Summary
Background
Insight into the mechanisms of organ engraftment and acquired tolerance has made it possible to facilitate these mechanisms, by tailoring the timing and dosage of immunosuppression in accordance with two therapeutic principles: recipient pretreatment, and minimum use of post-transplant immunosuppression. We aimed to apply these principles in recipients of renal and extrarenal organ transplants.
Methods
82 patients awaiting kidney, liver, pancreas, or intestinal transplantation were pretreated with about 5 mg/kg of a broadly reacting rabbit antithymocyte globulin during several hours. Post-transplant immunosuppression was restricted to tacrolimus unless additional drugs were needed to treat breakthrough rejection. After 4 months, patients on tacrolimus monotherapy were considered for dose-spacing to every other day or longer intervals.
Findings
We frequently saw evidence of immune activation in graft biopsy samples, but unless this was associated with graft dysfunction or serious immune destruction, treatment usually was not intensified. Immunosuppression-related morbidity was virtually eliminated. 78 (95%) of 82 patients survived at 1 year and at 13–18 months. Graft survival was 73 (89%) of 82 at 1 year and 72 (88%) of 82 at 13–18 months. Of the 72 recipients with surviving grafts, 43 are on spaced doses of tacrolimus monotherapy: every other day (n=6), three times per week (11), twice per week (15), or once per week (11).
Interpretation
The striking ability to wean immunosuppression in these recipients indicates variable induction of tolerance. The simple therapeutic principles are neither drug-specific nor organ-specific. Systematic application of these principles should allow improvements in quality of life and long-term survival after organ transplantation.
Introduction
Early loss of organ allografts to acute rejection has been almost eliminated by use of combinations of potent immunosuppressive drugs. However, chronic rejection has remained an unresolved problem. Furthermore, maintenance immunosuppression has continued to cause late morbidity and mortality. The ideal solution would be to make recipients tolerant to donor tissues.
We have suggested that extended organ engraftment under conventional immunosuppression is, in fact, a manifestation of partial tolerance,1 3 and that this tolerance could be made more complete by observation of two therapeutic principles:4 recipient pretreatment; and the least possible use of post-transplant immunosuppression. We aimed to systematically apply these principles in recipients of organ transplants.
Methods
Participants and protocol
Between July, 2001, and November, 2001, we recruited patients awaiting transplantation of the kidney, liver, intestine, or whole pancreas for whom there was sufficient time for pretreatment before transplantation. We excluded those who had insufficient time for pretreatment.
The regimen of immunosuppression was submitted to the University of Pittsburgh institutional review board, which judged it to be within the boundaries of standard treatment. The protocol was then remanded to the Presbyterian University Hospital innovative practices committee and to the pharmacy and therapeutics committee, with approval by both. All patients provided standard informed consent. In addition, separate informed consent was obtained for studies of immune variables not routinely obtained in our conventional practice. Data integrity, and safety and efficacy monitoring, were assured by establishment of a formal review every week of all cases.
Procedures
The generic protocol (all organs) stated a need for pretreatment with an infusion of 5 mg/kg of a broadly reactive rabbit antithymocyte globulin (thymoglobulin; Sangstat, Menlo Park, CA, USA) over the several hours immediately preceding transplantation; we gave participants 1–2 g methylprednisolone concomitantly to prevent cytokine reactions. Twice-daily monotherapy with tacrolimus was begun the day after transplantation, with a target trough concentration of 10 µg/L. We added other agents (prednisone, sirolimus, muromonab-CD3) as needed for control of rejection, and for as brief a period as possible.
To encourage protocol adherence, we explained the treatment rationale to workers in the clinical services in formal educational sessions throughout the accrual of cases. Despite these efforts, violations of the therapeutic algorithm were not rare, especially in the pancreas and intestine subgroups. Principal violations consisted of either systematically obtaining high trough concentrations of tacrolimus or adding multiple drugs to tacrolimus during the early post-transplant period. In some cases, the monitoring committee promptly aborted escalation of immunosuppression, which was not possible in other cases because of opposition by the clinical team. Even when a protocol violation was thought by consensus to have taken place, no cases were eliminated from analysis.
Beginning at 4 months, patients who had been on tacrolimus monotherapy for the preceding 60 days were considered for weaning. After obtaining a satisfactory graft biopsy sample, we consolidated the twice-daily doses of tacrolimus to one daily dose for a few days or weeks. We then spaced the daily doses to every other day and subsequently to longer intervals in selected individuals. In patients whose spacing reached one dose per week, we did not advise drug discontinuance.
We terminated weaning if rejection was diagnosed on the basis of substantial deterioration of graft-specific function tests, and confirmed by biopsy samples showing an unacceptable amount of immune activation or destruction. If abnormalities were not promptly reversed by steroid bolus treatment, we resumed daily tacrolimus. If necessary, late rejections were treated by addition of short courses of other agents as needed, in the same way as for treatment of early post-transplant rejection. After re-establishing control, resumption of spaced weaning was considered. The intent throughout was to find the minimum amount of immunosuppression consistent with the avoidance of irreversible graft damage.
Immunological monitoring was not used to guide weaning. However, in kidney recipients with more than 1 year follow-up, we did in-vitro studies: mixed lymphocyte reactivity, cytotoxicity-mediated lysis, limiting dilution assay for donor-specific precursor cytolytic T cells, and the ELISPOT test for frequency of γ-interferon-producing cells (Mabtech, Cincinnati, OH, USA).
We obtained graft biopsy specimens for suspicion of rejection, or before weaning. In addition to conventional haematoxylin and eosin histopathology, we used special stains when indicated to study blood vessels (Verhoff van Gieson), quantify extent of interstitial fibrosis (Masson Trichrome), or visualise other points of interest. When the donor and recipient were of opposite sexes, we analysed lymphoid collections in the biopsy samples by in-situ hybridisation, with X and Y chromosome probes. We placed a few specimens in optimum cold-temperature compound (Miles Laboratory, Elkhart, IN, USA) and snap froze them for delineation of donor and recipient HLA phenotypes with immunocytochemical methods.
