Abstract
Current treatments for high-grade lymphoma often have curative potential, but unfortunately many cases relapse and develop therapeutic resistance. Thus, there remains a need for novel therapeutics that can target the residual cancer cells whose phenotypes are distinct from the bulk tumor and that are capable of reforming tumors from very few cells. Oncolytic viruses offer an approach to destroy tumors by multiple mechanisms, but they can not effectively reach residual disease or micrometastases, especially within the lymphatic system. To address these limitations, we have generated immune cells infected with oncolytic viruses as a therapeutic strategy that can combine effective cellular delivery with synergistic tumor killing. In this study, we tested this approach against minimal disease states of lymphomas characterized by the persistence of cancer cells which display stem cell-like properties and resistance to conventional therapies. We found that the immune cells were capable of trafficking to and targeting residual cancer cells. The combination biotherapy used prevented relapse by creating a long-term, disease-free state, with acquired immunity to the tumor functioning as an essential mediator of this effect. Immune components necessary for this acquired immunity were identified. We further demonstrated that the dual-biotherapy could be applied before or after conventional therapy. Our approach offers a potentially powerful new way to clear residual cancer cells, showing how restoring immune surveillance is critical for maintenance of a disease-free state.
Keywords: oncolytic vaccinia, lymphoma, CIK cells, Immune cell therapy
Introduction
We recently described a novel combination approach for the treatment of cancer(1), utilizing a tumor-targeting immune cell (cytokine induced killer; CIK cells)(2, 3), as a carrier vehicle to systemically deliver an oncolytic vaccinia virus (vvDD)(4-6) to tumors. It was found these two therapies could synergize in their tumor targeting and killing in large primary tumors, where immune cell therapies are typically less effective(7). Here we sought to examine the ability of this dual biotherapy to treat minimal lymphoma disease states, characterized by the presence of residual cancer cells with stem cell-like properties and increased resistance to conventional therapies. Because the last remaining cancer cells following treatment, or those that initiate metastasis are likely to represent a heterogeneous population distinct from the cell types that constitute the majority of the tumor, therapies that target and destroy cancer cells by multiple distinct mechanisms, such as the CIK-vvDD dual biotherapy will be needed to eradicate them. We therefore sought to determine for the first time, whether CIK-vvDD dual biotherapy could specifically target and destroy residual disease in addition to the bulk tumor. This property would potentially make this approach a powerful new tool in the prevention of relapse.
We incorporated a model of residual disease using lymphoma cells isolated from spontaneous tumors formed in a transgenic mouse model utilizing the Tet-OFF system to conditionally regulate MYC oncogene overexpression in the lymphoid compartment(8-13). These tumors regress upon MYC oncogene inactivation. However, sustained MYC inactivation results in a small population of cells capable of persisting and eventually relapsing subsequent to chromosomal rearrangement(14). These cells therefore have some stem-like or tumor-initiating properties. This model allowed the reliable and reproducible creation of a minimal disease state after tumor regression that resulted in relapse if left untreated. These conditions could not be recreated with standard xenografts models.
Here we demonstrate that CIK cells are able to target these cells, even after MYC inactivation in vitro and in vivo, and that CIK-vvDD dual biotherapy is able to clear the residual cells and induces an acquired immune response that prevents tumor relapse. This approach may therefore be applied to greatly enhance the effectiveness of conventional cancer therapies in a variety of settings, and represents a novel means to directly target cancer-initiating cells.
Materials and Methods
Cells and viruses
Lymphoma cell lines 6780, 6814 and 8946 have been described previously(14) and were obtained from a spontaneous tumor transgenic mouse model incorporating the tetracycline transactivating protein (tTA) driven by the immunoglobulin heavy chain enhancer element and the SRalpha promoter (EμSR-tTA) and the tetracycline-responsive minimal promoter (tet-o) driving MYC protooncogene elements, such that oncogenic MYC is overexpressed in the absence, but not the presence of doxycycline exclusively in hematopoietic cell lineages(9). Cells isolated from spontaneous tumors (primarily B-cell lymphomas) formed in these mice can be transferred into naïve mice to form tumors that will regress when doxycycline is present(14).
The cell lines were labeled with firefly luciferase by retroviral infection and luciferase expression verified. Cell morphology, growth rates and response to doxycycline exposure were compared to the unlabeled parental strains.
The preparation of CIK cells (an ex vivo expanded NK-T cell population(3, 15)) has been previously described(2), briefly, mouse splenocytes or human peripheral blood lymphocytes were treated with IFN-gamma for 24h before exposure to anti-CD3 antibody for 24h and expansion in the presence of IL2 for a period of 14 to 21 days. CIK cells expressing luciferase were produced from an FVB transgenic strain expressing luciferase from the beta-actin promoter (as previously described(16)).
The viral strains used in this work are produced from Western Reserve strain of vaccinia, with mutations in the viral thymidine kinase and viral growth factor genes(6). Versions of the resultant double deleted virus (vvDD) expressing either luciferase or GFP from a synthetic early/late viral promoter have been described previously(1). To produce the CIK-vaccinia dual biotherapy, CIK cells and vvDD were mixed at a multiplicity of infection of 1.0 for 5h prior to use.
