Abstract
Objective
The US Centers for Disease Control and Prevention (CDC) recently revised their HIV screening guidelines to promote testing and earlier entry to care. Prior analyses have examined the policy’s cost-effectiveness but have not evaluated its impact on government budgets.
Methods
We used a simulation model of HIV screening, disease, and treatment to determine the budget impact of expanded HIV screening to US government discretionary, entitlement, and testing programs. We estimated total and incremental testing and treatment costs over a five-year time horizon under current and expanded screening scenarios. We used CDC estimates of HIV prevalence and annual incidence, and considered variations in screening frequency, test return rates, linkage to care, test characteristics, and eligibility for government screening and treatment programs.
Results
Under current practice, 177,000 new HIV cases will be identified over five years. Expanded screening will identify an additional 46,000 cases at an incremental five-year cost of $2.7 billion. The financial burden of expanded HIV screening will fall disproportionately on discretionary programs that fund care for newly identified patients and will not be offset by entitlement program savings. Testing will represent a small proportion (18%) of the total budget increase. Costs are sensitive to the frequency of screening and the proportion linked to care.
Conclusions
The expanded HIV screening program will have a large downstream impact on government programs that fund HIV care. Expanded HIV screening will not meet early treatment goals unless government programs have sufficient budgets to expand testing and provide care for newly-identified cases.
INTRODUCTION
In September 2006, the US Centers for Disease Control and Prevention (CDC) revised their HIV screening guidelines to increase rates of detection and facilitate early entry into care. Benefits of earlier detection and entry into care include better outcomes for patients themselves and lowered rates of HIV transmission to others due to biological (decreased infectivity as a result of treatment) and behavioral (reductions in risk behavior due to knowledge and counseling) mechanisms (1-4). The revised CDC guidelines encourage routine testing in all healthcare settings for the general population and annual testing for high-risk populations (5). However, financing concerns are a major barrier to implementing the recommendations (6). If government testing and care programs are under-funded, expanded screening may result in large numbers of newly-identified cases who are unable to receive care.
The CDC considered cost-effectiveness evaluations of expanded screening policies in its recent decision (7). Previous analyses have indicated that expanded HIV screening is cost-effective in many settings (8-10) and alternative targeted screening strategies have also been advocated on efficiency grounds (11). However, efficient policies may not be affordable from a payer’s perspective if the stream of financial costs is larger than budget allocations (12). The budget requirements for government programs to provide HIV testing and appropriate medical care to the entire eligible US population are unknown (11). It is unclear how expanded screening in the US may differentially affect discretionary government programs funded by fixed annual appropriations versus entitlement programs with budgets that automatically expand as demand increases. This question is particularly timely given the requirement for reauthorization legislation in the US Congress for the Ryan White HIV/AIDS Program (RW) before December 2009, and national debates on health care reform.
We conducted a budget impact analysis of expanded HIV screening using a published simulation model of HIV screening, disease, and treatment and national data on HIV epidemiology, enrollment in public healthcare programs, and program eligibility. We forecasted the impact on government budgets for testing programs, discretionary treatment programs (such as RW), and entitlement programs (Medicaid and Medicare) under current practice and expanded screening scenarios over a five-year period. We excluded patients covered by the Veterans Administration (VA) because the VA has a distinct single-payer system. We also excluded patients with private insurance in order to focus on government budgets.
Overview of HIV Finance in the US
The predominant sources of government funding for HIV care in the US are the federal-funded Medicare and federal- and state-funded Medicaid entitlement programs, and the discretionary Ryan White HIV/AIDS Program (13-16). Although specific eligibility criteria vary across states, HIV-infected individuals generally qualify for Medicaid after they meet low-income and “disability” criteria. Individuals with sufficient work experience may qualify for Medicare by reaching age 65 or meeting income and “permanent disability” standards (15). RW is mandated to be a “payer of last resort” and targets uninsured and underinsured HIV-infected individuals, particularly those who have not yet progressed to AIDS. Its federal budget is set annually by Congress, and some state legislatures provide supplementary annual appropriations. The largest components of RW spending are state-administered AIDS Drug Assistance Programs (ADAPs), which finance HIV medications (14, 17). We distinguish between discretionary and entitlement programs in our budget impact analysis because entitlement program budgets automatically expand in response to increased case load. Due to their discretionary structure, RW budgets are more vulnerable to unexpected increases in the number eligible for care.
HIV patient eligibility for public insurance programs fluctuates over time. Patients detected early in their disease may not initially meet disability criteria to qualify for Medicare or Medicaid; however, they may later transition from a discretionary to an entitlement program with age or development of symptomatic illness. In contrast, patients identified late in their disease may be immediately eligible for an entitlement program.
METHODS
Analytic Overview
Wherever possible, we adhered to the ISPOR guidelines on budget impact analysis (12). We used a published Monte Carlo state transition simulation model of HIV testing, disease, and treatment to estimate incremental testing and treatment costs for prevalent and incident cohorts of HIV-infected individuals over a five-year time horizon. The testing cost calculations also include the cost of screening non-infected individuals. In conformity with the ISPOR principles (12), we selected a planning horizon that reflects the time frames used for budget planning in publicly financed HIV care (18). Our baseline horizon of five years matches the three to five year interval typical of RW reauthorizations. Recognizing that some readers may be interested in the budget impact over a longer time horizon, we alsoconsider a ten-year horizon in a sensitivity analysis. We examined two testing strategies: current practice (defined by completing a test, on average, every 10 years) and expanded screening (defined by completing a test, on average, every five years), and evaluated alternative testing frequencies in sensitivity analyses. The derivation of the testing time frames is described below. We also examined alternatives for the type of test, rate of return for test results and linkage to care, and likelihood of qualifying for a government testing and care program.
We report the following outcomes: the number identified over five years, the fraction of cases identified through presentation to care with testing versus clinical AIDS, mean CD4 count at diagnosis (a measure of immune function), the incremental quality-adjusted life years (QALYs), and costs to government programs. Costs were forecasted separately for programs that pay for HIV testing, discretionary treatment programs (including federal RW funds, state matching funds, and uncompensated care pools), and entitlement treatment programs (Medicare and Medicaid).
National data on health insurance coverage and HIV epidemiology were used to estimate the proportion of cases eligible for discretionary and entitlement programs at the time of HIV detection and over the subsequent five years. We report undiscounted dollar outlays in each budget period in 2009 dollars, which is consistent with ISPOR’s recommendation to report undiscounted costs (12, 19).
Populations and Program Eligibility
We focused on adults (>18 years) due to our objective to project costs to RW, Medicaid, Medicare, and uncompensated care pools. Although RW does fund some services for children and youth, these funds represent a small percentage of overall RW grants (20). The federal and state-funded State Children’s Health Insurance Program (SCHIP) would likely incur most of the costs of treating newly identified HIV cases among children and adolescents, and is not included in our budget analysis. Additionally, the clinical parameters in our model are derived from adult populations.
We tracked HIV-related treatment costs for all adults, including the elderly. We excluded testing costs for elderly adults (>64 years) because neither past screening efforts nor the revised guidelines target this group (5). All cost calculations also excluded patients for whom testing and treatment costs were likely to be financed through the VA or private insurance.
We used national estimates of insurance status to estimate the total number of non-elderly adults (aged 19 to 64 years) without private or VA insurance who would be eligible to receive HIV tests through a government testing program (21, 22). For modeling purposes, we assumed that only those eligible for public sector-financed care (excluding VA coverage) would receive a public sector-financed test. Sensitivity analyses include scenarios where additional costs are incurred by government programs to test or eventually treat individuals who are currently insured through private insurance or the VA. We used 2008 national HIV incidence data (approximately 56,000 new cases annually) (23) to project the total number of HIV-infected individuals potentially eligible for testing and linkage to care upon diagnosis. We used national HIV prevalence data (approximately 1.1 million prevalent cases, of whom 21percent do not know their infection status) (24) to calculate the number of prevalent cases currently aware of their infection and eligible for care. These data were also used to estimate the number of undetected prevalent cases potentially eligible for testing and linkage to care upon diagnosis. We assumed a constant incidence of new infections over the 5-year period (23). We estimated the fraction of all cases (detected and undetected) likely to qualify for government discretionary and entitlement programs using data from the HIV Research Network (HIVRN) (25). We used data on the incidence and prevalence of HIV among veterans to subtract those likely to receive care through the VA from our population estimates (26). Our calculations yield the following populations eligible for government-financed testing and treatment in 2009: 50,100,000 HIV-negative individuals; 711,000 prevalent cases aware of their infection; 189,000 undetected prevalent cases; and 46,000 incident cases. Further details of these calculations are provided in the technical appendix.
We assumed that treatment costs for newly diagnosed individuals without private or VA insurance are financed through discretionary programs until they qualify for Medicare by attaining age 65 or they experience an AIDS-defining opportunistic infection (OI), thereby qualifying them for Medicaid or Medicare. Newly diagnosed individuals may be immediately eligible for entitlement programs before presentation with an OI due to pregnancy, prior disability, or state-specific poverty-level expansions (14, 27). Because national estimates of this population are unavailable and the number is likely to be small, we did not account for such eligibility.
Table 1 displays the data sources used to generate demographic and clinical characteristics of cases eligible for government care upon diagnosis. We used a different set of characteristics for HIV-infected individuals in the three cohorts (prevalent aware, prevalent unaware, and incident cases).
Table 1.
