Abstract
PURPOSE
The goal of this study was to document how treatment with a bisphosphonate affects the bone tissue following dental extraction.
METHODS
Skeletally mature female beagle dogs were either untreated controls (CON) or treated with intravenous zoledronic acid (ZOL). Following the extraction of the 4th premolars, healing was allowed for 4 or 8 weeks. Properties of the extraction site were assessed using micro-computed tomography (micro-CT) and dynamic histomorphometry.
RESULTS
The initial infilling of the extraction socket with bone was not affected by ZOL but subsequent removal of this bone was significantly suppressed compared to CON. After 8-weeks of healing, the alveolar cortical bone adjacent to the extraction socket had a remodeling rate of ~50%/year in CON animals while ZOL-treated animals had a rate of < 1%/year. One ZOL-treated animal developed exposed bone post-extraction which eventually led to the formation of a sequestrum. Assessment of the sequestrum with micro-CT and histology showed that it had features consistent with those reported in humans with osteonecrosis of the jaw.
CONCLUSIONS
These results, showing significantly compromised post-extraction osseous healing as well as presence of exposed bone and development of a sequestrum in one ZOL animal, provide a building block toward understanding the pathophysiology of osteonecrosis of the jaw.
INTRODUCTION
Bisphosphonates (BPs) are a standard component in the treatment of various cancers. Over the past six years the condition known as osteonecrosis of the jaw (ONJ) has emerged as a serious side-effect of BP treatment (1). The majority of ONJ cases have manifested in cancer patients administered high doses of intravenous BPs (2–4) with a smaller number of cases reported in patients receiving lower doses of BPs for various conditions (1, 4). Despite a low incidence rate (5), ONJ remains a concern due to its unknown pathophysiology and the millions of patients who take BPs for various conditions.
The risk of ONJ is associated with compromised dental health with the majority of cases occurring in patients who have undergone oral surgery (1, 6–9). When the incidence of ONJ is separately assessed for various populations treated with BPs (osteoporosis, Paget’s disease, and cancer treatment patients), there is a consistent 10-fold increase in ONJ occurrence when dental extraction was concomitant with BP treatment (4{Hoff, 2008 #169). Invasive oral procedures such as dental extraction are some of the most common surgical procedures performed each year. The events following dental extraction are well-described and include the initial rapid formation of woven bone which is then, over time, removed by osteoclast activity (10–12). Following tooth extraction, the bone in and around the socket undergoes significant amounts of remodeling not only to remove the woven bone that forms within the socket but also to reshape the alveolar cortical bone in the surrounding region.
Most hypotheses concerning ONJ implicate remodeling suppression as an underlying tissue-level mechanism(6–7, 13–16). Bone remodeling serves to replace bone that is unfit for its duties, whether it is hyper-mineralized, contains microdamage, or has a disrupted osteocyte/canalicular network(17–18). The importance of remodeling in these conditions is illustrated by the fact that its inhibition leads to focal regions of bone matrix necrosis (19–20). If inhibition of remodeling occurs under conditions where significant remodeling is necessary, such as following tooth extraction, it is possible that the local bone could become necrotic.
The goal of this study was to document how intravenous treatment with the bisphosphonate zoledronic acid at doses similar to those given to cancer patients, affects the mandibular bone tissue following dental extraction. Specifically, we tested the hypothesis that zoledronic acid would significantly reduce remodeling in the mandible following tooth extraction and that this would slow the healing of the extraction socket bone.
METHODS
Animals
Twelve skeletally mature female beagles (~ 1 year old) were purchased from Marshall Farms USA (North Rose, NY) and housed for the duration of the experiment in environmentally controlled rooms at Indiana University School of Medicine’s AAALAC accredited facility. All animal procedures were approved prior to the study by the IU School of Medicine Animal Care and Use Committee.
Animals were assigned to untreated control (CON; n=6) or zoledronic acid (ZOL; n=6) treatment groups. ZOL (Zometa®) was administered via intravenous infusion at a dose of 0.06 mg/kg, which corresponds to the 4 mg dose used in cancer patients, adjusted on a mg/kg basis (21). ZOL was infused every 2 weeks which is roughly twice as frequently as used clinically. This dosing frequency was chosen to maximize drug exposure during the relatively short 3 month experimental period. IV administration was done in accordance with previously published protocols from our lab (22). Briefly, animals were sedated and an over-the-needle catheter was inserted into the leg vein (rotated for each infusion between the cephalic and saphenous) with the drug administration in a 40 mL total volume over a 15 minute period. Control animals (CON) were untreated throughout the experimental period.
One month after the first ZOL dose, all animals (ZOL and CON) underwent dental extraction of the 4th right premolar; one month later all animals underwent extraction of the 4th left premolar. This study design allowed two different post-extraction times (four and eight weeks) to be studied in the same animals. The 4 week time-period was chosen as it is twice as long as the duration of normal epithelial closure in untreated beagle dogs; the 8 week duration has been shown to be sufficient for complete healing of extractions in untreated beagle dogs (10).
For all dental extractions, animals received pre-surgical antibiotics (Cefazolin 20mg/kg IV (100mg/ml) and then were anesthetized (Propofol (10mg/ml) 8mg/kg IV) and intubated with continuous isofluorane for the duration of the surgery. Animals also received a pre-operative intra-oral regional block (Bupivicaine 0.1ml/Lidocaine 1% 0.1ml) to the inferior alveolar nerve prior to surgery. The epithelial attachment of the tooth was severed with a blunt probe and then the crown on the tooth was split down the middle using a high speed dental drill. Each of the two roots was removed individually by gripping forceps to the crown and then rocking back and forth in the buccal-lingual direction. In the case where the root was fractured during extraction (see results for incidence among groups), the root was left intact. Following extraction the gingival tissue was closed using absorbable suture. Post-operative analgesia (Buprenorphine 0.01mg/kg SQ) was given starting 4–8 hours after surgery.
