Abstract
The factors responsible for the induction of allergic disease at an early age have not been completely identified. Therefore a major research focus is their identification to elaborate recommendations for prevention of sensitization in high-risk or atopic children. This review analyzes known or suspected reasons for sensitization in pregnant women and infants from both clinical and experimental animal studies. Recent studies and meta-analyses could not confirm the protective effect of an allergen-poor diet on the part of the mother during pregnancy and lactation. Likewise, the type of bottle feeding or the introduction of solid food into the child’s diet might not significantly influence the development of atopy, allergy, or asthma in the child’s life. Disappointingly, the few preventive measures remaining to reduce the risk of allergic sensitization and atopic diseases in mother and child are the avoidance of smoking and alcohol consumption during pregnancy and lactation and the avoidance of the impairment of gastric function. Further studies are urgently needed to address the influence of certain foods and nutrients, as well as environmental factors, for prevention of allergic diseases in the low- or high-risk infant.
Keywords: Allergy, pregnancy, lactation, atopy, newborn, acid-suppression, prevention
Type I allergies affect a high number of persons in industrialized countries worldwide. Regarding sensitization during pregnancy, the investigation of prevalence numbers is very scarce, except for sporadic reports on the Web (eg, www.allergyclinic.co.nz/guides/28.html) claiming that allergies are the most common diseases during pregnancy, with a prevalence as high as 20%. The incidence of sensitization in young children was assessed in the Multicenter Allergy Study performed in Germany, which revealed that sensitization to food allergens was abundant in 10% of 1-year-old infants but present in only 3% of 6-year-old children.1 In contrast, sensitization to inhalant allergens increased with age, from 1.5% at 1 year of age to 8% at 6 years of age.
Some connected diseases, such as asthma, seem to have reached a plateau in many European countries.2 However, prevalences of allergic diseases in Austria are high, which is similar to the situation in many other Western countries, such as Finland,3 and numbers for childhood asthma, atopic eczema, and allergic rhinitis among schoolchildren still showed an increase between 1995 and 2003.4 Also, rates of rhinoconjunctivitis have slightly increased worldwide.5
The responsible factors need to be revealed to be able to provide reasonable recommendations for the prevention of sensitization and allergy. Because sensitization can occur very early in life, measures already need to be taken by the mother during pregnancy and lactation, as well as for the newborn. Special consideration should be given to children born into both nonatopic or atopic families (ie, low-risk vs high-risk genetic background). In this context studies of epigenetics have to be forced to analyze connections of environmental and genetic modifications. Because studies have revealed that especially the genetic background and the homoeostasis of the TH1/TH2/regulatory T-cell response of the mother can affect the child’s immune response (Fig 1), the present article focuses on factors influencing the maternal rather than the paternal immune status and the subsequent effect on the immune response of the child.
FIG 1.
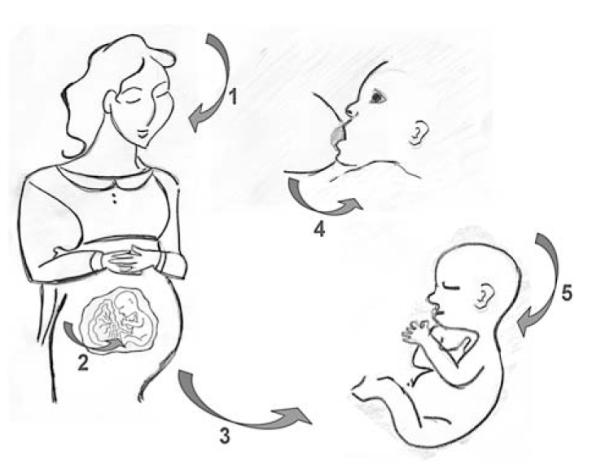
Risk factors for sensitization of mother and child can stem from the environment or be taken up through the diet (1 and 5). Maternofetal transfer can occur through the placenta (2) or during breast-feeding (4). The mode of delivery (3) might additionally play an important role for the immune status of the child.
RISK FACTORS FOR SENSITIZATION IN MOTHERHOOD: A CLINICAL POINT OF VIEW
The causes of allergies in general and of sensitization during pregnancy and in newborns in particular have not yet been entirely determined. However, some factors could be identified that on the one hand might contribute to the sensitization of the mother and the subsequent transfer of the risk of allergies to the offspring or on the other hand might induce sensitization directly in the newborn and young children.
Maternal diet during pregnancy
The food consumed by the mother during pregnancy can contribute to the immunologic profile of herself and the child.6 A Japanese study investigated the influence of the traditional Japanese diet, with a focus on seaweed, vegetables, fruit, antioxidants, fiber, and minerals, and found that seaweed intake was associated independently with a decreased rate of allergic rhinitis in the studied pregnant women.7 In parallel, also calcium, phosphorus, and magnesium decreased allergic diseases. In contrast, the intake of β-carotene was negatively associated with the appearance of allergic rhinitis in the same study. For vitamin D, 2 different hypotheses exist, arguing that excess of vitamin D in the form of supplementation might have contributed to the increase in allergic diseases, including food allergy.8-10 However, deficiency in vitamin D caused by reduced sunlight exposure might also be responsible for asthma and allergy increase, as indirectly concluded based on a 4 times more frequent epinephrine prescription in northern compared with southern regions of the United States.11 Furthermore, the maternal intake of vitamin D reduced the risk of recurrent wheeze in children at the age of 3 years.12 Contrasting results emerged from the International Study of Asthma and Allergies in Childhood study, which concentrated on the correlation of latitude, attitude, and annual temperature with the prevalence of symptoms of asthma, rhinitis, and atopic eczema in Brazilian children.13 This study was performed by means of questionnaires and showed that the prevalence of asthma and eczema was even higher in regions closer to the equator. However, this might be due to successful community intervention programs, as in Australia, which aim to reduce sunlight exposure because of the linked risks.14 Taken together, the different data on vitamin D status and, indirectly, on sunlight exposure are inconclusive, and therefore more interventional studies are needed.15
Exposure to tobacco smoke
One of the most discussed risk factors for induction of sensitization in all population and age groups is smoking, either in active form or through environmental exposure. In a mouse study it could be directly shown that passive exposure to tobacco smoke is able to induce a higher risk for sensitization against allergens.16 Also, in our human study of elderly adults, smoking was an independent risk factor for induction of IgE against respiratory allergens17 and might also contribute to sensitization against contact allergens.18 However, concise numbers in pregnant women are missing.
Alcohol
Alcohol consumption in adults is a documented risk factor for increased specific IgE levels against food antigens and aeroallergens.17,19,20 High continuous alcohol intake through a liquid diet in a mouse model was shown to have a detrimental influence on the induction of high total IgE and TH2-type cytokine levels, as well as a suppressive effect on the TH1-type antibody IgG2a.21 However, controversial studies exist also for this factor, showing that alcohol does not increase skin test reactivity in students of high social class.22 Studies explicitly performed in pregnant women are still missing.
Antiacid medication taken by the mother
The change in hormone status during pregnancy and the growing fetus often lead to heartburn, reflux, and stomachache in the mother. Approximately 70% are affected by these symptoms in their last trimester.23 These women are therefore prone to taking antiacid drugs to treat these symptoms. However, our human and animal studies have revealed that acid suppression and the resulting increased pH in the stomach lead to an increased risk for sensitization to food.24,25 Results from a mouse model indicated that the sensitization of the mother related to antiacid medications consecutively leads to an enhanced risk of allergy in the newborn.26 The mechanism could be that an allergic mother can transfer numerous factors through the placenta or breast milk and even through the transamniotic route. Examples include intact maternal IgE in amniotic fluid27; maternal DNA in cord blood28; leukocytes; chemokines, such as IL-8, RANTES, IFN-γ–inducible protein 10, or monokine induced by IFN-γ29; allergens30-32; and antibodies.27,33 In parallel, the offspring of sensitized mother mice show lower amounts of IFN-γ–producing cells, resulting in a further bias toward a TH2 immune response.34 The sensitization capacity of antiacid drugs could be confirmed in a very recent study performed as an investigation of 3 databases of human data in Sweden, which found a positive correlation of antiacid medication taken by the mother during pregnancy with the induction of allergy and asthma in the child.35,36
RISK FACTORS FOR SENSITIZATION IN THE CHILD: CLINICS AND MECHANISMS
Several factors held responsible for the development of sensitization or allergy in the child stem from clinical observations (Table I).37-56 For a number of them, mechanistic explanations have been provided also.
TABLE I.