We coded biopsy findings into standardised organ-specific categories—eg, the Banff system for the kidney5 and the modified Banff system for the liver6 and other organs. In the kidney classification, the ascending scale of rejection is: 0 (none), BL (borderline), 1A (mild), 1B (mild), 2A (moderate), 2B (moderate), and 3 (severe).
We then developed a rejection profile for each of the first 12 months for the entire population of kidney recipients. Patients with no biopsy samples and no clinical evidence of rejection were deemed rejection-free. When we obtained biopsy specimens we gave them Banff grades on a scale of absent to severe rejection, using the worst score in a given month for graphic display if multiple biopsy procedures took place in that month. Similar analyses were done for the other kinds of organ recipients.
Statistical analysis
We expressed graft function and other variables as mean (SD). We compared relevant subgroups with analysis of variance and t tests. No patients were lost to follow-up during the 13–18 months of post-transplant observations. Because of unequal starting times (early and late July, 2001), maximum follow-up was 18 months for the intestine and pancreas recipients and 17 months for the liver and kidney recipients.
We delineated populations into four categories in accordance with treatment after transplantation that was deemed necessary by members of the clinical service. Category 1 consisted of patients in any given month who received no drug other than tacrolimus. Patients in category 2 had additional one or two boluses of methylprednisolone. In category 3, we added either three or more steroid boluses to baseline tacrolimus, or gave a short course of oral prednisone or sirolimus. Category 4 consisted of recipients who were on daily double-drug or triple-drug immunosuppression for at least half the stipulated month—eg, tacrolimus and sirolimus with or without prednisone or additional antibody treatment.
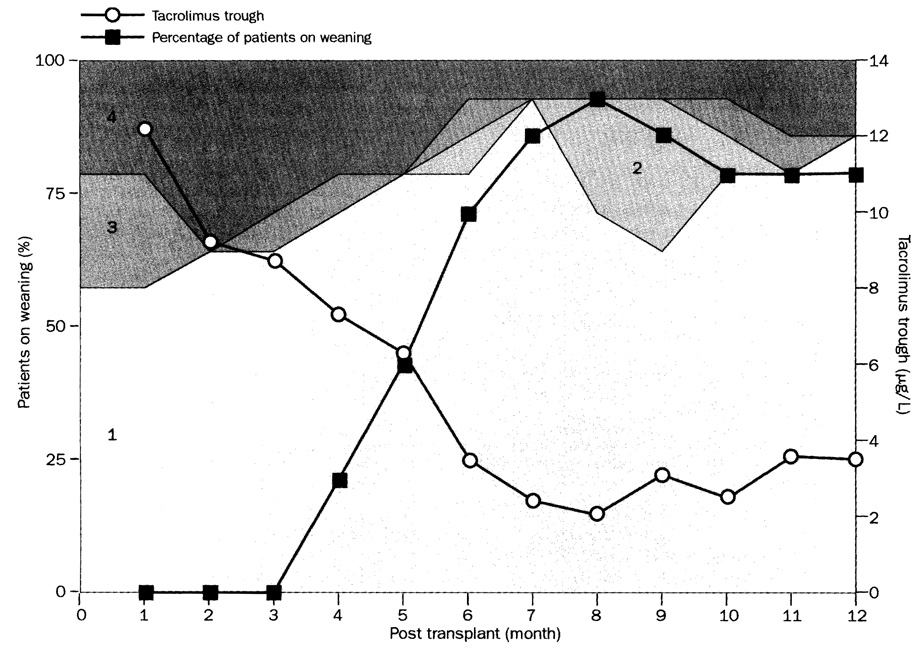
We superimposed the percentage of weaning patients on a graphic treatment summary, thus showing the correlation of overall treatment with weaning. The reference group consisted of kidney recipients. Since data elements are not organ specific, similar summaries for recipients of liver and other kinds of extrarenal organ allografts could be produced for independent study or for comparison with the kidney transplant reference population.
Role of the funding source
There was no external sponsor of the study, which was designed, initiated, and managed by the authors, who were responsible for data collection, data analysis, preparation of the report, and the decision to submit it for publication.
Results
We recruited 103 adults awaiting transplantation; entry was precluded for 21 recipients because not enough time was available for pretreatment. Thus, we studied 82 patients (50 kidney, 17 liver, 14 pancreas, 11 bowel; ten patients received kidney and pancreas transplants). 35 (43%) of the 82 recipients had an adjunct infusion of 1·9–9·1 × 108/kg donor bone marrow cells; this cohort was defined by availability of bone marrow cells.
Pretreatment effects
Similar to results of extensive studies in non-human primates,7 antithymocyte globulin induced a striking fall of all T-cell subsets to near zero within a few hours in all kinds of organ recipients and a less pronounced fall of other peripheral mononuclear cells, neutrophils, and platelets. Striking T-cell depletion of recipient lymph nodes sampled at surgery was also noted. Recovery of peripheral blood changes began in 1 or 2 weeks and was complete by 6 months. Well-known side-effects of antilymphoid globulins were noted, including chills, fever, headaches, diarrhoea, abdominal pain, shortness of breath, and hypotension or hypertension, which responded to treatment.
Kidney transplantation
Mean age of the 50 kidney recipients was 52·5 years (SD 13·2); these patients were not selected low-risk patients. Eight (16%) were undergoing retransplantation. Of the 50 kidney allografts, 40 were obtained from cadavers and the remaining ten were from unrelated (n=3) or related (7) live donors. Mean age of the adult cadaver donors was 49·9 years (SD 10·1), indicating both the ageing population of Pittsburgh and the need to systematically use marginal organs. Ten of the 40 recipients of cadaver kidneys also were given the donor pancreas (see below). 20 kidney recipients had 1·9–9·1 × 108/kg donor bone marrow cells infused within 24 h after organ implantation.8 Of the six HLA antigens tested, a mean of 3·6 (SD 1·7) were mismatched (range 0–6). No positive lyrnphocytotoxic crossmatches were reported, but seven (14%) recipients were presensitised—ie, panel-reactive antibodies greater than 20%.
