Abstract
We have compared the contributions of gap junctional communication and chemical signaling via H2O2 to NO-independent relaxations evoked by the Ca2+ ionophore A23187 and acetylcholine (ACh) in rabbit ilio-femoral arteries. Immunostaining confirmed the presence of connexins (Cxs) 37 and 40 in the endothelium and Cxs 40 and 43 in smooth muscle. Maximal endothelium-dependent subintimal smooth muscle hyperpolarizations evoked by A23187 and ACh were equivalent (≈20 mV) and almost abolished by an inhibitory peptide combination targeted against Cxs 37, 40, and 43. However, maximal NO-independent relaxations evoked by A23187 were unaffected by such peptides, whereas those evoked by ACh were depressed by ≈70%. By contrast, the enzyme catalase, which destroys H2O2, attenuated A23187-induced relaxations over a broad range of concentrations, but only minimally depressed the maximum response to ACh. Catalase did not affect A23187- or ACh-evoked hyperpolarizations. After loading with an H2O2-sensitive probe, A23187 caused a marked increase in endothelial fluorescence that correlated temporally with relaxation, whereas only a weak delayed increase was observed with ACh. In arteries without endothelium, the H2O2-generating system xanthine/xanthine oxidase induced a catalase-sensitive relaxation that mimicked the gap junction-independent response to A23187 as it was maximally equivalent to ≈80% of induced tone, but associated with a smooth muscle hyperpolarization <5 mV. We conclude that myoendothelial gap junctions underpin smooth muscle hyperpolarizations evoked by A23187 and ACh, but that A23187-induced relaxation is dominated by extracellular release of H2O2. Endothelium-derived H2O2 may thus be regarded as a relaxing factor, but not a hyperpolarizing factor, in rabbit arteries.
Keywords: connexin, A23187, acetylcholine
Acetylcholine (ACh) and the Ca2+ ionophore A23187 both evoke endothelium-dependent smooth muscle hyperpolarizations and relaxations that are not mediated by NO or vasoactive prostanoids. However, the mechanisms of relaxation activated by these agents may involve different pathways. In rabbit arteries, NO/prostanoid-independent relaxations to ACh are attenuated by connexin (Cx)-mimetic peptides and glycyrrhetinic acid derivatives that interrupt gap junctional communication, whereas analogous responses to A23187 are insensitive to such inhibitors (1-5). By contrast, “sandwich” bioassay studies with closely apposed endothelium-intact and -denuded strips of rabbit superior mesenteric or ilio-femoral artery demonstrate that A23187, but not ACh, promotes the release of a diffusible factor that is capable of causing relaxation (2, 5, 6). Such observations highlight controversies over the nature of NO- and prostanoid-independent responses, i.e., whether they are mediated by a transferable endothelium-derived hyperpolarizing factor (EDHF) or electrotonic conduction of endothelial hyperpolarization into and through the vascular media.
In some arteries, H2O2 has been implicated as a freely transferable EDHF on the basis that NO- and prostanoid-independent responses induced by ACh or bradykinin are attenuated by the enzyme catalase, which destroys this reactive oxygen species (7-9). However, this finding has not been universal, and many reports have failed to demonstrate a role for endothelium-derived H2O2 in the regulation of vascular tone after stimulation by agonists (10-14). In the present study, we have therefore exploited differences in the mechanisms that underpin relaxations to ACh and A23187 in rabbit arteries to evaluate the relative contributions of gap junctional communication and H2O2 to EDHF-type responses. Relaxation was correlated with measurements of subintimal smooth muscle membrane potential, and the role of gap junctions was assessed with peptides homologous to the gap 26 and 27 domains of the first and second extracellular loops of Cxs 37, 40, and 43. Such peptides interrupt intercellular communication in a Cx-selective fashion without exerting nonspecific effects on endothelial or smooth muscle cells (15-19), and thereby attenuate the electrotonic spread of ACh-evoked endothelial hyperpolarization into the media via gap junctions (4). Endothelial H2O2 production was assessed by sandwich bioassay and confocal imaging after loading of the endothelium with the H2O2-sensitive probe dihydrodichlorofluorescein (7, 20). Taken together, the findings suggest that the relative contributions of gap junctions and endothelium-derived H2O2 to relaxation may not simply be species- and/or vessel-specific, but may depend on the mode of endothelial stimulation.