Inputs and source data for simulation model to project budget impact of expanded HIV screening to public payers
| Variable | Base Case (Range) | Sources |
|---|---|---|
| General | ||
| Discount rate | 0% | (13) |
|
| ||
| Number of HIV-negative individuals who will be screened, at start of simulation | ||
| Na | 50,100,000 (upper 55,100,000) | See appendix |
|
| ||
| Characteristics of prevalent cases aware of their infection b | ||
| Na | 711,000 (upper 747,000) | See appendix |
| Age (mean) | 41 years | (60) |
| Female | 30% | (25, 60) |
| CD4 at simulation entry (mean, std) | 390 (260) cells/mm3 | (60) |
| Viral load | Published natural history data | (61), see appendix |
| Prior OI experience | Published natural history data | (61), see appendix |
|
| ||
| Characteristics of prevalent cases unaware of their infection c | ||
| Na | 189,000 (upper 198,000) |
See appendix |
| Age (mean) | 41 years | (60) |
| Female | 30% | (60) |
| CD4 at simulation entry (mean, std) | Published natural history data | (61, 62), see appendix |
| Viral load | Published natural history data | (61), see appendix |
| Prior OI experience | None | Assumption |
|
| ||
| Characteristics of incident cases d | ||
| Na | 45,800 per year (upper 48,100) | See appendix |
| Age (mean) | 33 years | (63), see appendix |
| Female | 30% | (42) |
| CD4 at simulation entry (mean, std) | 534 (164) cells/mm3 | (62) |
| Viral load | >100,000 copies/mL | Assumption, see appendix |
| Prior OI experience | None | Assumption, see appendix |
|
| ||
| Test characteristics | ||
| Probability of monthly test receipt | ||
| Current practice (%) | 0.83% (0%-1.67%) | (45) |
| Expanded (%) | 1.67% (0.83%-8.33%) | See text |
| Probability detected case linked to caree |
80% (50%-100%) | (36, 49, 50) |
| Rapid test characteristics | ||
| Sensitivity pre-seroconversion | 0.1% | Calculated |
| Specificity pre-seroconversion | 99.6% | (64) |
| Specificity post-seroconversion | 99.9% | (64) |
| HIV-positive test return rate | 97% (90%-100%) | (48) |
| HIV-negative test return rate | 97% (90%-100%) | (48) |
| ELISA test characteristics | ||
| Sensitivity pre-seroconversion | 0.1% | Calculated |
| Sensitivity post-seroconversion | 99.6% | (65) |
| Specificity pre-seroconversion | 99.9% | (65) |
| HIV-positive test return rate | 75% (50%-100%) | (48) |
| HIV-negative test return rate | 67% (50%-100%) | (48) |
|
| ||
| Testing costs | ||
| HIV test kit, administration, and laboratory analysis | ||
| Rapid test | $12.23 | (33) |
| ELISA | $7.05 | (33) |
| Confirmatory testing for positive results | ||
| Rapid test | $44.28 | (33) |
| ELISA | $52.72 | (33) |
| Pre-test counseling | $0 ($7.76) | (33) |
| Post-test counseling for negative test result |
$7.53 | (33) |
| Post-test linkage and counseling for positive test result |
$14.61 | (33) |
| Administrative cost for non-return for results |
$9.02 | Assumption (0.5 hours of administrative staff time and $1.00 for mail and phone reminders) |
All calculations exclude individuals eligible for testing and care through the Veterans Administration or private insurance.
Aware is defined as aware of their infection and currently in care through discretionary or entitlement programs, at the start of the simulation.
Unaware is defined as being unaware of their infection at the start of the simulation, and only eligible for government-financed care upon detection.
Incident cases are uninfected at the start of the simulation, and are only eligible for government-financed care upon detection.
See technical appendix for a more detailed description of the linkage to care probability and the test return rate.
The mean age, gender, and CD4 distribution for the prevalent aware cohort were obtained from summary data of all patients in the HIVRN study (28). We assumed that prior to ART, the distribution of viral loads after acute HIV infection for each CD4 stratum was similar to that of the Multicenter AIDS Cohort Study (MACS) cohort (29). We determined the distribution of prior OIs for each CD4 stratum through an initialization cohort, in which we simulated healthy cohorts with the same mean age and gender proportions until they reached each CD4 stratum (30). We assigned these patients to discretionary and entitlement programs based on insurance status reported in HIVRN.
By definition, demographic characteristics of prevalent unaware cases are unknown. We assumed that the age and gender of “prevalent unaware” cases were similar to that of “prevalent aware” cases. Untreated disease lasts on average ten years (120 months), of which the first two months are in the acute state, the last two years (24 months) are in the symptomatic chronic state, and the remainder of time (94 months) is spent in the asymptomatic chronic state. An undetected patient thereby had a 1.7% chance of being in the acute state (2/120), 78.3% chance of being in the asymptomatic chronic state (96/120), and 20% chance of being in the symptomatic chronic state (24/120) (8, 10). The mean CD4 distribution of acute cases was estimated from published studies of individuals with primary infection (31), and the mean CD4 distribution of chronic cases was estimated from the MACS cohort (29). We assumed that no patients had a history of OIs, as they otherwise would have been identified and linked to care upon presentation.
It is difficult to derive population characteristics of incident cases because these cases are not immediately detected. We derived the mean age of new cases through back calculation. Past research has estimated that on average, there is a duration of eight years between infection and presentation to care (32). We subtracted this value from data on prevalent cases to calculate the mean age of 33 years for the incident cohort (10). All individuals in this cohort entered the model at the time of infection, during an acute state of illness. Clinical characteristics included no OI history and a very high viral load (>100,000 copies/mL). The mean CD4 distribution of this cohort was estimated from published studies of individuals with primary infection (31). After individuals progressed past the acute state (approximately two months), their viral load decreased and patients were moved to a lower viral load stratum. The viral load distribution was derived from the MACS cohort (29).
Model Description
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) Model is a widely published computer simulation of HIV disease and treatment (8, 33-35). It contains two components. The Screening Module simulates an HIV screening program and determines when each simulated HIV-infected patient will become detected through testing or presentation to care with an AIDS-defining OI. For those detected through testing, the Screening Module additionally determines whether they were effectively linked to care. The Disease Module tracks the clinical progress of all patients, irrespective of whether they have been detected; however, only patients that have been successfully detected and linked to care through testing or AIDS-defining presentation are eligible for treatment.
The Screening Module allows user-defined inputs on test characteristics, testing frequency, linkage to care, and costs. We simulated cohorts of currently unidentified incident and prevalent cases likely to qualify for government care upon detection, as described above.
In the base case, patients were screened using the rapid HIV test. Many health departments have implemented rapid tests, and there is evidence of a shift from conventional (ELISA) to rapid technologies (36) because patients can receive preliminary results at the time of testing.
The Disease Module uses a Monte Carlo state-transition framework to track the natural history of illness in simulated patients with user-specified care. Data sources and details are described in the technical appendix. Simulated patients undergo monthly transitions among health states, categorized by chronic illness, acute illness, and death. Monthly probabilities of events include changes in CD4 counts and HIV viral load, the development of an OI, adverse drug reactions, and death related to OIs, chronic AIDS, or non-AIDS-related causes. Summary statistics are collected for each simulated patient on age, mean projected survival, cause of death, OIs, the length of time spent in each health state, and cost. Simulated patients may receive antiretroviral therapy, medications for treatment and prevention of OIs, and ongoing routine care.
Cost Inputs
Table 1 summarizes the sources used to tabulate costs. We adapted testing costs from a recent analysis conducted by CDC researchers (37), which updates earlier studies (38, 39). Cost estimates are consistent with recent literature that reports economic outcomes of HIV testing in emergency departments (40-43). All screened individuals were assigned the cost of the test, irrespective of their HIV status. Those with preliminary positive tests (true and false positives) additionally incurred the cost of a confirmatory test. All individuals were assigned the cost of post-test counseling, which differed by test result. Post-test counseling costs for HIV-infected persons included costs to facilitate linkage to care. Not all individuals received their test results; those who did not receive them did not incur costs for confirmatory testing and post-test counseling.
Prior to the CDC’s revised screening guidelines, pre-test counseling was encouraged. The current guidelines promote opt-out testing, without separate written consent and pre-test counseling (5, 7). In practice, legal requirements for pre-test counseling and written consent vary by state (44). Furthermore, some testing sites may continue to offer pre-test counseling in the absence of a requirement. In order to simulate the CDC guidelines as closely as possible, we excluded pre-test counseling costs from the base case analysis of both screening scenarios. We included these costs in a sensitivity analysis, and we assumed that providers’ decisions to offer pre-test counseling were independent of the testing frequency. The CDC’s revised recommendations for opt-out testing are controversial (6, 11). We assumed that if individuals who did not receive pre-test counseling changed their risk behavior, the cost impact to government programs would be minimal in our five-year time frame, although they may become significant in future years.
Pharmaceutical costs were calculated using published average wholesale prices (45), which were adjusted for the average state Medicaid reimbursement rate by weighting state-specific Medicaid discounts by AIDS prevalence (46). Costs of laboratory tests were derived from the Medicare fee schedule (47). Medical care utilization of patients at different stages of HIV disease were obtained from data collected by the HIV Research Network (HIVRN) (46). Costs of inpatient services were derived from the University HealthSystem Consortium database (46, 48).
Healthcare Cost Projections
We conducted separate simulations for three groups of patients and aggregated the results. The first group is the 711,000 prevalent cases eligible for entitlement and discretionary programs and receiving care in 2009. The second group is the 189,000 prevalent cases whose infection is undetected in 2009 and who will not incur costs to the government programs until they are detected and linked to care. The third group is the 46,000 incident cases eligible for government care each year, who will also only incur treatment costs upon detection and linkage to care.
Screening Strategies
To model current practice, we estimated that on average, individuals receive a test every 10 years, equivalent to a 0.83 percent chance of being tested each month and 5.0 million tests annually. This estimate is derived from a CDC analysis of national health data surveys (49), although other surveys suggest that current screening rates may be higher (50). We validated our estimate by comparing our model results to CDC estimates that 39 percent of HIV-infected individuals received an AIDS diagnosis within a year of their first HIV test (51). We found that our current practice of once every 10 years reasonably approximated these data on late presentation to care.
To model expanded screening, we assumed that on average, individuals are offered and accept a test every five years, equivalent to a 1.67 percent monthly chance of testing and 10.0 million tests annually. This represents a two-fold increase in testing from current practice. Although this test rate does not match the CDC’s recommendation for routine screening in the general population and repeat annual testing for high-risk populations (5), we believe that it best represents the population effect of reasonable efforts to implement the policy. Prior analyses of expanded HIV testing have also used a five-year testing frame (8, 9).
