Abstract
Adjuvants are compounds that can increase and/or modulate the intrinsic immunogenicity of an antigen and elicit strong and long lasting immune responses. During the last 80 years many adjuvants have been used in experimental settings, but due to various shortcomings of most of them only aluminum compounds made it into regular clinical usage. However, during the last years promising candidates have arisen that may finally adjunct or displace aluminum substances as main adjuvant. This review summarizes information on adjuvants currently used in clinical as well as in experimental settings.
Keywords: Adjuvant, Immune response, Aluminum, MF59, Monophosphoryl lipid A, Poly-lactid-co-glycolid acid, Virosomes, Freund’s adjuvant, Nanoparticles, Cholera toxin B, Flagellin
1. Introduction
Adjuvants are applied to enhance the ability of a vaccine to elicit strong and durable immune responses. The existence of the adjuvant effect in general was described in 1926 [1]. Adjuvants have been in practical use as compounds in vaccines for more than 80 years [2-4]. With the usage of such substances or molecules, less antigen and fewer injections are needed [5]. Furthermore, adjuvants can influence the balance of the induced antibody- and cell-mediated immunity. In the past, most vaccine formulations had been whole-cell or virus-based. These live vaccines induce both humoral and cell-based immunity per se [6]. Therefore, support by adjuvants relying on humoral effects was sufficient. In contrast, to reduce irrelevant reactions the new generation of vaccines has a more defined composition and often consists of subunit vaccines such as small peptides with lower immunogenicity. To treat intracellular pathogens and other antigens, new vaccine formulations have to be able to induce strong cellular responses, including T helper (Th) 1 cells and sometimes cytotoxic T lymphocytes (CTLs) in addition to antibodies [7]. Furthermore, it is feasible to provide protection against pathogens in mucosal tissues by boosting Th17 cells. Th17 cells are produced in the presence of IL-23 and are characterized by production of IL-17. They are an important modulator of inflammation and CD4+T cell recall or memory response and seem to be responsible for host defense against bacterial, fungal and viral pathogens at mucosal surfaces [8,9]. Therefore, new adjuvants, providing the above-mentioned properties, are needed.
2. Signal hypotheses
According to the two-signal hypothesis [10], in addition to the antigen-specific signal 1 from the T cell receptors, co-stimulation (signal 2) is required to activate naive antigen-specific T cells (Fig. 1). An intensively investigated co-stimulatory molecule on T cells is CD28, which interacts with CD80 and CD86. Another costimulator expressed by T cells is ICOS (inducible costimulator), which interacts with B7RP-1 (ICOS-L) [11].
Fig. 1.

Mechanisms of adjuvants. The initiation of Th cell responses requires three signals, referred to as signal 0, signal 1 and signal 2. In theory, adjuvants can act on each of these three signals. Most of the recently developed specific adjuvants, such as Toll-like receptor (TLR) agonists, are called type A adjuvants (e.g. MPL). Adjuvants and formulations targeting antigen processing cells (APCs) or favoring antigen capture are called type B adjuvants (e.g. aluminum hydroxide), acting on signal 1, as their effect is mediated by enhanced antigen presentation to T cells. Specific ligands of co-stimulatory molecules, like the CD28 agonist TGN1412, can enhance signal 2 and are called type C adjuvants. Signal 3 represents mediators delivered from the APC to the T cell, e.g. the cytokine IL-12.
This two-signal hypothesis has been extended by Janeway [12] and Matzinger [13,14] in different ways. On the one hand Janeway introduced “signal 0”, representing infectious non-self (for example bacteria), which causes signal 2 to be produced. Signal 0 is generated, e.g. through binding of pathogens to Toll-like receptors (TLRs) and brings cells into a general alarm condition. On the other hand Matzinger proposed the “danger theory”. This theory does not discriminate between self and non-self, but between dangerous and non-dangerous. According to Matzinger, the immune system is not concerned with the origin of the antigen, but with the ability to cause tissue damage. While bacterial colonisation, not associated with tissue damage, does not represent “danger”, bacterial infection and, e.g. surgical procedures were proposed to induce an immune response via the danger signal [15]. Danger signals in this context are endogenous, non-foreign alarm signals, i.e. released by damaged tissue, including mammalian DNA, RNA, heat shock proteins, interferon-α, interleukin-1b, CD40-L, and breakdown products of hyaluron [16]. Recently, it has been found that both, conserved bacterial motives (e.g. present on LPS) and non-foreign alarm signals are specifically recognized by the same TLRs on APCs. Considering these findings, both theories seem to overlap here, although their basic assumptions, whether “microbial non-self” or “danger” is the crucial criterion, are fundamentally different [16].
TLRs play a key role in the innate immune system and many of recently developed adjuvants act via TLR-dependent pathways. They are one of the most important mechanisms of the immune system to distinguish self from non-self. TLRs are pattern-recognition receptors (PRRs), which normally interact with pathogen-associated molecular patterns (PAMPs), e.g. found on LPS of bacterial cell membranes. TLRs are members of a larger super-family that also includes the IL-1 receptor. While the cytoplasmatic region of these proteins are of high homology (Toll/IL-1R domain), the extracellular part differs markedly [17]. So far, 13 members of the TLR family have been identified in mammals, each detecting different PAMPs [18]. They are found on LPS from bacteria, double-stranded RNA of viruses or unmethylated CpG islands of bacterial and viral DNA [19]. After binding their ligand, TLRs dimerize and activate a downstream signalling cascade. This cascade includes adaptor proteins like the myeloid differentiation primary-response protein 88 (MyD88) and the Toll-IL 1 receptor domain-containing adapter inducing interferon-β (TIRF), as well as IL-1R-associated kinases (IRAKs), transforming growth factor-β (TGF-β)-activated kinase (TAK1), TAK1-binding protein 1 (TAB1), TAB2 and tumor-necrosis factor (TNF)-receptor-associated factor 6 (TRAF6). Finally, this cascade leads to a translocation of NF-κ B into the nucleus causing the expression of its target genes [17].
Although there is a lot of evidence that TLR receptors and downstream signals are crucial for inducing immunity, the exact mechanisms are still under discussion. It has been shown that mice deficient in the critical signalling components for TLR (MyD88−/−; TrifLps2/Lps2) still show specific humoral immune responses to allergens applied in four typical adjuvants: incomplete Freund’s adjuvant (IFA), complete Freund’s adjuvant (CFA), alum and the TLR4/TLR2-agonist MPL [20]. Furthermore, the adjuvant flagellin promoted specific antibody levels in mice lacking TLR5 (specific for flagellin) compareable to those in WT mice. In MyD88−/− mice, the ability of flagellin to promote humoral immunity was reduced, but not eliminated [21]. These findings suggest, at least in case of humoral immunity, that TLR signalling may not be as crucial as previously reported.
