Abstract
INTRODUCTION:
Severe cognitive impairment follows thyroid hormone deficiency during the neonatal period. The role of nitric oxide (NO) in learning and memory has been widely investigated.
METHODS:
This study aimed to investigate the effect of hypothyroidism during neonatal and juvenile periods on NO metabolites in the hippocampi of rats and on learning and memory. Animals were divided into two groups and treated for 60 days from the first day of lactation. The control group received regular water, whereas animals in a separate group were given water supplemented with 0.03% methimazole to induce hypothyroidism. Male offspring were selected and tested in the Morris water maze. Samples of blood were collected to measure the metabolites of NO, NO2, NO3 and thyroxine. The animals were then sacrificed, and their hippocampi were removed to measure the tissue concentrations of NO2 and NO3.
DISCUSSION:
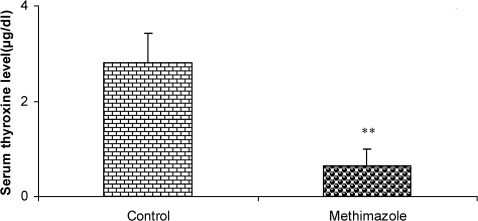
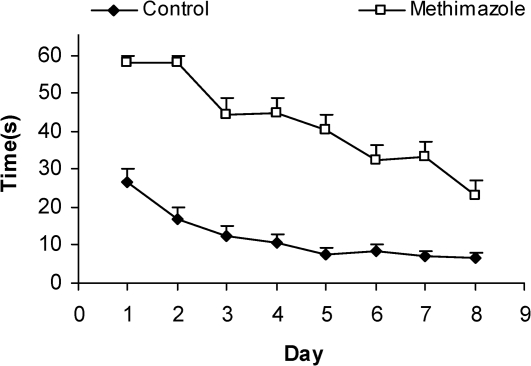
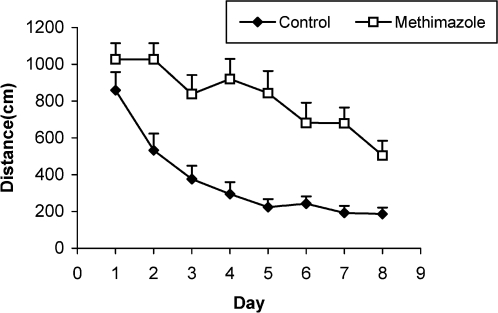
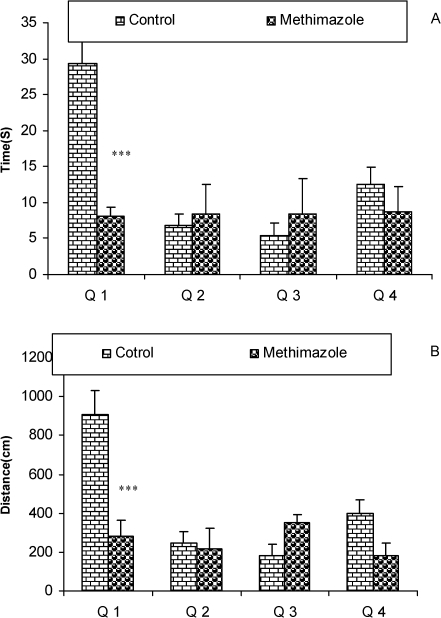
Compared to the control group's offspring, serum thyroxine levels in the methimazole group's offspring were significantly lower (P<0.01). In addition, the swim distance and time latency were significantly higher in the methimazole group (P<0.001), and the time spent by this group in the target quadrant (Q1) during the probe trial was significantly lower (P<0.001). There was no significant difference in the plasma levels of NO metabolites between the two groups; however, significantly higher NO metabolite levels in the hippocampi of the methimazole group were observed compared to controls (P<0.05).
CONCLUSION:
These results suggest that the increased NO level in the hippocampus may play a role in the learning and memory deficits observed in childhood hypothyroidism; however, the precise underlying mechanism(s) remains to be elucidated.
Keywords: Hypothyroidism, Learning, Memory, Nitric Oxide, Offspring
INTRODUCTION
Many studies have shown a significant role for thyroid hormones in the development and maturation of the mammalian central nervous system.1,2 These hormones regulate axonal and dendritic growth and synapse formation.3 Growth retardation and severe cognitive impairment are known complications following thyroid hormone deficiency in the prenatal and neonatal periods.4 It has been shown that hypothyroidism is associated with changes in gene expression in both the central and peripheral nervous system.5 The inability to produce long-term potentiation (LTP) in the rat hippocampus and impaired learning and memory in both rats and humans are among the functional consequences of hypothyroidism.6 Other studies have suggested that hypothyroidism affects behavioral conditions and is accompanied by emotional symptoms, including lethargy and dysphoria.7-11 The results of other studies have shown that several cognitive deficits, including attention and memory processing deficits, general intelligence and visual-spatial skills, are induced by hypothyroidism.9,10 Hypothyroidism has a lesser effect on auditory attention, motor skills, language and set-shifting.7,10,12 In addition, there is evidence indicating that even subclinical hypothyroidism is associated with depressive symptoms and cognitive impairment.13
Nitric oxide (NO), a free radical gas, is known to play critical roles in biologic systems;14 It acts like a diffusible intercellular signaling molecule in the brain and spinal cord.15 NO synthase (NOS) is the enzyme that produces NO from l-arginine. NO can act as an important mediator in synaptic plasticity, LTP and the consolidation of LTP.16-18 In addition, there are reports that suggest a relationship between NMDA (N-methyl-D-aspartate)receptors and the NO system in learning and memory.19,20 Several studies have shown that NOS inhibitors impair the consolidation of memory21,22 and block the induction of LTP.21-24 There is, however, some evidence showing that l-arginine, an NO precursor, improves memory formation and reverses the effect of NOS inhibition.25 There are also reports indicating that NO donors activate both LTP and long-term depression.23,26 These results suggest the involvement of NO in learning and memory processes.
