Abstract
Indoleamine 2,3-dioxygenase (IDO) is generally considered to be immunosuppressive but recent findings suggest this characterization oversimplifies its role in disease pathogenesis. Recently, we showed that IDO is essential for tumor outgrowth in the classical two-stage model of inflammatory skin carcinogenesis. Here, we report that IDO loss did not exacerbate classical inflammatory responses. Rather, IDO induction could be elicited by environmental signals and tumor promoters as an integral component of the inflammatory tissue microenvironment even in the absence of cancer. IDO loss had limited impact on tumor outgrowth in carcinogenesis models that lacked an explicit inflammatory tumor promoter. In the context of inflammatory carcinogenesis where IDO was critical to tumor development, the most important source of IDO was radiation-resistant non-hematopoietic cells, consistent with evidence that loss of the IDO regulatory tumor suppressor gene Bin1 in transformed skin cells facilitates IDO-mediated immune escape by a cell autonomous mechanism. Taken together, our results identify IDO as an integral component of ‘cancer-associated’ inflammation that tilts the immune system toward tumor support. More generally, they promote the concept that mediators of immune escape and cancer-associated inflammation may be genetically synonymous.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-010-0891-4) contains supplementary material, which is available to authorized users.
Keywords: Indoleamine 2,3-dioxygenase; Immunosuppression; Carcinogenesis
Introduction
The earliest descriptions of cancer histopathology in the 1800s by Virchow and others noted striking hallmarks of inflammation [1]. Yet despite extensive evidence that chronic inflammation can contribute to cancer development, gaps in knowledge persist concerning the requisite elements that define the tumor-promoting aspects of ‘cancer-associated’ inflammation. Indeed, it remains unclear whether specific distinctions can be drawn between this chronic inflammatory condition versus generalized aspects of inflammation as they are traditionally understood. The importance of developing a molecular and cellular definition of ‘cancer-associated inflammation’ in relation to ‘physiologic’ inflammation has been encapsulated by an NCI workshop in this area [2].
Inflammation, triggered by infection or tissue damage, is characterized by resident activation of innate immune functions and infiltration of immune cells into affected tissues and local lymph nodes (LNs). Most infections provoke inflammatory responses that stimulate effective T cell immunity and create long-term T cell memory. In contrast, chronic infections elicit responses that are associated with active T cell suppression and memory T cell exhaustion. With tissue damage, ‘alarmins’ or other ‘danger’ signals released by dying cells in the absence of infection can provoke a sterile inflammation. Inflammatory states associated with chronic infection or tissue damage can provide growth factors and cytokines that contribute to the intrinsic growth deregulation, survival, and movement of pre-malignant cells [3, 4], while also shaping the extrinsic microenvironment to restrict immune surveillance [5–8]. Although seemingly counterintuitive, active suppression of adaptive immunity clearly occurs in such pro-inflammatory settings, such that by the time tumors are overtly manifested both the tumor microenvironment and the TDLNs harbor potent T cell suppressor functions [9–11]. In considering how tumor cells acquire the ability to exploit inflammation yet subvert immune surveillance, one would predict that mechanisms that integrate immune escape within the context of inflammation would promote carcinogenesis.
One mechanism of immune escape that has been linked to cancers is elevation of the tryptophan catabolizing enzyme indoleamine 2,3-dioxygenase (IDO). There are two IDO family members encoded by independent genes; however, unlike Ido1, the physiological relevance of the recently identified Ido2 gene has yet to be investigated [12, 13]. Through its ability to initiate catabolism of the essential amino acid tryptophan, IDO is capable of exerting directly suppressive effects on T cells as well as activating suppressive populations of regulatory T cells [14]. Striking evidence supporting its role in the establishment of acquired tolerance to neoantigens was provided by the demonstration that pharmacological inhibition of IDO promoted rejection of allogeneic concepti through a T cell-mediated process [15, 16]. Thus, IDO has generally been considered to be an immunosuppressive actor in vivo.
In cancer, IDO activation occurs commonly in the tumor and/or tumor-draining lymph nodes (TDLNs) and pharmacological inhibition elicits T cell-dependent antitumor responses [14, 17, 18]. Recently, we published the first direct genetic validation of IDO as a critical element in de novo tumorigenesis [19]. In a classical two-stage carcinogenesis model, where tumors are initiated with the mutagen 7,12-dimethylbenz[a]anthracene (DMBA) and promoted with the pro-inflammatory phorbol ester 12-O-tetradecanoylphorbol-13-acetate (TPA), mice lacking both alleles of the Ido1 gene were shown to be highly resistant to the formation of benign skin papillomas and their subsequent progression to malignant skin carcinomas. Here, we have extended our investigations of the impact that IDO loss has on inflammation and cancer to more comprehensively ascertain its involvement at the interface of immune function and tumor formation. Our findings reveal that, rather than being a fundamental element of the tumorigenic process per se, IDO acts instead as an integral component of chronic inflammation that is required to support tumor development.
Materials and methods
Transgenic mouse husbandry
Ido1 homozygous null BALB/c and C57BL/6 congenic mice were gifts of A. Mellor (Medical College of Georgia). Strain-matched wild-type BALB/c and C57BL/6 mice were purchased from NCI-Frederick. Bin1 mosaic null and control mice generated in our laboratory have been described previously [20]. Genotyping and Bin1 status in transgenic mice was performed as described previously [20].