Materials and Methods
Mechanical Responses. Iliac arteries were obtained from male New Zealand White rabbits (2-2.5 kg) killed with sodium pentobarbitone (120 mg/kg; i.v.), and rings 2-3 mm wide were suspended in a myograph containing oxygenated (95% O2, 5% CO2) Holmans buffer (120 mM NaCl/5 mM KCl/2.5 mM CaCl2/1.3 mM NaH2PO4/25 mM NaHCO3/11 mM glucose/10 mM sucrose) at 37°C. In sandwich bioassay experiments, arterial rings 2-3 mm wide were denuded of endothelium, cut into strips, and pierced with a needle ≈2 mm from each end. These strips were introduced into the lumen of rings of endothelium-intact iliac artery, and the tissues were sutured together and mounted in a myograph with the pierced denuded strips hooked onto the vessel mountings. Both types of preparation were maintained at a resting tension of ≈2 mN during a 1-h equilibration period before incubation with the NO synthase inhibitor NG-nitro-l-arginine methyl ester (L-NAME, 300 μM) and the cyclooxygenase inhibitor indomethacin (10 μM) for 40 min. Constriction was then induced by phenylephrine (PE, 1 μM), and cumulative concentration-relaxation curves to A23187 or ACh were constructed under control conditions and after incubation with the Cx-mimetic peptides 37,43Gap27, 40Gap27, and 43Gap26 (amino acid sequences SRPTEKTIFII, SRPTEKNVFIV, VCYDKSFPISHVR, respectively) or catalase for 40 min. Relaxations to A23187 were also studied in the presence of the NO scavengers hemoglobin (20 μM) and 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO, 300 μM) in both ring and sandwich preparations. Hemoglobin was obtained by lysis of red cells in HPLC grade water and purified on a Sephadex G25 column. In further experiments with endothelium-denuded rings in the presence of L-NAME and indomethacin, cumulative concentration-relaxation curves were constructed for xanthine in the presence of 100 milliunits/ml of xanthine oxidase (XO) and for H2O2 after incubation with catalase (2,000 units/ml), superoxide dismutase (500 units/ml; xanthine curve only), the Kv channel blocker 4-aminopyridine (300 μM), the SKCa and IKCa/BKCa channel blockers apamin and charybdotoxin in combination (100 nM each), the KATP channel blocker glibenclamide (10 μM), the soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ, 10 μM), the adenylyl cyclase inhibitor 2′,5′-dideoxyadenosine (2′,5′-ddA, 500 μM), and the cell-permeant iron chelator 1,2 dimethyl-3-hydroxypyridin-4-one (deferiprone, 1 mM; xanthine curve only). All agents were obtained from Sigma, except 2′,5′-ddA (Affiniti, Nottingham, U.K.) and deferiprone (Calbiochem).
Microelectrode Studies. Iliac artery strips were held adventitia down in an organ chamber superfused (2 ml/min at 37°C) with oxygenated Holmans solution containing 300 μM L-NAME and 10 μM indomethacin, and subintimal smooth muscle membrane potential was recorded with glass capillary microelectrodes (tip resistance 60-110 MΩ) filled with 3 M KCl, as described (4). Drugs were administered directly into the organ chamber under conditions of no flow. In experiments involving xanthine/XO (X/XO) and H2O2 smooth muscle membrane potential was recorded in strips of endothelium-denuded iliac artery.
Immunohistology. Iliac arteries were cryopreserved in OCT compound (Agar Scientific, Stansted, U.K.) and cooled by liquid N2, and cryosections were prepared and immunostained as described (18). Sections were labeled with the following primary antibodies: for Cx 43 a mouse mAb generated against amino acids 252-270 (Chemicon, 5 μg/ml) was used; for Cxs 37 and 40 rabbit polyclonal antibodies, respectively prepared against specific sequences of 16 and 19 aa, were used (Alpha Diagnostics, San Antonio, TX, 5 μg/ml). The secondary antibodies were goat anti-mouse-conjugated Alexa 488 or goat anti-rabbit-conjugated Alexa 546 (Molecular Probes, 1:500 dilution) according to the primary antibody. Sections were mounted in Fluorsave (Calbiochem) and imaged with a Leica TCS 4D confocal laser scanning microscope equipped with an argon/krypton laser.
H2O2 Imaging. Femoral artery rings, prepared as above, were incubated in oxygenated Holmans buffer (37°C) containing 300 μM L-NAME and 10 μM indomethacin for 40 min. Subsequently, 2′,7′-dihydrodichlorofluorescein diacetate (H2DCF-DA, Molecular Probes, 5 μM) was introduced into the buffer for 30 min followed by washout. The rings were then stimulated by either 3 μM A23187 or 3 μM ACh for intervals up to 3 min before fixation in PBS (0.1 M) containing 0.2% glutaraldehyde and 2% formalin for 90 min. After mounting onto microscope slides under Fluorsave the intimal surface of the preparations was then imaged with a Leica TCS 4D confocal laser scanning system to detect dichlorofluorescein, which is fluorescent, and retained intracellularly after cleavage of the acetate moieties of H2DCF-DA and oxidation by H2O2 (7, 20).
Statistics. EC50 values are expressed as mean with 95% confidence intervals; results are otherwise given as mean ± SEM, where n denotes the number of animals studied for each data point. Data were compared by the Student t test for paired or unpaired data as appropriate, and concentration-response curves were assessed by one-way ANOVA followed by the Bonferroni multiple comparisons test with GraphPad (San Diego) PRISM 3.03. P < 0.05 was considered significant.
