Abstract
A focal and transitory inflammation induced by injection of complete Freund's adjuvant (CFA) in the submandibular skin of mice elicits pain behavior that persists for several weeks after the initial inflammation has resolved. Chronic pain, assessed as tactile hypersensitivity to stimulation with von Frey filaments, was evident from 1–7 weeks following CFA injection, although inflammation at the injection site was resolved by 3–4 weeks. In contrast, there were no changes in tactile sensitivity in the paw (un-injected site for comparison), no alterations in open field behavior and no differences in a functional observation battery evident in CFA-treated mice compared to controls (saline-injected) or to baseline (before CFA injection). Neither strain (Balb/c vs. C57BL/6) nor sex differences in baseline tactile threshold were significant in the submandibular skin. CFA-induced tactile hypersensitivity was also not a function of strain or sex. A single intraperitoneal injection of the gap junction blocker carbenoxolone (CBX) restored normal tactile thresholds in CFA-treated mice when administered at the peak of inflammation (1 week), after significant resolution of inflammation (3 weeks) or after total resolution of inflammation (4 and 5 weeks) without altering the tactile threshold of control subjects, tactile threshold in the paw or open field behavior. Thus, in this novel model of post-inflammatory pain, transitory inflammation induced persistent sex- and strain-independent behavioral hypersensitivity that was reversed by the gap junction blocker CBX, suggesting neuronal and/or glial plasticity as a major component of the chronic pain.
Keywords: Trigeminal ganglion, orofacial pain, tactile sensitivity, gap junction, mouse strain, sex difference, chronic pain, von Frey
INTRODUCTION
Methods of inducing chronic pain in rodents generally fall into one of several categories: nerve damage (axotomy, nerve crush, neuropathies), joint damage and/or inflammation (injection of adjuvants, formalin, etc.) and subcutaneous or intra-muscular injection of inflammatory or pain-causing agents (formalin, substance P, adjuvants, etc.). Although each of these models has advantages, the injection of complete Freund’s adjuvant (CFA) into the submandibular skin is a new model with unique characteristics that provides several advantages. First, the submandibular skin is glabrous and can thus be tested with von Frey filaments – a standard method for assessment of tactile sensitivity and mechanical allodynia in both rodents and humans [1–4]. Second, the trigeminal ganglion innervating the submandibular region is readily identifiable and is easy to access and excise, thus permitting concomitant analysis of underlying molecular mechanisms of changes in nociception [5]. Third, no surgery is required, and fourth, as shown in this study, the animals do not exhibit other signs of stress or pain, but only show hypersensitivity upon focal stimulation. In addition, the inflammation resolves comparatively quickly and results in no discernible tissue damage. These characteristics may foster the dissection of the mechanisms and timing of events initiating and maintaining chronic pain without the complications of long-term inflammation and persistent stimulation that can confound the distinction between factors that engender and maintain chronic pain [6].
Chronic orofacial pain is common, afflicting about 25% of the adult population [7]. It is likely that mechanisms responsible for chronic orofacial pain have much in common with other types of chronic pain [8–10]. Initial inflammatory processes are thought to lead to sensitization and hyperexcitability of primary afferent neurons, which in turn send nociceptive signals to the CNS producing hypersensitivity that persists long after the initial insult [11–13]. Thus, the elucidation of the molecular mechanisms in this model and the development of novel therapeutics would be expected to have potentially broad application to other types of chronic pain.
In recent years it has become clear that changes in gap junction expression and activation and gap junction-mediated coupling may play a role in chronic pain [14–17]. Thus, we investigated the potential analgesic effects of the gap junction blocker carbenoxolone (CBX). CBX has been used previously to demonstrate that gap junction-mediated coupling plays a role in several pathological states, including pain [18–20].
We here examine changes in tactile sensitivity after CFA injection into the submandibular skin in male and female Balb/c or C57BL/6 mice for 7 weeks. We show that this results in transitory and local inflammation and elicits tactile hypersensitivity (pain) persisting long after inflammation has resolved, independent of mouse strain and sex. We also demonstrate that the gap junction blocker CBX reverses tactile hypersensitivity during both acute and chronic pain states.
METHODS
Animal Subjects, Inflammation Model and Drug Administration
The present studies were approved by the Albert Einstein College of Medicine Animal Care and Use Committee and conform to the guidelines of the International Association for the Study of Pain and to the NIH standards for the care and use of laboratory animals. Every effort was made to minimize animal suffering and to reduce the number of animals used in our study. Male and female mice (Balb/c or C57BL/6), 2–4 months old, were used and strain, sex and number of animals are indicated in each of the figure legends or in the results. Inflammation was induced by injecting 20 µl of 1:3 complete Freund’s adjuvant (CFA; Sigma, St. Louis, MO, USA) solution in 0.9% saline into the submandibular skin of mice. Controls were injected with 20 µl of saline alone. Both, CFA and saline were injected into the midline of the submandibular skin (corresponding to the human chin), which is innervated by bilateral mandibular branches of the trigeminal ganglia.
The gap junction blocker carbenoxolone (CBX; 10, 25, 50 or 100 mg/kg of body weight in 0.9% saline, volume 50–200 µl; Sigma, St. Louis, MO, USA) was injected intraperitoneally (i.p.) at a single time, 1, 3, 4 or 5 weeks after CFA administration. Controls were injected i.p. with equal volumes of 0.9% saline. CBX or saline (i.p.) injections were performed 1 h prior to the behavioral testing.
Assessment of Inflammation
Inflammation in the submandibular skin of mice was assessed before subcutaneous CFA or saline injection (Pre) and at 1–5 weeks after CFA injection. Mice were sacrificed by CO2 asphyxiation, the submandibular skin was removed and fixed overnight in 4% paraformaldehyde in PBS, pH 7.4 at 4°C. The skin was kept in 70% ethanol until paraffin embedding. Skin inflammation was characterized by hematoxylin-eosin (H&E) staining of 5 µm-thick sections and revealed all types of inflammatory cells. Every 4th section was stained with H&E for morphological analysis, and a total of 6–10 sections from each skin tissue were examined by microscopy to detect the inflamed area. Measurements were performed by investigators blind with respect to test conditions. Data are expressed as the number of total white blood cells in a 1 mm2 area averaged over 6 sections.
Tactile Sensitivity Testing
Either the submandibular skin or the plantar surface of the hind paw (as a control) was stimulated with von Frey filaments (Stoelting, Wood Dale, IL, USA) to determine tactile sensitivity. As CFA and saline were injected into the middle of the submandibular skin, which is innervated equally by both trigeminal ganglia, contralateral stimulation could not provide a control; instead the hind paw was chosen to evaluate tactile sensitivity in an un-injected area. Ascending force intensities of von Frey filaments were applied to investigate tactile sensitivity [1]. Each filament is designed to produce a given force (in g) precisely upon buckling. Minimum force to evoke a withdrawal response and the tactile threshold were determined by applying each filament 10 times with 5–30 s random intervals to the submandibular skin in all subjects first. Tactile threshold of the paw was determined subsequently, approximately 1 h after testing the submandibular skin. A response to a given stimulus was defined as rapid withdrawal of the head or hind paw when stimuli were applied and the number of responses to each stimulus intensity was determined. Tactile threshold was defined as a withdrawal response in 8 of the 10 trials to a given stimulus intensity. A higher number of responses across stimulus intensities indicates a lower tactile threshold and is interpreted as increased tactile sensitivity.