Bone marrow transplant
Four-week-old BALB/c recipient mice were lethally irradiated with 770 Rad from a cesium irradiator and reconstituted by retro-orbital injection of 1 × 107 bone marrow cells harvested from 6-week-old donor mice. After allowing 6 weeks for the transplanted bone marrow to repopulate the host hematopoietic system, host mice were enrolled in the two-stage protocol of inflammatory skin carcinogenesis. Reconstitution efficiency at 6 weeks was confirmed using an atypical congenic BALB/c CB17 strain (Taconic) that harbors the C57BL/Ka IgH-1b immunoglobulin heavy chain allele (typical BALB/c is IgH-1a), which can be monitored on B lymphoid cells by an allele-specific antibody. Briefly, blood was collected from animals transplanted with CB17 bone marrow cells, erythrocytes were removed by hypotonic lysis, and the remaining peripheral blood lymphocytes were stained for cell surface expression of IgH-1b using standard protocols [21]. The antibodies used for this experiment were DS-1-PE (IgM derived from Igh-C[a]) and AF6-78-APC (IgM derived from Igh-C[b]). Samples were analyzed on a FACS Canto II flow cytometer using FACSDIVA software (BD Biosciences), in which 10,000 events for each sample were collected and gated for live lymphocytes based on forward and side scatter.
Carcinogenesis
For skin carcinogenesis, the shaved dorsal skin of 6–8-week-old mice was exposed to a single topical application of 400 nmol 7,12-dimethylbenz[a]anthracene (DMBA) in 200 μl acetone. For complete carcinogenesis, the initial DMBA dose was followed by weekly doses of 400 nmol in the same volume for 20 weeks. For two-stage inflammatory carcinogenesis, the initial DMBA dose was followed by topical application of 10 μg 12-O-tetradecanoylphorbol-13-acetate (TPA) 1 week later, as described previously [19], with biweekly applications continuing the entire course of the protocol. All animals were observed weekly and tumors that arose were monitored by caliper measurements and recorded. At the time of killing, tumors were isolated and fixed in 10% neutral-buffered formalin for histopathological analysis by hematoxylin and eosin staining, using standard methods. For TPA treatment alone, shaved mice were treated three times with 10 μg TPA on days 0, 3 and 7 and blood was collected on day 9 for serum kynurenine analysis as described [19]. For mammary gland carcinogenesis, female mice received s.c. implants in the intrascapular area of two compressed pellets of 20 mg medroxyprogesterone acetate (MPA; Hormone Pellet Press). Three weeks later, the first of four weekly doses of 50 mg/kg DMBA were administered p.o. in cottonseed oil with the three subsequent doses delivered 1, 3 and 4 weeks later. On this regimen, mammary tumors occurred with a frequency of ~100% as noted previously [22].
Skin abrasion wounding
After anesthetization, mouse dorsal fur was clipped and the skin depilated with Nair for 3–4 min before rinsing under warm water. Mice were dried and kept warm on heating pad. The lower dorsal skin was then swabbed with 70% isopropyl alcohol, and a 2 cm2 area on the lower back was gently abraded with a motorized felt wheel leaving the abraded area shiny, pink, and bloodless. Two hours before killing, all mice were injected i.p. with BrdU (Sigma) at a dose of 100 μg/g body weight. Skin tissue was collected from killed mice and fixed in 10% neutral-buffered formalin for histopathological analysis by hematoxylin and eosin staining, using standard methods.
Tumor graft assay and IDO inhibitor treatment
Outgrowth and IDO inhibitor response of tumors formed after subcutaneous engraftment of Myc+Hras1-transformed Bin1 −/− primary keratinocytes (MR KECs) was analyzed as described previously [17]. Briefly, MR KECs were injected s.c. above the femoral muscle of wild-type or Ido1 −/− C57BL/6 host mice and 1 week later animals were treated p.o. by oral gavage twice daily with 400 mg/kg of d,l-1-methyl-tryptophan (d,l-1MT) as described [23].
Cellular IDO regulation
Human U937 cells were seeded at 5 × 105 cells per well in RPMI 1640 containing penicillin/streptomycin, 55 μM β-mercaptoethanol, and 10% heat-inactivated fetal bovine serum. The following day endogenous IDO was induced by the addition of various stimuli for 24 h at which time culture media were harvested for kynurenine assay as described [24].
Results
IDO does not exhibit general anti-inflammatory characteristics
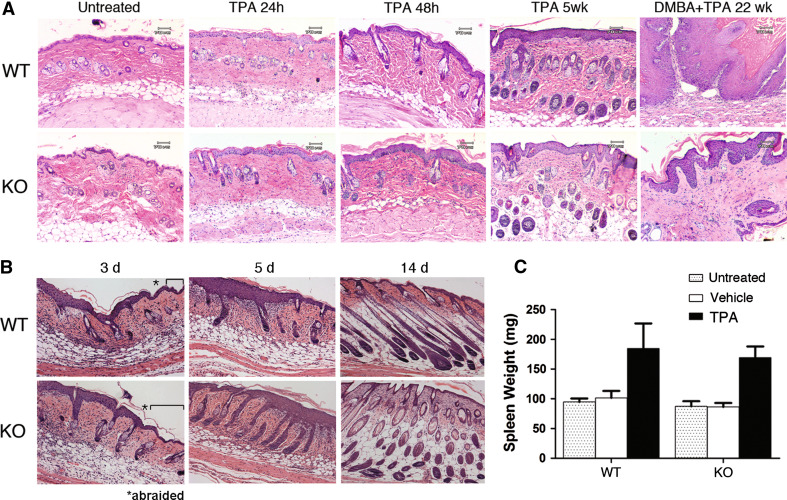
Generalized immunosuppressive mechanisms are actively required to keep inflammatory processes in check as evidenced by the development of severe multi-organ inflammation in mouse strains that are genetically deficient in key components of this regulatory system, such as FOXP3 or CTLA4 [25–28]. Although IDO action is generally considered to be immunosuppressive, no apparent evidence of spontaneous inflammation was observed as a consequence of homozygous disruption of the Ido1 gene in mice [29]. While IDO ablation may be insufficient to disrupt immune homeostasis, it might exaggerate the inflammatory response accompanying contact dermatitis or normal wound healing. To evaluate these possibilities, we compared the response of wild-type (WT) and Ido1-nullizygous (Ido1 −/−) mice to skin abrasion wounds or cutaneous application of TPA, the pro-inflammatory tumor-promoting agent used in the two-stage inflammatory skin carcinogenesis model. We observed no histologically discernable difference in the inflammatory skin response 24 and 48 h after a single application of TPA or after 5 weeks of TPA applications on the same schedule as used in the carcinogenesis regimen (Fig. 1a). Using a felt wheel, dorsal skin was abraded to the dermal layer and wound healing was followed by histological examination for several days afterward. We observed no difference in wound healing in mice lacking IDO (Fig. 1b). To further rule out the possibility that IDO loss simply de-represses the general inflammatory response to TPA, we confirmed that the characteristic ability of TPA to trigger accumulation of inflammatory cells in the spleen was unaffected in Ido1 −/− mice, as reflected in a comparable increase in spleen weights to wild-type controls (Fig. 1c). Thus, even in the context of active inflammation associated with skin wounding or TPA treatment, there is no apparent indication that IDO exerts any anti-inflammatory effect.