Results
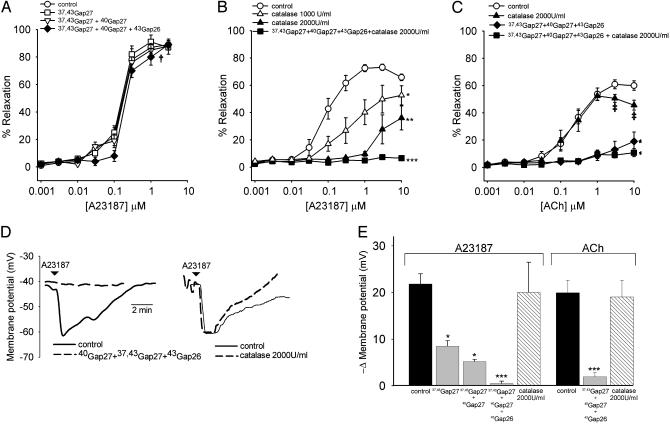
Ring Experiments. EDHF-type relaxations evoked by A23187 reached a maximum of 89.2 ± 2.1% of PE-induced tone at a concentration of 3 μM with an EC50 value of 242 nM (208-287) (n = 13; Fig. 1A). Incubation with either 37,43Gap 27 (900 μM) or the combination of 37,43Gap 27 + 40Gap 27 (450 μM each) did not affect maximal relaxations, and EC50 values were unchanged at 228 nM (192-270) and 256 nM (202-316), respectively (n = 5 in each case, Fig. 1 A). The triple peptide combination 37,43Gap 27 + 40Gap 27 + 43Gap 26 (300 μM each) also failed to affect maximal relaxation (n = 5, Fig. 1 A), but caused a small, but significant, rightward shift in EC50 to 325 nM (283-378) (P < 0.05). In a separate series of experiments, incubation with catalase at concentrations of 1,000 and 2,000 units/ml depressed maximal relaxations to A23187 from 73.1 ± 2.4% to 52.5 ± 5.5% and 36.2 ± 6.2%, respectively, with EC50 values increasing from 126 nM (83-176) to 382 nM (326-498) and 1150 nM (720-1580) (P < 0.05 and P < 0.01, n = 7 and 5, respectively; Fig. 1B). Residual relaxations observed in the presence of 2,000 units/ml catalase were abolished in the additional presence of 37,43Gap 27 + 40Gap 27 + 43Gap 26 (300 μM each) (n = 4 and P < 0.001; Fig. 1B). EDHF-type relaxations evoked by ACh were maximal at a concentration of 3 μM, exhibiting a 60.8 ± 3.3% decrease in PE-induced tone and an EC50 value of 292 nM (260-345) (n = 13, Fig. 1C). In the presence of catalase (2,000 units/ml), maximal relaxation and the EC50 value for ACh were 50.6 ± 2.9% and 273 nM (233-328), although responses differed statistically from control only at high ACh concentrations (n = 5; Fig. 1C and Fig. 5, which is published as supporting information on the PNAS web site). After incubation with the triple peptide combination 37,43Gap 27 + 40Gap 27 + 43Gap 26 (300 μM each) the maximal relaxation evoked by ACh was reduced to 19.5 ± 6.8% of PE-induced tone with an increase in EC50 value to 962 nM (896-1094), with further addition of catalase (2,000 units/ml) abolishing this residual relaxation (P < 0.01 and n = 4 for both; Fig. 1C). Neither A23187 nor ACh induced relaxation in rings from which the endothelium was removed by gentle abrasion (data not shown).
Fig. 1.
Concentration-response curves for EDHF-type relaxations of rabbit iliac arteries evoked by A23187 and ACh and associated changes in subintimal smooth muscle membrane potential. (A) Cx-mimetic peptides targeting Cxs 37, 40, and 43 did not affect maximal relaxations to A23187, either singly or in combination, but caused a small rightward shift in the response curve when used as the triple combination 37,43Gap27 + 40Gap27 + 43Gap26 (†, P < 0.05). Total peptide concentration was 900 μM in all experiments. (B and C) Catalase caused a concentration-dependent depression of relaxation to A23187, with residual responses observed in the presence of 2,000 units/ml catalase being abolished by 37,43Gap27 + 40Gap27 + 43Gap26. By contrast, relaxations to ACh were inhibited by 37,43Gap27 + 40Gap27 + 43Gap26, but minimally affected by catalase. ‡, P < 0.05 for specific ACh concentrations compared with control; *, P < 0.05; **, P < 0.01, and ***, P < 0.001 for whole curves. (D) Representative traces showing that the triple peptide combination abolished smooth muscle hyperpolarizations evoked by A23187, whereas catalase was without effect. (E) Histograms summarizing maximal smooth muscle membrane potential changes. The triple peptide combination was a more effective inhibitor of smooth muscle hyperpolarizations evoked by 3 μM A23187 than 37,43Gap27 or 37,43Gap27 + 40Gap27, and almost abolished the hyperpolarizing response to 3 μM ACh. Catalase (2,000 units/ml) did not affect the hyperpolarizing response to either A23187 or ACh. *, P < 0.05 and ***, P < 0.001 compared with control.
