Summary
Background
von Willebrand factor (VWF) released from endothelial cells is rich in ultra-large (UL) multimers that are intrinsically active in binding platelets, whereas plasma-type VWF multimers require shear stress to be activated. This functional difference may be attributed to thiols exposed on the surface of plasma-type VWF multimers, but not on ULVWF multimers. Shear stress induces the exposed thiols to form disulfide bonds between laterally apposed plasma-type VWF multimers, leading to enhanced VWF binding to platelets.
Objectives
We tested a hypothesis that ADAMTS-13 has a disulfide bond-reducing activity that regulates shear-induced thiol-disulfide exchange of VWF.
Methods
Thiol blocking agents and active thiol bead capturing were used to identify and locate this activity; along with truncated ADAMTS-13 mutants.
Results
ADAMTS-13 contains a disulfide-bond–reducing activity that primarily targets disulfide bonds in plasma-type VWF multimers induced by high shear stress or formed with thiol beads, but not on disulfide bonds in native multimeric structures. Cysteine thiols targeted by this activity are in the VWF C-domain and are known to participate in shear-induced thiol-disulfide exchange. ADAMTS-13 contains cysteine thiols that remain exposed after being subjected to hydrodynamic forces. Blocking these active thiols eliminates this reducing activity and moderately decreases ADAMTS-13 activity in cleaving ULVWF strings anchored to endothelial cells under flow conditions, but not under static conditions. The activity is located in the C-terminal region of ADAMTS-13.
Conclusions
This novel disulfide-bond-reducing activity of ADAMTS-13 may prevent covalent lateral association and increased platelet adherence of plasma-type VWF multimers induced by high fluid shear stress.
Introduction
Upon vascular injury, platelets adhere to exposed subendothelial matrix to initiate a hemostatic plug and arrest bleeding. The process is initiated by the interaction between von Willebrand factor (VWF) affixed to the subendothelium and the GP Ib-IX-V complex on the surface of platelets. VWF is a large multimeric glycoprotein that is synthesized in megakaryocytes/platelets and endothelial cells as monomers. In the endoplasmic reticulum and Golgi apparatus of these cells, monomers first form dimers through C-terminal disulfide bonds (1;2) and then multimerize through N-terminal disulfide linkages (3;4). Once synthesized, VWF multimers are stored in the Weibel-Palade bodies of endothelial cells and α-granules of megakaryocytes/platelets (5-7). VWF packed into these storage granules are secreted upon stimulation as multimers enriched in ultra-large (UL) forms that are hyperactive and capable of forming spontaneous high-strength ligand-receptor bonds with the GP Ib-IX-V complexes on platelets (8;9). This ULVWF hyperactivity is in contrast to VWF multimers circulating in blood, which bind platelets only after activation by high fluid shear stress, modulators, or immobilization on solid surfaces. ULVWF and plasma-type VWF multimers may, therefore, have different conformations or structural folding that make the former constitutively active and the latter inactive unless its conformation is changed by pathological levels of shear stress.
In the absence of vascular injury, secreted ULVWF multimers are anchored to endothelial cells and rapidly cleaved by the zinc metalloprotease ADAMTS-13 (A Disintegrin And Metalloprotease with Thrombospondin type 1 repeats 13) into the range of multimer sizes found in normal plasma (10-13). ADAMTS-13 cleaves a single peptide bond at position Y1605-M1606 in the VWF A2 domain in vitro. The cleavage under static conditions requires plasma-type VWF multimers to be partially denatured (10;11), unless a VWF A2 polypeptide (14) or a short A2 peptide of 73 amino acids is used as the substrate (15). ADAMTS-13 is, however, able to cleave endothelial cell anchored ULVWF strings atop activated endothelial cells independent of denaturing agents and hydrodynamic forces (16;17). The observations support the notion that plasma-type VWF and ULVWF multimers are conformationally or structurally distinct.
We have recently shown that thiols are exposed on the surface of plasma-type VWF multimers, but buried in ULVWF multimers (18). High fluid shear stress may induce a globular-to-elongated conformation change and/or lateral apposition of plasma-type VWF multimers (19). This may facilitate the formation of new disulfide bonds between the exposed thiols of sheared, laterally-associated plasma-type VWF multimers to enhance VWF binding to platelets (16;18). ULVWF secreted by and anchored to endothelial cells may be elongated by fluid shear stress to also expose thiols capable of forming disulfide bonds with circulating plasma-type VWF multimers (20). VWF multimers consist of cysteine-rich monomers linked by reversible disulfide bonds and form inter-multimer disulfide bonds upon exposure to high fluid shear stress in plasma. This tentative mechanism of thiol-disulfide bond exchange suggests that the adhesion activity of plasma-type VWF multimers is redox-regulated by several potential agents (i.e. glutathione and thrombospondin (21;22)). The plasma has also been reported to contain a protein with both protease and reductase activity towards VWF multimers (23). These observations led us to investigate whether ADAMTS-13 contributes disulfide-bond-reducing activity to VWF multimers.
Materials and Methods
All experiments were conducted using plasma-type VWF multimers (referred as VWF) purified from human cryoprecipitate (11) unless otherwise specified.
Preparation and purification of recombinant ADAMTS-13 and mutants
Recombinant (r) ADAMTS-13 was prepared as previously described (24). CHO cells expressing wild type ADAMTS-13 were maintained in Dulbecco Modified Eagle Medium (Invitrogen, Carlsbad, CA) containing 500 μg/ml hygromycin (final concentration) and 10% fetal bovine serum at 37°C with 95% air and 5% CO2. The conditioned medium was collected after cells were incubated in Opti PRO™ serum-free medium (Invitrogen) for 24–48 hr and rADAMTS-13 was purified from a Ni-NTA column (Invitrogen) and verified by immunoblotting using a goat anti-human ADAMTS-13 antibody (Bethyl laboratories, Montgomery, TX). The purity of rADAMTS-13 was 80–90% according to SDS-PAGE with Coomassie blue staining (data not shown). The activity of rADAMTS-13 was tested in a static assay using purified plasma VWF multimers as the substrate (12). To exclude the potential effects from the co-purified contaminants or ADAMTS-13 fragments, the conditioned medium from Sham transfected cells underwent the same purification process and was used as control (referred as buffer control) and a recombinant full-length ADAMTS-13 from stably transfected CHO cells and purified to homogeneity by a conventional multi-step purification (Baxter Innovations GmbH, Vienna, Austria) was also tested.
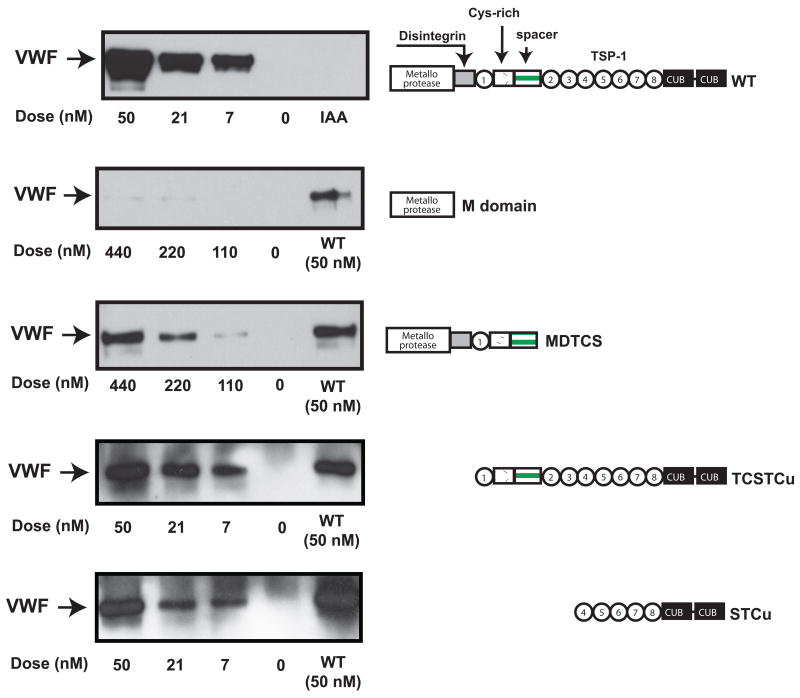
Four truncation mutants were generated by polymerase chain reaction (PCR): catalytic domain only (M) and three ADAMTS-13 constructs that lacked of: 1) TSP-1 motif 2-8 and CUB domains (MDTCS); 2) catalytic and disintegrin domains (TCSTCu); or 3) the sequence N-terminal to the fourth TSP-1 motif (STCu). The PCR reaction consisted of a cycle of 95°C for 5 min followed by 25–35 cycles of 1 min at 94°C for template denaturing, 1 min at 50–56° C for primer annealing, and 3 min at 72°C for primer extension. The PCR products were individually cloned into the expression vector pSecTag2 and verified by direct DNA sequencing. The truncation mutants were expressed in CHO cells, purified with a Ni-NTA column as described above, and quantified by a protein assay kit (Bio-Rad, Hercules, CA).