After 13–17 months, survival of patients who received a kidney transplant was 49 (98%) of 50, with graft survival of 47 (94%). A complication of anaesthesia led to the only death (after 2 days) in this group, with loss of a live donor graft. Two cadaveric kidneys were lost—one to a delayed Shwartzman reaction9 after 90 days. The other loss happened at 240 days, mainly because of progression of donor disease that was present in a pretransplant wedge biopsy sample. Patients’ survival to date, graft survival, and 1-year serum creatinine concentrations did not differ significantly between the cadaver kidney alone, kidney with pancreas, and live-donor subgroups (table 1). These results were not affected by additional donor bone-marrow cell infusion (table 2).
Table 1.
13–17 month patients’ and kidney graft survival and 1-year serum creatinine in the 47 recipients with functioning grafts
| n | Patients’ survival | Graft survival | Creatinine (µmol/L; mean [SD]) | |
|---|---|---|---|---|
| Category | ||||
| Cadaver kidney | ||||
| Alone | 30 | 30(100%) | 28 (93%) | 168·0 (61·9) |
| With pancreas | 10 | 10 (100%) | 10 (100%) | 150·3 (17·7) |
| Live donor kidney | 10 | 9 (90%) | 9 (90%) | 150·3 (88·4) |
| Total | 50 | 49 (98%) | 47 (94%) | 159·1 (61·9) |
Table 2.
Cadaver kidney transplantation without and with donor bone marrow infusion
| n | Patients’ survival at 13–17 months | Graft survival at 13–17 months | Creatinine (µmol/L; mean [SD]) at 1 year | |
|---|---|---|---|---|
| Cadaver kidney | ||||
| No bone marrow | 20 | 20 (100%) | 19 (95%) | 154·7 (56·6) |
| Bone marrow | 20 | 20 (100%) | 19 (95%) | 169·7 (80·4) |
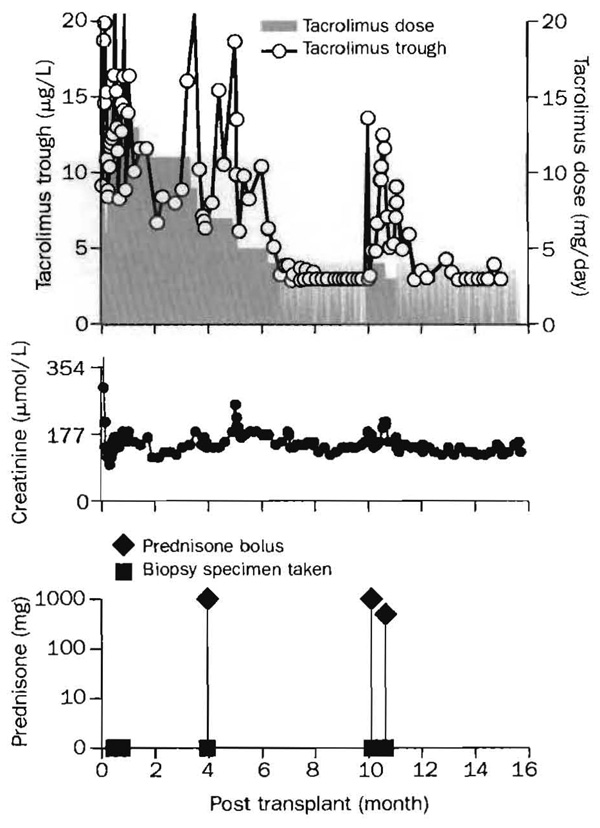
Spaced dosing was instituted 3·9–11·9 months after transplantation in 39 (83%) of the 47 renal recipients. Doctor’s or patient’S anxiety about spaced dosing frequently delayed its initiation, especially when cadaveric grafts had ischaemia-reperfusion injury and poor primary function. In other cases, histopathological evidence of immune activation in the preweaning biopsy specimen caused procrastination by the clinical staff. In one patient, for example, a clinical and biopsy-proved Banff 1A (mild) rejection after 20 days was treated with two boluses of methylprednisolone (figure 1). When further biopsy samples in the 3rd and 4th month showed continued Banff 1A rejection (figure 2), weaning was not started. No additional treatment was given, however, because renal function was stable. Despite similar histopathological findings at 7 months, spacing of tacrolimus doses to every other day was begun, and by the end of the year the dose frequency was one per week (figure 1).
Figure 1. Course of treatment of a cadaveric-kidney recipient.
Biopsy-proved rejection (Banff 1A) in the third week was treated with boluses of 1·0 g and 0·5 g prednisone. Similar findings in later biopsy specimens were not associated with renal function changes and were not treated. Instead, weaning was begun at 7 months. At 16·5 months, treatment was one dose per week tacrolimus.
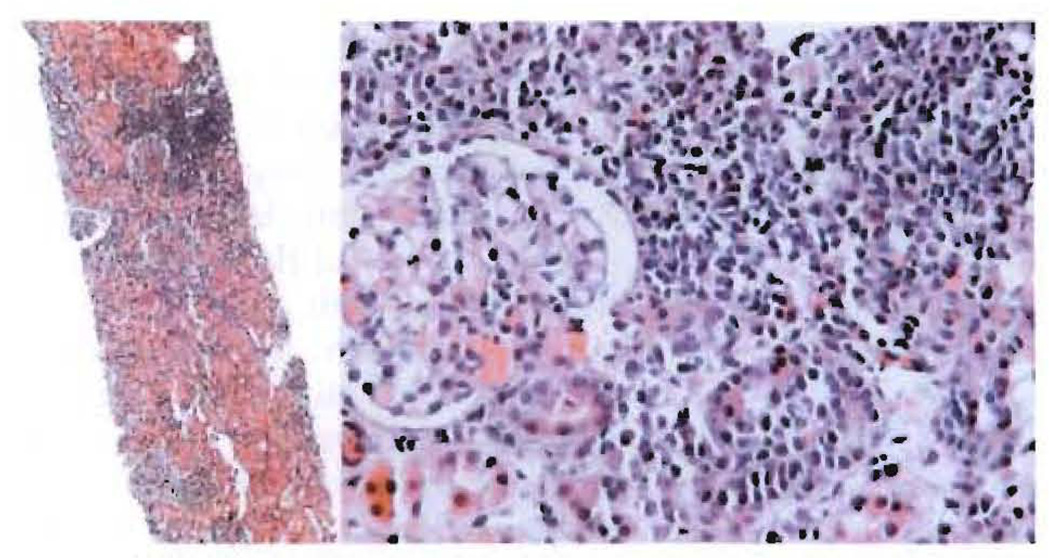
Figure 2. Biopsy specimen of cadaveric-kidney transplant.