In the base case for current practice and expanded screening, we assumed that 97 percent of patients received their rapid test result (52), and that 80 percent of all identified cases were successfully linked to care (53, 54).
Sensitivity Analyses
To examine if our results were robust to parameter uncertainty, we conducted extensive sensitivity analyses, listed in Table 1. One set of analyses used the ELISA test, which differs from the rapid test with respect to costs and the rate of receipt of test results. We varied the testing frequency, from a minimum of “no testing” to a maximum of “annual testing.” We varied the rate of receipt of rapid test results from 90 percent to 100 percent, and receipt of ELISA results from 50 percent to 100 percent. We estimated a range of linkage to care probabilities (for identified cases who had received their results), from perfect linkage (100 percent) to 50 percent linkage. We assessed the cost impact of including pre-test counseling. We calculated the impact of a 10 percent increase in the population eligible for government-financed testing and care if additional costs are incurred by government programs to test and treat individuals who are currently privately insured but have incomplete coverage or lose coverage in the future. Finally, we considered a ten-year time horizon.
RESULTS
Table 2 displays clinical characteristics of newly-identified HIV-infected adults eligible for government-financed testing and care (excluding the VA), for the base case of each screening scenario. If testing continues at an average frequency of once every ten years, 177,000 cases (116,000 prevalent; 61,000 incident) will be identified from 2009 to 2013. Over the course of their lifespan, 68 percent of currently unidentified prevalent cases and 49 percent of incident cases will receive a diagnosis after presenting to care with an AIDS-defining OI. If expanded testing increases testing to once every five years, an additional 46,000 cases (17,000 prevalent; 29,000 incident) will be identified from 2009 to 2013. The fraction of cases receiving a diagnosis as a result of an AIDS-defining OI will drop to 58 and 32 percent for prevalent and incident cases, respectively. The mean CD4 count at detection (a measure of HIV disease progression) will be higher under expanded screening for all cases, reflecting earlier detection.
Table 2.
Clinical characteristics of newly detected HIV-infected individuals eligible for care through discretionary and entitlement programsa
| Current Practice | Expanded Screening | |
|---|---|---|
| Number identified over 5-year period | ||
| Prevalent cases in year 1 (N) | 54,343 | 63,747 |
| Prevalent cases in year 2 (N) | 18,362 | 24,062 |
| Prevalent cases in year 3 (N) | 17,276 | 19,755 |
| Prevalent cases in year 4 (N) | 14,759 | 15,106 |
| Prevalent cases in year 5 (N) | 11,366 | 10,651 |
| Total prevalent cases in period (N) | 116,107 | 133,321 |
| Incident cases in year 1 (N) | 4,099 | 6,701 |
| Incident cases in year 2 (N) | 8,379 | 13,258 |
| Incident cases in year 3 (N) | 12,340 | 18,764 |
| Incident cases in year 4 (N) | 16,086 | 23,417 |
| Incident cases in year 5 (N) | 19,618 | 27,361 |
| Total incident cases in period (N) | 60,523 | 89,501 |
|
| ||
| Mechanism of detection, prevalent cases | ||
| Screening (%) | 19.7 | 33.1 |
| Opportunistic infection (%) | 68.3 | 57.8 |
| Never detected (%) | 12.0 | 9.1 |
|
| ||
| Mechanism of detection, incident cases | ||
| Screening (%) | 39.3 | 60.2 |
| Opportunistic infection (%) | 49.0 | 32.3 |
| Never detected (%) | 11.7 | 7.5 |
|
| ||
| CD4 count at detection | ||
| Prevalent (mean cells/mm3) | 122 | 140 |
| Incident (mean cells/mm3) | 251 | 312 |
|
| ||
| Incremental quality-adjusted survival per personb | ||
| Prevalent cases (ΔQALYs) | -- | 2.0 |
| Incident cases (ΔQALYs) | -- | 3.2 |
Clinical characteristics are different from Table 1 input parameters. Table 1 input parameters refer to the actual but unobserved characteristics at the start of the simulation. Table 2 input parameters refer to observed clinical characteristics upon detection. CD4 counts are lower in Table 2 because it takes time for HIV-infected cases to become detected, during which time CD4 counts generally fall. “Prevalent aware” cases are not included in this table because they have already been detected.
These numbers refer to the quality-adjusted survival over the newly detected cases’ lifetime, and not just the five-year time horizon of the budget impact analysis.
Table 3 lists the projected total testing and care costs to public payers, under each screening scenario. Costs are separated by program type (testing, discretionary, and entitlement) and then summed at the bottom of the table. In the base case, continued testing at the current rate will incur a total cost of $83.7 billion over five years. Expanded screening will incur an additional cost of $2.7 billion, for a total of $86.4 billion. Five-year testing costs will increase from $504 million to $1.0 billion. Budget projections are dominated by treatment costs. Testing costs represent a small fraction of total costs (0.6 percent and 1.2 percent for the current practice and expanded screening scenarios, respectively) and 18.3 percent of additional costs for expanded screening. Five-year projected costs to discretionary programs will increase by $2.9 billion (from $26.0 billion to $28.9 billion), which will be partially offset by a savings of $624 million in the entitlement program budget. In both scenarios, most costs will be incurred by entitlement programs.
Table 3.
Projected five-year costs (in millions) to US government testing, discretionary, and entitlement programs for HIV screening and care, 2009-2013
| Total 5-Year Government Budget Impact (Millions) | |||
|---|---|---|---|
| Current Practice | Expanded Screening | Incremental Cost | |
| Government-financed testing programs | |||
| Base case (rapid test) | $504.2 | $1,006.8 | $502.6 |
| ELISA test | $383.6 | $766.4 | $382.7 |
| Inclusion of pre-test counseling | $699.1 | $1,396.5 | $697.4 |
|
| |||
| Discretionary programs | |||
| Base case | $26,030.0 | $28,899.1 | $2,869.1 |
| Perfect rates of test return and linkage to care | $26,897.9 | $30,319.7 | $3,421.9 |
| Low rates of test return and linkage to carea | $24,611.1 | $26,518.5 | $1,907.4 |
|
| |||
| Entitlement programs | |||
| Base case | $57,128.4 | $56,504.8 | -$623.6 |
| Perfect rates of test return and linkage to care | $56,938.5 | $56,196.2 | -$742.3 |
| Low rates of test return and linkage to carea | $57,451.9 | $57,030.9 | -$421.0 |
|
| |||
| Total costs | |||
| Base case | $83,662.6 | $86,410.7 | $2,748.1 |
| Ten percent increase in eligible population | $92,028.9 | $95,051.7 | $3,022.9 |
| High-cost scenariob | $84,535.5 | $87,912.4 | $3,376.9 |
| Low-cost scenarioc | $82,446.6 | $84,315.8 | $1,869.2 |
80% test return rate 80% and 50% linkage to care probability
Rapid test with pre-test counseling and perfect rates of test return and linkage to care
ELISA test with no pre-test counseling and low rates of test return and linkage to care
Under a ten-year time horizon, 158,000 cases will be identified under current practice (19,000 prevalent; 139,000 incident) in the second 5-year period from 2014 to 2018. Over time, an increasing number of incident cases are identified via current practice as they progress through HIV disease and develop OIs. However, continuing expanded screening for the additional 5 years yields an additional 31,000 cases identified between 2014 and 2018. Compared to current practice, expanded screening will yield incremental costs of $1.1 billion to testing programs and $10.9 billion to discretionary programs, and incremental savings of $2.8 billion to entitlement programs.
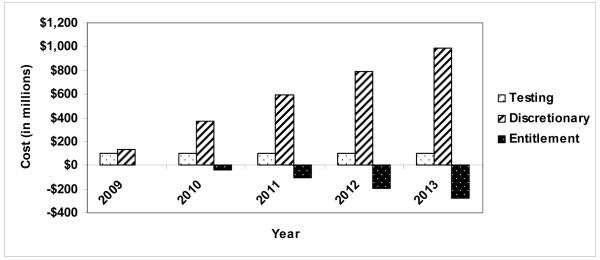
Figure 1 shows annual incremental costs for people identified through expanded screening. In each budget year, expanded screening would incur an additional screening program cost of approximately $101 million. This incremental cost remains constant. The projected annual incremental cost of care to discretionary budgets would rise throughout the period, from $133 million in 2009 to $983 million in 2013. Entitlement programs will experience cost savings, which will increase over time. We project annual savings of $2 million in 2009 and $280 million in 2013. Incremental costs to discretionary programs are not fully offset by cost savings to entitlement programs.
Figure 1.
Incremental costs (in millions) to US government testing, discretionary, and entitlement programs, comparing current practice and expanded screening, 2009-2013.
Figure 2 displays the projected pharmaceutical costs to discretionary programs under current and expanded screening in comparison to the 2007 RW AIDS Drug Assistance Program (ADAP) budget (inflated to $2009), including federal and state contributions (17). In the first year, pharmaceutical costs to discretionary programs would be about $3.2 billion under both scenarios, in comparison to the 2007 ADAP budget of $1.5 billion. By 2013, the annual pharmaceutical cost would increase by $0.2 billion under current screening and $0.8 billion under expanded screening.
Figure 2.
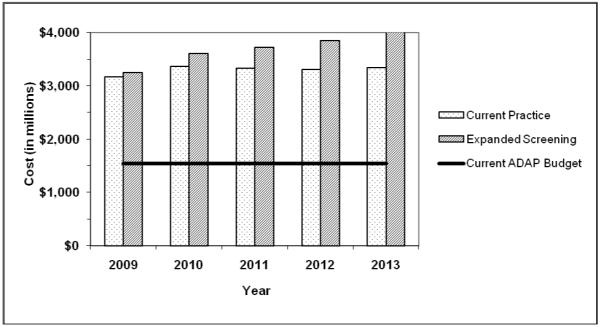
Projected pharmaceutical costs (in millions) to US discretionary programs under current practice and expanded screening, 2009-2013, compared to current ADAP budget.