A further extension of the two-signal hypothesis is “signal 3”, representing signals delivered from the APC to the T cell, e.g. IL-12 [22]. These signals determine the differentiation of T cells into certain effector cells (e.g. Th1 cell, Th2 cell or CTL).
3. Mechanisms of action
In general, adjuvants have various effector mechanisms (Fig. 1). Non-specific adjuvants (e.g. aluminum hydroxide) enhance antigen presentation by building a depot at the injection site, leading to a high local antigen concentration and therefore improving uptake by antigen presenting cells (APCs) [23]. Furthermore, antigen recognition may be accelerated by direct stimulation of immune cells [24]. These so-called type B adjuvants interact with APCs and antigens in an unspecific manner and their effect is based on an amplification of signal 1. They are still in common use and represent important substances especially in clinical settings.
Unlike type B adjuvants, type A adjuvants have a specific mechanism of action. Most of the recently developed type A adjuvants, e.g. monophosphoryl lipid A (MPL), are specific agonists for TLRs. Hence, they act primarily on signal 0, and indirectly on signal 2 by activating APCs and triggering the secretion of cytokines such as IL-12. In addition, TLR agonists can act on signal 1 by favoring efficient presentation of the co-administered antigen [7].
Finally, there are type C adjuvants. Their mode of function is based on an enhancement of signal 2 through interaction with co-stimulatory molecules on APCs. There have been efforts to introduce type C adjuvants into clinical use, but until now their applicability is to be estimated as marginal. A well-known example is TGN1412, a novel superagonist anti-CD28 monoclonal antibody that directly stimulates T cells. Originally intended to treat B cell chronic lymphocytic leukemia and rheumatoid arthritis, it was applied to 6 patients enrolled in a clinical phase I study. Shortly after application all patients needed intensive care due to cardiovascular shock and acute respiratory distress syndrome caused by cytokine storm [25].
4. Application routes
Depending on the intended characteristic of immune response, adjuvant formulations can be designed for application via various routes like intraperitoneal, intramuscular, intradermal, subcutaneous, mucosal or oral (Table 1). While both, the intraperitoneal and the subcutaneous route cause production of IgM, IgG and/or IgE antibodies, the latter may lead to higher antibody levels [26]. The mucosal route (e.g. intranasal or oral) is preferentially used to elicit IgA antibodies. Intranasal application is especially used to gain mucosal protection, e.g. in pre-clinical trials against sexually transmitted diseases like HIV and in experimental animal studies against allergy [27]. Oral application is suitable for mucosal protection as well and offers a suitable and safe form of vaccination [28]. Although currently the intramuscular route is most commonly used, in certain settings the intradermal route may be more effective, especially in former i.m. vaccine non-responders [29].
Table 1.
Application routes of vaccine formulations in clinical and/or experimental studies
| Application route | Disease (adjuvant used) | Reference |
|---|---|---|
| Intramuscular | HPV (MPL), hepatitis A/B (aluminum) | [106,107] |
| Oral | Rotavirus (PLG particles), | [108] |
| ETEC (CTB) | [92] | |
| Intranasal | HIV (CTA1-DD) | [109] |
| Subcutaneous | Yellow fewer (no adjuvant) | [110] |
| Intradermal | Hepatitis B (aluminum) | [29] |
| Intraperitoneal | Typhus (no adjuvant) | [111] |
5. Adjuvants in clinical use
In contrast to the numerous adjuvants in experimental settings, only few, discussed in the following sections, are currently approved for usage in humans. Besides the widely used aluminum compounds, several other adjuvants have recently made it into clinical usage.
5.1. Aluminum hydroxide (type B adjuvant)
Aluminum compounds have been in use as adjuvants for more than 80 years. While principally there are many different aluminum compositions, nowadays aluminum hydroxide and aluminum phosphate are the most commonly used adjuvants [4].
The immuno-modulating and immuno-stimulating effects of aluminum compounds result from several mechanisms. The basic effect is the absorption of the antigen. The major forces for this absorption are electrostatic and hydrophobic attraction, and ligand exchange [30]. This causes a delayed clearing of the complexed antigen from the injection site by building a depot, from which the antigen is released very slowly [24]. The high local concentration of the antigen consecutively stimulates the uptake by antigen-processing cells [23].
Furthermore, the absorption to aluminum causes a potentiation of the immune response via direct stimulation of immune cells. For example, macrophages can be directly activated by the foreign particulate character of aluminum hydroxide and thereby adapt a mature dendritic cell-like phenotype with an enhanced ability to present the concomitantly applied antigen to Th cells [31]. The direct stimulation of macrophages may depend on the intracellular innate immune response system called Nalp3 inflammasome [32].
Contrarily, it has been shown that an absorbance to the adjuvant, which is too strong, may negatively interfere with the antigen processing. This leads to an impaired presentation to T cells and results in a weaker immune response [33].
Aluminum hydroxide is considered as effective adjuvant that induces Th2 responses in mice, causing induction of IgE and IgG1 antibodies. In mice the class switch to IgE and IgG1 is accompanied by secretion of a characteristic cytokine pattern (IL-4, IL-5, IL-6, IL-9, IL-10 and IL-13). Human Th1 and Th2 cells produce similar cytokine patterns, but the synthesis of IL-2, IL-6, IL-10 and IL-13 is not as tightly restricted to a single subset as in mouse T cells [34].
One of the shortcomings of aluminum is the low capacity to stimulate cellular immune responses [35], which are needed for clearing intracellular pathogens like Mycobacterium tuberculosis, HIV and plasmodia.
Although aluminum is regarded as safe, there are several potential side effects. Reports about severe local effects including sterile abscesses, eosinophilia, myofasciitis and granuloma formation have arisen [36-38] as well as Th2 immune response induction in humans—a disadvantage when treating allergy [39]. Secondary, there is evidence that aluminum compounds may have a detrimental influence on the incidence of Alzheimer’s disease [40]. In animal trials aluminum has been shown not only to act as Th2 adjuvant when given parenterally [37], but also when applied orally [41-43].
5.2. Monophosphoryl lipid A (MPL) (type A adjuvant)
To avoid the aforementioned possible side effects of aluminum and, more importantly, to achieve long lasting cell-mediated immune responses, intensive research on development of new adjuvants has been going on. MPL is isolated from the lipopolysaccharide (LPS) of Salmonella minnesota R595 and retains much of the immunostimulatory properties of the parent lipopolysaccharide without the inherent toxicity [44]. This is achieved by sequential acid and base hydrolysis to remove saccharide groups and all but one phosphate present in LPS [45].