Smooth Muscle Membrane Potential. In arteries with intact endothelium incubation with either 37,43Gap 27 (900 μM) or the peptide combinations 37,43Gap 27 + 40Gap 27 (450 μM each) or 37,43Gap 27 + 40Gap 27 + 43Gap 26 (300 μM each) did not affect the resting membrane potential of subintimal smooth muscle cells, which was -42.4 ± 4.8 mV (n = 7) compared with -40.8 ± 4.0 mV, -43.6 ± 5.2 mV, and -45.5 ± 2.0 mV in the presence of the peptides, respectively (n = 3 for each data set). However, 37,43Gap 27 and the double peptide combination 37,43Gap 27 + 40Gap 27 attenuated hyperpolarizations evoked by 3 μM A23187 with maximal changes in membrane potential being reduced from -21.8 ± 2.2 mV (n = 7) to -8.5 ± 1.2 mV and -5.2 ± 0.4 mV, respectively (P < 0.05 and n = 3 for both; Fig. 1E). The hyperpolarization evoked by 3 μM ACh was -19.8 ± 3.2 mV (n = 6) and not significantly different from the electrical response to 3 μM A23187. Incubation with the triple combination 37,43Gap 27 + 40Gap 27 + 43Gap 26 abolished A23187- and ACh-evoked subintimal smooth muscle hyperpolarizations (P < 0.001 and n = 3 for both), whereas catalase (2,000 units/ml) was without effect (n = 3 for both; Fig. 1 D and E). No hyperpolarizing response was observed in rings denuded of their endothelium (data not shown).
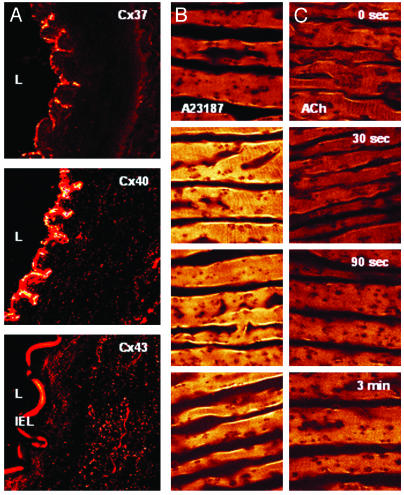
Confocal Microscopy. Immunostaining revealed heterogeneity in the distribution of Cx protein in gap junction plaques. Cx 37 was present only in the endothelium and Cx 43 was present only in the media, whereas staining for Cx 40 was evident in both cell layers (Fig. 2A). Acute administration of 3 μM A23187 caused a marked increase in endothelial cell fluorescence of arteries loaded with dihydrodichlorofluorescein within 30 s, whereas only a slight increase in fluorescence was apparent 3 min after exposure to 3 μM ACh (n = 4, Fig. 2 B and C).
Fig. 2.
(A) Transverse sections of rabbit iliac artery stained with primary antibodies to Cxs 37, 40, and 43 and secondary antibodies of goat anti-mouse-conjugated Alexa 488 or goat anti-rabbit-conjugated Alexa 546 as appropriate. Punctate fluorescence in the media and endothelium indicates the presence of gap junction plaques. Note autofluorescence of internal elastic lamina (IEL) in panel stained for Cx 43 (Bottom), but absence of endothelial Cx protein. L = lumen. (B) Time course of endothelial fluorescence induced by 3 μM A23187 in femoral arterial rings loaded with the H2O2-sensitive probe dihydrodichlorofluorescein. (C) Only a small and relatively delayed increase in fluorescence was observed after administration of 3 μM ACh. The ridged structure apparent in B and C reflects the intimal folds evident in A. (Magnifications: ×40 in A Top and Middle, B, and C; ×50 in A Bottom.)
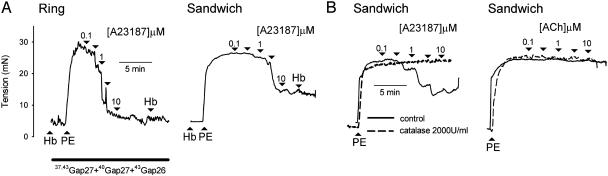
Bioassay Experiments. Preincubation with hemoglobin (20 μM) or carboxy-PTIO (300 μM) did not alter EDHF-type relaxations evoked by A23187 either in sandwich preparations or in rings incubated with the triple peptide combination 37,43Gap 27 + 40Gap 27 + 43Gap 26 (300 μM each) (n = 3 in each case; Figs. 3A and 5A). In such experiments additional administration of hemoglobin or carboxy-PTIO did not reverse the maximal relaxation evoked by A23187 (n = 3 in each case; Figs. 3A and 5). EDHF-type relaxations of sandwich preparations induced by A23187 were maximal at a concentration of 10 μM with a 52.4 ± 4.2% decrease in PE-induced tone (n = 3; Figs. 3B and 5). After incubation with catalase (2,000 units/ml), relaxations were abolished (P < 0.005, n = 3; Fig. 3B). By contrast, ACh failed to evoke an EDHF-type relaxation either in the absence or presence of catalase (n = 3; Fig. 3B). After administration of each concentration of A23187 relaxation generally attained its maximal level within 30 s, thus correlating with the time course of the fluorescence also induced by the ionophore (Figs. 2 and 3).
