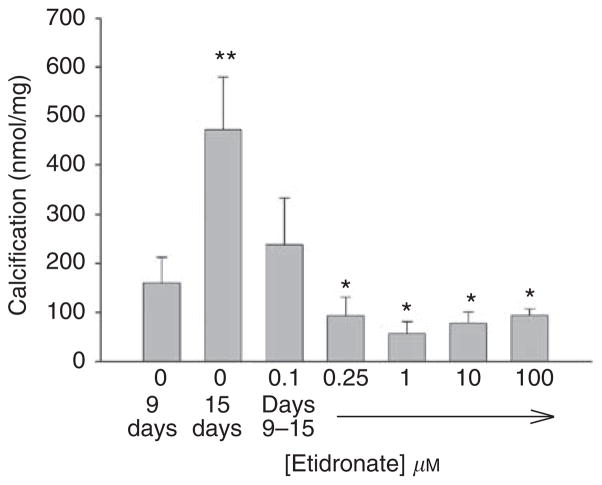
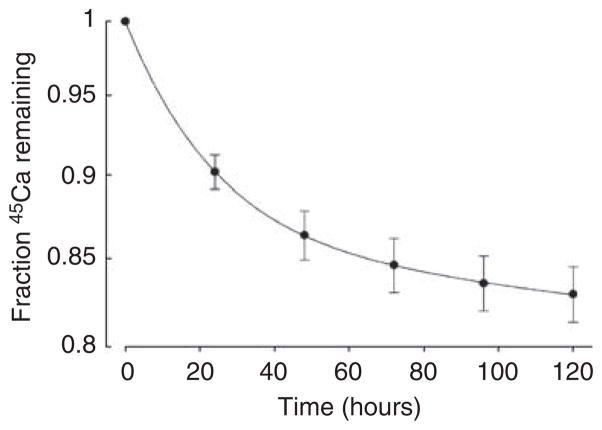
Additional studies were performed to determine whether bisphosphonates could also arrest or reverse existing calcification. To examine the effect on progression of calcification, aortas were cultured with alkaline phosphatase for 9 days in the absence of etidronate and then cultured an additional 6 days with alkaline phosphatase and varying concentrations of etidronate (Figure 7). Culture for an additional 6 days without etidronate resulted in a further 195% increase in calcification (P<0.02). This increase was prevented by etidronate but at higher concentrations (approximately 10-fold) than required to prevent de novo calcification. There was no further reduction at etidronate concentrations above 0.25 μM. Although calcification at 15 days was less than the calcification at 9 days at maximum concentrations of etidronate, suggesting reversal, the difference was not significant. To examine reversibility of calcification directly, release of 45Ca was measured in precalcified aortas. The results are shown in Figure 8, with the fraction of 45Ca remaining fitting a biexponential decline (r>0.99). Approximately 14% of the calcium exhibited a half-life of 16.0±0.8 h while the bulk of the calcium had a much longer half-life of 2920±351 h. Neither half-life was altered by 30 μM etidronate (14.0±0.8 h and 2980±421 h).
Figure 7. Arrest of aortic calcification by etidronate.
Aortas were cultured with alkaline phosphatase and 45Ca for 9 days and then for an additional 6 days with alkaline phosphatase in the presence of varying concentrations of etidronate. Calcium content of the aortas was measured as described in ‘Materials and Methods’. Results are the means±s.e. of at least eight aortic rings. *P<0.001 versus 15 days without etidronate; **P<0.02 versus 9 days.
Figure 8. Washout of calcium from calcified vessels.
Injured aortas were calcified for 9 days in high-phosphate DMEM prior to washout as described in ‘Materials and Methods’. Results are expressed as the fraction of 45Ca remaining in the aortas and are the means±s.e. of determinations in eight aortic rings.
