Abstract
Solitary rectal ulcer syndrome (SRUS) is a rare condition in children. We report a case of SRUS in an 8-year old Saudi girl who presented with recurrent rectal bleeding, intermittent mucosal prolapse, and passage of mucus per rectum. Colonoscopy revealed multiple polypoid mass lesions with histopathological features of SRUS. The polypoid variant of SRUS is very rare in children and may be confused with rectal malignant or inflammatory conditions.
Keywords: Polypoid, Rectal prolapse, Rectal bleeding, Child, Solitary rectal ulcer syndrome, Saudi Arabia
INTRODUCTION
Solitary rectal ulcer syndrome (SRUS) is a rare benign disease of the rectum, which predominately affects young adults aged between 30 and 50 years with a prevalence of 1 in 100 000 people per year[1,2]. SRUS usually presents with a symptom complex of rectal bleeding, passage of mucus and straining on defecation, tenesmus, perineal and abdominal pain, sensation of incomplete defecation, constipation and rectal prolapse[3]. SRUS is rare in children and its description is largely limited to case reports[4-14]. The underlying etiology of SRUS is not fully understood but it is likely to be secondary to ischemic changes in the rectum associated with paradoxical contraction of the pelvic floor and external anal sphincter muscles and with rectal prolapse[15]. The macroscopic appearance of the rectal lesion may vary from hyperemia to ulceration or a polypoid lesion that can mimic carcinoma[16], although the histological findings are characteristic, with fibromuscular obliteration of the lamina propria and disorientation of muscle fibers[17]. We report the case of a young girl who presented with a polypoid mass lesion of the rectum representing a SRUS variant.
CASE REPORT
An 8-year old Saudi girl was referred to our pediatric gastroenterology clinic with a 2-year history of recurrent rectal bleeding, passage of mucus, and intermittent rectal prolapse during defecation. In spite of receiving regular lactulose, the bleeding had not resolved. There was no history of fecal incontinence or self-digitation, nor of weight loss, fever, arthralgia, skin rash, abdominal pain, change in appetite or daily activity, or bleeding. The results of physical examination were unremarkable apart from pallor. Digital rectal examination revealed an irregular broad based polypoid lesion palpated on the rectum about 5 cm from the anal verge. Her anthropometric measurements were at the 25th percentile for weight and 50th percentile for height. The laboratory findings revealed hypochromic and microcytic anemia (hemoglobin 6.7 g/dL, hematocrit 23 %, mean corpuscular volume 54 fl, mean cell hemoglobin 15.6 pg, platelets count 704 × 103/mm3), normal erythrocyte sedimentation rate (15 mm/h), and normal coagulation profile. White blood cell count was 10 600/mm3; liver function tests, and serum proteins were normal. Perinuclear antineutrophil cytoplasmic antibody and anti-saccharomyces cerevisiae antibody were negative. Stool examination for ova, parasites, and cultures were repeatedly negative. Colonoscopy revealed multiple polypoid mass lesions in the rectum located at 5 cm from the anal verge with circumferential distribution. The mucosal surface of these lesions was ulcerated and covered with exudates. The surrounding mucosa was smooth with absence of the normal vascular pattern (Figure 1A and B). The remaining colon up to the cecum was normal. Several mucosal biopsies were obtained from the lesions. Histopathological examination revealed focal ulcerations of the lining mucosa with granulation tissue formation. There was smooth muscle fiber expansion between glands up to the submucosa which was perpendicular to the glands (Figure 2A and B). There was no evidence of cryptitis or crypt abscesses. The crypt architecture was maintained, with no findings of granuloma, atypia or malignancy.
Figure 1.
