Abstract
Background
Inflammatory events within the intestinal muscularis, including macrophage activation and leukocyte recruitment, have been demonstrated to participate in causing postoperative ileus. Recently, glycine has gained attention due to its beneficial immunomodulatory effects in transplantation, shock and sepsis.
Objective
The purpose of this study was to determine if pre-operative glycine administration would attenuate postoperative ileus in rodents.
Methods
Muscularis glycine receptors were investigated by immunohistochemistry. Gastrointestinal motility was assessed by in vivo transit distribution histograms with calculated geometric center analysis and jejunal circular smooth muscle contractility in a standard organ bath. The impact of glycine on the muscularis inflammatory responses to surgical manipulation of the intestine were measured by real time PCR, nitric oxide Griess reaction, prostaglandin ELISA, Luminex and histochemistry.
Results
Glycine-gated chloride channels were immunohistochemically localized to muscularis macrophages and postoperative infiltrating leukocytes. Pre-operative glycine treatment significantly improved postoperative gastrointestinal transit and jejunal circular muscle contractility. Pre-operative glycine injection significantly reduced the induction of IL-6, TNF-α, iNOS and ICAM-1 mRNAs, which was associated with the attenuation in postoperative leukocyte recruitment. Nitric oxide and prostanoid release from the postsurgical inflamed muscularis was diminished by glycine. The secretion of the inflammatory proteins IL-6, MCP-1 and MIP-1α were also significantly decreased by glycine pretreatment.
Conclusion
The data indicate that pre-operative glycine reduces postoperative ileus via the early attenuation of primal inflammatory events within the surgically manipulated gut wall. Therapeutic modulation of resident macrophages by glycine is a potential novel pharmacological target for the prevention of postoperative ileus.
Keywords: Glycine, macrophage, leukocytes, inflammation, postoperative ileus
INTRODUCTION
Postoperative ileus, remains a major clinical problem with significant deleterious individual and economic impact (1;2). Ileus has been widely accepted as a near inevitable physiological consequence of abdominal surgery (1). However, the time required for postoperative recovery of the gastrointestinal tract hinders recent efforts to minimize the length of hospital stay and reduce perioperative costs (3). Clinical progress regarding the amelioration of postoperative motility has been achieved by technical modifications such as the implementation of minimally invasive surgery and changes in perioperative management, particularly the early onset of enteral nutrition (4-9). Additionally, new encouraging pharmaceuticals have been suggested to improve gastrointestinal motor function after surgery (10-14). However, clinical therapeutic approaches are supportive in nature and do not target the mechanistic etiology of postoperative ileus (2). Consequently, therapeutic agents that specifically target pathophysiological mechanisms in the development of postoperative gastrointestinal dysmotility are needed to overcome the clinical problem of iatrogenic ileus.
Previous work has emphasized local muscularis inflammatory events as a major etiological factor in the development of postoperative ileus (15). In this scenario, resident muscularis macrophages act as potent immunological sentinels that react to external stimuli initiating a complex inflammatory cascade. Activated macrophages then express a multitude of proinflammatory cytokines and chemokines, which are associated with the induction of adhesion molecules in the muscularis microvasculature and the recruitment of immune-competent leukocytes from the systemic circulation. Recruited leukocytes as well as activated resident macrophages both produce kinetically active synthases, such as the inducible nitric oxide synthase (iNOS) and the inducible cyclo-oxygenase isoform (COX-2), that via the release of nitric oxide (NO) and prostaglandins directly modulate postoperative smooth muscle contractile activity. Although pharmacological agents designed to inhibit these individual mediators show beneficial effects in rodent models, these downstream terminal inflammatory pathway end-products do not address early primal initiating events that can be region and species specific indicating they are likely to be less effective in the clinic setting. Thus, a specific pharmacological approach should ideally target the initiation of the complex inflammatory cascade.
The dietary or intravenous application of glycine has been demonstrated to prevent inflammatory complications in several experimental models, such as ischemia/reperfusion, transplantation, shock, endotoxemia and diabetes (16-22). Glycine itself is very non-noxious and non-toxic with an LD50 of 2600 mg/kg for intravenous glycine. Glycine, but not valine, has been shown to significantly blunt the inflammatory reaction of macrophages and neutrophils via binding to specific glycine-gated chloride channels, which modulates intracellular calcium concentrations (23;24). As noted above, both of these cell populations are mechanistically involved in the development of the inflammatory cascade within the intestinal muscularis (25;26). Based on this, we hypothesized that pre-operative glycine administration would prevent postoperative ileus via the attenuation of muscularis inflammatory events. Therefore, the objectives were 1) to identify glycine-gated chloride channels within the intestinal muscularis, 2) to define the functional relevance of glycine pretreatment on the development of postoperative ileus and 3) to determine the impact of glycine on the development of the postoperative inflammatory cascade.
MATERIALS AND METHODS
Animals and Operative Procedures
Male ACI (black agouti) rats (180-220 g) and C57Bl/6 mice (20-30 g) were obtained from Harlan (Indianapolis, IN). The experimental design was approved by the University of Pittsburgh IACUC. Glycine (85 or 170 mg/kg) was injected intravenously via the penile vein 1 hour prior to surgery. This dose has shown to increase glycine serum levels sevenfold and to be effective in the prevention of reperfusion injury in rat liver transplantation (17). Age matched animals were treated with vehicle (normal saline) or valine (263.5 mg/kg, osmotic control). The glycine receptor is known to be activated by a range of simple amino acids including glycine, β-alanine and taurine. Valine was used as the osmotic control substance for glycine because both are non-polar and neutral amino acids. The entire intestine was subjected to a standardized degree of surgical manipulation as described previously (25). Briefly, the intestine was carefully eventrated and spread out onto a gauze sponge moistened with 0.9% saline. The intestine was gently compressed along its entire length using a rolling motion of two moist cotton-tipped applicators. To ensure even manipulation of all sections of the small and large intestine, this procedure was repeated three times. After completion of the standardized gut manipulation procedure, the intestine was returned to the peritoneal cavity and the incision was closed in two layers using running 4-0 silk suture.
Functional Studies
Mouse and rat in vivo gastrointestinal transit was measured after 24 hours postoperatively by evaluating the gastrointestinal distribution of orally administered fluorescein-labeled dextran (Molecular Probes, Eugene, OR). The geometric center (GC) was calculated as previously described (N = 4-9) (27). Briefly, mice were orally fed 10 μl and rats 20 μl of FITC-Dextran of 70,000 MW (FD70) dissolved in distilled water (6.25 mg/ml). Ninety minutes later, the animals were sacrificed with an overdose of isoflurane inhalation. The entire gastrointestinal tract, from lower esophageal sphincter to distal colon, was excised and divided into 15 segments: stomach, small intestine (10 segments of equal length), cecum, and colon (three segments of equal length). For mice the luminal content of each segment was collected into a small tube and suspended in 800 μl of distilled water. The intestinal chyme in each tube was mixed vigorously and then clarified by centrifugation. The supernatants were collected and fluorometrically assayed for the FD70 concentration. The distribution of FD70 along the gastrointestinal tract was summarized by calculating the geometric center (GC) for the distribution of the fluorescein-labeled dextran using the following formula; GC=∑ (% of total fluorescent signal per segment × segment number)/100. Mechanical in vitro activity of rat mid-jejunal circular muscle was evaluated at 24 hours postoperatively using bethanechol stimulation as described previously (rats: N = 5-11) (25).
mRNA Expression Analysis
Postoperative mediator mRNA expressions in the isolated rat small intestinal muscularis were analyzed using SYBR Green two-step RT-PCR as previously described, (N = 4-6) (28-34). Based on our previous observation that postoperative mediator induction reaches a maximum between 2 and 6 hours postoperatively, comparative mRNA analysis was performed at 3 hours. The sequences of the real-time-PCR primers are listed in Table 1. Relative quantification was performed using the comparative CT method as described by Schmittgen et al. (35).
Table 1.