DISCUSSION
Both etidronate and pamidronate prevented aortic calcification in uremic rats, confirming previous studies of bisphosphonates in the same model18 and other models6,16,17 of vascular calcification. Inhibition of calcification cannot be explained by changes in the circulating phosphate or calcium levels. Etidronate completely prevented calcification at 8 μmol/kg per day despite plasma calcium and phosphate levels that were almost identical to levels in untreated rats. Although the plasma calcium concentration was decreased at the highest dose of etidronate, any effect on calcification would have been offset by the concurrent increase in phosphate concentration. Plasma calcium and phosphate levels were reduced at the two highest doses of pamidronate compared with untreated rats, but the differences were not significant. Furthermore, pamidronate completely suppressed calcification at the lowest dose despite no change in plasma calcium or phosphate concentration. Although a decrease in plasma calcium might have been expected based on clinical experience, the bisphosphonates had little effect on bone resorption and the inhibition of bone formation would have countered any decrease in circulating calcium levels. Bisphosphonates also failed to lower calcium levels in a previous study in uremic rats.21 The higher phosphate and lower calcium levels at the highest etidronate dose probably reflect worse renal failure as indicated by the higher urea level. This is consistent with reports of renal failure with high doses of bisphosphonates.22
The fact that the potency of different bisphosphonates in previous studies of vascular calcification paralleled their reported antiresorptive potencies in vivo has been taken as evidence that medial vascular calcification is linked to bone resorption. However, no bone histology has been presented to support this hypothesis. The relative potencies of etidronate and pamidronate in the current study also paralleled the reported potencies for inhibition of bone resorption, but bone histology did not support a link between inhibition of vascular calcification and inhibition of bone resorption. The effect of bisphosphonate on indices of bone resorption was small and is consistent with a previous study in rats with renal ablation.21 Doses of bisphosphonates that completely suppressed aortic calcification did not significantly reduce any index of bone resorption. Furthermore, there was no consistent correlation between aortic calcium content and any index of bone resorption in the entire cohort of uremic rats.
The small increases in parameters of bone resorption compared with the larger increases in bone formation rate in the untreated uremic rats do not necessarily indicate a net increase in bone in this model. Bone resorption cannot be directly measured and an increase in bone volume was not observed histologically. Furthermore, this model is superimposed on a skeleton that continues to increase during the life of the rats. This together with the short duration of the model may explain why bone volume did not decrease with uremia.
The mechanism by which bisphosphonates reduce bone resorption and why the effect varies so widely among different compounds is not completely understood. The fact that the different potencies in vivo are not always reproduced in vitro15 suggests that differences in pharmacokinetics may contribute. Bioavailability can vary due to differences in binding to proteins, soft tissues, and bone15,23 and the pharmacokinetics of subcutaneous administration as used in rats have not been examined. Although the inhibition of bone resorption has been documented in several conditions associated with increased bone turnover, there has been only one previous study of bisphosphonate therapy in uremic osteodystrophy.21 Weekly treatment with ibandronate in partially nephrectomized rats did not alter osteoclast numbers or erosion surface but did reduce erosion depth. However, as in the present study, there were greater reductions in bone formation. As formation and resorption are normally coupled, the decrease in bone formation could represent a restoration of normal bone turnover. This is unlikely since, in this study, formation was reduced to levels well below control (nonuremic) animals. The results of these two studies suggest that caution must be used in extrapolating reported potencies of bisphosphonates for bone resorption and bone formation to bone metabolism in renal failure. As rat bone lacks the haversian system present in humans, the results cannot necessarily be extrapolated to patients with renal failure.
Inhibition of aortic calcification actually correlated better with inhibition of bone formation, which was almost completely suppressed by bisphosphonate. The potency of different bisphosphonates in inhibiting bone formation varies much less than the potency for inhibiting bone resorption,15,23 owing to the fact that inhibition of mineralization is a direct effect on hydroxyapatite formation related to the pyrophosphate-like backbone15 and not influenced as much by the side groups.23 The results in uremic rats showing decreased bone formation and mineralizing surface without a decrease in osteoblast number is consistent with this mineralizing defect. The fact that osteoid thickness did not increase is probably due to the short duration of treatment. The greater potency of pamidronate compared to etidronate in vivo may relate to pharmacokinetics since it is less potent than etidronate on bone in vitro23 and was less effective at inhibiting calcification of aortas in vitro in the current study. The frequent (daily) dosing combined with decreased clearance due to renal failure may also have contributed to the potency of pamidronate in vivo as continuous presence of pamidronate versus intermittent exposure greatly increases inhibition of bone mineralization in vitro.23 The correlation between aortic calcification and bone formation suggests that the two are linked, but it is also possible that inhibition of vascular calcification is a direct effect of bisphosphonates on hydroxyapatite formation that also occurs in the bone.