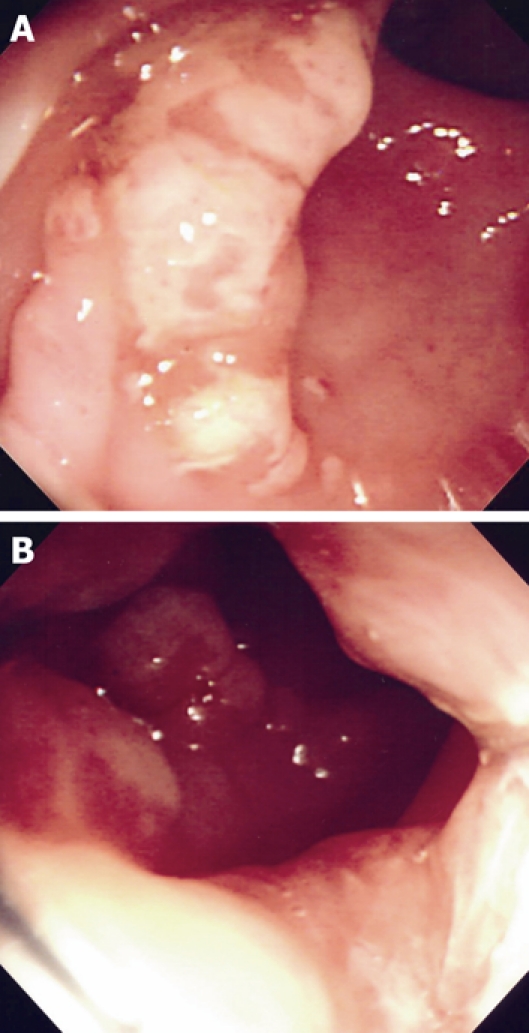
Colonoscopic examination. A: Polypoid mass with surface ulceration and surrounding mucosal erythema; B: Multiple polypoid mass lesions at the rectum.
Figure 2.
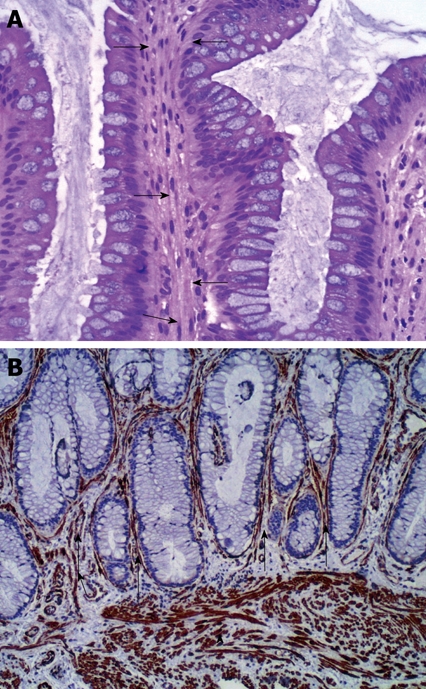
Histopathological examination. A: The rectal mucosa showing smooth muscle fibers proliferation perpendicular to the muscularis mucosa and extending between the glands (arrows) (HE stain × 40); B: Smooth muscle proliferation in the muscularis mucosa (arrow head as internal control)) and extending in between the mucosal glands (arrows) (Immunohistochemistry, smooth muscle actin, × 100).
Following the diagnosis of SRUS, general measures to reduce straining during defecation, were commenced as well as a stool softener (Macrogol 3350). Subsequent trials of corticosteroid and mesalazine enemas produced no improvement. She has recently been commenced on sucralfate enemas prior to rectopexy.
DISCUSSION
SRUS is rarely reported in children because it is difficult to recognize both the macroscopic and histopathological changes during childhood[3]. Even in adults the condition may go unrecognized or, more commonly, misdiagnosed for several years[18]. A prolonged period of misdiagnosis may have important consequences, such as anemia secondary to massive bleeding or poor appetite in a growing child[1]. This patient had low hemoglobin that required blood transfusion. Anemia is not consistently present in SRUS[4-14]. The severity of blood loss, the duration of the disease, as well as local factors related to the lesion may influence the development of anemia.