The relationship between thyroid hormones and the NO system has been well documented.23,26-28 There is evidence that thyroid hormones are involved in regulating NO synthase gene expression in the brain. Considering the aforementioned findings, this study aimed to investigate the effect of hypothyroidism induced during the neonatal and juvenile periods on learning and memory of offspring and on NO metabolite concentrations in the hippocampus.
MATERIALS AND METHODS
Animals and treatments
Twenty pregnant female Wistar rats (8 weeks old and weighing 200 ± 20 g) were kept in separate cages at 22 ± 2 °C in a room with a 12 h light/dark cycle (light on at 7:00 am). Offspring were randomly divided into two groups and treated according to the experimental protocol from the first day after birth through the first two months of life. Rats in the control group received normal drinking water, whereas the second group received the same drinking water supplemented with 0.03% methimazole (Sigma, USA) to induce hypothyroidism.29 After 60 days, seven male offspring of each group were randomly selected and tested in the Morris water maze (MWM).
In the methimazole group, hypothyroidism was confirmed by testing serum thyroxine concentration levels using the radioimmunoassay method (Daisource, T4- RIA - CT).
Animal handling and all related procedures were carried out in accordance with the rules set by the Mashhad University of Medical Sciences Ethical Committee.
Morris water maze apparatus and procedures
A circular black pool (136 cm diameter, 60 cm high, 30 cm deep) was filled with water (20–24 °C). A circular platform (10 cm diameter, 28 cm high) was placed within the pool and was submerged approximately 2 cm below the surface of the water in the center of the southwest quadrant. Outside the maze, fixed visual cues were present at various locations around the room (i.e., a computer, hardware and posters). An infrared camera was mounted above the center of the maze and an infrared LED was attached to each rat for motion tracking. Before each experiment, each rat was handled daily for 3 days and habituated to the water maze for 30 sec without a platform. The animals performed four trials on each of the eight consecutive days, and each trial began with the rat being placed in the pool and released facing the side wall at one of four positions (the boundaries of the four quadrants, labeled North (N), East (E), South (S) and West (W). Release positions were randomly predetermined. For each trial, the rat was allowed to swim until it found and remained on the platform for 15 seconds. If 60 seconds had passed and the animal had not found the platform, it was guided to the platform by the experimenter and allowed to stay on the platform for 15 sec. The rat was then removed from the pool, dried and placed in its holding bin for 5 min. The time latency to reach the platform and the length of the swimming path were recorded by a video tracking system.29,32-34 On the ninth day, the platform was removed, and the animals were allowed to swim for 60 s. The time spent in the target quadrant (Q1) and the traveled path were compared between groups. All measurements were performed during the first half of the light cycle.
Biochemical assessment
After the last session of the MWM test, blood samples were taken from all rats to determine hypothyroidism status and to measure the NO metabolites NO2 and NO3 (Griess reagent method). The animals were then sacrificed, and hippocampi were removed and submitted to NO metabolite measurements in the tissue. The Griess reaction was adapted to assay nitrates as previously described.35 Briefly, standard curves for nitrates (Sigma, St. Louis, Missouri, USA) were prepared, and samples (50 µl plasma and 100 µl tissue suspension) were added to the Griess reagent. The proteins were subsequently precipitated by the addition of 50 µl of 10% trichloroacetic acid (Sigma). The contents were then vortex-mixed and centrifuged, and the supernatants were transferred to a 96-well flat-bottomed microplate. Absorbance was read at 520 nm using a microplate reader, and final values were calculated from standard calibration plots.36
Statistical analysis
All data are expressed as mean ± SEM. Swim time latency and the length of the traveled path over the eight training days were analyzed by repeated measures analysis of variance ANOVA. The time spent in the target quadrant (Q1) and the length of the swimming path in this quadrant was compared using unpaired t-tests. Comparison of serum thyroxin levels and plasma levels of NO metabolites was carried out using unpaired t-tests. The criterion for statistical significance was P<0.05.
RESULTS
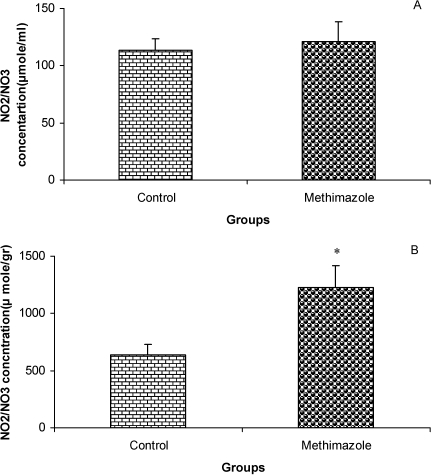
The serum thyroxine concentration in methimazole-treated animals was significantly lower compared to that of control animals (P<0.01; Fig. 1). In addition, the time latency and the length of the swimming path over the eight training days were significantly higher in offspring of the methimazole group (P<0.001; Figs. 2 and 3, respectively). In the probe trial, the time spent in the target quadrant (Q1) by the offspring of the methimazole group was significantly lower compared to controls (P<0.001; Fig. 4A). Furthermore, the length of the traveled path in Q1 by animals in the methimazole group was shorter than for those in the control group (P<0.001; Fig. 4B). There were no significant differences in the time spent or the length of the swim path in the other quadrants between the two groups. In addition, there were no significant differences between the plasma concentrations of NO2 or NO3; however, the concentration of NO metabolites in the hippocampi of the methimazole group offspring was higher than that of the control animals (P<0.05; Figs. 5A and 5B, respectively).
Figure 1.