Fig. 1.
IDO is not anti-inflammatory. a Skin histology after TPA treatment alone. Similar epidermal thickening is observed in wild-type and Ido1 −/− animals at 24 and 48 h after a single application of TPA and remains evident through 5 weeks of repeated treatment. For comparison, the last panels illustrate histology of DMBA + TPA-treated skin at 22 weeks including papilloma formation in the wild-type animal. b Skin histology after abrasion wounding. Brackets indicate the nonabraded region 3 days after wounding with the abraded region noted beyond the asterisk. KO, Ido1 −/− mice. c Spleen weight after TPA treatment alone. Mice were exposed to TPA alone as noted in (b), killed on day 9, and wet weight of spleens dissected at necropsy determined. Standard error in the data presented is shown
TPA directly induces cellular IDO activity and synergizes with the pro-inflammatory mediators IFN-γ, LPS, and IL-1β
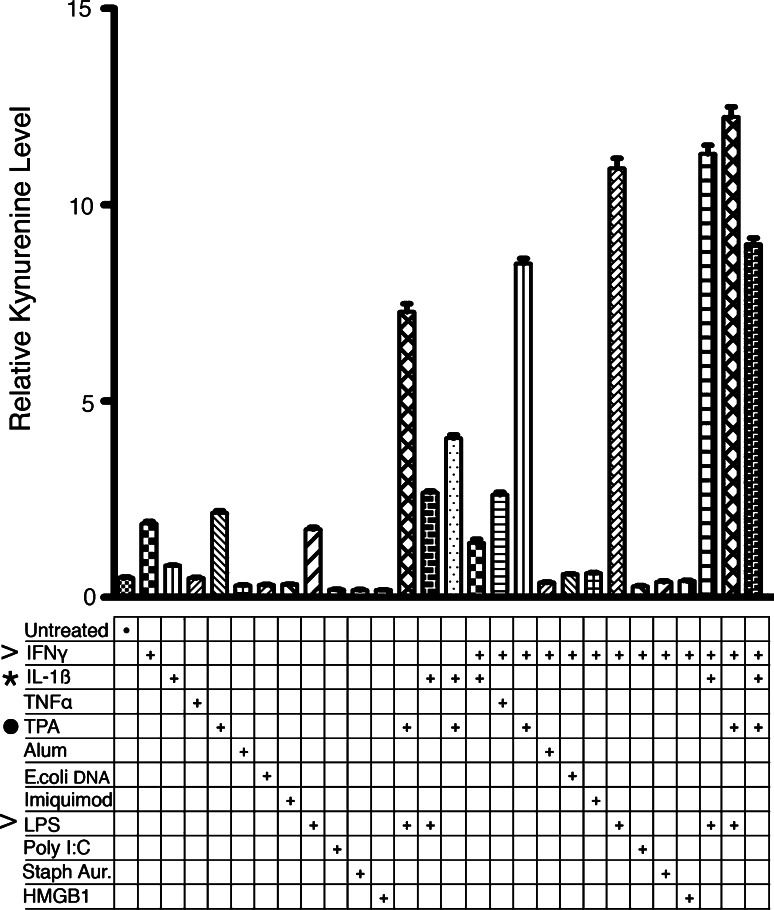
TPA exposure induces IDO activity in treated animals as demonstrated by elevated levels of the downstream catabolite kynurenine in the lymph nodes of TPA-exposed WT but not Ido1 −/− mice [19]. This induction of IDO activity might simply reflect an indirect consequence of inflammatory signals generated by chronic exposure to the pro-inflammatory chemical TPA. Alternately, TPA might act directly through protein kinase C activation or another signaling pathway to induce IDO. To assess these different possibilities, we examined the ability of TPA to directly stimulate IDO activity in cultured U937 myeloid cells in vitro. In this system, IDO can be induced by the Th1 cytokine interferon-γ (IFN-γ) or by the TLR4 ligand lipopolysaccharide (LPS), while the combination of the two produces a synergistic level of induction [24], providing positive controls for comparison. Briefly, U937 cells were treated for 24 h with 50 ng/ml TPA in the absence or presence of known IDO and/or immune-activating stimuli, followed by quantitation of the catabolic product kynurenine in cell culture supernatants using an HPLC/MS/MS-based detection method as described [24]. Several observations were made (Fig. 2). First, TPA directly activated IDO as effectively as the known activators IFN-γ and LPS. Second, TPA acted synergistically with either IFN-γ or LPS to superactivate IDO nearly as effectively as the powerful combination of IFN-γ and LPS. Third, IL-1β, a central pro-inflammatory cytokine, potentiated IDO induction by TPA with some apparent specificity as there was only weak enhancement of LPS induction with IL-1β and none at all with IFN-γ. While TPA acted less robustly with IL-1β than it did with IFN-γ or LPS, the stimulus from TPA and IL-1β was as strong as from TNF-α plus IFN-γ, a well-established combination for cooperative cytokine induction of IDO [30]. Lastly, TPA further enhanced IDO superinduction by the combination of IFN-γ and LPS. These data suggest that, in addition to the role of endogenous signals associated with TPA-elicited inflammation, TPA may also contribute directly to IDO induction in vivo and that, by acting synergistically, these signaling mechanisms are likely to be distinct and complementary.