VWF multimer analysis
VWF (40 nM) was incubated with or without rADAMTS-13 (7 nM) for 5 hrs at 37°C in 1XPBS in the absence of urea and barium. The mixture was separated by 1% SDS-agarose gel electrophoresis at pH 7.4, followed by immunoblotting with an anti-VWF antibody (DAKO, Carpinteria, CA) (24;25). The VWF loading was standardized based on VWF concentrations measured using an ELISA kit (Ramco Laboratory, Stafford, TX).
Detection of thiols in VWF and ADAMTS-13
Two techniques were used to detect thiols in ADAMTS-13 and VWF (18). First, maleimide-PEO2-Biotin (MPB, Pierce Biotechnology, Rockford, IL), which is a thiol-reactive agent with a hydrophilic polyethylene oxide spacer arm that reacts with cysteine thiols to form stable carbon-sulfur bonds, was used to globally and irreversibly label thiols in VWF and ADAMTS-13. Briefly, MPB (100 μM) was incubated with target proteins for 15–20 min at room temperature (RT) and the reaction was then quenched by 200 μM GSH. The maleimide-tagged proteins were separated by 6% SDS-PAGE under reducing conditions and detected by immunoblotting using HRP-conjugated streptavidin (Pierce Biotechnology). Control samples were first treated for 30 min at room temperature with 10 mM iodoacetamide (IAA, Sigma Aldrich, St. Louis, MO), which acetylates cysteine thiols and prevents their interaction with MPB. Unbound IAA was removed by dialysis against 1 L of 1XPBS overnight at 4°C. For direct labeling ADAMTS-13 thiols in plasma, MPB (100 mM) was incubated with 1 mL of dialyzed citrated human plasma (1XPBS, pH 7.4, overnight at 4°C). After dialysis, the labeled proteins were precipitated with streptavidin-coupled sepharose beads, separated on 6% SDS-PAGE, and immunoblotted with a polyclonal ADAMTS-13 antibody (Bethyl Laboratory). Plasma control was pretreated IAA (100 mM, excess IAA removed by dialysis) before MPB labeling and underwent the same precipitation procedure.
Second, thiolpropyl sepharose™ 6B beads (GE Healthcare Piscataway, NJ) covalently coupled to reactive 2-thiopyridyl disulfide groups were used to react with thiols in target proteins to form mixed disulfide bonds (18). Because of their large size (∼90 μm), the beads primarily react with thiols exposed on the surface of native proteins. Briefly, 5 mg of washed beads suspended in 1 ml of 1XPBS (pH 7.4) were incubated with 10 μg VWF, 5 μg/ml rADAMTS-13, or 1 ml of dialyzed citrated plasma for 15–20 min at RT with constant rotation. The beads with captured proteins were extensively washed with PBS to remove non-specifically bound proteins and then incubated with 20 mM dithiothreitol (DTT) for 10 min at RT to release covalently captured proteins, which were detected by immunoblotting. To test for disulfide-bond–reducing activity, rADAMTS-13 (instead of DTT) was incubated with VWF-bound beads for 1 hr at 37°C. One technical concern is that 2-thiopyridyl disulfide groups coupled to sepharose beads may have a mild disulfide bond-reducing activity. This is unlikely because the pKa of –SH on 2-thiopyridone is ∼10 and that of the adjacent -NH is ∼5.2. By conducting experiments at pH ∼7.4, the –SH groups are expected to be protonated and unable to attack disulfide bonds. Nevertheless, this possibility is addressed by conducting experiments in parallel using MPB labeling.
Effect of rADAMTS-13 on shear-induced thiol-disulfide exchange of VWF
A cone and plate viscometer (RS1, HAAKE, Karlsruhe, Germany), used previously to demonstrate shear-induced thiol-disulfide exchange in VWF (18), was employed to investigate whether rADAMTS-13 regulates the shear-induced thiol-disulfide exchange. The cone and plate were coated with 10% bovine serum albumin (BSA) to reduce non-specific adhesion to the metal surface. VWF (5 μg/ml, 20 nM calculated based on 250 kDa molecular mass) was loaded onto the plate in the presence or absence of 7 nM rADAMTS-13 (a molar ratio of 3:1, near the physiological ratio found in plasma) or an equal volume of buffer control. VWF was also exposed to shear stress in the presence of 1% of BSA as specificity control. The mixture was exposed to a constant shear stress of 100 dyn/cm2 at 37°C for 3 min and then mixed with thiol beads for covalent chromatography. To specifically measure ADAMTS-13 disulfide bond-reducing activity, VWF was also incubated with rADAMTS-13 at 37°C for 1 hr after VWF exposure to shear stress (before thiol bead capturing).
Effect of ADAMTS-13 on mixed disulfide bonds between VWF and thiol beads
VWF was first induced to form mixed disulfide bonds with thiol beads (18) and bead-bound VWF was then incubated with different concentrations of rADAMTS-13 or mutants in 1X PBS for 1 hour at 37°C with constant rotation. After removing beads by centrifugation, the supernatant was collected and separated by 6% SDS-PAGE under reducing conditions followed by immunoblotting with a polyclonal anti-VWF antibody (Dako). For control, rADAMTS-13 was tested after being treated with 10 mM IAA for 30 min at room temperature to block active thiols (excess IAA was removed by overnight dialysis with 1L of 1XPBS). Also as control, either rADAMTS-13 or IAA-treated rADAMTS-13 (both at 35 nM) was used to release BSA (1% loading) captured to thiol beads under conditions identical to those used for VWF. BSA was detected by coomassie blue staining.
Peptide mass analysis by MALDI/TOF/TOF
VWF (160 nM) captured by thiol beads was incubated with 2 μg/ml trypsin overnight at 37°C. After extensive washing, beads were incubated with 50 nM rADAMTS-13 for 1 hr at 37°C. rADAMTS-13 was subsequently removed by passing the solution through a spin-concentrator with a 100-kDa–cut-off membrane. To identify specifically cysteine residues in the tryptic peptide that form mixed disulfide bonds with thiol beads, the VWF tryptic peptides bound to the beads were treated with 10 mM IAA for 30 min at RT before being released by rADAMTS-13. To test the ability of rADAMTS-13 to reduce disulfide bonds formed between apposed VWF multimers through shear-induced thiol-disulfide exchange, VWF was exposed to 100 dyn/cm2 for 3 min at 37°C (18). The sheared VWF was incubated with rADAMTS-13 for 1 hr at 37°C, and then digested with sepharose bead-coupled trypsin overnight at 37°C. The reaction mixture was centrifuged at 1000 × g to remove trypsin-coupled beads. The mass of thiol-containing tryptic VWF peptides released by rADAMTS-13 from thiol beads or from sheared VWF after incubation with rADAMTS-13 was analyzed by a reflective mode MALDI/TOF/TOF mass spectrometer (4700 Proteomics Analyzer, Applied Biosystems, Foster City, CA).
ADAMTS-13 activity under static and flow conditions
rADAMTS-13 was first incubated with 5 mM N-ethylmaleimide (NEM) or 10 mM IAA for 15–30 min at room temperature to block thiols irreversibly. The treated rADAMTS-13 was dialyzed with 1 L of 1X PBS overnight at 4°C to remove unbound NEM or IAA and tested for cleaving ULVWF strings anchored to the surface of cultured endothelial cells and plasma VWF multimers under flow and static conditions, respectively (16;26).