(Left) Needle biopsy sample of the allograft in figure 1 at 4 months; haematoxylin and eosin staining ×40. (Right) Banff grade 1A (mild) rejection; haematoxylin and eosin staining ×200. Repeat biopsy specimens taken in subsequent months were closely similar.
In 25 (64%) of the 39 kidney recipients in whom weaning was begun, intermittent dosing has now been administered for 5–11 months (mean 9 [SD 1·5]), without (n=21; figure 1) or with (4; figure 3) short interruptions. At 14–17 months post-transplantation, the dose intervals of these 25 patients were every other day (n=1), three times a week (6), two times a week (11), and once a week (7).
Figure 3. Course of treatment of a cadaveric-kidney recipient with pre-existing nephropathy.
Five biopsy specimens taken over a period of 10 months had Banff rejection grades of either borderline or 1, prompting steroid boluses. Tacrolimus doses were first reduced from daily to two times per week (months 6·5–10), then temporarily restored to daily, and eventually established at three times per week.
Rate of weaning was different in kidney-alone and kidney-pancreas recipients. Weaning was attempted in 29 (78%) of 37 recipients of kidney-alone grafts, and remained in effect in 21 (72%) of these at 13–18 months. By contrast, spaced weaning that began at comparable postoperative times in all ten kidney-pancreas recipients was continued in only four (40%). These kidney-pancreas recipients still on weaning began dose-spacing 4–11 months after transplantation, and after a further 4–11 months, the dose frequency was three times per week (n=3) or once per week (1). All four patients have serum creatinine concentration less than 132·6 µmol/L.
In eight kidney-alone recipients in whom weaning was suspended, time from transplantation to start of dose spacing was 5·5–11·9 months (mean 8·1 [SD 2·7]), and duration of weaning was 1·9–9·0 months (5·0 [2·7]). The primary reason for return to daily treatment was a rise of serum creatinine from a mean of 159 [26·5] µmol/L (range 114·9–203·3) to a peak of 336 [194·5] µmol/L (range 141·4–707·2). Renal biopsy samples taken at the same time had borderline or Banff 1 rejection grades. All eight patients were treated by resumption of daily tacrolimus (or in one patient its replacement by daily sirolimus), and temporary addition of other immunosuppressants as needed. In seven of the eight patients, serum creatinine concentrations were promptly restored to baseline (range 114·9–221·0) with commensurate reduction in Banff biopsy sample scores. Serum creatinine concentration in the exceptional patient rose, despite treatment, to 663 µmol/L. The patient remained dialysis-free at 17 months. Chronic nephropathy, which was the dominant finding in multiple biopsy samples, had been present in pretransplant wedge biopsy specimens of both kidneys of the cadaver donor. After following a similar course, the mate kidney failed after 240 days.
In the six patients who underwent kidney and pancreas transplantation and who did not continue weaning of tacrolimus, the retreat from weaning after dose-spacing for 1–7·5 months was prompted by rises in serum lipase rather than in serum creatinine or by loss of pancreas function. Lipase changes were viewed with alarm, and precipitated multiple kidney and pancreas biopsy procedures. When evidence of mildly destructive immune activation was reported in one or the other organ (or both), daily tacrolimus was resumed and second and third drugs were added for lengthy periods. During the succeeding months, serum creatinine concentrations rose in four patients from previously normal amounts—in one patient to 442 µmol/L.
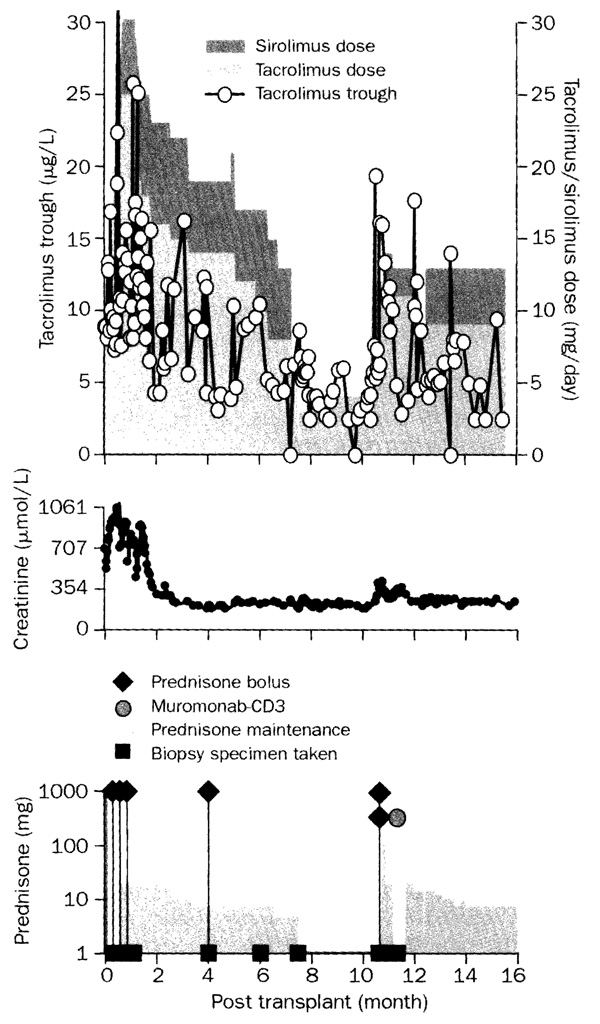
Figure 4 is a month-by-month summary of the immunosuppression during the first 12 months of the 47 kidney recipients (of the starting 50), whose renal grafts still functioned. In each of the first 12 months, 38–42 (81–90%) of recipients were on tacrolimus monotherapy (category 1) or nearly so (categories 2 and 3). The other five to nine patients had multiple drug treatment during months 3–12 and were in category 4. Five of the nine also had been treated with multiple drugs during the first 30–60 days, and thus were in category 4 from the outset. Early use of multiple agents in category 4 patients usually indicated a compulsion to treat—ie, a protocol violation—rather than a genuine indication for intensified immunosuppression. For example, the cadaveric kidney in the patient depicted in figure 5 functioned poorly at first because of biopsy-documented severe reperfusion injury. By the third week, further biopsy samples also showed evidence of mild (Banff 1A) rejection. Because of the clinical team’s conviction that recovery from the combined insult could not take place without heavy immunosuppression, continuous sirolimus and prednisone were added to tacrolimus. The kidney did recover, but efforts to return to tacrolimus monotherapy 7 months later led to rejection. On Jan 1, 2003, 39 (83%) of the 47 patients with functioning kidneys—including 25 on spaced weaning—were receiving one drug: tacrolimus (n=32) or sirolimus (7). Three were on double-drug treatment with tacrolimus and prednisone (n=2) or mycophenolate mofetil (1), whereas five were on triple-drug regimens.