Nucleotide sequences of oligonucleotide primers. Slopes and correlation coefficients of the primer standard curves.
| Target gene |
Primer sequences (5′to 3′) | MgCl2 | Slope | Correlation- coefficient |
|---|---|---|---|---|
| GAPDH | Sense: ATGGCACAGTCAAGGCTGAGA | 3 | −3.197 | 0.996 |
| Antisense: CGCTCCTGGAAGATGGTGAT | ||||
| COX-2 | Sense: CTCTGCGATGCTCTTCCGAG | 5 | −3.494 | 0.991 |
| Antisense: AAGGATTTGCTGCATGGCTG | ||||
| ICAM-1 | Sense: CGTGGCGTCCATTTACACCT | 3 | −3.404 | 0.991 |
| Antisense: TTAGGGCCTCCTCCTGAGC | ||||
| IL-6 | Sense: GCCCTTCAGGAACAGCTATGA | 5 | 3.360 | 0.997 |
| Antisense: TGTCAACAACATCAGTCCCAAGA | ||||
| iNOS | Sense: GGAGAGATTTTTCACGACACCC | 3 | −3.518 | 0.999 |
| Antisense: CCATGCATAATTTGGACTTGCA | ||||
| TNF-α | Sense: GGTGATCGGTCCCAACAAGGA | 5 | −3.021 | 0.997 |
| Antisense: CACGCTGGCTCAGCCACTC |
Histochemistry and Immunohistochemistry
Leukocyte infiltration was quantified on rat whole-mounts of the intestinal muscularis as described previously using Hanker-Yates histochemistry (Polysciences, Warrington, PA) (N = 6-8) (36). For glycine-gated chloride channel immunohistochemistry, rat jejunal whole mounts were incubated for 18 hours at 4°C with a mouse-anti-rat monoclonal glycine receptor antibody (1:50) (Alexis Corp, San Diego, CA), followed by 3x washes in 0.05 mol/l phosphate-buffered saline solution (PBS). Each specimen was then incubated with a Cy3 donkey-anti-mouse secondary antibody (1:500) for 4 hours at 4°C and washed again three times in 0.05M PBS. Secondary antibodies without glycine receptor antibody preincubation were used in parallel in all staining procedures to ensure specificity. Macrophages were visualized by using ED2 immunohistochemistry (Serotec Inc., Raleigh, NC)., as previously described (37).
Tissue culture and determination of nitrite and prostanoid release in supernatant
The rat small intestine and colon were processed for tissue culture, (N = 5-8). Briefly, the isolated muscularis (80-120 g) was incubated for 24 hours (37°C, 5% CO2) in 6-well tissue culture plates and 500 μl aliquots of supernatant were collected, frozen in liquid nitrogen and stored at −80°C. The generation of NO from the intestinal muscularis was measured as nitrite production using the Griess reaction (38). The muscularis release of prostanoids was assayed by ELISA (Cayman Chemicals, Ann Arbor, MI). Each standard and supernatant sample was analyzed in duplicate, and the mean value was normalized to the tissue weight.
Luminex Multiplex Bead Immunoassays
The release of 20 inflammatory analytes into the mouse tissue culture supernatant, obtained as above, was quantified with a Luminex 100™ using microsphere-based multiplexing technology. The mouse cytokine twenty plex immune kit was comprised of analyte specific components for the simultaneous measurement of the following mouse cytokines (FGF-basic, IL-1α, IL-1ß, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17, TNF-α, IFN-γ, GM-CSF, KC, MCP-1, MIG, MIP-1α, IP-10 and VEGF) (Invitrogen, Carlsbad, CA).
Solutions and Data Analysis
A standard KRB was used as described previously (27;36). KRB constituents, bethanechol, glycine, L-valine, urea, 3% hydrogen peroxide, HBSS and DMEM-F12 were purchased from Sigma Chemical Co. (St.Louis, MO). Results are expressed as mean ± standard error of the mean (SEM). The data were analyzed using a SPSS two-way analysis of variance (ANOVA) and statistical significance was determined using Tukey post-hoc group comparisons (p-values < 0.05 were considered significant).
RESULTS
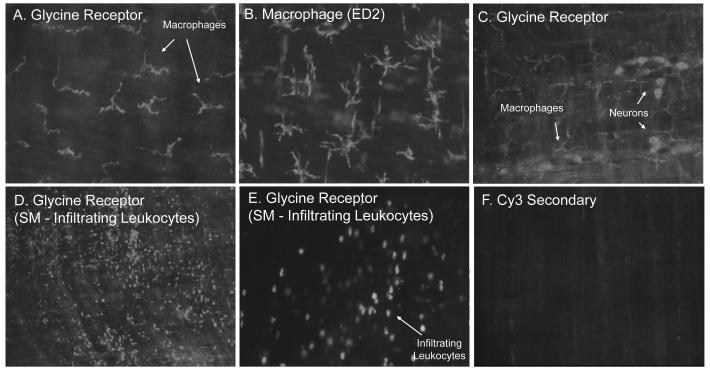
We first investigated the localization of glycine receptors within the rat intestinal muscularis using immunohistochemistry. As shown in Figure 1, Panel A, muscularis whole-mounts from control animals exhibited glycine-gated chloride channel immunoreactivity prominently on stellate shaped cells. The distribution and morphology of these cells were similar to that of resident muscularis macrophages and immunohistochemical staining with the rat macrophage marker ED2 confirmed the identification of these cells as resident muscularis macrophage-like cells (Figure 1, Panel B). Glycine receptor immunoreactivity was also recognized in a subpopulation of neurons in the myenteric plexus (Figure 1, Panel C). Under normal physiological circumstances infiltrating leukocytes are nearly absent in the intestinal muscularis. However, after a local or systemic injury to the gut, such as surgical manipulation (39), endotoxemia (40;41), ischemia/reperfusion injury (42) or hemorrhagic shock (43), a significant number of leukocytes are recruited into the intestinal muscularis. Thus, we also stained for glycine receptor immunoreactivity 24 hours after surgical manipulation of the intestine to determine the presence of the glycine receptor on infiltrating leukocytes. As depicted in Figures 1, Panel D and Panel E, whole-mounts after intestinal manipulation showed pronounced receptor-like immunoreactivity on round to oblong shaped cells with a distribution and morphology resembled those of infiltrating leukocytes. Staining procedures with the secondary antibody but without glycine receptor antibody preincubation revealed no specific immunoreactivity either on macrophages or neurons in control whole-mounts (Figure 1, Panel F).
Figure 1.
Panels A-F. Fluorescence micrographs of rat jejunal whole-mounts immunohistochemically stained with a specific antibody against the rat glycine receptor with the secondary conjugated to Cy3. Panel A.) Muscularis whole-mounts from control rats expressed a highly fluorescent immunoreactive signal to the glycine receptor on stellate shaped cells. Panel B.) Using macrophage specific ED2 immunohistochemistry, these cells were identified as macrophages. Panel C.) In addition to macrophages, enteric neuronal cells presented with glycine receptor-like immunoreactivity. Panels D and E.) Numerous glycine receptor positive infiltrating leukocytes were observed 24 hours after intestinal manipulation. Panel F.) No specific immunoreactivity was visualized using a control Cy3 staining without primary antibody incubation. (original magnification: A, C, E and F=200X; B=250X; D=100X).
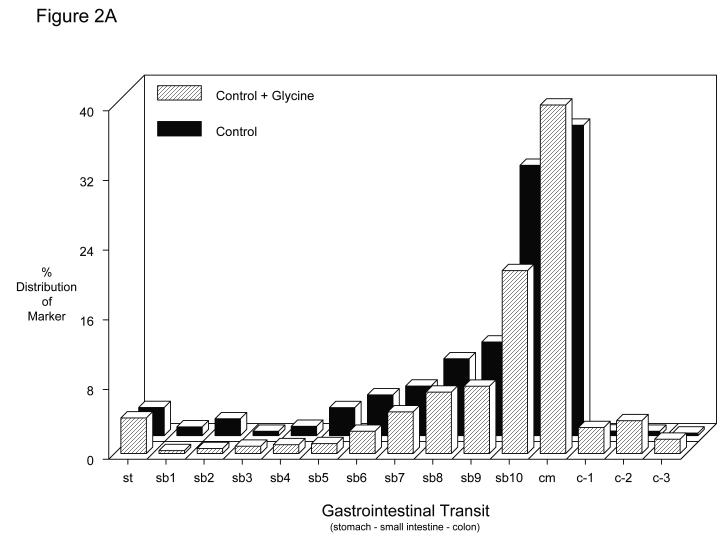
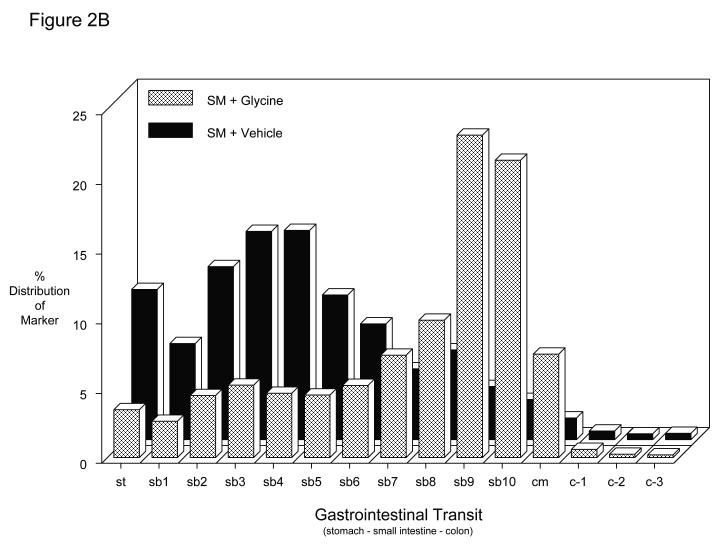
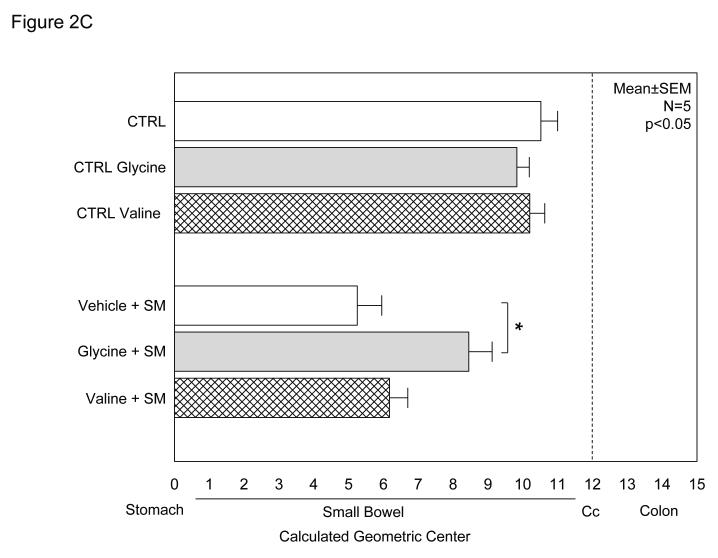
The potential of glycine pretreatment to prevent postoperative inflammatory dysmotility was analyzed in vivo by measuring gastrointestinal transit distribution histograms and in vitro using standard mechanical organ bath techniques. As plotted in the histogram of Figure 2A, non-manipulated vehicle controls and glycine treated rats presented with similar transit distribution patterns of the non-absorbable transit marker two hours after oral administration (vehicle: GC = 10.5 ± 0.5; glycine: GC = 9.8 ± 0.4 – Figure 2C). Valine also had no effect on control gastrointestinal transit measurements (GC = 10.2 ± 0.3). As previously shown surgical manipulation causes a delay in intestinal transit among the vehicle injected animals (GC = 5.2 ± 0.7). In this group, the fluorescence marker was retained within the proximal intestine yielding a delayed geometric center (Figures 2B and 2C). However, bowel manipulated rats that had received glycine (170 mg/kg) pretreatment before surgery showed a significantly improved postoperative transit (Figures 2B) exhibiting a calculated geometric center of 8.5 ± 0.7 (Figures 2C). In contrast, the manipulated amino acid particle control pretreatment group demonstrated significant ileus with valine having no significant beneficial effect on postoperative gastrointestinal transit (GC = 6.2 ± 0.5), compared to manipulated vehicle-injected rats (Figure 2C) (two-way ANOVA with Tukey post-hoc analysis for treatment groups).