Two static assays were also performed. The first was to measure the cleavage of plasma-type VWF multimers in the presence of urea and barium as previously described (24). The second was to measure the cleavage of ULVWF strings on endothelial cells in the absence of flow (27).
Thiol quantification
Thiols in ADAMTS-13 were quantified using 4, 4′-dithiopyridine as the labeling agent (28;29) with minor modifications. The chemical modification of thiols by 4, 4′-dithiopyridine, which interacts with thiols to form a 4-thiolpyridone chromophor with an extinction coefficient of 19,800 M-1cm-1 at 324 nm. Briefly, 2.5 μM purified rADAMTS-13 (>90% purity) was suspended in 1XPBS containing 5 M Urea and incubated with a 100-fold molar excess of 4, 4′-dithiopyridine for 2 hr at RT. The amino acid cysteine (Sigma Aldrich, St. Louis, MO) was used to set the calibration standard.
Statistical Analysis
All quantitative experimental data are presented as mean ± SEM. The Student's t-test was used for data analysis and a p value less than 0.05 was considered to be statistically significant.
Results
ADAMTS-13 prevented shear-induced thiol-disulfide exchange of VWF
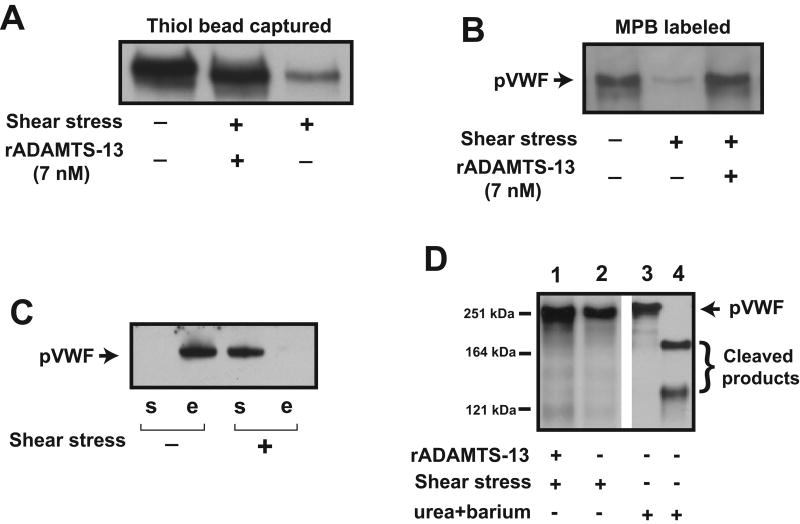
We have previously shown that shear stress induces thiol-disulfide exchange that significantly increases binding of plasma-type VWF multimers to platelets (18). In this study, we investigated the VWF thiol-disulfide state after shear exposure in the presence of rADAMTS-13 to determine whether the metalloprotease affects the exchange. VWF was exposed to 100 dyn/cm2 shear stress in the presence and absence of rADAMTS-13. The samples were subsequently incubated with either thiol beads to capture VWF via mixed disulfide bonds or MPB to directly label thiols. Consistent with our earlier report, VWF was captured by thiol beads (Figure 1A) or labeled with MPB (Figure 1B) before, but not after shear exposure. VWF was, however, captured by thiol beads and labeled with MPB after shear exposure when rADAMTS-13 was present (Figure 1A & B). In contrast, BSA did not prevent the shear-induced thiol-disulfide exchange in VWF (Figure 1C). Under this experimental protocol, rADAMTS-13 did not cleave VWF (Figure 1D) or coimmunoprecipitate with VWF (supplemental Figure 1A), suggesting that rADAMTS-13 prevents the shear-induced thiol-disulfide bond exchange of VWF and this change in thiol content was not due to VWF cleavage or VWF forming a complex with ADAMTS-13.
Figure 1. ADAMTS-13 on shear-induced thiol-disulfide exchange of VWF.
(A) VWF (20 nM) was exposed to a 100-dyn/cm2 shear stress in the presence and absence of rADAMTS-13 (7 nM) for 3 min at 37°C and incubated with thiol beads for 15–20 min. VWF captured by beads was released by 20 mM DTT and detected by a polyclonal anti-VWF antibody under reducing conditions. (B) VWF multimers were incubated with MPB before and after shear exposure (and with and without rADAMTS-13), and MPB-labeled VWF was detected by HRP-streptavidin. (C) Thiol beads captured VWF before (detected in DTT elute [“e” fraction]), but not after (detected in supernatant [“s” fraction]) being exposed to fluid shear stress in the presence of 1% BSA. (D) VWF was exposed to 100 dyn/cm2 shear stress in the presence (lane 1) or absence (lane 2) of 7 nM of rADAMTS-13, separated on 6% SDS-PAGE, and immunoblotted with a polyclonal VWF antibody. As a control, VWF was subjected to a standard static assay of VWF cleavage at a low–ionic-strength in the presence of 1 mM BaCl2 and 1.5 M urea for 0 (lane 3) and 16 hrs (lane 4) at 37°C. The figure represents 3-5 separate experiments.
ADAMTS-13 reduced shear-induced disulfide bonds in VWF
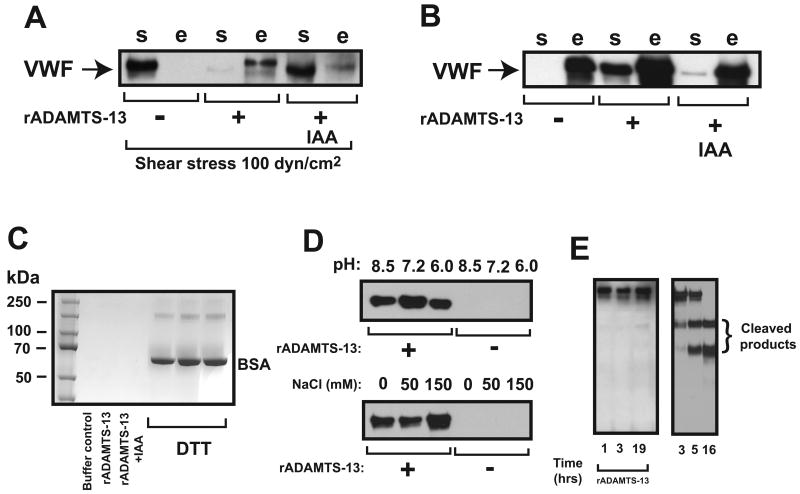
ADAMTS-13 could prevent the shear-induced transition of thiols to disulfide bonds in VWF by 1) interfering shear-induced lateral association of VWF multimers or 2) reducing newly formed disulfide bonds after shear exposure. To distinguish between these two possibilities, we first exposed VWF to shear stress to induce thiol-to-disulfide exchange and then incubated the sheared VWF with rADAMTS-13 to rule out steric hindrance effect of rADAMTS-13 on VWF during shear exposure. As shown in Figure 2A, thiol beads captured ∼ 5% and 40–60% of sheared VWF (detected in DTT eluate [“e” fraction]) when VWF was incubated with buffer control and rADAMTS-13, respectively, suggesting that rADAMTS-13 reduced disulfide bonds formed between apposed plasma-type VWF multimers under shear stress. Consistent with the possibility, rADAMTS-13 also released VWF captured by thiol beads (Figure 2B). This activity of ADAMTS-13 was thiol-dependent because it was significantly blocked by IAA (Figure 2A and 2B). It was also substrate-specific because ADAMTS-13 did not release BSA that has been captured to thiol beads (Figure 2C). ADAMTS-13 was active in releasing VWF from thiol beads at pH 6.0 to 8.5 and ionic strength from 0 to 150 mM NaCl, but the activity was highest at pH 7.2 and an ionic strength of 150 mM NaCl (Figure 2D). VWF released from thiol beads by rADAMTS-13 remained as ∼250-kDa monomers under reducing conditions. When incubated with rADAMTS-13 in 1XPBS buffer in the absence of Ca2+, barium, and urea for up to 19 hrs at 37°C, whereas VWF was 100% cleaved after 16 hr incubation with barium activated rADAMTS-13 in the presence of 1.5 M urea and 20 mM of NaCl (Figure 2E).
