Abstract
Pediatric bipolar disorder is a severe and impairing illness. Characterizing the impact of pediatric bipolar disorder on cognitive function might aid in understanding the phenomenology of the disorder. While previous studies of pediatric bipolar disorder have reported deficits in cognitive control and reward behavior, little is understood about how affective processes influence behavioral control. Relative to prior studies using manual-response paradigms, eye movement tasks provide a more precise assessment of reward sensitivity and cognitive and motor control. The current study compares 20 youths with bipolar disorder (mean age = 13.9 years ± 2.22) and 23 healthy subjects (mean age = 13.8 years ± 2.49) on a mixed pro–antisaccade task with monetary incentives. On both types of saccades, participants were presented with three types of incentives: those where subjects can win money, lose money, or neither win nor lose money. Impaired reward processing was found in youths with bipolar disorder relative to controls, particularly on antisaccades. This difference was reflected in lower error rates during incentive trials in the control but not in the bipolar disorder group. By comparison, no group differences were found on prosaccade trials. The results provide further evidence for deficits in cognitive and reward processing in bipolar disorder.
Keywords: bipolar disorder, reward, antisaccade, cognitive control, emotion, ADHD
Introduction
Bipolar disorder (BD) in children and adolescents is a disabling psychiatric illness that impairs social and academic functioning. However, little is understood about the pathophysiology of the disorder. In youths with BD, clear deficits are seen in measures of cognitive control (Bearden et al., 2007; Dickstein et al., 2007; McClure et al., 2005; Pavuluri et al., 2006). For example, we found that patients with BD had difficulty inhibiting a prepotent response and substituting an alternate one (Dickstein et al., 2007; McClure et al., 2005), evidencing deficits in shifting cognitive ‘set’ and impaired inhibitory control. In a functional magnetic resonance imaging (fMRI) study of the stop-signal task, Leibenluft et al. (2007) found that relative to controls, youths with BD demonstrated distinct neural activation patterns associated with failed inhibitory processes. Patients with BD also are impaired on other neuropsychological measures of cognitive control such as set shifting, working memory, and verbal learning (Pavuluri et al., 2006).
In addition, preliminary studies suggest deficits of reward-related processes in youths with BD relative to healthy controls (Dickstein et al., 2004; Dickstein et al., 2007; Gorrindo et al., 2005; Rich et al., 2007). Whereas studies indicate reward deficits in youths with BD on attention tasks (Rich et al., 2007) and response reversal tasks (Dickstein et al., 2004; Dickstein et al., 2007; Gorrindo et al., 2005), no performance impairment was found on an affect-modulated startle task (Rich et al., 2005), or reward-associated decision-making tasks (Ernst et al., 2004; Rau et al., 2008). The most convincing finding for deficits in reward processing in pediatric BD emerged on an affective variant of the Posner attention task. Specifically, Rich et al. (2007) reported that children with BD differed from controls in failing to improve their performance under incentives, particularly negative incentives (avoiding punishment). While that study paired incentive conditions with a task measuring attentional processing, here we examine inhibitory control processes and their modulation by incentives in BD. We used a saccadic eye movement task, since such tasks provide exquisite measures of control processes that are sensitive to age and incentives (Hardin et al., 2007; Jazbec et al., 2005; Leigh and Kennard, 2004; Luna et al., 2001; Mueller et al., 2006). The antisaccade task requires the inhibition of a prepotent saccade (the prosaccade) to a peripherally appearing stimulus and the execution of an eye movement in the opposite direction (Munoz and Everling, 2004).
The aim of the current study was to compare youths with BD and healthy controls on a modified version of the monetary incentive antisaccade task (Jazbec et al., 2005). With respect to reward processes, we expected to find reduced sensitivity to incentives in BD, based on data from the affective Posner task (Rich et al., 2007). Also, in a previous study of healthy adults and adolescents, incentive effects tended to be larger on antisaccade than prosaccade trials (Jazbec et al., 2006). Thus, we hypothesized that incentives would impact antisaccade performance (as measured by latency and accuracy) in controls but not patients with BD.