The fact that both bisphosphonates also prevented calcification of rat aortas in culture clearly demonstrates direct inhibition independent of the bone. Inhibition of the aortic calcification induced by alkaline phosphatase in vitro is consistent with the fact that bisphosphonates can substitute for the pyrophosphate removed by alkaline phosphatase and are resistant to the action of this enzyme. Bisphosphonates also inhibited calcification of injured aortas cultured in the absence of alkaline phosphatase, although higher concentrations were required. The mechanism of the calcification in injured aortas is not clear but upregulation of alkaline phosphatase does occur.7 Calcification is usually more robust in injured aortas, which may require greater quantities of bisphosphonates as inhibition likely requires binding to the hydroxyapatite.24 The greater potency of etidronate compared with pamidronate in vitro is consistent with results in the bone23 and indicates a direct physicochemical action of bisphosphonates on hydroxyapatite formation. Pamidronate contains a side group on the carbon atom that imbues it with an additional, intracellular action that is thought to be responsible for the greater inhibition of bone resorption.15 This clearly does not enhance inhibition of aortic calcification in vitro and appears to interfere with the physicochemical action.
In addition to preventing de novo calcification in vitro, bisphosphonates were also effective in arresting progression of established calcification. This required significantly greater amounts, which may be related to the extent of calcification. Bisphosphonates bind avidly to hydroxyapatite24 and this is probably the mechanism by which calcification is blocked. Thus the amount required to prevent calcification is probably dependent on the amount of hydroxyapatite present, which has been demonstrated in vitro with PPi.5 Although etidronate blocked further calcification, it did not directly reverse calcification. The kinetics of calcium release from calcified vessels revealed two pools. The majority of the calcium was released very slowly with a half-life of 40–120 days. This undoubtedly represents apatitic calcium, which is highly insoluble under physiologic conditions.25 The remainder of the calcium was released more rapidly and probably represents amorphous calcium phosphate or brushite, which is far more soluble.26 Although etidronate did not increase the rate of either pool, reversal of calcification could occur over an extended period of time as the calcifications dissolve when further hydroxyapatite formation is prevented. The lack of effect on dissolution is in contrast to the previously reported inhibition of hydroxyapatite dissolution by bisphosphonates at similar concentrations.27 Possible explanations for this difference are that the previous study used chemically prepared hydroxyapatite, was not performed in a biological system, and was performed in the absence of calcium or phosphate. Physiologic concentrations of calcium and phosphate were used in the present studies to mimic conditions in vivo and to maintain the health of the cells. Thus, some or all of the 45Ca release could represent exchange rather than net release of calcium.
Alkaline phosphatase has a critical role in calcification by virtue of hydrolyzing PPi.7,28 Although bisphosphonates are not hydrolyzable, they could interact with the catalytic site and interfere with PPi hydrolysis, resulting in higher PPi levels and inhibition of calcification. Such inhibition has been reported but required millimolar concentrations.20 In the current studies, pamidronate did not alter the rate of hydrolysis of PPi by aortas, indicating that this is not a mechanism for preventing calcification. Another key protein is matrix Gla protein (MGP), an inhibitor of vascular calcification.29 Despite a report that bisphosphonates reduce expression of mRNA for MGP in cultured smooth muscle cells,19 no changes were detected in cultured aortas. There was also no change in the amount of mRNA for osteopontin, another inhibitor.30 The increased abundance of these mRNAs during culture under calcifying conditions is consistent with the increased protein expression that is observed (Lomashvili KA and O’Neill WC, unpublished studies). Etidronate also did not alter the content of mRNA for runx2, an osteogenic transcription factor that has been hypothesized to drive smooth muscle calcification,31 or osterix, a bone-specific transcription factor. Of note, the abundance of both mRNAs was very low in aortas and did not increase under calcifying conditions. These negative data all point to direct inhibition of hydroxyapatite formation as the mechanism for bisphosphonate action in cultured aortas.