MPL acts as an agonist at TLR4 and TLR2 on APCs [7,46]. While the signalling cascade via TLR4 induced by LPS is mediated by the adaptor proteins MyD88 and TRIF together, MPL shows a tendency towards function via TRIF over MyD88. This was shown in a T cell stimulation experiment, where antigen plus LPS released both MyD88-dependent mediators (IFN-γ, IL-1β, IL-6, MIP-1α) and TRIF-dependent mediators (G-CSF, IP-10, MCP-1, RANTES), whereas antigen plus MPL induced mainly the latter [47].
MPL stimulates the production of the Th1 cytokines IL-2 and IFN-γ [46] and antibody class IgG2a, and to a lesser extent also the Th2 cytokines IL-4 and IL-5 and antibody classes IgE and IgG1 in mice [37]. Furthermore, it is capable of up-regulating human leukocyte antigen (HLA)-DR, CD80, CD86, CD40 and activation marker CD83 expressed on dendritic cells in vitro [48]. Several studies have demonstrated the ability of MPL to activate human monocytes and macrophages in vitro [46].
In contrast to most type B adjuvants like aluminum hydroxide, the type A adjuvant MPL showed acceptable results as Th1 adjuvant and revealed its safety in animal and human studies [49,50]. It is currently being evaluated in numerous human trials and has already been used in some vaccine formulations like a hepatitis B vaccine and a HSV-II vaccine formulation [51-53]. Currently, MPL is routinely used as adjuvant in the HPV vaccine Cerverix®[54].
5.3. MF59 (type B adjuvant)
Besides MPL, one of the most promising candidates to successfully replace aluminum in the future is MF59 [55,56]. This oil-in-water-emulsion consists of 5% squalene, 0.5% Tween 80 and 0.5% sorbitan trioleate (Span 85®). Detailed information on the mechanisms of action is still lacking. Unlike aluminum compounds, MF59 does not inhibit the distribution of the co-administered antigen. MF59 interacts with APCs at the injection site, disperses slowly to the draining lymph nodes, where it is most concentrated two days after injection. In lymph nodes it is endocytosed by lymph node-resident cells that have the characteristics of APCs [57]. This may increase the efficiency of antigen presentation [58]. Furthermore, chemoattraction of macrophages to the site of injection has been shown [57]. On the one hand MF59 has been demonstrated to be capable of inducing high IgE and moderate IgG antibody titers accompanied by the secretion of Th2 cytokines IL-5 and IL-6 in Th2-biased BALB/c mice [59]. On the other hand in rhesus macaques MF59 triggered the release of Th1 cytokines IFN-γ and IL-2 [60]. In general MF59 elicits a predominant Th1 response which promotes efficient generation of cytotoxic T cells, providing this adjuvant with a marked humoral as well as cellular potency [61]. Compared to aluminum compounds, MF59 application rendered higher immune responses [62,63]. Furthermore, clinical trials demonstrated that MF59 is safe to be used in humans as seen in a clinical study on a H5N1 vaccine formulation (Novartis) [64]. Currently, MF59 is routinely used as adjuvant in the influenza vaccine Fluad® (Novartis) [65], as well as in the experimental vaccine for avian influenza A/H9N2 virus [66] and HBV [67].
5.4. Liposomal adjuvants (type B adjuvants)
The umbrella term liposome-like particle covers several substances like transfersomes, proteosomes, archaesomes, niosomes, cochleates, liposomes and virosomes that all are based on a common principle [68].
Liposome-like particles are vesicles of varying size consisting primarily of a thin layer of amphipathic phospholipids, similar to those of cell membranes. Due to the chemical properties of phospholipids they automatically form a continuous, spherical lipid bilayer with an aqueous inner compartment, that can be loaded with antigens. The only exception is cochleates, which consist of rolled up bilayer sheets without internal volume [68].
As an example of liposome-like particles, immunopotentiating reconstituted influenza virosomes (IRIVs) will be discussed here [69]. IRIVs are influenza viruses cleared from genetic material (virus-like particle structures) with a mean diameter of 150 nm [70]. The first step in producing IRIVs is to inactivate the stem influenza virus with beta-propiolactone. Subsequently the obtained antigens (neuraminidase (NA) and hemagglutinin (HA)) are mixed with the phospholipid component lecithin and several viral phospholipids, adding also octaethyleneglycol monododecylether. Finally the viral antigens NA and HA are embedded through the spontaneous formation of the IRIVs during the stepwise removal of detergent [71]. In general the mechanism of action of IRIVs is very similar to natural influenza viruses. Whereas the IRIV particle retains the cell binding and membrane fusion properties of the original virus, the ability to replicate is lost. The immunogenic property of IRIVs results from the presence of biologically active influenza antigen HA in their membrane, which mediates an interaction with Ig receptors on B lymphocytes. Furthermore the properties of IRIVs can be modified to emphasize either a stimulation of CD4+T cells (MHC II pathway) or CD8+ cells (MHC I pathway). This is achieved by choosing the suitable orientation of the antigen on IRIVs. Antigens attached to the surface of IRIVs promote endocytosis of the particle and are proteolysed inside endosomes of APCs. This initializes the presentation of the antigen in the MHC II-dependent pathway and leads to CD4+T cell activation. On the contrary, antigens embedded inside IRIVs are transported directly to the cytosol of APCs. Thereafter the antigen is translocated to the endoplasmic reticulum, where it is linked to newly produced MHC I complexes [72]. Not all of the injected antigen strictly follows either the MHC I or the MHC II pathway. This finally leads to a stimulation of both T helper cells (CD4+) and CTLs (CD8+) with an emphasis on either of the two [71]. All together this balanced Th1/Th2 response makes IRIVs an excellent adjuvant [70].
Virosomes have been shown in clinical trials, assessing various hepatitis A vaccines, to be comparable to aluminum adjuvants in terms of safety and to be superior in terms of immunogenicity. Currently, virosomes are routinely used as adjuvant in the hepatitis A vaccine Epaxal® (Baxter) [73].
6. Adjuvants in experimental use
Additionally to the discussed adjuvants already in clinical application, there are numerous candidates used in experimental settings. Many of them, introduced in the following sections, show excellent adjuvant efficacy, but lack appropriate safety properties.