Serum thyroxine concentrations (µg/dl) in offspring of methimazole and control groups. The animals in the control group received regular drinking water, whereas the methimazole group received the water supplemented with 0.03% methimazole. Data are shown as mean ± SEM (n = 7 in each group). **P<0.01 compared to controls.
Figure 2.
Comparison of swim time latency (sec) to find the platform between offspring of the methimazole and control groups. The latency was significantly higher in offspring of rats in the methimazole group compared to controls (P<0.001). Data are shown as mean ± SEM of seven animals per group.
Figure 3.
Comparison of the length of the swimming path (cm) between methimazole-treated animals and controls using a repeated measure ANOVA. The length of the swimming path in the offspring of the methimazole group was significantly higher than that of controls (P<0.001). Data are shown as mean ± SEM of seven animals per group.
Figure 4.
The results of the time (sec) spent (4A) and the length of the swimming path (cm) (4B) in each quadrant during the probe trial on day 9 (24 h after the last secession of learning). Data are shown as mean ± SEM of seven animals per group. The platform was removed, and the time spent and the length of the swim path in the target quadrant (Q1) was compared between the groups. The time spent and the length of the swim path in Q1 by the offspring of the methimazole group was significantly lower compared to controls. ***P<0.001 compared to controls.
Figure 5.
Comparison of plasma NO metabolite levels (A) and the concentrations of these metabolites in hippocampal tissue (B) between offspring of the methimazole and control groups. Data are shown as mean ± SEM of seven animals per group. There were no significant differences between the plasma concentrations of NO2 or NO3; however, the concentration of NO metabolites in the hippocampi of the methimazole group offspring was higher than that of the control animals. *P<0.05 compared to controls.
DISCUSSION
Neonatal hypothyroidism has been clearly shown to be related to cognitive dysfunction.9-11,37,38 For example, studies in humans have shown that a temporal deficiency in thyroid hormones during developmental periods impairs cognitive functions such as attention, learning and memory.39 Experimental hypothyroidism in developing rats has also been shown to result in impaired learning and memory.40,41 In this study we induced hypothyroidism in rats, resulting in impaired learning and memory during the neonatal and juvenile periods.
Hypothyroidism impairs hippocampal-dependent learning and short- and long-term memory.42,43 In addition, hypothyroidism impairs early and late phases of LTP.44 The exact mechanisms underlying the induced deficits in memory and LTP have not been elucidated. Many studies have demonstrated that cognitive dysfunction in hypothyroidism is likely due to abnormal brain development, diminished inter-neuronal connectivity and, in particular, impaired synaptic plasticity in the hippocampus.45 It has been shown that maturation and function of the hippocampus are dependent on thyroid hormones.43 Transient hypothyroidism has even been shown to impair synaptic transmission and plasticity in the adult hippocampus.46,47 It has also been reported that impairment of LTP and memory due to hypothyroidism correlates with changes in c-jun and c-fos protein expression and extracellular signal regulated kinases (ERKs) levels in the hippocampus during crucial periods of brain development.48,49 Changes in the expression of other proteins such as synapsin I, synaptotagmin I, and syntaxin may also be involved in hypothyroidism-induced memory deficits.50 It has also been suggested that thyroid hormone deficiency results in changes in brain regions such as the dorsal hippocampal-mPFC pathway and changes in the glutamate release, which can lead to cognitive disturbances.51-53 Moreover, hypothyroidism has been shown to induce oxidative stress in the hippocampus.54
The relationship between oxidative stress, neuronal damage and cognitive dysfunction has been well documented.55-58 In addition, it is well known that neonatal hypothyroidism is accompanied by a delay in myelinogenesis and a decrease in the number of myelinated axons.59,60 In the present study, we have shown that a deficiency of thyroid hormones during lactation and in the neonatal period could impair learning of offspring in the MWM test. The animals in the hypothyroid group had significantly higher time latency to find the platform during every day of training (Fig. 2), indicating a deleterious effect of the lack of thyroid hormones on spatial learning processes. The results presented here also confirm that methimazole-induced hypothyroidism during the neonatal and juvenile periods impairs spatial memory given that the animals in the hypothyroid group spent less time in the target quadrant compared to the control group when tested on the ninth day (probe trial; Fig. 4). The results of the present study are consistent with findings from our previous study, in which we found an impairment in the MWM in adult rats with methimazole-induced hypothyroidism over a 180-day period.29 It has also been reported that treatment of thyroidectomized adult rats with thyroxine improved radial arm water maze tasks and LTP in the CA1 area of the hippocampus.61 These researchers showed that cyclic-AMP response element binding protein (CREB) and mitogen-activated protein kinases (MAPKs) may contribute to the impairment of hippocampal-dependent learning and memory; however, the authors suggested that calcium-calmodulin-dependent NOS and brain-derived neurotrophic factor (BDNF) may not have roles in this process.61
The free radical gas NO has been associated with different forms of learning and memory and in several forms of synaptic plasticity thought to be involved in memory formation. This association has been widely confirmed via pharmacological studies, which have utilized a variety of substances and methods to inhibit NO synthase. Results from knockout studies in mice have revealed that mice deficient in eNOS and nNOS expression exhibit impaired LTP.20,61-65 It has also been reported that nitrergic neurons, the neurons that produce NO, increase in number after spatial learning in rats, which can be interpreted as upregulation induced by behavioral training.63 This evidence implies that NO participates in the memory process.63 In contrast to these results, which indicate a positive role for NO in learning and memory, it has been shown that inhibition of NOS does not prevent the induction of LTP or impair spatial memory. Thus, these findings suggest that NO does not play an important role in memory and learning.66 Here, we have examined the relationship between NO and hypothyroidism. We found that both hypothyroidism and impaired MWM performance were present in the methimazole group.