Figure 2.
A-C. Gastrointestinal transit histograms and calculated geometric center histogram for the distribution of fluorescein-labeled dextran along the rat gastrointestinal tract 2 hours after oral administration (SM: intestinal manipulation, St = stomach, SB = small bowel, CM = cecum, C = colon). 2A.) Gastrointestinal transit distribution histograms for control and glycine pretreated control showing the progression of the transit marker to the distal regions of the small intestine over a period of 2 hours. 2B.) Gastrointestinal transit distribution histograms for vehicle and glycine pretreated animals which had been surgically manipulated 24 hours prior to measurement. Vehicle pretreatment resulted in a marked delay in gastrointestinal transit. Glycine pretreatment significantly improved postoperative gastrointestinal transit with the fluorescent marker transported more caudally compared to the vehicle pretreated manipulated rodents (N = 6 each). 2C.) Histogram demonstrating calculated transit geometric centers (GC) for six groups of rats. Control vehicle, glycine and valine pretreated non-operated rats had a geometric center approximately 10 indicating transit near to the cecum. Surgical manipulation (SM) with vehicle treatment significantly reduced the GC, while this reduction was prevented by glycine immunomodulation pretreatment. In contrast, valine did not significantly improve gastrointestinal transit. (N = 6 each, mean±SEM; two-way ANOVA with Tukey post-hoc group comparisons with * = p-values < 0.05).
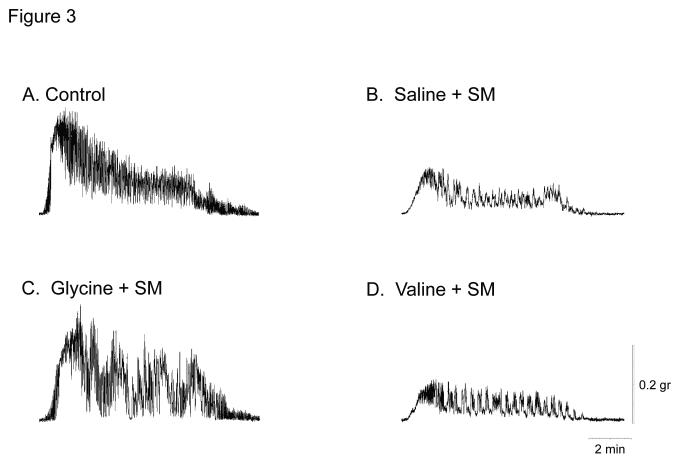
Next, we investigated the impact of glycine pretreatment directly to the rat jejunal muscularis by measuring in vitro spontaneous and bethanechol stimulated contractile activity. As illustrated in Figure 3A, bethanechol (100 μM) stimulated control vehicle pretreated full thickness circular muscularis strips to generate a robust tonic contracture with overlying large phasic contractions. Intestinal manipulation caused a marked reduction in the contractile response to bethanechol and a diminished tonic component. In contrast, glycine pretreatment attenuated the postoperative reduction in contractile activity (Figure 3A, Panel C), whereas valine pretreatment had no effect on the postoperative motor function of the circular muscularis (Figure 3A, Panel D).
Figure 3.
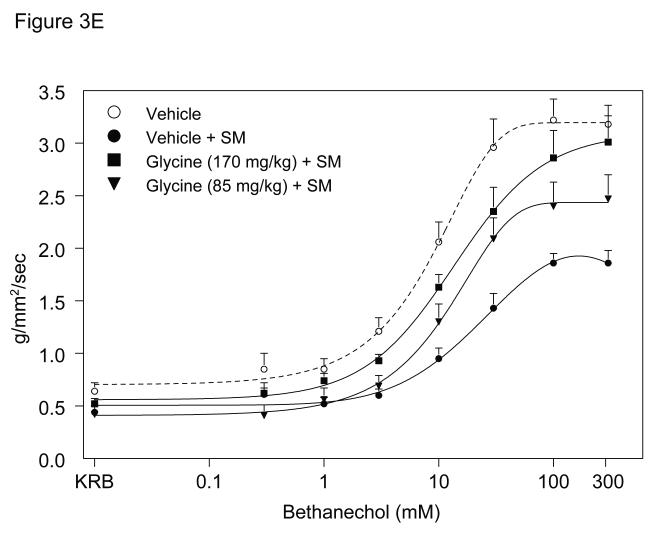
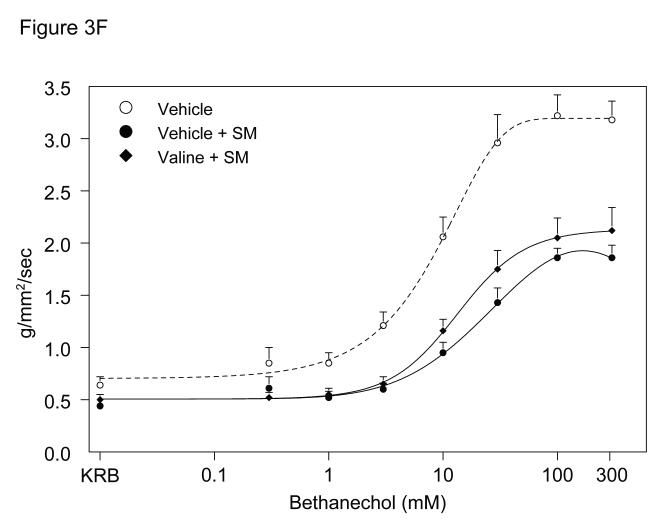
A. Representative bethanechol stimulated (100 μM) rat jejunal circular smooth muscle contractile activity recorded in an organ bath 24 hours after surgical manipulation (SM). A vigorous contractile response is observed in vehicle injected rat controls (Panel A). Intestinal manipulation in vehicle animals caused a distinct reduction of the contractile response to bethanechol (Panel B), whereas manipulated rats after glycine pretreatment (Panel C) presented with only a mild reduction in bethanechol stimulated contractile activity. Valine pretreated animals also exhibited a distinct reduction of the contractile response to bethanechol (Panel D). Figure 3, Panel E and F, shows bethanechol dose response curves of jejunal circular smooth muscle contractile activity 24 hours after surgical manipulation (SM) with glycine or valine pretreatment. ( ○ ) shows a robust control response curve calculated from control vehicle jejunal muscles. Surgical manipulation with saline pretreatment significantly decreased muscle contractility ( ● ). Glycine pretreatment at both 85 mg/kg ( ▼) and 170 mg/kg ( ■ ) dose dependently prevented the suppression in muscle contractility. Figure 3, Panel F, illustrates that valine pretreatment did not markedly change in vitro muscle contractility. (n = 8 per group, data are expressed as mean ± SEM).