Cell Viability Assays
In some experiments cell lines were treated with increasing doses of irradiation or chemotherapy (paclitaxel, SIGMA); 7 days after irradiation or 3 days after addition of chemotherapy cell viability was determined by MTS assay (Promega).
Animal models
Tumors were formed by implantation of lymphoma cells either subcutaneously (1×106 cells) or into the peritoneal cavity (5×105 cells) of FVB or NOD SCID mice (Jackson laboratories). Tumor growth was determined by caliper measurement or in vivo bioluminescence imaging (BLI, see below), with treatment initiated either once primary tumors had established (reached 100mm3, or had displayed increasing BLI signal for two consecutive images), or at time points as described in the legends to figures. Tetracycline-mediated regression of tumors was achieved by addition of doxycycline (50 ng/ml) to the drinking water (water changed every 24h). In some experiments BALB/c mice were injected subcutaneously with 1×107 A20 lymphoma cells (ATCC).
Animals were treated with 1×107 PFU of vvDD; 1×107 CIK cells, or 1×107 PFU of vvDD pre-mixed with 1×107 CIK cells via tail vein injection. In some experiments vvDD and CIK cells were delivered together without the pre-infection step. Cyclophosphamide was delivered through intraperitoneal injection (100mg/kg).
BLI was performed using an IVIS200 system (Caliper LifeSciences) following intraperitoneal injection of 200 ul of 30mg/ml luciferin substrate and anesthesia with 3% isoflurane. Images were analyzed using LivingImage software (Caliper LifeSciences).
Immune assays
In the cytotoxic T-lymphocyte (CTL) kill assay, CIK effector cells were mixed with lymphoma target cells expressing luciferase in effector to target ratios of 5:1 to 50:1 in triplicate and incubated for 12h. Target cell survival was measured by BLI, and compared to target cells alone (100% viable) or effector cells alone (0% viable).
The degranulation assay was performed as previously described(17). Briefly, lymphocytes were mixed with antigens in the presence of GolgiStop (BD Biosciences) and anti-CD107a-FITC antibody or anti-CD154-PE (BD Pharmingen). After 6h cells were washed and counterstained with CD8-PE and CD4-FITC respectively. Percentages of CD4 or CD8 cells undergoing de-granulation (i.e. CD154 or CD107a cell surface stains respectively) in response to different stimuli were quantified by flow cytometry (FACScaliber, BD Pharmingen).
Cytokine levels were assessed via intracellular staining. Lymphocytes were mixed with target antigens in the presence of GolgiStop. Cells were fixed, permeabilized (BD cytofix/cytoperm) and stained with conjugated antibodies to IL-4, IL-10 (both from Biolegend), IFN-gamma (eBioscience) or IL-2 (Caltag), as well as surface stains to CD4 and CD8. Percentages of CD4 or CD8 cells staining positive for different cytokines were determined by flow cytometry.
Anti-vaccinia neutralizing antibody titers were determined in plasma samples obtained from treated mice. Serial dilutions were mixed with 1000PFU of vvDD-luciferase virus for 2h, before being transferred to A2780 cells (ATCC) in 96-well plates. Luciferase signals from vaccinia-infected cells were determined after 24h. Neutralizing antibody levels were determined as the dilution that resulted in neutralization of 50% of the luciferase-expressing virus.
ELISAs were run according to the manufacturer's instructions (IP-10 and I-TAC ELISA, RnD Systems).
qRT-PCR was run on cDNA samples obtained from cells; collection and processing of cDNA was according to manufacturer's instructions, and qRT-PCR was run in 96-well array formats using commercial kits (Th1-Th2-Th3 kits, SuperArray, part of SA Biosciences).
Cells were also stained for several additional surface markers before analysis by flow cytometry. These included MULT-1 (R&D Systems), Rae-1 (BD Pharmingen) and Sca-1 (BD Pharmingen)
Statistical analysis
Unpaired and paired Student's T-tests were run to determine statistical significance (defined as p<0.05).