Fig. 3.
(A) Representative traces from ring and sandwich preparations showing that neither preincubation with 20 μM hemoglobin nor the additional acute administration of 20 μM hemoglobin inhibited relaxation to A23187. (B) Representative traces from sandwich bioassay experiments confirming that A23187-evoked relaxations were sensitive to catalase, whereas ACh did not evoke detectable release of H2O2.
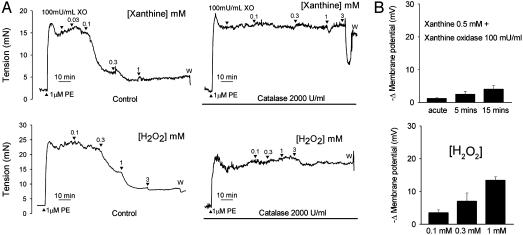
Responses to X/XO and Authentic H2O2. Relaxations evoked by xanthine in the presence of 100 milliunits/ml of XO attained a maximum of 80.4 ± 3.5% of PE-induced tone at a xanthine concentration of 1 mM with an EC50 value of 99 μM (86-115) (n = 9; Fig. 4A). Incubation with catalase prevented this relaxant response to xanthine, except at the highest concentrations used when a transient fall in tone was observed (Fig. 4A). Administration of 4-aminopyridine, the combination of charybdotoxin and apamin, glibenclamide, ODQ, 2′,5′-ddA, superoxide dismutase, or deferiprone did not significantly affect maximum relaxations or EC50 values (Fig. 6, which is published as supporting information on the PNAS web site). Relaxations evoked by authentic H2O2 reached a maximum of 83.4 ± 3.3% of PE-induced tone at a concentration of 1 mM with an EC50 value of 92 μM (74-116) (n = 9, Fig. 4A). Incubation with catalase abolished H2O2-induced relaxations (Fig. 4A). Incubation with 4-aminopyridine, charybdotoxin plus apamin, glibenclamide, ODQ, or 2′,5′-ddA did not significantly affect maximum relaxations or EC50 values (Fig. 6).
Fig. 4.
(A) Concentration-dependent relaxations of endothelium-denuded iliac artery rings evoked by enzymatically generated (xanthine plus XO) and authentic H2O2. Relaxations to both sources of H2O2 were effectively abolished by catalase. (B) Histograms showing that X/XO reduced smooth muscle membrane potential by <5 mV, and that significant hyperpolarization was observed only after administration of supraphysiological concentrations of authentic H2O2.
In endothelium-denuded arterial strips administration of 0.5 mM xanthine and 100 milliunits/ml XO caused a small acute smooth muscle hyperpolarization of -1.2 ± 0.2 mV, which increased slightly to -2.5 ± 0.8 mV and -4.0 ± 1.2 mV in repeat recordings after 5 and 15 min, respectively (n = 3; Fig. 4B). In similar preparations H2O2 evoked smooth muscle hyperpolarizations of -3.5 ± 0.8 mV, -7.0 ± 2.5 mV, and -13.4 ± 1.1 mV 15 min after exposure to concentrations of 100 μM, 300 μM, and 1 mM, respectively (n = 3; Fig. 4B).
Discussion
The major finding of the present study is that gap junctions and H2O2 both may participate in the genesis of NO/prostanoid-independent relaxations of rabbit ilio-femoral arteries, but that the relative contribution of these signaling mechanisms depends on the mode of endothelial stimulation. In rings constricted by phenylephrine in the presence of L-NAME and indomethacin, maximal relaxations to A23187 and ACh were observed at concentrations of 3 μM and were equivalent to ≈80% and ≈60% of developed tone, respectively. Such concentrations of A23187 and ACh induced similar smooth muscle hyperpolarizations of ≈20 mV in arterial strips impaled via their intimal surface. Immunostaining confirmed the presence of Cxs 37 and 40 in the endothelium and Cxs 40 and 43 in the media of the vessels studied, and subintimal hyperpolarizations to A23187 or ACh were abolished by a triple peptide combination, 37,43Gap27 plus 40Gap27 plus 43Gap26, targeted to these three Cx subtypes, thus confirming the central role of myoendothelial gap junctions in permitting the electrotonic spread of endothelial hyperpolarization into the media (4). Previous studies have suggested that more than one Cx subtype may participate in the EDHF phenomenon (16, 18), and the triple peptide combination was found to be a more effective inhibitor of A21387-evoked smooth muscle hyperpolarizations than either 37,43Gap27 alone or 37,43Gap27 plus 40Gap27 at the same overall concentration (900 μM).