Figure 2. ADAMTS-13 on newly formed disulfide bonds.
(A) Sheared VWF (20 nM) was incubated with buffer control; IAA treated or untreated rADAMTS-13 (7 nM) and then mixed with thiol beads (5 mg). VWF was captured by the thiol beads when it was exposed to shear stress in the presence (detected in bead eluate, “e” fraction), but not in the absence of rADAMTS-13 (detected in supernatant, “s” fraction). IAA significantly blocked this rADATMS-13 activity. (B) VWF captured to thiol beads was incubated with buffer control, 15 nM IAA-treated or untreated rADAMTS-13 for 1 hr at 37℃. VWF was released from rADAMTS-13 treated (detected in supernatant, “s” fraction), but not buffer-treated beads (detected in DTT eluate, “e” fraction). Releasing VWF from thiol beads was significantly reduced when IAA treated ADAMTS-13 was used. (C) BSA captured by thiol beads was first treated with buffer control, rADAMTS-13 or IAA-treated rADAMTS-13 (35 nM). After collection of supernatant, BSA bound to thiol beads was eluted with DTT. BSA in both supernatant and DTT eluate was separated on 6% SDS-PAGE and detected by coomassie blue staining (rADAMTS-13 failed to release BSA from the beads (rADAMTS-13 was not visible on the gel image because a > 60 fold dilution of the supernatant [rADAMTS-13 < 1 ng]). rADAMTS-13 did not release BSA from thiol beads. (D) rADAMTS-13 (35 nM) was incubated with VWF captured onto thiol beads at various pH and ionic strength for 1 hr at 37°C and VWF released from the beads was separate by 6% SDS-PAGE and detected by immunoblotting with an ADAMTS-13 antibody. (E) VWF captured to thiol beads was incubated with rADAMTS-13 in 1XPBS without Ca2+, urea and barium for up to 19 hrs at 37°C, separated by 6% SDS-PAGE, and detected with a VWF antibody under reducing conditions. As control, VWF was cleaved by rADAMTS-13 in a standard static assay. The figure represents 3–5 independent experiments.
ADAMTS-13 targeted cysteine thiols in the VWF C domain
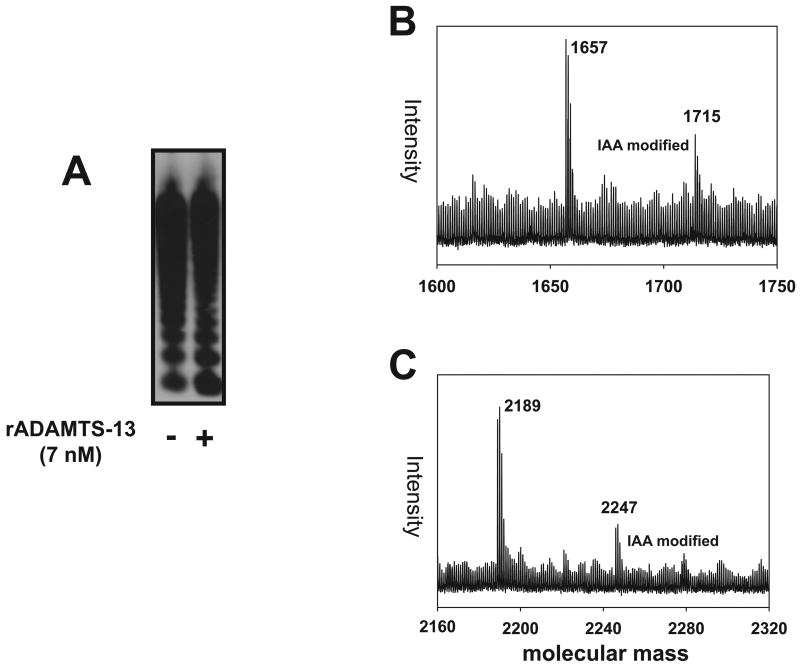
The disulfide-bond reducing activity of ADAMTS-13 could target structural disulfide bonds that maintain VWF multimeric structure and/or new disulfide bonds that are formed between VWF multimers after exposure to high shear stress or between VWF and thiol beads. To clarify the ADAMTS-13 target(s), VWF was incubated with rADAMTS-13 for 1 hr at 37°C in 1xPBS buffer without urea and barium, an experimental condition used to detect the disulfide bond-reducing activity of ADAMTS-13. As shown in Figure 3A, there is no detectable difference in VWF multimer patterns before and after rADAMTS-13 treatment, suggesting that ADAMTS-13 does not reduce disulfide bonds that maintain VWF multimeric structure under the experimental conditions.
Figure 3. ADAMTS-13 on thiols in the C domain of VWF.
(A) VWF (20 nM) was incubated with rADAMTS-13 (7 nM) in 1X PBS buffer for 1 hr at 37°C in the absence of urea and barium, separated by 1% SDS agarose gel electrophoresis under non-reducing conditions, and immunoblotted with a VWF antibody. The figure represents 3 separate experiments. In (B) & (C), tryptic cysteine-containing VWF peptides bound to thiol beads were first treated with IAA (excess IAA was removed by dialysis with 1L 1XPBS overnight at 4°C) and then released from thiol beads by rADAMTS-13. The released tryptic peptides were analyzed by mass spectrometry. MALDI/TOF/TOF detected two VWF tryptic peptides that were modified by IAA and in each of these peptides, only one cysteine was acetylated by IAA as determined by increased molecular mass from 1657 Da to 1715 Da (B) and from 2189 Da to 2247 Da (C), respectively. The second cysteine in each tryptic peptide formed mixed disulfide bonds with thiol beads and was therefore not modified by IAA.
We have previously identified exposed and active thiols in four peptides that are derived from the VWF C and D3 domains (18). To determine if any of these thiols forms disulfide bonds with thiol beads and is reduced by rADAMTS-13, we digested VWF captured to thiol beads with trypsin and the bead-bound tryptic peptides were then released by rADAMTS-13 by disulfide bond reduction. The two most prominent thiol-containing tryptic peptides released by rADAMTS-13 were from the C2 domain (Table 1) and were among those released by the reducing agent DTT (18). We then determined whether these thiols were involved in the shear-induced thiol-disulfide exchange between laterally apposed VWF multimers. VWF was exposed to high fluid shear stress to induce thiol-disulfide exchange and then incubated with rADAMTS-13 for 1 hr at 37°C. The mixture was then subjected to digestion with sepharose-bead–coupled trypsin. The tryptic peptides of sheared VWF were subjected to analysis by MALDI mass spectrometry. The same two thiol-containing VWF peptides listed in Table 1 were again detected.
Table 1. Thiol-containing tryptic VWF peptides released by rADAMTS-13.
| Tryptic peptide sequence | Molecular mass | Location (domain)* |
|---|---|---|
| SVGSQWASPENPCLINECVR | 2189.01 | 2516–2535 (C2) |
| SGFTYVLHEGECCGR | 1657.71 | 2479–2493 (C2) |
the sequence locations include the signal peptide and propeptide sequences
Since each of the two tryptic peptides contains two cysteine residues (Table 1), we determined whether one or both cysteine residues in each peptide are in thiol form. VWF tryptic peptides were treated with IAA before being released from thiol beads with ADAMTS-13. Mass spectrometry identified a portion of each peptide that was modified by IAA at a single cysteine (Figure 3B & C), suggesting that both cysteine residues in each peptide are in thiol form.