Results
In vitro and in vivo targeting of lymphoma cell lines by CIK cells is possible even after MYC inactivation
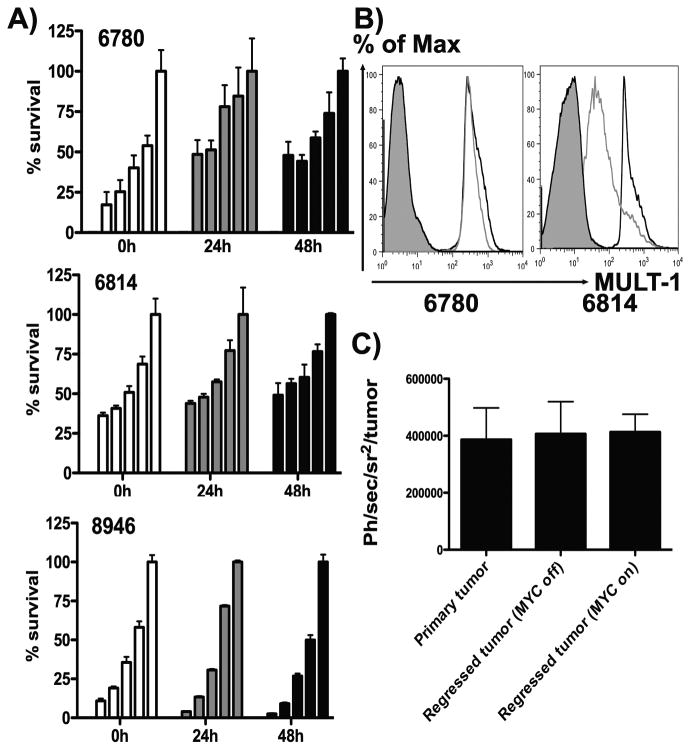
MYC inactivation of lymphoma cells cultured from spontaneous tumors formed in the EμSR-tTA tet-o-Myc transgenic mouse in vitro leads to cell cycle arrest, reversion to a ‘normal’ lymphocytic morphological phenotype and eventually senescence and apoptosis [14]. We found MYC inactivation (50 mg/ml doxycycline) and differentiation back into a pre-malignant phenotype did not result in evasion from CIK-mediated killing for any of the cell lines tested (6780, 8946 and 6814) (Fig 1a). CIK cells efficiently killed the lymphoma cells after loss of the neoplastic phenotype, but did not kill normal lymphocytes (data not shown). Because CIK cells recognize and destroy their tumor targets through recognition of NKG2D ligands (Rae-1, H-60 or MULT-1 in the mouse), we looked to determine the levels of cell surface expression of these ligands with and without MYC expression. Although different lymphomas from the same transgenic strain displayed different NKG2D ligand expression patterns and the loss of MYC expression generally resulted in reduction in cell surface levels of these ligands, significant levels of MULT-1 were typically maintained (Fig 1b).
Fig 1. Targeting of pre-malignant lymphomas with CIK cells.
(A) 6780, 6814 or 8946 lymphoma cell lines expressing luciferase were treated with doxycycline to inactivate the MYC oncogene for the times indicated on the x-axis. At each time, effector (CIK cell) to tumor target mixes of 50:1, 25:1, 10:1 and 5:1 and tumor cells only, respectively, were left for 12 hours before BLI was used to determine tumor cell survival. (B) 6780 and 6814 cells treated with doxycycline for 48h, were stained with MULT-1 antibody, and mean fluorescence intensity determined by flow cytometry (black – no doxycycline; grey - with doxycycline; solid – isotype). (C) Trafficking of CIK cells to lymphoma. 6780 cells were injected subcutaneously in FVB mice and then underwent (i) no treatment; (ii) sustained MYC inactivation; (iii) MYC inactivation until tumors were no longer palpable, followed by MYC reactivation. All animals were treated iv with CIK cells expressing luciferase (isolated from FVB-luciferase transgenic animals) and imaged 72h later. Bioluminescence signal from the region of the tumor was determined (n=5/group), mean and standard deviation are shown.
MYC repression in vivo resulted in lymphoma regression, and implanted tumors become undetectable after 14-21 days, but relapses frequently occur after sustained repression (of up to 30 weeks), implying that residual tumor cells are retained for extended periods. We used bioluminescence imaging (BLI) to determine that CIK cells were equally effective at trafficking to the site of a regressed tumor as they were at trafficking to primary tumors and were able to target the minimal residual disease states whether MYC expression was re-activated or if it was continually repressed (Fig 1c).
CIK-vvDD dual biotherapy prevents relapse from residual lymphoma cells persisting after MYC inactivation
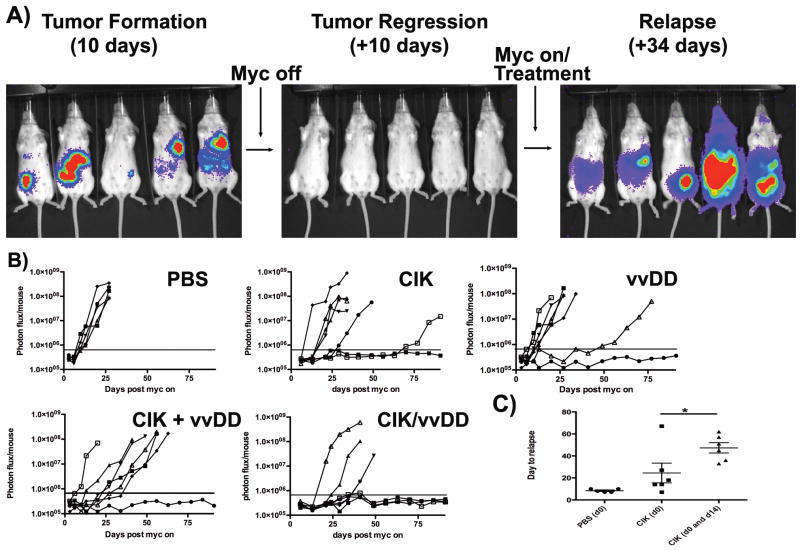
Mice with intraperitoneal 6780 lymphomas underwent MYC oncogene inactivation leading to tumor regression (Fig 2a), until they were no longer detectable by imaging (indicating less than 1000 tumor cells remained Fig S1). MYC inactivation was continued for a further 7 days and then animals were treated intravenously with (i) PBS; (ii) vvDD (oncolytic vaccinia virus); (iii) CIK cells (iv) CIK cells and vvDD or (iv) CIK-vvDD dual biotherapy (vvDD pre-infected into CIK cells). Immediately after treatment, MYC expression was re-introduced. Animals treated with PBS reproducibly relapsed after 7-10 days (fig 2ab). This system therefore represents an experimental model of therapeutic clearance of detectable tumor, with a few remaining tumor cells leading to relapse. Because of the small number of lymphoma cells mediating this relapse, they must have tumor initiating properties. Animals in this minimal disease state treated with CIK cells alone demonstrated a relapse rate of 85% (6 of 7) with a general delay to relapse (from an average of 8 days to 27 days) (Fig 2b). Interestingly, this prolongation of disease-free state corresponded to the approximate period of time (about 21 days) we expect the CIK cells to remain resident within the host (18). It was found that repeat treatment with a second dose of CIK cells 14 days after initial treatment could further delay the average time to relapse to 48 days (Fig 2c), but overall survival was not improved. Animals treated with a single intravenous injection of oncolytic virus demonstrated a similar overall rate of relapse (6 of 7) as CIK treatment. However, in this group there was typically no delay to relapse, indicating that the virus, which cannot actively target tumor cells, was unable to reach the residual disease in the majority of the animals. Animals treated with both CIK and vvDD displayed similar relapse kinetics as CIK cells alone. Finally, animals treated with the dual biotherapy (CIK-vvDD), displayed a significantly lower rate of relapse (3 of 7, p=0.001). It therefore appears that the ability of the CIK cells to home to the residual tumor cells coupled with the additional killing mechanisms provided by delivery of oncolytic virus resulted in greatly improved clearance of the minimal residual.