Methods
Participants
Twenty youths with BD and 23 healthy youths completed the study. The participants were enrolled in an ongoing research protocol of the National Institute of Mental Health (NIMH). Patients were recruited through advertisements placed in local parenting magazines, on support groups’ websites, and distributed to psychiatrists nationwide. Participants for the healthy control group were recruited by advertisements in the local newspapers. The study was approved by the Institutional Review Board of the NIMH. Parents provided written informed consent, and youths’ written informed assent, prior to participation. Diagnoses were made using the Kiddie Schedule for Affective Disorders and Schizophrenia for school-age children (K-SADS) (Kaufman et al., 1997) administered to children and parents by clinicians with established inter-rater reliability (k > 0.90). Additionally, the Children's Depression Rating Scale (CDRS) (Emslie et al., 1990) and the Young Mania Rating Scale (YMRS) (Young et al., 1978) were completed by patients and their parents. Measures of IQ were collected with the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). Youths with BD met ‘narrow phenotype’ criteria, in that all patients had a history of at least one hypomanic or manic episode (≥ 4 days of hypomania or ≥7 days for mania) with abnormally elevated mood or grandiosity, and at least three symptoms listed under criterion ‘B’ for manic episodes. Exclusion criteria for BD group were defined by an IQ < 80, unstable medical illness, substance abuse within the past two months, pregnancy, or co-morbid neurological disorders. Inclusion criteria for control subjects consisted of no prior or current history of psychiatric or medical disorders based on K-SADS interview with parent and child separately and IQ > 80.
Apparatus
Eye movements were recorded from the left eye using a remote mounted eye tracker with a sampling rate of 60 Hz (Applied Science Laboratories; ASL Inc., Bedford, MA). Participants were required to make one of two eye movements: either an eye movement towards (prosaccades) or an eye movement away (antisaccades) from a white target asterisk that was subtending 0.5° in visual angle (Figure 1).
Figure 1.
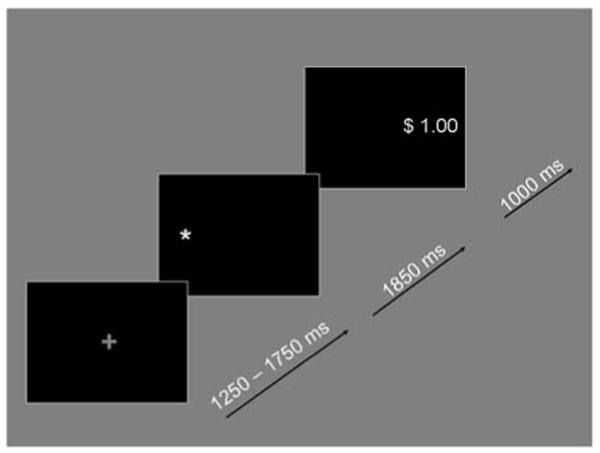
Example of a trial in which an antisaccade is required during the reward condition (grey plus sign) followed by the white target asterisk and the correct feedback location with positive feedback assuming a correct eye movement.
In addition, each eye movement was paired with one of three incentive conditions. Prior to the target, a cue indicated the type of incentive and the type of saccade participants had to perform. If the color of the cue was grey an antisaccade was required. If it was white, participants had to execute a prosaccade. The cue itself consisted of either a ‘+’, a ‘−’, or a ‘0′. During the reward condition a ‘+’ sign indicated that participants could win $1 if they executed a correct eye movement, in the punishment condition a ‘−’ sign indicated that they would lose $1 for an incorrect eye movement, and in the neutral or no incentive condition a ‘0′ indicated no gain or punishment regardless of whether the eye movement was correct or incorrect. In order to examine potential differences of valence, statistical analyses directly compared reward and punishment conditions. No significant effect of valence was found (F(1,46) = 0.086, ns). Consequently, to increase statistical power, positive and negative incentives were pooled during the main analysis. Participants were given immediate feedback as to whether they had won $1 (displayed in green) or lost $1 (displayed in red). Stimuli were presented on a black background. Each trial started with the cue, which was presented for 1250–1750 ms and which indicated the type of incentive and the type of saccade, both of which were randomized. Once the cue disappeared, the white target asterisk was displayed for 1850 ms on either the left-hand or right-hand side approximately 6.15° to the centrally presented cue. Then, the feedback display (subtending roughly 1.8° in visual angle) was presented for 1000 ms on the side where the correct eye movement should have occurred.
