Summary
“Oncogene addiction” refers to the process of tumor cell death that can occur after inactivation of a single oncogene. In this issue of Cancer Cell, Rakhra, et al. argue that complete tumor clearance after molecular targeted therapies requires a functioning immune system, pointing the way toward radically new combination therapies.
Tumor cells generally contain many genetic and epigenetic abnormalities that trigger manifold cellular pathways leading to the uncontrolled growth and metastases of transformed cells. Given the complex disruption of the cancer genome and epigenome, it may seem surprising that the inactivation of a few or even one single oncogene can profoundly affect the maintenance and growth of a tumor cell mass. Yet this perplexing phenomenon often occurs and has been referred to as “oncogene addiction.”
Oncogene addiction provides the rationale for molecularly targeted therapies, which have substantially been validated in both mice and in humans with cancer. In a transgenic mouse system employing a tetracycline regulatable c-Myc, the withdrawal of this oncogene can lead to sudden tumor cell death (Felsher and Bishop, 1999). Another example is the withdrawal of Oct3/4 in a mouse model of choriocarcinoma (Gidekel et al., 2003).
Examples that this is a “real” phenomenon in patients with cancer comes from treatments using monoclonal antibodies such trastuzumab (Herceptin), which targets the HER2 receptor tyrosine kinase in patients with HER2-positive breast cancer. Small molecule drugs like imatinib (Gleevec) can also interfere with the functioning of various oncogenic protein kinases by targeting the BCR-ABL in chronic myeloid leukemia and KIT in gastrointestinal stromal tumors. Erlotinib (Tarceva) and gefitinib (Iressa) both target the epidermal growth factor receptor (EGFR) and have some activities in varieties of cancers. Melanoma patients can be successfully treated with PLX4032, an inhibitor of the serine–threonine protein kinase B-RAF, which disrupts the mitogen-activated protein (MAP) kinase pathway (Flaherty et al., 2010). In addition, a subgroup of patients with non-small-cell lung cancers expressing an oncogenic fusion of the EML4 and anaplastic lymphoma kinase (ALK) genes can be successfully treated with crizotinib, a small-molecule inhibitor of the ALK tyrosine kinase (Kwak, 2010).
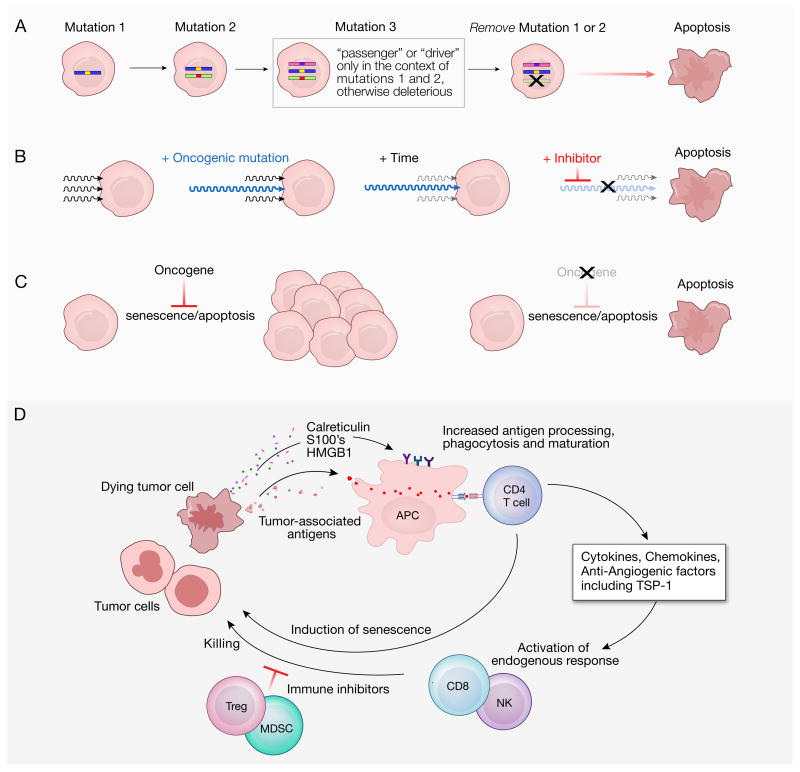
Although these treatments are generally not curative, the striking effectiveness of some single agent inhibitors of oncogenic proteins in humans and in animal models has led to the quest to understand the mechanisms underlying the phenomena of oncogene addiction (Figure 1). The partial or complete tumor regression associated with oncogene withdrawal is accompanied by cell cycle arrest, tumor cell differentiation, senescence and apoptosis (Kaelin, 2005). While the precise mechanisms involved remain largely speculative, several theories have been forwarded. Cancer cells accumulate mutations resulting from various types of genetic damage including polyploidy, aneuploidy, endoreduplication (duplication of the genome without mitosis), DNA breaks, translocations and all of the epigenetic abnormalities that accompany these problems. Continued survival of cancer cells is achieved only when they acquire mutations that are neutral (“passenger”) or beneficial (“driver”). In the cumulative mutation or ‘house of cards’ model, some of these alterations might be lethal in the absence of preceding mutations, so removing the activity of an early oncogene enables programmed death to occur (Figure 1A). Another theory purports that a single oncogenic signaling pathway comes to dominate over