Results of sensitivity analyses are shown in Table 3. Using ELISA tests will decrease testing costs to $384 million and $766 million for current practice and expanded screening, due to the lower test cost and return rate. Inclusion of pre-test counseling will increase testing costs to $699 million and $1.4 billion for current practice and expanded screening. Perfect rates of test return and linkage to care would increase total care costs to $83.8 billion for current practice ($26.9 billion discretionary; $56.9 billion entitlement) and $86.5 billion for expanded screening ($30.3 billion discretionary; $56.2 billion entitlement). Low rates of test return and linkage to care would decrease total care costs to $82.1 billion for current practice ($24.6 billion discretionary; $57.5 billion entitlement) and $83.5 billion for expanded screening ($26.5 billion discretionary; $57.0 billion entitlement).
Combining these sensitivity analyses, we estimate that the high-cost scenario (rapid test with pre-test counseling and perfect rates of test return and linkage to care) will incur total testing and care costs of $84.5 billion and $87.9 billion for current practice and expanded screening, for an incremental cost of $3.4 billion. The low-cost scenario (ELISA test with no pre-test counseling and low rates of test return and linkage to care) will incur total costs of $82.4 billion and $84.3 billion for current practice and expanded screening, for an incremental cost of $1.9 billion.
If there is a 10 percent increase in the population eligible for government-financed testing and care, total costs will increase to $92.0 billion under current practice and $95.1 billion under expanded screening, for an incremental cost of $3.0 billion.
DISCUSSION
Both cost-effectiveness and budget impact analysis can inform policy decisions (12, 55, 56). Due to the fragmented US healthcare delivery system, the societal perspective used in CEA may not apply to public payers. Healthcare dollars are often earmarked for specific purposes, and decision-makers have limited capacity to determine how and when to spend budgets.
The CDC considered economic evidence in formulating its recent recommendation to increase HIV testing in routine care (7) but did not estimate total costs (11). Peer-reviewed literature has described the potential of the CDC’s screening guidelines to be cost-effective (8, 9), as well as the cost-effectiveness of other testing policies (11). However, government budget outlays for the CDC’s policy are unknown. We projected the budget impact of doubling testing rates from once every ten years to once every five years. Although testing every five years does not perfectly match the CDC recommendation, our aim was to project the budget impact of the policy as it is likely to be implemented.
Expanded screening will increase the costs of HIV testing and care to government programs by $2.7 billion over five years. This increase is large in comparison to the annual RW budget ($2.1 billion per year) (20). It is smaller relative to the total entitlement budget for HIV care (annual federal budgets of $6.3 billion for Medicaid and $3.2 billion for Medicare) (16, 27). In both current practice and expanded screening strategies, HIV testing represents a small fraction of total costs. The downstream pathway of costs triggered by expanded screening and the cost-shifting that will occur between entitlement and discretionary programs are important budget concerns.
Discretionary programs such as RW will be most affected by expanded screening. Pharmaceutical costs will be the main driver of budget increases, as antiretroviral medications represent nearly three-fourths of lifetime HIV care costs (46). An expanded screening program will impose an additional pharmaceutical cost to discretionary programs of $1.9 billion over the five-year period, which is equivalent to 25 percent of the current ADAP budget if it remained constant during the time period. RW has been flat-funded since 2000 despite higher HIV case counts (57). Congress earmarks a portion of RW funds for ADAP and many states supplement their federal allocations with local contributions (20). However, budget constraints have already forced many states to implement ADAP cost-containment strategies such as wait lists, copayments, reduced drug formularies, and restricted eligibility (17).
As the expanded screening program increases HIV care costs to discretionary programs, costs to entitlement programs will decrease slightly. Cost savings to entitlement programs will be very small in the first year but will increase over time. Some cost savings are due to earlier detection of cases, with averted OIs and hospitalizations. Additionally, earlier detection will cause cost-shifting from entitlement to discretionary programs. On average, newly-identified cases will incur fewer treatment costs than previously diagnosed cases, owing to the fact that they will tend to be younger and healthier, less likely to initiate antiretroviral therapy, and more likely to be initially assigned to discretionary programs.
We project that an increase of $503 million in the budget for government-financed testing programs would be required to implement expanded screening, which is larger than the $53 million budget increase proposed for all CDC HIV prevention and surveillance work in the next fiscal year (58). If expanded screening were implemented consistent with the CDC guidelines, the policy may not be feasible without additional funds from state and local governments.
RW is scheduled for reauthorization later this year. Activists argue that RW, particularly ADAP, is chronically under-funded (59); this is confirmed by our results which show that even under current rates of testing, projected discretionary pharmaceutical costs surpass the ADAP budget. If expanded HIV screening increases demand for discretionary programs as we project, RW will be further under-funded and unable to cover the costs of those eligible for services. Expanding Medicaid services to low-income individuals who have not yet progressed to AIDS may alleviate some demand for RW (16). Congress has intermittently considered – but not enacted – the Early Treatment for HIV Act, which would give states the option to extend Medicaid benefits (60). Although entitlement programs generally respond more readily to increased demand, it may be politically challenging to enact legislation to shift costs from discretionary to entitlement programs. Policy-makers may want to consider the downstream care costs of expanded HIV screening as they deliberate RW’s reauthorization.
Limitations
One set of assumptions may have underestimated costs. We did not account for new drugs or technologies, or future changes in treatment guidelines towards earlier initiation of antiretroviral therapy, all of which may increase care costs. We assumed that privately insured individuals retain their coverage, are not under-insured, and are ineligible for government-funded care. We further assumed a constant percentage of newly-identified individuals with private or VA coverage. However, as the epidemic continues to move disproportionately into underserved populations, fewer new cases will have existing coverage. Individuals with private insurance may transition to public programs as they exhaust their benefits or age. Additionally, the weak economy has increased the number of individuals eligible for government programs, as individuals lose employer-based coverage (61). It may be infeasible for government-funded HIV testing centers to exclude individuals with existing coverage. We addressed these limitations through a sensitivity analysis in which we increased the population eligible for government-financed testing and care. Our analysis did not include the budget impact of testing and care costs for youth, even though the CDC guidelines included this group. We also did not explicitly budget the costs of scaling up outreach programs such as the National HIV Testing and Mobilization Campaign (62), because the focus of the CDC recommendations are routine screening in healthcare settings.
Other assumptions may have overestimated costs. We did not model patients dropping out of care or discontinuing antiretroviral therapy. Results did not incorporate financial benefits of preventing HIV transmission to potential partners. While any reduction in the number of secondary infections will likely reduce long-term treatment costs, the magnitude of these cost reductions over a five-year horizon may not be perceptible (10). We did not account for potential economies of scale with higher testing rates.
Our data sources have several limitations. First, it is difficult to obtain precise population estimates of HIV-infected individuals in the VA. Second, although HIVRN includes sites from multiple regions, its data may not be nationally representative. Third, existing observational data do not allow us to assess whether the mix of unaware and aware cases differs by insurance status.
Another limitation is our failure to report year-by-year changes in the demographic composition (e.g., age and sex) of new HIV cases identified. These would provide a fuller description of the impact of alternative screening strategies.
Our analysis ignores the fact that individuals self-select into HIV testing. There is reason to believe that there exists a population of individuals who chronically refuse HIV testing. This group might be large if the CDC guidelines were attempted in earnest. In the emergency department context, for example, it is hard to test more than 10 to 20 percent of potentially eligible patients (40, 53). Differences in risk characteristics between refusers and accepters are currently unknown. Furthermore, there are limited data from these settings on test acceptance conditional on prior refusal. The budget impact of hard-to-test populations warrants further analysis.
All budgets were estimated on an aggregate national basis, rather than to specific states. Because Medicare is a federal program, administrative decisions are often made at the national level. In contrast, RW and Medicaid are primarily administered by states. Consequently, there is substantial interstate variation in program management and service delivery. Variation in eligibility for RW and Medicaid may result in cost-shifting across these programs at the state level. Additionally, the actual unit cost of services and medications to government programs differs nationally due to variation in pharmaceutical prices obtained by state ADAPs, the extent to which state programs pay insurance premiums to fund individuals’ care rather than incurring direct costs, client cost-sharing, and reimbursement rates to providers (17, 63). We ignored interstate variation because our goal was to predict aggregate US costs, rather than the budget impact for specific states. Assessing the budget impact of interstate differences in program administration is an important area of future research.
Finally, the budget impact of expanded HIV screening may be influenced by the recent health reform legislation. Nearly all U.S. residents living with HIV will be insured after 2014. Those below 133% of the federal poverty level will be eligible for Medicaid, and the remainder will most likely be enrolled in the insurance exchange, have employer-based coverage, be enrolled in Medicare, or have access through federally qualified health centers (FQHC). We anticipate that RW budgets will be affected. Although our analysis is relevant to current decision-making, this major policy change warrants future research.
CONCLUSIONS
Expanded screening can identify new HIV cases and facilitate early entry to care, but also treating these individuals will increase budget requirements for government programs by $2.7 billion. The burden will fall disproportionately on discretionary programs, because persons identified with HIV early are less likely to be immediately eligible for entitlement programs. Expanded HIV screening will not meet early treatment goals unless government programs have sufficient budgets to conduct testing and provide care for newly-identified cases.
Acknowledgements
We are grateful to Kenneth Freedberg for making available the CEPAC Model and its associated resources, Caroline Sloan for her technical assistance, and Mark Schlesinger, Patricia Keenan, and Haiqun Lin for helpful comments on an earlier draft.
Financial support: This work was supported by the National Institute on Drug Abuse (R01DA015612), the Agency for Health Research and Quality (T32HS017589), the National Institute of Mental Health (R01MH65869 and R01MH073445), the National Institute of Allergy and Infectious Diseases (R37 AI042006), and the Doris Duke Charitable Foundation (Clinical Scientist Development Award).
TECHNICAL APPENDIX
Expanded HIV Screening in the U.S.: What Will It Cost Government Discretionary and Entitlement Programs? A Budget Impact Analysis
Introduction
This technical appendix supplements the methodological details in the manuscript text. We provide additional information on the Disease Module and Screening Module, detail the source data for the Disease Module, describe the incident and prevalent cohorts modeled in the analysis and derive their population estimates, describe HIV screening protocols and detail how these costs were tabulated in the analysis, and clarify how we differentiated entitlement and discretionary costs. Portions of the Disease Module description have been previously published elsewhere (1-4).