Risk factors for sensitization in the child
| Factor | Influence on risk of sensitization in the child | Reference |
|---|---|---|
| Diet of mother during pregnancy | ||
| n-6 PUFA biased | • Higher risk of eczema in the child during first 2 years of life | 37, 38 |
| Celery and citrus fruits | • Higher risk of food sensitization | 39 |
| Vegetable oil, raw sweet pepper, citrus fruit | • Increase risk of sensitization against inhalant antigens | 37 |
| Daily consumption of nuts | • Higher risk of wheezing, dyspnea, steroid use, and asthma | 39 |
| Overall high energy and lipid intake | • Higher risk of sensitization, asthma, or both | 40, 41 |
| Probiotics (eg, Lactobacillus rhamnosus LGG) | • No effect on the outcome of atopic dermatitis at 2 years of age, increased risk of recurrent wheezing episodes |
53 |
| Smoke exposure of mother during pregnancy | Higher total and specific IgE levels, eosinophils, airway disease, and wheezing episodes |
42-45 |
| Alcohol consumption | Higher IgE levels | 46 |
| Antibiotic treatment of child | Increased wheezing treated with inhaled corticosteroids, skin test reactivity, development of specific IgE |
46, 47 |
| Insufficient exposure to environmental bacteria during pregnancy and early life, rare acute respiratory tract infections within first 9 months of child’s life |
Development of allergic diseases more likely, asthma, hay fever, inhalant sensitization, and total IgE level higher |
55, 56 |
| Preterm birth, low birth weight | Decreased expiratory volume, increased bronchial hyperresponsiveness, and decreased lung function in adolescent age |
49 |
| Exposure route to an antigen: environmental contact through skin |
Leads to sensitization | 50 |
| Breast-feeding performed exclusively for longer than 9 months |
Increases risk for atopic diseases (atopic dermatitis, food hypersensitivity) in high-risk children |
54 |
| Birth by caesarean section | Increases risk for allergic rhinitis and atopy; higher number of wheezing episodes and food-specific IgE level |
51, 52 |
Diet of the mother during pregnancy
During pregnancy, a diet enriched in n-6 poly-unsaturated fatty acids (PUFAs), which are contained, for example, in margarine and vegetable oils, seems to be more likely to induce eczema in the child during the first 2 years than n-3 PUFAs, which are contained in fish, at least when consumed during the last 4 weeks of pregnancy.37,38 Possible mechanisms might be that higher levels of n-3 PUFAs are associated with reduced neonatal oxidative stress, lower production of inflammatory leukotriene B4 and IL-2, reduced production of cytokines, and enhanced production of protein kinase c-ζ (inverse association with allergic diseases) by T cells, as shown in human57 and murine58 studies. However, it was also observed that conjugated forms of n-6 PUFAs, such as conjugated linoleic acid (CLA), which are present in milk, cream, butter, and meat of ruminants, can have anti-inflammatory properties.59 These effects could be mediated through binding of CLA to peroxisome proliferator-activated receptor γ, the ligand-dependent interaction of which dramatically inhibits cellular immune responses and production of inflammatory mediators.60,61 In line with this, Jaudszus et al62 showed in a murine model that the cis-9, trans-11 CLA prevented allergen-specific antibody production (IgE, IgG1, and IgG2a), airway reactivity in vivo, and airway inflammation (IL-5, bronchoalveolar lavage cells, eosinophils, and mucus production). Furthermore, CLA acquired through the diet also prevented the downregulation of the peroxisome proliferator-activated receptor γ receptor in the lung (shown in mRNA levels) and reduced the concentration of eicosanoid precursory fatty acids. On the other hand, not only the absolute content but also the ratio of n-6/n-3 PUFAs might influence the development of either tolerance or sensitization to food because in a rat model a ratio of 9:1 in the mothers’ diet prevented tolerance induction in neonatal rats, which otherwise could be achieved when the diet was n-3 PUFA biased.63 Fish oil supplementation during pregnancy has further been shown to increase the levels of the long-chain n-3 PUFA docosahexaenoic acid and eicosapentaenoic acid in breast milk up to 6 weeks after delivery in a randomized controlled human trial.64 Other human studies showed again that fish or fish oil consumption decreases eczema39 or at least the severity of atopic dermatitis in high-risk children at 1 year of age.65 Regarding other food groups, diets rich in celery and citrus fruits seem to increase the risk for food sensitization, whereas vegetable oils, raw sweet pepper, and citrus fruits increase sensitization to inhalant antigens in the progeny of the human birth cohort study Influences of Lifestyle-related Factors on the Immune System and the Development of Allergies in Childhood (LISA).37 It was further found that daily, but not sporadic, consumption of nuts during pregnancy was positively associated with childhood wheeze, dyspnea, steroid use, and asthma symptoms, which continued to be significantly related to persistent wheeze up to the age of 8 years. Apart from certain diet variants or food components, an overall high intake of energy and lipids, such as spreadable fat and vegetable oils, during pregnancy seems to increase the risk of sensitization, asthma, or both in the infants.40,41 In contrast, the consumption of proteins, carbohydrates, and milk/milk products might have a positive effect on the prevention of allergic diseases. More recent reviews, however, state that for children without first-degree relatives with an allergy (ie, parents and siblings), there is no benefit of special nutritional interventions during pregnancy, during lactation,66 or in the first year of life.67
Exposure to tobacco smoke
In blood samples of neonates whose mothers smoked during pregnancy, levels of TH2 cytokines were higher compared with those from nonsmoking mothers.68 Moreover, smoke exposure enhanced total and specific IgE levels,42,43 eosinophil counts,44 airway disease,43 positive skin prick test responses,69 and wheezing episodes/asthma in later life.45,70,71 The risk of induction of specific IgE against inhalant or food allergens was especially increased when a child born to parents without an allergy was exposed to environmental tobacco smoke during the first 2 months of life.72 Alarmingly, in the birth cohort study done on the Isle of Wight, about 43% of the children were exposed to environmental smoke in the first year of life,73 and a French study showed that 20% of the children were exposed to tobacco smoke in utero.74 In contrast to the study of Lannero et al72 mentioned above, the latter study revealed that prenatal exposure was more associated with sensitization than postnatal exposure, that especially maternal allergies and maternal exposure to smoke were associated with higher levels of IgE specific for house dust mite in children, and that sensitization to food antigens was not associated with smoke exposure.74
Exposure to alcohol
Not only smoking but also alcohol consumption of the mother during pregnancy was associated with higher IgE levels in children and an enhanced risk of atopic dermatitis in high-risk infants of a Danish national birth cohort.46
Treatment with antibiotics
In children at 6 and 12 months of age, treatment with antibiotics in the neonatal period was found to be an independent risk factor for wheezing that was treated with inhaled corticosteroids at 12 months of age.47 Confirming these results, Johnson et al48 revealed that atopy, defined as positive skin test reactivity, and development of any specific IgE at 6 to 7 years of age were associated with the use of antibiotics in the first 6 months of life. The commonly accepted mechanism is the disturbance of the commensal intestinal flora by antibiotics and therefore a disturbed development of the gut immune system.75
Insufficient exposure to environmental bacteria
The hygiene hypothesis states that insufficient exposure to environmental bacteria during pregnancy and early life might mislead immune responses toward a TH2 bias also after birth. Important to note is that a TH2 environment is indispensable for a successful pregnancy. However, this dominant TH2 milieu has to be counterbalanced after birth either by downregulating TH2 responses or by upregulating TH1 responses, which most effectively can be achieved through contact with environmental microorganisms during birth (vaginal and fecal) and breast-feeding (skin). Efficient intestinal colonization of the newborn with bacteria seems to be dependent on these contacts and crucial for the development of mucosal immunity.76,77 In accordance with these assumptions, the prenatal application of microbial compounds, such as LPS, before conception and during pregnancy in a murine model has been proved to prevent respiratory allergy parameters, to enhance TH1 cytokine–producing cells, and to reduce IgE and IgG1 levels in the offspring.78 Likewise, the application of LPS during pregnancy and to newborn mice prevented airway inflammation and reduced eosinophil and lymphocyte counts, as well as IgE, IL-5, and IL-13 production, in parallel to an upregulation of IFN-γ, soluble CD14, and Toll-like receptors 2, 4, and 9 as signs of a TH1 response.79 These results are further underlined by the observation that oral tolerance can only be induced in the presence of the normal gastrointestinal microflora but not in germ-free mice.80 In accordance with the hygiene hypothesis, more frequent acute respiratory tract infections within the first 9 months of the child’s life seem to reduce asthma, eczema, hay fever, atopic sensitization, and total IgE levels, as reported from a historical cohort study.81
Diet of the mother during lactation
As mentioned above, not the avoidance of allergy-inducing food during pregnancy and lactation but, on the contrary, allergen exposure might be necessary for development of tolerance induction and prevention of malnutrition of mother and child.82 This is contradictory to early studies suggesting that sensitization (eg, against peanut, milk, and egg) can occur through breast milk and be responsible for allergic reactions to antigens on a presumably first encounter.83-86 In the same studies, however, it is noted that mothers of sensitized children did not consume significantly different amounts of the respective food during pregnancy, and more importantly, children of mothers avoiding certain foods were not protected against sensitization.84 Moreover, a Cochrane Database review found no strong evidence for an allergen-avoidance diet during pregnancy or lactation, even in high-risk breast-feeding mothers, for the prevention of atopy in the child and therefore demanded further studies.82 Furthermore, the induction of oral tolerance could be accomplished in a mouse model when the antigen was transferred from the mother to the neonate through milk, even without the transfer of immunoglobulins,87 again confirming the importance of antigen contact for tolerance induction in the child.
Prematurity and low birth weight
Prematurity and low birth weight are not associated with a change in risk of food allergy in childhood.49 These factors might even be preventive for the development of atopic dermatitis.88 The risk of sensitization against other allergens, such as aeroallergens, has not been investigated, except in one study in which a substantially decreased expiratory volume, increased bronchial hyperresponsiveness, and a number of risk factors for a decrease in lung function were observed in adolescents (mean age, 17.7 years) who were born extremely preterm (gestational age ≤28 weeks).89
Exposure route to antigens
According to the dual-allergen-exposure hypothesis,50 the exposure route to an antigen (especially a food antigen) might critically determine the outcome of the immune response with environmental contact, for instance through the skin leading to sensitization, whereas oral uptake of larger amounts could induce tolerance to the food protein in the child. An exposure of the child predominantly through the environment in the absence of food consumption therefore might increase the risk of food allergy.