ADAMTS-13 contained free thiols
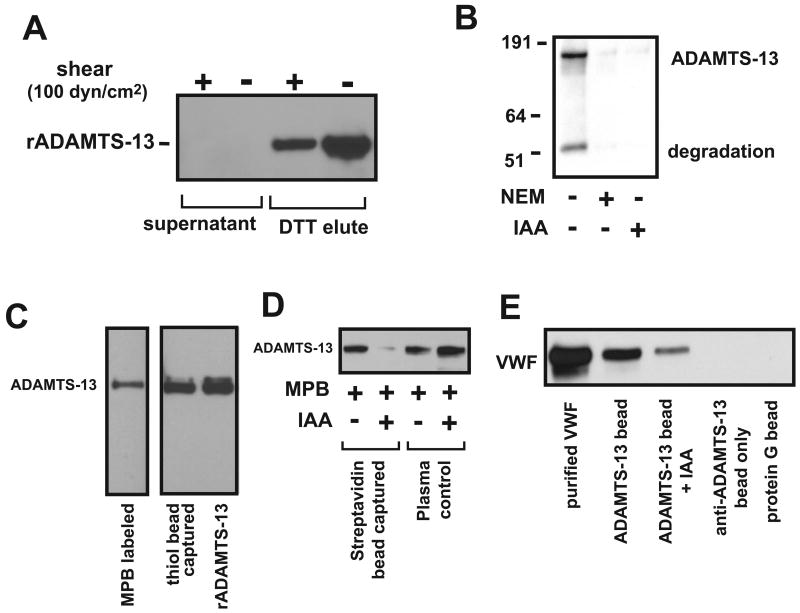
The data in Figures 1–3 demonstrate that rADAMTS-13 reduces disulfide bonds formed between apposing plasma-type VWF multimers that are exposed to high fluid shear stress and between VWF and thiols on beads. A prerequisite for this disulfide-bond–reducing activity is that ADAMTS-13 contains cysteine thiol(s) necessary to reduce a disulfide bond. Moreover, the active thiols should be surface-exposed and accessible to the substrate without profound conformational changes. Because of their large size (∼90 μm), the active thiol beads may only form mixed disulfide bonds with thiols exposed on the surface of ADAMTS-13 under non-denaturing conditions. As shown in Figure 4A, thiol beads captured 100% of rADAMTS-13 that was then released by DTT and detected by immunoblotting, independent of exposure to shear stress. The rADAMTS-13 band in the post-shear sample may have been decreased because of non-specific adhesion of the metalloprotease to the viscometer metal plate, a commonly observed phenomenon. rADAMTS-13 was also labeled with MPB, and the labeling was blocked by 10 mM IAA or 5 mM NEM (Figure 4B). These results suggest that rADAMTS-13 contains surface-exposed thiols that remain after exposure to high fluid shear stress. We then determined whether thiols were present in native ADAMTS-13, or produced during purification of rADAMTS-13. Figure 4C shows that ADAMTS-13 from dialyzed citrated plasma was directly captured by thiol beads or labeled with MPB. Streptavidin beads did not capture ADAMTS-13 when plasma was pretreated with IAA that blocked MPB labeling (Figure 4D). Furthermore, ADAMTS-13 immunoprecipitated with an ADAMTS-13 antibody (against the CUB domains) coupled to protein G sepharose beads was active in releasing VWF from thiol beads and this activity was again blocked by IAA (Figure 4E).
Figure 4. Surface-exposed thiols in ADAMTS-13.
(A) rADAMTS-13 (35 nM) was incubated with thiol beads before and after exposure to 100-dyn/cm2 shear stress for 3 min at 37°C, separated by 6% SDS-PAGE, and detected by immunoblotting with an anti-Myc antibody. rADAMTS-13 was detected in DTT eluate (thiol form), but not in supernatant (disulfide bond form). (B) rADAMTS-13 was first treated with NEM or IAA for 10 min at RT and subjected to dialysis in 1L of 1XPBS to remove excess NEM or IAA. The treated and untreated rADAMTS-13 was then labeled with MPB, separated by 6% SDS-PAGE, and probed with HRP-streptavidin. (C) Normal human plasma (1 ml) was incubated with 100 mM of MPB or captured by 5 mg of thiol beads for 15–20 min at RT. MPB labeled plasma proteins were precipitated by streptavidin beads, released by boiling in SDS sample buffer, separated on 6% SDS-PAGE, and immunoblotted with an anti-ADAMTS-13 antibody (left lane). ADAMTS-13 captured with thiol beads was released by 20 mM DTT, separated on 6% SDS-PAGE, and blotted with an ADAMTS-13 antibody (middle lane). Unlabeled ADAMTS-13 was used as a control (right lane). (D) Normal human plasma (1 ml) was incubated with MPB before and after it was pretreated with 10 mM of IAA. ADAMTS-13 from labeled plasma was captured by streptavidin-coupled sepharose beads, separated on 6% SDS-PAGE, and immunoblotted with an ADAMTS-13 antibody. Because MPB failed to label IAA-treated plasma, streptavidin beads did not precipitate ADAMTS-13 from plasma (lane 2). Plasma without IAA treatment was used as control. (E) Normal human plasma (1 ml) was incubated with an ADAMTS-13 antibody coupled to protein G sepharose beads before and after it was treated with 10 mM of IAA. The bead-captured plasma ADAMTS-13 was able to release VWF from thiol beads. The figure represents 3–6 separate experiments.
Four truncation mutants were used to map the regions of ADAMTS-13 that are responsible for VWF disulfide bond-reducing activity. As shown in Figure 5, the catalytic domain polypeptide was inactive in releasing VWF from thiol beads, whereas MDTCS was 10-fold less active that WT rADAMTS-13. Two N-terminal truncation mutants were more effective than MDTCS, but less active than the full length ADAMTS-13. The order of activity in releasing VWF from thiol beads was WT > TCSTCu > STCu > MDTCS > M, suggesting that the activity is primarily located in the C-terminal domains.
Figure 5. Reducing activity of ADAMTS-13 truncation mutants.
VWF multimers captured with thiol beads were incubated with various concentrations of full-length rADAMTS-13, M, MDTCS, TCSTCu, or STCu domain for 1 hr at 37°C. VWF released from the beads was separated by 6% SDS-PAGE and detected by immunoblotting using an anti-VWF antibody. A full-length rADAMTS-13 protein (50 nM) was used as a positive control and IAA-treated rADAMTS-13 as a negative control for WT rADAMTS-13. The figure represents 3-5 separate experiments.
rADAMTS-13 was found to have ∼6 thiols/molecule after 20 min incubation with 4, 4′-dithiopyridine as the thiol labeling agent. The number of thiols increased to 24-28 after 2 hr incubation in the presence of the denaturing agent urea (5 M, supplemental Figure 1B). Mass spectrometry also detected 6 surface exposed thiols and 3 were in the CUB-1 domain (Table 2). Quantitative densitometry determined that recombinant and native ADAMTS-13 contained a comparable number of thiols (supplemental Figure 1C). The densitometry instead of 4, 4′-dithiopyridine was used to avoid thiols being generated during ADAMTS-13 purification because highly purified ADAMTS-13 is required for the 4, 4′-dithiopyridine labeling
Table 2. Free thiols in ADAMTS-13 detected by mass spectrometry*.
| Sequence | Mass | Location | Domain |
|---|---|---|---|
| AGLAWSPCSR | 1047.5040 | 258-267 | catalytic domain |
| CVEAQGSLLK | 1047.5503 | 769-778 | 3rd TSP-1 motif |
| VPVQEELCGLASKPGSR | 1769.9214 | 926-942 | 5th TSP-1 motif |
| LLPGPQENSVQSSACGR | 1742.8490 | 1178-1194 | CUB-1 |
| GPGQADCAVAIGRPLGEVVT LR | 2179.1651 | 1207-1228 | CUB-1 |
| CGRPGGGVLLR | 1084.6044 | 1275-1285 | CUB-1 |
The peptides were repeatedly detected in four separate sample analyses.