Disease Module Overview
The Cost-Effectiveness of Preventing AIDS Complications (CEPAC) Model is a widely-published computer-based model of chronic HIV disease (1-5). It uses a Monte Carlo state-transition framework to track the natural history of illness in individuals. “State-transition” means that the model characterizes patients’ natural history of illness as a sequence of monthly transitions from one health state to another. “Monte Carlo” refers to a random number generator and set of estimated probabilities that are used to determine the sequence of transitions among health states.
Clinically, HIV-infected individuals experience a rapid rise in the HIV viral load within the first few weeks of exposure. Acute infection may manifest with flu-like symptoms in up to half of patients. However, this syndrome is self-limited and often does not present to medical attention or lead to HIV diagnosis. With resolution of acute infection symptoms, viral load declines to a “steady state.” Undetected HIV-infected individuals then remain in a period of asymptomatic infection with progressive immunologic decline and continued viral replication for almost a decade. In the absence of HIV testing during the asymptomatic period, patients generally present to medical care with an opportunistic infection (OI) or other HIV-related illness such as renal insufficiency. Patients whose HIV infection is detected may benefit from antiretroviral therapy (ART), which acts to suppress the viral load, increase immune system function, and deter poor HIV-related outcomes (6).
The CEPAC Model simulates the clinical disease process as follows. Simulated patients undergo monthly transitions among mutually exclusive health states, categorized by primary HIV infection, chronic illness, acute illness, and death. States are further subdivided into six unique CD4 strata (>500 cells/mm3, 301-500 cells/mm3, 201-300 cells/mm3, 101-200 cells/mm3, 50-100 cells/mm3, and ≤50 cells/mm3) and seven unique HIV RNA (viral load) strata (>100,000 copies/mL, 30,001-100,000 copies/mL, 10,001-30,000 copies/mL, 3,001-10,000 copies/mL, 501-3,000 copies/mL, 51-500 copies/mL, and ≤50 copies/mL). Patients are at risk for the following opportunistic infections (OI) that lead to temporary states of acute illness: Pneumocystis jiroveci pneumonia (PCP), toxoplasmosis, Mycobacterium avium complex (MAC) disease, disseminated fungal infection, cytomegalovirus, and bacterial and other infections. Monthly probabilities of events include changes in CD4 counts and viral load, the development of OIs, adverse reactions to medications, and death. The viral load level determines the rate of CD4 cell decline in the absence of antiretroviral therapy (ART) or in patients who have failed ART, and the CD4 count predicts the monthly risk of OIs.
Investigators may specify the number, efficacy, and cost of sequential regimens of ART. In the model, ART decreases viral load. The reduction in viral load promotes CD4 rebound, thereby decreasing the risk of developing an OI.
Summary statistics are collected for each simulated patient on all clinical events, the length of time spent in each health state, and the associated costs and quality of life. A large cohort (usually around one million) is simulated until estimates are stable. Investigators input user-defined initial population distributions of demographic and clinical characteristics, including age, sex, CD4 count, and viral load. Investigators also specify the number of sequential lines of available ART, starting criteria for drug therapy, efficacy, and cost of treatment. Other user-defined clinical inputs include the timing and effectiveness of prophylaxis and treatment for OIs and the frequency of ongoing laboratory monitoring (CD4 counts and HIV RNA tests) and routine clinical visits. Table 4 lists additional details on these inputs. There are over a thousand inputs in total and we do not reproduce them all here. A full set of inputs is available to interested readers upon request.
Table 4.
Inputs and source data for efficacy and cost of antiretroviral regimens, OI treatments, and routine outpatient care
| Variable | Baseline Value | Reference |
|---|---|---|
| Efficacy of antiretroviral therapy (% HIV RNA suppressed to < 400 copies/mL at 48 weeks) | ||
| 1st line | 73 | (19) |
| 2nd line | 62 | (21) |
| 3rd line | 52 | (21) |
| 4th line | 55 | (20) |
| 5th line | 34 | (24) |
| 6th line OBR only | 13 | (24) |
| Cost of antiretroviral therapy ($USD/month) | ||
| 1st line | 1,278 | (53) |
| 2nd line | 1,953 | (53, 54) |
| 3rd line | 1,961 | (53, 54) |
| 4th line | 2,480 | (53, 54) |
| 5th line | 3,801 | (53, 54) |
| 6th line | 1,738 | (53, 54) |
| Opportunistic infections and routine care | ||
| Timing of prophylaxis and treatment for OIs | National guidelines | (28) |
| Frequency of ongoing laboratory monitoring | Every 3 months | (28) |
| Frequency of routine clinical visits | Every 3 months | (28) |
Screening Module Overview
A separate Screening Module has been designed to simulate the clinical impact of counseling, testing, and referral (CTR) on incident and prevalent patients who are unaware of their infection status at the start of the simulation (3, 7). The Screening Model is a front-end structure that determines when each simulated patient will become detected through screening or presentation to care with an OI. For those detected through screening, the Screening Module additionally determines whether they were linked to care. All patients (detected and undetected) have their clinical outcomes tracked through the Disease Module. However, only those who have been successfully detected and linked to care are eligible for treatment and incur costs. The Screening Module allows for input assumptions on testing frequency, linkage to care, rates of return for results, clinical characteristics of undetected populations, test performance characteristics (such as sensitivity and specificity), and cost.
National testing rates were translated into monthly probabilities of test receipt using a binomial approximation. For example, an annual test was simulated with a monthly test probability of 1/12. We assumed that all undetected patients with an OI would present to care and learn of their infection status during the treatment episode.
Source Data for Disease Module
All clinical data for the Disease Module were derived from published literature, including the Multicenter AIDS Cohort Study (MACS), randomized clinical trials, and cohort studies. Data from MACS were used to model the natural history of disease (8), including the probability of chronic AIDS death for each health state, the probability of primary infection with an OI, and the probability of death from an OI. Cohort studies were used to derive the probability of secondary infection with an OI (9-16). The monthly probability of non-AIDS deaths was calculated from the U.S. life expectancy tables (17). The monthly baseline CD4 decline was derived from clinical literature (18). The efficacy of each antiretroviral regimen was estimated from published clinical trials (19-25). The full set of parameters is available from the authors upon request.
Patients initiated ART at CD4 < 350 or upon development of an OI, in accordance with DHHS guidelines (26). Patients were offered six sequential lines of therapy, with decreasing effectiveness. Patients who were observed to fail on one line of therapy were switched to the subsequent line in the following month. Failure was defined as an observed increase in viral load or a 50 percent decrease in CD4 count for two consecutive months. Data on the efficacy of the ART regimens (measured by rates of viral suppression) were derived from clinical trials (19-25). We reduced the efficacy of ART regimens, as reported in clinical trials, by 15 percent to account for lower efficacy between observational cohorts and clinical trials (19, 27). Prior to the start of each new regimen, patients received an HIV drug resistance test, in accordance with DHHS guidelines (26). Patients received OI prophylaxis therapy in accordance with national recommendations (28). Data on the efficacy of OI treatments and the probabilities of prophylaxis-related toxicity were derived from clinical trials (13, 29-36). Viral load and CD4 counts were measured every three months, in accordance with the guidelines (26).
Characteristics of Incidence and Prevalent Cohorts
We modeled three distinct cohorts: 1) prevalent cases aware of their infection status at the start of the simulation (“prevalent aware”), 2) prevalent cases unaware of their infection at the start of the simulation (“prevalent unaware”), and 3) incident cases. The first cohort was modeled using the Disease Model. The latter two cohorts were modeled using both the Screening Model and Disease Model; patients in these cohorts were only eligible for treatment upon successful detection and linkage to care. We assumed that all incident cases are unaware of their infection until they are detected in the Screening Model. To calculate the number of patients eligible for government programs, we used national estimates of prevalence and incidence and subtracted the number of HIV-infected individuals likely to receive care through private insurance or the Veterans Administration (VA), as described below.
Prevalent Cases Currently Aware of Their Infection
The CDC estimates that there are 1,106,000 cases of HIV in the U.S., of whom 21% do not know their infection status (37). This yields 874,000 prevalent cases aware of their infection (“prevalent aware”) and a maximum of 232,000 prevalent cases who could potentially be identified through a screening program (“prevalent unaware”). The VA cares for approximately 33,400 cases (38). Of patients enrolled in HIVRN sites, 15.4% have private insurance (pooled across years) (39). The HIVRN sample excludes veterans. We calculate the number of “prevalent aware” cases with private insurance as 15.4%*(874,000-33,400) = 129,000. The number of “prevalent aware” cases currently eligible for care through discretionary or entitlement programs is the total population (874,000) minus those receiving care through the VA (33,400) and private insurance (129,000), which equals 711,000.
The actual number of individuals who seek government-financed care may be larger than our estimate if there is a decrease in the number of individuals with private or VA insurance, or if some individuals are under-insured. We address this variable with a sensitivity analysis in which we increase the population eligible for testing and care by 10 percent. The upper bound for the prevalent aware estimate is 747,000.
Prevalent Cases Currently Unaware of Their Infection
We assumed that the fraction of “prevalent unaware” cases that would be eligible for care through the VA or private insurance would be similar to the payer distribution of “prevalent aware” cases. Patients without private insurance may have fewer encounters with the healthcare system, in which they might receive an HIV test. If this were true, “prevalent unaware” cases would be more likely to be eligible for discretionary or entitlement programs, compared to “prevalent aware” cases. Our assumption of a similar payer distribution would thereby result in a conservative estimate of the budgetary impact to public payers. We addressed this issue in a sensitivity analysis, in which we increased the number of cases eligible for publicly financed healthcare by 10 percent.