Breast-feeding, formula diet, and introduction of solid food
Reduced breast-feeding and early solid food introduction have been repeatedly discussed as confounders to allergy development. However, Muraro et al66 concluded in their review of observational and interventional studies that only in high-risk patients is exclusive breast-feeding for at least 4 to 6 months or with extensively hydrolyzed formula in parallel to avoidance of solid food and cow’s milk an effective preventive measure for allergic diseases, at least for atopic dermatitis/eczema and cow’s milk allergy in the first 2 years of life.90 It was further recently confirmed by an expert group set up by the Section on Pediatrics of the European Academy of Allergology and Clinical Immunology, who published an updated review on dietary prevention of allergic diseases91 (an editorial for comparison of recommendations to previous reports was published by Sicherer and Burks92), that breast-feeding might decrease the wheezing episodes associated with respiratory tract infections in children younger than 4 years.93,94 Comparing breast-feeding and different formula nutrition in a review of clinical reports, approximately 9% of newborns were found to have atopic dermatitis symptoms despite having been breast-fed.67 In an intervention study with 945 high-risk children in Germany (the German Infant Nutritional Intervention Study), allergic manifestation at 12 months of age was investigated after application of different formula diets.95 Approximately 15% of the newborns fed cow’s milk formula were affected by atopic dermatitis. Interestingly, an extensively hydrolyzed whey-based diet resulted in similarly high numbers of cases of atopic dermatitis as did cow’s milk–based formula (13%). On the contrary, partially hydrolyzed whey-based formula could decrease this percentage to 9.1%, and casein-based extensively hydrolyzed formula could decrease it to 7.1%. These preventive effects with hydrolyzed formula were found to be true for the development of atopic dermatitis, allergic manifestation (defined as any of physician-diagnosed atopic dermatitis, urticaria, and food allergy/intolerance), or both up to the age of 6 years in high-risk children having at least 1 parent or sibling with a history of allergic diseases.96,97 However, the development of asthma could not be prevented by any of the applied formulas in this study. In agreement with this, another working group, which reviewed studies on the capacity of hydrolyzed formula for the prevention of atopic diseases in high-risk children, concluded that hypoallergenic preparations are more likely to delay then prevent (only mild) symptoms.98 Summing up the present evidence, the authors of a review concluded that at present the insufficient data do not warrant any dietary intervention for the child beyond 4 to 6 months of age for protection against the development of atopic diseases.67
The previously assumed negative effect of an early introduction of solid food (before 4 months of age) could not be confirmed in a systematic review of several human randomized-controlled, case-control, and cohort studies.99 The only conclusion drawn was that early solid feeding might increase the risk of eczema, but there was missing evidence to link early feeding of solid foods to the development of persistent asthma, persistent food allergy, allergic rhinitis, or animal dander allergy. Therefore the authors made the Salomon-like proposal that physicians should temper parental concern on the one hand and educate parents that early solid foods do not confer a nutritional advantage for the child on the other hand. However, the risk for induction of celiac disease was 5 times higher when babies were fed a gluten-comprising diet within the first 3 months of age compared with a later introduction of cereals.100
Mode of birth
Birth by cesarean section has long been discussed to enhance the risk of sensitization in the child. A recent study confirmed this assumption by showing that cesarean section leads to increased numbers of patients with allergic rhinitis and atopy among children of atopic parents.51 The reason could be the lack of contact with environmental bacteria during delivery. Another working group showed that a higher number of wheezing episodes and food-specific IgE levels occurs during the first 2 years of life in children delivered by cesarean section.52 However, the same study could not confirm the abovementioned association of cesarean delivery and allergic rhinoconjunctivitis.
Epigenetic influences (gene-environment interactions)
The influence of single constituents from the environment or food might not have a direct effect on the immune response but rather act in an epigenetic form, meaning that changes in gene expression inheritable over several generations can occur without alterations in DNA sequences.101 Thereby these modifications influence the fetal epigenome already during the gestational phase in utero and play an important role in the fetal basis of adult disease susceptibility.102 The mechanisms include chromatin remodeling, changes in histone structure and acetylation state, DNA CpG-methylation, and transgene silencing on the transcriptional or posttranscriptional level, which all in turn can affect the transcription of genes.103,104 Examples regarding sensitization are the DNA methylation/demethylation state of genes for transcriptional factors, cytokine expression, antibody production, or T-lymphocyte differentiation into either TH1, TH2, or regulatory T lineages.105-109 Certain epigenetic changes affecting the allergen-specific TH2 response can therefore subsequently increase asthma and allergic diseases.101,104 In a very recent murine study, the gene Runt-related transcription factor 3 (Runx3), which is responsible for negative regulation of allergic airway disease, was found to be excessively methylated (ie, silenced) in the offspring of C57Bl/6J mice when the diet of the mother was supplemented with methyl donors, such as folic acid, vitamin B12, choline, L-methionine, zinc, and betaine, during gestation.110 Because folic acid represents one of those methyl donors, it is tempting to speculate that the increase in asthma and the worldwide recommendations for supplementation of folate in the preconeptional and gestational phases are related to each other. The observed increased allergy parameters in airways could not be revealed when the diet was given later in lactation or adulthood, pointing toward a certain time window during which epigenetic modification has far-reaching consequences for sensitization.
Apart from the diet, a variety of other environmental epigenetic influences have been revealed. For example, diesel exhaust particles from wood smoke and road traffic or tobacco smoke were shown in mouse models to induce hypermethylation at several sites of the IFN-γ promoters and hypomethylation at the CpG-408 site of the IL-4 promoter, both correlating significantly with changes in levels of IgE.111,112 Regarding tobacco smoke, one suggestive human study exists showing that grandmaternal smoking influences asthma in 5-year-old grandchildren.113 Other epigenetic factors are xenobiotic chemicals, endocrine disruptors, heavy metals,102 or low-dose radiation. Certainly, because this is a very new but fast-paced field, epigenetic influences need to be controlled in human clinical studies, especially when considering the major effect on public health.
PREVENTIVE MEASURES FOR AVOIDING ALLERGY INDUCTION
Primary prevention depends on the identification and consecutive omission or avoidance of factors that are responsible for sensitization (Table II). As more and more contradictory studies emerge, preventive measures based on sufficient epidemiologic evidence, clinical evidence, or both are urgently needed.
TABLE II.
Preventive measures to avoid sensitization
| Factor | Handling |
|---|---|
| Exposure to tobacco smoke | Avoidance by mother and child |
| Alcohol intake | Avoidance during pregnancy and lactation |
| Diet during pregnancy and lactation | Well balanced; no special diet necessary, except for allergic mother or allergic child |
| Allergen contact (eg, animal and dust) | Avoidance only if allergy already exists in mother or child |
| Solid food introduction | Avoidance up to the age of 4 months |
| Drugs | Avoidance of nonprescription drugs, self-medication, and dietary supplements; application of drugs and other substances only when recommended or prescribed by a physician |
Exposure to tobacco smoke
It can be trusted that exposure to tobacco smoke should under any circumstances be strictly avoided in both the active and passive forms. This is especially important for pregnant asthmatic patients, in whom morbidity is independent of and additive to morbidity that is due to asthma.
Alcohol intake
It is further clear that alcohol intake should be avoided by the pregnant mother, not only because of the allergy-inducing risk but also because of the well-known toxic effects for mother and fetus.
Diet of the mother during pregnancy
The diet of the expectant mother should be well balanced and consist of as many different nutrients as possible because existing literature suggests that antigens taken early in life through the oral route are needed for tolerance induction in the child.114,115 Therefore no special diet is required during pregnancy, except when the mother herself is already allergic, and then a specific food allergen–free diet is unavoidable. If atopy or allergy history exists in closely related family members (parents and siblings; ie, high-risk children), the avoidance of foods such as peanuts, nuts, fish, eggs, and sesame in the last 3 months of pregnancy might have a protective effect for the child116; however, also in this case of high-risk families, contradictory studies have emerged (reviewed in detail by Greer et al67).
Numerous individual aliments or nutrients have been described to prevent atopic diseases, such as apples and fish, vitamin D,12 vitamin E (which decreases cord blood mononuclear cell stimulation capacity),117 zinc,118 antioxidants, and different prebiotics and probiotics.119-122 For instance, the supplementation with Lactobacillus reuteri for the mother (daily from gestational week 36 until delivery) and the child (from birth until 12 months of age) resulted in less IgE-associated eczema during the second year of life (8% vs 20% in nonsupplemented children), especially in children of mothers with a history of allergy.121 Also, skin prick test reactivity was less common in the treated compared with the placebo group. Confirming these observations, another human interventional study proved the immunoprotective potency of probiotics given during pregnancy and lactation on the development of atopic eczema in the first 2 years of the baby’s life, and again the effect was more pronounced in children with increased IgE levels in cord blood.123 An experimental study in a mouse model of respiratory allergy revealed that an underlying mechanism of the positive effects of probiotic supplementation in early life is related to the induction of regulatory T cells (TGF-β production, Foxp3 positive).124 Similar results were obtained in animal studies, in which supplementation with perinatal Lactobacillus rhamnosus GG122 or LPS78 reduced expression of TH2 cytokines in splenocytes and allergic airway and peribronchial inflammation in offspring derived from supplemented mother mice. Providing prebiotic or probiotic substances for mother and child125 (mouse model122) might force the colonization of symbiotic bacteria. However, a recent placebo-controlled human trial showed no effect of probiotic Lactobacillus rhamnosus GG consumption during pregnancy (4-6 weeks before delivery and 6 months postnatally) on the prevention of atopic dermatitis at the age of 2 years in high-risk children but was even associated with an increased risk of recurrent episodes of wheezing.53 These results implicate that probiotics might not be generally useful for the primary prevention of atopic diseases.