We also found a significant increase in hippocampal NO metabolites in the methimazole-treated group. The association between hypothyroidism and increased NO levels in the brain has been previously investigated. One study indicated that maternal thyroid hormone deficiency during the early gestational period resulted in a significant elevation in neuronal nitric oxide synthase (nNOS) expression with associated neuronal death in the embryonic rat neocortex.67 It has also been shown that nNOS acts as a negative regulator of neurogenesis.67-69 The inhibitory effect of NO on neurogenesis may be due to a reduction in the proliferative potential of neural precursor cells70 or a defect in the survival of newly generated neurons after differentiation.71 There is evidence showing that thyroid hormone deficiency increases cell death.67 The relationship between nNOS and poor survival of neurons and thyroid hormone deprivation has also been suggested.67 It has been shown that even a moderate and transient decrease in maternal thyroxine significantly increases nNOS expression, which is reversible by hormone replacement.67,72 In contrast, another report showed that propylthiouracil (PTU)-treated animals exhibited reduced NOS activity that could be rescued by T4 administration.72
It has also been shown that hypothyroidism changes the pattern of NOS activity in a tissue-dependent manner. NOS activity was significantly increased in both the right and left ventricles, but it was significantly reduced in the aorta, whereas in the vena cava, renal cortex and medulla, the enzyme activity was non-significantly higher compared to controls.73 The results of the present study also showed no difference in NO metabolites between the hypothyroid group and controls in peripheral blood samples. It might be suggested that an increase in NO in the hippocampus is due to stimulatory effects of thyroid hormone deprivation on nNOS activity. Although some studies have suggested NO to be a neurotransmitter that plays an important role in enhancing memory, others have claimed that its excess in the hippocampus may result in memory deficits.74 NO has been shown to have bidirectional effects. Under normal physiological conditions, it acts as an important neuronal messenger; however, when NO increases to high concentrations, it has a toxic effect that can lead to neuronal death.75-77 A common example of the toxic effect of NO in the CNS is due to glutamate neurotransmission and the activation N-methyl-D-aspartate (NMDA) receptors, which leads to significant increases in intracellular calcium, followed by stimulation of neuronal NOS.77,78
Finally, other findings have suggested selective enhancement of reactive oxygen species (ROS) and lipid peroxidation in the amygdala and hippocampus of rats after three weeks of treatment with methimazole. These authors showed a significant increase in NOS activity,54 suggesting a correlation between increased NOS activity and elevated oxidative stress. It may therefore be suggested that increased NO production in the hippocampus during hypothyroidism acts as an oxidative stressor, which may explain the deficits in learning and memory observed here.
CONCLUSIONS
In this study, we found that hypothyroidism induced during the neonatal and juvenile periods resulted in impaired learning and memory of rats tested in the Morris water maze. In addition, we found an increase in NO metabolites in the hippocampus, suggesting that the impairment of memory in hypothyroidism may be due to increases in NOS and NO.
ACKNOWLEDGEMENTS
We would like to thank the Vice Chancellor of Research of Mashhad University of Medical Sciences for financial support.
REFERENCES
- 1.Timiras PS, Nzekwe EU. Thyroid hormones and nervous system development. Biol Neonate. 1989;55:376–85. doi: 10.1159/000242941. 10.1159/000242941 [DOI] [PubMed] [Google Scholar]
- 2.Vallortigara J, Alfos S, Micheau J, Higueret P, Enderlin V. T3 administration in adult hypothyroid mice modulates expression of proteins involved in striatal synaptic plasticity and improves motor behavior. Neurobiol Dis. 2008;31:378–85. doi: 10.1016/j.nbd.2008.05.015. 10.1016/j.nbd.2008.05.015 [DOI] [PubMed] [Google Scholar]
- 3.Hosoda R, Nakayama K, Kato-Negishi M, Kawahara M, Muramoto K, Kuroda Y. Thyroid hormone enhances the formation of synapses between cultured neurons of rat cerebral cortex. Cell Mol Neurobiol. 2003;23:895–906. doi: 10.1023/B:CEMN.0000005318.53810.de. 10.1023/B:CEMN.0000005318.53810.de [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porterfield SP, Hendrich CE. The role of thyroid hormones in prenatal and neonatal neurological development--current perspectives. Endocr Rev. 1993;14:94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi K, Tsuji R, Yoshioka T, Kushida M, Yabushita S, Sasaki M, et al. Effects of hypothyroidism induced by perinatal exposure to PTU on rat behavior and synaptic gene expression. Toxicology. 2005;212:135–47. doi: 10.1016/j.tox.2005.04.012. 10.1016/j.tox.2005.04.012 [DOI] [PubMed] [Google Scholar]