Procedure
Children were seated in a chair about 26 inches away from the screen in an illuminated room with standard fluorescent lights. A chin-rest was used to minimize movement. However, in addition to the chin-rest, a magnetic head tracker (Ascension Technology Inc., Milton, VT) was also used to ensure uninterrupted recording of eye movements if children moved their heads. Participants completed three runs of 48 randomly intermixed trials (= 144 total). Subjects started with $0.00 and could win up to $4.80 per run. In between runs, participants were re-calibrated if necessary. Participants were then sent a check for the amount of their winnings.
Statistical analysis
Two saccade parameters were analyzed: accuracy (overall level of performance, as measured by percent of trials that were errors) and latency (response time of correct responses in ms). A correct response was defined by its direction and latency. Anticipatory (latency < 80 ms) or late (latency > 700 ms) saccades, i.e. judged to not be in response to the target, were discarded. Saccade threshold criterion was set at 30/s. Each saccade parameter was entered separately into a three-way repeated measures analysis of variance (ANOVA), with group (Controls vs. BD) as the one between-subjects factor and with two within-subjects factors: saccade type (prosaccades vs. antisaccades) and incentive (incentive vs. no incentive). Our hypothesis that incentives would enhance performance in controls, but not patients, on antisaccade trials only would be confirmed by the presence of a significant three-way interaction for our two variables of interest (accuracy and latency).
For those clinical characteristics that differed between patients and controls, we conducted a series of post-hoc analyses to examine the potential impact of mood state, medications, and comorbid illnesses. To test whether mood state could account for our results, we compared euthymic patients (n = 9) with controls. We also conducted correlational analyses between YMRS and CDRS scores and the variables showing significant between-group effects. To account for possible medication effects, repeated measures ANOVAs were performed with the same within-group factors as in the main analyses (saccade type by incentive) but with medication status (on vs. off a particular category of medication) as the between-group factor. We also examined Pearson's product-moment coefficient correlations between saccadic performance and each subject's number of prescribed medications. To ensure that our findings were not confounded by comorbid illnesses, the main analyses were repeated twice: once after removing patients with comorbid attention-deficit hyperactivity disorder (ADHD), and once after removing patients with comorbid anxiety disorder.
Results
Sample
Sample characteristics are detailed in Table 1. Briefly, BD and controls did not differ on age, sex, or IQ. Among BD, 1 of 20 (5%) was currently depressed (YMRS ≤ 12 and CDRS ≥ 40), 8 of 20 (40%) were euthymic (YMRS ≤ 12 and CDRS < 40), 3 of 20 (15%) were hypomanic (YMRS = 13–24 and CDRS < 40) and 4 of 20 (20%) were mixed (YMRS ≥ 12 and CDRS ≥ 40). No patient was manic (YMRS ≥ 25 and CDRS < 40). The most common comorbid illnesses (Table 1) were ADHD (50%), Anxiety Disorders (30%), and Oppositional Defiant Disorder (35%). At time of testing, 10% of the patients (n = 2) were medication-free.
Table 1. Demographic and clinical characteristics for the patient and control groups.
| Control (n = 23) | Bipolar (n = 20) | |
|---|---|---|
| Gender* (% male) | 43.48 | 65.00 |
| Age* (years; mean, SD) | 13.80 (2.49) | 13.90 (2.22) |
| IQ* (mean, SD) | 114.27 (12.78) | 107.40 (14.14) |
| Ethnicity* | ||
| Caucasian | 18 | 16 |
| African American | 3 | 1 |
| Mixed race | 2 | 3 |
| YMRS (mean, SD) | - | 12.20 (6.61) |
| CDRS (mean, SD) | - | 30.95 (10.14) |
| Comorbid diagnoses (n / %) | - | |
| ADHD | - | 10 (50) |
| Anxiety disorders# | - | 8 (40) |
| ODD | - | 7 (35) |
| Current Medications (n / %) | ||
| Number of medications (Mean/SD) | - | 3.80 (2.63) |
| None | - | 2 (10) |
| Lithium | - | 6 (30) |
| Stimulants | - | 8 (40) |
| Antidepressants | - | 8 (40) |
| Atypical antipsychotics | - | 14 (70) |
| Anticonvulsants | - | 16 (80) |
No difference in IQ (t(40) = 1.65, p = 0.11) or Age (t(41) = −0.14, p = 0.89), gender (x2(1) = 1.58, p = 0.16), or ethnicity (x2(2) = 1.11, p = 0.57).