Fig. 2.
Tumor promoter TPA directly activates IDO expression and cooperates with pro-inflammatory regulators IFN-γ, LPS, and IL-1β to superactivate IDO expression. Human U937 cells were untreated or treated with 100 ng/ml TPA in the absence or presence of known immune and/or IDO-activating stimuli. Culture media were harvested 24 h later to quantitate relative levels of kynurenine by LC/MS as analyzed in triplicate. The stimuli included IFN-γ (100 ng/ml), TGF-β (50 ng/ml), IL-1β (5 ng/ml), TNF-α (10 ng/ml), alum (100 μg/ml), genomic E. coli DNA (9.5 μg/ml), imiquimod (3 μg/ml), lipopolysaccharide (LPS; 100 ng/ml), poly dI:dC (10 μg/ml), heat-killed S. aureus (1.5 × 108 bacteria/ml), or HMGB1 (3 μg/ml). Additive effects are apparent between TPA (dot) and the stronger IDO inducers IFN-γ and LPS (carots) or the weaker inducer IL-1β (asterisk). The experiment was repeated once with similar results
IDO is not critical for carcinogenesis in the absence of pro-inflammatory tumor promotion
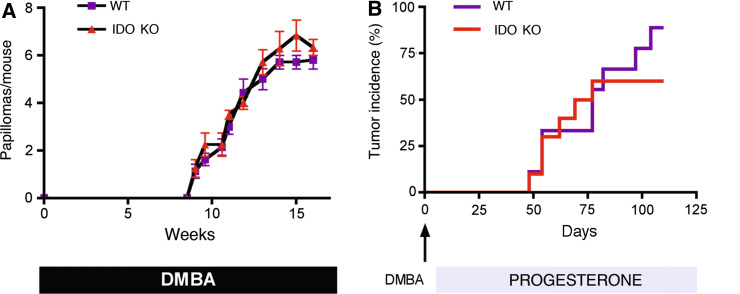
We previously demonstrated that Ido1 −/− mice are resistant to inflammatory skin carcinogenesis. The classic two-stage protocol involves a single topical application of the carcinogen 7,12-dimethylbenz[a]anthracene (DMBA), which oncogenically activates Hras1, plus regular biweekly topical applications of the pro-inflammatory phorbol ester 12-O-tetradecanoylphorbol-13-acetate [TPA (also known as PMA)], which elicits a chronic inflammatory response that promotes neoplastic development from within the pool of mutagen-initiated cells. While IDO is clearly needed to support the outgrowth of tumors generated by this protocol, one could not distinguish whether IDO plays a generalized role in cancer pathogenesis or a more specialized role in defining the cancer-promoting character of the chronic inflammation elicited by TPA. To further explore the degree to which IDO plays a generalized role in tumor development, we compared the cancer susceptibility of Ido1 −/− mice on other carcinogenesis protocols which lacked an explicit pro-inflammatory tumor promoter. For complete skin carcinogenesis, tumors were induced by repetitive weekly applications of DMBA alone. For mammary carcinogenesis, tumors were induced by orally administering DMBA to mice that had also received a subcutaneous implant of a time-release pellet of the synthetic progesterone mimetic MPA. On both protocols, we found that Ido1 deletion did not affect the incidence, kinetics, multiplicity, or stage of tumors formed (Fig. 3). Thus, IDO status did not affect tumor formation in the context of a one-stage protocol in which DMBA was used on its own to initiate and promote tumorigenesis, or in a two-stage protocol in which tumor promotion was achieved through use of a non-inflammatory hormone. These results support the interpretation that the role of IDO in the carcinogenic process is to shape a pathogenic inflammatory state that supports tumor formation, but that IDO is not fundamentally required for tumorigenesis.
Fig. 3.