The mice were placed on a wire grid platform with 0.4 cm square grids through which von Frey filaments were inserted from below, and applied to the surface of the submandibular skin or the hind paws with enough force so that the filaments buckled. Applying the filaments from underneath enabled us to stimulate the submandibular skin without touching the whiskers, and to the plantar surface of the paw. A wire top (wire mesh desktop organizer cup; Staples) was placed over the animal with 3–5 cm clearance above the head and 3–5 cm clearance around the body to restrict the animal's movement sufficiently to permit accurate placement of the filaments (Supplemental Fig. 2). This did not restrain the mice or interfere with head or paw withdrawal responses. The animals were allowed to acclimatize to this environment for 20–30 min prior to testing. The filament was applied only when the mouse was stationary, standing on all paws and holding its head straight. Tactile responses were assessed before any injection (Pre) and on 1–7 weeks post injection of either CFA or saline. In all behavioral tests, testers were blind to the condition of the subjects. A withdrawal response could not be reliably elicited or scored in 4 of the approximately 120 total subjects during baseline testing; these mice were therefore removed from the study.
Experimental Design
Influence of Strain and Sex on Tactile Sensitivity
In our initial study, the number of responses across the entire range of stimulus intensities and the mean baseline threshold of each strain (Balb/c or C57BL/6) and sex was established before subcutaneous CFA or saline injection. Data, illustrated as the number of responses across the entire range of stimulus intensities, were analyzed in a repeated measures ANOVA (JMP, SAS Cary, NC). Thresholds were analyzed in a 2-way ANOVA. Subsequently, CFA or saline was injected once into the submandibular skin of the mice and tactile sensitivity was tested weekly or bi-weekly for a total of 7 weeks. Threshold data in these studies were analyzed using a mixed linear model with random (within) and fixed (between) factors. This design was chosen because for numerous reasons not all animals completed the entire 7-week time course and such results with missing data is not most appropriately analyzed in a repeated measures model. The mixed design preserves the number of subjects analyzed at multiple time points.
Reliability
Cohort effects on tactile sensitivity were evaluated in three sets of experiments performed by three different testers. Effects of CFA on tactile threshold one week post injection were compared to baseline levels in repeated measures ANOVA tests.
Alteration of Hypersensitivity by Carbenoxolone
As neither sex nor strain significantly interacted with the levels of CFA-induced tactile hypersensitivity post injection (see supplementary data), the effects of a single injection of CBX (100 mg/kg of body weight, i.p.) were investigated at 1, 3, 4 or 5 weeks after CFA injection in only male C57BL/6 mice. In all the studies, CFA-induced tactile hypersensitivity was compared in mice following injection of CBX or saline at a single time point. The threshold before injection (Pre) and the threshold of saline injected controls did not differ from each other, and were combined and designated as CBX- to contrast with CBX+ (CBX injected) mice. In addition, at the 4-week time point, we included a full 2-way design with a total of 4 treatment groups (+ or − CFA and + or − CBX). In these studies, effects of CFA and CBX were compared in 2-way ANOVAs with post hoc student’s t-tests.
Functional Observation Battery and Open Field Analysis
Following determination of tactile threshold, animals underwent testing in a functional observation battery (FOB) based on the commonly used Primary Screen and SHIRPA Protocols [21, 22]. Briefly, this is a standard high-throughput but quantitative assessment of behaviors in a comprehensive range of functional domains, including activity and arousal, reflexes and autonomic function, sensory and motor function. The behavioral assessments included locomotor activity and exploration in the open field (grid cross and number of rears), the corneal blink response, toe and tail pinch, Preyer reflex, pelvic and tail elevation, number of blinks in 1 min, number of bouts of facial grooming in the total test period (10 min), and orientation response to whisker stimulation and whisker mobility. Mice were transferred from the home cage to a clean rat cage, where observations began with transfer arousal (latency to move one full body length after transfer). Immediately thereafter, number of grid crosses and rears were counted for 1 min. For the other measures, ordinal scores were recorded, such that 0 was considered normal by a trained observer and negative numbers indicated hypo-reactivity and positive numbers indicated hyper-reactivity and ranged from −3 to +3. In the case of animals injected with CBX, we also performed a full open field analysis (6 min total test) analyzed with Viewer automated tracking software (Biobserve, Bonn, Germany).
Analysis and Statistics
For behavioral studies, mice were tested in a mixed effects design that offers several advantages regarding the power of the statistics and interpretation. Where indicated, comparisons are made to controls subjects (saline injected) and to the subject’s own baseline (pre-CFA) injections. In this way we could test the subjects longitudinally so that we could assess the chronic nature of the responses in CFA treated mice compared to their own baseline in addition to comparing this group to controls, and thus assess the influence both between and within subject factors. Tactile threshold data of CFA-injected subjects were compared to either their own baseline or to controls using a mixed linear design with both random (within subjects) and fixed (between subjects) factors (JMP, SAS Cary, NC). This design was chosen instead of the more commonly used repeated measures to account for the loss of some subjects at several time points (e.g. for histology). This design was also used to analyze the number of responses to each stimulus intensity over time in order to properly account for the 2 repeated measure (time and stimulus intensity).
Comparisons were also made longitudinally between saline treated controls and CFA injected mice using a repeated measures analysis in a separate cohort of mice.
In some cases, mice were injected with CBX. These data were also analyzed compared to either baseline (pre-CBX) at all time points. To ensure that CBX had no effect on tactile threshold of saline injected subject, CBX injections were compared in both CFA treated mice and saline treated controls at one time point.
RESULTS
Time Course of Inflammation in the Submandibular Skin Following CFA Injection
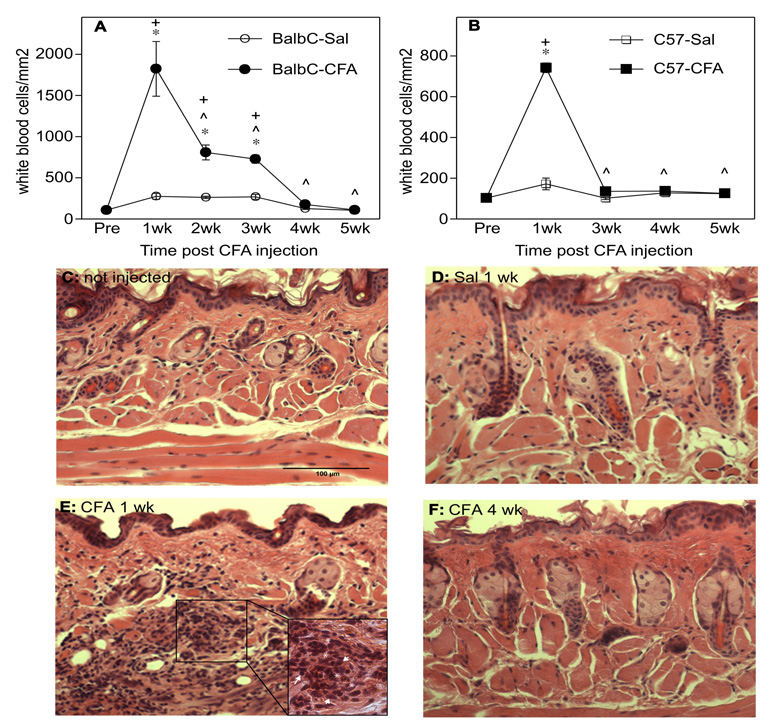
Inflammation can induce persistent neuronal activity in sensory ganglia, which is one putative basis of tactile hypersensitivity and pain. In our model of orofacial pain, inflammation was induced by the injection of complete Freund’s adjuvant (CFA) into the submandibular skin of Balb/c and C57BL/6 mice and total white blood cells were quantified over a 5-week time course (Fig. 1A–F). 1 week post injection all CFA-injected mice showed marked inflammatory cell infiltration in the submandibular skin (saline vs. CFA, p<0.005), which was mainly comprised by polymorphic neutrophils and macrophages. At later time points (2–3 wks) inflammation was significantly reduced (CFA 1 week vs. CFA 3 weeks, p<0.05), though it was still elevated compared to controls (saline vs. CFA, p<0.005) with lymphocytes present but fewer neutrophils and macrophages. Inflammation subsided completely by 4–5 weeks (Fig. 1A and B). It is noteworthy that significantly more inflammatory cells infiltrated the submandibular skin of Balb/c than of C57BL/6 mice 1 week after CFA injection (p<0.001). There were significant, but minimal, inflammatory changes in response to saline injection. These data show that CFA injection caused a localized and transitory inflammation in the submandibular region in both mouse strains.