Figure 4. Four categories of treatment in the first 12 months for 47 kidney recipients whose grafts still function after 13–17 months.
Numbers represent category of treatment. 81–90% of patients in any given month were on tacrolimus monotherapy (category 1) or nearly so (category 2 and 3).
Figure 5. Protocol violation in recipient of a cadaver kidney that sustained a severe ischemia-reperfusion injury.
Daily sirolimus (doses stacked on tacrolimus doses) and prednisone boluses plus daily steroid treatment were added to tacrolimus in the third week because of biopsy findings of mild (Banff 1A) rejection. A return to tacrolimus monotherapy after 7 months was succeeded by a Banff 2A (moderate) rejection and restoration of multiple drug immunosuppression.
Immunological studies were done on ten kidney recipients with more than 1 year follow-up. Seven of the ten recipients had been on spaced doses of tacrolimus for 6–10 months. One of the other three was receiving daily tacrolimus, and two (including the patient depicted in figure 5) were on multiple drugs. By mixed lymphocyte reactivity testing, the two patients with daily multiple drugs had near total suppression of responses to donor and third-party alloantigens, and to mitogens. By contrast, results of the mixed lymphocyte reactivity assay showed retention of vigorous responses against third-party alloantigens and mitogens by all seven patients on spaced tacrolimus doses and by the patient on daily tacrolimus. Moreover, donor-specific responses were fully intact by mixed lymphocyte reactivity testing in six of these eight patients. Further studies were done in five of these recipients. In three, cytotoxicity-mediated lysis assay showed 0, 0, and 2% donor-cell killing; in all three, the frequency of precursor cytolytic T cells was shown by the limiting dilution assay to be fewer than 1/300 000. Cell-killing was low (10% and 15%) in the other two patients with frequencies of precursor cytolytic T cells of 1/60 000 and 1/150 000. ELISPOT tests were done in two of the five patients. Both recipients had a very low frequency of γ-interferon cells in response to donor-specific stimulation.
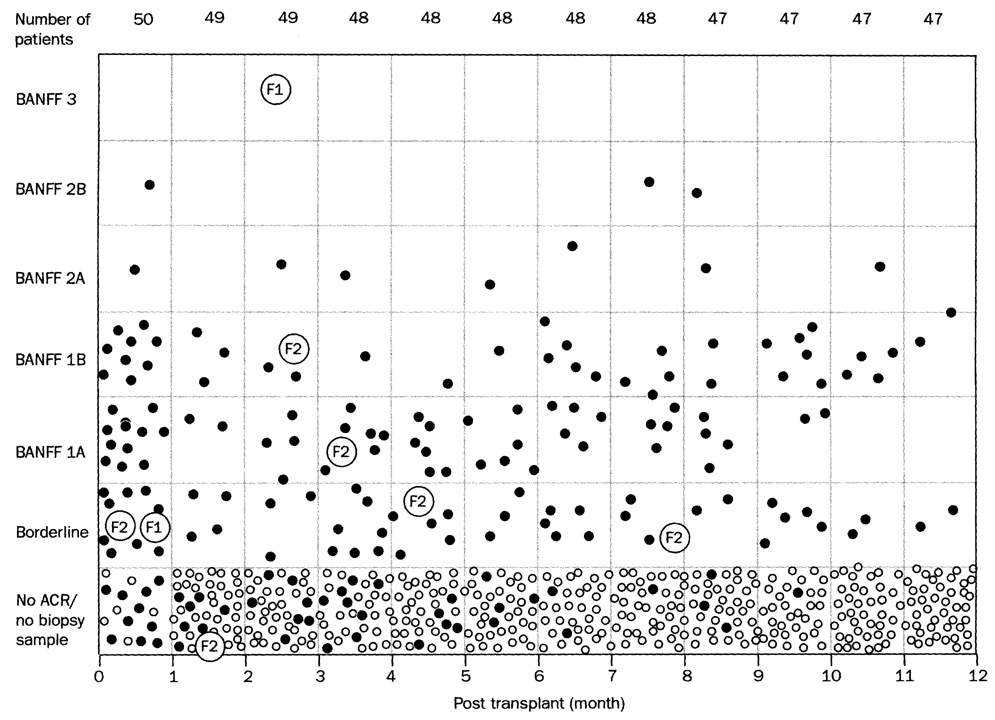
The frequency of biopsy specimens generated by clinical suspicion of rejection was greatest during the first 30 days (figure 6). During this period and throughout the rest of the year, most diagnosed rejections were graded as borderline or Banff 1. The renal graft that survived the coagulopathy of an aborted intraoperative Shwartzman reaction had a deceptively encouraging first biopsy sample, but developed complete cortical necrosis at 90 days (figure 6). The recipient who received the graft with pre-existing severe nephropathy had worsening of this underlying pathology before loss of the graft at 240 days, but with only mild acute rejection.
Figure 6. Banff grades of allograft biopsy specimens obtained in 50 kidney recipients.
Solid circles represent recipients in whom biopsy sample was taken. Small open circles depict patients with no biopsy sample and no clinical evidence of rejection. Large open circles depict biopsy procedures of kidneys lost at 90 (F1) and 240 (F2) days. ACR=acute cellular rejection.