ADAMTS-13 thiols affected cleaving ULVWF under flow conditions
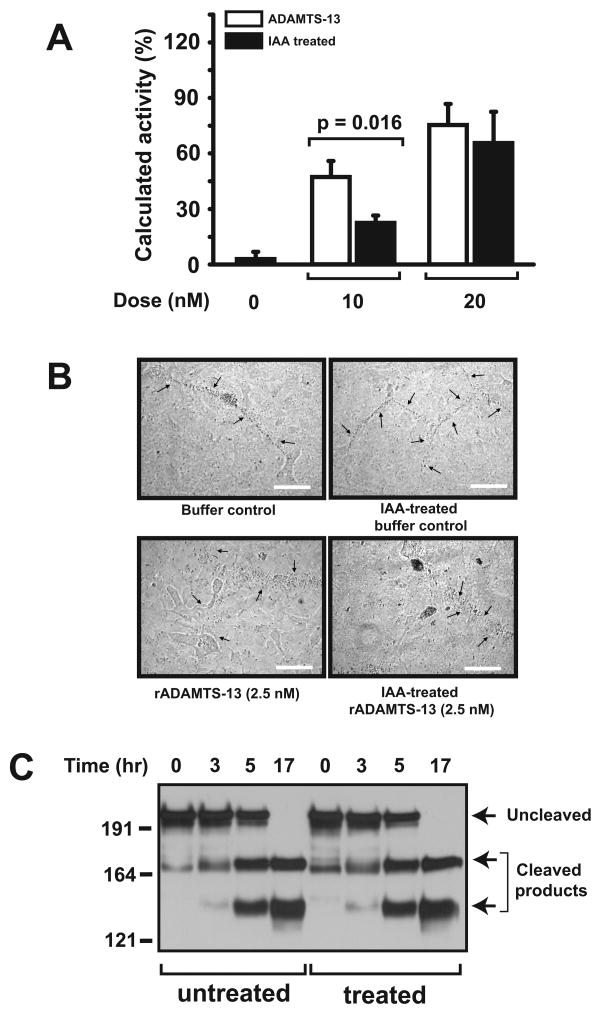
We quantified ADAMTS-13 cleavage of UL VWF-cleaving activity of rADAMTS-13 treated with IAA or NEM. At 20 nM, IAA-treated or untreated rADAMTS-13 had comparable activities in cleaving platelet-decorated ULVWF strings under a 2.5-dyn/cm2 shear stress (64.5 ± 8.5% vs. 73.8 ± 8.7%, Figure 6A). However, at 10 nM, IAA-treated ADAMTS-13 was less active than untreated ADAMTS-13 (22.9 ± 8.7% vs. 46.3 ± 11.1%). The concentrations of ADAMTS-13 used in the assay were higher than ∼7 nM found in normal plasma. However, the increased levels are consistent with our earlier report that higher concentrations of ADAMTS-13 are required to cleave platelet-decorated ULVWF strings under constant flow (16). In contrast, we did not detect a difference in cleaving these ULVWF strings under static conditions between IAA-treated and untreated rADAMTS-13 (Figure 6B) (20). Furthermore, IAA-treated and untreated rADAMTS-13 were also comparably active in cleaving VWF under static conditions in the presence of urea and barium (Figure 6C). The results were confirmed with NEM-treated ADAMTS-13 (data not shown).
Figure 6. Effect of blocking thiols in rADAMTS-13 on VWF cleavage.
(A) HUVECs were cultured to confluence and then stimulated with 25 mM histamine. Washed platelets with rADAMTS-13 (10 or 20 nM) before and after being treated with 10 mM of IAA (free IAA was removed by dialysis) were perfused over the activated endothelial cells at a 2.5-dyn/cm2 shear stress for 3 min. The number of ULVWF strings over 10 continuous review fields was then counted and ADAMTS-13 activity was calculated as a percentage of ULVWF strings cleaved during perfusion (Student's t-test, n = 6). (B) Stimulated HUVECs were incubated with lyophilized human platelets in the presence of buffer control, IAA-treated buffer control, rADAMTS-13, or IAA-treated rADAMTS-13. Platelet-decorated ULVWF strings are visible under a light microscope (200 ×, top two panels), but cleaved and undetectable in the presence of IAA-treated and untreated rADAMTS-13 (bottom two panels, bar = 25 μm). (C) Purified plasma VWF multimers were first partially denatured with urea and then incubated with barium-activated rADAMTS-13 with and without IAA treatment. Aliquots were taken at different incubation intervals to detect cleaved VWF fragments by immunoblotting. The figure represents 4 separate experiments.
Discussion
We have presented evidence to demonstrate that ADAMTS-13 contains a disulfide-bond–reducing activity. First, ADAMTS-13 prevents the shear-induced thiol-disulfide exchange of plasma-type VWF multimers (Figure 1A & B) and reduces disulfide bonds formed between apposed plasma-type VWF multimers after VWF exposure to high fluid shear stress (Figure 2). ADAMTS-13 also releases VWF covalently captured onto thiols beads through mixed disulfide bonds (Figure 2). This disulfide bond-reducing activity is the strongest at a pH of 7.2 and an ionic strength 150 mM of NaCl (Figure 2). The specificity of this activity was confirmed by its inactivation when rADAMTS-13 was treated with the thiol-blocking agent IAA; and by the failure of ADAMTS-13 to release BSA, a thiol-containing protein, from thiol beads (Figure 2C). This activity is independent of proteolytic activity because ADAMTS-13 did not cleave VWF under experimental condition used for reducing VWF disulfide bond (Figure 1 & 2) and truncation mutants that lack of the catalytic domain remain active (Figure 5).
Second, treating VWF multimers with rADAMTS-13 in the absence of urea and barium does not alter the VWF multimer patterns (Figure 3A), indicating that the disulfide-bond-reducing activity does not disrupt VWF multimeric structures. Instead, it specifically reduces disulfide bonds formed between thiols located in the VWF C-domain (Table 1 and Figure 3B and C). This indicates that ADAMTS-13 reduces disulfide bonds formed between laterally associated VWF multimers under the influence of fluid shear stress (Figure 2). We have previously shown that nine cysteine residues in the VWF C-domain are in thiol forms that are exposed on the surface of the quaternary structure of VWF multimers, and are capable of forming disulfide bonds upon exposure to high shear stress (18). In this study, we show that some of these thiols (in two tryptic VWF peptides) are targeted by rADAMTS-13 (Table 1, Figure 3). One of the two peptides (SGFTYVLHEGECCGR) is homologous with the crossveinless VWF C type repeat (CV- 2-VWC)(30). However, unlike CV- 2-VWC, the two vicinal cysteine residues in the VWF are in thiol forms. This result suggests possibility that ADAMTS-13 also interacts with the VWF C (or adjacent) domains to facilitate disulfide bond-reduction. This possibility is supported by the observation that the C-terminal domains of ADAMTS-13 are important for binding to (24;31), but not required for cleaving VWF (32;33). Zanardelli S, et al further shows that ADAMTS-13 binds to the D4 domain through its C-terminal TSP-1 motifs and CUB domains (34), the location of most exposed thiols (Table 2).
Third, recombinant and plasma ADAMTS-13 contains surface-exposed thiols both before and after shear exposure (Figure 4). ADAMTS-13 contains 75 cysteine residues (13) and its disulfide bond structure remains unknown. Using a 4, 4′-dithiopyridine labeling assay, we detected ∼6 thiols/ADAMTS-13 after 20 min incubation, but the number of thiols increased to 24-28 after the metalloprotease was incubated with 5M urea for 2 hrs (supplemental Figure 1B). These results suggest that there may be ∼6 thiols exposed on the surface of ADAMTS-13, with the others buried within the tertiary structure of ADAMTS-13. Results from testing four truncation mutants further locate the disulfide bond-reducing activity to the C-terminal domains of ADAMTS-13 (Figure 4). Consistent with data from the thiol labeling assay, mass spectrometry also detected 6 thiol-containing tryptic peptides of ADAMTS-13 in the absence of urea (each contains 1 thiol). Five of them are located in the C-terminal TSP-1 motifs and CUB-1 domain (Table 2). It is not clear if some or all of these thiols are involved in the VWF disulfide bond-reducing activity, the disulfide bond reducing activity may involve thiols that are distant in linear sequence, but become proximal in the ADAMTS-13 tertiary structure.