6.1. Freund’s adjuvant (type B adjuvant)
Freund’s adjuvant is an example for water-in-oil emulsions. This adjuvant is based on the observation of Jules Freund, that guinea pigs infected with Mycobacterium tuberculosis (MT) develop higher antibody titers after immunization than non-infected animals. In consecutive experiments, he found a similar effect when mixing protein antigen with killed MT in a water-in-oil emulsion [74].
As in some cases MT is not needed, two versions of the Freund adjuvant were developed—CFA including dried MT and IFA without MT. These formulations are potent adjuvants possessing the ability to elicit both Th1 (CFA) and Th2 (IFA) responses. Mycobacteria, which are accounted for the Th1 response of CFA, induce also Th17 cells in murine cell culture and in vivo. This is accompanied by elevated IL-6, IL-23 and TGF-β levels [75].
While CFA has generally been regarded as toxic for use in humans because of the killed MT, IFA was used successfully in numerous human trials in the 1950s. However, due to several safety concerns based on animal trials, IFA was discontinued as vaccine adjuvant in humans [76].
6.2. Nanoparticles (type B adjuvants)
Nanoparticles consist of different material, for instance biodegradable polymers like poly-lactid-co-glycolid acid (PLGA) and have adjuvant properties due to their depot effect [77] and particle character [78]. Antigens entrapped by those particles are released in a delayed continuous or pulsatile manner [79]. Furthermore, the adjuvant effect can be influenced by coating microspheres with various substances. For example, wheat germ agglutinin or Aleuria aurantia lectin, non-toxic lectins, specifically bind carbohydrate moieties on the glycocalyx of intestinal enterocytes and the mucus layer [80,81]. Thus, coating of PLGA-nanoparticles with lectins enhances the uptake and therefore the immune response on the oral/mucosal route.
Furthermore, they have been shown to stimulate both, mucosal and systemic immunity [77]. In general, PLGA particles show a marked humoral immune response assisted by a very clear Th1 bias [82]. Furthermore, those particles were effective in down-regulating ongoing Th2-responses in a BALB/c mouse model of type I allergy [83]. In pre-clinical studies, nanoparticles induced antibody titers comparable to those of aluminum salts after systemic immunization. Although biodegradable polymers have been tested for toxicity and safety in extensive animal studies, they have not made it into clinical use as adjuvants yet [84].
In contrast to biodegradable nanoparticles, non-degradable nanoparticles consist of latex, gold, silica or polystyrene that remain at the site of injection for extended periods of time [85]. This leads to a long-lasting presentation and an enhanced immunogenicity of the co-delivered antigen.
Gold particles have already been used as adjuvants in several phase I studies (hepatitis B and malaria) as part of a particlemediated epidermal delivery system. In these trials gold particles were coated with DNA vaccines and delivered into the epidermal skin layer using a so-called “gene gun”. Applying these needle-free devices, DNA is “shot” directly into keratinocytes and professional APCs of the epidermis. This stimulates CD4+ and CD8+T cells and leads to a predominant Th1 response [86].
Although non-degradable nanoparticles have been tested in phase I studies and reveal a low incidence of side effects, further studies are needed until these substances can be used in routine vaccine formulations.
6.3. Toxin-derived adjuvants (type B adjuvants)
The most relevant adjuvant among toxin-derived adjuvants is cholera toxin (CT). It is a potent oral and parenteral adjuvant that belongs to the class of bacterial toxins produced by Vibrio cholerae. It consists of the two main subunits A and B. The catalytically active heterodimeric subunit A is responsible for CT’s toxicity and leads to severe gastrointestinal symptoms, especially diarrhea. Subunit B has the ability to bind to the ganglioside receptor GM-1 of intestinal epithelial cells and allows toxins to enter the cells [87]. To avoid harmful effects of the toxic subunit A (CTA), normally only the nontoxic pentameric subunit B (CTB) is used as adjuvant (Fig. 2). This can either be achieved by purifying CTB from CT or by producing recombinant CTB. CTB is commonly reported to elicit a Th2 or a mixed Th1/Th2 response with a bias towards Th2 [88]. It is used as treatment in animal settings that serve as models of human autoimmune diseases because many of these diseases are accompanied by a bias towards Th1. For example, experimental autoimmune encephalomyelitis is treated by feeding the CTB-conjugated specific autoantigen myelin basic protein; or autoimmune uveitis is treated by feeding CTB-conjugated retinal autoantigen interphotoreceptor retinoid-binding protein. Hence the Th1-based response to the autoantigens is skewed to a nonpathogenic phenotype [89]. Besides the typical Th2 response achieved with CTB adjuvants there are sporadic reports of Th1 shifts [90]. Furthermore, in a murine experiment CT has been shown to be capable of inducing Th17 responses, accompanied by an accumulation of neutrophils in the lung of treated mice and an augmentation of IL-6. This response is primarily based on CT’s subunit CTB, while CTA per se shows only little Th17-driving activity [91]. The cause of this variability of the T helper cell response may lie in the nature of the antigen/allergen [90].
Fig. 2.
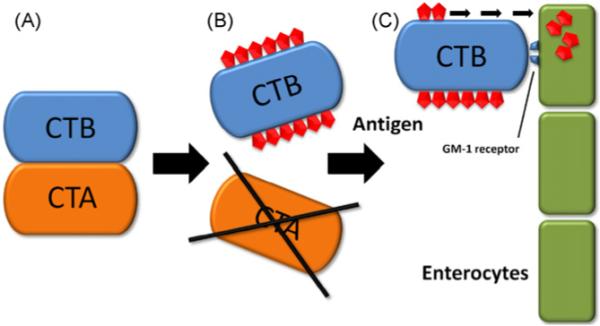
Cholera toxin. (A) Cholera toxin consists of the two main subunits, cholera toxin A subunit (CTA) and cholera toxin B subunit (CTB). CTB is accountable for the adjuvant capabilities, while CTA’s toxicity causes severe gastrointestinal symptoms. (B) Thus, during production of vaccines/adjuvants, CTA is cleaved off and CTB is linked to the antigen of choice. (C) CTB binds to the GM-1 receptor on enterocytes and enhances recognition of the linked antigen by the immune system.
Besides using CTB for treating experimental autoimmune diseases, it has already been successfully tested in a clinical trial assessing safety and immunogenicity of an oral ETEC vaccination in children [92].