A recent multicenter, randomized, double-blind, placebo-controlled trial with 311 apparently healthy mothers detected that supplementation with fish oil during pregnancy is associated with decreased mRNA levels of TH2-related molecules in the cord blood (IL-4, IL-13, and CCR4), reduced numbers of natural killer cells and CCR3+CD8+ T cells, and decreased levels of inflammatory cytokines (IL-1 and IFN-γ) in maternal blood.124 The authors speculate that both effects are mediated by TGF-β, levels of which were increased in the fish oil–supplemented group in both maternal peripheral blood and cord blood.
Despite these encouraging effects of different nutrients, human studies showed that there was no longer an association between maternal intake of most foods during pregnancy and asthma and respiratory and allergic outcomes in 5-year-old children,39,82,116,127,128 and it appeared that the protective effect in children of atopic parents was primarily true only during the first year of life.116
Avoidance of allergens during pregnancy
Avoidance of allergens other than allergy-provoking foods (eg, cats, dogs, horses, and house dust) is only necessary if patients are already sensitized. However, allergen avoidance might not be preventive or suitable in all cohorts.129 Furthermore, to prevent sensitization in the fetus/newborn, allergen avoidance might not be effective during pregnancy because the priming of specific TH2 cells seems to occur entirely after birth, at least for house dust mite specificity.130
Diet of the mother during lactation
Also during lactation, a special diet by the mother or, if not breast-feeding, hypoallergenic formula nutrition for the baby should only be considered in case of an established high risk of atopy or existing allergy and individual circumstances.82,127 This is especially true because studies have shown that even food avoidance by the mother might not substantially reduce the allergen contact of the child.131 In this respect the government recommendations have to be formulated and distributed very carefully because it was found that peanut allergy avoidance was overdone by pregnant women: although only recommended for women with atopy risk, 65% of pregnant women who had heard or read about the recommendation had avoided peanuts during pregnancy.39,132
Diet of the newborn
To date, breast-feeding is strongly recommended by midwives and physicians. Some positive constituents in milk have been described for the prevention of allergic disorders or pathogen-related diseases in neonates. For instance, the content of oligosaccharides in the milk of healthy mothers seems to reduce the risk of allergic disorders through prebiotic effects on the intestinal flora.119 Animal studies have shown a downregulation of TH2 responses and allergic airway parameters in mice by feedings with oligosaccharides.133,134 The TH2-reducing effect was also shown in human studies, in which supplementation of infants with oligosaccharides resulted in a significantly reduced IgE serum level and a reduced incidence of atopic dermatitis in 6-month-old high-risk children.135
Maternal milk also has the potency to induce oral tolerance to food and environmental antigens by means of immunosuppressive activity.126,136 There was no difference between milk from mothers with or without atopic dermatitis regarding the fatty acid composition; however, the content of TGF-β was much lower in patients with dermatitis.137 Furthermore, the work by Zizka et al136 proved that colostrum and milk from allergic or healthy mothers do induce similar in vitro proliferation and anti-body production by cord blood lymphocytes of neonates; however, these in vitro reactions were significantly greater in cells from children of allergic mothers.
Taking all the studies together, there is not enough evidence that breast-feeding might be more protective against allergies than bottle feeding. Despite the contrasting results regarding protection from allergic diseases, there are of course a number of other advantages of breast-feeding. In addition to the delivery of pathogen-specific IgG or IgA antibodies,138,139 which contribute to the primary protection of the immature immune system of the child, breast-feeding provides the necessary factors for colonization and maturation of the neonatal gastrointestinal tract and its immune defense mechanisms, respectively. Nevertheless, it should not be done exclusively for longer than 9 months because this increases the risk for atopic diseases, such as atopic dermatitis and food hypersensitivity, in children with a family history of allergy.54
Another topic of controversial discussion is the introduction of solid food into the nutrition of the baby. It has been suggested that the introduction of solid foods in general should be postponed until 6 months of age and the introduction of allergy-provoking food even further: milk products until 12 months, hen’s eggs until 24 months, and peanuts, tree nuts, fish, and seafood until at least 36 months.140-142 On the other hand, contact with and exposure to antigens might be necessary in early life to educate the immune system in the direction of tolerance, which is an active and specific process like sensitization.
Therefore the overall literature on delayed introduction of solids leads to conflicting results, and some authors suggest that there is not enough evidence to recommend delay of solid food introduction beyond the age of 4 months.90
Antiacid treatment
In recent studies we have found that antiacid drugs can induce sensitization in adults.17,24,25 Further human studies are needed, and although this is not included in the World Health Organization guidelines yet, it seems advisable to start with nonmedication treatment, such as avoiding high amounts of food shortly before bedtime, sleeping with an elevated upper body, and avoiding lots of coffee, sweets, and fatty foodstuffs. Also, smoking should be stopped by the mother for prevention of reflux.
SUMMARY
The list of preventive measures for pregnant women to avoid allergy induction for themselves and their growing children is short because of increasing numbers of controversial studies. Exposure to tobacco smoke in active or passive forms, as well as alcohol consumption, should be avoided. No special diet for the mother during pregnancy and lactation is necessary, except when either the mother or the child has a diagnosed sensitization against food antigens. Avoidance of other allergen sources, such as pets, house dust, contact allergens, or drugs, is necessary for mother and child only when sensitization is already diagnosed. Breast-feeding is recommended during the first 4 months and should in any case be no longer than 9 months exclusively. Special hypoallergenic formulas (extensively hydrolyzed protein formula or amino acid based formula) should be used for high-risk children to prevent the development of atopic dermatitis or if the child is already symptomatic. All nonprescription drugs (eg, acid-suppressing drugs) should be avoided during pregnancy and lactation, unless recommended by the attending physician.
FUTURE PROSPECTS
More clinical human studies are urgently needed for the development of final and evidence-based recommendations to prevent allergy induction. First, it is necessary to reveal the effect of single nutrients and their composition in different foods on the development of allergic diseases separately in mother and child. Furthermore, the effect of environmental factors on the occurrence of allergic diseases, allergenicity of proteins (eg, through food processing and environmental factors), or both needs to be elucidated. The definition of possible time windows in unborn/newborn/young children for provoking or preventing allergy (eg, special diet of the mother during pregnancy and time of introduction of solid food) is another aspect to be clarified, with a special focus on epigenetic influences. Last but not least, the effects of different kinds of drugs (eg, antiacid medication and antibiotics) and dietary supplements on the immune status of mother or child are largely unknown and should be revealed. Even more challenging, all the abovementioned problems and questions need to be separately investigated on the atopic or nonatopic family background.
Acknowledgments
We thank Verena Schöll for designing the graphic arts in Fig 1.
Supported by Hertha Firnberg stipends T283-B13 and SFB F1808-B13 of the Austrian Science Fund (FWF).
Abbreviations used
- CLA
Conjugated linoleic acid
- PUFA
Polyunsaturated fatty acid
Footnotes
Disclosure of potential conflict of interest: I. Pali-Schöll has received research support from the Austrian Science Fund. The rest of the authors have declared that they have no conflict of interest.