To label active bone remodeling sites, animals were injected with calcein (5 mg/kg, intravenous)using a 2-12-2-5 schedule (labels were injected on two consecutive days, twelve days were allowed to pass, another two consecutive days of label were given, and then the animals were euthanized five days later. Animals were euthanized by intravenous administration of sodium pentobarbital and the right and left mandibles, right 9th rib, and right tibia were dissected free and placed in 70% ethanol for analysis.
In vivo extraction site healing (oral lesion visualization)
All animals were assessed daily post-extraction for exposed bone in the oral cavity. Our criterion for classification of ONJ was if exposed bone existed at the extraction site 4 weeks post-extraction. This time-period was chosen as it is two-times longer than the reported duration of normal epithelial closure in untreated beagles (10).
Micro-computed tomography of extraction site
The extraction sites of both the left and right hemimandibles were isolated by making parallel buccal-lingual cuts using a diamond wire saw (Histosaw; Delaware Diamond Knives). Each entire extraction site was scanned using high resolution micro-computed topography at a resolution of 8 microns (Skyscan 1172, Belgium). Raw data projection images were reconstructed and analyzed using manufacturer provided software (Skyscan, NRecon and CTAN programs). For each extraction site (right and left), both sockets were assessed and the data averaged to get a single value for each site. For those animals where roots were fractured, only the socket with a successful complete extraction was analyzed. Volume and morphology of the bone within the socket was assessed in three dimensions by using a region of interest to isolate the socket from the surrounding bone followed by thresholding of this region to separate mineralized tissue from the marrow. Outcome parameters of the extraction socket bone consisted of bone volume/tissue volume (BV/TV, %) and bone surface/total volume (BS/TV, 1/mm).
Histological Processing
Numerous skeletal sites were processed for cortical bone histological analysis: the extraction sites of both the left and right hemimandibles (following microCT scanning), a second region from each hemimandible (3rd premolar - to examine mandible properties distant from the extraction site) (Figure 1), a portion of the rib (located at the greatest curvature), and a portion of the tibia (~4 cm proximal to the distal end).
Figure 1.
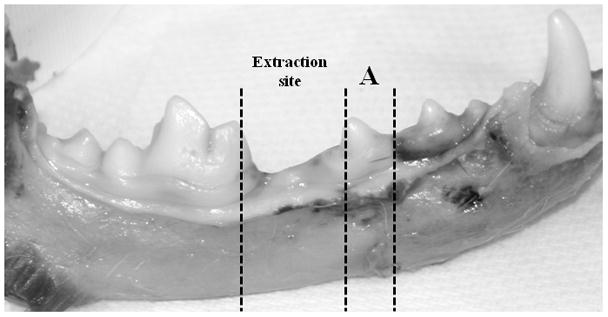
Regions of histological assessment of intracortical bone remodeling in the mandible. Both the extraction site and an adjacent non-extraction site (3rd premolar (A)) were assessed histologically.
All tissue segments were stained with basic fuchsin in order to assess bone matrix necrosis (19). Using 1% basic fuchsin dissolved in increasing concentrations of ethanol (80%, 95%, and 100%), specimens were stained for 48 hours per step. Following basic fuchsin staining, bones were embedded in plastic and then sectioned (~100 μm thick) using a diamond wire saw (Histosaw; Delaware Diamond Knives). Two sections, separated by ~400 μm, were cut for the rib and tibia and the 3rd premolar region of the right and left hemimandbile. For the region of bone surrounding the extraction sites that had healed for 4 weeks, two sections were collected by making parallel buccal-lingual cuts that passed through the center of one root socket. For the extraction sites that had healed for 8 weeks, parallel buccal-lingual cuts were made to make sections throughout one of the sockets (typically giving 8–12 sections per site). This greater number of sections was used in order to thoroughly examine the bone surrounding the entire socket at this time point for bone for matrix necrosis.
Histological Assessment
Histological measurements were made using a semiautomatic analysis system (Bioquant OSTEO 7.20.10, Bioquant Image Analysis Co.) attached to a microscope (Nikon Optiphot 2 microscope, Nikon) with a fluorescent light source. For assessment of intra-cortical bone formation rate, two cross-sections were assessed at each skeletal site with data from the sections combined to calculate the outcome parameters. For mandible sections, data were collected separately for alveolar bone regions (defined as bone above the most distally observed portion of the tooth root or extraction socket) and non-alveolar bone regions (below the tooth root) (19, 22). The cortical bone of the entire cross section of the rib and tibia was assessed.
Under ultraviolet light, the bone area (B.Ar), number of labeled osteons (L.Os.#, osteons with either single or double label), the total length of osteonal labeled surface (LS), and the mean inter-label width (Ir.L.Wi) were measured. Mineral apposition rate (MAR, um/day) was calculated as Ir.L.Wi/12, where 12 is the number of days between labels. Intra-cortical bone formation rate (BFR, %/year) was calculated as (MAR × (LS/2)/B.Ar × 100) × 365. For animals with only single labeled osteons, a value of 0.3 was used for MAR (23); if no label was present MAR was considered to be a missing value and BFR was considered to be zero, reflecting the absence of bone formation (22). All measures and calculations are similar to those reported previously (22) and are in accordance with ASBMR recommended standards (24).