Generalized anxiety disorder, social anxiety disorder, and/or social phobia.
ADHD: Attention-deficit hyperactivity disorder; CDRS: Children's Depression Rating Scale; ODD: Oppositional Defiant Disorder; SD: standard deviation; YMRS: Young Mania Rating Scale.
Saccades
Accuracy
As hypothesized, there was a significant three-way interaction of incentive, saccade and group (F(1,41) = 6.44, p = 0.015) Figure 2. The two-way incentive by group interaction was also significant (F(1,41) = 4.79, p < 0.05). A significant main effect of group indicated higher accuracy rates for the control group relative to the BD group (F(1,41) = 5.98, p = 0.02) Figure 2. In addition, the main effect of saccade (F(1,41) = 133.35, p < 0.001) indicated more correct prosaccades than antisaccades. To understand the three-way interaction, ANOVAs were conducted for the two different saccade types separately.
Figure 2.
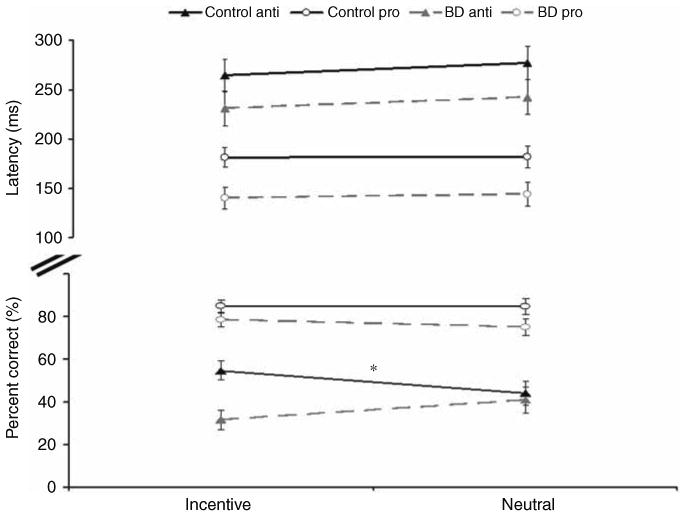
Figure shows the significant three-way interaction in accuracy and displays group means for latencies (in ms, upper panel) and accuracy rates (in %, lower panel) split according to group, saccade and incentive type. Error bars denote standard error. Asterisk indicates significant effect of incentive in the control group in the accuracy rates.
For accuracy on antisaccade trials, results revealed a significant interaction of incentive and group (F(1,41) = 8.06, p < 0.01). This interaction reflected an improvement with incentives for controls (t(22) = 3.02, p < 0.01) (from 44.2% correct for neutral trials to 54.7% correct for incentive trials), but no effect of incentives on performance in BD (40.8% correct for neutral trials and 31.6% correct for incentive trials) (t(19) = 1.47, p = 0.15). The group main effect was also significant (F(1,41) = 4.24, p <0.05), indicating a higher number of correct antisaccades for the control group (48.7%) relative to BD (35.3%).
For prosaccades, the analysis failed to reveal a significant interaction between group and incentive (F(1,41) = 0.47, ns) and instead revealed a trend toward a main effect of group indicating slightly lower accuracy for BD than controls (F(1,41) = 3.57, p = 0.066). These findings suggest that controls improve their performance selectively on antisaccades when incentives are introduced, but subjects with BD do not.
Latency
For latency, the three-way interaction between group, incentive and saccade was not significant (F(1,41) = 0.09, ns) Figure 2. However, there was a significant main effect of group indicating faster latencies for the BD than the control group (F(1,41) = 4.12, p < 0.05). As expected, antisaccades were characterized by slower latencies than prosaccades (F(1,41) = 177.18, p < 0.001). There were no significant main effects of incentive (F(1,41) = 2.18, ns) or interactions with group (group by incentive: F(1,41) = 0.02, ns; group by saccade: F(1,41) = 0.18, ns).