the others because its efficiency results in a diminishing of selection pressure on other pathways to maintain collateral signaling pathways (Figure 1B). This “dominant oncogene” theory requires the rapid decay of these collateral growth factor signaling pathways. In yet a third theory, dubbed “oncogene amnesia,” the removal of the dominant oncogene enables the reinstatement of normal physiologic programs whereby a tumor becomes subject to checkpoint induced apoptosis (Felsher, 2008) (Figure 1C). This hypothesis takes as an assumption that the original pathways controlling cellular death are at least partially intact after being subjected to the oncogenic process.
Figure 1.
Theories of oncogene addiction. A. In the “cumulative mutations” model, tumors accrue mutated oncogenes, some of which are “driver” and some of which are “passenger.” Any change must be neutral or beneficial to the tumor cell’s viability. However, some mutations could occur that are beneficial only in the context of existing mutations but are otherwise harmful. Withdrawal of an early oncogene signal in this scenario would induce senescence or death, like a molecular “house of cards.” B. In the “dominant oncogene” model, tumors receive signals from multiple growth factor pathways but become dependent on one of these. With time, other cell growth pathways become less subject to selection and suffer mutations or epigenetic changes that attenuate or obliterate their functions. Removal of the dominant growth factor signal then triggers apoptosis. Note that arrows represent intracellular signaling pathways. C. The “oncogene amnesia” model, and intact oncogene signal (shown at left) limits cell death. Thus, pathways that would normally induce senescence and death are held in check by the activities of the oncogene, but are re-instated upon withdrawal of the oncogene’s activity (shown at right). D. How mechanisms of immune activation might explain oncogene addiction. When cells experience apoptosis associated with inactivation of an oncogene they might release tumor-associated antigens, which come in the form of tissue differentiation antigens, cancer/testis antigens, or products of mutated genes expressed by the transformed cells. Additional immune activating signals including calreticulin, the S100 family of proteins, and (in secondary necrosis) the high mobility group box 1 protein (HMGB1) can be emitted by some dying cells. Resulting activation of host antigen presenting cells (APC) could in turn activate CD4+ T cells capable of producing a host of immunoregulatory and anti-angiogenesis molecules including thrombospondin-1 (TSP1). CD4+ T cells recognizing processed tumor-associated antigens also can activate CD8+ T cells and natural killer (NK) cells leading to more tumor killing. The activities of immune cells can be inhibited by myeloid-derived suppressor cells (MDSC) and by regulatory T cells (Tregs). However, the administration of tumor-reactive T cells in combination with ablation of regulatory cells can result in long-term durable tumor regression in mice and humans.
Most theories regarding these phenomena have assumed that oncogene addiction involves cell autonomous mechanisms, but in this issue of Cancer Cell, Rakhra, et al. argue in favor of a mechanism that is not entirely cell autonomous (Rakhra, 2010). By doing experiments using a series of immune-deficient animals, the authors trace the key immune cells to be CD4+ T cells. The authors focus on the role of thrombospondin-1 (TSP1), a pleiotropic glycoprotein that is produced by many cells including immune cells and platelets and has major roles in angiogenesis, inflammation and wound healing.