Bone matrix necrosis of the cortical bone was assessed by bright-field microscopy as previously described (19). These analyses were conducted on two cross-sections for most skeletal sites (rib, tibia, non-extraction mandible site, extraction site healed for 4 weeks). For cortical bone surrounding the extraction site allowed to heal for 8 weeks a greater number of sections (8–12) were assessed for necrosis. Regions of bone void of basic fuchsin stain >500 μm2 were considered necrotic.
Statistics
Statistical tests were performed using SAS software (SAS Institute, Inc.). Parameters were compared between CON and ZOL animals using two-sample t-tests. Difference in extraction site properties were compared between time-points (4- and 8-weeks post-extraction) within each treatment group using paired t-tests. Histological parameters of the non-extracted, 4 weeks of healing, and 8 weeks of healing mandible regions were compared within treatment using a one way ANOVA with LSD post-hoc tests. For all tests, p < 0.05 was considered to be statistically significant.
RESULTS
There was no difference between groups in baseline (CON: 7.1 ± 0.4; ZOL: 6.8 ± 0.4; p = 0.60) or final (CON: 7.4 ± 0.5; ZOL: 6.9 ± 0.4; p = 0.49) body weights.
Dental extraction efficacy
All animals had at least one complete root extraction of both the right and left 4th premolars. Of the sites that had 8 weeks to heal, 2 of the 6 CON animals and all 6 ZOL-treated animals had both roots successfully removed. Of the sites that had 4 weeks to heal, 4 of the 6 CON animals and 5 of the 6 ZOL-treated animals had both roots successfully removed. Only sockets with complete root extractions were assessed.
ZOL-treatment does not alter initial socket bone formation but slows its remodeling
There was no significant difference between groups in the amount (BV/TV) or morphology (BS/TV) of the newly formed bone within the socket after 4 weeks of healing (Figure 2). After 8 weeks of healing, there was significantly more bone (+26%), with higher BS/TV (+62%), in the socket of ZOL-treated animals relative to CON. Compared to the 4 week time-point, BV/TV was significantly higher (+28%) and BS/TV significantly lower (−45%) in CON animals at week 8. This bone was predominantly at the surface of the socket (condensed cortical bone) with little remaining trabecular bone (Figure 2). The ZOL-treated animals also had higher BV/TV at 8-weeks post-extraction compared to 4 weeks (+78%) although the BS/TV was lower (−16%). In contrast to the CON animals, the bone in ZOL-treated animals remained in the socket and had not condensed to the surface cortex.
Figure 2.
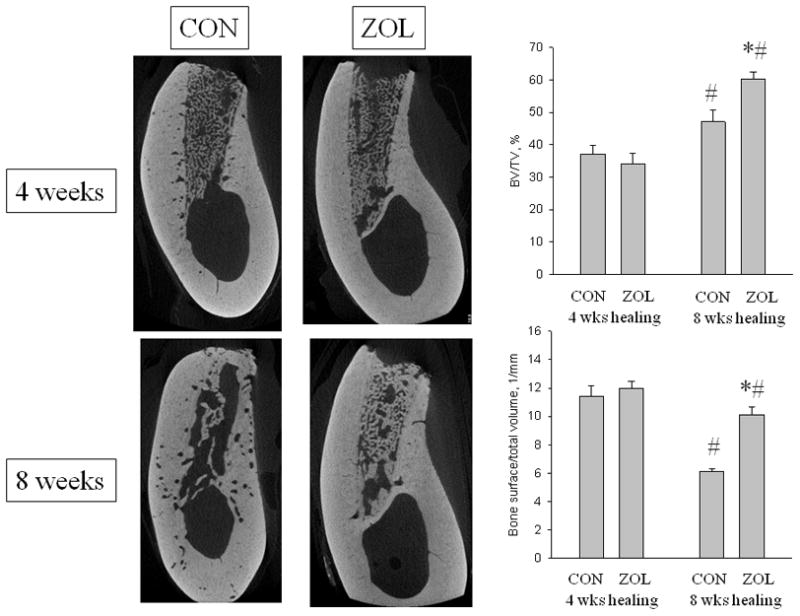
Micro-computed tomography assessment of the extraction site. Representative images through the center of the extraction socket in control (CON and zoledronic acid-treated (ZOL) animals after either 4 or 8 weeks of healing. After 4 weeks the sites appear similar in the two groups while after 8 weeks there is clear morphological difference. Three-dimensional analysis of the entire socket region support these qualitative images by showing that after 4 weeks of healing there was no difference in bone volume/tissue volume (BV/TV, %) or bone surface/total volume between the two groups. After 8 weeks of healing, BV/TV was significantly higher in both treatment groups compared to the 4 week time-point yet was higher in ZOL compared to CON. Bone surface/total volume was significantly lower at 8 weeks compared to 4 weeks in both groups, with ZOL significantly higher than CON. p < 0.05 versus 4 week time-point within group (#) or versus CON-treatment within time point (*).
ZOL-treatment severely suppresses intracortical remodeling post-extraction
At all skeletal sites examined, intracortical bone formation rate (BFR) was significantly lower (≥ 93%) in ZOL animals compared to CON (Figure 3, Tables 1 and 2). At the 4 week extraction sites, alveolar and non-alveolar BFRs were lower in the ZOL animals compared to CON (p = 0.002) with neither rate being significantly different from the non-extraction site. In 8 week extraction sites the alveolar BFR was lower in the ZOL animals compared to CON (−99%, p < 0.001) with both groups having higher alveolar BFR compared to 4 weeks (+123% in CON; +594% in ZOL). There was no evidence of bone matrix necrosis in any region of the mandible of the CON or ZOL animals.