REFERENCES
- 1.Kulig M, Bergmann R, Klettke U, Wahn V, Tacke U, Wahn U. Natural course of sensitization to food and inhalant allergens during the first 6 years of life. J Allergy Clin Immunol. 1999;103:1173–9. doi: 10.1016/s0091-6749(99)70195-8. [DOI] [PubMed] [Google Scholar]
- 2.Anderson HR, Gupta R, Strachan DP, Limb ES. 50 years of asthma: UK trends from 1955 to 2004. Thorax. 2007;62:85–90. doi: 10.1136/thx.2006.066407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haahtela T, von Hertzen L, Makela M, Hannuksela M. Finnish Allergy Programme 2008-2018—time to act and change the course. Allergy. 2008;63:634–45. doi: 10.1111/j.1398-9995.2008.01712.x. [DOI] [PubMed] [Google Scholar]
- 4.Schernhammer ES, Vutuc C, Waldhor T, Haidinger G. Time trends of the prevalence of asthma and allergic disease in Austrian children. Pediatr Allergy Immunol. 2008;19:125–31. doi: 10.1111/j.1399-3038.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 5.Bjorksten B, Clayton T, Ellwood P, Stewart A, Strachan D. Worldwide time trends for symptoms of rhinitis and conjunctivitis: phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19:110–24. doi: 10.1111/j.1399-3038.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 6.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–74. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 7.Miyake Y, Sasaki S, Ohya Y, Miyamoto S, Matsunaga I, Yoshida T, et al. Dietary intake of seaweed and minerals and prevalence of allergic rhinitis in Japanese pregnant females: baseline data from the Osaka Maternal and Child Health Study. Ann Epidemiol. 2006;16:614–21. doi: 10.1016/j.annepidem.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Wjst M, Hypponen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62:1085–6. doi: 10.1111/j.1398-9995.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 9.Wjst M, Dold S. Genes, factor X, and allergens: what causes allergic diseases? Allergy. 1999;54:757–9. doi: 10.1034/j.1398-9995.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- 10.Milner JD, Stein DM, McCarter R, Moon RY. Early infant multivitamin supplementation is associated with increased risk for food allergy and asthma. Pediatrics. 2004;114:27–32. doi: 10.1542/peds.114.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Camargo CA, Jr, Clark S, Kaplan MS, Lieberman P, Wood RA. Regional differences in EpiPen prescriptions in the United States: the potential role of vitamin D. J Allergy Clin Immunol. 2007;120:131–6. doi: 10.1016/j.jaci.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 12.Camargo CA, Jr, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. Am J Clin Nutr. 2007;85:788–95. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sole D, Wandalsen GF, Camelo-Nunes IC, Naspitz CK. Prevalence of symptoms of asthma, rhinitis, and atopic eczema among Brazilian children and adolescents identified by the International Study of Asthma and Allergies in Childhood (ISAAC)—phase 3. J Pediatr (Rio J) 2006;82:341–6. doi: 10.2223/JPED.1521. [DOI] [PubMed] [Google Scholar]
- 14.McGlade JP, Gorman S, Zosky GR, Larcombe AN, Sly PD, Finlay-Jones JJ, et al. Suppression of the asthmatic phenotype by ultraviolet B-induced, antigen-specific regulatory cells. Clin Exp Allergy. 2007;37:1267–76. doi: 10.1111/j.1365-2222.2007.02750.x. [DOI] [PubMed] [Google Scholar]
- 15.Schauber J, Gallo RL. Vitamin D deficiency and asthma: not a strong link—yet. J Allergy Clin Immunol. 2008;121:782–3. doi: 10.1016/j.jaci.2007.12.1170. author reply 3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penn AL, Rouse RL, Horohov DW, Kearney MT, Paulsen DB, Lomax L. In utero exposure to environmental tobacco smoke potentiates adult responses to allergen in BALB/c mice. Environ Health Perspect. 2007;115:548–55. doi: 10.1289/ehp.9780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakos N, Schöll I, Szalai K, Kundi M, Untersmayr E, Jensen-Jarolim E. Risk assessment in elderly for sensitization to food and respiratory allergens. Immunol Lett. 2006;107:15–21. doi: 10.1016/j.imlet.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Dotterud LK, Smith-Sivertsen T. Allergic contact sensitization in the general adult population: a population-based study from Northern Norway. Contact Dermatitis. 2007;56:10–5. doi: 10.1111/j.1600-0536.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Quintela A, Vidal C, Gude F. Alcohol-induced alterations in serum immunoglobulin e (IgE) levels in human subjects. Front Biosci. 2002;7:e234–44. doi: 10.2741/A919. [DOI] [PubMed] [Google Scholar]
- 20.Linneberg A, Hertzum I, Husemoen LL, Johansen N, Jorgensen T. Association between alcohol consumption and aeroallergen sensitization in Danish adults. Clin Exp Allergy. 2006;36:714–21. doi: 10.1111/j.1365-2222.2006.02507.x. [DOI] [PubMed] [Google Scholar]
- 21.Linneberg A, Roursgaard M, Hersoug LG, Larsen ST. Effects of alcohol consumption on the allergen-specific immune response in mice. Alcohol Clin Exp Res. 2008;32:553–6. doi: 10.1111/j.1530-0277.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 22.Assing K, Bodtger U, Linneberg A, Malling HJ, Poulsen LK. Association between alcohol consumption and skin prick test reactivity to aeroallergens. Ann Allergy Asthma Immunol. 2007;98:70–4. doi: 10.1016/S1081-1206(10)60862-9. [DOI] [PubMed] [Google Scholar]
- 23.Marrero JM, Goggin PM, de Caestecker JS, Pearce JM, Maxwell JD. Determinants of pregnancy heartburn. Br J Obstet Gynaecol. 1992;99:731–4. doi: 10.1111/j.1471-0528.1992.tb13873.x. [DOI] [PubMed] [Google Scholar]
- 24.Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, et al. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–60. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- 25.Untersmayr E, Bakos N, Schöll I, Kundi M, Roth-Walter F, Szalai K, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656–8. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- 26.Schöll I, Ackermann U, Ozdemir C, Blümer N, Dicke T, Sel S, et al. Anti-ulcer treatment during pregnancy induces food allergy in mouse mothers and a Th2-bias in their offspring. FASEB J. 2007;21:1264–70. doi: 10.1096/fj.06-7223com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jarrett EE, Hall E. IgE suppression by maternal IgG. Immunology. 1983;48:49–58. [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer M, Orescovic I, Schoell WM, Bianchi DW, Pertl B. Detection of maternal deoxyribonucleic acid in umbilical cord plasma by using fluorescent polymerase chain reaction amplification of short tandem repeat sequences. Am J Obstet Gynecol. 2002;186:117–20. doi: 10.1067/mob.2002.118306. [DOI] [PubMed] [Google Scholar]
- 29.Michie CA, Tantscher E, Schall T, Rot A. Physiological secretion of chemokines in human breast milk. Eur Cytokine Netw. 1998;9:123–9. [PubMed] [Google Scholar]
- 30.Szepfalusi Z, Loibichler C, Pichler J, Reisenberger K, Ebner C, Urbanek R. Direct evidence for transplacental allergen transfer. Pediatr Res. 2000;48:404–7. doi: 10.1203/00006450-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 31.Szepfalusi Z, Loibichler C, Hanel-Dekan S, Dehlink E, Gerstmayr M, Pichler J, et al. Most of diaplacentally transferred allergen is retained in the placenta. Clin Exp Allergy. 2006;36:1130–7. doi: 10.1111/j.1365-2222.2006.02559.x. [DOI] [PubMed] [Google Scholar]
- 32.Loibichler C, Pichler J, Gerstmayr M, Bohle B, Kisst H, Urbanek R, et al. Materno-fetal passage of nutritive and inhalant allergens across placentas of term and pre-term deliveries perfused in vitro. Clin Exp Allergy. 2002;32:1546–51. doi: 10.1046/j.1365-2222.2002.01479.x. [DOI] [PubMed] [Google Scholar]
- 33.Victor JR, Jr, Fusaro AE, Duarte AJ, Sato MN. Preconception maternal immunization to dust mite inhibits the type I hypersensitivity response of offspring. J Allergy Clin Immunol. 2003;111:269–77. doi: 10.1067/mai.2003.39. [DOI] [PubMed] [Google Scholar]
- 34.Uthoff H, Spenner A, Reckelkamm W, Ahrens B, Wolk G, Hackler R, et al. Critical role of preconceptional immunization for protective and nonpathological specific immunity in murine neonates. J Immunol. 2003;171:3485–92. doi: 10.4049/jimmunol.171.7.3485. [DOI] [PubMed] [Google Scholar]
- 35.Dehlink E, Yen E, Leichtner AM, Hait EJ, Fiebiger E. First evidence of a possible association between gastric acid suppression during pregnancy and childhood asthma: a population-based register study. Clin Exp Allergy. 2009;39:246–53. doi: 10.1111/j.1365-2222.2008.03125.x. [DOI] [PubMed] [Google Scholar]
- 36.Kemp AS. Allergy and gastric acid suppression. Clin Exp Allergy. 2009;39:176–8. doi: 10.1111/j.1365-2222.2008.03141.x. [DOI] [PubMed] [Google Scholar]