The direct effect of glycine pretreatment on the jejunal muscularis externa 24 hours after surgery was explored by generating complete bethanechol dose response curves for control vehicle, glycine (85 mg/kg), glycine (170 mg/kg) and valine (263.5 mg/kg), as shown in Figures 3E and 3F. Jejunal circular muscles from non-manipulated, vehicle pretreated rats showed a progressive increase in the contractile force in response to increasing bethanechol stimulation and generated contractions with a mean contractile force of 3.2 ± 0.2 g/mm2/s at 100 μM bethanechol. Twenty-four hour pretreatment of control un-operated rats with glycine (170 mg/kg) or valine (263.5 mg/kg) had no effect on bethanechol responses compared to vehicle treated animals (3.3 ± 0.4 and 2.9 ± 0.3 g/mm2/s at 100μM, respectively). In contrast, intestinal manipulation caused a significant 42% reduction in the contractile responses (1.9 ± 0.1 g/mm2/s at 100 μM) (t-test, p<0.05). Muscle strips from manipulated glycine pretreated rats presented with a significant, dose dependent improvement in bethanechol stimulated contractile activity, compared to manipulated vehicle pretreated animals (85mg/kg glycine: 2.4 ± 0.3 g/mm2/s and 170 mg/kg glycine: 2.9 ± 0.3 g/mm2/s at 100 μM bethanechol). And as illustrated in Figure 3F, there was no significant difference in muscle contractility of manipulated valine pretreated animals (2.1 ± 0.2 g/mm2/s at 100 μM) versus manipulated vehicle pretreated animals (N = 8 per group, mean±SEM; two-way ANOVA with Tukey post-hoc group comparisons for treatment groups).
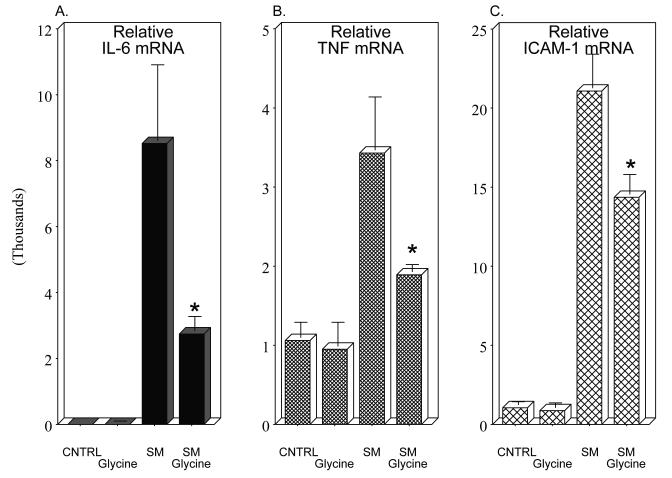
We next investigated the impact of pre-operative glycine treatment on the induction of prototypical inflammatory mediators in the early postoperative course. We focused on the mRNAs of the multifunctional cytokines IL-6 and TNF-α, and the leukocyte adhesion mediator ICAM-1. RT-PCR for IL-6 at 3 hours postoperatively in rat muscularis samples of the saline treated manipulated rats entered the exponential amplification phase earlier than the saline controls demonstrating a higher template concentration (8521-fold). And as shown in Figure 4, Panel A, glycine pretreated animals significantly attenuated the postoperative induction of IL-6 mRNA. A similar glycine attenuation of the postoperative induction of a molecular inflammatory response was also obtained for TNF-α and ICAM-1 mRNAs (Figure 4, Panels B and C). Mediator mRNA levels from non-operated vehicle rats did not differ from non-operated glycine injected animals and valine pretreatment of surgically manipulated animals had no effect on IL-6, TNF-α or ICAM-1 mRNA levels (N=4 each).
Figure 4.
Histograms of real-time two-step RT-PCR analysis of inflammatory mediator mRNA expressions 3 hours postoperatively (SM: intestinal manipulation). The mRNA of the three inflammatory indicators were quantified (IL-6, TNF-α and ICAM-1) in unoperated vehicle controls, glycine controls and after surgical manipulation with and without glycine pretreatment of the surgically manipulated animals. Each inflammatory mRNA was markedly induced by surgical manipulation and this induction was significantly blunted by glycine pretreatment (n = 6-8 per group, mean ± SEM; F-test and Bonferroni post-hoc group comparisons with * = p-values < 0.05).
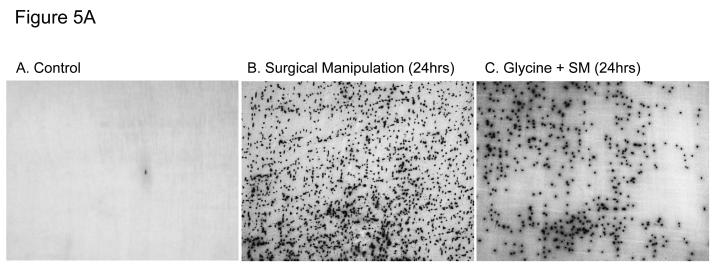
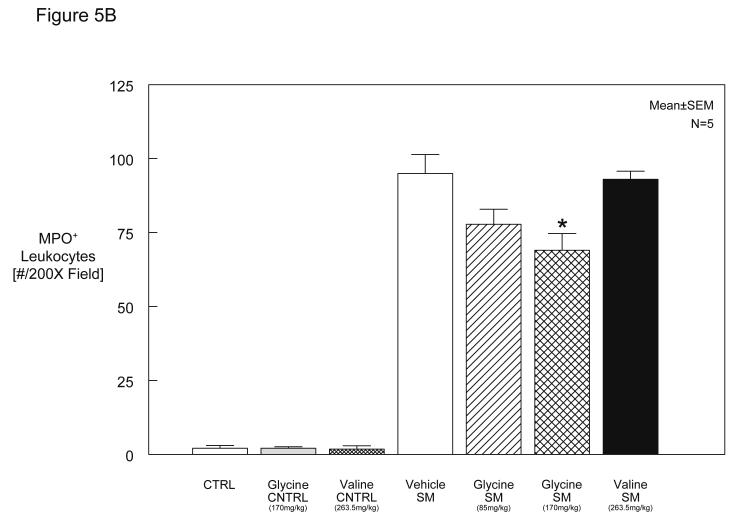
We hypothesized that preoperative exogenous glycine administration would reduce the postoperative recruitment of leukocytes into the manipulated small intestinal muscularis. As illustrated in Figure 5A and plotted in the histogram of Figure 5B, vehicle, glycine or valine injected rats without intestinal manipulation presented with only few MPO positive cells (vehicle: 2 ± 0.9, glycine: 2 ± 0.5 cells/field at 200X magnification). In accordance with our previous observations (25;26), Figure 5A, Panel B, demonstrates that surgical manipulation of the small intestine results in a massive infiltration of neutrophils into the muscularis (95 ± 6.4 cells/field at 200X magnification). However, as illustrated in (Figure 5A, Panel C) glycine pretreatment at 170 mg/kg resulted in a moderate but significant 27% reduction of infiltrating leukocytes compared to manipulated, vehicle pretreated rats (69 ± 5.7 cells/field at 200X). Leukocyte infiltrates were reduced by 18% in rats that had received 85 mg/kg glycine preoperatively (78 ± 5.1 cells/field at 200X). In contrast, pretreatment with valine had no significant impact on the number of infiltrating cells in manipulated animals (93 ± 2.7 cells/field at 200X) (Figure 5B).
Figure 5.
A and 5B. Figure 5A illustrates myeloperoxidase (MPO) staining of rat jejunal muscularis whole-mounts demonstrating few cells in the control (Panel A) and significant leukocyte recruitment after intestinal manipulation (Panel B) and a diminution in neutrophil recruitment with glycine pretreatment of surgically manipulated animals (Panel C). (A, 100X; B-C, 40X). Figure 5B shows a histogram of quantified infiltrating MPO-positive leukocytes in muscularis whole mounts from vehicle, glycine or valine injected control rats versus surgically manipulated rats pretreated with vehicle/saline, glycine (85 mg/kg and 170 mg/kg) or valine (263.5 mg/kg) pretreatment (SM = surgical manipulation). Glycine pretreatment significantly attenuates postoperative leukocyte recruitment into the intestinal muscularis (n = 6-8 per group, data are expressed as mean ± SEM; F-test and Bonferroni post-hoc group comparisons with * = p-value < 0.05).
In the present study, rat jejunal iNOS mRNA was upregulated 197.1-fold postoperatively at 3 hours compared to non-operated saline treated controls. This surgical induction was significantly suppressed by glycine pretreatment. Actual comparative CT values for the jejunum was calculated to be: Control+Saline=1.1±0.21, Control+Glycine=1.1±0.24, SM+Saline=197.1±82.09 and SM+Glycine=16.9±2.07.