As experience of the clinical team accumulated, secondary immunosuppressants were not added to tacrolimus in response to most bordeline grades and many Banff 1 scores if renal function was stable and no other clinical evidence of rejection was present. In many putative benign biopsy samples, lymphoid aggregates were present with nearby low-grade tubulitis, but with normal intervening tissue. The finding is typified by the 4-month biopsy specimen (figure 2) that caused the weaning delay for the patient whose course is shown in figure 1. In 16 biopsy samples from 14 patients, the lymphoid aggregates in grafts from opposite-sex donors were analysed by X and Y chromosome probes: in 11 of the biopsy samples, 1–9% of cells were donor-derived. The aggregates in figure 2 were 9% donor.
Liver transplantation
17 patients received liver transplants for chronic end-stage hepatic disease. ABO-identical allografts were obtained from 15 cadavers and two live volunteers. The mean number of HLA mismatches was 4·4 (SD 1·3), and in one case, the lymphocytotoxic crossmatch was positive. Four of the cadaveric recipients were also given donor bone marrow cells.
Three (17·6%) of the 17 liver transplant recipients died of primary graft non-function (after 2 days), complications from sarcoidosis (171 days), and femoralartery haemorrhage after an interventional radiological procedure (181 days). No livers were lost to rejection. Immunosuppression for the first year (figure 7) was similar to the pattern for kidney recipients. Mean bilirubin concentration at 1 year of the 14 recipients was 18·8 µmol/L (SD 14·7). From the fourth month onward, between 10 and 12 of the 14 liver recipients needed only spaced monotherapy (figure 7). Late rejections during and after the first year usually were treated with one or two boluses of prednisone or reduction of the intervals between tacrolimus doses.
Figure 7. Four categories of treatment in the first 12 months for 14 liver recipients who survive after 13–17 months.
Note that the proportion of people who needed category 3 and 4 immunosuppression during the first 60 days was comparable with that in the kidney recipients (figure 4).
Minimum use of immunosuppression has greatly facilitated management of patients with confounding factors, of which the most common was recurrent hepatitis (five of 14 survivors). At 13–17 months, dose frequencies were every other day (n=2), three per week (4), two per week (2), and one per week (3).
Pancreas transplantation
The 14 patients, who had been insulin dependent for many years, received crossmatch-negative cadaveric allografts which were transplanted alone (n=4) or with the kidney from the same donor (n=10, see above). Two of the four pancreas-alone recipients, and six of the ten pancreas-kidney recipients, also had bone marrow cell infusion. One of four pancreas-alone grafts was lost to rejection. Another that was transplanted with kidney was removed after 5 months because of arterial thrombosis, without harm to the renal graft.
In what we deemed protocol violations, the pancreas recipients were frequently treated with multiple drugs in response to rises in lipase concentration (see above). Nevertheless, five of 12 patients with normally functioning pancreas (table 3) are on spaced doses of tacrolimus monotherapy: every other day (n=1), three times a week (2), and once a week (2).
Table 3.
Patients’ and graft survival and incidence of weaning in intestine and pancreas recipients at end of study
| n | Survival |
Spaced weaning/ surviving grafts |
||
|---|---|---|---|---|
| Patient | Graft | |||
| Transplant | ||||
| Intestine | 11 | 11 (100%) | 8(73%) | 6/8 (75%) |
| Pancreas | 14 | 14 (100%) | 12 (86%) | 5/12 (42%) |
| Total | 25 | 25 | 20 (80%) | 11/20 (55%) |
Intestine transplantation
All 11 bowel recipients had chronic intestinal failure from non-neoplastic diseases. Small intestinal allografts were transplanted alone in nine patients or as a component of multivisceral allografts that included the liver in two. Mean HLA mismatches were 4·7 (SD 0·9). Nine allografts, including the two that consisted of multiple organs, had ex-vivo bowel irradiation (7·5 Gy). Recipients of these nine grafts were given an infusion of 2·4–9·0 × 108/kg donor bone marrow cells within the subsequent 24 h.10 The two recipients of non-irradiated intestine were not given adjunct bone marrow.
All 11 patients, and eight (73%) of the 11 grafts, survived. Difficulties in dosing resulted in a mean tacrolimus trough concentration of 20·4 µg/L (SD 2·1) during the first 30 days. Although this was twice the stipulated target and thus a systematic protocol violation, six of the eight patients with surviving grafts are on spaced-doses of tacrolimus: every other day (n=3), three times a week (1), and twice a week (2). Two of the three patients who lost intestine-only grafts had successful retransplantation, leaving only one of the 11 dependent on parenteral hyperalimentation.
Discussion
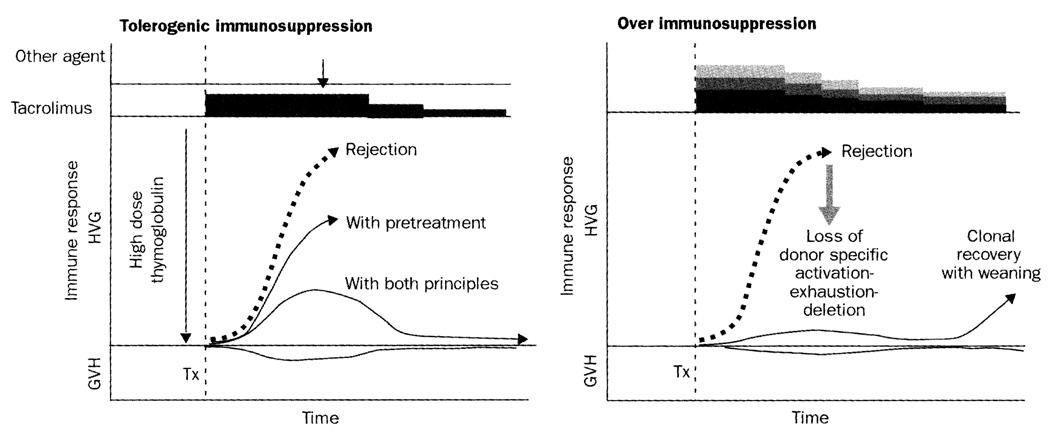
Our findings suggest that improvements in clinical transplantation might be within easy grasp by simple modification of the timing and dosage of immunosuppression. Clinical or histopathological evidence of immune activation has been widely thought to be categorically undesirable, and the equivalent of rejection. Instead, rejection and tolerance are stages of the same continuum in the notion from which our treatment algorithm derived.2–4,11 Thus, immune activation at any point on the immune response curve shown in figure 8 could theoretically represent a stage in the evolution of immunological tolerance rather than predicting graft loss. In this view, organ engraftment entails the same mechanisms as bone-marrow engraftment, beginning with contemporaneous host-versus-graft and graft-versus-host reactions. These responses reach peak intensity in the first few weeks post-transplant, and might be manifested clinically as graft-versus-host disease, rejection, or both simultaneously.