IDO is not critical for carcinogenesis in the absence of an explicit pro-inflammatory driver. a IDO is not critical for skin carcinogenesis in the absence of TPA-induced inflammation. Mice were enrolled on the standard protocol of complete carcinogenesis involving repetitive exposure to carcinogen DMBA alone and tumor incidence was determined. Standard error in the data presented is shown. b IDO is not critical for DMBA-induced mammary carcinogenesis. Mice were enrolled on a standard protocol of mammary carcinogenesis involving i.p. administration of DMBA and continuous exposure to the progesterone mimetic medroxyprogesterone acetate. WT, wild-type mice; KO, Ido1 −/− mice. (n = 10 all groups)
IDO expression outside the radiation-sensitive hematopoietic cell compartment is important for pro-inflammatory tumor promotion
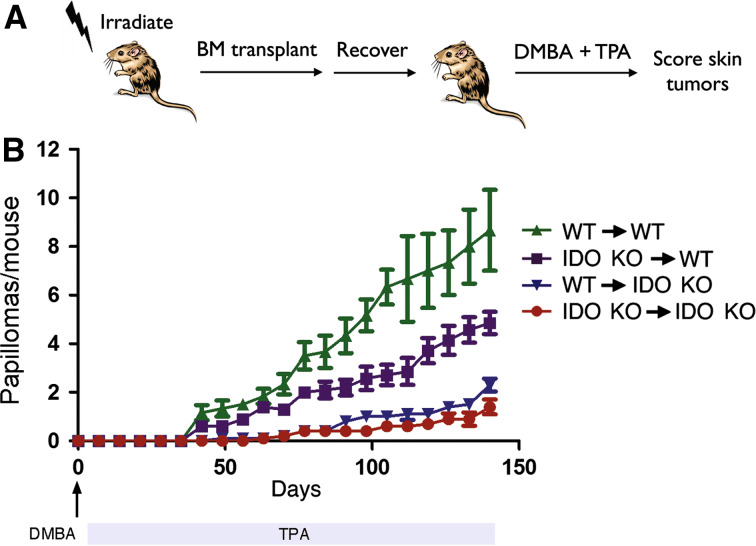
As noted earlier, TPA exposure was shown to promote skin carcinogenesis in an IDO-dependent manner while also stimulating a population of IDO-expressing, T cell-suppressive dendritic cells within the draining lymph nodes [19]. However, a direct causal connection was not established between these two effects of TPA treatment. To assess the relevance of IDO expression within the hematopoietic lineage (the source for dendritic cells), we took advantage of the inherent radiation sensitivity of hematopoietic cells to reconstitute this lineage by bone marrow transplantation (Fig. 4a). Briefly, lethally irradiated Ido1 −/− mice were reconstituted with WT bone marrow (non-hematopoietic cells are Ido1 −/−) and their susceptibility to inflammatory skin carcinogenesis was compared to WT BALB/c mice reconstituted with Ido1 −/− bone marrow (hematopoietic cells are Ido1 −/−). As controls, WT mice were reconstituted with WT bone marrow and Ido1 −/− mice were reconstituted with Ido1 −/− bone marrow. Control chimeric mice recapitulated the differences in susceptibility between non-reconstituted WT and Ido1 −/− mice, with Ido1 −/− chimeras demonstrating resistance to papilloma formation (Fig. 4b). In contrast, the host phenotype tended to dominate in the experimental chimeric mice that received reciprocal bone marrow engraftment (Fig. 4b). Thus, while not as susceptible as WT mice engrafted with WT bone marrow, WT mice engrafted with Ido1 −/− bone marrow remained more susceptible to tumor formation than Ido1 −/− mice engrafted with WT bone marrow, which in turn were nearly as resistant as Ido1 −/− mice engrafted with Ido1 −/− bone marrow. A comparable outcome was obtained in an experiment of reciprocal bone marrow transplants in which successful immune replenishment by the donor bone marrow was confirmed using IgM allotype-marked mice (Suppl. Fig. 6a, b). Thus, while the expression of IDO within radiosensitive hematopoietic cells may contribute to some degree to skin tumor susceptibility, the outcome of these bone marrow chimera experiments suggests that IDO expressed in radioresistant non-hematopoietic cells of the host plays a more consequential role.
Fig. 4.
IDO functions primarily in non-hematopoietic cells to support inflammatory skin carcinogenesis. a Experimental design. Four-week-old recipient mice were lethally irradiated and reconstituted with bone marrow from 6-week-old donor mice. All control and reciprocal transplant possibilities in wild-type and Ido1 −/− mice were performed. After allowing 6 weeks for the transplanted bone marrow to repopulate the hematopoietic system, host mice were subjected to two-stage inflammatory skin carcinogenesis. b Papilloma formation in bone marrow chimeric mice. Legend indicates host-to-donor configuration in each cohort (n = 10). Standard error is indicated for each of the incidence curves. KO, Ido1 −/− mice
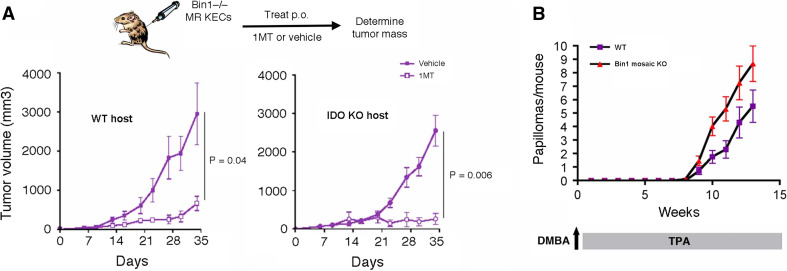
We further evaluated the non-hematopoietic contribution of IDO to tumor formation by extending an earlier analysis of the effects of ablating the IDO regulatory tumor suppressor gene Bin1. We have shown previously that Myc+Hras1-transformed keratinocytes (MR KECs) that are genetically nullizygous for Bin1 exhibit superinduction of IDO and aggressive tumorigenicity compared to Bin1-expressing MR KECs [17]. Inhibiting IDO activity with 1MT abolished the benefits of Bin1 loss to tumor formation in this context, suggesting that IDO dysregulation was responsible for driving the aggressive growth of Bin1-null MR KECs. However, a potential caveat to this interpretation was the possibility that tumor inhibitory effects of 1MT may be unrelated to inhibition of IDO in the transformed cells but instead related to inhibition of IDO in host immune cells [19]. To attempt to rule out this possibility, we compared the ability of 1MT to suppress the outgrowth of Bin1-null MR KECs in wild-type or Ido1 −/− host animals, where in the latter case IDO was expressed only in the engrafted transformed cells. Notably, both the outgrowth and the 1MT response of the transformed cells were similar whether or not host IDO was present (Fig. 5a). This result provides direct evidence that IDO activity within tumor cells can be sufficient to promote T cell-targeted immune escape and tumor outgrowth.