Figure 3.
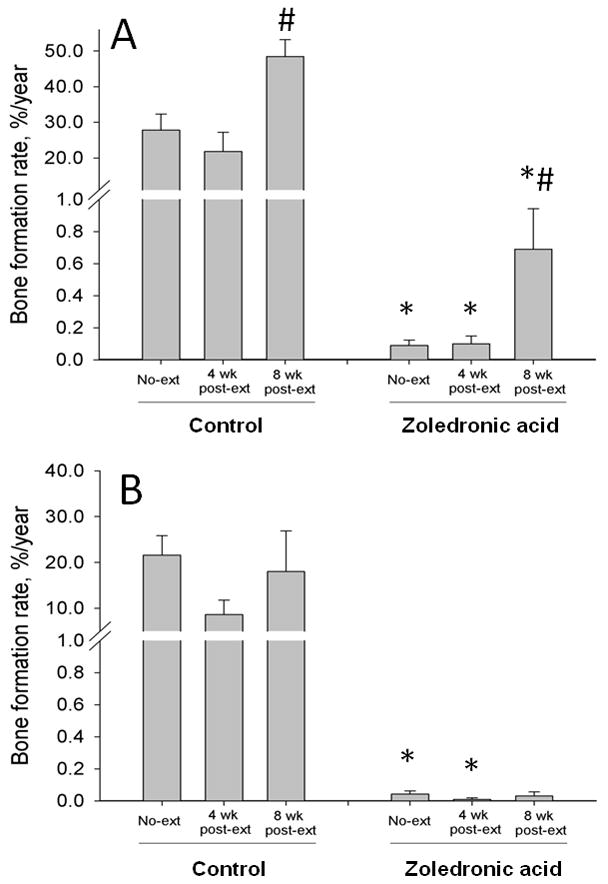
Histological assessment of intra-cortical bone formation rate in the mandible. Bone formation rate (BFR, %/year), measured using fluorochrome labels administered toward the end of the experiments, was separately assessed in the alveolar (A) and non-alveolar (B) bone at a region distant from the extraction site, at the extraction site allowed to heal for 4 weeks, and at the extraction site allowed to heal for 8 weeks. At all regions analyzed, the BFR was significantly lower in zoledronic acid-treated (ZOL) animals compared to controls (CON). In both CON and ZOL groups, there was no difference in BFR at 4 weeks post extraction yet significantly higher rates at 8 weeks post-extraction in the alveolar region. p < 0.05 versus non-extraction and 4 week healing region within treatment (#) or versus CON-treatment within region (*).
TABLE 1.
Dynamic histomorphometry data of intra-cortical mandible bone
| Control | Zoledronic acid | |||||
|---|---|---|---|---|---|---|
| Non-extraction site | 4weeks healing | 8 weeks healing | Non-extraction site | 4weeks healing | 8weeks healing | |
| Alveolar region | ||||||
| Labeled osteon number, #/mm2 | 1.69 ± 0.34 | 1.51 ± 0.38 | 2.38 ± 0.29 | 0.07 ± 0.03* | 0.10 ± 0.04* | 0.74 ± 0.22*# |
| Mineral apposition rate, μm/day | 2.37 ± 0.17 | 2.20 ± 0.18 | 2.66 ± 0.21 | 0.25 ± 0.05* | 0.25 ± 0.05* | 0.3 ± 0* |
| Bone formation rate, %/year | 27.8 ± 4.5 | 21.8 ± 5.4 | 48.5 ± 4.6# | 0.1 ± 0.03* | 0.1 ± 0.05* | 0.7 ± 0.25*# |
| Non-alveolar region | ||||||
| Labeled osteon number, #/mm2 | 1.21 ± 0.18 | 0.49 ± 0.16 | 1.22 ± 0.57 | 0.04 ± 0.02* | 0.02 ± 0.01* | 0.05 ± 0.04* |
| Mineral apposition rate, μm/day | 2.53 ± 0.12 | 1.81 ± 0.40 | 2.23 ± 0.50 | 0.25 ± 0.05* | 0.05 ± 0.05* | 0.1 ± 0.06* |
| Bone formation rate, %/year | 21.6 ± 4.2 | 3.6 ± 3.1 | 18.0 ± 8.9 | 0.04 ± 0.02* | 0.01 ± 0.01* | 0.03 ± 0.03 |
Data presented as mean ± SE. p < 0.05 versus non-extraction and 4 week healing region within treatment (#) or versus CON-treatment within region (*).
TABLE 2.
Dynamic histomorphometry of intra-cortical bone
| Control | Zoledronic acid | p value | |
|---|---|---|---|
| Rib | |||
| Labeled osteon number, #/mm2 | 2.51 ± 0.47 | 0.55 ± 0.09 | 0.002 |
| Mineral apposition rate, μm/day | 1.71 ± 0.35 | 0.66 ± 0.27 | 0.030 |
| Bone formation rate, %/year | 19.7 ± 5.6 | 1.5 ± 0.9 | 0.009 |
| Tibia | |||
| Labeled osteon number, #/mm2 | 0.20 ± 0.02 | 0.06 ± 0.02 | 0.001 |
| Mineral apposition rate, μm/day | 1.56 ± 0.54 | 0.36 ± 0.13 | 0.055 |
| Bone formation rate, %/year | 2.0 ± 1.0 | 0.1 ± 0.1 | 0.076 |
Data presented as mean ± SE.
Exposed bone with sequestrum formation in a ZOL-treated animal
One animal developed exposed bone in this study – this occurred following the first extraction in an animal that was treated with ZOL. This animal had been treated with two doses of ZOL prior to extraction, the second being 6 days prior to extraction. The animal received its third dose of ZOL 7 days following the first extraction and the fourth dose 22 days post-extraction.