The clinical presentation of SRUS is diverse. Patients commonly present with obstructed defecation, rectal bleeding or prolapsed rectal mucosa either overt or occult[3]. Histopathological examination is key to the diagnosis of SRUS. A combination of fibromuscular obliteration of the lamina propria, crypt distortion, and surface serration can establish the diagnosis in most cases[16].
In adults, 25%-32% of SRUS may appear as polypoid lesions[5,19]. The SRUS-polypoid variant may lead to serious misdiagnosis as its appearance may be confused with an inflammatory polyp, hyperplastic polyps, or rectal carcinoma[19,20]. Our patient had multiple polypoid lesions that were circumferential with an ulcerated surface that mimiced rectal cancer in its appearance. Among the cases reported in children, the polypoid variant is very rare and has previously been reported in only two patients[6,11].
Rectal prolapse is associated with 16%-59% of SRUS in adults[1,2]. Our patient also had intermittent rectal prolapse, as previously reported in children with SRUS[6,9,11,21]. Rectal prolapse may be occult, and defecography may help in its diagnosis[7].
Therapeutic experience in children with SRUS, is limited, with widely varying reported treatment protocols and poorly documented clinical outcomes. Conservative measures have included avoidance of straining, use of high fiber diet and intermittent use of laxatives. Local sucralfate, sulfasalazine or steroid enemas have been reported to be effective[1,11,14]. Children with overt rectal prolapse who failed medical treatment may benefit from rectopexy[6,11,21].
In conclusion, the presence of a rectal polypoid mass with ulceration in a child with obstructed defecation and rectal bleeding should raise the suspicion of SRUS. Clinicians and surgical pathologists should be aware of the features of SRUS, so that it is not confused with other conditions.
Acknowledgments
We thank Associate Professor Anthony Catto-Smith, Royal Children Hospital, Australia for editorial assistance
Footnotes
Peer reviewer: Ming-Xu Da, MD, Department of General Surgery, Gansu People’s Hospital, 160 Donggang West Road, Lanzhou 730000, Gansu Province, China
S- Editor Wang JL L- Editor Hughes D E- Editor Yang C
References
- 1.Madigan MR, Morson BC. Solitary ulcer of the rectum. Gut. 1969;10:871–881. doi: 10.1136/gut.10.11.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin CJ, Parks TG, Biggart JD. Solitary rectal ulcer syndrome in Northern Ireland. 1971-1980. Br J Surg. 1981;68:744–747. doi: 10.1002/bjs.1800681021. [DOI] [PubMed] [Google Scholar]
- 3.Keshtgar AS. Solitary rectal ulcer syndrome in children. Eur J Gastroenterol Hepatol. 2008;20:89–92. doi: 10.1097/MEG.0b013e3282f402c1. [DOI] [PubMed] [Google Scholar]
- 4.Sondheimer JM, Slagle TA, Bryke CR, Hill RB. Solitary rectal ulcer syndrome in a teenaged boy. J Pediatr Gastroenterol Nutr. 1985;4:835–838. doi: 10.1097/00005176-198510000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa-Colon R, Younoszai MK, Mitros FA. Solitary ulcer syndrome of the rectum in children. J Pediatr Gastroenterol Nutr. 1989;8:408–412. doi: 10.1097/00005176-198904000-00027. [DOI] [PubMed] [Google Scholar]
- 6.Godbole P, Botterill I, Newell SJ, Sagar PM, Stringer MD. Solitary rectal ulcer syndrome in children. J R Coll Surg Edinb. 2000;45:411–414. [PubMed] [Google Scholar]