Similarly to the CTB approach, CTA1-DD, a fusion protein consisting of the ADP-ribosylating part of CT (CTA1) and of the Ig-binding and B cell targeting fragment D of Staphylococcus aureus protein A, has been tested in pre-clinical settings [93,94]. An advantage of CTA1-DD over CTB may be its ability to elicit a strong IgA immunity, whereas CTB-conjugated antigens have been reported to promote tolerance in certain settings [95,96]. Furthermore, CTA1-DD may have fewer side effects, because with CTA1-DD no binding to the nervous tissue of the olfactory bulbs has been detected, whereas strong binding was observed with CTB [97].
6.4. Flagellin
Flagellin is a structural component of the filament of bacterial flagella. Purified or recombinant flagellin induces inflammatory responses on treated cells in vitro or in vivo after systemic administration [98]. Although the exact mechanism of action is still under discussion [21], the adjuvant effect of flagellin is, at least in part dependent on a high-affinity interaction with TLR5 on CD11+ dendritic cells [99]. It has been shown that the genetic fusion product comprising flagellin and the antigen of choice enhances the antigen’s ability to induce a specific immune response [100]. Immunization of mice with the recombinant flagellin-OVA fusion protein induces both humoral and cell-based immunity, comparable or better than responses to OVA mixed with CFA [101]. In an intranasal influenza vaccination model in BALB/c mice, elevated specific IgG and IgA serum titers could be observed, accompanied by Th1 (IFN-γ) and Th2 (IL-4) cytokines, whereas no increase of specific antibody levels in the mucosal tissue was found [102]. Another study used flagellin fused with the MT protein p27 and compared it to Freund’s adjuvant and to a CpG motif containing DNA adjuvant. Flagellin led to the strongest cellular response among tested substances, accompanied by high IFN-γ secretion, indicating a Th1 biased response [103]. Furthermore, flagellin has been recently shown to promote Th17 differentiation in a certain subset of dendritic cells (CD11chigh/CD11bhigh) [104]. Currently, flagellin is used in a clinical phase II trial as component of an influenza vaccine [105].
7. Conclusion
During the last 80 years many adjuvants have been developed and have been used extensively in experimental settings. Nevertheless, until a few years ago the only adjuvants approved for clinical use in humans have been aluminum compounds. Due to their missing ability of eliciting potent T cell-based responses and the possibility of side effects, research for adjuvants without these shortcomings was intensified. Among the vast number of candidates a few promising substances (MPL, MF59 and virosomes) have been designed during the last years, which finally made it into clinical trials and are currently in use as compounds of vaccines.
Acknowledgements
Research for this article was financially supported by Hertha Firnberg stipend T283-B13 (IP-S) and SFB F1808-B13 of the Austrian Science Fund. RB was supported by a research stipend of the Medical University of Vienna. The Austrian Nano-Initiative co-financed this work as part of the Nano-Health project.
Abbreviations
- ADP
adenosine diphosphate
- APCs
antigen presenting cells
- CT
cholera toxin
- CTA
cholera toxin A subunit
- CTA1-DD
cholera toxin A1 subunit-D dimer
- CTB
cholera toxin B subunit
- CTL
cytotoxic T lymphocytes
- ETEC
enterotoxigenic Escherichia coli
- HA
hemagglutinin
- HBV
hepatitis B virus
- HIV
human immune deficiency virus
- HPV
human papillomavirus
- HSV
Herpes simplex virus
- ICOS
inducible costimulator
- IL
interleukin
- IRIV
immunopotentiating reconstituted influenza virosome
- LPS
lipopolysaccharide
- MPL
monophosphoryl lipid A
- MT
Mycobacterium tuberculosis
- MyD88
myeloid differentiation primary-response protein 88
- NA
neuraminidase
- PLG(A)
polylactic-co-glycolic (acid)
- Th
T helper
- TLR
toll-like receptor
- TRIF
Toll-IL 1 receptor domain-containing adapter inducing interferon-β
References
- [1].Glenny AT, Pope CG, Waddington H, Wallace U. Immunological notes XVII to XXIV. J Pathol. 1926;29:31–40. [Google Scholar]
- [2].Friedewald WF. Enhancement of the immunizing capacity of influenza virus vaccines with adjuvants. Science. 1944;99:453–4. doi: 10.1126/science.99.2579.453. [DOI] [PubMed] [Google Scholar]
- [3].Hoyt A, Thompson MA, Moore FJ, Smith CR. The effect of adjuvants on a nonviable antituberculosis vaccine and on live BCG. Am Rev Respir Dis. 1965;91:565–74. doi: 10.1164/arrd.1965.91.4.565. [DOI] [PubMed] [Google Scholar]
- [4].Lindblad EB. Aluminium adjuvants—in retrospect and prospect. Vaccine. 2004;22:3658–68. doi: 10.1016/j.vaccine.2004.03.032. [DOI] [PubMed] [Google Scholar]
- [5].Salk JE, Laurent AM, Bailey ML. Direction of research on vaccination against influenza: new studies with immunologic adjuvants. Am J Public Health Nations Health. 1951;41:669–77. doi: 10.2105/ajph.41.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ellis RW. Technologies for the design, discovery, formulation and administration of vaccines. Vaccine. 2001;19:2681–7. doi: 10.1016/s0264-410x(00)00504-1. [DOI] [PubMed] [Google Scholar]
- [7].Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–17. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- [8].Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, et al. Il-23 and il-17 in the establishment of protective pulmonary cd4+t cell responses after vaccination and during mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–77. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- [9].Khader SA, Gaffen SL, Kolls JK. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009;2:403–11. doi: 10.1038/mi.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gill RG, Coulombe M, Lafferty KJ. Pancreatic islet allograft immunity and tolerance: the two-signal hypothesis revisited. Immunol Rev. 1996:74–96. doi: 10.1111/j.1600-065x.1996.tb00900.x. [DOI] [PubMed] [Google Scholar]
- [11].Coyle AJ, Gutierrez-Ramos JC. The expanding B7 superfamily: increasing complexity in costimulatory signals regulating T cell function. Nat Immunol. 2001;2:203–9. doi: 10.1038/85251. [DOI] [PubMed] [Google Scholar]