To estimate the number of “prevalent unaware” cases eligible for care through the VA upon detection, we assumed that the probability of awareness of HIV status among veterans is similar to the U.S. population (79%). The 33,400 “prevalent aware” VA cases would correspond to 33,400*(0.79/0.21) = 8,900 “prevalent unaware” VA cases. As described above, we estimated the number of “prevalent unaware” cases with private insurance as 15.4%*(232,000-8,900) = 34,400. The number of “prevalent unaware” cases currently eligible for care through discretionary or entitlement programs is the total population (232,000) minus those likely to receive care through the VA (8,900) and private insurance (34,000), which equals 189,000. In our sensitivity analysis, we used an upper bound of 198,000.
Incident Cases
We used the CDC’s recently updated estimates of national HIV incidence (revised from 40,000 to 56,300 new cases annually) (40). If the fraction of incident cases receiving care through the VA and private insurance is similar to the fraction among “prevalent aware” cases, then 2,200 of incident cases would be eligible for VA care and 8,300 would be eligible for private insurance upon detection. The number of incident cases currently eligible for care after detection through discretionary or entitlement programs is the total population of incident cases (56,300) minus those likely to receive care through the VA (2,200) and private insurance (8,300), which equals 45,800. The number of individuals who seek a government-financed test may be larger if it is difficult in practice to exclude those with alternate insurance sources from public testing facilities. In our sensitivity analysis, we used an upper bound of 48,100.
In contrast to the two static prevalent cohorts, a new incident cohort arrives during each budget year. To estimate the budgetary impact of incident cohorts, one run was performed to capture annual costs for a single newly-infected cohort. These costs were repeated for each subsequent budget year, with the arrival of a new incident cohort. The total cost in each year was the summation of the annual costs of the cohorts. Similar to cost calculations on the prevalent unaware cohort, we only tabulated costs of incident cases who have been identified. New cases are not linked to care in the model until detection.
Characteristics of Individuals Eligible for Screening
We tabulated HIV screening costs for non-elderly adults eligible for discretionary or entitlement programs only (aged 19 to 64) in order to reflect the revised guidelines’ focus on this group (41). We tabulated treatment costs for all adults (aged 19 or greater) eligible for these government programs, including the elderly. As less than 3 percent of HIV-infected individuals are 65 or older (42), seniors comprise a small share of total treatment costs. However, the effectiveness of current therapies makes it possible for those infected and identified to live into late adulthood, thereby incurring costs to Medicare.
Data from the Henry J. Kaiser Family Foundation (KFF) were used to derive the number of non-elderly adults eligible for a government-financed HIV test (43). Of the 261,400,000 non-elderly individuals (including children) in the U.S., 16% (41,800,000) are enrolled in Medicaid or other public insurance and 17% (44,400,000) are uninsured. Of the 78,600,000 children in the U.S., 29% (22,800,000) have Medicaid or other publicly-funded care and 11% (8,600,000) are uninsured. Subtracting the number of children from the former estimates yields 19,000,000 non-elderly adults (aged 19 to 64) enrolled in Medicaid or other public health programs and 35,800,000 non-elderly uninsured adults.
We used data from the VA (44) to remove the number of non-elderly veterans from this estimate. The VA estimates there are 7,800,000 veterans enrolled in the VA healthcare system, of whom 39% (3,100,000) are elderly. We assumed that the number of veterans under 19 years of age was negligible. Subtracting the VA non-elderly population (4,700,000) from the KFF estimates yielded a total of 50,100,000 non-elderly adults in the U.S. who would be eligible for an HIV test financed through a public program. In practice, program coordinators at HIV prevention centers may be unable to either exclude those with private or VA insurance from HIV testing or obtain reimbursement through these sources. We addressed this in a sensitivity analysis, in which we increased the number of cases eligible for government-financed testing to 55,100,000.
Calculation of Screening Costs
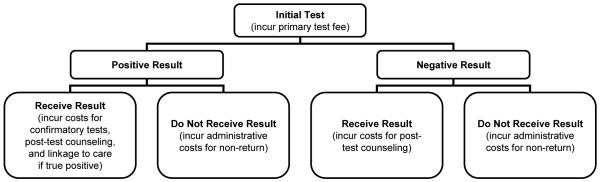
There are several reasons why HIV-infected individuals may not access care: first, they may not know they are infected, and second, they may know their infection status but have not been linked to care. We account for both of these issues in the screening module. As discussed above, 21 percent of individuals do not know they have been infected (37). This is incorporated into our estimates through the N calculations described above. Some individuals receive a test but do not return for results, and this test return varies by test type (45). Of those who are identified, clinical data suggest that approximately 80 percent are linked to care (46-48). Incomplete test receipt and linkage to care is incorporated into the screening module input parameters and is described in greater detail below.
Screening Protocols
In the base case, we modeled a one-step rapid test with two steps for a positive result, as described in prior cost-effectiveness analyses (49-51). After a patient consents to testing, a provider collects a specimen and a laboratory technician processes the sample. If the result is positive, a second specimen is collected and sent to an outside laboratory for a Western blot confirmatory analysis. Patients must return to receive results during a second visit. All patients receive post-test counseling at the time of their test results. For HIV-positive patients, post-test counseling includes assistance with identifying follow-up care. Approximately three percent of patients do not wait to receive results of their rapid test during the initial visit (45), which we indicate in Table 1 as a 97 percent “test return rate.” Of those who receive their positive rapid test results, four-fifths are successfully linked to follow-up HIV specialty care (46, 48), which we indicate in Table 1 as an 80 percent “probability detected case linked to care.” We assumed confirmatory tests had perfect sensitivity and specificity, an assumption used in other cost analyses (51).
In a sensitivity analysis, we modeled a two-step enzyme-linked immunosorbent assay (ELISA) with four steps for a positive initial result (49-51). In contrast to the rapid test, patients do not receive preliminary results during the first visit. Upon consent, a provider collects a specimen which is sent to an outside laboratory for analysis. If the specimen tests positive, a technician will repeat the ELISA twice (for a total of three ELISA tests) and perform a subsequent Western blot. Patients must return for a second visit to receive their results and post-test counseling. Due to the two-visit process, there is a higher rate of non-return for the ELISA than the rapid test: approximately three-fourths of those with positive readings and two-thirds of those with negative readings return for their results (45). Table 1 differentiates between the test return rates for positive and negative results. We assumed that those who received their positive ELISA test results had the same probability of being linked to care (80%) as those who received their positive rapid test results.
Screening Costs
The assignment of screening costs is displayed graphically in Figure 3. All patients incurred the initial screening costs. HIV-infected individuals in the acute state (the first two months of infection) were assumed to remain undetected because they do not have detectable antibodies (6), unless they received a false positive antibody result and were subsequently identified by confirmatory testing. This occurred with probability 0.1%, obtained by subtracting the test specificity (99.9%) from 1. A small fraction (0.4%, determined by the test sensitivity) received a false negative result. All individuals incurred the costs of post-test counseling upon receipt of their positive or negative test results. Patients testing positive on the initial test incurred costs for additional laboratory testing, post-test counseling, and linkage to care. All costs were derived from micro-cost data presented by Farnham (51), adjusted for inflation using the medical care component of the CPI (52). Government payers incurred additional administration costs for patients who did not return for their results, which included mailing reminder letters or making phone calls. We assumed these costs totaled 30 minutes of staff time and $1 for materials (mailings and phone calls).
Figure 3.
Assignment of HIV screening costs for budget impact analysis.
Screening costs for HIV-negative individuals were calculated deterministically based on test characteristics. All patients incurred the initial screening costs. The small fraction (0.1%, determined by the test specificity) receiving a false positive result incurred costs for subsequent confirmatory testing and post-test counseling but not linkage to care. Patients testing negative on the initial test incurred post-test counseling costs if they received their results. Patients who did not return for their results incurred an administration cost. All costs except for the non-return cost were derived from Farnham (51).
Differentiating Between Discretionary and Entitlement Costs
As described in the manuscript, we assumed that the treatment costs for all currently undetected patients (incident and “prevalent unaware”) were incurred by discretionary programs until they developed an OI or attained age 65, at which point their care was financed through an entitlement program. We used these markers as a proxy for gaining eligibility for Medicaid or Medicare on the basis of disability or age.
We allowed currently detected patients (“prevalent aware”) to start the simulation in either a discretionary or entitlement program. As with the incident and “prevalent unaware” cohorts, patients enrolled in a discretionary program became eligible for an entitlement program when they developed an OI or attained age 65. We used national data to assign the fraction of the “prevalent aware” cohort to each program. Among patients currently enrolled in HIVRN, 28.7% receive care financed by RW or are uninsured, and 55.9% receive care through Medicare or Medicaid (39). Using the calculation method described above, we estimated that 241,000 of the “prevalent aware” cases were eligible for care through a discretionary program at the start of the simulation, and that the remaining 470,000 were immediately eligible for care through Medicare or Medicaid.
We differentiated between discretionary and entitlement costs using two sets of simulations. The first set of simulations tracked all costs. The second set of simulations used identical inputs except that costs terminated in the month following detection with an OI or attainment of age 65. This second set of simulations represented costs to discretionary programs. The difference between these cost outlays represents the costs to entitlement programs.