While the socket appeared somewhat abnormal in the days following extraction (discolored), on day 11 post-extraction exposed bone was first noticed in the socket (Figure 4). This exposed bone persisted through day 23 post-extraction. On day 30 post-extraction, at the time of the second extraction, a sequestrum was observed protruding from the soft tissue (which had healed) at the extraction site. This bone fragment was plucked free, with little resistance, after which the site briefly bled. Thereafter, the site appeared normal throughout the remainder of the study. The sequestrum was subjected to high resolution microCT to observe its morphology (Figure 5). The CT analysis provided a means of assessing the sequestrum volume (1.60 mm3) while also revealing the presence of highly scalloped surfaces – qualitatively similar to the morphology of human BRONJ tissue previously assessed in our laboratory. Histological analysis of the sequestrum revealed a high prevalence of osteoclasts as well as a near complete absence of osteocytes (Figure 5).
Figure 4.
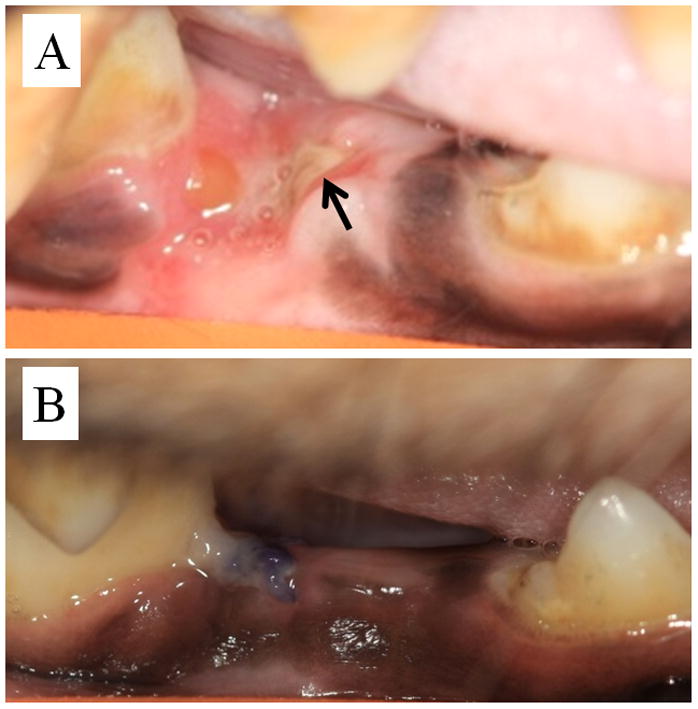
Photomicrograph of zoledronic acid-treated (A) and control (B) extraction site 11 days after surgery. Exposed bone can be observed in the zoledronic acid-treated animal (black arrow) while the extraction site in the control animal is completely covered with mucosa.
Figure 5.
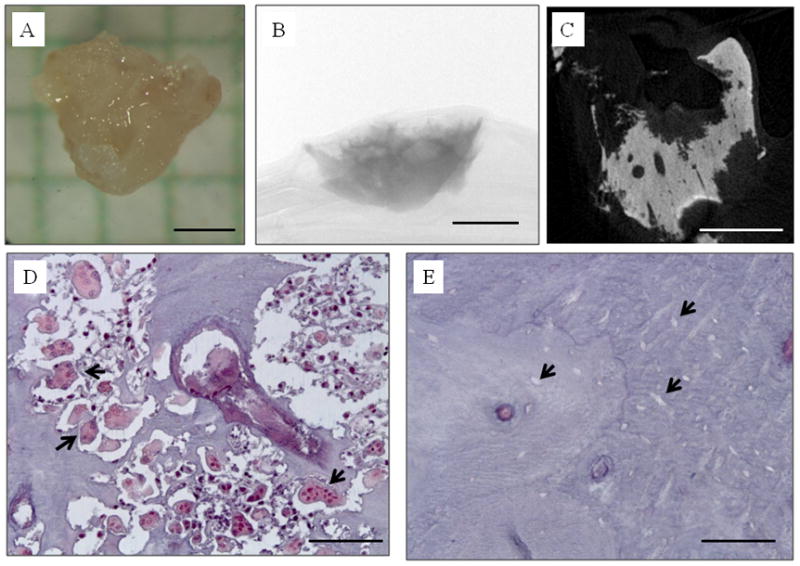
The exposed bone in the zoledronic acid-treated animal eventually manifested a sequestrum which was removed from the soft tissue mucosa at the extraction site. (A) photograph; (B) two-dimensional micro CT image through the center of the sequestrum showing highly scalloped surfaces; C) two-dimensional micro CT projection image of the entire specimen; D) photomicrograph of histological section through the sequestrum stained with tartrate-resistant acid phosphatase (TRAP) to visualize osteoclasts (select, but not all, osteoclasts identified with black arrowheads); E) photomicrograph of histological section through the sequestrum stained with TRAP showing empty osteocyte lacunae (select, but not all, empty osteocyte lacunae identified with black arrowheads). Scale bars = 1 mm for A–C, 100 μm for D and E.
DISCUSSION
With one premolar extraction site allowed to heal for 4 weeks and the contralateral site allowed to heal for 8 weeks, the current study provides some insight into the dynamic process of dental extraction osseous healing and how its affected with bisphosphonate treatment. Our micro CT data clearly document that this high-dose zoledronic acid treatment does not compromise the amount of bone that initially fills the socket – a process driven by bone modeling. This finding is in agreement with others who have studied similar outcomes in rodents treated with bisphosphonates and undergoing dental extraction (25–26). As our earliest duration of assessment was four weeks post-extraction, it is possible that differences in healing could have occurred very early in the healing process.