We next investigated whether glycine pretreatment reduced the release of nitric oxide metabolites (nitrite) from the manipulated rat jejunum, and we also assayed their release from the isolate colonic muscularis as well. In accordance with our previous results (44), nitrite production was significantly increased by 11.4-fold and 10.9-fold in jejunal and colonic organ culture supernatants of manipulated vehicle pretreated rats compared to vehicle controls (t-test). While glycine (170 mg/kg) had no effect on Griess reaction measurements from the unmanipulated muscularis, the muscularis of rats that had received glycine (170 mg/kg) 1 hour before manipulation released significantly less nitrite compared to manipulated vehicle pretreated animals (2.7-fold and 2.5-fold increase over control for jejunum and colon). Valine pretreatment had no significant impact on nitrite release from the cultured manipulated muscularis of the jejunum or colon (12.0-fold and 9.5-fold, respectively) (two-way ANOVA with Tukey post-hoc group comparisons for treatment groups). Actual normalized NO Griess reaction measurements (μM/g tissue) were calculated for the jejunum and colon to be: Control=22.0±2.8 and 16.7±2.4, Control Glycine=18.0±5.2 and 14.3±4.1, Control Valine=14.9±7.8 and 11.89±6.5, Saline SM=251.3±78.34 and 182.1±23.74, Glycine SM=58.7±15.89 and 42.7±10.72 and Valine SM=265.5±23.92 and 158.5±23.39.
Prostanoids produced through the increased expression of COX-2 have also been shown to be important in causing postoperative ileus (45;46). Indeed, the effect of glycine shown above for iNOS mRNA expression was also mimicked for COX-2 mRNA induction. COX-2 was significantly induced postoperatively at 3 hours by surgical manipulation (3.2-fold, t-test). Glycine pretreatment (170 mg/kg) tended to alleviate the postoperative upregulation (2.3 fold), but was not significant at this single time point by F-test. Actual comparative CT values for the rat jejunum was calculated to be: Control=1.1±0.29, Control+Glycine=1.4±0.29, SM+Saline=3.2±0.48 and SM+Glycine=2.4±0.37.
We also measured prostaglandin release from the manipulated rat jejunal and colonic muscularis over a period of 24 hours in organ culture. As previously shown (45) and in concurrence with the postoperative upregulation in COX-2 mRNA, manipulation increased prostanoid release into the culture media from the jejunal (5.9-fold) and colonic (3.1-fold) organ cultured muscularis. While the muscularis of rats that had received glycine (170 mg/kg) before manipulation released significantly less prostanoids over the 24 hours period of incubation compared to saline pretreated manipulated animals (0.9-fold and 0.7-fold for jejunum and colon). Although, there was a trend for valine pretreatment to decrease prostanoid release from the cultured manipulated muscularis of the jejunum and colon (3.4-fold and 1.5-fold increases from control, respectively), this was not significant (one-way ANOVA with Bonferroni post-hoc group comparisons for treatment groups). Actual normalized ELISA measurements of prostaglandin values (ng/ml/mg) were as follows for the jejunum and colon: Control=131.1±41.20 and 185.4±41.94, Saline SM=773.5±258.62 and 585.9±144.36, Glycine SM=119.1±24.74 and 128.5±56.91 and Valine SM=448.2±163.78 and 282.8±138.15. Interestingly, glycine postsurgical treatment of the already inflamed muscularis externa was not able to alter NO or prostaglandin release indicating that pretreatment is necessary for a reduction in the intestinal inflammatory response. Mediator mRNA levels from non-operated vehicle rats did not differ from non-operated glycine injected animals.
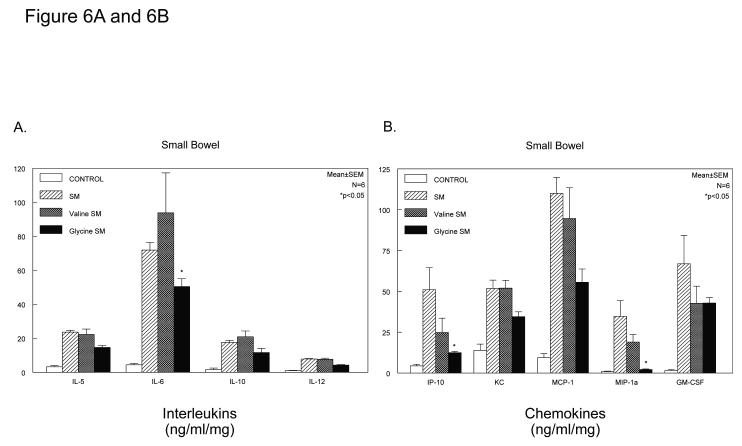
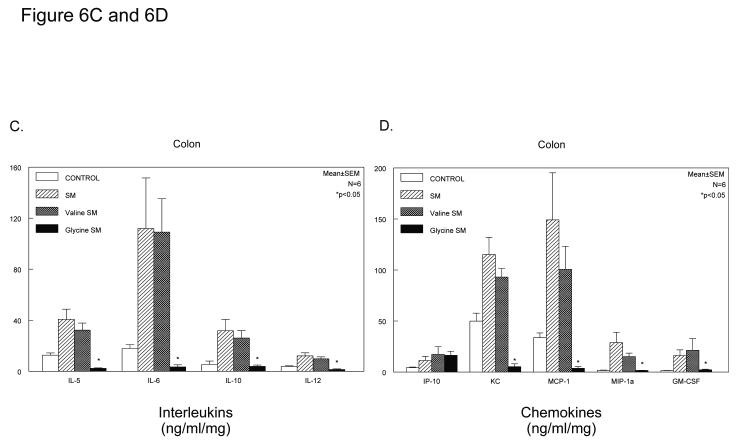
A broad inhibition of the molecular inflammatory response was further investigated in C57Bl/6 mice, which also demonstrated a significant protection from the development of postoperative ileus by glycine (geometric centers: control=11.0±0.44, SM=4.1±0.18, SM+saline=4.1±.5 and SM+glycine 170mg/kg=10.3±.7). Four groups of mice were analyzed using multiplex Luminex technology to measure the release of 20 inflammatory mediators in the media from the organ cultured muscularis of the jejunum and colon (FGF-basic, IL-1α, IL-1ß, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-13, IL-17, TNF-α, IFN-γ, GM-CSF, KC, MCP-1, MIG, MIP-1α, IP-10 and VEGF). As shown in Figure 6, Panel A for the jejunum and Figure 6, Panel C for the colon, glycine caused a decrease in the release of interleukins 5, 6, 10 and 12 with the response being more significantly pronounced in measurements from the colonic muscularis. A series of chemokines were also measured by the Luminex panel of inflammatory mediators (IP-10, KC, MCP-1, MIP-1α, VEGF and GM-CSF). These data shown in Figure 6, Panels B and D demonstrate a trend for the inhibition of the production of these proteins by glycine with again the inhibition being more dramatic in the media from the colonic muscularis (one-way ANOVA with Bonferroni post-hoc group comparisons for treatment groups). Glycine or valine did not significantly alter the release of inflammatory mediators from the unoperated muscularis compared to control vehicle treatment.
Figure 6.
Histograms depicting glycine quelling of interleukins (Panel A) and chemokines (Panel B) released into the organ culture media of manipulated mouse jejunal muscularis externa. All interleukins and chemokines tended to be quelled with statistical significance reached by IL-6, IP-10 and MIP1-α. Histograms depicting glycine quelling of interleukins (Panel C) and chemokines (Panel D) released into the organ culture media of manipulated mouse colonic muscularis externa. All interleukins and chemokines tended to be quelled with statistical significance reached by IL-5, IL-6, IL-10, IL-12, KC, MCP-1, MIP1-α and GM-CSF. (n = 5 per group, data are expressed as mean ± SEM; F-test and Bonferroni post-hoc group comparisons with * = p-value < 0.05).
DISCUSSION
The interpretation of postoperative ileus as the result of a local intestinal muscularis inflammatory response with the coexpression of kinetically active mediators suggests that an anti-inflammatory pharmacological approach may alleviate postoperative ileus. In fact, we have previously shown that specific inhibition of iNOS or COX-2 can each significantly ameliorate postoperative smooth muscle dysfunction. However, the complexity of the inflammatory response within the intestinal muscularis, particularly the involvement of many different mediators or metabolites with functional impact on smooth muscle and enteric neural function, requires a pharmacological approach that interrupts primal key events of the inflammatory cascade. Thus, we investigated whether pre-operative application of the anti-inflammatory acting amino acid glycine could prevent the development of ileus (47). Our results clearly demonstrate for the first time that L-glycine pretreatment results in the significant prevention of postoperative ileus and an alleviation of postoperative smooth muscle dysfunction via a suppression in numerous inflammatory responses within the postsurgical intestinal muscularis externa.