Finally, blocking thiols in ADAMTS-13 had a moderate effect on cleaving endothelial cell anchored ULVWF strings under flow, but not static conditions. Blocking thiols also had no effect on cleaving plasma-type VWF multimers under static conditions in the presence of urea and barium (Figure 6). One explanation for the discrepancy is that blocking thiols alters the on-rate of ADAMTS-13-VWF interaction necessary for tethering the metalloprotease to ULVWF strings under flowing conditions. Nevertheless, the disulfide bond reducing activity is independent of ADAMTS-13's proteolytic activity because 1) a truncation ADAMTS-13 mutant lacking the C-terminal domains, where the disulfide bond reducing activity is likely located, remains active in cleaving ULVWF strings and plasma –type VWF multimers under flow and static conditions, respectively (32) and 2) the proteolytically inactive N-terminal truncated mutants remained active in reducing VWF disulfide bonds (Figure 2E and Figure 5).
In summary, we have shown that ADAMTS-13 contains a novel VWF disulfide-bond–reducing activity that is distinct from its proteolytic activity. This reducing activity does not affect disulfide bonds that maintain VWF multimeric structures, but selectively targets inter-molecular disulfide bonds between VWF multimers that are formed when VWF multimers are exposed to high fluid shear stress. The physiological significance of this ADAMTS-13 activity remains to be further investigated, but one may speculate a role in preventing shear-induced VWF thiol-disulfide exchange that promotes covalent lateral association of plasma-type VWF multimers into fibrillar structures with enhanced binding to platelets (21). The tentative mechanism is consistent with reports that VWF multimers undergo conformational changes (19), become laterally associated (35;36), and are activated to agglutinate platelets (37;38) under pathological levels of fluid shear stress. It is also supported by a recent report that VWF multimers in their native conformation forms a complex with ADAMTS-13 in plasma without undergoing cleavage (39).
Supplementary Material
(A) VWF was incubated with rADAMTS-13 (with and without IAA treatment to block thiols) before and after exposure to 100 dyn/cm2 of shear stress for 3 min at 37°C. VWF was then immunoprecipitated with a polyclonal VWF antibody, subjected to SDS-PAGE under reducing conditions, and immunoblotted with a monoclonal anti-Myc antibody. rADAMTS-13 was not immunoprecipitated by the VWF antibody either before or after exposure to shear stress. rADAMTS-13 (lane 1) was directly loaded on the gel to serve as control. (B) Purified rADAMTS-13 (2.5 μM) was incubated with a 100-fold molar excess of 4, 4′-dithiopyridine and the thiol modification recorded at 324 nm. Six thiols were calculated to be modified within 20 min, but the number of modified thiols increased to ∼26 after 2 hrs incubation in the presence of 5M urea. (C) MPB-labeled ADAMTS-13 was purified from citrate normal human plasma using an ADAMTS-13 antibody coupled affinity column (Aminolink Plus, Thermo Scientific), separated by 6% SDS-PAGE, and detected with streptavidin-HRP. MPB labeling was compared to the standard curve of rADAMTS-13 by densitometry at three different protein loadings. The figure represents 2-4 separate experiments.
Footnotes
This work was supported by NIH grants HL71895, HL085769, and research grants from the Mary R. Gibson Foundation and the Mabel and Everett Hinkson Memorial Fund.
Author contributions
Hui-Chun Yeh: Designed and conducted research experiments and contributed to manuscript writing.
Zhou Zhou: Conducted research experiments and contributed to manuscript writing.
Hiuwan Choi, Senem Tekeoglu, Will May III, Christina Wang, and Nancy Turner: Conducted research experiments.
Fritz Scheiflinger: Provided critical reagents and contributed to manuscript writing.
Joel L. Moake: Formulated hypothesis and wrote the manuscript.
Jing-fei Dong: Formulated hypothesis, designed experiments, and wrote the manuscript.
Conflict of Interest Statement
Dr. Fritz Scheiflinger works for Baxter Innovations GmbH
References
- 1.Voorberg J, Fontijn R, Calafat J, Janssen H, van Mourik JA, Pannekoek H. Assembly and routing of von Willebrand factor variants: the requirements for disulfide-linked dimerization reside within the carboxy-terminal 151 amino acids. J Cell Biol. 1991;113:195–205. doi: 10.1083/jcb.113.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isaacs NW. Cystine knots. Curr Opin Struct Biol. 1995;5:391–395. doi: 10.1016/0959-440x(95)80102-2. [DOI] [PubMed] [Google Scholar]
- 3.Wagner DD, Lawrence SO, Ohlsson-Wilhelm BM, Fay PJ, Marder VJ. Topology and order of formation of interchain disulfide bonds in von Willebrand factor. Blood. 1987;69:27–32. [PubMed] [Google Scholar]
- 4.Marti T, Rosselet SJ, Titani K, Walsh KA. Identification of disulfide-bridged substructures within human von Willebrand factor. Biochemistry. 1987;26:8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- 5.Tsai HM, Nagel RL, Hatcher VB, Seaton AC, Sussman II. The high molecular weight form of endothelial cell von Willebrand factor is released by the regulated pathway. Br J Haematol. 1991;79:239–245. doi: 10.1111/j.1365-2141.1991.tb04528.x. [DOI] [PubMed] [Google Scholar]
- 6.Sporn LA, Marder VJ, Wagner DD. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986;46:185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- 7.Brouland JP, Egan T, Roussi J, Bonneau M, Pignaud G, Bal C, Vaiman M, Andre P, Herve P, Mazmanian GM, Drouet L. In vivo regulation of von willebrand factor synthesis: von Willebrand factor production in endothelial cells after lung transplantation between normal pigs and von Willebrand factor-deficient pigs. Arterioscler Thromb Vasc Biol. 1999;19:3055–3062. doi: 10.1161/01.atv.19.12.3055. [DOI] [PubMed] [Google Scholar]
- 8.Arya M, Anvari B, Romo GM, Cruz MA, Dong JF, McIntire LV, Moake JL, Lopez JA. Ultralarge multimers of von Willebrand factor form spontaneous high-strength bonds with the platelet glycoprotein Ib-IX complex: studies using optical tweezers. Blood. 2002;99:3971–3977. doi: 10.1182/blood-2001-11-0060. [DOI] [PubMed] [Google Scholar]
- 9.Moake JL, Chow TW. Increased von Willebrand factor (vWf) binding to platelets associated with impaired vWf breakdown in thrombotic thrombocytopenic purpura. J Clin Apheresis. 1998;13:126–132. doi: 10.1002/(sici)1098-1101(1998)13:3<126::aid-jca6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Furlan M, Robles R, Lammle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87:4223–4234. [PubMed] [Google Scholar]
- 11.Tsai HM. Physiologic cleavage of von Willebrand factor by a plasma protease is dependent on its conformation and requires calcium ion. Blood. 1996;87:4235–4244. [PubMed] [Google Scholar]
- 12.Levy GG, Nichols WC, Lian EC, Foroud T, McClintick JN, McGee BM, Yang AY, Siemieniak DR, Stark KR, Gruppo R, Sarode R, Shurin SB, Chandrasekaran V, Stabler SP, Sabio H, Bouhassira EE, Upshaw JD, Jr, Ginsburg D, Tsai HM. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature. 2001;413:488–494. doi: 10.1038/35097008. [DOI] [PubMed] [Google Scholar]
- 13.Zheng X, Chung D, Takayama TK, Majerus EM, Sadler JE, Fujikawa K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J Biol Chem. 2001;276:41059–41063. doi: 10.1074/jbc.C100515200. [DOI] [PubMed] [Google Scholar]
- 14.Cruz MA, Whitelock J, Dong JF. Evaluation of ADAMTS-13 activity in plasma using recombinant von Willebrand Factor A2 domain polypeptide as substrate. Thromb Haemost. 2003;90:1204–1209. doi: 10.1160/TH03-06-0398. [DOI] [PubMed] [Google Scholar]