Compared with controls, patients exhibited both overall faster latencies and lower accuracy rates. This performance pattern may be explained by a speed–accuracy trade-off. To examine this possibility, we performed correlations between latencies and accuracy rates for neutral antisaccades and incentive antisaccades, examining each group separately. There was no significant correlation between accuracy rates and incentive trials (BD: r2(20) = 0.11, p = 0.64, controls: r2(23) = −0.1, p = 0.56). For neutral trials, there was a negative correlation between accuracy rates and latency for BD (r2(20) = −0.51, p = 0.02), but not for controls (r2(23) = −0.09, p = 0.68). However, the negative correlation (shorter latency associated with higher accuracy) argued against a speed–accuracy trade-off.
Associations with co-morbid illnesses, medication, and mood state
To examine the potential impact of mood state, medication and co-morbidity on the present results, we performed additional post-hoc analyses on antisaccade accuracy. The direct comparison of euthymic patients vs. controls revealed the same group by incentive by saccade interaction as the main analyses (F(1,30) = 8.11, p < 0.01), as well as a significant incentive by group interaction (F(1,30) = 6.84, p = 0.01) and a significant main effect of saccade (F(1,30) = 89.97, p < 0.001). There were also no significant correlations of antisaccade accuracy with YMRS (r2(22) < 0.21, p > 0.69) or CDRS (r2(22) < −0.13, p >0.55) scores. These data suggest that mood state did not account for the findings in the primary analysis.
With regards to medication effects, there was no significant correlation between antisaccade accuracy and the number of prescribed medications (r2(22) < −0.20, p > 0.37). In addition, a direct comparison between antisaccade performance and medication status (patients on vs. off a given class of medication) did not reveal any significant performance differences for lithium, stimulants, antidepressants, antipsychotics or anticonvulsants (all F(1,9) ≤ 4.29, p ≥ 0.068). However, these analyses were relatively underpowered because of the small number of patients in each group.
To ensure that findings were not driven by comorbid disorders, the analyses were repeated first without the patients with comorbid ADHD (n = 10), then without the patients with comorbid anxiety (n = 14), and finally without patients with comorbid psychotic features (n = 16). The findings remained significant in all analyses. Without comorbid anxiety: group by incentive by saccade interaction: (F(1,35) = 5.71, p < 0.05; group by incentive: F(1,35) = 4.34, p < 0.05); without comorbid ADHD: a trend for group by incentive by saccade (F(1,31) = 3.61, p = 0.067; group by incentive F(1,31) = 4.60, p < 0.05); without comorbid psychosis: group by incentive by saccade: (F(2,74) = 6.58, p = 0.002; group by incentive F(2,74) = 2.38, p = 0.099). These findings suggest that the observed effects were not driven by either co-morbid anxiety, co-morbid ADHD, or co-morbid psychosis.
Discussion
A saccadic eye-movement task was used to examine core cognitive and reward processes in children meeting DSM-IV criteria for BD. Findings revealed that, in contrast to the healthy control group, the BD group did not improve their antisaccade performance under incentives. This deficit did not appear to be accounted for by mood state, medication, or comorbid illnesses. On prosaccade trials, neither group improved their performance under incentives.
Previous studies using an affective version of the Posner task reported lack of incentive-related performance improvement, i.e. lack of shortened reaction time, measured after trials that resulted in punishment (Rich et al., 2007). Likewise, in the current study, patients with BD differed from controls in that they did not improve their antisaccade accuracy in response to incentives. Also, as noted in the introduction, antisaccade tasks have similar task demands as the stop-signal-change task, which has been used previously in youths with BD. In the stop-signal-change task, a colored cue indicates that a prepotent response has to be inhibited (similar to the inhibition of a prosaccade) and an alternative one substituted (similar to the generation of an antisaccade). Previous findings on the stop-signal-change task, as well as on tasks requiring simple and complex response-reversals (Dickstein et al., 2007) demonstrated impaired performance in children with BD relative to controls, i.e. slowed change response times and higher error rates. Similarly, here the BD group also exhibited impairments on a task requiring both inhibition and the execution of an alternative response, as seen in lower accuracy rates on antisaccade trials relative to control children. Thus, performance on the saccade task revealed incentive-related deficits in cognitive control in children with BD relative to their healthy peers. These data extend previous findings in both attentional tasks and tasks requiring manual response reversals to situations that require strong cognitive control within a reward-driven context in the oculomotor domain.