- 7.Kiriştioğlu I, Balkan E, Kiliç N, Doğruyol H. Solitary rectal ulcer syndrome in children. Turk J Pediatr. 2000;42:56–60. [PubMed] [Google Scholar]
- 8.Baskonus I, Maralcan G, Gokalp A, Sanal I. Solitary rectal ulcer syndrome: an unusual cause of rectal stricture. Case report. Chir Ital. 2001;53:563–566. [PubMed] [Google Scholar]
- 9.Ertem D, Acar Y, Karaa EK, Pehlivanoglu E. A rare and often unrecognized cause of hematochezia and tenesmus in childhood: solitary rectal ulcer syndrome. Pediatrics. 2002;110:e79. doi: 10.1542/peds.110.6.e79. [DOI] [PubMed] [Google Scholar]
- 10.Martín de Carpi J, Vilar P, Varea V. Solitary rectal ulcer syndrome in childhood: a rare, benign, and probably misdiagnosed cause of rectal bleeding. Report of three cases. Dis Colon Rectum. 2007;50:534–539. doi: 10.1007/s10350-006-0720-1. [DOI] [PubMed] [Google Scholar]
- 11.Dehghani SM, Haghighat M, Imanieh MH, Geramizadeh B. Solitary rectal ulcer syndrome in children: a prospective study of cases from southern Iran. Eur J Gastroenterol Hepatol. 2008;20:93–95. doi: 10.1097/MEG.0b013e3282f1cbb6. [DOI] [PubMed] [Google Scholar]
- 12.K C S, Sharma S, Basnet B, Mishra AK. Solitary rectal ulcer syndrome: uncommon cause of rectal bleeding in children. JNMA J Nepal Med Assoc. 2008;47:238–240. [PubMed] [Google Scholar]
- 13.Pai RR, Mathai AM, Magar DG, Tantry BV. Solitary rectal ulcer syndrome in childhood. Trop Gastroenterol. 2008;29:177–178. [PubMed] [Google Scholar]
- 14.Kumar M, Puri AS, Srivastava R, Yachha SK. Solitary rectal ulcer in a child treated with local sulfasalazine. Indian Pediatr. 1994;31:1553–1555. [PubMed] [Google Scholar]
- 15.Mackle EJ, Parks TG. The pathogenesis and pathophysiology of rectal prolapse and solitary rectal ulcer syndrome. Clin Gastroenterol. 1986;15:985–1002. [PubMed] [Google Scholar]
- 16.Wong WM, Lai KC, Shek TW, Lam SK. Self-inflicted rectal ulcer with exuberant granulation tissue: a lesion that mimics carcinoma. Gastrointest Endosc. 2002;55:951–952. doi: 10.1067/mge.2002.124556. [DOI] [PubMed] [Google Scholar]
- 17.Malik AK, Bhaskar KV, Kochhar R, Bhasin DK, Singh K, Mehta SK, Datta BN. Solitary ulcer syndrome of the rectum--a histopathologic characterisation of 33 biopsies. Indian J Pathol Microbiol. 1990;33:216–220. [PubMed] [Google Scholar]
- 18.Tjandra JJ, Fazio VW, Petras RE, Lavery IC, Oakley JR, Milsom JW, Church JM. Clinical and pathologic factors associated with delayed diagnosis in solitary rectal ulcer syndrome. Dis Colon Rectum. 1993;36:146–153. doi: 10.1007/BF02051170. [DOI] [PubMed] [Google Scholar]
- 19.Chong VH, Jalihal A. Solitary rectal ulcer syndrome: characteristics, outcomes and predictive profiles for persistent bleeding per rectum. Singapore Med J. 2006;47:1063–1068. [PubMed] [Google Scholar]
- 20.Sztarkier I, Benharroch D, Walfisch S, Delgado J. Colitis cystica profunda and solitary rectal ulcer syndrome-polypoid variant: Two confusing clinical conditions. Eur J Intern Med. 2006;17:578–579. doi: 10.1016/j.ejim.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Bonnard A, Mougenot JP, Ferkdadji L, Huot O, Aigrain Y, De Lagausie P. Laparoscopic rectopexy for solitary ulcer of rectum syndrome in a child. Surg Endosc. 2003;17:1156–1157. doi: 10.1007/s00464-002-4285-3. [DOI] [PubMed] [Google Scholar]