- 6.Lee PR, Brady D, Koenig JI. Thyroid hormone regulation of N-methyl-D-aspartic acid receptor subunit mRNA expression in adult brain. J Neuroendocrinol. 2003;15:87–92. doi: 10.1046/j.1365-2826.2003.00959.x. 10.1046/j.1365-2826.2003.00959.x [DOI] [PubMed] [Google Scholar]
- 7.Burmeister LA, Ganguli M, Dodge HH, Toczek T, DeKosky ST, Nebes RD. Hypothyroidism and cognition: preliminary evidence for a specific defect in memory. Thyroid. 2001;11:1177–85. doi: 10.1089/10507250152741037. 10.1089/10507250152741037 [DOI] [PubMed] [Google Scholar]
- 8.Capet C, Jego A, Denis P, Noel D, Clerc I, Cornier AC, et al. [Is cognitive change related to hypothyroidism reversible with replacement therapy?] Rev Med Interne. 2000;21:672–8. doi: 10.1016/s0248-8663(00)80022-3. 10.1016/S0248-8663(00)80022-3 [DOI] [PubMed] [Google Scholar]
- 9.Smith JW, Evans AT, Costall B, Smythe JW. Thyroid hormones, brain function and cognition: a brief review. Neurosci Biobehav Rev. 2002;26:45–60. doi: 10.1016/s0149-7634(01)00037-9. 10.1016/S0149-7634(01)00037-9 [DOI] [PubMed] [Google Scholar]
- 10.Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413–8. doi: 10.1001/archinte.158.13.1413. 10.1001/archinte.158.13.1413 [DOI] [PubMed] [Google Scholar]
- 11.Haggerty JJ, Jr, Garbutt JC, Evans DL, Golden RN, Pedersen C, Simon JS, et al. Subclinical hypothyroidism: a review of neuropsychiatric aspects. Int J Psychiatry Med. 1990;20:193–208. doi: 10.2190/ADLY-1UU0-1A8L-HPXY. 10.2190/ADLY-1UU0-1A8L-HPXY [DOI] [PubMed] [Google Scholar]
- 12.Miller KJ, Parsons TD, Whybrow PC, Van Herle K, Rasgon N, Van Herle A, et al. Verbal memory retrieval deficits associated with untreated hypothyroidism. J Neuropsychiatry Clin Neurosci. 2007;19:132–6. doi: 10.1176/jnp.2007.19.2.132. [DOI] [PubMed] [Google Scholar]
- 13.McDermott MT, Ridgway EC. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab. 2001;86:4585–90. doi: 10.1210/jcem.86.10.7959. 10.1210/jc.86.10.4585 [DOI] [PubMed] [Google Scholar]
- 14.Yildiz Akar F, Ulak G, Tanyeri P, Erden F, Utkan T, Gacar N. 7-Nitroindazole, a neuronal nitric oxide synthase inhibitor, impairs passive-avoidance and elevated plus-maze memory performance in rats. Pharmacol Biochem Behav. 2007;87:434–43. doi: 10.1016/j.pbb.2007.05.019. 10.1016/j.pbb.2007.05.019 [DOI] [PubMed] [Google Scholar]
- 15.Susswein AJ, Katzoff A, Miller N, Hurwitz I. Nitric oxide and memory. Neuroscientist. 2004;10:153–62. doi: 10.1177/1073858403261226. 10.1177/1073858403261226 [DOI] [PubMed] [Google Scholar]
- 16.Boger RH. The pharmacodynamics of L-arginine. J Nutr. 2007;137(6 Suppl 2):1650S–5S. doi: 10.1093/jn/137.6.1650S. [DOI] [PubMed] [Google Scholar]
- 17.Yamada K, Nabeshima T. Changes in NMDA receptor/nitric oxide signaling pathway in the brain with aging. Microsc Res Tech. 1998;43:68–74. doi: 10.1002/(SICI)1097-0029(19981001)43:1<68::AID-JEMT10>3.0.CO;2-W. 10.1002/(SICI)1097-0029(19981001)43:1<68::AID-JEMT10>3.0.CO;2-W [DOI] [PubMed] [Google Scholar]
- 18.Yamada K, Tanaka T, Zou LB, Senzaki K, Yano K, Osada T, et al. Long-term deprivation of oestrogens by ovariectomy potentiates beta-amyloid-induced working memory deficits in rats. Br J Pharmacol. 1999;128:419–27. doi: 10.1038/sj.bjp.0702811. 10.1038/sj.bjp.0702811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60–7. doi: 10.1016/0166-2236(91)90022-m. 10.1016/0166-2236(91)90022-M [DOI] [PubMed] [Google Scholar]
- 20.Prast H, Philippu A. Nitric oxide as modulator of neuronal function. Prog Neurobiol. 2001;64:51–68. doi: 10.1016/s0301-0082(00)00044-7. 10.1016/S0301-0082(00)00044-7 [DOI] [PubMed] [Google Scholar]
- 21.Kopf SR, Benton RS, Kalfin R, Giovannini MG, Pepeu G. NO synthesis inhibition decreases cortical ACh release and impairs retention of a conditioned response. Brain Res. 2001;894:141–4. doi: 10.1016/s0006-8993(00)03148-6. 10.1016/S0006-8993(00)03148-6 [DOI] [PubMed] [Google Scholar]
- 22.Yildirim M, Marangoz C. Effects of nitric oxide on passive avoidance learning in rats. Int J Neurosci. 2004;114:597–606. doi: 10.1080/00207450490430471. 10.1080/00207450490430471 [DOI] [PubMed] [Google Scholar]
- 23.Bohme GA, Bon C, Stutzmann JM, Doble A, Blanchard JC. Possible involvement of nitric oxide in long-term potentiation. Eur J Pharmacol. 1991;199:379–81. doi: 10.1016/0014-2999(91)90505-k. 10.1016/0014-2999(91)90505-K [DOI] [PubMed] [Google Scholar]
- 24.Bon CL, Garthwaite J. On the role of nitric oxide in hippocampal long-term potentiation. J Neurosci. 2003;23:1941–8. doi: 10.1523/JNEUROSCI.23-05-01941.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Majlessi N, Choopani S, Bozorgmehr T, Azizi Z. Involvement of hippocampal nitric oxide in spatial learning in the rat. Neurobiol Learn Mem. 2008;90:413–9. doi: 10.1016/j.nlm.2008.04.010. 10.1016/j.nlm.2008.04.010 [DOI] [PubMed] [Google Scholar]