This study demonstrates that inhibition of uremic vascular calcification by bisphosphonates correlates more strongly with inhibition of bone formation than inhibition of bone resorption. Although it is possible that there is a direct link between bone formation and vascular calcification, the in vitro data indicate that bisphosphonates directly inhibit hydroxyapatite formation at both locations. This suggests that doses of bisphosphonates necessary to prevent vascular calcification will also impair bone formation, thus limiting their therapeutic potential. However, aortic calcification was prevented at a dose of pamidronate that did not suppress bone formation below normal levels, indicating that a therapeutic window may exist. The lowest dose of pamidronate used in this study (0.03 mg/kg per day for 28 days) is similar to the dose in humans to inhibit bone resorption (90 mg monthly). However, subcutaneous administration may not be equivalent to intravenous injection in humans and pamidronate clearance may be significantly retarded by renal failure. Also, this model of renal failure and vascular calcification in rats may not be applicable to humans with renal failure because of the nature of the renal lesion, the short duration of uremia, and the differences between rat and human bone. Although it is difficult to compare these doses, it is likely that the doses, which inhibit vascular calcification are substantially higher than the antiresorptive doses currently used in humans. Additional studies with careful attention to drug bioavailability, dynamics of vascular calcification, and changes in bone metabolism will be required to determine whether bisphosphonates are clinically useful in preventing uremic vascular calcification. Although this study does not provide evidence for a causal relationship between bone metabolism and vascular calcification, it does not refute this putative link. As bisphosphonates have direct effects on vascular calcification, they cannot be used to address this hypothesis.
MATERIALS AND METHODS
Animal studies
Chronic renal failure was produced by feeding adenine to rats.32,33 This results in crystallization of 2,8-dihydroxyadenine in the tubules and interstitium with resulting interstitial nephritis and fibrosis and all the metabolic abnormalities of chronic renal failure.32 Male Sprague-Dawley rats were obtained from Charles River Laboratories and fed a standard rodent diet until body weight exceeded 300 g. Rats were then fed a 2.5% protein diet (Harlan Teklad, Madison, WI, USA), which enhances vascular calcification,18 supplemented with phosphate. This diet contained 1.06% calcium and 0.92% phosphorus. The adenine content was 0.75% for the first 7 days and 0.5% thereafter. Control rats were fed the identical diet without adenine but restricted in amount so that their weight loss matched that of the adenine-fed rats. Etidronate (Sigma-Aldrich, St Louis, MO, USA) and pamidronate (Novartis, Basel, Switzerland) were administered in saline, buffered to pH 7.4, by daily subcutaneous injection starting on the first day of the adenine diet. The doses of etidronate were 0.17, 1.7, and 17 mg/kg per day (0.8, 8, and 80 μmol/kg per day) and the doses of pamidronate were 0.03, 0.3, and 3 mg/kg per day (0.08, 0.8, and 8 μmol/kg per day). There were seven rats in each group with renal failure and six pair-fed rats without renal failure. Rats were killed after 28 days and aortas were perfused with saline, harvested, and cleaned. After drying and weighing, the aortas were extracted overnight in 1 M HCl and calcium in the extract was measured colorimetrically by the cresolphthalein method.34 Plasma urea was measured colorimetrically by the urease-glutamate dehydrogenase method (Sigma-Aldrich, St Louis, MO), plasma phosphate was measured colorimetrically by the molybdate method,35 and plasma calcium was measured by the cresolpthalein method.34 Plasma intact parathyroid hormone levels were determined using a rat enzyme-linked immunoassay (Scantibodies Inc., Santee, CA, USA).