The restriction of the incentive effect to antisaccade in healthy subjects suggests that incentives affect tasks that require strong cognitive control as opposed to responses to prepotent stimuli. An absence of an incentive effect during antisaccades in the patient group thus indicates a deficit in the circuits that mediate incentive-related facilitation of cognitive control. In fact, the BD group exhibited slightly higher, albeit non-significant, accuracy rates in the neutral relative to the incentive condition, which may suggest a potential interference effect of incentives on motivational behavior rather than an increase in motivation. However, the failure to detect an incentive effect on prosaccade trials in either group might reflect a floor effect, i.e. no room for improvement. Future studies will need to clarify this issue.
Previous studies of eye movements in adult patients with BD have reported increased antisaccade errors for BD relative to controls (Tien et al., 1996) and impaired pursuit eye movements (Sweeney et al., 1999), but no differences in saccadic latencies (Fukushima et al., 1990). Consistent with the findings by Tien et al. (1996), youths with BD in the present study exhibited higher error rates relative to controls; however, unlike previous report, we found decreased saccadic latencies in patients with BD. Although we could not find a specific effect of a potential speed–accuracy trade-off on the reward manipulation, it is possible that youths with BD generally responded faster due to a lack of inhibitory control, resulting in increased impulsive responding.
Prior studies have found some evidence of perturbed reward processing in pediatric mood disorders (Dickstein et al., 2004; Dickstein et al., 2007; Forbes et al., 2007). Studies in youths with BD suggest that patients only exhibit deficits in reward processing in the presence (Gorrindo et al., 2005) but not absence (Rau et al., 2008) of a concurrent response flexibility condition, and indicates that youths with BD do not exhibit problems in learning stimulus-reward associations per se. In a previous study of children with anxiety or depression that used the same eye movement paradigm as here, both patients and controls exhibited reduced error rates with incentives during antisaccade trials (Jazbec et al., 2005). These findings suggest that BD presents deficits in responses to incentives that are not seen in unipolar depression or anxiety disorders.
The current data need to be interpreted with caution, particularly in view of the potential confounding factors of medication and comorbidity. First, a possible contribution of pharmacological treatment to the effects reported in this study cannot be ruled out, given that previous studies found performance improvement, i.e. reduced antisaccade error rates, with atypical antipsychotic medication (Burke and Reveley, 2002) and speeded prosaccade response times with anticonvulsants (Remler et al., 1990; Tedeschi et al., 1989). Although we could not detect effects of medication on antisaccade performance, conclusions from these post-hoc analyses have to be considered tentative. Second, most patients also exhibited comorbid disorders. For example, half of the current sample exhibited co-morbid ADHD; ADHD has also been found to be associated with perturbed antisaccade performance (Klein et al., 2003). However, patients with ADHD commonly show reduced peak velocities (Munoz et al., 2003) and prolonged latencies (Klein et al., 2003; Munoz et al., 2003). In the present study, patients with BD exhibited faster, not slower, latencies relative to controls. Moreover, when we directly compared affected children without ADHD with healthy peers, while the three-way interaction was reduced to a trend, the group by incentive interaction remained significant, suggesting that our results were not driven by comorbid ADHD. Similarly, we could not detect effects of comorbid anxiety on the present findings. To formally evaluate the possible effects of comorbid disorders or medication on cognitive function, future studies should include a comparison group of ADHD patients and/or medication-free patients. Finally, given the relatively small sample size of BD subjects and a restricted range of symptom scores, it seems unlikely that we had sufficient statistical power to detect symptom correlations with performance.
In summary, the current data suggest that patients with BD evidence deficits in reward processing specifically in interaction with inhibitory control. The antisaccade task appears to be a useful paradigm that is sensitive to perturbations of cognitive control and reward processing in pediatric BD. The next step will be to investigate the neural correlates of these perturbations using fMRI.
Acknowledgments
This work was supported by the intramural program of the NIMH, NIH.
Footnotes
Reprints and permissions: sagepub.co.uk/journalsPermissions.nav
Conflict of interest: None of the authors has a conflict of interest to declare.