Fig 2. Treatment of minimal residual disease.
(A) Experimental procedure; FVB mice received intraperitoneal injections of 6780 cells expressing luciferase with subsequent tumor formation followed by BLI. Once tumors reached 1×108 Ph/Sec, MYC was inactivated to induce tumor regression. Once disease had been undetectable for at least 7 days, animals were treated with a single iv injection of (i) PBS; (ii) CIK cells; (iii) vvDD; (iv) CIK and vvDD or (v) vvDD pre-infected into CIK cells. At the same time doxycycline treatement was stopped (n=8/group, except PBS group, n=5). Subsequent relapse was followed by BLI. (B) Plots of individual animals' BLI signal over time after treatment is shown. Horizontal bars represent lower limits of detection. (C) Average day to relapse (first detectable BLI signal above background) after 6780-luciferase minimal disease was treated with CIK cells delivered iv at day 0 or day 0 and day 14 after doxycycline removal. PBS group 5 of 5 relapsed; CIK(d0) 6 of 7 relapsed; CIK(d0 &d14) 6 of 7 relapsed (* p=0.047).
Lymphomas capable of relapsing from minimal disease states also display increased resistance to radio- and chemotherapy
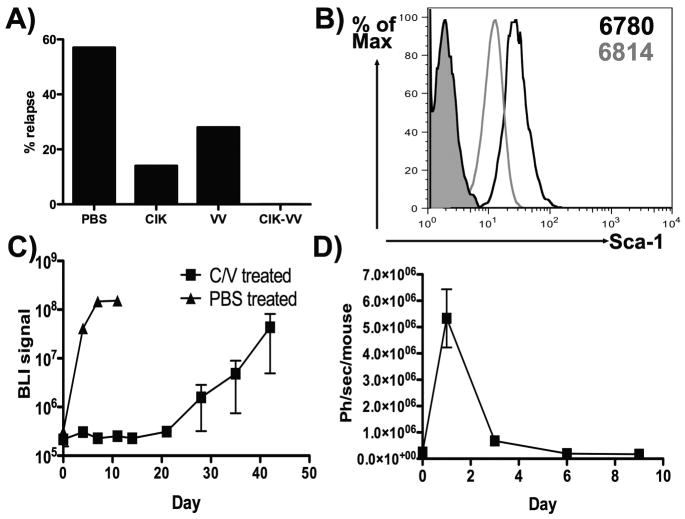
In a second lymphoma cell line (6814; isolated from a second mouse from the same transgenic strain), it was found that subsequent to regression, only 5 of 7 of the PBS treated mice actually relapsed once MYC expression was resumed (Fig 3a). It therefore appears these cells possess reduced stem-like qualities. Although no defined ‘cancer stem cell’ or cancer-initiating cell markers exist for murine lymphomas, it was found that stem cell antigen-1 (Sca-1) was upregulated on the surface of the lymphoma cells that reliably relapsed (6780) relative to the 6814 line (Fig 3b). In this second tumor model, only 1 of 7 CIK treated mice, 2 of 7 vaccinia treated and 0 of 7 dual biotherapy treated animals relapsed (Fig 3a). Interestingly, the Sca-1hi 6780 cells, that display the greatest rates of relapse, were also found to be significantly more resistant to both radiotherapy and chemotherapy (paclitaxel treatment) than the 6814 cells and so possess other properties often associated with tumor-initiating cells (Fig S2).
Fig 3.