Fig. (1).
The time course of inflammation assessed as density of white blood cells in H&E-stained sections before (Pre) and for 5 weeks (wk) after CFA injection (A). In Balb/c mice, inflammation peaked at 1 week after CFA injection, was significantly reduced 2–3 weeks after CFA injection, and was completely resolved 4–5 weeks after CFA injection when compared to either Pre injection values or compared to saline (Sal) injected controls at the same time points. (B) In C57BL/6 mice inflammation also peaked at 1 week, but was significantly resolved by 3 weeks. * indicates significant differences from Pre. ^ indicates significant differences from CFA 1 week. + indicates significant differences from saline at a given time point. Panels C–F illustrate representative staining from non-injected Balb/c mice (C), staining 1 week post-Sal injection (D), 1 week post-CFA injection (E) and 4 weeks post-CFA injection in Balb/c mice (F). Insert in E shows polymorphonuclear neutrophils (*), macrophages (arrowheads) and lymphocytes (arrow) infiltrating the submandibular skin. Scale bar=100 µm
Effects of Strain and Sex on Baseline Tactile Sensitivity
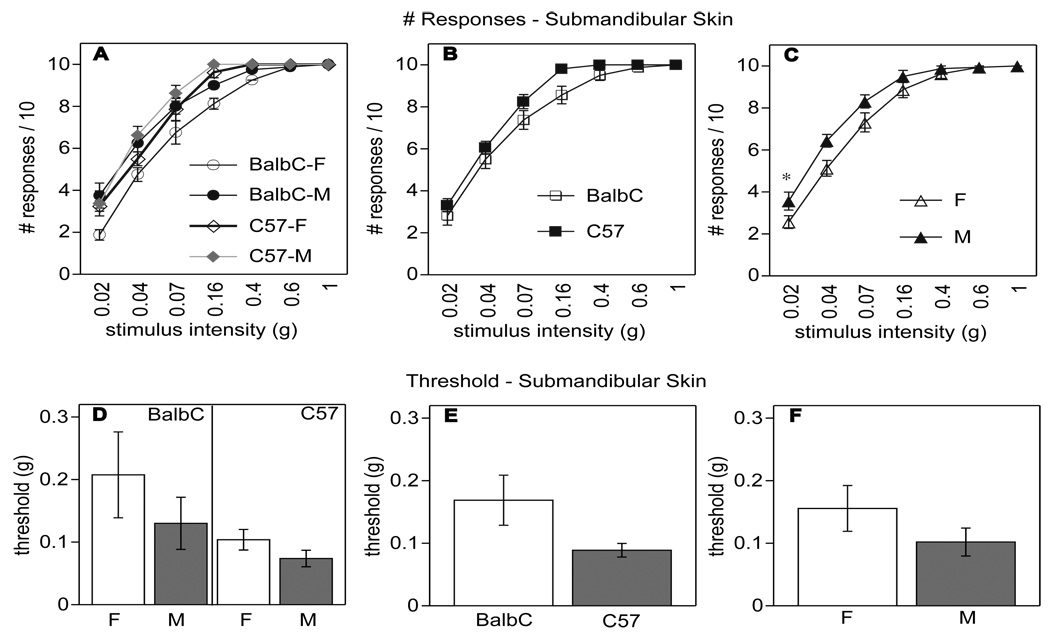
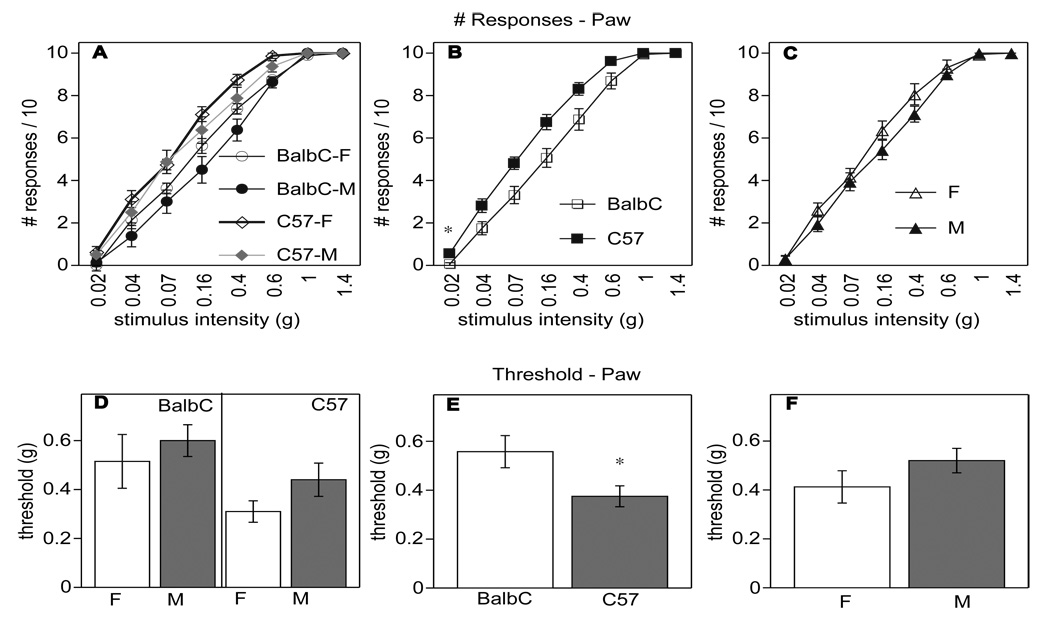
Behavioral differences in sensitivity to tactile stimulation in the submandibular skin and in the hind paw of Balb/c and C57BL/6 mice of either sex were assessed using von Frey filaments. We analyzed the number of responses across all stimulus intensities in the submandibular skin (Fig. 2A–C) and in the hind paw (as a control, Fig. 3A–C), and based on these data we established baseline (Pre) values of the tactile threshold, defined as the stimulus level resulting in 8/10 withdrawal responses (Fig. 2D–F for submandibular skin, Fig. 3D–F for hind paw).
Fig. (2).
Influence of strain and sex on baseline tactile sensitivity in the submandibular skin assessed with von Frey filaments: n=8 in each condition for this and the following graph. Number (#) of withdrawal responses out of 10 stimulations across all stimulus intensities in Balb/c and C57BL/6 mice are shown in panels A–C. Strain did not influence the number of responses in the submandibular skin (B), but sex affected this measure significantly (C: p<0.04). Tactile thresholds for submandibular skin in Balb/c and C57BL/6 mice of both sexes are shown in panels D–F as the stimulus intensity resulting in 8/10 withdrawal responses. There were no significant effects of strain (E) or sex (F) on baseline tactile threshold in the submandibular skin. There were no interactions between sex and strain.
Fig. (3).
Influence of strain and sex on baseline (before CFA or saline injection) tactile sensitivity in the hind paw assessed with von Frey filaments. Number (#) of withdrawal responses is shown in panels A–C. Strain (B) significantly influenced the number of responses evoked in the paw (p<0.005), but there was no significant effect of sex (C). Tactile threshold for paw is shown in panels D–F. There was also a significant effect of strain on the paw tactile threshold (E, p<0.03), but no main effect of sex (F). There were no interactions between sex and strain.
Although it appeared that C57BL/6 mice had a higher tactile sensitivity (lower threshold) than Balb/c mice, the main effect of strain did not reach significance in the number of responses across stimulus intensities or in baseline tactile threshold in the submandibular skin (Fig. 2B and E; p<0.06 in each analysis; n=8 per group). However, in the paw, C57BL/6 mice exhibited a higher baseline tactile sensitivity than Balb/c mice (Fig. 3E: significant effect of strain p<0.005 on threshold and Fig. 3B, significant effect of strain on number of responses across stimulus intensities p<0.03).