In marked contrast to its similar inhibitory effects on smooth muscle hyperpolarizations evoked by A23187 and ACh, the triple peptide combination 37,43Gap27 plus 40Gap27 plus 43Gap26 differentially affected mechanical responses to these agents. There was no significant reduction in the maximum A23187-induced relaxation, and only a small increase in the EC50 value of the concentration-relaxation curve, thus indicating that gap junctions play an almost negligible role in the overall mechanical response to the ionophore. By contrast, the triple peptide combination depressed maximal relaxations to ACh by ≈70% and caused a 3-fold increase in the EC50 value for this agonist. These observations were explained by bioassay experiments with sandwich preparations in which A23187, but not ACh, was shown to evoke the release of a transferable relaxing factor. Because there can be no direct electrical endothelial-smooth muscle coupling between the “donor” endothelium and “detector” smooth muscle in such vessel composites, gap junctions appear central to the EDHF-type response to ACh, whereas a transferable factor released by A23187 normally overrides a conducted smooth muscle hyperpolarization also evoked by the ionophore. Although previous workers have hypothesized that residual NO activity may contribute to EDHF-type relaxations (21), A23187-induced relaxations in ring and sandwich preparations were unaffected by the NO scavengers hemoglobin and carboxy-PTIO, consistent with evidence that A23187 does not elevate cGMP levels in rabbit iliac arteries incubated with 300 μM L-NAME (3, 5).
Functional experiments with catalase in conjunction with confocal imaging of the endothelium after loading with the H2O2-sensitive probe dihydrodichlorofluorescein provided clear evidence that the factor released by A23187 was H2O2. Catalase attenuated A23187-induced relaxations of intact rings in a concentration-dependent fashion and abolished relaxation in sandwich preparations. The ≈35% residual relaxation still evident in rings in the presence of high concentrations of catalase (2,000 units/ml) was subsequently abolished by 37,43Gap27 plus 40Gap27 plus 43Gap26, thereby indicating that electrotonically conducted relaxant mechanisms activated by the ionophore are normally masked by a larger smooth muscle response to endothelium-derived H2O2. By contrast, H2O2 played a minimal role in the response to ACh as control relaxations were depressed only by catalase at agonist concentrations >3 μM, and residual ACh-evoked relaxations observed in the presence of the triple peptide combination were <20% of induced tone, although they, too, were abolished by catalase. Consistent with these findings, in arteries loaded with dihydrodichlorofluorescein, A23187 produced a marked increase in endothelial fluorescence within 30 s that matched the time course of the mechanical response to this ionophore, whereas a limited increase in fluorescence was evident with ACh only after 3 min. Notably, subintimal hyperpolarizations to A23187 and ACh were unaffected by catalase, indicating that endothelium-derived H2O2 cannot be considered as a hyperpolarizing factor. Confirmation that exogenous H2O2 can mimic the response to A23187 was obtained in endothelium-denuded arteries exposed to X/XO, which generates H2O2 directly and via dismutation of the superoxide anions also produced by this system (22). X/XO thus evoked catalase-sensitive endothelium-independent relaxations that were maximally equivalent to ≈80% of induced tone but were associated with smooth muscle hyperpolarizations that were <5 mV, and therefore much smaller than the electrical response to A23187 and ACh.
Previous studies have shown that the ambient levels of H2O2 generated by X/XO at concentrations of 0.5 mM and 500 milliunits/ml attain a maximal level of ≈4 μM in physiological buffer and may be even lower (≈1.5 μM) in the presence of endothelial cells because of their endogenous catalase activity (23). This finding contrasts with the present finding that relaxations of endothelium-denuded iliac artery rings evoked by authentic H2O2 exhibited a threshold at 100-300 μM with a maximum at ≈1 mM, i.e., concentrations that are considerably higher than those likely to result from administration of A23187 or X/XO. Furthermore, the hyperpolarizations induced by authentic H2O2 at 100-300 μM were of the order of ≈5 mV and therefore much smaller than those induced by A23187. Many previous studies have also shown that authentic H2O2 induces direct smooth muscle relaxation/hyperpolarization only at supraphysiological concentrations in the range of 0.1 to 1 mM (7, 10, 20, 24-26). This apparent discrepancy between the vascular effects of endogenous, XO-generated and authentic H2O2 has been noted by other workers (20), and, speculatively, could reflect higher biological activity of “nascent” H2O2 formed by enzyme systems in close proximity to its site of action.