Figure 8. Mechanisms of immunosuppression.
(Left) Conversion of rejection (thick dashed arrow) to an immune response that can be exhausted and deleted by combination of pretreatment and minimalistic post-transplant immunosuppression. (Right) If the clonal response is eliminated by excessive post-transplant immunosuppression, exhaustion-deletion shown on the left is precluded, and subsequent graft survival is permanently dependent on immunosuppression. GVH=graft-versus-host; HVG=host-versus-graft; Tx=transplantation.
The usually dominant host-versus-graft response of the organ recipient is induced by migration to host lymphoid organs of the graft’s passenger leucocytes.2–4,11 If the response cannot eliminate the mobile donor leucocytes (and the source organ graft), it may be exhausted and deleted (figure 8). The response can be reduced into a more easily deletable range by pretreatment with many immunosuppressive modalities.12,13 This was accomplished in our patients with a polyclonal antithymocyte globulin.7 Other antilymphoid antibody preparations are expected to be effective, including the humanised monoclonal antibody, alemtuzumab, used for cadaver-kidney recipients by Calne and colleagues14 as part of a steroid-sparing regimen based on ciclosporin.
However, pretreatment alone is not enough to permit alloengraftment in most experimental models, or in humans. The aim of minimum post-transplant immunosuppression is to further reduce the clonal response with just enough treatment to prevent irreversible immune damage to the graft (figure 8), but not with such heavy treatment that the donor-specific clonal exhaustion-deletion is precluded. Thus, the common practice of beginning multiagent immunosuppression at the time of transplantation is potentially antitolerogenic (figure 8). Results of direct experimentation have shown, in various models, that tacrolimus or ciclosporin,15–17 prednisone,18 antilymphoid antibodies,13,19 and irradiation13 in the early post-transplant period can abrogate production of tolerance. Moreover, immunosuppression theoretically can break partial tolerance. This occurrence was suspected in several of our pancreas recipients who were given a possibly unwarranted burst of late immunosuppression.
In the idea of immunosuppression-aided organ engraftment depicted in figure 8, the extent of weaning is thought to parallel the completeness with which tolerance was achieved. This conclusion is lent support by results of in-vitro immunological studies at 1 year in kidney recipients who were on spaced doses of tacrolimus. Moreover, our definition of tolerance as an active antigen-dependent process2–4,11,20 is congruent with the frequent biopsy finding of immune activation (sometimes including low-grade immune destruction) in well-functioning allografts. The presence after 60 days of many donor cells in lymphoid aggregates of such allografts was reminiscent of experimental data suggesting that a transplanted organ might be a privileged repository for mobile donor leucocytes,21,22 whose late traffic between non-lymphoid locations and lymphoid organs is a prerequisite for maintenance of variable tolerance induced at the outset.3,4
The amount of immunosuppression needed during the critical first 60 days of most active tolerance was essentially the same in our kidney and liver recipients. This observation does not lend support to the widely held view that the transplanted liver induces a weaker response than other organs.23 Instead, it is consistent with the hypothesis that hepatic grafts are unusually tolerogenic because of the greater content of immunostimulatory—ie, inducing—passenger leucocytes.2–4,11,20 Not surprisingly, the eventual rate of spaced weaning was highest in the liver recipients.
The load of donor leucocytes can be increased in recipients of less leucocyte-endowed organs by infusion of donor bone-marrow cells. This extra treatment improves allograft survival in many experimental models. However, it has had little effect in clinical trials under conventional immunosuppression8,24,25 and did not confer an advantage in our small subgroup of bone marrow-infused recipients under immunosuppression that was designed to facilitate tolerance mechanisms. These results do not rule out the possibility of promoting tolerance in organ recipients by donor leucocyte augmentation (including stem-cell transplantation), but they suggest that the timing and dosage of immunosuppression might be more important than donor-cell dose in determining the outcome.
Our ultimate objective has been to find the lowest maintenance drug doses consistent with stable graft function. We emphasise that this needs more sophisticated care and surveillance than conventional use of high-dose prophylactic immunosuppression. The optimum approach to weaning has yet to be fully defined. Instead of gradually reducing daily amounts of tacrolimus in our patients, we have raised the intervals between doses. It has been learned from historical26 and other experience27 that the need for continued immunosuppression might approach or reach zero. In rats however, Murase and colleagues showed that maintenance doses of tacrolimus, given as infrequently as once per week, can sustain low-level microchimerism and prevent otherwise inexorable chronic rejection of organ allografts.11 In view of such evidence, weaning beyond once a week doses of tacrolimus has not been recommended to our patients.
Acknowledgments
We thank Terry L Mangan for administrative and secretarial assistance; Cynthia Eubanks, Anna Marie Rabier, and Cyndi Shelkey of the Transplant Institute Informatics Division, who were responsible for data accrual and collation; Robert J Weber of the School of Pharmacy, who had a role in quality assurance of drugs; Amit Basu, Tom Cacciarelli, David Geller, Ashok Jain, Wallis Marsh, George Mazariegos, Jerry McCauley, Jorge Reyes, Henkie Tan, and Andrew Yeager, who all contributed materially to clinical care; William Rudert, Diana Metes, nurses, and staff of the Transplantation Institute, who provided advice and invaluable assistance throughout. The work was supported by NIH grant DK 29961.