- [12].Janeway C. Immunogenicity signals 1,2,3…, and 0. Immunol Today. 1989;10:283–6. doi: 10.1016/0167-5699(89)90081-9. [DOI] [PubMed] [Google Scholar]
- [13].Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- [14].Matzinger P. The immune system’s role in graft loss: theoretic considerations. Transplant P. 1997;29 doi: 10.1016/s0041-1345(97)00848-8. [DOI] [PubMed] [Google Scholar]
- [15].Medzhitov R. Approaching the asymptote: 20 years later. Immunity. 2009;30:766–75. doi: 10.1016/j.immuni.2009.06.004. [DOI] [PubMed] [Google Scholar]
- [16].Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–5. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- [17].Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- [18].Kawai T, Akira S. Toll-like receptor and rig-I-like receptor signaling. Ann N Y Acad Sci. 2008;1143:1–20. doi: 10.1196/annals.1443.020. [DOI] [PubMed] [Google Scholar]
- [19].Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–5. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- [20].Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–8. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sanders CJ, Franchi L, Yarovinsky F, Uematsu S, Akira S, Nunez G, et al. Induction of adaptive immunity by flagellin does not require robust activation of innate immunity. Eur J Immunol. 2009;39:359–71. doi: 10.1002/eji.200838804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- [23].HogenEsch H. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine. 2002;20(Suppl 3):34–9. doi: 10.1016/s0264-410x(02)00169-x. [DOI] [PubMed] [Google Scholar]
- [24].Gupta RK, Rost BE, Relyveld E, Siber GR. Adjuvant properties of aluminum and calcium compounds. Pharm Biotechnol. 1995;6:229–48. doi: 10.1007/978-1-4615-1823-5_8. [DOI] [PubMed] [Google Scholar]
- [25].Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med. 2006;355:1018–28. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- [26].van Houten NE, Zwick MB, Menendez A, Scott JK. Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide. Vaccine. 2006;24:4188–200. doi: 10.1016/j.vaccine.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Repa A, Kozakova H, Hudcovic T, Stepankova R, Hrncir T, Tlaskalova-Hogenova H, et al. Susceptibility to nasal and oral tolerance induction to the major birch pollen allergen Bet v 1 is not dependent on the presence of the microflora. Immunol Lett. 2008;117:50–6. doi: 10.1016/j.imlet.2007.11.025. [DOI] [PubMed] [Google Scholar]
- [28].Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- [29].Rahman F, Dahmen A, Herzog-Hauff S, Böcher WO, Galle PR, Löhr HF. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology. 2000;31:521–7. doi: 10.1002/hep.510310237. [DOI] [PubMed] [Google Scholar]
- [30].Iyer S, Robinett RSR, HogenEsch H, Hem SL. Mechanism of adsorption of hepatitis B surface antigen by aluminum hydroxide adjuvant. Vaccine. 2004;22:1475–9. doi: 10.1016/j.vaccine.2003.10.023. [DOI] [PubMed] [Google Scholar]
- [31].Rimaniol AC, Gras G, Verdier F, Capel F, Grigoriev VB, Porcheray F, et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine. 2004;22:3127–35. doi: 10.1016/j.vaccine.2004.01.061. [DOI] [PubMed] [Google Scholar]
- [32].Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–6. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hansen B, Sokolovska A, HogenEsch H, Hem SL. Relationship between the strength of antigen adsorption to an aluminum-containing adjuvant and the immune response. Vaccine. 2007;25:6618–24. doi: 10.1016/j.vaccine.2007.06.049. [DOI] [PubMed] [Google Scholar]
- [34].Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–46. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- [35].Agger EM, Cassidy JP, Brady J, Korsholm KS, Vingsbo-Lundberg C, Andersen P. Adjuvant modulation of the cytokine balance in mycobacterium tuberculosis subunit vaccines: immunity, pathology and protection. Immunology. 2008;124:175–85. doi: 10.1111/j.1365-2567.2007.02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–96. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- [37].Brunner R, Wallmann J, Szalai K, Karagiannis P, Kopp T, Scheiner O, et al. The impact of aluminium in acid-suppressing drugs on the immune response of BALB/c mice. Clin Exp Allergy. 2007;37:1566–73. doi: 10.1111/j.1365-2222.2007.02813.x. [DOI] [PubMed] [Google Scholar]
- [38].Schöll I, Kopp T, Bohle B, Jensen-Jarolim E. Biodegradable PLGA particles for improved systemic and mucosal treatment of type I allergy. Immunol Allergy Clin North Am. 2006;26:349–64. doi: 10.1016/j.iac.2006.02.007. [DOI] [PubMed] [Google Scholar]
- [39].Spazierer D, Skvara H, Dawid M, Fallahi N, Gruber K, Rose K, et al. T helper 2 biased de novo immune response to keyhole limpet hemocyanin in humans. Clin Exp Allergy. 2009;39:999–1008. doi: 10.1111/j.1365-2222.2008.03177.x. [DOI] [PubMed] [Google Scholar]
- [40].Martyn CN, Osmond C, Edwardson JA, Barker DJP, Harris EC, Lacey RF. Geographical relation between Alzheimer’s disease and aluminium in drinking water. Lancet. 1989;1:59–62. [PubMed] [Google Scholar]
- [41].Brunner R, Wallmann J, Szalai K, Karagiannis P, Altmeppen H, Riemer AB, et al. Aluminium per se and in the anti-acid drug sucralfate promotes sensitization via the oral route. Allergy. 2009;64:890–7. doi: 10.1111/j.1398-9995.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- [42].Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, et al. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–60. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- [43].Schöll I, Ackermann U, Ozdemir C, Blumer N, Dicke T, Sel S, et al. Anti-ulcer treatment during pregnancy induces food allergy in mouse mothers and a Th2-bias in their offspring. FASEB J. 2007 Apr;21(4):1264–70. doi: 10.1096/fj.06-7223com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Freytag LC, Clements JD. Mucosal adjuvants. Vaccine. 2005;23:1804–13. doi: 10.1016/j.vaccine.2004.11.010. [DOI] [PubMed] [Google Scholar]
- [45].Thompson BS, Chilton PM, Ward JR, Evans JT, Mitchell TC. The low-toxicity versions of LPS, MPL adjuvant and RC529, are efficient adjuvants for CD4+ T cells. J Leukoc Biol. 2005;78:1273–80. doi: 10.1189/jlb.0305172. [DOI] [PubMed] [Google Scholar]