Although a more fulsome investigation of the roles of CD4+ T cells will undoubtedly be forthcoming, they are known to play a central role in orchestrating many elements of the adaptive immune system (Muranski and Restifo, 2009). CD4+ T cells accomplish this by direct interaction with other immune cells, and by producing immunomodulatory cytokines and chemokines. CD4+ T cells can activate panoply of immune cells, including CD8+ T cells and natural killer (NK) cells, whose activities can be inhibited by myeloid-derived suppressor cells (MDSC) and by regulatory T cells (Tregs). When given in combination with anti-tumor T cells, ablation of these immunoregulatory cells can enable complete and durable tumor destruction in mice and humans with large, established cancers (Gattinoni et al., 2006).
One scenario for how oncogene addiction might be linked to immune eradication is illustrated in Figure 1D. It seems plausible that removal of a single oncogene might start the process of tumor cell death by a cell-intrinsic mechanism thereby releasing tumor-associated antigens and immune modulators that activate antigen presenting cells (APC). The MHC class II pathway, which presents antigens to CD4+ T cells, is particularly efficient at processing extracellular antigens (Muranski and Restifo, 2009). The mechanism of tumor cell death resulting in antigen presentation and immune activation is similar in many ways to the tumor immunogenicity that can be induced after the use of some chemotherapies (Zitvogel et al., 2008).
These results call for more study of the mechanisms of tumor regression after oncogene withdrawal using immunologically intact animals, but point to areas of concern as well. The tumors studied by Rakhra, et al. in this report may represent highly immunogenic variants because they are engineered to express the immunologically “foreign” proteins of the Tet regulatory protein and inducible human MYC protein. In addition, the authors may have inadvertently increased the immunogenicity of the transplanted tumors by inserting the luciferase enzyme, which might itself function as a “non-self” protein. Tumor immunologists might view insertion of this artificial genetic information as something akin to introduction of a foreign protein such as chicken ovalbumin into a tumor, which dramatically increases its immunogenicity. The artificial aspects of this sort of immunity might not translate well into human trials, where there is ample time for the immune system to become activated and select against highly immunogenic tumor-associated epitopes. In addition, human trials of molecular targeted therapies do not thus far hint at having a strong immune-activating component, where expected immune-type side effects would likely include fevers, headaches, myalgias and even hypotension.
In conclusion, the fields of molecularly targeted therapy and immunotherapy of cancer still seem worlds apart, but additional experiments are clearly warranted. The use of animal models with intact host immune systems could enable the study of potential combination therapies. Thomas Henry Huxley wrote how in science “many a beautiful theory [is] killed by an ugly fact.” To accurately determine the fate of combination therapies using molecular targeted therapies together with immune-based manipulations, future experiments should be performed using spontaneous tumors in the non-transplantation setting or tumor systems with less intrinsic immunogenicity. Such combinations may enable the more frequent induction of long-term durable cancer regression.
Acknowledgments
The author would like to thank Yardena Samuels, Simon Turcotte, James Yang and Pawel Muranski for helpful discussions; Megan Bachinski for editorial input; and Lydia Kibiuk for drawing the figure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Felsher DW. Oncogene addiction versus oncogene amnesia: perhaps more than just a bad habit? Cancer Res. 2008;68:3081–3086. doi: 10.1158/0008-5472.CAN-07-5832. discussion 3086. [DOI] [PubMed] [Google Scholar]
- Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, O’Dwyer PJ, Lee RJ, Grippo JF, Nolop K, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–393. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidekel S, Pizov G, Bergman Y, Pikarsky E. Oct-3/4 is a dose-dependent oncogenic fate determinant. Cancer Cell. 2003;4:361–370. doi: 10.1016/s1535-6108(03)00270-8. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol. 2009;21:200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhra K. CD4+ T-cells contribute to the remodeling of the microenvironment required for sustained tumor regression upon ocogene inactivation. Cancer Cell. 2010 doi: 10.1016/j.ccr.2010.10.002. Current Issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]