Bone histomorphometry
Rats received intraperitoneal injections of calcein (2 mg) on days 20 and 25. At the time of killing, one femur was removed, cleaned of attached tissue, and placed in absolute ethanol. After dehydration, samples were embedded in methyl methacrylate and eight sections were cut serially at 4 μm thickness with a heavy-duty microtome. This thickness allows analysis of the same histologic features in slides prepared for light and fluorescent microscopy. Every other section was stained with the modified Masson–Goldner trichrome technique whereas the other sections were reserved for fluorescence microscopy. Static and dynamic parameters of bone structure, formation, and resorption were measured at a standardized site below the growth plate using a semi-automatic method.36,37
Aortic calcification in vitro
Aortas were cultured from adult, male Sprague–Dawley rats as described earlier.7 Briefly, aortas were dissected from surrounding tissue and perfused with physiologic saline under sterile conditions. After the adventitia was carefully removed with scissors to avoid any mechanical trauma, 2–3 mm rings were placed in high-phosphate DMEM medium containing 3.75 U/ml of calf intestinal alkaline phosphatase and incubated at 37°C in 5% CO2 with medium changes every 3 days. In studies comparing potencies of bisphosphonates, 25 μM warfarin was added to maximize calcification. Calcification was also produced by rubbing aortas with a cotton swab followed by culture without alkaline phosphatase. The total concentrations of calcium and phosphate were 1.8 and 3.8 mM respectively. To quantitate calcification, 45Ca was added to the medium and incorporation into the aortic rings was measured after extracting the rings in 3 M HCl.
Calcium release
Injured aortas were cultured for 9 days in high-phosphate DMEM containing 45Ca. The aortas were washed several times in low-phosphate DMEM and then placed in the same medium for 4 h to wash out unbound 45Ca. Medium was then changed and samples were removed at the indicated times for measurement of 45Ca. After 5 days, 45Ca content of the aortas was measured as above.
Pyrophosphate hydrolysis
This was measured as described earlier9 by incubating freshly isolated aortas with 1 μM sodium pyrophosphate and [32P]PPi (3 μCi/ml) and then adding ammonium molybdate (to bind orthophosphate) followed by extraction with isobutanol/petroleum ether (4:1) to separate phosphomolybdate from pyrophosphate. The rings were then dried and weighed.
Quantitative polymerase chain reaction
Aortas were frozen in liquid nitrogen, powdered and then extracted with a guanidinium and phenol solution on ice with additional homogenization. Chlorophorm was added and RNA extracted by centrifugation. cDNA was synthesized using 2 μg of total RNA from each sample with iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) and quantitative PCR performed with an iCycler iQ Detection System using iQ SYBR− Green Supermix (Bio-Rad). To avoid amplification of genomic DNA, primers for QPCR were designed to flanking at least on intron. Sequences of the primer pairs (F: forward; R: reverse) were as follow. Osteopontin: CTGTCTCCCGGTGAAAGTG (F), GTCATCCGTTTCTTCAGAGG (R); matrix gla protein: AGGCAGACTCACAGGACACC (F), CATTTCTCCGGTTGGTGAAG (R); Runx 2: CTTCACAAATCCTC CCCAAG (F), GAGGCGGTCAGAGAACAAAC (R); Osterix: TCTC CATCTGCCTGACTCCT (F), TTCTTTGTGCCTCCTTTTCC (R).
Statistics
The Mann–Whitney U-test was applied to nonparametric data (aortic calcium content and bone parameters). Differences between groups for all other data were examined by Student’s t-test.
Acknowledgments
This work was supported by Grant DK069681 from the National Institutes of Health and a grant from the Genzyme Renal Innovations Program (to Dr O’Neill), and Nephrology Fellowship and Junior Faculty Grants from Amgen (to Dr Lomashvili). Portions of this work have previously been presented in abstract form at the American Society of Nephrology Annual Meeting 2006 and 2008.
Footnotes
DISCLOSURE
All the authors declared no competing interests.