Female mice of both strains showed significantly lower number of responses across stimulus intensities (Fig. 2C; p<0.04) in the submandibular skin; however, there was no difference in tactile threshold between sexes (Fig. 2F, no significant effect of sex). Moreover, no significant effects of sex were obtained in the paw on either measure (Fig. 3C and F). Thus, tactile sensitivity in the submandibular skin was not substantially influenced by strain, nor did sex prove to have a major influence.
Time Course of Tactile Hypersensitivity Following CFA-Induced Transitory Inflammation
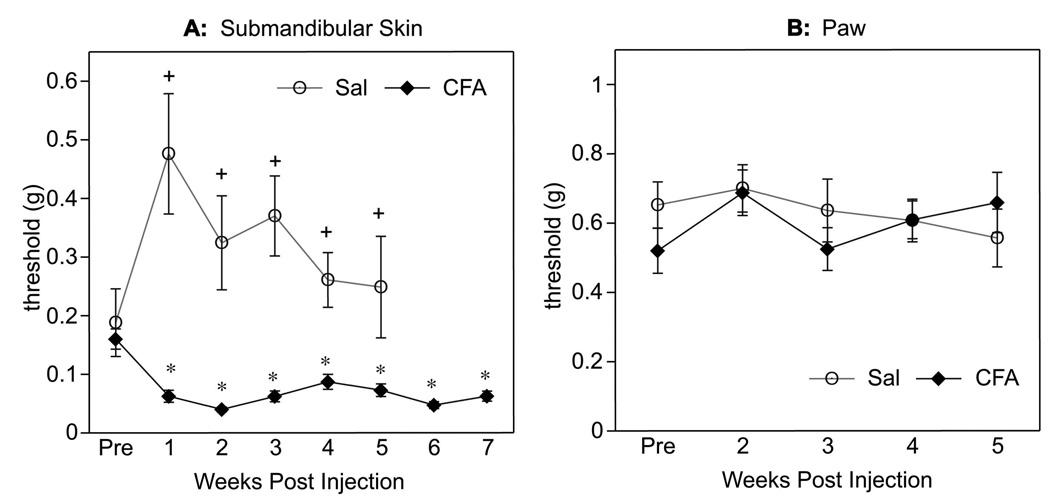
Following a single injection of CFA or saline under the submandibular skin, the tactile sensitivity of Balb/c and C57BL/6 mice of both sexes was tested every 1–2 weeks for 7 weeks (Fig. 4). One week post injection, tactile threshold in the submandibular skin of CFA injected mice was significantly lower compared both to baseline thresholds (before CFA injection, Pre) and to saline-injected controls (Fig. 4A) demonstrating CFA-induced tactile hypersensitivity. This tactile hypersensitivity persisted for the entire testing period, illustrated as a significant decrease of the tactile threshold in CFA-treated mice persisting up to 5 weeks compared to saline-injected controls (p<0.001) and up to 7 weeks compared to baseline (Pre) thresholds (p<0.007). However, the CFA injection into the submandibular skin had no effect on tactile sensitivity in the hind paw at any time point (Fig. 4B), indicating that pain behavior was restricted to the injection site. This marked and consistent CFA-induced hypersensitivity of the submandibular skin was evident during acute inflammation and persisted long after inflammation resolved (Fig. 1).
Fig. (4).
Tactile thresholds in the submandibular skin (A) and paw (B) after CFA or saline injection into the submandibular skin over 7 weeks. Data from both strains are combined. CFA reduced tactile thresholds in the submandibular skin for up to 7 weeks, but had no effect on paw tactile thresholds at any time point. * indicates significant differences between Pre and post CFA injections (p<0.007). + indicates significant differences between CFA- and saline-injected mice (p<0.001). Samples sizes were: n=16–28 for saline (1–5 weeks); n=68 for CFA (Pre and 1 week) and n=20–44 for CFA (2–7 weeks).
It should be noted that despite the individual variability and potential strain and sex effects in the baseline tactile threshold, no effects of strain or sex on the tactile threshold of the submandibular skin were evident after CFA injection (see Supplemental Fig. 1A and B).
Despite the significant differences in submandibular skin inflammation and tactile sensitivity between CFA- and saline-injected animals, all animals appeared to be of normal general health and demonstrated no other behavioral changes as assessed in the open field test and by a functional observation battery (see Table 1 for representative data). This included similar activity and arousal levels, similar corneal and startle reflexes and similar responses to toe and tail pinch. Furthermore, responses to whisker stimulation were of similar magnitude and character and the animals did not display site-specific grooming.
Table 1.
Assessment of CFA Effects on Mouse Behavior Using a Functional Observation Battery. Locomotor Activity (Grid Cross), Exploration in the Open Field (Number of Rears), the Preyer Reflex, Animal Weight and Transfer Arousal were Quantitatively Assessed Over a Time Course of 6 Weeks After CFA or Saline Injection. There were no Significant Differences Between Groups at Any Time Point. n=6 Per Condition
| Activity - # Grid Crosses | ||||||
|---|---|---|---|---|---|---|
| Week | Saline | CFA | ||||
| Mean | SE | Mean | SE | |||
| Pre | 14.6 | ± | 4.4 | 11.4 | ± | 4.3 |
| 1 wk | 16.2 | ± | 2.0 | 17.8 | ± | 3.5 |
| 4 wk | 16.1 | ± | 5.4 | 17.4 | ± | 4.3 |
| 6 wk | 17.6 | ± | 2.8 | 15.8 | ± | 1.2 |
| Exploration - # Rears | ||||||
| Week | Saline | CFA | ||||
| Mean | SE | Mean | SE | |||
| Pre | 1.8 | ± | 0.6 | 1.6 | ± | 0.7 |
| 1 wk | 0.8 | ± | 0.3 | 1.5 | ± | 0.9 |
| 4 wk | 1.9 | ± | 0.9 | 1.4 | ± | 0.7 |
| 6 wk | 1.4 | ± | 0.6 | 0.8 | ± | 0.9 |
| Preyer Reflex | ||||||
| Week | Saline | CFA | ||||
| Mean | SE | Mean | SE | |||
| Pre | 1.9 | ± | 0.1 | 1.7 | ± | 0.2 |
| 1 wk | 1.8 | ± | 0.2 | 2 | ± | 0 |
| 4 wk | 1.6 | ± | 0.2 | 1.8 | ± | 0.2 |
| 6 wk | 1.8 | ± | 0.2 | 2 | ± | 0 |
| Weight (g) | ||||||
| Week | Saline | CFA | ||||
| Mean | SE | Mean | SE | |||
| Pre | 22.9 | ± | 0.3 | 23.2 | ± | 0.4 |
| 1 wk | 22.9 | ± | 0.2 | 22.7 | ± | 0.3 |
| 4 wk | 24.8 | ± | 0.1 | 25.3 | ± | 0.3 |
| 6 wk | 25.9 | ± | 0.2 | 27 | ± | 0.6 |
| Transfer Arousal (Sec) | ||||||
| Week | Saline | CFA | ||||
| Mean | SE | Mean | SE | |||
| Pre | 17.6 | ± | 2.9 | 19.0 | ± | 6.6 |
| 1 wk | 11.8 | ± | 2.4 | 11.3 | ± | 3.1 |
| 4 wk | 8.4 | ± | 2.9 | 8.2 | ± | 2.8 |
| 6 wk | 3.8 | ± | 0.2 | 4.0 | ± | 0.8 |
Reliability of Tactile Sensitivity Assessment and CFA Effects
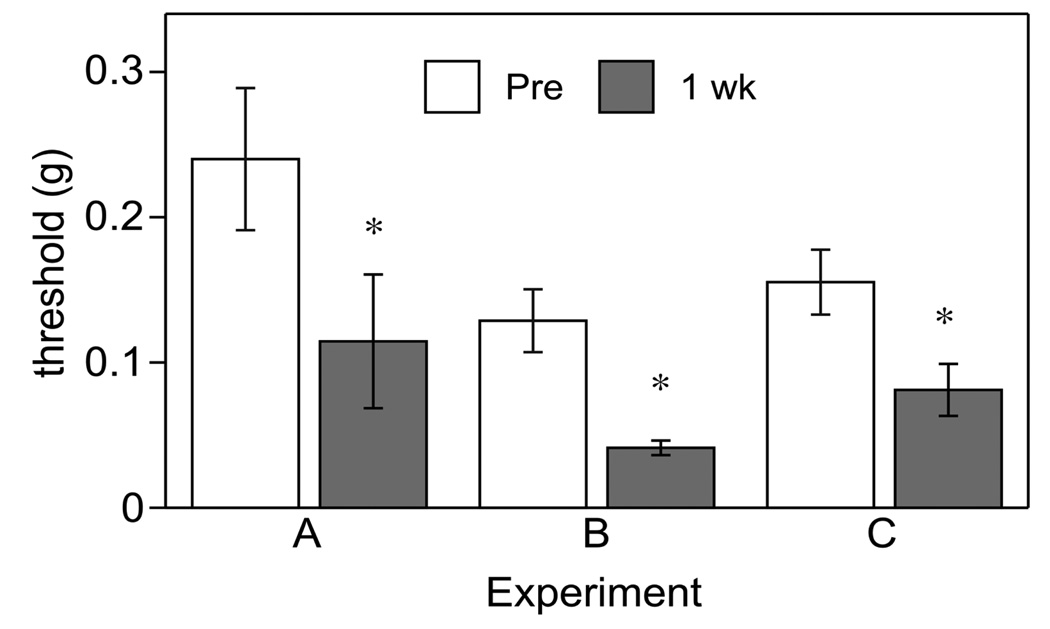
We performed three separate experiments, in which three different testers (A–C) evaluated tactile thresholds of the submandibular skin before (Pre) and 1 week after CFA injection in different cohorts of Balb/c (Fig. 5, Experiment A&B) or C57BL/6 mice (Fig. 5, Experiment B&C). All cohorts reliably demonstrated tactile hypersensitivity of the submandibular skin after CFA administration. Data from different experimenters in the same strain of mice were also reliable.