The most striking effect of zoledronic acid treatment on dental healing in the current study is in the remodeling of the initial bone that formed within the socket. This is consistent with data from fracture healing studies in which the initial callus forms normally but its remodeling is significantly compromised (27–28). The higher bone volume in extraction sockets allowed to heal for 8 weeks compared to 4 weeks in the presence of zoledronic acid suggests continued formation of bone within the socket; this is contrasted with control animals in which bone volume increases due to consolidation of bone on the apical surface of the socket with removal of the woven bone within the socket (Figure 2). These data lead to the question of whether the socket in BP-treated individuals will continue to form bone and eventually fill up or, if at some point, osteoclast-mediated removal will occur as it does in the callus post-fracture. If the socket eventually fills with bone, it will be important to understand if this has any significant implications.
Similar to our previous findings, both with long-term oral alendronate treatment (19) and short-term intravenous zoledronic acid treatment (22), this current work documents significant suppression of intracortical bone remodeling in the mandible and other skeletal sites. One important advancement provided by the current work is that it shows ZOL does not allow the normal increase in remodeling of the adjacent alveolar cortical bone post- extraction. Since rodents do not normally undergo intra-cortical remodeling, such data are not available from studies using rats and mice. Following tooth extraction, the cortical bone in humans near the socket undergoes significant amounts of remodeling to reshape the alveolar cortical bone in the surrounding region. With ZOL treatment, although the intra-cortical bone formation rate was significantly higher 8 weeks post-extraction compared to the mandible site distant from the extraction and the extraction site at 4 weeks post-extraction, the rate was still less than 1%/year. The consequences of this dramatically lower remodeling rate in the bone surrounding the extraction site are unknown.
Given the short-term nature of this experiment, we did not expect to see osteonecrosis of the jaw. However, one animal in the study (in the ZOL group) developed exposed bone following the first of the two dental extractions. The exposed bone was present for < 4 weeks, a shorter timeframe than we had set a priori for defining ONJ. Although this timeframe was based on normal healing in untreated dogs (10), it was somewhat arbitrary. That is, it is unclear how to translate the human definition of ONJ - having exposed bone for > 8 weeks (1) – to a timeframe in dogs. As such we believe the presence of exposed bone at three weeks post extraction is noteworty. Similar to the human condition of ONJ, the animal with exposed bone in our study eventually formed a sequestrum. Using CT, the sequestrum that formed in this animal appeared morphologically similar to those from humans with ONJ (13) and histological analysis revealed a striking number of osteoclasts and mostly empty lacunae. These findings are consistent with human ONJ specimens (29). It is important to note that the existence of a sequestrum is not exclusive to ONJ, thus its existence in this instance is not proof of ONJ. The occurrence of exposed bone in our study after just two doses of ZOL is more rapid than the majority of clinical cases (5, 30) and healed more quickly than is routinely observed clinically (31). It is difficult to know how to relate the timing of onset and resolution of the exposed bone in the current study to the human condition.
The development of an animal model to study ONJ is essential to advance understanding of this condition (1). Exposed bone associated with bisphosphonate treatment (both with and without dental extraction) has now been documented in several rat and mouse studies (25, 32–39). These rodent studies each employed different definitions of mucosal or bone necrosis making it difficult to compare among them. One common feature, however, is that in most of these studies, roughly 10–20% of animals treated with BP alone developed necrotic bone; higher percentages of animals displayed necrotic regions when BP was combined with vitamin d deficiency (38) or dexamethasone (32, 34). It is also noteworthy that in most of these studies a percentage of control animals had necrosis - perhaps indicative of the trauma induced during the difficult extraction procedure in such small animals. Continued work with one or more of these animal models will prove useful in future studies aimed at understanding ONJ pathophyisology.
The results of this study should be considered within the context of some aspects of the study design. The dose of zoledronic acid, while similar to that given to cancer patients on a mg/kg basis (21), was administered more frequently (every 2 weeks) than is routinely given clinically (every 3–4 weeks). Whether or not the effects on extraction site healing and the development of exposed bone is a function of this more frequent dosing is not clear, although the level of remodeling suppression with such a schedule is comparable to that which occurs with dosing every 4 weeks (22). The control animals in the current study were not injected with vehicle. Comparing mandible BFR of the current study’s CON animals to a group of similar aged IV vehicle-treated animals from our previous work (22) documents similar alveolar BFR (p = 0.47) suggesting the absence of VEH injections in the current study are unlikely to have affected our results. A number of animals had roots fractured during extraction. We cannot discount that the response in those animals was different from those where both roots were successfully removed. While there was no significant difference in either CT or histological variables for controls with two successful root extractions the sample sizes of the various groups (complete vs incomplete root removal within each treatment and timepoint) are not powered to sufficiently detect a difference if one existed. Also important to note, these dogs did not have cancer, were not immunologically compromised, nor were the teeth that were extracted infected, as is routinely the case clinically. If and how any of these factors influence the results is not known.
In conclusion we show that the combination of high dose zoledronic acid can significantly alter the later stages of post-extraction osseous healing and are sufficient to produce exposed bone and sequestrum formation. These data advance our understanding of how bisphosphonate treatment affects the bones of the oral skeleton and suggest the dog may be a useful model to study the pathogenesis of ONJ.