References
- 1.O’Neill WC. Vascular calcification: not so crystal clear. Kidney Int. 2007;71:282–283. doi: 10.1038/sj.ki.5002119. [DOI] [PubMed] [Google Scholar]
- 2.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 3.Min H, Morony S, Sarosi I, et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J Exp Med. 2000;192:463–474. doi: 10.1084/jem.192.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell RGG, Bisaz S, Fleisch H. Pyrophosphate and diphosphates in calcium metabolism and their possible role in renal failure. Arch Intern Med. 1969;124:571–575. [PubMed] [Google Scholar]
- 5.Meyer JL. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231:1–8. doi: 10.1016/0003-9861(84)90356-4. [DOI] [PubMed] [Google Scholar]
- 6.Francis MD, Russell RGG, Fleisch H. Diphosphonates inhibit formation of calcium phosphate crystals in vitro and pathologic calcification in vivo. Science. 1969;165:1264–1266. doi: 10.1126/science.165.3899.1264. [DOI] [PubMed] [Google Scholar]
- 7.Lomashvili KA, Cobbs S, Hennigar RA, et al. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15:1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 8.Lomashvili KA, Khawandi W, O’Neill WC. Reduced plasma pyrophosphate levels in hemodialysis patients. J Am Soc Nephrol. 2005;16:2495–2500. doi: 10.1681/ASN.2004080694. [DOI] [PubMed] [Google Scholar]
- 9.Lomashvili KA, Garg P, Narisawa S, et al. Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int. 2008;73:1024–1030. doi: 10.1038/ki.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goding JW. Ecto-enzymes: physiology meets pathology. J Leukocyte Biol. 2000;67:285–308. doi: 10.1002/jlb.67.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belli SI, van Driel IR, Goding JW. Identification and characterization of a soluble form of the plasma cell membrane glycoprotein PC-1 (5′-nucleotide phosphodiesterase) Euro J Biochem. 1993;217:421–428. doi: 10.1111/j.1432-1033.1993.tb18261.x. [DOI] [PubMed] [Google Scholar]
- 12.Goding JW, Terkeltaub RA, Maurice M, et al. Ecto-phosphodiesterase/pyrophosphatase of lymphocytes and non-lymphoid cells: structure and function of the PC-1 family. Immunological Reviews. 1998;161:11–26. doi: 10.1111/j.1600-065x.1998.tb01568.x. [DOI] [PubMed] [Google Scholar]
- 13.Schibler D, Russell GG, Fleisch H. Inhibition by pyrophosphate and polyphosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci. 1968;35:363–372. [PubMed] [Google Scholar]
- 14.Fleisch H, Russell RGG, Bisaz S, et al. The inhibitory effect of phosphonates on the formation of calcium phosphate crystals in vitro and on aortic and kidney calcification in vivo. Euro J Clin Invest. 1970;1:12–18. doi: 10.1111/j.1365-2362.1970.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 15.Fleisch H. Bisphosphonates: mechanisms of action. Endocrine Rev. 1998;19:80–100. doi: 10.1210/edrv.19.1.0325. [DOI] [PubMed] [Google Scholar]
- 16.Potokar M, Schmidt-Dunker M. The inhibitory effect of new diphosphonic acids on aortic and kidney calcification in vivo. Atherosclerosis. 1978;30:313–320. doi: 10.1016/0021-9150(78)90124-7. [DOI] [PubMed] [Google Scholar]
- 17.Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21:817–824. doi: 10.1161/01.atv.21.5.817. [DOI] [PubMed] [Google Scholar]
- 18.Price PA, Roublick AM, Williamson MK. Artery calcification in uremic rats is increased by a low protein diet and prevented by treatment with ibandronate. Kidney Int. 2006;70:1577–1583. doi: 10.1038/sj.ki.5001841. [DOI] [PubMed] [Google Scholar]
- 19.Saito E, Wachi H, Sato F, et al. Treatment with vitamin K2 combined with bisphosphonates synergistically inhibits calcification in cultured smooth muscle cells. J Atheroscler Thromb. 2007;14:317–324. doi: 10.5551/jat.e501. [DOI] [PubMed] [Google Scholar]