- 37.Sausenthaler S, Koletzko S, Schaaf B, Lehmann I, Borte M, Herbarth O, et al. Maternal diet during pregnancy in relation to eczema and allergic sensitization in the offspring at 2 y of age. Am J Clin Nutr. 2007;85:530–7. doi: 10.1093/ajcn/85.2.530. [DOI] [PubMed] [Google Scholar]
- 38.Devereux G, Seaton A. Diet as a risk factor for atopy and asthma. J Allergy Clin Immunol. 2005;115:1109–18. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]
- 39.Willers SM, Devereux G, Craig LC, McNeill G, Wijga AH, El-Magd W Abou, et al. Maternal food consumption during pregnancy and asthma, respiratory and atopic symptoms in 5-year-old children. Thorax. 2007;62:772–8. doi: 10.1136/thx.2006.074187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ushiyama Y, Matsumoto K, Shinohara M, Wakiguchi H, Sakai K, Komatsu T, et al. Nutrition during pregnancy may be associated with allergic diseases in infants. J Nutr Sci Vitaminol (Tokyo) 2002;48:345–51. doi: 10.3177/jnsv.48.345. [DOI] [PubMed] [Google Scholar]
- 41.Fitzsimon N, Fallon U, O’Mahony D, Loftus BG, Bury G, Murphy AW, et al. Mothers’ dietary patterns during pregnancy and risk of asthma symptoms in children at 3 years. Ir Med J. 2007;100(suppl):27–32. [PubMed] [Google Scholar]
- 42.Kulig M, Luck W, Wahn U. The association between pre- and postnatal tobacco smoke exposure and allergic sensitization during early childhood. Multicentre Allergy Study Group. Germany. Hum Exp Toxicol. 1999;18:241–4. doi: 10.1191/096032799678839987. [DOI] [PubMed] [Google Scholar]
- 43.el-Nawawy A, Soliman AT, el-Azzouni O, Amer el S, Demian S, el-Sayed M. Effect of passive smoking on frequency of respiratory illnesses and serum immunoglobulin-E (IgE) and interleukin-4 (IL-4) concentrations in exposed children. J Trop Pediatr. 1996;42:166–9. doi: 10.1093/tropej/42.3.166. [DOI] [PubMed] [Google Scholar]
- 44.Ronchetti R, Macri F, Ciofetta G, Indinnimeo L, Cutrera R, Bonci E, et al. Increased serum IgE and increased prevalence of eosinophilia in 9-year-old children of smoking parents. J Allergy Clin Immunol. 1990;86:400–7. doi: 10.1016/s0091-6749(05)80104-6. [DOI] [PubMed] [Google Scholar]
- 45.Horak E, Morass B, Ulmer H. Association between environmental tobacco smoke exposure and wheezing disorders in Austrian preschool children. Swiss Med Wkly. 2007;137:608–13. doi: 10.4414/smw.2007.11870. [DOI] [PubMed] [Google Scholar]
- 46.Linneberg A, Petersen J, Gronbaek M, Benn CS. Alcohol during pregnancy and atopic dermatitis in the offspring. Clin Exp Allergy. 2004;34:1678–83. doi: 10.1111/j.1365-2222.2004.02101.x. [DOI] [PubMed] [Google Scholar]
- 47.Alm B, Erdes L, Mollborg P, Pettersson R, Norvenius SG, Aberg N, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics. 2008;121:697–702. doi: 10.1542/peds.2007-1232. [DOI] [PubMed] [Google Scholar]
- 48.Johnson CC, Ownby DR, Alford SH, Havstad SL, Williams LK, Zoratti EM, et al. Antibiotic exposure in early infancy and risk for childhood atopy. J Allergy Clin Immunol. 2005;115:1218–24. doi: 10.1016/j.jaci.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Liem JJ, Kozyrskyj AL, Huq SI, Becker AB. The risk of developing food allergy in premature or low-birth-weight children. J Allergy Clin Immunol. 2007;119:1203–9. doi: 10.1016/j.jaci.2006.12.671. [DOI] [PubMed] [Google Scholar]
- 50.Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121:1331–6. doi: 10.1016/j.jaci.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 51.Pistiner M, Gold DR, Abdulkerim H, Hoffman E, Celedon JC. Birth by cesarean section, allergic rhinitis, and allergic sensitization among children with a parental history of atopy. J Allergy Clin Immunol. 2008;122:274–9. doi: 10.1016/j.jaci.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Negele K, Heinrich J, Borte M, von Berg A, Schaaf B, Lehmann I, et al. Mode of delivery and development of atopic disease during the first 2 years of life. Pediatr Allergy Immunol. 2004;15:48–54. doi: 10.1046/j.0905-6157.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 53.Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics. 2008;121:e850–6. doi: 10.1542/peds.2007-1492. [DOI] [PubMed] [Google Scholar]
- 54.Pesonen M, Kallio MJ, Ranki A, Siimes MA. Prolonged exclusive breastfeeding is associated with increased atopic dermatitis: a prospective follow-up study of unselected healthy newborns from birth to age 20 years. Clin Exp Allergy. 2006;36:1011–8. doi: 10.1111/j.1365-2222.2006.02526.x. [DOI] [PubMed] [Google Scholar]
- 55.Renz H, Blumer N, Virna S, Sel S, Garn H. The immunological basis of the hygiene hypothesis. Chem Immunol Allergy. 2006;91:30–48. doi: 10.1159/000090228. [DOI] [PubMed] [Google Scholar]
- 56.Hersoug LG. A reformulation of the hygiene hypothesis: maternal infectious diseases confer protection against asthma in the infant. Med Hypotheses. 2006;67:717–20. doi: 10.1016/j.mehy.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 57.Prescott SL, Irvine J, Dunstan JA, Hii C, Ferrante A. Protein kinase Czeta: a novel protective neonatal T-cell marker that can be upregulated by allergy prevention strategies. J Allergy Clin Immunol. 2007;120:200–6. doi: 10.1016/j.jaci.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 58.Jolly CA, Jiang YH, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids suppress murine lymphoproliferation, interleukin-2 secretion, and the formation of diacylglycerol and ceramide. J Nutr. 1997;127:37–43. doi: 10.1093/jn/127.1.37. [DOI] [PubMed] [Google Scholar]
- 59.O’Shea M, Bassaganya-Riera J, Mohede IC. Immunomodulatory properties of conjugated linoleic acid. Am J Clin Nutr. 2004;79(suppl):1199S–206S. doi: 10.1093/ajcn/79.6.1199S. [DOI] [PubMed] [Google Scholar]
- 60.Yu Y, Correll PH, Heuvel JP Vanden. Conjugated linoleic acid decreases production of pro-inflammatory products in macrophages: evidence for a PPAR gamma-dependent mechanism. Biochim Biophys Acta. 2002;1581:89–99. doi: 10.1016/s1388-1981(02)00126-9. [DOI] [PubMed] [Google Scholar]
- 61.Moya-Camarena SY, Heuvel JP Vanden, Blanchard SG, Leesnitzer LA, Belury MA. Conjugated linoleic acid is a potent naturally occurring ligand and activator of PPARalpha. J Lipid Res. 1999;40:1426–33. [PubMed] [Google Scholar]
- 62.Jaudszus A, Krokowski M, Mockel P, Darcan Y, Avagyan A, Matricardi P, et al. Cis-9,trans-11-conjugated linoleic acid inhibits allergic sensitization and airway inflammation via a PPARgamma-related mechanism in mice. J Nutr. 2008;138:1336–42. doi: 10.1093/jn/138.7.1336. [DOI] [PubMed] [Google Scholar]
- 63.Korotkova M, Telemo E, Yamashiro Y, Hanson LA, Strandvik B. The ratio of n-6 to n-3 fatty acids in maternal diet influences the induction of neonatal immunological tolerance to ovalbumin. Clin Exp Immunol. 2004;137:237–44. doi: 10.1111/j.1365-2249.2004.02527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunstan JA, Mitoulas LR, Dixon G, Doherty DA, Hartmann PE, Simmer K, et al. The effects of fish oil supplementation in pregnancy on breast milk fatty acid composition over the course of lactation: a randomized controlled trial. Pediatr Res. 2007;62:689–94. doi: 10.1203/PDR.0b013e318159a93a. [DOI] [PubMed] [Google Scholar]
- 65.Dunstan JA, Mori TA, Barden A, Beilin LJ, Taylor AL, Holt PG, et al. Fish oil supplementation in pregnancy modifies neonatal allergen-specific immune responses and clinical outcomes in infants at high risk of atopy: a randomized, controlled trial. J Allergy Clin Immunol. 2003;112:1178–84. doi: 10.1016/j.jaci.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 66.Muraro A, Dreborg S, Halken S, Host A, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Part III: critical review of published peer-reviewed observational and interventional studies and final recommendations. Pediatr Allergy Immunol. 2004;15:291–307. doi: 10.1111/j.1399-3038.2004.00127.x. [DOI] [PubMed] [Google Scholar]
- 67.Greer FR, Sicherer SH, Burks AW. Effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics. 2008;121:183–91. doi: 10.1542/peds.2007-3022. [DOI] [PubMed] [Google Scholar]
- 68.Noakes PS, Holt PG, Prescott SL. Maternal smoking in pregnancy alters neonatal cytokine responses. Allergy. 2003;58:1053–8. doi: 10.1034/j.1398-9995.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 69.Lindfors A, van Hage-Hamsten M, Rietz H, Wickman M, Nordvall SL. Influence of interaction of environmental risk factors and sensitization in young asthmatic children. J Allergy Clin Immunol. 1999;104:755–62. doi: 10.1016/s0091-6749(99)70284-8. [DOI] [PubMed] [Google Scholar]
- 70.Sharma SK, Banga A. Prevalence and risk factors for wheezing in children from rural areas of north India. Allergy Asthma Proc. 2007;28:647–53. doi: 10.2500/aap.2007.28.3059. [DOI] [PubMed] [Google Scholar]