Fig. (5).
Assessment of tactile thresholds of the submandibular skin before (Pre) and 1 week after CFA injection in 3 experiments each performed by a different experimenter (A,B,C) using different cohorts demonstrated the reliability of the CFA-induced tactile sensitivity and internal validity. * indicates significant differences between tactile thresholds Pre and 1 week after CFA injection (p<0.05). Experimenter A tested male Balb/c mice, B used male and female Balb/c mice and C57BL/6 mice, C used only male C57BL/6 mice.
The Gap Junction Blocker Carbenoxolone (CBX) Reverses Tactile Hypersensitivity in CFA-Injected Mice
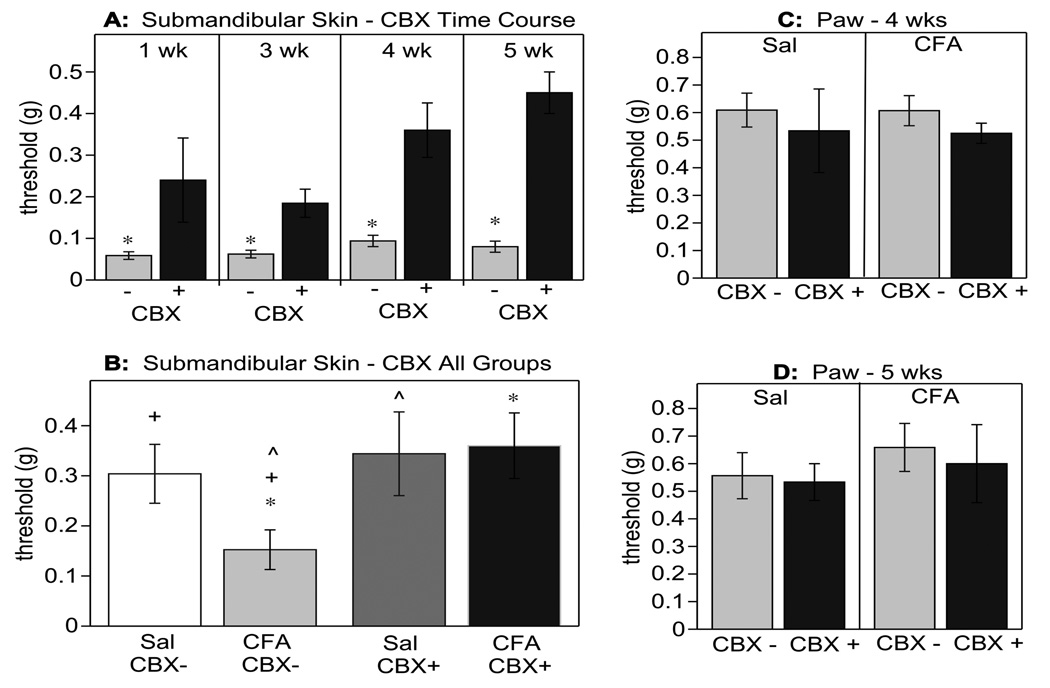
CBX significantly inhibited the CFA-induced tactile hypersensitivity in the submandibular skin (Fig. 6A and B, p<0.01 for CFA/CBX− vs. CFA/CBX+). A single i.p. injection of CBX or saline was administered at different time points to determine if the effectiveness of CBX was a function of inflammation status. The effect of CBX was evident when tested 1 week after CFA administration, at the peak of inflammation (Fig. 6A). CBX was also effective at 3, 4 or 5 weeks after initial CFA administration (Fig. 6A), when the inflammation was either substantially or totally resolved (Fig. 1), indicating that the analgesic action of CBX was independent of the extent of inflammation. A full factorial design was also performed comparing i.p. CBX (CBX+) and control (saline, CBX−) injections in CFA-treated or control (saline) subjects (Fig. 6B). Note that systemic injection of CBX had no effect on tactile threshold of the submandibular skin in control mice (Sal/CBX+, Fig. 6B). In addition, tactile thresholds in the paw were not affected by CBX when examined 4 weeks (Fig. 6C) or 5 weeks after CFA injection (Fig. 6D).
Fig. (6).
The gap junction blocker carbenoxolone (CBX; 100 mg/kg, i.p.) reversed CFA-induced tactile hypersensitivity. (A) CBX significantly increased tactile thresholds when injected once at 1,3,4 or 5 weeks after CFA administration (* p<0.01). Tactile sensitivity was always determined 1 h after i.p. injection of CBX or saline (control). (B) The significantly lower tactile threshold evident in CFA-treated mice (CFA/CBX−) compared to controls (Sal/CBX− and Sal/CBX+) was restored to control levels after CBX injection (CFA/CBX+; p<0.01 for all comparisons). * indicates significant differences between CFA/CBX− and CFA/CBX+. + indicates significant differences between Sal/CBX−and CFA/CBX−. ^ indicates significant differences between CFA/CBX− and Sal/CBX+. Note that CBX did not alter the threshold in control mice (Sal/CBX+ and Sal/CBX−). The paw threshold was not altered by CBX in saline or CFA treated mice 4 weeks (C) or 5 weeks (D) after CFA administration.