Fig. 5.
IDO can exert a tumor cell autonomous function to support inflammatory skin carcinogenesis. a Tumor cell autonomous role of IDO activity in tumor formation by transformed Bin1 −/− skin epithelial cells. Bin1 −/− keratinocytes transformed by the Myc and Hras1 oncogenes (MR KECs) were injected s.c. into syngeneic immunocompetent mice and tumor formation was monitored by caliper measurements as described [17]. Seven days after tumor cell engraftment the IDO inhibitor 1MT was administered b.i.d. by oral gavage at 400 mg/kg as described previously [47]. Standard error for the data is provided for each cohort (n = 5). b Bin1 ablation increases susceptibility to inflammatory skin cancer. Bin1 mosaic null mice have been described previously [20]. Mice were enrolled on the two-stage inflammatory skin carcinogenesis protocol and papilloma formation was monitored as before (n = 10). The results presented include data collected from two separate trials. Standard error is indicated for each of the incidence curves
The primary keratinocytes that were used to establish the transformed MR KEC cell lines used in these tumor isograft studies were of similar origin to the epithelial precursors that form neoplastic papillomas in the DMBA + TPA inflammatory skin carcinogenesis model. Based on the MR KEC isograft studies, we anticipated that the loss of Bin1 would elevate susceptibility in this de novo model of IDO-dependent skin tumor formation as well. This hypothesis was tested in Bin1 mosaic null mice that bypass the embryonic lethality that occurs in animals with a constitutive homozygous Bin1 gene deletion [20]. As predicted, on the classical two-stage protocol, Bin1 mosaic null mice displayed an increased incidence of papilloma formation relative to wild-type control mice (Fig. 5b). Overall, our results prompt the conclusion that the primary contribution of IDO to inflammatory skin carcinogenesis is mediated through its expression in non-hematopoietic stroma or tumor cells.
Discussion
How it is that chronic inflammatory disease supports the development and progression of many adult cancers is an area of active investigation. The findings of this study specifically point to IDO activity as a key defining element of ‘cancer-associated’ inflammation that supports malignant development and progression by enabling immune escape. As a positive modifier of cancer at this level, IDO may condition the microenvironment of an initiated tumor at its early stages to engender local tissue support. More generally, our findings support the idea, suggested previously [31], that mediators of immune escape in cancer may be genetically synonymous with defining elements of cancer-associated inflammation. This concept offers explanative appeal in its ability to molecularly define ‘cancer-associated’ inflammation as a chronic inflammatory state that includes the involvement of one or more key mediators of immune escape. At present, immune escape mechanisms tend to be considered an acquired consequence of the selective pressures imposed by immunoediting. However, if immune escape mechanisms are instead integral elements that define the tumorigenic potential of a chronic inflammatory reaction, as our findings suggest, then the collective status of immune escape mediators inherent within an inflammatory microenvironment may dictate the propensity of that microenvironment to support cancer development. We believe that the modifier effects engendered by IDO may extend to other epithelial tumors, given emerging evidence in our group that IDO ablation can blunt Ras-induced inflammation-associated carcinogenesis in tissues beyond the skin (unpublished observations). From this perspective, reports that IDO dysregulation has prognostic impact in various cancers are provocative to consider [32–39]. Genetic polymorphisms in IDO or the IDO pathway affecting its levels or activity in different individuals may also influence risks in the development or progression of inflammation-associated cancers [13, 40, 41].
Importantly, the role of IDO in the context of chronic inflammation appears to be more complex than simply to act as an immunosuppressive brake. The results of this study show instead IDO acting as an integral component of the inflammatory milieu that alters its pathogenic capacity to support tumor outgrowth, without discernibly altering the severity of classical inflammatory responses in other settings. These results establish IDO as representative of a novel class of immune modifier elements that uniquely define cancer-associated inflammation through their ability to support tumoral immune escape. The successful implementation of strategies to defeat immune escape [42] is now widely regarded as being a key to improving immunotherapy responses. However, a major concern with regard to the feasibility of targeting immunosuppressive mechanisms in order to elicit productive antitumor responses is the likelihood that such therapies will also produce serious autoimmune side effects. Indeed, recent clinical trials along these lines tend to support the conclusion that dose-limiting, immune-related adverse events correlate with therapeutic efficacy. In this regard, specifically targeting pathogenic determinants of cancer-associated inflammation, such as IDO, may have the potential to effectively circumvent mechanism-based side effects associated with more generalized immunosuppressive strategies.