(A) Experiment repeated as in Fig 2 with a second tumor cell line (6814). Percentages of animals that displayed relapse by 90 days after treatment are shown (n=7/group). (B) The mean fluorescence intensity of 6780 and 6814 cells stained with Sca-1 antibody, along with isotype (solid). (C) Experiment repeated as in Fig 2 with 6780-luciferase cells, only implantation was into NOD SCID mice. Relapse of mice treated iv with PBS or vvDD infected into CIK cells (C/V) is shown (Day0 = time of treatment and removal of doxycycline from regressed tumors) (n=3/group). (B) Animals from Fig 2 that were treated with CIK-vvDD dual therapy and that displayed no relapse by 90 days (n=4) were rechallenged with 6780-luciferase cells (1×106 IP). Subsequent BLI signal is shown.
Prevention of relapse and the creation of a long-term disease-free state by CIK-vvDD therapy requires an adaptive immune response
When the experiment in Fig 2A was repeated using 6780 cells implanted into NOD SCID mice, PBS treatment resulted in the same timing and rate of relapse as in the immunocompetent animals (Fig 3c). However, when residual disease in immunodeficient mice was treated with CIK-vvDD dual biotherapy, treated animals displayed a delay to relapse but did all relapse, closely mirroring the response of the CIK treated group in immunocompetent animals. This implies that the ability to create a long-term disease-free state is dependent on an intact host immune response and surprisingly is not simply due to the additional tumor-killing ability provided by vvDD. We therefore looked to determine whether mice displaying complete responses following CIK-vvDD dual biotherapy were resistant to re-challenge. Mice bearing 6780 or 6814 tumors (from Figs 2 & 3A) that had demonstrated long-term (greater than 90 days) disease free states after treatment were re-challenged with the same cell lines. It was found that the tumors initially treated with CIK-vvDD dual biotherapy were able to reject re-challenge (Fig 3D and S3). It was also seen that the (Sca-1lo) 6814 tumors that did not relapse in the PBS control group were susceptible to re-challenge (Fig S3), confirming that a lack of regenerative qualities in this cell line is the cause of the lower rates of relapse rather than any increased immunogenicity of these tumor cells. Single therapies in this tumor model that led to complete responses were also typically not protective.
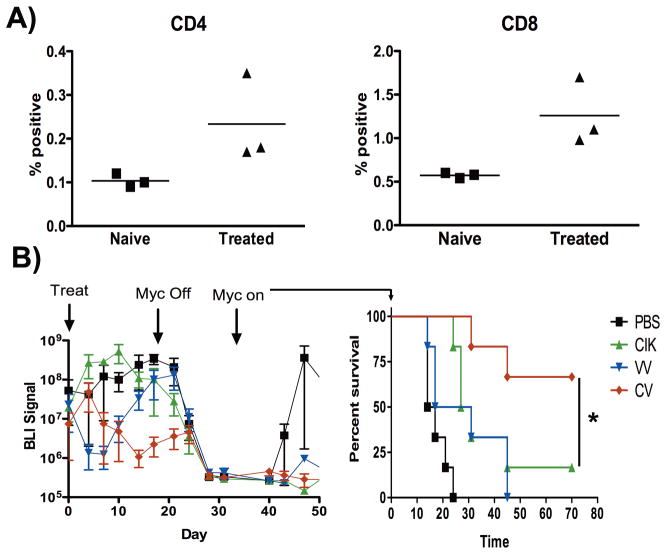
To determine the basis of the immune involvement in rejection of re-challenge, animals were left for a further 90 days, before the remaining animals (n=3 of 6780 tumors) were sacrificed. Splenocytes recovered from these mice were exposed to the same lymphoma cells ex vivo and numbers of CD4+ and CD8+ cells recognizing antigens on these cells were determined by de-granulation assay. Increased levels of CD3+CD4+ and CD3+CD8+ splenocytes recognizing the tumor antigens (relative to splenocytes from naïve controls), indicated the presence of a long-term memory T-cell response (fig 4A).
Fig 4.
(A) Mice that rejected re-challenge with 6780-luciferase cells (Fig 3D) were sacrificed after 90 days. Splenocytes were exposed to 6780 cells and de-granulation determined by CD107a or CD154 surface staining relative to naïve controls (for CD8 and CD4 cells respectively)(p=0.029 and 0.011 for CD4 and CD8). (B) FVB mice implanted IP with 6780-luciferase cells were treated when large primary tumors had formed with iv injections of (i) PBS (black); (ii) CIK cells (green); (iii) vvDD (blue); (iv) vvDD infected into CIK cells (red line), and subsequent tumor growth followed by BLI. When tumor BLI signal reached 1×108 Ph/sec/mouse, tumors were regressed through repression of MYC expression. Once tumors had regressed to undetetectable levels for >7 days, the MYC repression was removed, and relapse followed by BLI (survival curves are shown, with mice sacrificed when tumor burden reached 1×108 Ph/sec/mouse)(n=6/group)(*p<0.05).
In a correlative experiment, animals with large primary 6780 tumors implanted into the peritoneal cavity were treated with single intravenous injections of PBS, CIK cells, vvDD, or CIK-vvDD dual biotherapy at non-curative doses. Although the dual biotherapy treated group displayed the greatest response to treatment (Fig 4B), ultimately all animals displayed tumor re-growth. At this point the MYC oncogene was inactivated (to model a subsequent curative therapy). As before, tumors regressed to undetectable levels (by BLI) before the MYC oncogene was reactivated (with no further treatment). PBS treated animals relapsed as before. The CIK and vvDD single therapy groups also displayed a high rate of relapse. However, 4 of 6 of the CIK-vvDD treated animals did not relapse, indicating that the dual biotherapy is capable of inducing a systemic host immune response targeting tumor antigens even in large primary tumors and that this therapeutic strategy may be effective in an adjuvant setting prior to debulking surgery or chemotherapy.