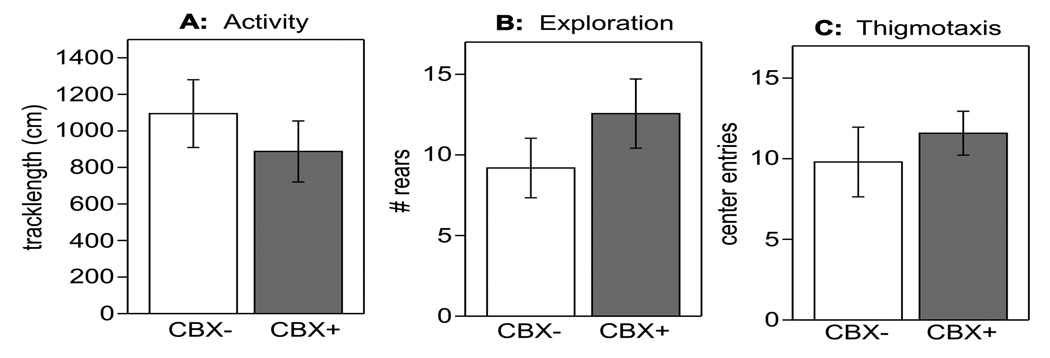
Thus, although CBX restored normal tactile thresholds after CFA administration in the submandibular skin it did not alter the tactile threshold of control mice or tactile threshold in the paw. Locomotor activity was also not affected by the systemic injection of CBX and thus was not likely to confound assessment of the withdrawal response. Furthermore, CBX did not influence behavior assessed in the open field test (Fig. 7), in which we investigated activity (Fig. 7A), exploration (Fig. 7B) and thigmotaxis (Fig. 7C) immediately after the evaluation of tactile sensitivity. Despite the normal behavior of all mice on the day of CBX injections and the 2 following days, 40–50% of 100 mg/kg CBX injected animals died within 3–5 days, whereas no mortality was seen in control animals.
Fig. (7).
A single i.p. injection of CBX (100 mg/kg) did not affect activity (total track length: A), exploration (number of rears: B) or thigmotaxis (anxiety-like behavior: C) in CFA treated subjects assessed in an open field test. Behavior of mice in the FOB and open field were immediately determined after tactile sensitivity tests. n=16 per condition.
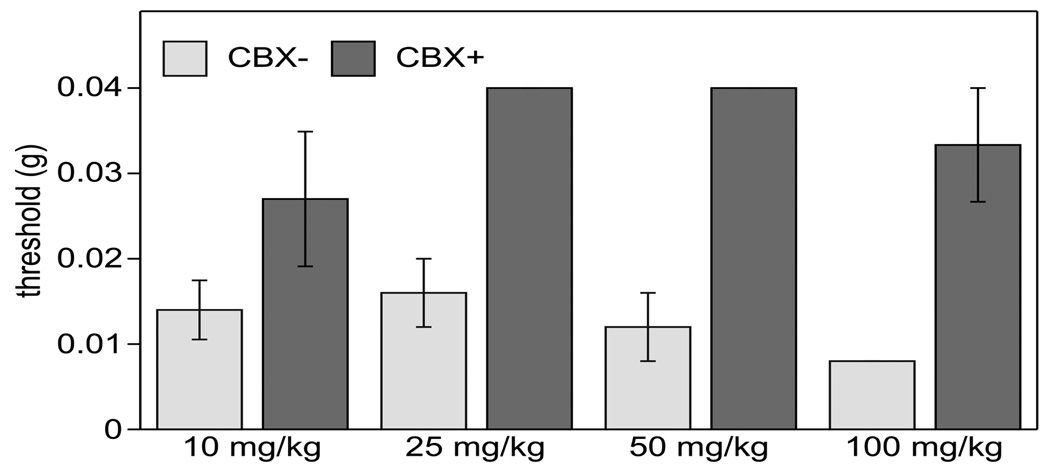
The administration of lower doses of CBX (10–50 mg/kg) was as effective in reducing tactile hypersensitivity as the high CBX dose (100 mg/kg), but did not result in animal mortality (Fig. 8).
Fig. (8).
Low doses of CBX (10, 25 and 50 mg/kg) are effective at reversing CFA-induced tactile hypersensitivity. Tactile sensitivity was determined 1 h after i.p. injection of CBX or saline (control). n=3–4 per group.
DISCUSSION
We here demonstrate that injection of CFA into the submandibular skin of mice results in tactile hypersensitivity that persists long after the resolution of inflammation. Furthermore, the gap junction blocker carbenoxolone (CBX) reversed the CFA-induced hypersensitivity. Other behaviors (paw threshold, voluntary activity, etc.) were unaffected by CFA treatment or by CBX, indicating the specific and localized characteristic of the pain, despite its chronic nature, and the focal nature of the effects of CBX on pain. Furthermore, there was no discernible tissue damage as assessed by H&E staining. Lastly, this peripheral locus is readily accessible to translational behavioral measures (unlike in joint models) and projects to a well-characterized ganglion and anatomical region. These characteristics allow an examination of the specific pain locus with regard to molecular and physiological changes and affords the opportunity to distinguish between peripheral and central pain mechanisms in prospective studies, as it is easy to excise the ganglion.
In our model, inflammatory processes may initiate a cascade of events very soon after CFA injection, evoking hypersensitivity that persisted after cessation of inflammation, therefore representing a model of post-inflammatory pain. Other studies investigating post-inflammatory pain exist [23, 24], but only few have examined orofacial pain [25, 26] or conducted a systematic and comprehensive analysis of behaviors analogous to those used for testing in humans. The ability to distinguish between the mechanisms that initiate and maintain chronic pain is one of the many advantages of this model. Further advantages include reliability, a robust and truly chronic behavioral response and behavioral measures that are highly translational [1–4]. Longitudinal measures and sensitive within subject analyses can be performed, increasing the statistical power. The general behavior of the mice is unaffected, and thus, though the behavioral manifestation of pain (greatly reduced tactile threshold) is robust and reliable, this response is specific and only evident upon stimulation.
Although it is clear that inflammation can engender chronic pain, several lines of evidence indicate that a continued inflammatory response is not required to maintain chronic pain [6, 24]. First, the time course of inflammation and the time course of the tactile hypersensitivity induced by CFA administration diverge significantly. Second, the extent of tactile hypersensitivity is independent of the extent of inflammation. Finally, CBX is equivalently efficacious regardless the inflammatory state. The inflammatory response induced by CFA peaked after 1 week, resolved after 3 weeks and was completely abolished after 4 weeks as determined with H&E staining, which marks all inflammatory cells, including those that infiltrate the tissue at later stages [27]. In contrast, the tactile hypersensitivity remained stable for a 7-week duration. The extent of inflammation, which was lower in C57BL/6 than in Balb/c mice, also did not alter the CFA-induced tactile hypersensitivity in either strain. Strain differences in inflammatory cell infiltration have been previously reported [28]. Lastly, the gap junction blocker CBX was equally effective at reversing the CFA-induced tactile hypersensitivity when administered at the peak of inflammation, after significant resolution of inflammation or after complete absence of inflammation. Taken together, these data indicate that inflammation initiates rapid changes after injury or insult, such as altered gap junction expression and intercellular coupling [29, 30], which are maintained long after inflammatory processes have ceased.
The robust effects of CBX are consistent with a role for gap junction-mediated coupling in any of several mechanisms thought to underlie chronic pain, including neuronal hyperactivity, calcium waves and increase in coupling of satellite glia cells [5, 14, 31–33]. Previous reports support this hypothesis, as injury and/or inflammation result in increased gap junction expression [16, 34, 35] and in gap junction-mediated coupling in sensory ganglia that innervate the site of injury [33, 36, 37] that are concomitant with increased neuronal firing and tactile hypersensitivity in numerous pain models [5, 14, 31]. The development of chronic pain has been associated with increased excitability of sensory neurons [31] and changes in intercellular signaling between neurons and/or glia cells, which is partly mediated by gap junctions [16]. Furthermore, other groups have reported analgesic effects of CBX [19, 20, 38] in rodent models of acute and chronic pain. Finally, preventing injury-induced gap junction expression via RNA interference reduces pain [16]. However these studies have generally focused on relatively acute pain (1–2 weeks), whereas in the present study CBX was effective for up to 5 weeks after the initial insult.