- [46].Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–7. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- [47].Mata-Haro V, Cekic C, Martin M, Chilton PM, Casella CR, Mitchell TC. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science. 2007;316:1628–32. doi: 10.1126/science.1138963. [DOI] [PubMed] [Google Scholar]
- [48].Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, et al. Monophosphoryl lipid A activates both human dendritic cells and T cells. J Immunol. 2002;168:926–32. doi: 10.4049/jimmunol.168.2.926. [DOI] [PubMed] [Google Scholar]
- [49].Baldrick P, Richardson D, Elliott G, Wheeler AW. Safety evaluation of monophosphoryl lipid A (MPL): an immunostimulatory adjuvant. Regul Toxicol Pharmacol. 2002;35:398–413. doi: 10.1006/rtph.2002.1541. [DOI] [PubMed] [Google Scholar]
- [50].Baldrick P, Richardson D, Wheeler AW, Woroniecki SR. Safety evaluation of a new allergy vaccine containing the adjuvant monophosphoryl lipid A (MPL) for the treatment of grass pollen allergy. J Appl Toxicol. 2004;24:261–8. doi: 10.1002/jat.981. [DOI] [PubMed] [Google Scholar]
- [51].Vandepapeliere P, Rehermann B, Koutsoukos M, Moris P, Garcon N, Wettendorff M, et al. Potent enhancement of cellular and humoral immune responses against recombinant hepatitis B antigens using AS02A adjuvant in healthy adults. Vaccine. 2005;23:2591–601. doi: 10.1016/j.vaccine.2004.11.034. [DOI] [PubMed] [Google Scholar]
- [52].Kundi M. New hepatitis B vaccine formulated with an improved adjuvant system. Expert Rev Vaccines. 2007;6:133–40. doi: 10.1586/14760584.6.2.133. [DOI] [PubMed] [Google Scholar]
- [53].Bernstein DI, Aoki FY, Tyring SK, Stanberry LR, St-Pierre C, Shafran SD, et al. Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clin Infect Dis. 2005;40:1271–81. doi: 10.1086/429240. [DOI] [PubMed] [Google Scholar]
- [54].Petäjä T, Keränen H, Karppa T, Kawa A, Lantela S, Siitari-Mattila M, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health. 2009;44:33–40. doi: 10.1016/j.jadohealth.2008.10.002. [DOI] [PubMed] [Google Scholar]
- [55].Stephenson I, Nicholson KG, Hoschler K, Zambon MC, Hancock K, DeVos J, et al. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. N Engl J Med. 2008;359:1631–3. doi: 10.1056/NEJMc0805274. [DOI] [PubMed] [Google Scholar]
- [56].O’Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6:699–710. doi: 10.1586/14760584.6.5.699. [DOI] [PubMed] [Google Scholar]
- [57].Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–22. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- [58].Dupuis M, Murphy TJ, Higgins D, Ugozzoli M, van Nest G, Ott G, et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186:18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- [59].Valensi JM, Carlson JR, Van Nest GA. Systemic cytokine profiles in BALB/c mice immunized with trivalent influenza vaccine containing MF59 oil emulsion and other advanced adjuvants. J Immunol. 1994;153:4029–39. [PubMed] [Google Scholar]
- [60].Verschoor EJ, Mooij P, Oostermeijer H, van der Kolk M, ten Haaft P, Verstrepen B, et al. Comparison of immunity generated by nucleic acid-, MF59-, and ISCOM-formulated human immunodeficiency virus type 1 vaccines in rhesus macaques: evidence for viral clearance. J Virol. 1999;73:3292–300. doi: 10.1128/jvi.73.4.3292-3300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Radosevic K, Rodriguez A, Mintardjo R, Tax D, Bengtsson KL, Thompson C, et al. Antibody and T-cell responses to a virosomal adjuvanted H9N2 avian influenza vaccine: impact of distinct additional adjuvants. Vaccine. 2008;26:3640–6. doi: 10.1016/j.vaccine.2008.04.071. [DOI] [PubMed] [Google Scholar]
- [62].Vajdy M, Selby M, Medina-Selby A, Coit D, Hall J, Tandeske L, et al. Hepatitis C virus polyprotein vaccine formulations capable of inducing broad antibody and cellular immune responses. J Gen Virol. 2006;87:2253–62. doi: 10.1099/vir.0.81849-0. [DOI] [PubMed] [Google Scholar]
- [63].Wack A, Baudner BC, Hilbert AK, Manini I, Nuti S, Tavarini S, et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26:552–61. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- [64].Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, et al. MF59-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS ONE. 2009;4 doi: 10.1371/journal.pone.0004384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Li R, Fang H, Li Y, Liu Y, Pellegrini M, Podda A. Safety and immunogenicity of an MF59-adjuvanted subunit influenza vaccine in elderly Chinese subjects. Immun Ageing. 2008;5:2–12. doi: 10.1186/1742-4933-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Atmar RL, Keitel WA, Patel SM, Katz JM, She D, El Sahly H, et al. Safety and immunogenicity of nonadjuvanted and MF59-adjuvanted influenza A/H9N2 vaccine preparations. Clin Infect Dis. 2006;43:1135–42. doi: 10.1086/508174. [DOI] [PubMed] [Google Scholar]
- [67].Heineman TC, Clements-Mann ML, Poland GA, Jacobson RM, Izu AE, Sakamoto D, et al. A randomized, controlled study in adults of the immunogenicity of a novel hepatitis B vaccine containing MF59 adjuvant. Vaccine. 1999;17:2769–78. doi: 10.1016/s0264-410x(99)00088-2. [DOI] [PubMed] [Google Scholar]
- [68].Kersten GF, Crommelin DJ. Liposomes and iscoms. Vaccine. 2003;21:915–20. doi: 10.1016/s0264-410x(02)00540-6. [DOI] [PubMed] [Google Scholar]
- [69].Felnerova D, Viret JF, Glück R, Moser C. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 2004;15:518–29. doi: 10.1016/j.copbio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- [70].Wilschut J. Influenza vaccines: the virosome concept. Immunol Lett. 2009;122:118–21. doi: 10.1016/j.imlet.2008.11.006. [DOI] [PubMed] [Google Scholar]
- [71].Glück R, Metcalfe IC. Novel approaches in the development of immunopotentiating reconstituted influenza virosomes as efficient antigen carrier systems. Vaccine. 2003;21:611–5. doi: 10.1016/s0264-410x(02)00567-4. [DOI] [PubMed] [Google Scholar]
- [72].Bungener L, Serre K, Bijl L, Leserman L, Wilschut J, Daemen T, et al. Virosome-mediated delivery of protein antigens to dendritic cells. Vaccine. 2002;20:2287–95. doi: 10.1016/s0264-410x(02)00103-2. [DOI] [PubMed] [Google Scholar]