- 26.Shibuki K, Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991;349:326–8. doi: 10.1038/349326a0. 10.1038/349326a0 [DOI] [PubMed] [Google Scholar]
- 27.Hermenegildo C, Medina P, Peiro M, Segarra G, Vila JM, Ortega J, et al. Plasma concentration of asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, is elevated in hyperthyroid patients. J Clin Endocrinol Metab. 2002;87:5636–40. doi: 10.1210/jc.2002-020905. 10.1210/jc.2002-020905 [DOI] [PubMed] [Google Scholar]
- 28.Ueta Y, Levy A, Chowdrey HS, Lightman SL. Hypothalamic nitric oxide synthase gene expression is regulated by thyroid hormones. Endocrinology. 1995;136:4182–7. doi: 10.1210/endo.136.10.7545100. 10.1210/en.136.10.4182 [DOI] [PubMed] [Google Scholar]
- 29.Hosseini M, Hadjzadeh MA, Derakhshan M, Havakhah S, Rassouli FB, Rakhshandeh H, et al. The beneficial effects of olibanum on memory deficit induced by hypothyroidism in adult rats tested in Morris water maze. Arch Pharm Res. 2010;33:463–8. doi: 10.1007/s12272-010-0317-z. 10.1007/s12272-010-0317-z [DOI] [PubMed] [Google Scholar]
- 30.Ampong B, Honda H, Kogo H. Effect of hypothyroidism on beta-adrenoceptor-mediated relaxation in the rat thoracic aortae. A time-dependent study. Vascul Pharmacol. 2002;38:149–55. doi: 10.1016/s1537-1891(02)00166-0. 10.1016/S1537-1891(02)00166-0 [DOI] [PubMed] [Google Scholar]
- 31.Leal AL, Pantaleao TU, Moreira DG, Marassi MP, Pereira VS, Rosenthal D, et al. Hypothyroidism and hyperthyroidism modulates Ras-MAPK intracellular pathway in rat thyroids. Endocrine. 2007;31:174–8. doi: 10.1007/s12020-007-0029-4. 10.1007/s12020-007-0029-4 [DOI] [PubMed] [Google Scholar]
- 32.Monteiro SC, Matte C, Bavaresco CS, Netto CA, Wyse AT. Vitamins E and C pretreatment prevents ovariectomy-induced memory deficits in water maze. Neurobiol Learn Mem. 2005;84:192–9. doi: 10.1016/j.nlm.2005.08.002. 10.1016/j.nlm.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 33.Saffarzadeh F, Eslamizade MJ, Nemati Karimooy HA, Hadjzadeh MA, Khazaei M, Hosseini M. The effect of L-Arginin on Morris water maze tasks of ovariectomized rats. Acta Physiol Hung. 2010;97:216–23. doi: 10.1556/APhysiol.97.2010.2.8. 10.1556/APhysiol.97.2010.2.8 [DOI] [PubMed] [Google Scholar]
- 34.Alaei H, Moloudi R, Sarkaki AR. Effects of treadmill running on mid-term memory and swim speed in the rat with Morris water maze test. J Bodyw Mov Ther. 2008;12:72–5. doi: 10.1016/j.jbmt.2007.05.004. 10.1016/j.jbmt.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 35.Nahrevanian H, Dascombe MJ. Nitric oxide and reactive nitrogen intermediates during lethal and nonlethal strains of murine malaria. Parasite Immunol. 2001;23:491–501. doi: 10.1046/j.1365-3024.2001.00406.x. 10.1046/j.1365-3024.2001.00406.x [DOI] [PubMed] [Google Scholar]
- 36.Nahrevanian H, Najafzadeh M, Hajihosseini R, Nazem H, Farahmand M, Zamani Z. Antileishmanial effects of trinitroglycerin in BALB/C mice infected with Leishmania major via nitric oxide pathway. Korean J Parasitol. 2009;47:109–15. doi: 10.3347/kjp.2009.47.2.109. 10.3347/kjp.2009.47.2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooistra L, Laane C, Vulsma T, Schellekens JM, van der Meere JJ, Kalverboer AF. Motor and cognitive development in children with congenital hypothyroidism: a long-term evaluation of the effects of neonatal treatment. J Pediatr. 1994;124:903–9. doi: 10.1016/s0022-3476(05)83178-6. 10.1016/S0022-3476(05)83178-6 [DOI] [PubMed] [Google Scholar]
- 38.Vara H, Munoz-Cuevas J, Colino A. Age-dependent alterations of long-term synaptic plasticity in thyroid-deficient rats. Hippocampus. 2003;13:816–25. doi: 10.1002/hipo.10132. 10.1002/hipo.10132 [DOI] [PubMed] [Google Scholar]
- 39.Kopp P, Kitajima K, Jameson JL. Syndrome of resistance to thyroid hormone: insights into thyroid hormone action. Proc Soc Exp Biol Med. 1996;211:49–61. doi: 10.3181/00379727-211-43951. [DOI] [PubMed] [Google Scholar]
- 40.Akaike M, Kato N, Ohno H, Kobayashi T. Hyperactivity and spatial maze learning impairment of adult rats with temporary neonatal hypothyroidism. Neurotoxicol Teratol. 1991;13:317–22. doi: 10.1016/0892-0362(91)90077-a. 10.1016/0892-0362(91)90077-A [DOI] [PubMed] [Google Scholar]
- 41.Hashimoto K, Curty FH, Borges PP, Lee CE, Abel ED, Elmquist JK, et al. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc Natl Acad Sci U S A. 2001;98:3998–4003. doi: 10.1073/pnas.051454698. 10.1073/pnas.051454698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerges NZ, Alzoubi KH, Park CR, Diamond DM, Alkadhi KA. Adverse effect of the combination of hypothyroidism and chronic psychosocial stress on hippocampus-dependent memory in rats. Behav Brain Res. 2004;155:77–84. doi: 10.1016/j.bbr.2004.04.003. 10.1016/j.bbr.2004.04.003 [DOI] [PubMed] [Google Scholar]