Although H2O2 has been postulated to mediate relaxation by hyperpolarizing vascular smooth muscle through the activation of KCa, KATP, or Kv channels, the functional contribution of such channels to H2O2-induced relaxation may vary considerably between vessels (7-9, 26, 27). Indeed, in the rabbit arteries used in the present study selective blockers of these different K+ channel subtypes had no effect on H2O2-evoked relaxations whether these were evoked by X/XO or authentic H2O2. In some vessels, H2O2 can also reduce smooth muscle tone by promoting accumulation of the cyclic nucleotide cGMP (28), and also in theory cAMP (29), but again inhibitors of soluble guanylyl cyclase and adenylyl cyclase (ODQ and 2′,5′-ddA) failed to mimic the effects of catalase on the response to X/XO or authentic H2O2 in rabbit arteries. It may therefore be hypothesized that the relaxant effects of H2O2 in rabbit arteries involves effects on the smooth muscle contractile machinery. Indeed, in the endothelium-denuded rabbit aorta and canine tracheal smooth muscle, H2O2 is thought to mediate relaxation by depressing the sensitivity of the contractile apparatus to Ca2+, thereby overriding a paradoxical elevation in smooth muscle intracelluar Ca2+ concentration caused by H2O2 itself (30, 31). We confirmed that neither superoxide anions nor hydroxyl radicals generated intracellularly from H2O2 via the Fenton reaction contribute to relaxation in rabbit arteries because superoxide dismutase and the cell-permeant iron chelator deferiprone failed to affect relaxations evoked by X/XO.
In conclusion, we have provided evidence that NO/prostanoid-independent relaxations of rabbit iliac arteries evoked by A23187 are mediated principally by H2O2, but that this reactive oxygen species cannot be regarded as an EDHF because the catalase-sensitive component of the response to the ionophore was not associated with smooth muscle hyperpolarization. Rather, direct smooth muscle relaxant effects of H2O2 mask the functional consequences of an associated A23187-evoked endothelial hyperpolarization conducted electrotonically into the vessel wall via gap junctions. This finding contrasts with the mechanisms that underpin NO/prostanoid-independent relaxations evoked by ACh, which are dominated by electrotonic signaling, with only a minor contribution from endothelium-derived H2O2 being evident at high agonist concentrations. These findings can be generalized because catalase also attenuates NO/prostanoid-independent relaxations to A23187 in the rabbit superior mesenteric artery (see Fig. 5) in which sandwich experiments have confirmed release of an endothelium-derived relaxing factor distinct from NO after stimulation by A23187 but not ACh (2). In theory, endothelial production of H2O2 in the presence of L-NAME and indomethacin could involve NAD(P)H oxidases, lipoxygenases, cytochrome P450 epoxygenases, and components of the mitochondrial electron transport chain (28). Although endothelial NOS may generate superoxide anions (and thus H2O2) in diseased arteries with relative tetrahydrobiopterin deficiency (32), this enzyme is unlikely to contribute to the ability of A23187 to stimulate the production of H2O2 under the present experimental conditions because L-NAME inhibits the production of superoxide by this enzyme (33). Further research is therefore needed to characterize the pathway(s) responsible for the formation of H2O2 by endothelial cells under different experimental conditions.
Supplementary Material
Acknowledgments
The study was supported in part by the British Heart Foundation.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: ACh, acetylcholine; Cx, connexin; EDHF, endothelium-derived hyperpolarizing factor; L-NAME, NG-nitro-L-arginine methyl ester; PE, phenylephrine; carboxy-PTIO, 2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; 2′,5′-ddA, 2′,5′-dideoxyadenosine; XO, xanthine oxidase; X/XO, xanthine/XO.