Despite these data, it must be noted that CBX is neither selective with respect to its blockade of gap junctions formed of specific connexins [39, 40], nor selective for gap junction inhibition, as it directly affects voltage-gated Ca2+ channels [41], NMDA-evoked currents [42] and Pannexin1/2 hemichannels [43–45]. It is therefore possible that CBX may exert its analgesic effect via any of these mechanisms, although these several possible loci of action are not mutually exclusive. In addition we administered CBX systemically and we therefore have no direct evidence that CBX acted selectively on the primary peripheral targets - the trigeminal ganglia. It is not clear if CBX can cross the blood-brain barrier, as contradictory evidence has been reported [46–48]. However, there is evidence that CBX reduces neuronal excitability in isolated dorsal root ganglia from a murine inflammatory pain model [14] and thus at least does not require a central mode of action, although CNS effects are not precluded. However, in our model, CBX did not alter paw threshold, activity, exploration or anxiety-like behavior, thus it is plausible that at the dose administered the effects of CBX are restricted to reduction of mechanisms causing tactile hypersensitivity at the locus of pain induction in the trigeminal ganglion.
Surprisingly, we found a high mortality rate (about 40%) in mice 3 to 5 days after the injection of 100 mg/kg CBX, although no mortality was observed when lower doses (CBX 10–50 mg/kg) were administered. Few other studies reported higher mortality in animals 72 hours after repeated CBX injections [18, 49]. The lack of reports on long-term CBX actions might be due to the fact that in most of these studies animals were killed shortly after CBX injection. Unlike these studies, we applied only a single systemic injection of CBX, and no acute effects of CBX were apparent 24–72 hours afterwards. The half life of CBX in humans after oral intake is 13–16 hours [50], indicating that CBX levels are highest within a few hours after injection. No CBX side effects appeared during this time in our model as assessed by tactile sensitivity in the paw and open field test. Although CBX has already been used as an FDA approved gastric ulcer treatment for many years [51], this nevertheless does not exclude the possibility that CBX blocks gap junctions in critical organs, such as heart, liver and kidney, which might result in delayed mortality. The systemic administration of CBX in humans revealed no or very rare adverse CNS effects and no major side effects were seen [52, 53]. This may indicate differences in responses of mice and humans to CBX, or different pharmacokinetics based on the route of administration and whether there is a first pass through the liver. Despite this caution, gap junction blockers might be putative novel agents in pain treatment, as we found CBX to be effective also in lower doses without resulting in mortality. Currently, highly selective gap junction blockers are not available. However, there is a major effort to develop blockers that will be selective for specific connexins, and some progress has already taken place in this direction [54, 55]. It is thus anticipated that novel blockers will eventually be available for therapeutic use. Trigeminal ganglia are favorable targets for analgesics as, unlike most parts of the central nervous system, they are accessible to substances in the circulation [32] and to direct injection.
In summary, we presented here a new model of post-inflammatory orofacial pain in mice. We demonstrated that injection of CFA administered under the submandibular skin resulted in a transient inflammatory response and persistent behavioral hypersensitivity, which, despite the cessation of inflammation 3 weeks post CFA injection, was maintained over a period of 7 weeks, suggesting neuronal and/or glial plasticity as a major component of the ongoing hypersensitivity. In recent years, it has been proposed that gap junction-mediated coupling in central as well as in peripheral sites contribute to pathologic pain. Our data also highlight the positive action of the clinically relevant molecule CBX to reduce hypersensitivity during both the acute and chronic phase of orofacial pain.
ACKNOWLEDGEMENTS
We thank the Histotechnology and Comparative Pathology Core Facility for training and advice. This work was supported by NS041282 to D.C.S., BSF (20073/1) to M.H., M.G. and D.C.S. and ISF (212/08) to M.H.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Web site along with the published article.
This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/-licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
REFERENCES
- 1.Lambert GA, Mallos G, Zagami AS. Von Frey's hairs--a review of their technology and use--a novel automated von Frey device for improved testing for hyperalgesia. J Neurosci Methods. 2009;177:420–426. doi: 10.1016/j.jneumeth.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Mulder GB, Pritchett K. Rodent analgesiometry: the hot plate, tail flick and Von Frey hairs. Contemp Top Lab Anim Sci. 2004;43:54–55. [PubMed] [Google Scholar]
- 3.Keizer D, van Wijhe M, Post WJ, Wierda JM. Quantifying allodynia in patients suffering from unilateral neuropathic pain using von frey monofilaments. Clin J Pain. 2007;23:85–90. doi: 10.1097/01.ajp.0000210950.01503.72. [DOI] [PubMed] [Google Scholar]
- 4.Wolff B. Methods of testing pain mechanism in normal man. In: Wall PD, Melzack R, editors. Textbook of pain. Edinburgh: Churchill Livingstone; 1984. pp. 186–194. [Google Scholar]
- 5.Suadicani SO, Cherkas PS, Zuckerman J, Smith DN, Spray DC, Hanani M. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol. 2010;6(1):43–51. doi: 10.1017/S1740925X09990408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson AW, Medhurst SJ, Dixon CI, et al. An animal model of chronic inflammatory pain: pharmacological and temporal differentiation from acute models. Eur J Pain. 2006;10:537–549. doi: 10.1016/j.ejpain.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Gremillion HA. The prevalence and etiology of temporomandibular disorders and orofacial pain. Tex Dent J. 2000;117:30–39. [PubMed] [Google Scholar]
- 8.Graff-Radford SB. Temporomandibular disorders and other causes of facial pain. Curr Pain Headache Rep. 2007;11:75–81. doi: 10.1007/s11916-007-0026-6. [DOI] [PubMed] [Google Scholar]
- 9.Svensson P. Muscle pain in the head: overlap between temporomandibular disorders and tension-type headaches. Curr Opin Neurol. 2007;20:320–325. doi: 10.1097/WCO.0b013e328136c1f9. [DOI] [PubMed] [Google Scholar]
- 10.Maixner W, Fillingim R, Booker D, Sigurdsson A. Sensitivity of patients with painful temporomandibular disorders to experimentally evoked pain. Pain. 1995;63:341–351. doi: 10.1016/0304-3959(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 11.Guo W, Wang H, Watanabe M, et al. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27:6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benoliel R, Wilensky A, Tal M, Eliav E. Application of a pro-inflammatory agent to the orbital portion of the rat infraorbital nerve induces changes indicative of ongoing trigeminal pain. Pain. 2002;99:567–578. doi: 10.1016/S0304-3959(02)00272-5. [DOI] [PubMed] [Google Scholar]
- 13.DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- 14.Huang TY, Belzer V, Hanani M. Gap junctions in dorsal root ganglia: possible contribution to visceral pain. Eur J Pain. 2010;14:49. doi: 10.1016/j.ejpain.2009.02.005. 1–11. [DOI] [PubMed] [Google Scholar]
- 15.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohara PT, Vit JP, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J Neurophysiol. 2008;100:3064–3073. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledda M, Blum E, De Palo S, Hanani M. Augmentation in gap junction-mediated cell coupling in dorsal root ganglia following sciatic nerve neuritis in the mouse. Neuroscience. 2009;164:1538–1545. doi: 10.1016/j.neuroscience.2009.09.038. [DOI] [PubMed] [Google Scholar]