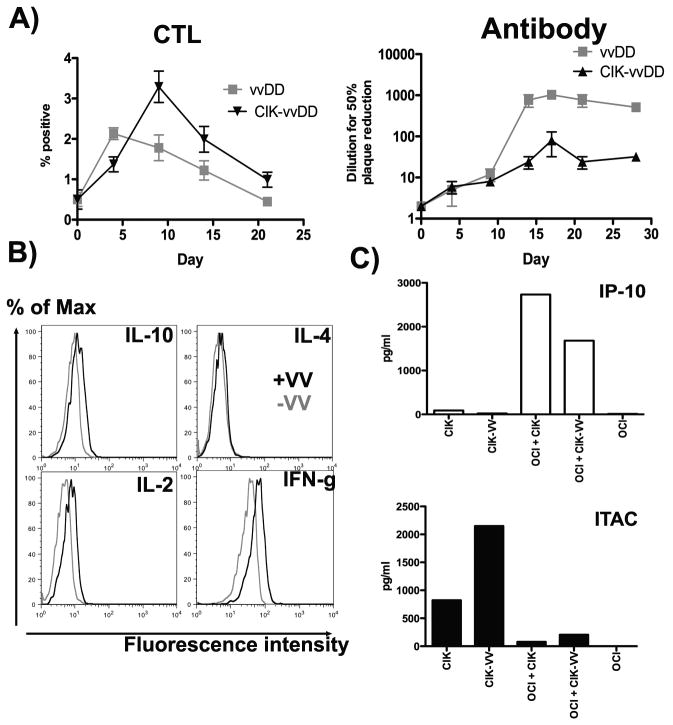
Pre-infection of CIK cells with vvDD, leading to a Th1 skewing of the immune response is critical for optimizing the induction of anti-tumor immunity
The presence of CIK cells co-localized with vaccinia infection therefore appears to direct the immune response. This was further tested; first, the immune response targeting the virus itself was examined after treatment of subcutaneous 6780 tumors with vvDD or vvDD-CIK. It was seen that levels of CD4+ cells capable of degranulation in response to virus ex vivo (CD154 positive) were increased when vvDD-CIK treatment was used relative to vvDD alone, and that the levels of neutralizing antibody produced were decreased under the same conditions (Fig 5A). The immune response induced against the virus therefore appeared skewed towards a Th1 response when CIK cells were present. In a second experiment, intracellular cytokine staining was used to determine the levels of Th1-associated cytokines (IL12 and IFNgamma) and Th2-associated cytokines (IL4 and IL10) produced by CIK cells. It was found that alone CIK cells produced high levels of IFN-gamma, low levels of IL12 and IL10, and no IL4, (Fig 5B), while CIK cells pre-infected with vvDD produced increased levels of IFN-gamma and IL12, skewing the response towards the Th1 arm, and explaining why pre-infected CIK cells were better able to induce a cellular anti-tumor immune response than were CIK cells and virus delivered together without the pre-infection step. A qRT-PCR array was also performed with a panel of Th1/Th2 cytokines. This confirmed that CIK cells produced more IL12 and IFNgamma than IL4 and IL10 (data not shown). Overall, very few genes were up-regulated as a result of viral infection of CIK cells and the only genes significantly up-regulated included IL-2, CD28 and IL-7, indicating again that pre-infected CIK cells may act as more potent instigators of a cellular adaptive immune response. Finally, we looked at the production of chemokines known to attract Tc1 cells through binding to CXCR3 [CXCL10 (IP-10) and CXCL11 (I-TAC)](Fig 5C). It was found that CIK cell production of CXCL11 was increased when the cells were infected with vvDD. CXCL10 was only weakly produced by infected or uninfected CIK cells. However, when the CIK cells (with or without viral infection) came into contact with lymphoma cells, significant amounts of this chemokine were produced. It would therefore appear that the combination of cytokines and chemokines produced when infected CIK cells are located within the tumor would induce a cellular (Th1) host immune response.
Fig 5.
(A) FVB mice with subcutaneous 6780 tumors were treated IT with vvDD or vvDD-CIK. Mice were sacrificed at indicated times and (i) degranulation assays run as before to determine levels of CD4+ cells activated by exposure to vvDD infected cells (n=3/group)(p<0.05) or (ii) vvDD neutralizing antibody assays were run on mouse plasma. (B) Human CIK cells alone, or following vvDD infection (MOI of 1.0 for 24h) underwent intracellular cytokine stains for the indicated cytokines, and mean fluorescence intensities determined (grey = CIK cells alone; black = CIK cells infected with vvDD); (D) ELISAs were run for IP-10 (CXCL10) or I-TAC (CXCL11) on the media from (i) human CIK cells; (ii) CIK cells infected with vvDD; (iii) OCI-Ly8 lymphoma cells exposed to CIK cells; (iv) OCI-Ly8 cells exposed to CIK cells infected with vvDD; (v) OCI-Ly8 cells alone.