- 71.Palvo F, Toledo EC, Menin AM, Jorge PP, Godoy MF, Sole D. Risk Factors of Childhood Asthma in Sao Jose do Rio Preto, Sao Paulo, Brazil. J Trop Pediatr. 2008;54:253–7. doi: 10.1093/tropej/fmn007. [DOI] [PubMed] [Google Scholar]
- 72.Lannero E, Wickman M, van Hage M, Bergstrom A, Pershagen G, Nordvall L. Exposure to environmental tobacco smoke and sensitisation in children. Thorax. 2008;63:172–6. doi: 10.1136/thx.2007.079053. [DOI] [PubMed] [Google Scholar]
- 73.Venter C, Pereira B, Grundy J, Clayton CB, Roberts G, Higgins B, et al. Incidence of parentally reported and clinically diagnosed food hypersensitivity in the first year of life. J Allergy Clin Immunol. 2006;117:1118–24. doi: 10.1016/j.jaci.2005.12.1352. [DOI] [PubMed] [Google Scholar]
- 74.Raherison C, Penard-Morand C, Moreau D, Caillaud D, Charpin D, Kopferschmitt C, et al. Smoking exposure and allergic sensitization in children according to maternal allergies. Ann Allergy Asthma Immunol. 2008;100:351–7. doi: 10.1016/S1081-1206(10)60598-4. [DOI] [PubMed] [Google Scholar]
- 75.Levy J. The effects of antibiotic use on gastrointestinal function. Am J Gastroenterol. 2000;95(suppl):S8–10. doi: 10.1016/s0002-9270(99)00808-4. [DOI] [PubMed] [Google Scholar]
- 76.Kirjavainen PV, Gibson GR. Healthy gut microflora and allergy: factors influencing development of the microbiota. Ann Med. 1999;31:288–92. doi: 10.3109/07853899908995892. [DOI] [PubMed] [Google Scholar]
- 77.Bjorksten B. Environmental influences on the development of the immune system: consequences for disease outcome. Nestle Nutr Workshop Ser Pediatr Program. 2008;61:243–54. doi: 10.1159/000113498. [DOI] [PubMed] [Google Scholar]
- 78.Blumer N, Herz U, Wegmann M, Renz H. Prenatal lipopolysaccharide-exposure prevents allergic sensitization and airway inflammation, but not airway responsiveness in a murine model of experimental asthma. Clin Exp Allergy. 2005;35:397–402. doi: 10.1111/j.1365-2222.2005.02184.x. [DOI] [PubMed] [Google Scholar]
- 79.Gerhold K, Avagyan A, Seib C, Frei R, Steinle J, Ahrens B, et al. Prenatal initiation of endotoxin airway exposure prevents subsequent allergen-induced sensitization and airway inflammation in mice. J Allergy Clin Immunol. 2006;118:666–73. doi: 10.1016/j.jaci.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 80.Sudo N, Sawamura S, Tanaka K, Aiba Y, Kubo C, Koga Y. The requirement of intestinal bacterial flora for the development of an IgE production system fully susceptible to oral tolerance induction. J Immunol. 1997;159:1739–45. [PubMed] [Google Scholar]
- 81.Zutavern A, von Klot S, Gehring U, Krauss-Etschmann S, Heinrich J. Pre-natal and post-natal exposure to respiratory infection and atopic diseases development: a historical cohort study. Respir Res. 2006;7:81. doi: 10.1186/1465-9921-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kramer MS, Kakuma R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Cochrane Database Syst Rev. 2006;(3):CD000133. doi: 10.1002/14651858.CD000133.pub2. [DOI] [PubMed] [Google Scholar]
- 83.Hourihane JO, Dean TP, Warner JO. Peanut allergy in relation to heredity, maternal diet, and other atopic diseases: results of a questionnaire survey, skin prick testing, and food challenges. BMJ. 1996;313:518–21. doi: 10.1136/bmj.313.7056.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gerrard JW, Perelmutter L. IgE-mediated allergy to peanut, cow’s milk, and egg in children with special reference to maternal diet. Ann Allergy. 1986;56:351–4. [PubMed] [Google Scholar]
- 85.Duchen K, Bjorksten B. Sensitization via the breast milk. Adv Exp Med Biol. 1991;310:427–36. doi: 10.1007/978-1-4615-3838-7_55. [DOI] [PubMed] [Google Scholar]
- 86.Warner JO. Food allergy in fully breast-fed infants. Clin Allergy. 1980;10:133–6. doi: 10.1111/j.1365-2222.1980.tb02090.x. [DOI] [PubMed] [Google Scholar]
- 87.Verhasselt V, Milcent V, Cazareth J, Kanda A, Fleury S, Dombrowicz D, et al. Breast milk-mediated transfer of an antigen induces tolerance and protection from allergic asthma. Nat Med. 2008;14:170–5. doi: 10.1038/nm1718. [DOI] [PubMed] [Google Scholar]
- 88.Buhrer C, Grimmer I, Niggemann B, Obladen M. Low 1-year prevalence of atopic eczema in very low birthweight infants. Lancet. 1999;353:1674. doi: 10.1016/S0140-6736(98)03975-0. [DOI] [PubMed] [Google Scholar]
- 89.Halvorsen T, Skadberg BT, Eide GE, Roksund OD, Carlsen KH, Bakke P. Pulmonary outcome in adolescents of extreme preterm birth: a regional cohort study. Acta Paediatr. 2004;93:1294–300. [PubMed] [Google Scholar]
- 90.Gdalevich M, Mimouni D, David M, Mimouni M. Breast-feeding and the onset of atopic dermatitis in childhood: a systematic review and meta-analysis of prospective studies. J Am Acad Dermatol. 2001;45:520–7. doi: 10.1067/mjd.2001.114741. [DOI] [PubMed] [Google Scholar]
- 91.Host A, Halken S, Muraro A, Dreborg S, Niggemann B, Aalberse R, et al. Dietary prevention of allergic diseases in infants and small children. Pediatr Allergy Immunol. 2008;19:1–4. doi: 10.1111/j.1399-3038.2007.00680.x. [DOI] [PubMed] [Google Scholar]
- 92.Sicherer SH, Burks AW. Maternal and infant diets for prevention of allergic diseases: understanding menu changes in 2008. J Allergy Clin Immunol. 2008;122:29–33. doi: 10.1016/j.jaci.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 93.Oddy WH. Breastfeeding and asthma in children: findings from a West Australian study. Breastfeed Rev. 2000;8:5–11. [PubMed] [Google Scholar]
- 94.Kull I, Almqvist C, Lilja G, Pershagen G, Wickman M. Breast-feeding reduces the risk of asthma during the first 4 years of life. J Allergy Clin Immunol. 2004;114:755–60. doi: 10.1016/j.jaci.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 95.von Berg A, Koletzko S, Grubl A, Filipiak-Pittroff B, Wichmann HE, Bauer CP, et al. The effect of hydrolyzed cow’s milk formula for allergy prevention in the first year of life: the German Infant Nutritional Intervention Study, a randomized double-blind trial. J Allergy Clin Immunol. 2003;111:533–40. doi: 10.1067/mai.2003.101. [DOI] [PubMed] [Google Scholar]
- 96.von Berg A, Koletzko S, Filipiak-Pittroff B, Laubereau B, Grubl A, Wichmann HE, et al. Certain hydrolyzed formulas reduce the incidence of atopic dermatitis but not that of asthma: three-year results of the German Infant Nutritional Intervention Study. J Allergy Clin Immunol. 2007;119:718–25. doi: 10.1016/j.jaci.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 97.von Berg A, Filipiak-Pittroff B, Kramer U, Link E, Bollrath C, Brockow I, et al. Preventive effect of hydrolyzed infant formulas persists until age 6 years: longterm results from the German Infant Nutritional Intervention Study (GINI) J Allergy Clin Immunol. 2008;121:1442–7. doi: 10.1016/j.jaci.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 98.Brand PL, Vlieg-Boerstra BJ, Dubois AE. Dietary prevention of allergic disease in children: are current recommendations really based on good evidence? Pediatr Allergy Immunol. 2007;18:475–9. doi: 10.1111/j.1399-3038.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 99.Tarini BA, Carroll AE, Sox CM, Christakis DA. Systematic review of the relationship between early introduction of solid foods to infants and the development of allergic disease. Arch Pediatr Adolesc Med. 2006;160:502–7. doi: 10.1001/archpedi.160.5.502. [DOI] [PubMed] [Google Scholar]
- 100.Norris JM, Barriga K, Hoffenberg EJ, Taki I, Miao D, Haas JE, et al. Risk of celiac disease autoimmunity and timing of gluten introduction in the diet of infants at increased risk of disease. JAMA. 2005;293:2343–51. doi: 10.1001/jama.293.19.2343. [DOI] [PubMed] [Google Scholar]
- 101.Miller RL, Ho SM. Environmental epigenetics and asthma: current concepts and call for studies. Am J Respir Crit Care Med. 2008;177:567–73. doi: 10.1164/rccm.200710-1511PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord. 2007;8:173–82. doi: 10.1007/s11154-007-9042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller RL. Prenatal maternal diet affects asthma risk in offspring. J Clin Invest. 2008;118:3265–8. doi: 10.1172/JCI37171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bousquet J, Jacot W, Yssel H, Vignola AM, Humbert M. Epigenetic inheritance of fetal genes in allergic asthma. Allergy. 2004;59:138–47. doi: 10.1046/j.1398-9995.2003.00359.x. [DOI] [PubMed] [Google Scholar]
- 105.Kim HP, Leonard WJ. CREB/ATF-dependent T cell receptor-induced FoxP3 gene expression: a role for DNA methylation. J Exp Med. 2007;204:1543–51. doi: 10.1084/jem.20070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee DU, Agarwal S, Rao A. Th2 lineage commitment and efficient IL-4 production involves extended demethylation of the IL-4 gene. Immunity. 2002;16:649–60. doi: 10.1016/s1074-7613(02)00314-x. [DOI] [PubMed] [Google Scholar]