References
- 1.Freedberg K, Losina E, Weinstein M, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 2.Freedberg K, Scharstein J, Seage G, III, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–36. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 3.Paltiel A, Weinstein M, Kimmel A, et al. Expanded screening in the United States -- an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 4.Walensky R, Paltiel A, Losina E, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006:194. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein M, Goldie S, Losina E, et al. Use of genotype resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–50. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 6.Kahn J, Walker B. Acute human immunodeficiency virus type I infection. N Engl J Med. 1998;339:33–39. doi: 10.1056/NEJM199807023390107. [DOI] [PubMed] [Google Scholar]
- 7.Paltiel A, Walensky R, Schackman B, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Muticenter AIDS Cohort Study (MACS) Public Dataset: Release PO4. National Technical Information Service; Springfield, VA: 1995. [Google Scholar]
- 9.Bozzette S, Larsen R, Chiu J, et al. California Collaborative Treatment Group A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:580–84. doi: 10.1056/NEJM199102283240902. [DOI] [PubMed] [Google Scholar]
- 10.Cole S, Hernan M, Robins J, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–94. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 11.Drew W, Ives D, Lalezari J, et al. Syntex Cooperative Oral Ganciclovir Study Group Oral ganciclovir as maintenance treatment for Cytomegalovirus retinitis in patients with AIDS. N Engl J Med. 1995;333:615–20. doi: 10.1056/NEJM199509073331002. [DOI] [PubMed] [Google Scholar]
- 12.Dube M, Sattler F, Torriani F, et al. A randomized evaluation of ethambutol for prevention of relapse and drug resistance during treatment of Mycobacterium avium complex bacteremia with clarithromycin-based combination therapy. J Infect Dis. 1997;176:1225–32. doi: 10.1086/514116. [DOI] [PubMed] [Google Scholar]
- 13.Ioannidis J, Cappelleri J, Skolnik P, et al. A meta-analysis of the relative efficacy and toxicity of Pneumocystis carinii prophylactic regimens. Arch Intern Med. 1996;156:177–88. [PubMed] [Google Scholar]
- 14.Pedrol E, Gonzalez-Clemente J, Gatell J, et al. Central nervous sytem toxoplasmosis in AIDS patients: efficacy of an intermittent maintenance therapy. AIDS. 1990;4:511–17. doi: 10.1097/00002030-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Powderly W, Saag M, Cloud G, et al. The NIAID AIDS Clinical Trials Group. Mycoses Study Group A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1992;326:793–98. doi: 10.1056/NEJM199203193261203. [DOI] [PubMed] [Google Scholar]
- 16.Wheat J, Hafner R, Wulfsohn M, et al. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118:610–16. doi: 10.7326/0003-4819-118-8-199304150-00006. [DOI] [PubMed] [Google Scholar]
- 17.Arias E. US life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PubMed] [Google Scholar]
- 18.Mellors J, Munoz A, Giorgi J, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 19.Gallant J, DeJesus E, Arribas J, et al. Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med. 2006;354:251–60. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 20.Grinsztejn B, Nguyen B, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–69. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 21.Johnson M, Grinsztejn B, Rodriguez C, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–94. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 22.Johnson M, Grinsztejn B, Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20:711–18. doi: 10.1097/01.aids.0000216371.76689.63. [DOI] [PubMed] [Google Scholar]
- 23.Lalezari J, Goodrich J, DeJesus E, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada; Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. 2007. [Google Scholar]
- 24.Nelson M, Arasteh K, Clotet B, et al. Durable efficacy of enfuvirtide over 48 weeks in heavily treatment-experienced HIV-1-infected patients in the T-20 versus optimized background regimen only 1 and 2 clinical trials. J Acquir Immune Defic Syndr. 2005;40:404–12. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 25.Pozniak A, Gallant J, DeJesus E, et al. Tenofovir disoproxil fumarate, emtricitabine, and efavirenz versus fixed-dose zidovudine/lamivudine and efavirenz in antiretroviral-naive patients: virologic, immunologic, and morphologic changes--a 96-week analysis. J Acquir Immune Defic Syndr. 2006;43:535–40. doi: 10.1097/01.qai.0000245886.51262.67. [DOI] [PubMed] [Google Scholar]
- 26.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2008. [Google Scholar]
- 27.Moore R, Keruly J, Gebo K, et al. An improvement in virologic response to highly active antiretroviral therapy in clinical practice from 1996 through 2002. J Acquir Immune Defic Syndr. 2005;39:195–98. [PubMed] [Google Scholar]
- 28.Panel on Guidelines for the Prevention. Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents . Guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. 2008. [Google Scholar]
- 29.Benson C, Williams P, Cohn D, et al. The AIDS Clinical Trials Group 196/Terry Beirn Community Programs for Clinical Research on AIDS 009 Protocol Team Clarithromycin or rifabutin alone or in combination for primary prophylaxis of Mycobacterium avium complex disease in patients with AIDS: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2000;181:1289–97. doi: 10.1086/315380. [DOI] [PubMed] [Google Scholar]
- 30.Carr A, Tindall B, Brew B, et al. Low-dose trimethoprim-sulfamethoxazole prophylaxis for toxoplasmic encephalitis in patients with AIDS. Ann Intern Med. 1992;117:106–11. doi: 10.7326/0003-4819-117-2-106. [DOI] [PubMed] [Google Scholar]
- 31.El-Sadr W, Murphy R, Yurik T, et al. Community Program for Clinical Research on AIDS and the AIDS Clinical Trials Group Atovaquone compared with dapsone for the prevention of Pneumocystis carinii pneumonia in patients with HIV infection who cannot tolerate trimethoprim, sulfonamides, or both. N Engl J Med. 1998;339:1889–95. doi: 10.1056/NEJM199812243392604. [DOI] [PubMed] [Google Scholar]
- 32.Girard P, Landman R, Gaudebout C, et al. The PRIO Study Group Dapsone-pyrimethamine compared with aerosolized pentamidine as primary prophylaxis against Pneumocystis carinii pneumonia and toxoplasmosis in HIV infection. N Engl J Med. 1993;328:1514–20. doi: 10.1056/NEJM199305273282102. [DOI] [PubMed] [Google Scholar]
- 33.Havlir D, Dube M, Sattler F, et al. California Collaborative Treatment Group Prophylaxis against disseminated Mycobacterium avium complex with weekly azithromycin, daily rifabutin, or both. N Engl J Med. 1996;335:392–98. doi: 10.1056/NEJM199608083350604. [DOI] [PubMed] [Google Scholar]
- 34.Leport C, Raffi F, Matheron S, et al. Treatment of central nervous system toxoplasmosis with pyrimethamine/sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am J Med. 1998;84:94–100. doi: 10.1016/0002-9343(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 35.Lidman C, Berglund O, Tynell E, et al. Aerosolized pentamidine as primary prophylaxis for Pneumocystis carinii pneumonia: efficacy, mortality and morbidity. AIDS. 1994;8:935–40. doi: 10.1097/00002030-199407000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Nightingale S, Cameron D, Gordin F, et al. Two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993;329:828–33. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention HIV prevalence estimates - United States, 2006. MMWR Morb Mortal Wkly Rep. 2008;57:1073–76. [PubMed] [Google Scholar]
- 38.Fultz S, Skanderson M, Mole L, et al. Development of a “virtual” cohort using the national VA health information system. Med Care. 2006;8:S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 39.Fleishman J, Gebo K, Reilly E, et al. Hospital and outpatient health services utilization among HIV-infected adults in care 2000-2002. Med Care. 2005;43:40–52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 40.Hall H, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–29. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention . CDC releases revised HIV testing recommendations in healthcare settings. 2006. [Google Scholar]
- 42.Centers for Disease Control and Prevention . HIV/AIDS surveillance report, 2004. Vol. 16. US Department of Health and Human Services; Atlanta, GA: 2005. [Google Scholar]
- 43.Henry J, Kaiser Family Foundation . Health insurance coverage in America, 2007 (chartbook) 2007. [Google Scholar]
- 44.National Center for Veterans Analysis and Statistics . VA benefits and healthcare utilization. 2008. [Google Scholar]
- 45.Centers for Disease Control and Prevention Update: HIV counseling and testing using rapid tests - United States, 1995. MMWR Morb Mortal Wkly Rep. 1998;47:211–15. [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention Rapid HIV testing in emergency departments - three U.S. sites, January 2005 - March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597–601. [PubMed] [Google Scholar]
- 47.Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention guidelines. J Acquir Immune Defic Syndr. 2007;46:395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 48.Lyss S, Branson B, Kroc K, et al. Detecting unsuspected HIV infection with a rapid whole-blood HIV test in an urban emergency department. J Acquir Immune Defic Syndr. 2007;44:442–53. doi: 10.1097/QAI.0b013e31802f83d0. [DOI] [PubMed] [Google Scholar]
- 49.Ekwueme D, Pinkerton S, Holtgrave D, et al. Cost comparison of three HIV counseling and testing technologies. Am J Prev Med. 2003;25:112–21. doi: 10.1016/s0749-3797(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 50.Farnham P, Grosky R, Holtgrave D, et al. Counseling and testing for HIV prevention: costs, effects, and cost-effectiveness of more rapid screening tests. Public Health Rep. 1996;111:44–53. [PMC free article] [PubMed] [Google Scholar]
- 51.Farnham P, Hutchinson A, Samsom S, et al. Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep. 2008;123:51–62. doi: 10.1177/00333549081230S307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.US Department of Labor, Bureau of Labor and Statistics . Consumer price index - medical care component. 2009. [Google Scholar]
- 53.Red Book. Medical Economics; Montvale, NJ: 2004. [Google Scholar]
- 54.Schackman B, Gebo K, Walensky R, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–97. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
REFERENCES