Footnotes
Contributors
T E Starzl, N Murase, A J Demetris, K Abu-Elmagd, M Trucco, and J J Fung generated the treatment protocol. Informatics were supervised by E A Gray, with the collaboration of N Murase and J Woodward. Directorship of the transplant services was as follows: R Shapiro, M Jordan, and V Scantlebury (kidney); J Fung, A Marcos, and B Eghtesad (liver); K Abu-Elmagd (intestine); and R Corry (deceased) and S Potdar (pancreas). Important elements of operative and posttransplant care were provided by P Fontes, T Gayowski, and J Bond. Pathological analyses for these services were done by P Randhawa (kidney), A J Demetris (liver), T Wu (intestine), and P Randhawa and M Nalesnik (pancreas). Tissue typing and immune monitoring was supervised by A Zeevi. All authors contributed to the planning and implementation of the study. As corresponding author, T E Starzl had full access to all data in the study and had final responsibility for the decision to submit for publication.
Conflict of interest statement
None declared.
References
- 1.Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385–395. [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Zinkernagel R. Antigen localization and migration in immunity and tolerance. N Engl J Med. 1998;339:1905–1913. doi: 10.1056/NEJM199812243392607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Starzl TE, Zinkernagel R. Transplantation tolerance from a historical perspective. Nat Rev Immunol. 2001;1:233–239. doi: 10.1038/35105088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raeusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. doi: 10.1046/j.1523-1755.1999.00299.x. [DOI] [PubMed] [Google Scholar]
- 6.and international panel. Demetris AJ, Batts KP, Dhillon AP. Banff schema for grading liver allograft rejection: an international consensus document. J Hepatol. 1997;25:658–663. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 7.Preville X, Flacher M, LeMauff B, et al. Mechansims involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460–460. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 8.Fontes P, Rao A, Demetris AJ, et al. Augmentation with bone marrow of donor leukocyte migration for kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–155. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starzl TE, Lerner RA, Dixon FJ, Groth CG, Brettschneider L, Terasaki PI. Shwartzman reaction after human renal transplantation. N Engl J Med. 1968;278:642–648. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: a decade of a single center experience. Ann Surg. 2001;234:404–416. doi: 10.1097/00000658-200109000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murase N, Starzl TE, Tanabe M, et al. Variable chimerism, graft versus host disease, and tolerance after different kinds of cell and whole organ transplantation from Lewis to Brown-Norway rats. Transplantation. 1995;60:158–171. doi: 10.1097/00007890-199507000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starzl TE, Weil R, III, Koep LJ. The pretreatment principle in renal transplantation as illustrated by thoracic duct drainage. In: Cummings NB, Klahr S, editors. Chronic renal disease. New York: Plenum Publishing; 1985. pp. 507–515. [Google Scholar]
- 13.Myburgh JA. Total lymphoid irradiation in transplantation: experimental background and results in 70 patients. In: Messmer K, Stein M, editors. Pathways in applied immunology. Berlin: Springer-Verlag; 1991. pp. 87–93. [Google Scholar]
- 14.Calne R, Friend P, Moffatt S, et al. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet. 1998;351:1701–1702. doi: 10.1016/S0140-6736(05)77739-4. [DOI] [PubMed] [Google Scholar]
- 15.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 16.Kirk AD, Burkly LC, Batty DS, et al. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686–693. doi: 10.1038/9536. [DOI] [PubMed] [Google Scholar]
- 17.Smiley ST, Csizmadia V, Gao W, Turka LA, Hancock WW. Differential effects of cyclosporine A, methylprednisolone, mycophenolate, and rapamycin on CD154 induction and requirement for NFκB: implications for tolerance induction. Transplantation. 2000;70:415–419. doi: 10.1097/00007890-200008150-00005. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Sun J, Sheil AG, McCaughan GW, Bishop GA. A short course of methylprednisolone immunosuppression inhibits both rejection and spontaneous acceptance of rat liver allografts. Transplanation. 2001;72:44–51. doi: 10.1097/00007890-200107150-00011. [DOI] [PubMed] [Google Scholar]
- 19.Myburgh JA, Smit JA, Meyers AM, Botha JR, Browde S, Thomson PD. Total lymphoid irradiation in renal transplantation. World J Surg. 1986;10:369–380. doi: 10.1007/BF01655296. [DOI] [PubMed] [Google Scholar]
- 20.Starzl TE, Demetris AJ, Trucco M, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology. 1993;17:1127–1152. [PMC free article] [PubMed] [Google Scholar]
- 21.Sakamoto T, Ye Q, Lu L, Demetris AJ, Starzl TE, Murase N. Donor hematopoietic progenitor cells in non myeloablated rat recipients of allogeneic bone marrow and liver grafts. Transplantation. 1999;67:833–840. doi: 10.1097/00007890-199903270-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichikawa N, Demetris AJ, Starzl TE, et al. Donor and recipient leukocytes in organ allografts of recipients with variable donor-specific tolerance: with particular reference to chronic rejection. Liver Transpl. 2000;6:686–702. doi: 10.1053/jlts.2000.19029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calne R, Davies H. Organ graft tolerance: the liver effect. Lancet. 1994;343:67–68. doi: 10.1016/s0140-6736(94)90809-5. [DOI] [PubMed] [Google Scholar]
- 24.Barber WH, Diethelm AG, Laskow DA, Deierhoi MH, Julian BA, Curtis JJ. Use of cryopreserved donor bone marrow in cadaver kidney allograft recipients. Transplantation. 1989;47:66–71. doi: 10.1097/00007890-198901000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Morales R, Carreno M, Mathew J, et al. Continuing observations on the regulatory effects of donor-specific bone marrow cell infusions and chimerism in kidney transplant recipients. Transplantation. 1998;65:956–965. doi: 10.1097/00007890-199804150-00016. [DOI] [PubMed] [Google Scholar]
- 26.Starzl TE. The saga of liver replacement, with particular reference to the reciprocal influence of liver and kidney transplantation (1955–1967) J Am Coll Surg. 2002;195:587–610. doi: 10.1016/s1072-7515(02)01498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takatsuki M, Uemoto SH, Inomata Y, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation. 2001;72:449–454. doi: 10.1097/00007890-200108150-00016. [DOI] [PubMed] [Google Scholar]