- 43.Gerges NZ, Alkadhi KA. Hypothyroidism impairs late LTP in CA1 region but not in dentate gyrus of the intact rat hippocampus: MAPK involvement. Hippocampus. 2004;14:40–5. doi: 10.1002/hipo.10165. 10.1002/hipo.10165 [DOI] [PubMed] [Google Scholar]
- 44.Alzoubi KH, Aleisa AM, Gerges NZ, Alkadhi KA. Nicotine reverses adult-onset hypothyroidism-induced impairment of learning and memory: Behavioral and electrophysiological studies. J Neurosci Res. 2006;84:944–53. doi: 10.1002/jnr.21014. 10.1002/jnr.21014 [DOI] [PubMed] [Google Scholar]
- 45.Rami A, Rabie A, Patel AJ. Thyroid hormone and development of the rat hippocampus: cell acquisition in the dentate gyrus. Neuroscience. 1986;19:1207–16. doi: 10.1016/0306-4522(86)90134-x. 10.1016/0306-4522(86)90134-X [DOI] [PubMed] [Google Scholar]
- 46.Gilbert ME. Alterations in synaptic transmission and plasticity in area CA1 of adult hippocampus following developmental hypothyroidism. Brain Res Dev Brain Res. 2004;148:11–8. doi: 10.1016/j.devbrainres.2003.09.018. 10.1016/j.devbrainres.2003.09.018 [DOI] [PubMed] [Google Scholar]
- 47.Gilbert ME, Paczkowski C. Propylthiouracil (PTU)-induced hypothyroidism in the developing rat impairs synaptic transmission and plasticity in the dentate gyrus of the adult hippocampus. Brain Res Dev Brain Res. 2003;145:19–29. doi: 10.1016/s0165-3806(03)00191-3. 10.1016/S0165-3806(03)00191-3 [DOI] [PubMed] [Google Scholar]
- 48.Dong J, Yin H, Liu W, Wang P, Jiang Y, Chen J. Congenital iodine deficiency and hypothyroidism impair LTP and decrease C-fos and C-jun expression in rat hippocampus. Neurotoxicology. 2005;26:417–26. doi: 10.1016/j.neuro.2005.03.003. 10.1016/j.neuro.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 49.Sui L, Anderson WL, Gilbert ME. Impairment in short-term but enhanced long-term synaptic potentiation and ERK activation in adult hippocampal area CA1 following developmental thyroid hormone insufficiency. Toxicol Sci. 2005;85:647–56. doi: 10.1093/toxsci/kfi095. 10.1093/toxsci/kfi095 [DOI] [PubMed] [Google Scholar]
- 50.Vara H, Martinez B, Santos A, Colino A. Thyroid hormone regulates neurotransmitter release in neonatal rat hippocampus. Neuroscience. 2002;110:19–28. doi: 10.1016/s0306-4522(01)00541-3. 10.1016/S0306-4522(01)00541-3 [DOI] [PubMed] [Google Scholar]
- 51.Shuaib A, Ijaz S, Hemmings S, Galazka P, Ishaqzay R, Liu L, et al. Decreased glutamate release during hypothyroidism may contribute to protection in cerebral ischemia. Exp Neurol. 1994;128:260–5. doi: 10.1006/exnr.1994.1135. 10.1006/exnr.1994.1135 [DOI] [PubMed] [Google Scholar]
- 52.Sui L, Wang F, Li BM. Adult-onset hypothyroidism impairs paired-pulse facilitation and longterm potentiation of the rat dorsal hippocampo-medial prefrontal cortex pathway in vivo. Brain Res. 2006 22;1096:53–60. doi: 10.1016/j.brainres.2006.04.042. 10.1016/j.brainres.2006.04.042 [DOI] [PubMed] [Google Scholar]
- 53.Hrabovszky E, Turi GF, Kallo I, Liposits Z. Expression of vesicular glutamate transporter-2 in gonadotropin-releasing hormone neurons of the adult male rat. Endocrinology. 2004;145:4018–21. doi: 10.1210/en.2004-0589. 10.1210/en.2004-0589 [DOI] [PubMed] [Google Scholar]
- 54.Cano-Europa E, Perez-Severiano F, Vergara P, Ortiz-Butron R, Rios C, Segovia J, et al. Hypothyroidism induces selective oxidative stress in amygdala and hippocampus of rat. Metab Brain Dis. 2008;23:275–87. doi: 10.1007/s11011-008-9099-0. 10.1007/s11011-008-9099-0 [DOI] [PubMed] [Google Scholar]
- 55.Gustaw-Rothenberg K, Lerner A, Bonda DJ, Lee HG, Zhu X, Perry G, et al. Biomarkers in Alzheimer's disease: past, present and future. Biomark Med. 2010;4:15–26. doi: 10.2217/bmm.09.86. 10.2217/bmm.09.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Head E. Oxidative damage and cognitive dysfunction: antioxidant treatments to promote healthy brain aging. Neurochem Res. 2009;34:670–8. doi: 10.1007/s11064-008-9808-4. 10.1007/s11064-008-9808-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de la Torre JC. Pathophysiology of neuronal energy crisis in Alzheimer's disease. Neurodegener Dis. 2008;5:126–32. doi: 10.1159/000113681. 10.1159/000113681 [DOI] [PubMed] [Google Scholar]
- 58.Jellinger KA. Recent advances in our understanding of neurodegeneration. J Neural Transm. 2009;116:1111–62. doi: 10.1007/s00702-009-0240-y. 10.1007/s00702-009-0240-y [DOI] [PubMed] [Google Scholar]
- 59.Barradas PC, Ferraz AS, Ferreira AA, Daumas RP, Moura EG. 2′3′Cyclic nucleotide 3′phosphodiesterase immunohistochemistry shows an impairment on myelin compaction in hypothyroid rats. Int J Dev Neurosci. 2000;18:887–92. doi: 10.1016/s0736-5748(00)00028-9. 10.1016/S0736-5748(00)00028-9 [DOI] [PubMed] [Google Scholar]