- 20.Vaisman DN, McCarthy AD, Cortizo AM. Bone-specific alkaline phosphatase activity is inhibited by bisphosphonates: role of divalent cations. Biol Trace Elem Res. 2005;104:131–140. doi: 10.1385/BTER:104:2:131. [DOI] [PubMed] [Google Scholar]
- 21.Geng Z, Monier-Faugere M-C, Bauss F, et al. Short-term administration of the bisphosphonate ibandronate increases bone volume and prevents hyperparathyroid bone changes in mild experimental renal failure. Clin Nephrol. 2000;54:45–53. [PubMed] [Google Scholar]
- 22.Markowitz GS, Fine PL, Stack JI, et al. Toxic acute tubular necrosis following treatment with zolendonate (Zometa) Kidney Int. 2003;64:281–289. doi: 10.1046/j.1523-1755.2003.00071.x. [DOI] [PubMed] [Google Scholar]
- 23.Van Beek E, Hoekstra M, Van de Ruit M, et al. Structural requirements for bisphosphonate actions in vitro. J Bone Miner Res. 1994;9:1875–1882. doi: 10.1002/jbmr.5650091206. [DOI] [PubMed] [Google Scholar]
- 24.Jung A, Bisaz S, Fleisch H. The binding of pyrophosphate and two diphosphonates by hydroxyapatite crystals. Calcif Tiss Res. 1973;11:269–280. doi: 10.1007/BF02547227. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman HW, Kleinberg I. Studies on the incongruent solubility of hydroxyapatite. Calcif Tissue Int. 1979;27:143–151. doi: 10.1007/BF02441177. [DOI] [PubMed] [Google Scholar]
- 26.Neuman WF, Neuman MW. The chemical dynamics of bone mineral. University of Chicago Press; Chicago: 1958. [Google Scholar]
- 27.Evans JR, Robertson WG, Morgan DB, et al. Effects of pyrophosphate and diphosphonates on the dissolution of hydroxyapatites using a flow system. Calcif Tissue Int. 1980;31:153–159. doi: 10.1007/BF02407176. [DOI] [PubMed] [Google Scholar]
- 28.Murshed M, Harmey D, Millan JL, et al. Unique coexpression in osteoblasts of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Development. 2006;19:1093–1104. doi: 10.1101/gad.1276205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix Gla protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 30.Wada T, McKee MD, Steitz S, et al. Calcification of vascular smooth muscle cell cultures. Inhibition by osteopontin. Circ Res. 1999;84:166–178. doi: 10.1161/01.res.84.2.166. [DOI] [PubMed] [Google Scholar]
- 31.Steitz SA, Speer MY, Curinga G, et al. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineagemarkers. Circ Res. 2001;89:1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 32.Yokozawa T, Zheng PD, Oura H, et al. Animal model of adenine-induced chronic renal failure in rats. Nephron. 1986;44:230–234. doi: 10.1159/000183992. [DOI] [PubMed] [Google Scholar]
- 33.Okada H, Kaneko Y, Yawata T, et al. Reversibility of adenine-induced renal failure in rats. Clin Exper Nephrol. 1999;3:82–88. [Google Scholar]
- 34.Moorehead WR, Biggs HG. 2-amino-2-methyl-1-propanol as the alkalizing agent in an improved continuous-flow cresolphthalein complexone procedure for calcium in serum. Clin Chem. 1974;20:1458–1460. [PubMed] [Google Scholar]
- 35.Cogan EB, Birrell GB, Griffith OH. A robotics-based automated assay for inorganic and organic phosphates. Anal Bioch. 1999;271:29–35. doi: 10.1006/abio.1999.4100. [DOI] [PubMed] [Google Scholar]
- 36.Malluche HH, Sherman D, Meyer W, et al. A new semiautomatic method for quantitative static and dynamic bone histology. Calcif Tissue Int. 1982;34:439–448. doi: 10.1007/BF02411282. [DOI] [PubMed] [Google Scholar]
- 37.Manaka RC, Malluche HH. A program package for quantitative analysis of histologic structure and remodeling dynamics of bone. Comput Programs Biomed. 1981;13:191–202. doi: 10.1016/0010-468x(81)90098-2. [DOI] [PubMed] [Google Scholar]