CIK-vvDD dual biotherapy also functions to clear minimal disease states produced following chemotherapy treatment
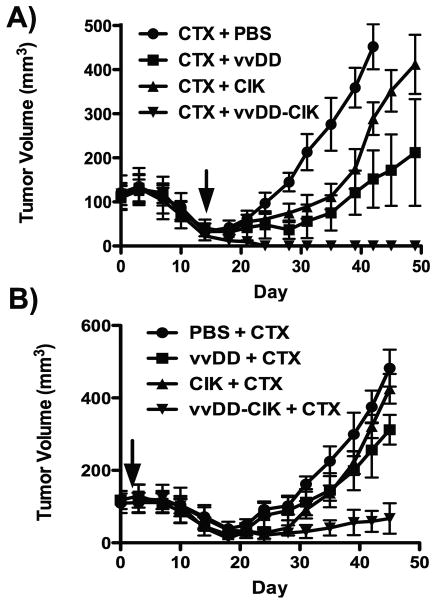
We looked to extend these observations beyond the tet-OFF regulated MYC system and model a more clinically accurate system. BALB/c mice were therefore implanted with A20 lymphoma cells and treated IV with vvDD; CIK or vvDD-CIK either 2d before or 14d after chemotherapy (cyclophosphamide, 100 mg/kg IP, as a common lymphoma treatment) (Fig 6). When the chemotherapy was applied first (Fig 6A), the biological therapies were applied at a point when tumors had regressed considerably from the time of initial treatment, but re-grew without any additional therapy. As before, the CIK and vvDD therapies used alone slowed or delayed, but could not prevent, tumor reformation. vvDD-CIK dual biotherapy was again the most effective and 8 of 8 mice remained disease free, despite the known immunosuppressive nature of cyclophosphamide.
Fig 6.
Combining dual biotherapy with chemotherapy. Mice (BALB/c) bearing subcutaneous A-20 tumors were treated IP with 100mg/kg cyclophosphamide (CTX) either 14d before (A) or 2d after (B) IV treatment with PBS, CIK, vvDD or vvDD-CIK as before (n=8/group), tumor volume determined by caliper measurement.
When the biological agents were added first (Fig 6B), modeling an adjuvant setting, single therapies (CIK or vvDD alone) resulted in only very minor additional benefit over the chemotherapy alone. The dual biotherapy again led to the greatest overall therapeutic benefit (with 3 of 8 complete responses).
Discussion
Therapies designed specifically to target the minimal disease remaining after a successful therapy or debulking surgery might provide a dramatic improvement in life expectancy for patients with cancers that are prone to relapse. However, such therapies must be able to both reach the last cancer cells, and be able to destroy these cells, which are frequently heterogeneous, and phenotypically distinct from the bulk tumor. Any therapies specifically designed to target these cells must therefore have the ability to destroy tumor cells through multiple mechanisms.
Here we extend our previous work targeting bulk tumors with a novel dual biotherapy (vvDD-CIK) to demonstrate that this therapeutic approach displays a profound ability to specifically clear the last remaining tumor cells and so prevent relapse. We also demonstrate for the first time that the critical factor for this process is the immunogenicity of the virus, aided by the release of cytokines and chemokines from the CIK cells that together can lead to potent CTL responses as an additional anti-tumor mechanism.
Because minimal residual disease is traditionally difficult to reliably and reproducibly recreate in pre-clinical models, we developed a novel system to test this therapy. In this model, regulated inactivation of oncogenic MYC leads to reversion of tumor cells to an undifferentiated phenotype in vitro, and tumor regression in vivo. Subsequent re-activation of MYC expression leads to tumor relapse, despite the residual number of tumor cells being very low (<1000). It was also found that an ability to reform tumors from a minimal disease state was not a universal property of spontaneous tumors formed in this transgenic mouse model, but did correlate with surface expression levels of the stem cell antigen (Sca-1) and increased resistance to both chemotherapy and radiotherapy.
CIK-vvDD biotherapy prevented relapse in more than half of the mice with a minimal disease state. Interestingly, CIK cells alone could delay relapse, but once they were no longer present the cancer returned. It would seem likely that the increased tumor-killing potential provided by vvDD released from CIK cells would be responsible for this ability to clear the last remaining cancer cells, however the fact that no such enhanced effect was seen in immunodeficient mice surprisingly implies that the additional benefits are in fact mediated by the host immune response. This represents for the first time a situation under which the directly oncolytic potential of the virus is not necessary, with its immunogenic potential being of paramount importance instead. This is not entirely unexpected however, as ourselves and others have reported that oncolytic viruses are capable of transitioning to a predominantly immune-mediated mechanism of tumor killing under some conditions (20). As CIK-mediated delivery of vvDD may not allow direct targeting of every remaining cancer cell by the therapeutic, the additional immune response raised against tumor antigens would be necessary for complete tumor clearance and long-term immune surveillance to prevent relapse.
It was further demonstrated that previously undescribed and unique characteristics of CIK cells infected with vvDD lead to the greatest induction of CTL, and likely also enhance the trafficking of these T-cells to the residual tumor cells. In particular a Th1 skewing of the immune response and increased levels of chemokines known to attract activated Tc1 cells were produced by, or in response to CIK cells infected with vvDD.