- 107.Shin HJ, Park HY, Jeong SJ, Park HW, Kim YK, Cho SH, et al. STAT4 expression in human T cells is regulated by DNA methylation but not by promoter polymorphism. J Immunol. 2005;175:7143–50. doi: 10.4049/jimmunol.175.11.7143. [DOI] [PubMed] [Google Scholar]
- 108.Fields PE, Kim ST, Flavell RA. Cutting edge: changes in histone acetylation at the IL-4 and IFN-gamma loci accompany Th1/Th2 differentiation. J Immunol. 2002;169:647–50. doi: 10.4049/jimmunol.169.2.647. [DOI] [PubMed] [Google Scholar]
- 109.van Panhuys N, Le Gros G, McConnell MJ. Epigenetic regulation of Th2 cytokine expression in atopic diseases. Tissue Antigens. 2008;72:91–7. doi: 10.1111/j.1399-0039.2008.01068.x. [DOI] [PubMed] [Google Scholar]
- 110.Hollingsworth JW, Maruoka S, Boon K, Garantziotis S, Li Z, Tomfohr J, et al. In utero supplementation with methyl donors enhances allergic airway disease in mice. J Clin Invest. 2008;118:3462–9. doi: 10.1172/JCI34378. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 111.Liu J, Ballaney M, Al-alem U, Quan C, Jin X, Perera F, et al. Combined inhaled diesel exhaust particles and allergen exposure alter methylation of T helper genes and IgE production in vivo. Toxicol Sci. 2008;102:76–81. doi: 10.1093/toxsci/kfm290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Samuelsen M, Nygaard UC, Lovik M. Allergy adjuvant effect of particles from wood smoke and road traffic. Toxicology. 2008;246:124–31. doi: 10.1016/j.tox.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 113.Li YF, Langholz B, Salam MT, Gilliland FD. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest. 2005;127:1232–41. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 114.Lack G. The concept of oral tolerance induction to foods. Nestle Nutr Workshop Ser Pediatr Program. 2007;59:63–72. doi: 10.1159/000098513. [DOI] [PubMed] [Google Scholar]
- 115.Salvatore S, Keymolen K, Hauser B, Vandenplas Y. Intervention during pregnancy and allergic disease in the offspring. Pediatr Allergy Immunol. 2005;16:558–66. doi: 10.1111/j.1399-3038.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- 116.Zeiger RS, Heller S, Mellon MH, Forsythe AB, O’Connor RD, Hamburger RN, et al. Effect of combined maternal and infant food-allergen avoidance on development of atopy in early infancy: a randomized study. J Allergy Clin Immunol. 1989;84:72–89. doi: 10.1016/0091-6749(89)90181-4. [DOI] [PubMed] [Google Scholar]
- 117.Devereux G, Barker RN, Seaton A. Antenatal determinants of neonatal immune responses to allergens. Clin Exp Allergy. 2002;32:43–50. doi: 10.1046/j.0022-0477.2001.01267.x. [DOI] [PubMed] [Google Scholar]
- 118.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo CA, Jr, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses in children at 2 y of age. Am J Clin Nutr. 2006;84:903–11. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boehm G, Stahl B. Oligosaccharides from milk. J Nutr. 2007;137(suppl):847S–9S. doi: 10.1093/jn/137.3.847S. [DOI] [PubMed] [Google Scholar]
- 120.Moore DC, Elsas PX, Maximiano ES, Elsas MI. Impact of diet on the immunological microenvironment of the pregnant uterus and its relationship to allergic disease in the offspring—a review of the recent literature. Sao Paulo Med J. 2006;124:298–303. doi: 10.1590/S1516-31802006000500013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Abrahamsson TR, Jakobsson T, Bottcher MF, Fredrikson M, Jenmalm MC, Bjorksten B, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174–80. doi: 10.1016/j.jaci.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 122.Blumer N, Sel S, Virna S, Patrascan CC, Zimmermann S, Herz U, et al. Perinatal maternal application of Lactobacillus rhamnosus GG suppresses allergic airway inflammation in mouse offspring. Clin Exp Allergy. 2007;37:348–57. doi: 10.1111/j.1365-2222.2007.02671.x. [DOI] [PubMed] [Google Scholar]
- 123.Rautava S, Kalliomaki M, Isolauri E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J Allergy Clin Immunol. 2002;109:119–21. doi: 10.1067/mai.2002.120273. [DOI] [PubMed] [Google Scholar]
- 124.Feleszko W, Jaworska J, Rha RD, Steinhausen S, Avagyan A, Jaudszus A, et al. Probiotic-induced suppression of allergic sensitization and airway inflammation is associated with an increase of T regulatory-dependent mechanisms in a murine model of asthma. Clin Exp Allergy. 2007;37:498–505. doi: 10.1111/j.1365-2222.2006.02629.x. [DOI] [PubMed] [Google Scholar]
- 125.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357:1076–9. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 126.Krauss-Etschmann S, Hartl D, Rzehak P, Heinrich J, Shadid R, Ramirez-Tortosa MD, et al. Decreased cord blood IL-4, IL-13, and CCR4 and increased TGF-beta levels after fish oil supplementation of pregnant women. J Allergy Clin Immunol. 2008;121:464–70. doi: 10.1016/j.jaci.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 127.Zeiger RS. Food allergen avoidance in the prevention of food allergy in infants and children. Pediatrics. 2003;111:1662–71. [PubMed] [Google Scholar]
- 128.Ferreira CT, Seidman E. Food allergy: a practical update from the gastroenterological viewpoint. J Pediatr (Rio J) 2007;83:7–20. doi: 10.2223/JPED.1587. [DOI] [PubMed] [Google Scholar]
- 129.Torrent M, Sunyer J, Munoz L, Cullinan P, Iturriaga MV, Figueroa C, et al. Earlylife domestic aeroallergen exposure and IgE sensitization at age 4 years. J Allergy Clin Immunol. 2006;118:742–8. doi: 10.1016/j.jaci.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 130.Rowe J, Kusel M, Holt BJ, Suriyaarachchi D, Serralha M, Hollams E, et al. Prenatal versus postnatal sensitization to environmental allergens in a high-risk birth cohort. J Allergy Clin Immunol. 2007;119:1164–73. doi: 10.1016/j.jaci.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 131.Vance GH, Lewis SA, Grimshaw KE, Wood PJ, Briggs RA, Thornton CA, et al. Exposure of the fetus and infant to hens’ egg ovalbumin via the placenta and breast milk in relation to maternal intake of dietary egg. Clin Exp Allergy. 2005;35:1318–26. doi: 10.1111/j.1365-2222.2005.02346.x. [DOI] [PubMed] [Google Scholar]
- 132.Dean T, Venter C, Pereira B, Grundy J, Clayton CB, Higgins B. Government advice on peanut avoidance during pregnancy—is it followed correctly and what is the impact on sensitization? J Hum Nutr Diet. 2007;20:95–9. doi: 10.1111/j.1365-277X.2007.00751.x. [DOI] [PubMed] [Google Scholar]
- 133.Vos AP, Haarman M, Buco A, Govers M, Knol J, Garssen J, et al. A specific prebiotic oligosaccharide mixture stimulates delayed-type hypersensitivity in a murine influenza vaccination model. Int Immunopharmacol. 2006;6:1277–86. doi: 10.1016/j.intimp.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 134.Vos AP, van Esch BC, Stahl B, M’Rabet L, Folkerts G, Nijkamp FP, et al. Dietary supplementation with specific oligosaccharide mixtures decreases parameters of allergic asthma in mice. Int Immunopharmacol. 2007;7:1582–7. doi: 10.1016/j.intimp.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 135.Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814–9. doi: 10.1136/adc.2006.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zizka J, Hrdy J, Lodinova-Zadnikova R, Kocourkova I, Novotna O, Sterzl I, et al. Effect of breast milk of healthy and allergic mothers on in vitro stimulation of cord blood lymphocytes. Pediatr Allergy Immunol. 2007;18:486–94. doi: 10.1111/j.1399-3038.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 137.Laiho K, Lampi AM, Hamalainen M, Moilanen E, Piironen V, Arvola T, et al. Breast milk fatty acids, eicosanoids, and cytokines in mothers with and without allergic disease. Pediatr Res. 2003;53:642–7. doi: 10.1203/01.PDR.0000055778.58807.C8. [DOI] [PubMed] [Google Scholar]
- 138.Kelleher SL, Lonnerdal B. Immunological activities associated with milk. Adv Nutr Res. 2001;10:39–65. doi: 10.1007/978-1-4615-0661-4_3. [DOI] [PubMed] [Google Scholar]
- 139.Cleary TG. Human milk protective mechanisms. Adv Exp Med Biol. 2004;554:145–54. doi: 10.1007/978-1-4757-4242-8_14. [DOI] [PubMed] [Google Scholar]
- 140.Fiocchi A, Assa’ad A, Bahna S. Food allergy and the introduction of solid foods to infants: a consensus document. Adverse Reactions to Foods Committee, American College of Allergy, Asthma and Immunology. Ann Allergy Asthma Immunol. 2006;97:10–20. doi: 10.1016/s1081-1206(10)61364-6. quiz 1, 77. [DOI] [PubMed] [Google Scholar]
- 141.Calder PC, Krauss-Etschmann S, de Jong EC, Dupont C, Frick JS, Frokiaer H, et al. Early nutrition and immunity—progress and perspectives. Br J Nutr. 2006;96:774–90. [PubMed] [Google Scholar]
- 142.Khakoo GA, Lack G. Introduction of solids to the infant diet. Arch Dis Child. 2004;89:295. doi: 10.1136/adc.2003.039016. [DOI] [PMC free article] [PubMed] [Google Scholar]