References
- Bearden CE, Glahn DC, Caetano S, et al. Evidence for disruption in prefrontal cortical functions in juvenile bipolar disorder. Bipolar Disord. 2007;9(Suppl 1):145–159. doi: 10.1111/j.1399-5618.2007.00453.x. [DOI] [PubMed] [Google Scholar]
- Burke JG, Reveley MA. Improved antisaccade performance with risperidone in schizophrenia. J Neurol Neurosurg Psychiatry. 2002;72:449–454. doi: 10.1136/jnnp.72.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DP, Nelson EE, McClure EB, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Treland JE, Snow J, et al. Neuropsychological performance in pediatric bipolar disorder. Biol Psychiatry. 2004;55:32–39. doi: 10.1016/s0006-3223(03)00701-7. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Weinberg WA, Rush AJ, Adams RM, Rintelmann JW. Depressive symptoms by self-report in adolescence: phase I of the development of a questionnaire for depression by self-report. J Child Neurol. 1990;5:114–121. doi: 10.1177/088307389000500208. [DOI] [PubMed] [Google Scholar]
- Ernst M, Dickstein DP, Munson S, et al. Reward-related processes in pediatric bipolar disorder: a pilot study. J Affect Disord. 2004;82(Suppl 1):S89–S101. doi: 10.1016/j.jad.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biol Psychiatry. 2007;61:633–639. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Fukushima J, Morita N, Fukushima K, Chiba T, Tanaka S, Yamashita I. Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. J Psychiatr Res. 1990;24:9–24. doi: 10.1016/0022-3956(90)90021-h. [DOI] [PubMed] [Google Scholar]
- Gorrindo T, Blair RJ, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am J Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. J Child Psychol Psychiatry. 2007;48:446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Exp Brain Res. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biol Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Klein CH, Raschke A, Brandenbusch A. Development of pro- and antisaccades in children with attention-deficit hyperactivity disorder (ADHD) and healthy controls. Psychophysiology. 2003;40:17–28. doi: 10.1111/1469-8986.00003. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am J Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Kennard C. Using saccades as a research tool in the clinical neurosciences. Brain. 2004;127:460–477. doi: 10.1093/brain/awh035. [DOI] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Munoz DP, et al. Maturation of widely distributed brain function subserves cognitive development. Neuroimage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- McClure EB, Treland JE, Snow J, et al. Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1644–1651. doi: 10.1176/appi.ajp.162.9.1644. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Jackson GM, Dhalla R, Datsopoulos S, Hollis CP. Enhanced cognitive control in young people with Tourette's syndrome. Curr Biol. 2006;16:570–573. doi: 10.1016/j.cub.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Armstrong IT, Hampton KA, Moore KD. Altered control of visual fixation and saccadic eye movements in attention-deficit hyperactivity disorder. J Neurophysiol. 2003;90:503–514. doi: 10.1152/jn.00192.2003. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Schenkel LS, Aryal S, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am J Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- Rau G, Blair KS, Berghorst L, et al. Processing of differentially valued rewards and punishments in youths with bipolar disorder or severe mood dysregulation. J Child Adolesc Psychopharmacol. 2008;18:185–196. doi: 10.1089/cap.2007.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remler BF, Leigh RJ, Osorio I, Tomsak RL. The characteristics and mechanisms of visual disturbance associated with anticonvulsant therapy. Neurology. 1990;40:791–796. doi: 10.1212/wnl.40.5.791. [DOI] [PubMed] [Google Scholar]
- Rich BA, Bhangoo RK, Vinton DT, et al. Using affect-modulated startle to study phenotypes of pediatric bipolar disorder. Bipolar Disord. 2005;7:536–545. doi: 10.1111/j.1399-5618.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psychiatry. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Haas GL, Keshavan MS, Mann JJ, Thase ME. Pursuit tracking impairments in schizophrenia and mood disorders: step-ramp studies with unmedicated patients. Biol Psychiatry. 1999;46:671–680. doi: 10.1016/s0006-3223(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Tedeschi G, Casucci G, Allocca S, et al. Computer analysis of saccadic eye movements: assessment of two different carbamazepine formulations. Eur J Clin Pharmacol. 1989;37:513–516. doi: 10.1007/BF00558133. [DOI] [PubMed] [Google Scholar]
- Tien AY, Ross DE, Pearlson G, Strauss ME. Eye movements and psychopathology in schizophrenia and bipolar disorder. J Nerv Ment Dis. 1996;184:331–338. doi: 10.1097/00005053-199606000-00001. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; 1999. [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]