Previously, glycine has been shown to be beneficial in the prevention of mucosal changes following ischemia/reperfusion injury (48;49). And a preoperative protective effect of glycine has been described in experimental liver manipulation, dissection and transplantation (17;50-52). These studies demonstrated that liver manipulation during harvesting activates Kupffer cells, which leads to the release of cytokines and the production of toxic radicals which impair liver function. Pretreatment of donors with glycine reduces Kupffer cell activation and thus manipulation and reperfusion injury (17). The activation of resident immunocompetent cells is also a key event in the development of postoperative ileus associated muscularis externa inflammation (25;37). However, in addition to macrophage activation, recruited leukocytes play a decisive role in the expression of inflammatory and kinetically active mediators which leads to intestinal muscularis dysfunction.
Pathophysiological responses of macrophages to external stimuli, such as lipopolysaccharide, are mediated through the activation of voltage dependent calcium channels which causes an influx of calcium (53). The increase of intracellular calcium in turn activates a multitude of signaling cascades, such as the transcription and translation of inflammatory mediators (24;54-56). It has been demonstrated that the immunomodulatory effects of glycine in Kupffer cells (57), alveolar macrophages (21), monocytes (18) and neutrophil granulocytes (23) are the result of a glycine receptor mediated increase in intracellular chloride, as it is often called the glycine-gated chloride channel (47). Glycine activates chloride channels in immunocompetent cells leading to chloride influx and membrane hyperpolarization (47). The change in membrane potential then inhibits the activation of voltage gated calcium channels and thus limits an increase in intracellular calcium (24). Here we shown that in the control intestinal muscularis, glycine receptors were immunohistochemically evident on the normally resident dense network of muscularis macrophages and on a distinct subpopulation of enteric neurons. In addition, whole mounts of manipulated animals exhibited glycine receptor immunoreactivity on infiltrating leukocytes. Consequently, the intestinal muscularis offers potential receptor targets for glycine in the initiation phase (macrophages), as well as in the later phase of leukocyte recruitment during the postoperative inflammatory response. Interestingly though, the glycine receptor on macrophage/monocyte/neutrophil and Kupffer cells are not identical. Macrophage/monocytes/and neutrophil glycine receptors consist of α2-, α4- and ß-subunits, whereas, the Kupffer cell glycine receptor is composed of α1-, α4 and ß-subunits (58). The functional significance of these α-subunit substitutions is currently not clear.
Our functional studies convincingly show that glycine prevented the delay in postoperative gastrointestinal transit in both rats and mice. Additionally, glycine pretreatment resulted in improved in vitro circular smooth muscle contractility demonstrating a localized effect within the muscularis externa itself as a significant basis for the overall improvement in gastrointestinal transit. The beneficial functional response of the gastrointestinal tract to exogenous glycine with the associated suppression in numerous postoperative molecular and cellular inflammatory events, confirms the immunosuppressive effect of glycine on the initial inflammatory phase within the intestinal muscularis. The impact of glycine on cytokine production and release was described earlier in endotoxin or shock models of the lung (21), the liver (59) and the musculoskeletal system (60). These studies focused mainly on the expression of the prototypical cytokine TNF-α (18). In line with these observations the present study demonstrates that glycine pretreatment prevents TNF-α mRNA induction within the rat intestinal muscularis, but TNF-α protein was not detectable in the mouse cultured supernatant (which is a consistent species difference between the postsurgical TNF-α response in rats and mice). In addition to TNF-α, glycine also attenuated rat IL-6 mRNA expression and decreased the release of this protein in the mouse from both the small intestinal and colonic muscularis externa. As previously shown the proinflammatory cytokine IL-6 is highly inducible to many models of abdominal trauma and it promotes leukocyte recruitment (61;62). However, in addition to IL-6, in the colonic muscularis the release of IL-5 and IL-12 were also suppressed. Both IL-6 and the adhesion molecule ICAM-1 play a major role in the development of leukocyte infiltration within the surgically altered intestinal muscularis (26). We report here that the mRNA expression of both mediators, as well as the magnitude of leukocyte infiltration was significantly reduced in glycine pretreated animals. By utilizing the mouse specific 20-plex assay, we were able to show additionally that in the mouse jejunal muscularis glycine decreased the release of the chemokines IP-10, MCP-1 and MIP-1α. While, in the mouse colonic muscularis the inhibition of chemokine production was even more dramatic with significant decreases in KC, MCP-1, MIP-1α and GM-CSF.
The combined suppression of these chemokines could all participate in the observed alleviation in leukocyte recruitment, as the therapeutic potential of glycine has also been described after hemorrhagic shock in the liver and the kidney (59) as well as for endotoxemia in joints (60), liver (63) and lung (20). Furthermore, mucosal myeloperoxidase activity is reduced in reperfusion injured intestine that had been pretreated with glycine (48;64). However, in these studies the specific role of glycine on the postoperative intestinal mucosa was not explored.
Based on our previous observations (44;65), we hypothesized that the observed improvement in postoperative motility would be due to a reduction in iNOS mRNA expression and a subsequently blunted NO release. In fact, we did measure a significant reduction in iNOS and NO release from the muscularis after glycine pretreatment compared to the untreated postoperative muscularis in the rat. This is supported by findings that glycine inhibited NO release and iNOS expression in Kupffer cells (17;66). As previously shown, prostaglandins also play an important role in the development of inflammatory smooth muscle dysfunction. Here again in the rat, glycine limited postoperative muscularis COX-2 expression and suppressed the generation of prostanoids from the pretreated muscularis of the jejunum and colon. Interestingly, glycine postsurgical treatment of the already inflamed muscularis externa was not able to alter NO or prostaglandin release indicating that pretreatment is necessary for a reduction in the intestinal inflammatory response.
As noted in the results, glycine receptors were present on a subpopulation of enteric neurons. Although, glycine has been reported to acutely modulate pain and alter the excitability of enteric neurons (67), in our experimental protocol glycine was given acutely 24 hours before functional motility measurements were performed. Therefore, it seems unlikely that a neural effect could explain the functional changes 24 hours when glycine levels would no longer be elevated.
Mast cells have been reported to be involved in the development of postoperative ileus {de Jonge, 2004 DEJONGE2004 /id;The, 2009 THE2009 /id}. However, we have reported previously that very few mast cells are constitutively present within the muscularis externa of the mouse and rat {Kalff, 1998 KALFF1998B /id}. Therefore, the contribution of mast cells to the molecular inflammatory responses within the muscularis extracts for qPCR at 3 hours after surgical manipulation would be only very minor. Also, we did not detect glycine receptors on un-identifiable cells within the muscularis. However, our study does not rule out an effect of glycine on mesenteric mast cells or mucosal mast cell populations.
In conclusion, the data demonstrate that glycine protects rodents from inflammatory postoperative ileus. Glycine pretreatment attenuated the molecular and cellular inflammatory events within the intestinal muscularis that occur after surgical manipulation of the intestine. Subsequently, postoperative alterations in smooth muscle contractile activity and the delay in postoperative gastrointestinal transit were minimized. We propose that preoperative glycine treatment would be a novel and inexpensive adjuvant to prevent the development of postoperative ileus.
ACKNOWLEDGEMENTS
This study was supported by National Institute of Health grants:R01-GM58241, R01-DK068610, P50-GM-53789, and DK02488 and a grant from the Deutsche Forschungsgemeinschaft (TU 116/2-1).