References
- 1.Chaytor, A. T., Evans, W. H. & Griffith, T. M. (1998) J. Physiol. (London) 508, 561-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hutcheson, I. R., Chaytor, A. T., Evans, W. H. & Griffith, T. M. (1999) Circ. Res. 84, 53-63. [DOI] [PubMed] [Google Scholar]
- 3.Taylor, H. J., Chaytor, A. T., Edwards, D. H. & Griffith, T. M. (2001) Biochem. Biophys. Res. Commun. 283, 583-589. [DOI] [PubMed] [Google Scholar]
- 4.Griffith, T. M., Chaytor, A. T., Taylor, H. J., Giddings, B. D. & Edwards D. H. (2002) Proc. Natl. Acad. Sci. USA 99, 6392-6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaytor, A. T., Taylor, H. J. & Griffith, T. M. (2002) Am. J. Physiol. 282, H1548-H1555. [DOI] [PubMed] [Google Scholar]
- 6.Plane, F., Pearson. T. & Garland, C. J. (1995) Br. J. Pharmacol. 115, 31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matoba, T., Shimokawa, H., Nakashima, M., Hirakawa, Y., Mukai, Y., Hirano, K., Kanaide, H. & Takeshita, A. (2000) J. Clin. Invest. 106, 1521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacza, Z., Puskar, M., Kis, B., Perciaccante, J. V., Miller, A. W. & Busija, D. W. (2002) Am. J. Physiol. 283, H406-H411. [DOI] [PubMed] [Google Scholar]
- 9.Rabelo, L. A., Cortes, S. F., Alvarez-Leite, J. I. & Lemos, V. S. (2003) Br. J. Pharmacol. 138, 1215-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beny, J.-L. & von der Weid, P. Y. (1991) Biochem. Biophys. Res. Commun. 176, 378-384. [DOI] [PubMed] [Google Scholar]
- 11.Pomposiello, S., Rhaleb, N. E., Alva, M. & Carretero, O. A. (1999) J. Cardiovasc. Pharmacol. 34, 567-574. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton, C. A., McPhaden, A. R., Berg, G., Pathi, V. & Dominiczak, A. F. (2001) Am. J. Physiol. 280, H2451-H2455. [DOI] [PubMed] [Google Scholar]
- 13.McNeish, A. J., Wilson, W. S. & Martin, W. (2002) Br. J. Pharmacol. 135, 1801-1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brandes, R. P., Schmitz-Winnenthal, F. H., Feletou, M., Godecke, A., Huang, P. L., Vanhoutte, P. M., Fleming, I. & Busse, R. (2000) Proc. Natl. Acad. Sci. USA 97, 9747-9752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaytor, A. T., Martin, P. E. M., Evans, W. H., Randall, M. D. & Griffith, T. M. (1999) J. Physiol. (London) 520, 539-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaytor, A. T., Martin, P. E. M., Edwards, D. H. & Griffith, T. M. (2001) Am. J. Physiol. 280, H2441-H2450. [DOI] [PubMed] [Google Scholar]
- 17.Berman, R. S., Martin, P. E. M., Evans, W. H. & Griffith, T. M. (2002) Microvasc. Res. 63, 115-128. [DOI] [PubMed] [Google Scholar]
- 18.Ujiie, H., Chaytor, A. T., Bakker, L. M. & Griffith, T. M. (2003) Stroke 34, 544-550. [DOI] [PubMed] [Google Scholar]
- 19.Sandow, S. L., Tare, M., Coleman, H. A., Hill, C. E. & Parkington, H. C. (2002) Circ. Res. 90, 1108-1113. [DOI] [PubMed] [Google Scholar]
- 20.Miura, H., Bosnjak, J. J., Ning, G., Saito, T., Miura, M. & Gutterman, D. D. (2003) Circ. Res. 92, e31-e40. [DOI] [PubMed] [Google Scholar]
- 21.Cohen, R. A., Plane, F., Najibi, S., Huk, I., Malinski. T. & Garland, C. J. (1997) Proc. Natl. Acad. Sci. USA 94, 4193-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fridovich, I. (1970) J. Biol. Chem. 245, 4053-4057. [PubMed] [Google Scholar]
- 23.Gonzalez-Flecha, B., Evelson, P., Ridge, K. & Sznajder, J. I. (1996) Biochim. Biophys. Acta 1290, 46-52. [PubMed] [Google Scholar]
- 24.Karasu, C. (1999) Free Radical Biol. Med. 27, 16-27. [DOI] [PubMed] [Google Scholar]
- 25.Hattori, T., Kajikuri, J., Katsuya, H. & Itoh, T. (2003) Eur. J. Pharmacol. 464, 101-109. [DOI] [PubMed] [Google Scholar]
- 26.Gao, Y. J., Hirota, S., Zhang, D. W., Janssen, L. J. & Lee, R. M. (2003) Br. J. Pharmacol. 138, 1085-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barlow, R. S., El-Mowafy, A. M. & White, R. E. (2000) Am. J. Physiol. 279, H475-H483. [DOI] [PubMed] [Google Scholar]
- 28.Wolin, M. S. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 1430-1442. [DOI] [PubMed] [Google Scholar]
- 29.Tan, C. M., Xenoyannis, S. & Feldman, R. D. (1995) Circ. Res. 77, 710-717. [DOI] [PubMed] [Google Scholar]
- 30.Iesaki, T., Okada, T., Shimada, I., Yamaguchi, H. & Ochi, R. (1996) Cardiovasc. Res. 31, 820-825. [DOI] [PubMed] [Google Scholar]
- 31.Perkins, W. J., Lorenz, R. R., Bogoger, M., Warner, D. O. Cremo, C. R. & Jones, K. A. (2003) Am. J. Physiol. 284, L324-L332. [DOI] [PubMed] [Google Scholar]
- 32.Landmesser, U., Dikalov, S., Price, S. R., McCann, L., Fukai, T., Holland, S. M., Mitch, W. E. & Harrison, D. G. (2003) J. Clin. Invest. 111, 1201-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia, Y., Tsai, A. L., Berka, V. & Zweier, J. L. (1998) J. Biol. Chem. 273, 25804-25808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.