- 15.Kokame K, Nobe Y, Kokubo Y, Okayama A, Miyata T. FRETS-VWF73, a first fluorogenic substrate for ADAMTS13 assay. Br J Haematol. 2005;129:93–100. doi: 10.1111/j.1365-2141.2005.05420.x. [DOI] [PubMed] [Google Scholar]
- 16.Dong JF, Moake JL, Nolasco L, Bernardo A, Arceneaux W, Shrimpton CN, Schade AJ, McIntire LV, Fujikawa K, Lopez JA. ADAMTS-13 rapidly cleaves newly secreted ultralarge von Willebrand factor multimers on the endothelial surface under flowing conditions. Blood. 2002;100:4033–4039. doi: 10.1182/blood-2002-05-1401. [DOI] [PubMed] [Google Scholar]
- 17.Dong JF. Cleavage of ultra-large von Willebrand factor by ADAMTS-13 under flow conditions. J Thromb Haemost. 2005;3:1710–1716. doi: 10.1111/j.1538-7836.2005.01360.x. [DOI] [PubMed] [Google Scholar]
- 18.Choi H, Aboulfatova K, Pownall HJ, Cook R, Dong JF. Shear-induced disulfide bond formation regulates adhesion activity of von willebrand factor. J Biol Chem. 2007;282:35604–35611. doi: 10.1074/jbc.M704047200. [DOI] [PubMed] [Google Scholar]
- 19.Siedlecki CA, Lestini BJ, Kottke-Marchant KK, Eppell SJ, Wilson DL, Marchant RE. Shear-dependent changes in the three-dimensional structure of human von Willebrand factor. Blood. 1996;88:2939–2950. [PubMed] [Google Scholar]
- 20.Li Y, Choi H, Zhou Z, Nolasco L, Pownall HJ, Voorberg J, Moake JL, Dong JF. Covalent regulation of ULVWF string formation and elongation on endothelial cells under flow conditions. J Thromb Haemost. 2008;6:1135–1143. doi: 10.1111/j.1538-7836.2008.02991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie L, Chesterman CN, Hogg PJ. Control of von Willebrand factor multimer size by thrombospondin-1. J Exp Med. 2001;193:1341–1349. doi: 10.1084/jem.193.12.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pimanda JE, Ganderton T, Maekawa A, Yap CL, Lawler J, Kershaw G, Chesterman CN, Hogg PJ. Role of thrombospondin-1 in control of von Willebrand factor multimer size in mice. J Biol Chem. 2004;279:21439–21448. doi: 10.1074/jbc.M313560200. [DOI] [PubMed] [Google Scholar]
- 23.Frangos JA, Moake JL, Nolasco L, Phillips MD, McIntire LV. Cryosupernatant regulates accumulation of unusually large vWF multimers from endothelial cells. Am J Physiol. 1989;256:H1635–H1644. doi: 10.1152/ajpheart.1989.256.6.H1635. [DOI] [PubMed] [Google Scholar]
- 24.Tao Z, Peng Y, Nolasco L, Cal S, Lopez-Otin C, Li R, Moake JL, Lopez JA, Dong JF. Recombinant CUB-1 domain polypeptide inhibits the cleavage of ULVWF strings by ADAMTS13 under flow conditions. Blood. 2005;106:4139–4145. doi: 10.1182/blood-2005-05-2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest. 1986;78:1456–1461. doi: 10.1172/JCI112736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tao Z, Anthony K, Peng Y, Choi H, Nolasco L, Rice L, Moake JL, Dong JF. Novel ADAMTS-13 mutations in an adult with delayed onset thrombotic thrombocytopenic purpura. J Thromb Haemost. 2006;4:1931–1935. doi: 10.1111/j.1538-7836.2006.02098.x. [DOI] [PubMed] [Google Scholar]
- 27.Turner N, Nolasco L, Dong JF, Moake J. ADAMTS-13 cleaves long von Willebrand factor multimeric strings anchored to endothelial cells in the absence of flow, platelets or conformation-altering chemicals. J Thromb Haemost. 2009;7:229–232. doi: 10.1111/j.1538-7836.2008.03209.x. [DOI] [PubMed] [Google Scholar]
- 28.Grassetti DR, Murray JF., Jr Determination of sulfhydryl groups with 2,2′- or 4,4′-dithiodipyridine. Arch Biochem Biophys. 1967;119:41–49. doi: 10.1016/0003-9861(67)90426-2. [DOI] [PubMed] [Google Scholar]
- 29.Grassetti DR, Murray JF., Jr The use of 2,2′-dithiobis-(5-nitropyridine) as a selective reagent for the detection of thiols. J Chromatogr. 1969;41:121–123. doi: 10.1016/0021-9673(64)80109-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhang JL, Qiu LY, Kotzsch A, Weidauer S, Patterson L, Hammerschmidt M, Sebald W, Mueller TD. Crystal structure analysis reveals how the Chordin family member crossveinless 2 blocks BMP-2 receptor binding. Dev Cell. 2008;14:739–750. doi: 10.1016/j.devcel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 31.Zhang P, Pan W, Rux AH, Sachais BS, Zheng XL. The cooperative activity between the carboxyl-terminal TSP1 repeats and the CUB domains of ADAMTS13 is crucial for recognition of von Willebrand factor under flow. Blood. 2007;110:1887–1894. doi: 10.1182/blood-2007-04-083329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tao Z, Wang Y, Choi H, Bernardo A, Nishio K, Sadler JE, Lopez JA, Dong JF. Cleavage of ultra-large multimers of Von Willebrand factor by C-terminal truncated mutants of ADAMTS-13 under flow. Blood. 2005;106:141–143. doi: 10.1182/blood-2004-11-4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng X, Nishio K, Majerus EM, Sadler JE. Cleavage of von Willebrand factor requires the spacer domain of the metalloprotease ADAMTS13. J Biol Chem. 2003;278:30136–30141. doi: 10.1074/jbc.M305331200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zanardelli S, Chion AC, Groot E, Lenting PJ, McKinnon TA, Laffan MA, Tseng M, Lane DA. A novel binding site for ADAMTS13 constitutively exposed on the surface of globular VWF. Blood. 2009;114:2819–2828. doi: 10.1182/blood-2009-05-224915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savage B, Sixma JJ, Ruggeri ZM. Functional self-association of von Willebrand factor during platelet adhesion under flow. Proc Natl Acad Sci U S A. 2002;99:425–430. doi: 10.1073/pnas.012459599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, Schneider MF. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci U S A. 2007;104:7899–7903. doi: 10.1073/pnas.0608422104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chow TW, Hellums JD, Moake JL, Kroll MH. Shear stress-induced von Willebrand factor binding to platelet glycoprotein Ib initiates calcium influx associated with aggregation. Blood. 1992;80:113–120. [PubMed] [Google Scholar]
- 38.Zhang JN, Bergeron AL, Yu Q, Sun C, McIntire LV, Lopez JA, Dong JF. Platelet Aggregation and Activation under Complex Patterns of Shear Stress. Thromb Haemost. 2002;88:817–821. [PubMed] [Google Scholar]
- 39.Feys HB, Anderson PJ, Vanhoorelbeke K, Majerus EM, Sadler JE. Multi-step binding of ADAMTS-13 to von Willebrand factor. J Thromb Haemost. 2009;7:2088–2095. doi: 10.1111/j.1538-7836.2009.03620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) VWF was incubated with rADAMTS-13 (with and without IAA treatment to block thiols) before and after exposure to 100 dyn/cm2 of shear stress for 3 min at 37°C. VWF was then immunoprecipitated with a polyclonal VWF antibody, subjected to SDS-PAGE under reducing conditions, and immunoblotted with a monoclonal anti-Myc antibody. rADAMTS-13 was not immunoprecipitated by the VWF antibody either before or after exposure to shear stress. rADAMTS-13 (lane 1) was directly loaded on the gel to serve as control. (B) Purified rADAMTS-13 (2.5 μM) was incubated with a 100-fold molar excess of 4, 4′-dithiopyridine and the thiol modification recorded at 324 nm. Six thiols were calculated to be modified within 20 min, but the number of modified thiols increased to ∼26 after 2 hrs incubation in the presence of 5M urea. (C) MPB-labeled ADAMTS-13 was purified from citrate normal human plasma using an ADAMTS-13 antibody coupled affinity column (Aminolink Plus, Thermo Scientific), separated by 6% SDS-PAGE, and detected with streptavidin-HRP. MPB labeling was compared to the standard curve of rADAMTS-13 by densitometry at three different protein loadings. The figure represents 2-4 separate experiments.