Burkhard Stoffels:
Performed the research
Analyzed the data
Andreas Türler:
Performed the research
Designed the research study
Analyzed the data
Joachim Schmidt:
Performed the research
Analyzed the data
Asad Nazir:
Performed the research
Analyzed the data
Takeshi Tsukamoto:
Performed the research
Analyzed the data
Beverley A. Moore:
Designed the research study
Christoph Schnurr:
Performed aspects of the research
Jörg C. Kalff:
Analyzed the data
Wrote the paper
Anthony J. Bauer:
Performed the research
Designed the research study
Contributed essential reagents or tools
Analyzed the data
Wrote the paper
Abbreviations
- FD70
fluorescence-labeled dextran of 70,000 MW
- CT
cycle threshold
- GAPDH
glyceraldehyde phosphate dehydrogenase
- GC
geometric center
- ICAM-1
intercellular adhesion molecule-1
- IL-6
interleukin-6
- iNOS
inducible nitric oxide synthase
- KRB
Krebs-Ringer buffer
- MPO
myeloperoxidase
- NO
nitric oxide
- PBS
phosphate-buffered saline
- RT-PCR
reverse transcriptase polymerase chain reaction
Footnotes
COMPETING INTERESTS: The authors acknowledge that a patent application has been submitted on aspects of this study. Inventors: Anthony J. Bauer, Burkhard Stoffels, Joachim Schmidt, R. Savanh Chanthaphavong and Andreas Tuerler
- Agents: LEYDIG VOIT & MAYER, LTD
- Assignees: University of Pittsburgh of the Commonwealth System of Higher Education
- Origin: CHICAGO, IL US
- IPC8 Class: AA61K3820FI
- USPC Class: 424 852
REFERENCES
- (1).Holte K, Kehlet H. Postoperative ileus: a preventable event. Br J Surg. 2000;87(11):1480–93. doi: 10.1046/j.1365-2168.2000.01595.x. [DOI] [PubMed] [Google Scholar]
- (2).Livingston EH, Passaro EP., Jr. Postoperative ileus. Dig Dis Sci. 1990;35(1):121–32. doi: 10.1007/BF01537233. [DOI] [PubMed] [Google Scholar]
- (3).Delaney CP, Wolff BG, Viscusi ER, et al. Alvimopan, for postoperative ileus following bowel resection: a pooled analysis of phase III studies. Annals of Surgery. 2007;245(3):355–63. doi: 10.1097/01.sla.0000232538.72458.93. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Chen HH, Wexner SD, Iroatulam AJ, et al. Laparoscopic colectomy compares favorably with colectomy by laparotomy for reduction of postoperative ileus. Dis Colon Rectum. 2000;43(1):61–5. doi: 10.1007/BF02237245. [DOI] [PubMed] [Google Scholar]
- (5).Piskun G, Kozik D, Rajpal S, et al. Comparison of laparoscopic, open, and converted appendectomy for perforated appendicitis. Surg Endosc. 2001;15(7):660–2. doi: 10.1007/s004640020072. [DOI] [PubMed] [Google Scholar]
- (6).Maxwell-Armstrong CA, Robinson MH, Scholefield JH. Laparoscopic colorectal cancer surgery. Am J Surg. 2000;179(6):500–7. doi: 10.1016/s0002-9610(00)00390-1. [DOI] [PubMed] [Google Scholar]
- (7).Bardram L, Funch-Jensen P, Jensen P, et al. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet. 1995;345(8952):763–4. doi: 10.1016/s0140-6736(95)90643-6. [DOI] [PubMed] [Google Scholar]
- (8).Basse L, Madsen L, Kehlet H. Normal gastrointestinal transit after colonic resection using epidural analgesia, enforced oral nutrition and laxative. Br J Surg. 2001;88(11):1498–500. doi: 10.1046/j.0007-1323.2001.01916.x. [DOI] [PubMed] [Google Scholar]
- (9).Moss G. Maintenance of gastrointestinal function after bowel surgery and immediate enteral full nutrition. II. Clinical experience, with objective demonstration of intestinal absorption and motility. JPEN J Parenter Enteral Nutr. 1981;5(3):215–20. doi: 10.1177/0148607181005003215. [DOI] [PubMed] [Google Scholar]
- (10).Taguchi A, Sharma N, Saleem RM, et al. Selective postoperative inhibition of gastrointestinal opioid receptors. N Engl J Med. 2001;345(13):935–40. doi: 10.1056/NEJMoa010564. [DOI] [PubMed] [Google Scholar]
- (11).de Jonge WJ, The FO, Lowenberg M, et al. P38 MAPK inhibitor semapimod reduces postoperative ileus via peripheral and central mechanisms. Gastroenterology. 2009;136(5):1841–2. doi: 10.1053/j.gastro.2008.12.075. [DOI] [PubMed] [Google Scholar]
- (12).De Winter BY, Boeckxstaens GE, De Man JG, et al. Effect of different prokinetic agents and a novel enterokinetic agent on postoperative ileus in rats. Gut. 1999;45(5):713–8. doi: 10.1136/gut.45.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Schmidt J, Stoffels B, Nazir A, et al. Alvimopan and COX-2 inhibition reverse opioid and inflammatory components of postoperative ileus. Neurogastroenterol Motil. 2008 doi: 10.1111/j.1365-2982.2007.01078.x. [DOI] [PubMed] [Google Scholar]
- (14).Wehner S, Straesser S, Vilz TO, et al. Inhibition of p38 mitogen-activated protein kinase pathway as prophylaxis of postoperative ileus in mice. Gastroenterology. 2009;136(2):619–29. doi: 10.1053/j.gastro.2008.10.017. [DOI] [PubMed] [Google Scholar]
- (15).Bauer AJ. Mentation on the immunological modulation of gastrointestinal motility. Neurogastroenterol Motil. 2008;20(Suppl 1):81–90. doi: 10.1111/j.1365-2982.2008.01105.x. [DOI] [PubMed] [Google Scholar]
- (16).Rivera CA, Wheeler MD, Enomoto N, et al. A choline-rich diet improves survival in a rat model of endotoxin shock. Am J Physiol. 1998;275(4 Pt 1):G862–G867. doi: 10.1152/ajpgi.1998.275.4.G862. [DOI] [PubMed] [Google Scholar]
- (17).Schemmer P, Bradford BU, Rose ML, et al. Intravenous glycine improves survival in rat liver transplantation. Am J Physiol. 1999;276(4 Pt 1):G924–G932. doi: 10.1152/ajpgi.1999.276.4.G924. [DOI] [PubMed] [Google Scholar]
- (18).Spittler A, Reissner CM, Oehler R, et al. Immunomodulatory effects of glycine on LPS-treated monocytes: reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999;13(3):563–71. doi: 10.1096/fasebj.13.3.563. [DOI] [PubMed] [Google Scholar]
- (19).Thurman RG, Schemmer P, Zhong Z, et al. Kupffer cell-dependent reperfusion injury in liver transplantation: new clinically relevant use of glycine. Langenbecks Arch Chir Suppl Kongressbd. 1998;115:185–90. [PubMed] [Google Scholar]
- (20).Wheeler MD, Rose ML, Yamashima S, et al. Dietary glycine blunts lung inflammatory cell influx following acute endotoxin. Am J Physiol Lung Cell Mol Physiol. 2000;279(2):L390–L398. doi: 10.1152/ajplung.2000.279.2.L390. [DOI] [PubMed] [Google Scholar]
- (21).Wheeler MD, Thurman RG. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am J Physiol. 1999;277(5 Pt 1):L952–L959. doi: 10.1152/ajplung.1999.277.5.L952. [DOI] [PubMed] [Google Scholar]
- (22).Cruz M, Maldonado-Bernal C, Mondragon-Gonzalez R, et al. Glycine treatment decreases proinflammatory cytokines and increases interferon-gamma in patients with type 2 diabetes. Journal of Endocrinological Investigation. 2008;31(8):694–9. doi: 10.1007/BF03346417. [DOI] [PubMed] [Google Scholar]
- (23).Wheeler M, Stachlewitz RF, Yamashina S, et al. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000;14(3):476–84. doi: 10.1096/fasebj.14.3.476. [DOI] [PubMed] [Google Scholar]
- (24).Wheeler MD, Ikejema K, Enomoto N, et al. Glycine: a new anti-inflammatory immunonutrient. Cell Mol Life Sci. 1999;56(9-10):843–56. doi: 10.1007/s000180050030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Kalff JC, Schraut WH, Simmons RL, et al. Surgical manipulation of the gut elicits an intestinal muscularis inflammatory response resulting in postsurgical ileus. Ann Surg. 1998;228(5):652–63. doi: 10.1097/00000658-199811000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Kalff JC, Carlos TM, Schraut WH, et al. Surgically induced leukocytic infiltrates within the rat intestinal muscularis mediate postoperative ileus. Gastroenterology. 1999;117(2):378–87. doi: 10.1053/gast.1999.0029900378. [DOI] [PubMed] [Google Scholar]
- (27).Kalff JC, Buchholz BM, Eskandari MK, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999;126(3):498–509. [PubMed] [Google Scholar]
- (28).Fink L, Seeger W, Ermert L, et al. Real-time quantitative RT-PCR after laser-assisted cell picking. Nat Med. 1998;4(11):1329–33. doi: 10.1038/3327. [DOI] [PubMed] [Google Scholar]
- (29).Kosuga K, Yui Y, Hattori R, et al. Cloning of an inducible nitric oxide synthase from rat polymorphonuclear cells. Endothelium. 1994;2:217–21. [Google Scholar]
- (30).Xu K, Robida AM, Murphy TJ. Immediate-early MEK-1-dependent stabilization of rat smooth muscle cell cyclooxygenase-2 mRNA by Galpha(q)-coupled receptor signaling. J Biol Chem. 2000;275(30):23012–9. doi: 10.1074/jbc.M001611200. [DOI] [PubMed] [Google Scholar]