Acknowledgments
We would like to thank the following individuals who contributed to this work: Binal Pandya for assistance with histological analyses, Mark Koivuniemi for CT scanning/analysis and histological sectioning, Nichole Leahy for histological sectioning, Keith Condon for histological preparation, and Carrie Pell and her staff for assistance with animal care. This work was supported by grants from the Showalter Foundation and the NIH (R21-DE019686 and S10-RR023710). Drs Allen and Burr serve as a consultant and receive research support from the Alliance for Better Bone health.
Footnotes
Potential conflicts: Dr. Allen and Dr. Burr receive research support and are consultants for Procter & Gamble Pharmaceuticals. Dr. Burr serves as a consultant for Amgen.
References
- 1.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 2.Lacy MQ, Dispenzieri A, Gertz MA, Greipp PR, Gollbach KL, Hayman SR, Kumar S, Lust JA, Rajkumar SV, Russell SJ, Witzig TE, Zeldenrust SR, Dingli D, Bergsagel PL, Fonseca R, Reeder CB, Stewart AK, Roy V, Dalton RJ, Carr AB, Kademani D, Keller EE, Viozzi CF, Kyle RA. Mayo clinic consensus statement for the use of bisphosphonates in multiple myeloma. Mayo Clin Proc. 2006;81:1047–1053. doi: 10.4065/81.8.1047. [DOI] [PubMed] [Google Scholar]
- 3.Woo SB, Hellstein JW, Kalmar JR. Systematic review: Bisphosphonates and osteonecrosis of the jaws. Annals of Internal Medicine. 2006;144:753–761. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 4.Mavrokokki T, Cheng A, Stein B, Goss A. Nature and frequency of bisphosphonate-associated osteonecrosis of the jaws in australia. J Oral Maxillofac Surg. 2007;65:415–423. doi: 10.1016/j.joms.2006.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu MM, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–836. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marx RE, Sawatari Y, Fortin M, Broumand V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J Oral Maxillofac Surg. 2005;63:1567–1575. doi: 10.1016/j.joms.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 7.Migliorati CA, Casiglia J, Epstein J, Jacobsen PL, Siegel MA, Woo SB. Managing the care of patients with bisphosponate-associated osteonecrosis - An American Academy of Oral Medicine position paper. Journal of the American Dental Association. 2005;136:1658–1668. doi: 10.14219/jada.archive.2005.0108. [DOI] [PubMed] [Google Scholar]
- 8.Zervas K, Verrou E, Teleioudis Z, Vahtsevanos K, Banti A, Mihou D, Krikelis D, Terpos E. Incidence, risk factors and management of osteonecrosis of the jaw in patients with multiple myeloma: a single-centre experience in 303 patients. British Journal of Haematology. 2006;134:620–623. doi: 10.1111/j.1365-2141.2006.06230.x. [DOI] [PubMed] [Google Scholar]
- 9.Vahtsevanos K, Kyrgidis A, Verrou E, Katodritou E, Triaridis S, Andreadis CG, Boukovinas I, Koloutsos GE, Teleioudis Z, Kitikidou K, Paraskevopoulos P, Zervas K, Antoniades K. Longitudinal Cohort Study of Risk Factors in Cancer Patients of Bisphosphonate-Related Osteonecrosis of the Jaw. J Clin Oncol. 2009 doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 10.Cardaropoli G, Araujo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites. An experimental study in dogs. J Clin Periodontol. 2003;30:809–818. doi: 10.1034/j.1600-051x.2003.00366.x. [DOI] [PubMed] [Google Scholar]
- 11.Kingsmill VJ. Post-extraction remodeling of the adult mandible. Crit Rev Oral Biol Med. 1999;10:384–404. doi: 10.1177/10454411990100030801. [DOI] [PubMed] [Google Scholar]
- 12.Amler MH, Johnson PL, Salman I. Histological and histochemical investigation of human alveolar socket healing in undisturbed extraction wounds. J Am Dent Assoc. 1960;61:32–44. doi: 10.14219/jada.archive.1960.0152. [DOI] [PubMed] [Google Scholar]
- 13.Allen MR, Burr DB. The Pathogenesis of Bisphosphonate-Related Osteonecrosis of the Jaw: So Many Hypotheses, So Few Data. J Oral Maxillofac Surg. 2009;67:61–70. doi: 10.1016/j.joms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 14.Ruggiero SL, Mehrotra B. Bisphosphonate-Related Osteonecrosis of the Jaw: Diagnosis, Prevention, and Management. Annual Review of Medicine. 2009;60:85–96. doi: 10.1146/annurev.med.60.063007.134350. [DOI] [PubMed] [Google Scholar]
- 15.Ruggiero SL, Drew SJ. Osteonecrosis of the jaws and bisphosphonate therapy. Journal of Dental Research. 2007;86:1013–1021. doi: 10.1177/154405910708601101. [DOI] [PubMed] [Google Scholar]
- 16.Ruggiero SL, Mehrotra B, Rosenberg TJ, Engroff SL. Osteonecrosis of the jaws associated with the use of bisphosphonates: A review of 63 cases. J Oral Maxillofac Surg. 2004;62:527–534. doi: 10.1016/j.joms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Burr DB. Targeted and nontargeted remodeling. Bone. 2002;30:2–4. doi: 10.1016/s8756-3282(01)00619-6. [DOI] [PubMed] [Google Scholar]
- 18.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 19.Allen MR, Burr DB. Mandible matrix necrosis in beagle dogs after 3 years of daily oral bisphosphonate treatment. J Oral Maxillofac Surg. 2008;66:987–994. doi: 10.1016/j.joms.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enlow DH. Osteocyte necrosis in normal bone. J Dent Res. 1966;45:213. doi: 10.1177/00220345660450011901. [DOI] [PubMed] [Google Scholar]