TPA acts as a molecular mimic of diacylglycerol (DAG), activating protein kinase C (PKC) isoenzymes as well as other DAG-binding proteins carrying the C1 domain [43]. DAG is a second messenger signaling lipid produced by phospholipase C (PLC) that regulates cellular activation. Our finding that TPA directly stimulates IDO expression suggests that IDO may be responsive to the internal activation status of cells, as well as to external pro-inflammatory signals within the tissue microenvironment. In fact, the synergistic cooperativity exhibited by TPA with the known IDO activators IFN-γ and bacterial LPS, as well as with the pro-inflammatory cytokine IL-1β, suggests that the signaling pathways activated by these agents are mechanistically distinct and complementary. Overall, our results highlight the likelihood that IDO thresholds achieved in inflammatory microenvironments via multiple regulatory inputs may positively modify the risk of tumor progression. Consistent with this concept, IDO is downregulated by a variety of anti-inflammatory agents that also exert anti-cancer effects, including aspirin [44], celecoxib [45, 46], and the simple NFκB-inhibitory compound ethyl pyruvate [29]. Thus, in addition to the pathobiological evidence that IDO and carcinogenesis susceptibility are linked through a specific aspect of the inflammatory process, the evidence that pro-inflammatory stimuli known to influence cancer development are also important regulators of IDO activity furthers the likelihood of a proximal relationship existing between IDO and cancer-associated inflammation.
Previous work in tumor isograft models has made a compelling case for a tumor non-cell autonomous role for IDO in supporting immune escape through its activity in a regulatory population of antigen-presenting dendritic cells (DCs) elevated within the TDLNs [7, 23, 47]. IDO+ regulatory DCs were similarly elevated in the draining lymph nodes of TPA-treated animals [19]. So, data implicating non-hematopoietic cells as the relevant source of IDO that supports DMBA + TPA skin carcinogenesis run counter to expectations. However, this outcome is consistent with other evidence that, for tumors formed by transformed Bin1−/− keratinocytes, where IDO is hyperresponsive to stimulation as a result of Bin1 deletion [17], IDO activity is critical for growth of engrafted transformed cells whether or not IDO is functional in the host. This evidence for a tumor cell autonomous mechanism argues that tumor cells can serve as a sufficient site of IDO modulating function in tumor growth. Taken together, these findings corroborate the immunoregulatory relevance of IDO expressed outside the hematopoietic compartment in tumor and/or tumor stromal cells. In future work, it will be valuable to gain deeper genetic and cellular insights with regard to the specific sites of IDO activity and how this contributes to tumoral immune escape within the context of cancer-associated inflammation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was supported by grants from the DoD Breast Cancer Research Program (A.J.M., G.C.P.), Pennsylvania Department of Health CURE/Tobacco Settlement Award (A.J.M.) and NIH grants CA82222 (G.C.P.), CA109542 (G.C.P.), and CA070739 (S.K.G.). Additional support was also provided by New Link Genetics Corporation, Dan Green Foundation, Lankenau Hospital Foundation, and the Main Line Health System (G.C.P.). A conflict of interest is declared by G.C.P., A.J.M., and J.B.D. who have intellectual property rights and financial interests in New Link Genetics Corporation which is developing IDO inhibitors for treatment of cancer and other diseases.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Peek RM, Mohla S, DuBois RN. Inflammation in the genesis and perpetuation of cancer: summary and recommendations from a National Cancer Institute-sponsored meeting. Cancer Res. 2005;65:8583–8586. doi: 10.1158/0008-5472.CAN-05-1777. [DOI] [PubMed] [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21(2):137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Gajewski TF. Failure at the effector phase: immune barriers at the level of the melanoma tumor microenvironment. Clin Cancer Res. 2007;13(18 Pt 1):5256–5261. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 7.Munn DH, Mellor AL. The tumor-draining lymph node as an immune-privileged site. Immunol Rev. 2006;213:146–158. doi: 10.1111/j.1600-065X.2006.00444.x. [DOI] [PubMed] [Google Scholar]
- 8.Koebel CM, Vermi W, Swann JB, Zerafa N, Rodig SJ, Old LJ, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. doi: 10.1038/nature06309. [DOI] [PubMed] [Google Scholar]
- 9.Sotomayor EM, Borrello I, Rattis FM, Cuenca AG, Abrams J, Staveley-O’Carroll K, et al. Cross-presentation of tumor antigens by bone marrow-derived antigen-presenting cells is the dominant mechanism in the induction of T-cell tolerance during B-cell lymphoma progression. Blood. 2001;98(4):1070–1077. doi: 10.1182/blood.V98.4.1070. [DOI] [PubMed] [Google Scholar]
- 10.Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Curr Opin Immunol. 2006;18(2):226–231. doi: 10.1016/j.coi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med. 2007;204(1):49–55. doi: 10.1084/jem.20062056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ball HJ, Sanchez-Perez A, Weiser S, Austin CJ, Astelbauer F, Miu J, et al. Characterization of an indoleamine 2,3-dioxygenase-like protein found in humans and mice. Gene. 2007;396(1):203–213. doi: 10.1016/j.gene.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 13.Metz R, DuHadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor IDO inhibitory compound D-1MT. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 14.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117(5):1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellor AL, Sivakumar J, Chandler PKS, Molina H, Mao D, et al. Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat Immunol. 2001;2:64–68. doi: 10.1038/83183. [DOI] [PubMed] [Google Scholar]
- 16.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 17.Muller AJ, DuHadaway JB, Sutanto-Ward E, Donover PS, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunomodulatory target of the tumor suppressor gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005;11:312–319. doi: 10.1038/nm1196. [DOI] [PubMed] [Google Scholar]