- (31).Northemann W, Braciak TA, Hattori M, et al. Structure of the rat interleukin 6 gene and its expression in macrophage-derived cells. J Biol Chem. 1989;264(27):16072–82. [PubMed] [Google Scholar]
- (32).Kita Y, Takashi T, Iigo Y, et al. Sequence and expression of rat ICAM-1. Biochim Biophys Acta. 1992;1131(1):108–10. doi: 10.1016/0167-4781(92)90107-b. [DOI] [PubMed] [Google Scholar]
- (33).Yoshimura T, Takeya M, Takahashi K. Molecular cloning of rat monocyte chemoattractant protein-1 (MCP-1) and its expression in rat spleen cells and tumor cell lines. Biochem Biophys Res Commun. 1991;174(2):504–9. doi: 10.1016/0006-291x(91)91445-i. [DOI] [PubMed] [Google Scholar]
- (34).Tso JY, Sun XH, Kao TH, et al. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985;13(7):2485–502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Schmittgen TD, Zakrajsek BA, Mills AG, et al. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285(2):194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- (36).Kalff JC, Schwarz NT, Walgenbach KJ, et al. Leukocytes of the intestinal muscularis: their phenotype and isolation. J Leukoc Biol. 1998;63(6):683–91. doi: 10.1002/jlb.63.6.683. [DOI] [PubMed] [Google Scholar]
- (37).Eskandari MK, Kalff JC, Billiar TR, et al. Lipopolysaccharide activates the muscularis macrophage network and suppresses circular smooth muscle activity. Am J Physiol. 1997;273(3 Pt 1):G727–G734. doi: 10.1152/ajpgi.1997.273.3.G727. [DOI] [PubMed] [Google Scholar]
- (38).Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131–8. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- (39).Turler A, Schnurr C, Nakao A, et al. Endogenous endotoxin participates in causing a panenteric inflammatory ileus after colonic surgery. Ann Surg. 2007;245(5):734–44. doi: 10.1097/01.sla.0000255595.98041.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Eskandari MK, Kalff JC, Billiar TR, et al. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. American Journal of Physiology. 1999;277(2:Pt 1):t-86. doi: 10.1152/ajpgi.1999.277.2.G478. [DOI] [PubMed] [Google Scholar]
- (41).Overhaus M, Moore BA, Barbato JE, et al. Biliverdin protects against polymicrobial sepsis by modulating inflammatory mediators. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2006;290(4):G695–G703. doi: 10.1152/ajpgi.00152.2005. [DOI] [PubMed] [Google Scholar]
- (42).Hierholzer C, Kalff JC, Audolfsson G, et al. Molecular and functional contractile sequelae of rat intestinal ischemia/reperfusion injury. Transplantation. 1999;68(9):1244–54. doi: 10.1097/00007890-199911150-00006. [DOI] [PubMed] [Google Scholar]
- (43).Hierholzer C, Kalff JC, Chakraborty A, et al. Impaired gut contractility following hemorrhagic shock is accompaied by IL-6 and G-CSF production and neutrophil infiltration. Dig Dis Sci. 2001;46(2):230–41. doi: 10.1023/a:1005524021552. [DOI] [PubMed] [Google Scholar]
- (44).Kalff JC, Schraut WH, Billiar TR, et al. Role of inducible nitric oxide synthase in postoperative intestinal smooth muscle dysfunction in rodents. Gastroenterology. 2000;118(2):316–27. doi: 10.1016/s0016-5085(00)70214-9. [DOI] [PubMed] [Google Scholar]
- (45).Schwarz NT, Kalff JC, Turler A, et al. Prostanoid production via COX-2 as a causative mechanism of rodent postoperative ileus. Gastroenterology. 2001;121(6):1354–71. doi: 10.1053/gast.2001.29605. [DOI] [PubMed] [Google Scholar]
- (46).Kreiss C, Birder LA, Kiss S, et al. COX-2 dependent inflammation increases spinal Fos expression during rodent postoperative ileus. Gut. 2003;52(4):527–34. doi: 10.1136/gut.52.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Zhong Z, Wheeler MD, Li X, et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Current Opinion in Clinical Nutrition & Metabolic Care. 2003;6(2):229–40. doi: 10.1097/00075197-200303000-00013. [Review] [80 refs] [DOI] [PubMed] [Google Scholar]
- (48).Lee MA, McCauley RD, Kong SE, et al. Pretreatment with glycine reduces the severity of warm intestinal ischemic-reperfusion injury in the rat. Ann Plast Surg. 2001;46(3):320–6. doi: 10.1097/00000637-200103000-00020. [DOI] [PubMed] [Google Scholar]
- (49).Iijima S, Shou J, Naama H, et al. Beneficial effect of enteral glycine in intestinal ischemia/reperfusion injury. J Gastrointest Surg. 1997;1(1):53–60. doi: 10.1007/s11605-006-0011-0. [DOI] [PubMed] [Google Scholar]
- (50).Schemmer P, Enomoto N, Bradford BU, et al. Activated Kupffer cells cause a hypermetabolic state after gentle in situ manipulation of liver in rats. Am J Physiol Gastrointest Liver Physiol. 2001;280(6):G1076–G1082. doi: 10.1152/ajpgi.2001.280.6.G1076. [DOI] [PubMed] [Google Scholar]
- (51).Schemmer P, Schoonhoven R, Swenberg JA, et al. Gentle in situ liver manipulation during organ harvest decreases survival after rat liver transplantation: role of Kupffer cells. Transplantation. 1998;65(8):1015–20. doi: 10.1097/00007890-199804270-00001. [DOI] [PubMed] [Google Scholar]
- (52).Schemmer P, Bunzendahl H, Klar E, et al. Reperfusion injury is dramatically increased by gentle liver manipulation during harvest. Transpl Int. 2000;13(Suppl 1):S525–S527. doi: 10.1007/s001470050394. [DOI] [PubMed] [Google Scholar]
- (53).Perera PY, Vogel SN, Detore GR, et al. CD14-dependent and CD14-independent signaling pathways in murine macrophages from normal and CD14 knockout mice stimulated with lipopolysaccharide or taxol. J Immunol. 1997;158(9):4422–9. [PubMed] [Google Scholar]
- (54).Clapham DE. Calcium signaling. Cell. 1995;80(2):259–68. doi: 10.1016/0092-8674(95)90408-5. [DOI] [PubMed] [Google Scholar]
- (55).Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392(6679):933–6. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- (56).Dolmetsch RE, Lewis RS, Goodnow CC, et al. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386(6627):855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- (57).Ikejima K, Qu W, Stachlewitz RF, et al. Kupffer cells contain a glycine-gated chloride channel. Am J Physiol. 1997;272(6 Pt 1):G1581–G1586. doi: 10.1152/ajpgi.1997.272.6.G1581. [DOI] [PubMed] [Google Scholar]
- (58).Froh M, Thurman RG, Wheeler MD. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2002;283(4):G856–G863. doi: 10.1152/ajpgi.00503.2001. [DOI] [PubMed] [Google Scholar]
- (59).Zhong Z, Enomoto N, Connor HD, et al. Glycine improves survival after hemorrhagic shock in the rat. Shock. 1999;12(1):54–62. doi: 10.1097/00024382-199907000-00008. [DOI] [PubMed] [Google Scholar]
- (60).Li X, Bradford BU, Wheeler MD, et al. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: role for glycine-gated chloride channel. Infect Immun. 2001;69(9):5883–91. doi: 10.1128/IAI.69.9.5883-5891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Romano M, Sironi M, Toniatti C, et al. Role of IL-6 and its soluble receptor in induction of chemokines and leukocyte recruitment. Immunity. 1997;6(3):315–25. doi: 10.1016/s1074-7613(00)80334-9. [DOI] [PubMed] [Google Scholar]
- (62).Wortel CH, van Deventer SJ, Aarden LA, et al. Interleukin-6 mediates host defense responses induced by abdominal surgery. Surgery. 1993;114(3):564–70. [PubMed] [Google Scholar]
- (63).Ikejima K, Iimuro Y, Forman DT, et al. A diet containing glycine improves survival in endotoxin shock in the rat. Am J Physiol. 1996;271(1 Pt 1):G97–103. doi: 10.1152/ajpgi.1996.271.1.G97. [DOI] [PubMed] [Google Scholar]
- (64).Mangino JE, Kotadia B, Mangino MJ. Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation. 1996;62(2):173–8. doi: 10.1097/00007890-199607270-00005. [DOI] [PubMed] [Google Scholar]
- (65).Eskandari MK, Kalff JC, Billiar TR, et al. LPS-induced muscularis macrophage nitric oxide suppresses rat jejunal circular muscle activity. Am J Physiol. 1999;277(2 Pt 1):G478–G486. doi: 10.1152/ajpgi.1999.277.2.G478. [DOI] [PubMed] [Google Scholar]
- (66).Mauriz JL, Matilla B, Culebras JM, et al. Dietary glycine inhibits activation of nuclear factor kappa B and prevents liver injury in hemorrhagic shock in the rat. Free Radic Biol Med. 2001;31(10):1236–44. doi: 10.1016/s0891-5849(01)00716-x. [DOI] [PubMed] [Google Scholar]
- (67).Neunlist M, Michel K, Reiche D, et al. Glycine activates myenteric neurones in adult guinea-pigs. Journal of Physiology. 2001;536(Pt:3):3–39. doi: 10.1111/j.1469-7793.2001.00727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]