- 21.Clemons MJ, Dranitsaris G, Ooi WS, Yogendran G, Sukovic T, Wong BYL, Verma S, Pritchard KI, Trudeau M, Cole DEC. Phase II Trial Evaluating the Palliative Benefit of Second-Line Zoledronic Acid in Breast Cancer Patients With Either a Skeletal-Related Event or Progressive Bone Metastases Despite First-Line Bisphosphonate Therapy. J Clin Oncol. 2006;24:4895–4900. doi: 10.1200/JCO.2006.05.9212. [DOI] [PubMed] [Google Scholar]
- 22.Allen MR, Kubek DJ, Burr DB. Cancer treatment dosing regimens of zoledronic acid result in near-complete suppression of mandible intracortical bone remodeling in beagle dogs. J Bone Miner Res. 2010;25:98–105. doi: 10.1359/jbmr.090713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foldes J, Shih MS, Parfitt AM. Frequency distributions of tetracycline-based measurements: implications for the interpretation of bone formation indices in the absence of double-labeled surfaces. J Bone Miner Res. 1990;5:1063–1067. doi: 10.1002/jbmr.5650051010. [DOI] [PubMed] [Google Scholar]
- 24.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone Histomorphometry - Standardization of Nomenclature, Symbols, and Units. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 25.Hikita H, Miyazawa K, Tabuchi M, Kimura M, Goto S. Bisphosphonate administration prior to tooth extraction delays initial healing of the extraction socket in rats. J Bone Miner Metab. 2009;27:663–672. doi: 10.1007/s00774-009-0090-6. [DOI] [PubMed] [Google Scholar]
- 26.Altundal H, Guvener O. The effect of alendronate on resorption of the alveolar bone following tooth extraction. Int J Oral Maxillofac Surg. 2004;33:286–293. doi: 10.1006/ijom.2002.0472. [DOI] [PubMed] [Google Scholar]
- 27.Li J, Mori S, Kaji Y, Mashiba T, Kawanishi J, Norimatsu H. Effect of bisphosphonate (incadronate) on fracture healing of long bones in rats. J Bone Miner Res. 1999;14:969–979. doi: 10.1359/jbmr.1999.14.6.969. [DOI] [PubMed] [Google Scholar]
- 28.McDonald MM, Dulai S, Godfrey C, Amanat N, Sztynda T, Little DG. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone. 2008 doi: 10.1016/j.bone.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Hansen T, Kunkel M, Springer E, Walter C, Weber A, Siegel E, Kirkpatrick CJ. Actinomycosis of the jaws--histopathological study of 45 patients shows significant involvement in bisphosphonate-associated osteonecrosis and infected osteoradionecrosis. Virchows Arch. 2007;451:1009–1017. doi: 10.1007/s00428-007-0516-2. [DOI] [PubMed] [Google Scholar]
- 30.Estilo CL, Van Poznak CH, Wiliams T, Bohle GC, Lwin PT, Zhou Q, Riedel ER, Carlson DL, Schoder H, Farooki A, Fornier M, Halpern JL, Tunick SJ, Huryn JM. Osteonecrosis of the maxilla and mandible in patients with advanced cancer treated with bisphosphonate therapy. Oncologist. 2008;13:911–920. doi: 10.1634/theoncologist.2008-0091. [DOI] [PubMed] [Google Scholar]
- 31.Ruggiero SL, Fantasia J, Carlson E. Bisphosphonate-related osteonecrosis of the jaw: background and guidelines for diagnosis, staging and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:433–441. doi: 10.1016/j.tripleo.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC. Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncology. 2009;45:164–172. doi: 10.1016/j.oraloncology.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Gotcher JE, Jee WS. The progress of the periodontal syndrome in the rice rat. II. The effects of a diphosphonate on the periodontium. J Periodontal Res. 1981;16:441–455. doi: 10.1111/j.1600-0765.1981.tb00995.x. [DOI] [PubMed] [Google Scholar]
- 34.Kikuiri T, Kim I, Yamaza T, Akiyama K, Zhang Q, Li Y, Chen C, Chen W, Wang S, Le AD, Shi S. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res. 2010 doi: 10.1002/jbmr.37. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Senel FC, Duman MK, Muci E, Cankaya M, Pampu AA, Ersoz S, Gunhan O. Jaw bone changes in rats after treatment with zoledronate and pamidronate. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2010;109:385–391. doi: 10.1016/j.tripleo.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, Yatani H, Yoneda T. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. Journal of Bone and Mineral Metabolism. 2010;28:165–175. doi: 10.1007/s00774-009-0128-9. [DOI] [PubMed] [Google Scholar]
- 37.Biasotto M, Chiandussi S, Zacchigna S, Moimas S, Dore F, Pozzato G, Cavalli F, Zanconati F, Contardo L, Giacca M, Di Lenarda R. A novel animal model to study non-spontaneous bisphosphonates osteonecrosis of jaw. J Oral Pathol Med. 2010 doi: 10.1111/j.1600-0714.2009.00878.x. (In press) [DOI] [PubMed] [Google Scholar]
- 38.Hokugo A, Christensen R, Chung E, Sung E, Felsenfeld AL, Sayre JW, Garrett N, Adams JS, Nishimura I. Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res. 2010 doi: 10.1002/jbmr.23. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senel FC, Duman MK, Muci E, Cankaya M, Pampu AA, Ersoz S, Gunhan O. Jaw bone changes in rats after treatment with zoledronate and pamidronate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010 doi: 10.1016/j.tripleo.2009.10.011. (In press) [DOI] [PubMed] [Google Scholar]