- 18.Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in immune suppression and cancer. Curr Cancer Drug Targets. 2007;7(1):31–40. doi: 10.2174/156800907780006896. [DOI] [PubMed] [Google Scholar]
- 19.Muller AJ, Sharma MD, Chandler PR, Duhadaway JB, Everhart ME, Johnson BA, III, et al. Chronic inflammation that facilitates tumor progression creates local immune suppression by inducing indoleamine 2,3 dioxygenase. Proc Natl Acad Sci USA. 2008;105(44):17073–17078. doi: 10.1073/pnas.0806173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang M, Boulden J, Katz JB, Wang L, Meyer TJ, Soler AP, et al. Bin1 ablation increases susceptibility to cancer during aging, particularly lung cancer. Cancer Res. 2007;67:7605–7612. doi: 10.1158/0008-5472.CAN-07-1100. [DOI] [PubMed] [Google Scholar]
- 21.Hardy RR, Carmack CE, Shinton SA, Kemp JD, Hayakawa K. Resolution and characterization of pro-B and pre-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–1225. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang M, Boulden J, Sutanto-Ward E, Duhadaway JB, Soler AP, Muller AJ, et al. Bin1 ablation in mammary gland delays tissue remodeling and drives cancer progression. Cancer Res. 2007;67:100–107. doi: 10.1158/0008-5472.CAN-06-2742. [DOI] [PubMed] [Google Scholar]
- 23.Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67(2):792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 24.Muller AJ, DuHadaway JB, Jaller D, Curtis P, Metz R, Prendergast GC. Immunotherapeutic suppression of IDO and tumor growth by ethyl pyruvate. Cancer Res. 2010;70(5):1845–1853. doi: 10.1158/0008-5472.CAN-09-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27(1):68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 26.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 27.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 28.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 29.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171(4):1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 30.Robinson CM, Shirey KA, Carlin JM. Synergistic transcriptional activation of indoleamine dioxygenase by IFN-gamma and tumor necrosis factor-alpha. J Interferon Cytokine Res. 2003;23(8):413–421. doi: 10.1089/107999003322277829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prendergast GC, Jaffee EM. Cancer immunologists and cancer biologists: why we didn’t talk then but need to now. Cancer Res. 2007;67(8):3500–3504. doi: 10.1158/0008-5472.CAN-06-4626. [DOI] [PubMed] [Google Scholar]
- 32.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12(4):1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 33.Chamuleau ME, van de Loosdrecht AA, Hess CJ, Janssen JJ, Zevenbergen A, Delwel R, et al. High INDO (indoleamine 2,3-dioxygenase) mRNA level in blasts of acute myeloid leukemic patients predicts poor clinical outcome. Haematologica. 2008;93(12):1894–1898. doi: 10.3324/haematol.13112. [DOI] [PubMed] [Google Scholar]
- 34.Ino K, Yoshida N, Kajiyama H, Shibata K, Yamamoto E, Kidokoro K, et al. Indoleamine 2,3-dioxygenase is a novel prognostic indicator for endometrial cancer. Br J Cancer. 2006;95(11):1555–1561. doi: 10.1038/sj.bjc.6603477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, et al. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11(16):6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 36.Pan K, Wang H, Chen MS, Zhang HK, Weng DS, Zhou J, et al. Expression and prognosis role of indoleamine 2,3-dioxygenase in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2008;134(11):1247–1253. doi: 10.1007/s00432-008-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urakawa H, Nishida Y, Nakashima H, Shimoyama Y, Nakamura S, Ishiguro N. Prognostic value of indoleamine 2,3-dioxygenase expression in high grade osteosarcoma. Clin Exp Metastasis. 2009;26(8):1005–1012. doi: 10.1007/s10585-009-9290-7. [DOI] [PubMed] [Google Scholar]
- 38.Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology. 2007;214(1):8–14. doi: 10.1159/000096906. [DOI] [PubMed] [Google Scholar]
- 39.Yoshikawa T, Hara T, Tsurumi H, Goto N, Hoshi M, Kitagawa J, et al. Serum concentration of L-kynurenine predicts the clinical outcome of patients with diffuse large B-cell lymphoma treated with R-CHOP. Eur J Haematol. 2009;84(4):304–309. doi: 10.1111/j.1600-0609.2009.01393.x. [DOI] [PubMed] [Google Scholar]
- 40.Arefayene M, Philips S, Cao D, Mamidipalli S, Desta Z, Flockhart D, et al. Identification of genetic variants in the human indoleamine 2,3-dioxygenase (IDO1) gene, which have altered enzyme activity. Pharmactogenet Genomics. 2009;19:464–476. doi: 10.1097/FPC.0b013e32832c005a. [DOI] [PubMed] [Google Scholar]
- 41.Witkiewicz AK, Costantino CL, Metz R, Muller AJ, Prendergast GC, Yeo CJ, et al. Genotyping and expression analysis of IDO2 in human pancreatic cancer: a novel, active target. J Am Coll Surg. 2009;208(5):781–787. doi: 10.1016/j.jamcollsurg.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6(8):613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 43.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7(4):281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 44.Sayama S, Yoshida R, Oku T, Imanishi J, Kishida T, Hayaishi O. Inhibition of interferon-mediated induction of indoleamine 2,3-dioxygenase in mouse lung by inhibitors of prostaglandin biosynthesis. Proc Natl Acad Sci USA. 1981;78(12):7327–7330. doi: 10.1073/pnas.78.12.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, et al. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol. 2006;177(4):2391–2402. doi: 10.4049/jimmunol.177.4.2391. [DOI] [PubMed] [Google Scholar]
- 46.Lee SY, Choi HK, Lee KJ, Jung JY, Hur GY, Jung KH, et al. The immune tolerance of cancer is mediated by IDO that is inhibited by COX-2 inhibitors through regulatory T cells. J Immunother. 2009;32(1):22–28. doi: 10.1097/CJI.0b013e31818ac2f7. [DOI] [PubMed] [Google Scholar]
- 47.Munn DH, Sharma MD, Hou D, Baban B, Lee JR, Antonia SJ, et al. Expression of indoleamine 2,3- dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 2004;114(2):280–290. doi: 10.1172/JCI21583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.