- 1.Marks G, Crepaz N, Senterfitt J, et al. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States. J Acquir Immune Defic Syndr. 2005;39:446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 2.Quinn T, Wawer M, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type I. N Engl J Med. 2000;342:921–29. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 3.Crepaz N, Lyles C, Wolitski R, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials [Editorial] AIDS. 2006;20:143–57. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 4.Barroso P, Schechter M, Gupta P, et al. Effect of antiretroviral therapy on HIV shedding in the semen. Ann Intern Med. 2000;133:280–84. doi: 10.7326/0003-4819-133-4-200008150-00012. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention CDC releases revised HIV testing recommendations in healthcare settings. 2006 [Google Scholar]
- 6.Bartlett J, Branson B, Fenton K, et al. Opt-out testing for human immunodeficiency virus in the United States: progress and challenges. JAMA. 2008;300:945–51. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 7.Branson B. Current HIV epidemiology and revised recommendations for HIV testing in health-care settings. J Med Virol. 2007;79:S6–S10. doi: 10.1002/jmv.20972. [DOI] [PubMed] [Google Scholar]
- 8.Paltiel A, Weinstein M, Kimmel A, et al. Expanded screening in the United States -- an analysis of cost-effectiveness. N Engl J Med. 2005;352:586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- 9.Sanders G, Bayoumi A, Biliar S, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–85. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- 10.Paltiel A, Walensky R, Schackman B, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145:797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- 11.Holtgrave D. Costs and consequences of the US Centers for Disease Control and Prevention’s recommendations for opt-out HIV testing. PLoS Med. 2007;4:e194. doi: 10.1371/journal.pmed.0040194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mauskopf J, Sullivan S, Annemans L, et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on Good Research Practice - Budget Impact Analysis. Value Health. 2007;10:336–47. doi: 10.1111/j.1524-4733.2007.00187.x. [DOI] [PubMed] [Google Scholar]
- 13.Henry J, Kaiser Family Foundation . Medicaid and HIV/AIDS fact sheet. 2006. [Google Scholar]
- 14.Henry J, Kaiser Family Foundation . The Ryan White Program. 2008. [Google Scholar]
- 15.Henry J, Kaiser Family Foundation Medicare and HIV/AIDS. 2009 [Google Scholar]
- 16.Committee on Public Financing and Delivery of HIV Care. Board on Health Promotion and Disease Prevention . Public finance and delivery of HIV/AIDS care: securing the legacy of Ryan White. National Academies Press; Washington, DC: 2005. [Google Scholar]
- 17.Henry J. Kaiser Family Foundation, National Alliance of State and Territorial AIDS Directors. National ADAP monitoring project annual report. 2008 [Google Scholar]
- 18.Schackman B, Freedberg K, Goldie S, et al. Budget impact of Medicaid Section 1115 demonstrations by early HIV treatment. Health Care Financ Rev. 2005;26:67–80. [PMC free article] [PubMed] [Google Scholar]
- 19.US Department of Labor, Bureau of Labor and Statistics . Consumer price index - medical care component. 2009. [Google Scholar]
- 20.Health Resources and Services Administration Ryan White HIV/AIDS Program website. 2009
- 21.Henry J, Kaiser Family Foundation . Health insurance coverage in America, 2007 (chartbook) 2007. [Google Scholar]
- 22.National Center for Veterans Analysis and Statistics . VA benefits and healthcare utilization. 2008. [Google Scholar]
- 23.Hall H, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300:520–29. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention . HIV/AIDS surveillance report, 2006. Vol. 18. US Department of Health and Human Services; Atlanta, GA: 2008. [Google Scholar]
- 25.Fleishman J, Gebo K, Reilly E, et al. Hospital and outpatient health services utilization among HIV-infected adults in care 2000-2002. Med Care. 2005;43:40–52. doi: 10.1097/01.mlr.0000175621.65005.c6. [DOI] [PubMed] [Google Scholar]
- 26.Fultz S, Skanderson M, Mole L, et al. Development of a “virtual” cohort using the national VA health information system. Med Care. 2006;8:S25–S30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 27.Henry J, Kaiser Family Foundation . Medicare and HIV/AIDS fact sheet. 2008. [Google Scholar]
- 28.Agency for Healthcare Research and Quality . HIVnet, HIV resource utilization data coordinating center (DCC) project. 2003. [Google Scholar]
- 29.Muticenter AIDS Cohort Study (MACS) Public Dataset: Release PO4. National Technical Information Service; Springfield, VA: 1995. [Google Scholar]
- 30.Paltiel A, Goldie S, Losina E, et al. Preevaluation of clinical trial data: the case of preemptive cytomegalovirus therapy in patients with human immunodeficiency virus. Clin Infect Dis. 2001;32:783–93. doi: 10.1086/319223. [DOI] [PubMed] [Google Scholar]
- 31.Walensky R, Goldie S, Sax P, et al. Treatment for primary HIV infection: projecting outcomes of immediate, interrupted, or delayed therapy. J Acquir Immune Defic Syndr. 2002;31:27–37. doi: 10.1097/00126334-200209010-00004. [DOI] [PubMed] [Google Scholar]
- 32.Samet J, Freedberg K, Savetsky J, et al. Understanding delay to medical care for HIV infection: the long-term non-presenter. AIDS. 2001;15:77–85. doi: 10.1097/00002030-200101050-00012. [DOI] [PubMed] [Google Scholar]
- 33.Freedberg K, Scharstein J, Seage G, III, et al. The cost-effectiveness of preventing AIDS-related opportunistic infections. JAMA. 1998;279:130–36. doi: 10.1001/jama.279.2.130. [DOI] [PubMed] [Google Scholar]
- 34.Freedberg K, Losina E, Weinstein M, et al. The cost effectiveness of combination antiretroviral therapy for HIV disease. N Engl J Med. 2001;344:824–31. doi: 10.1056/NEJM200103153441108. [DOI] [PubMed] [Google Scholar]
- 35.Paltiel A, Scharfstein J, Seage G, III, et al. A Monte Carlo simulation of advanced HIV disease: application to prevention of CMV infection. Med Decis Making. 1998;18:S93–S105. doi: 10.1177/0272989X98018002S11. [DOI] [PubMed] [Google Scholar]
- 36.National Alliance of State and Territorial AIDS Directors . Rapid HIV testing assessment. 2006. [Google Scholar]
- 37.Farnham P, Hutchinson A, Samsom S, et al. Comparing the costs of HIV screening strategies and technologies in health-care settings. Public Health Rep. 2008;123:51–62. doi: 10.1177/00333549081230S307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farnham P, Grosky R, Holtgrave D, et al. Counseling and testing for HIV prevention: costs, effects, and cost-effectiveness of more rapid screening tests. Public Health Rep. 1996;111:44–53. [PMC free article] [PubMed] [Google Scholar]
- 39.Ekwueme D, Pinkerton S, Holtgrave D, et al. Cost comparison of three HIV counseling and testing technologies. Am J Prev Med. 2003;25:112–21. doi: 10.1016/s0749-3797(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 40.Brown J, Shesser R, Simon G, et al. Routine HIV screening in the emergency department using the new US Centers for Disease Control and Prevention guidelines. J Acquir Immune Defic Syndr. 2007;46:395–401. doi: 10.1097/qai.0b013e3181582d82. [DOI] [PubMed] [Google Scholar]
- 41.Lyons M, Lindsell C, Ledyard H, et al. Emergency department HIV testing and counseling: an ongoing experience in a low-prevalence area. Ann Emerg Med. 2005;46:22–28. doi: 10.1016/j.annemergmed.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 42.Silva A, Glick N, Lyss S, et al. Implementing an HIV and sexually transmitted disease screening program in an emergency department. Ann Emerg Med. 2007;49:564–72. doi: 10.1016/j.annemergmed.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 43.Walensky R, Losina E, Malatesta L, et al. Effective HIV case identification through routine HIV screening at urgent care centers in Massachusetts. Am J Pub Health. 2005;95:71–73. doi: 10.2105/AJPH.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mahajan A, Stemple L, Shapiro M, et al. Consistency of state statutes with the Centers for Disease Control and Prevention HIV testing requirements for health care settings. Ann Intern Med. 2009;150:263–69. doi: 10.7326/0003-4819-150-4-200902170-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Red Book. Medical Economics; Montvale, NJ: 2004. [Google Scholar]
- 46.Schackman B, Gebo K, Walensky R, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–97. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 47.Centers for Medicare and Medicaid Services . Clinical laboratory fee schedule. 2008. [Google Scholar]
- 48.University HealthSystem Consortium . CDP online report. 2006. [Google Scholar]
- 49.Centers for Disease Control and Prevention Number of persons tested for HIV - United States, 2002. MMWR Morb Mortal Wkly Rep. 2004;53:1110–13. [PubMed] [Google Scholar]
- 50.Henry J, Kaiser Family Foundation . 2009 survey of Americans on HIV/AIDS: summary of findings on the domestic epidemic. 2009. [Google Scholar]
- 51.Centers for Disease Control and Prevention . HIV/AIDS surveillance report, 2004. Vol. 16. US Department of Health and Human Services; Atlanta, GA: 2005. [Google Scholar]
- 52.Centers for Disease Control and Prevention Update: HIV counseling and testing using rapid tests - United States, 1995. MMWR Morb Mortal Wkly Rep. 1998;47:211–15. [PubMed] [Google Scholar]
- 53.Centers for Disease Control and Prevention Rapid HIV testing in emergency departments - three U.S. sites, January 2005 - March 2006. MMWR Morb Mortal Wkly Rep. 2007;56:597–601. [PubMed] [Google Scholar]
- 54.Lyss S, Branson B, Kroc K, et al. Detecting unsuspected HIV infection with a rapid whole-blood HIV test in an urban emergency department. J Acquir Immune Defic Syndr. 2007;44:442–53. doi: 10.1097/QAI.0b013e31802f83d0. [DOI] [PubMed] [Google Scholar]
- 55.Cohen J, Stolk E, Niezen M. Role of budget impact in drug reimbursement decisions. J Health Polit Policy Law. 2008;33:225–47. doi: 10.1215/03616878-2007-054. [DOI] [PubMed] [Google Scholar]
- 56.Neil N, Walker D, Sesso R, et al. Gaining efficiencies: resources and demand for dialysis around the globe. Value Health. 2008;12:73–79. doi: 10.1111/j.1524-4733.2008.00414.x. [DOI] [PubMed] [Google Scholar]
- 57.Martin E, Pollack H, Paltiel A. Fact, fiction, and fairness: resource allocation under the Ryan White CARE Act. Health Aff. 2006;25:1103–12. doi: 10.1377/hlthaff.25.4.1103. [DOI] [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention . FY 2010 Justification of Estimates for Appropriation Committees. 2009. [Google Scholar]
- 59.Schmid C, McColl W. ADAP: securing HIV/AIDS drugs for the nation’s poor and uninsured. 2009. [Google Scholar]
- 60.Westmoreland T. 2008 Personal communication. [Google Scholar]
- 61.Henry J, Kaiser Family Foundation . Rising unemployment, Medicaid, and the uninsured. 2009. [Google Scholar]
- 62.Department of Health and Human Services Office of HIV/AIDS Policy National HIV Testing Mobilization Campaign website. 2009
- 63.Government Accountability Office . Ryan White CARE Act. Improved oversight needed to ensure AIDS Drug Assistance Programs obtain best prices for drugs. Washington, DC: 2006. [Google Scholar]