- 60.Guadano Ferraz A, Escobar del Rey F, Morreale de Escobar G, Innocenti GM, Berbel P. The development of the anterior commissure in normal and hypothyroid rats. Brain Res Dev Brain Res. 1994 16;81:293–308. doi: 10.1016/0165-3806(94)90315-8. 10.1016/0165-3806(94)90315-8 [DOI] [PubMed] [Google Scholar]
- 61.Alzoubi KH, Gerges NZ, Aleisa AM, Alkadhi KA. Levothyroxin restores hypothyroidisminduced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus. 2009;19:66–78. doi: 10.1002/hipo.20476. 10.1002/hipo.20476 [DOI] [PubMed] [Google Scholar]
- 62.Holscher C, Rose SP. An inhibitor of nitric oxide synthesis prevents memory formation in the chick. Neurosci Lett. 1992;145:165–7. doi: 10.1016/0304-3940(92)90012-v. 10.1016/0304-3940(92)90012-V [DOI] [PubMed] [Google Scholar]
- 63.Zhuo M, Laitinen JT, Li XC, Hawkins RD. On the respective roles of nitric oxide and carbon monoxide in long-term potentiation in the hippocampus. Learn Mem. 1998;5:467–80. [PMC free article] [PubMed] [Google Scholar]
- 64.Zhuo M, Laitinen JT, Li XC, Hawkins RD. On the respective roles of nitric oxide and carbon monoxide in long-term potentiation in the hippocampus. Learn Mem. 1999;6:63–76. [PMC free article] [PubMed] [Google Scholar]
- 65.Zhuo M, Small SA, Kandel ER, Hawkins RD. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260:1946–50. doi: 10.1126/science.8100368. 10.1126/science.8100368 [DOI] [PubMed] [Google Scholar]
- 66.Bannerman DM, Chapman PF, Kelly PA, Butcher SP, Morris RG. Inhibition of nitric oxide synthase does not prevent the induction of long-term potentiation in vivo. J Neurosci. 1994;14:7415–25. doi: 10.1523/JNEUROSCI.14-12-07415.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sinha RA, Pathak A, Mohan V, Bandyopadhyay S, Rastogi L, Godbole MM. Maternal thyroid hormone: a strong repressor of neuronal nitric oxide synthase in rat embryonic neocortex. Endocrinology. 2008;149:4396–401. doi: 10.1210/en.2007-1617. 10.1210/en.2007-1617 [DOI] [PubMed] [Google Scholar]
- 68.Cheng A, Wang S, Cai J, Rao MS, Mattson MP. Nitric oxide acts in a positive feedback loop with BDNF to regulate neural progenitor cell proliferation and differentiation in the mammalian brain. Dev Biol. 2003 15;258:319–33. doi: 10.1016/s0012-1606(03)00120-9. 10.1016/S0012-1606(03)00120-9 [DOI] [PubMed] [Google Scholar]
- 69.Fritzen S, Schmitt A, Koth K, Sommer C, Lesch KP, Reif A. Neuronal nitric oxide synthase (NOS-I) knockout increases the survival rate of neural cells in the hippocampus independently of BDNF. Mol Cell Neurosci. 2007;35:261–71. doi: 10.1016/j.mcn.2007.02.021. 10.1016/j.mcn.2007.02.021 [DOI] [PubMed] [Google Scholar]
- 70.Terada H, Nagai T, Kimura H, Kitahama K, Okada S. Distribution of nitric oxide synthase immunoreactive neurons in fetal rat brains at embryonic day 15 and day 19. J Chem Neuroanat. 1996;10:273–8. doi: 10.1016/0891-0618(96)00141-x. 10.1016/0891-0618(96)00141-X [DOI] [PubMed] [Google Scholar]
- 71.Samdani AF, Newcamp C, Resink A, Facchinetti F, Hoffman BE, Dawson VL, et al. Differential susceptibility to neurotoxicity mediated by neurotrophins and neuronal nitric oxide synthase. J Neurosci. 1997 15;17:4633–41. doi: 10.1523/JNEUROSCI.17-12-04633.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Serfozo Z, Kiss PB, Kukor Z, Lontay B, Palatka K, Varga V, et al. Thyroid hormones affect the level and activity of nitric oxide synthase in rat cerebral cortex during postnatal development. Neurochem Res. 2008;33:569–78. doi: 10.1007/s11064-007-9480-0. 10.1007/s11064-007-9480-0 [DOI] [PubMed] [Google Scholar]
- 73.Quesada A, Sainz J, Wangensteen R, Rodriguez-Gomez I, Vargas F, Osuna A. Nitric oxide synthase activity in hyperthyroid and hypothyroid rats. Eur J Endocrinol. 2002;147:117–22. doi: 10.1530/eje.0.1470117. 10.1530/eje.0.1470117 [DOI] [PubMed] [Google Scholar]
- 74.Cernak I, Wang Z, Jiang J, Bian X, Savic J. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 2001;15:593–612. doi: 10.1080/02699050010009559. 10.1080/02699050010009559 [DOI] [PubMed] [Google Scholar]
- 75.Dawson TM, Dawson VL, Snyder SH. A novel neuronal messenger molecule in brain: the free radical, nitric oxide. Ann Neurol. 1992;32:297–311. doi: 10.1002/ana.410320302. 10.1002/ana.410320302 [DOI] [PubMed] [Google Scholar]
- 76.Zhang J, Dawson VL, Dawson TM, Snyder SH. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994 Feb 4;263:687–9. doi: 10.1126/science.8080500. 10.1126/science.8080500 [DOI] [PubMed] [Google Scholar]
- 77.Reiter RJ. Oxidative damage in the central nervous system: protection by melatonin. Prog Neurobiol. 1998;56:359–84. doi: 10.1016/s0301-0082(98)00052-5. 10.1016/S0301-0082(98)00052-5 [DOI] [PubMed] [Google Scholar]
- 78.Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991;88:6368–71. doi: 10.1073/pnas.88.14.6368. 10.1073/pnas.88.14.6368 [DOI] [PMC free article] [PubMed] [Google Scholar]