Finally, it was shown that it was also possible to induce the immune response through treatment of bulk tumors, but that the benefits of this immune response were not seen until after the large (and presumably immunosuppressive) tumors had been removed. This implies that this approach can be applied in either an adjuvant or a neoadjuvant setting. This was confirmed in a second mouse model of residual disease, involving chemotherapy treatment to de-bulk lymphoma.
CIK-vvDD treatment of minimal residual disease, or even prior to other debulking therapeutic strategies therefore represents a novel approach for the treatment of cancer patients and a novel application for vvDD-CIK therapy, as well as providing a much needed means to clear the last remaining cancer cells that possess tumor-initiating properties and an increased resistance to chemotherapy and radiotherapy.
Supplementary Material
Acknowledgments
This work was supported by the Hillman Foundation, a Young Innovator award from The Pittsburgh Foundation and R01 CA140215 (Dr Thorne), and P50 CA114747 and R24 CA92862 (Dr Contag).
Footnotes
Conflict of interest; SHT holds stock in Jennerex Biotherapeutics.
References
- 1.Thorne SH, Negrin RS, Contag CH. Synergistic antitumor effects of immune cell-viral biotherapy. Science. 2006;311:1780–4. doi: 10.1126/science.1121411. [DOI] [PubMed] [Google Scholar]
- 2.Lu PH, Negrin RS. A novel population of expanded human CD3+CD56+ cells derived from T cells with potent in vivo antitumor activity in mice with severe combined immunodeficiency. J Immunol. 1994;153:1687–96. [PubMed] [Google Scholar]
- 3.Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:181–7. doi: 10.1016/j.bbmt.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Thorne SH, Hwang TH, O'Gorman WE, et al. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. J Clin Invest. 2007;117:3350–8. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 6.McCart JA, Ward JM, Lee J, et al. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001;61:8751–7. [PubMed] [Google Scholar]
- 7.Thorne SH, Contag CH. Integrating the biological characteristics of oncolytic viruses and immune cells can optimize therapeutic benefits of cell-based delivery. Gene Ther. 2008 doi: 10.1038/gt.2008.42. [DOI] [PubMed] [Google Scholar]
- 8.Wu CH, Sahoo D, Arvanitis C, Bradon N, Dill DL, Felsher DW. Combined analysis of murine and human microarrays and ChIP analysis reveals genes associated with the ability of MYC to maintain tumorigenesis. PLoS Genet. 2008;4:e1000090. doi: 10.1371/journal.pgen.1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 10.Giuriato S, Ryeom S, Fan AC, et al. Sustained regression of tumors upon MYC inactivation requires p53 or thrombospondin-1 to reverse the angiogenic switch. Proc Natl Acad Sci U S A. 2006;103:16266–71. doi: 10.1073/pnas.0608017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu CH, van Riggelen J, Yetil A, Fan AC, Bachireddy P, Felsher DW. Cellular senescence is an important mechanism of tumor regression upon c-Myc inactivation. Proc Natl Acad Sci U S A. 2007;104:13028–33. doi: 10.1073/pnas.0701953104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran PT, Fan AC, Bendapudi PK, et al. Combined Inactivation of MYC and K-Ras oncogenes reverses tumorigenesis in lung adenocarcinomas and lymphomas. PLoS One. 2008;3:e2125. doi: 10.1371/journal.pone.0002125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shachaf CM, Gentles AJ, Elchuri S, et al. Genomic and proteomic analysis reveals a threshold level of MYC required for tumor maintenance. Cancer Res. 2008;68:5132–42. doi: 10.1158/0008-5472.CAN-07-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karlsson A, Giuriato S, Tang F, Fung-Weier J, Levan G, Felsher DW. Genomically complex lymphomas undergo sustained tumor regression upon MYC inactivation unless they acquire novel chromosomal translocations. Blood. 2003;101:2797–803. doi: 10.1182/blood-2002-10-3091. [DOI] [PubMed] [Google Scholar]
- 15.Baker J, Verneris MR, Ito M, Shizuru JA, Negrin RS. Expansion of cytolytic CD8(+) natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon gamma production. Blood. 2001;97:2923–31. doi: 10.1182/blood.v97.10.2923. [DOI] [PubMed] [Google Scholar]
- 16.Cao YA, Bachmann MH, Beilhack A, et al. Molecular imaging using labeled donor tissues reveals patterns of engraftment, rejection, and survival in transplantation. Transplantation. 2005;80:134–9. doi: 10.1097/01.tp.0000164347.50559.a3. [DOI] [PubMed] [Google Scholar]
- 17.Rubio V, Stuge TB, Singh N, et al. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–82. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- 18.Edinger M, Cao YA, Verneris MR, Bachmann MH, Contag CH, Negrin RS. Revealing lymphoma growth and the efficacy of immune cell therapies using in vivo bioluminescence imaging. Blood. 2003;101:640–8. doi: 10.1182/blood-2002-06-1751. [DOI] [PubMed] [Google Scholar]
- 19.Kirn DH, Wang Y, Le Boeuf F, Bell J, Thorne SH. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 2007;4:e353. doi: 10.1371/journal.pmed.0040353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prestwich RJ, Ilett EJ, Errington F, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15:4374–81. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.