- 18.Ayer R, Chen W, Sugawara T, Suzuki H, Zhang JH. Role of gap junctions in early brain injury following subarachnoid hemorrhage. Brain Res. 2010;1315:150–158. doi: 10.1016/j.brainres.2009.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan L, Yuan H, Duan L, et al. Blocking the glial function suppresses subcutaneous formalin-induced nociceptive behavior in the rat. Neurosci Res. 2007;57:112–119. doi: 10.1016/j.neures.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Dublin P, Hanani M. Satellite glial cells in sensory ganglia: their possible contribution to inflammatory pain. Brain Behav Immun. 2007;21:592–598. doi: 10.1016/j.bbi.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Irwin S. Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse. Psychopharmacologia. 1968;13:222–257. doi: 10.1007/BF00401402. [DOI] [PubMed] [Google Scholar]
- 22.Rogers DC, Peters J, Martin JE, et al. SHIRPA, a protocol for behavioral assessment: validation for longitudinal study of neurological dysfunction in mice. Neurosci Lett. 2001;306:89–92. doi: 10.1016/s0304-3940(01)01885-7. [DOI] [PubMed] [Google Scholar]
- 23.Traub RJ, Tang B, Ji Y, Pandya S, Yfantis H, Sun Y. A rat model of chronic postinflammatory visceral pain induced by deoxycholic acid. Gastroenterology. 2008;135:2075–2083. doi: 10.1053/j.gastro.2008.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eijkelkamp N, Heijnen CJ, Willemen HL, et al. GRK2: a novel cell-specific regulator of severity and duration of inflammatory pain. J Neurosci. 2010;30:2138–2149. doi: 10.1523/JNEUROSCI.5752-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chudler EH, Anderson LC. Behavioral and electrophysiological consequences of deafferentation following chronic constriction of the infraorbital nerve in adult rats. Arch Oral Biol. 2002;47:165–172. doi: 10.1016/s0003-9969(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 26.Anderson LC, Vakoula A, Veinote R. Inflammatory hypersensitivity in a rat model of trigeminal neuropathic pain. Arch Oral Biol. 2003;48:161–169. doi: 10.1016/s0003-9969(02)00203-0. [DOI] [PubMed] [Google Scholar]
- 27.Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol. 2007;81:775–785. doi: 10.1189/jlb.0506356. [DOI] [PubMed] [Google Scholar]
- 28.Hoover-Plow JL, Gong Y, Shchurin A, Busuttil SJ, Schneeman TA, Hart E. Strain and model dependent differences in inflammatory cell recruitment in mice. Inflamm Res. 2008;57:457–463. doi: 10.1007/s00011-008-7062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chandross KJ. Nerve injury and inflammatory cytokines modulate gap junctions in the peripheral nervous system. Glia. 1998;24:21–31. doi: 10.1002/(sici)1098-1136(199809)24:1<21::aid-glia3>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Rouach N, Avignone E, Meme W, et al. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 31.Cherkas PS, Huang TY, Pannicke T, Tal M, Reichenbach A, Hanani M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain. 2004;110:290–298. doi: 10.1016/j.pain.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Hanani M. Satellite glial cells in sensory ganglia: from form to function. Brain Res Brain Res Rev. 2005;48:457–476. doi: 10.1016/j.brainresrev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Huang TY, Cherkas PS, Rosenthal DW, Hanani M. Dye coupling among satellite glial cells in mammalian dorsal root ganglia. Brain Res. 2005;1036:42–49. doi: 10.1016/j.brainres.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 34.Pannese E, Ledda M, Cherkas PS, Huang TY, Hanani M. Satellite cell reactions to axon injury of sensory ganglion neurons: increase in number of gap junctions and formation of bridges connecting previously separate perineuronal sheaths. Anat Embryol (Berl) 2003;206:337–347. doi: 10.1007/s00429-002-0301-6. [DOI] [PubMed] [Google Scholar]
- 35.Chang Q, Balice-Gordon RJ. Gap junctional communication among developing and injured motor neurons. Brain Res Brain Res Rev. 2000;32:242–249. doi: 10.1016/s0165-0173(99)00085-5. [DOI] [PubMed] [Google Scholar]
- 36.Hanani M, Huang TY, Cherkas PS, Ledda M, Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–283. doi: 10.1016/s0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- 37.Huang TY, Hanani M. Morphological and electrophysiological changes in mouse dorsal root ganglia after partial colonic obstruction. Am J Physiol Gastrointest Liver Physiol. 2005;289:G670–G678. doi: 10.1152/ajpgi.00028.2005. [DOI] [PubMed] [Google Scholar]
- 38.Spataro LE, Sloane EM, Milligan ED, et al. Spinal gap junctions: potential involvement in pain facilitation. J Pain. 2004;5:392–405. doi: 10.1016/j.jpain.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Davidson JS, Baumgarten IM. Glycyrrhetinic acid derivatives: a novel class of inhibitors of gap-junctional intercellular communication. Structure-activity relationships. J Pharmacol Exp Ther. 1988;246:1104–1107. [PubMed] [Google Scholar]
- 40.Spray DC, Rozental R, Srinivas M. Prospects for rational development of pharmacological gap junction channel blockers. Curr Drug Targets. 2002;3:455–464. doi: 10.2174/1389450023347353. [DOI] [PubMed] [Google Scholar]
- 41.Vessey JP, Lalonde MR, Mizan HA, Welch NC, Kelly ME, Barnes S. Carbenoxolone inhibition of voltage-gated Ca channels and synaptic transmission in the retina. J Neurophysiol. 2004;92:1252–1256. doi: 10.1152/jn.00148.2004. [DOI] [PubMed] [Google Scholar]
- 42.Chepkova AN, Sergeeva OA, Haas HL. Carbenoxolone impairs LTP and blocks NMDA receptors in murine hippocampus. Neuropharmacology. 2008;55:139–147. doi: 10.1016/j.neuropharm.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. doi: 10.1111/j.1471-4159.2004.02947.x. [DOI] [PubMed] [Google Scholar]
- 44.Iglesias R, Locovei S, Roque A, et al. P2X7 receptor-Pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol. 2008;295:C752–C760. doi: 10.1152/ajpcell.00228.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Locovei S, Bao L, Dahl G. Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci USA. 2006;103:7655–7659. doi: 10.1073/pnas.0601037103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leshchenko Y, Likhodii S, Yue W, Burnham WM, Perez Velazquez JL. Carbenoxolone does not cross the blood brain barrier: an HPLC study. BMC Neurosci. 2006;7:3. doi: 10.1186/1471-2202-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gareri P, Condorelli D, Belluardo N, et al. Antiabsence effects of carbenoxolone in two genetic animal models of absence epilepsy (WAG/Rij rats and lh/lh mice) Neuropharmacology. 2005;49:551–563. doi: 10.1016/j.neuropharm.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Gareri P, Condorelli D, Belluardo N, et al. Anticonvulsant effects of carbenoxolone in genetically epilepsy prone rats (GEPRs) Neuropharmacology. 2004;47:1205–1216. doi: 10.1016/j.neuropharm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 49.Manaenko A, Lekic T, Sozen T, Tsuchiyama R, Zhang JH, Tang J. Effect of gap junction inhibition on intracerebral hemorrhage-induced brain injury in mice. Neurol Res. 2009;31:173–178. doi: 10.1179/174313209X393591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baron JH, Gribble JN, Rhodes C, Wright PA. Serum carbenoxolone in patients with gastric and duodenal ulcer: Absorption, efficacy and side-effects. Gut. 1978;19:330–335. doi: 10.1136/gut.19.4.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turpie AG, Thomson TJ. Carbenoxolone sodium in the treatment of gastric ulcer with special reference to side-effects. Gut. 1965;6:591–594. doi: 10.1136/gut.6.6.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davies GJ, Rhodes J, Calcraft BJ. Complications of carbenoxolone therapy. Br Med J. 1974;3:400–402. doi: 10.1136/bmj.3.5927.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Montgomery RD. Side effects of carbenoxolone sodium: a study of ambulant therapy of gastric ulcer. Gut. 1967;8:148–150. doi: 10.1136/gut.8.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci USA. 2004;101:12364–12369. doi: 10.1073/pnas.0402044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Srinivas M, Spray DC. Closure of gap junction channels by arylaminobenzoates. Mol Pharmacol. 2003;63:1389–1397. doi: 10.1124/mol.63.6.1389. [DOI] [PubMed] [Google Scholar]