- [73].Dagan R, Amir J, Livni G, Greenberg D, Abu-Abed J, Guy L, et al. Concomitant administration of a virosome-adjuvanted hepatitis A vaccine with routine childhood vaccines at age twelve to fifteen months: a randomized controlled trial. Pediatr Infect Dis J. 2007;26:787–93. doi: 10.1097/INF.0b013e318060acbd. [DOI] [PubMed] [Google Scholar]
- [74].Chang JCC, Diveley JP, Savary JR, Jensen FC. Adjuvant activity of incomplete freund’s adjuvant. Adv Drug Del Rev. 1998;32:173–86. doi: 10.1016/s0169-409x(98)00009-x. [DOI] [PubMed] [Google Scholar]
- [75].McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–53. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- [76].Alving CR. Design and selection of vaccine adjuvants: animal models and human trials. Vaccine. 2002;20:S56–64. doi: 10.1016/s0264-410x(02)00174-3. [DOI] [PubMed] [Google Scholar]
- [77].Igartua M, Hernández RM, Esquisabel A, Gascón AR, Calvo MB, Pedraz JL. Enhanced immune response after subcutaneous and oral immunization with biodegradable PLGA microspheres. J Control Rel. 1998;56:63–73. doi: 10.1016/s0168-3659(98)00077-7. [DOI] [PubMed] [Google Scholar]
- [78].Schöll I, Boltz-Nitulescu G, Jensen-Jarolim E. Review of novel particulate antigen delivery systems with special focus on treatment of type I allergy. J Control Rel. 2005;104:1–27. doi: 10.1016/j.jconrel.2004.12.020. [DOI] [PubMed] [Google Scholar]
- [79].Sah H, Chien YW. Prolonged immune response evoked by a single subcutaneous injection of microcapsules having a monophasic antigen release. J Pharm Pharmacol. 1996;48:32–6. doi: 10.1111/j.2042-7158.1996.tb05872.x. [DOI] [PubMed] [Google Scholar]
- [80].Walter F, Schöll I, Untersmayr E, Ellinger A, Boltz-Nitulescu G, Scheiner O, et al. Functionalisation of allergen-loaded microspheres with wheat germ agglutinin for targeting enterocytes. Biochem Biophys Res Commun. 2004;315:281–7. doi: 10.1016/j.bbrc.2004.01.057. [DOI] [PubMed] [Google Scholar]
- [81].Roth-Walter F, Schöll I, Untersmayr E, Fuchs R, Boltz-Nitulescu G, Weissenböck A, et al. M cell targeting with aleuria aurantia lectin as a novel approach for oral allergen immunotherapy. J Allergy Clin Immunol. 2004;114:1362–8. doi: 10.1016/j.jaci.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [82].San Román B, Irache JM, Gómez S, Tsapis N, Gamazo C, Espuelas MS. Co-encapsulation of an antigen and CpG oligonucleotides into PLGA microparticles by TROMS technology. Eur J Pharm Biopharm. 2008;70:98–108. doi: 10.1016/j.ejpb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- [83].Schöll I, Weissenböck A, ouml F, rster-Waldl E, Untersmayr E, Walter F, Willheim M, et al. Allergen-loaded biodegradable poly(D,L-lactic-co-glycolic) acid nanoparticles down-regulate an ongoing Th2 response in the BALB/c mouse model. Clin Exp Allergy. 2004;34:315–21. doi: 10.1111/j.1365-2222.2004.01884.x. [DOI] [PubMed] [Google Scholar]
- [84].Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- [85].Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–28. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40:86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- [87].Blanchard TG, Lycke N, Czinn SJ, Nedrud JG. Recombinant cholera toxin B subunit is not an effective mucosal adjuvant for oral immunization of mice against helicobacter felis. Immunology. 1998;94:22–7. doi: 10.1046/j.1365-2567.1998.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Su S, Silver PB, Wang P, Chan C, Caspi RR. Cholera toxin prevents Th1-mediated autoimmune disease by inducing immune deviation. J Immunol. 2004;173:755–61. doi: 10.4049/jimmunol.173.2.755. [DOI] [PubMed] [Google Scholar]
- [89].Sun JB, Rask C, Olsson T, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc Natl Acad Sci USA. 1996;93:7196–201. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wiedermann U, Jahn-Schmid B, Lindblad M, Rask C, Holmgren J, Kraft D, et al. Suppressive versus stimulatory effects of allergen/cholera toxoid (CTB) conjugates depending on the nature of the allergen in a murine model of type I allergy. Int Immunol. 1999;11:1717–24. doi: 10.1093/intimm/11.10.1717. [DOI] [PubMed] [Google Scholar]
- [91].Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces Th17-dominated T-cell response to bystander antigens. PLoS One. 2009;4:e5190. doi: 10.1371/journal.pone.0005190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Qadri F, Ahmed T, Ahmed F, Begum YA, Sack DA, Svennerholm A. Reduced doses of oral killed enterotoxigenic escherichia coli plus cholera toxin B subunit vaccine is safe and immunogenic in bangladeshi infants 6–17 months of age: dosing studies in different age groups. Vaccine. 2006;24:1726–33. doi: 10.1016/j.vaccine.2005.08.110. [DOI] [PubMed] [Google Scholar]
- [93].Lycke N, Schön K. The B cell targeted adjuvant, CTA1-DD, exhibits potent mucosal immunoenhancing activity despite pre-existing anti-toxin immunity. Vaccine. 2001;19:2542–8. doi: 10.1016/s0264-410x(00)00487-4. [DOI] [PubMed] [Google Scholar]
- [94].Nyhus J, Kran AM, Sommerfelt MA, Baksaas I, Sorensen B, Kvale D. Multiple antigen concentrations in delayed-type hypersensitivity (DTH) and response diversity during and after immunization with a peptide-based HIV-1 immunotherapy candidate (vacc-4x) Vaccine. 2006;24:1543–50. doi: 10.1016/j.vaccine.2005.10.012. [DOI] [PubMed] [Google Scholar]
- [95].Sun JB, Holmgren J, Czerkinsky C. Cholera toxin B subunit: an efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–9. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Lycke N. ADP-ribosylating bacterial enzymes for the targeted control of mucosal tolerance and immunity. Ann N Y Acad Sci. 2004;1029:193–208. doi: 10.1196/annals.1309.036. [DOI] [PubMed] [Google Scholar]
- [97].Eriksson AM, Schön KM, Lycke NY. The cholera toxin-derived CTA1-DD vaccine adjuvant administered intranasally does not cause inflammation or accumulate in the nervous tissues. J Immunol. 2004;173:3310–9. doi: 10.4049/jimmunol.173.5.3310. [DOI] [PubMed] [Google Scholar]
- [98].Cuadros C, Lopez-Hernandez FJ, Dominguez AL, McClelland M, Lustgarten J. Flagellin fusion proteins as adjuvants or vaccines induce specific immune responses. Infect Immun. 2004;72:2810–6. doi: 10.1128/IAI.72.5.2810-2816.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bates JT, Uematsu S, Akira S, Mizel SB. Direct stimulation of TLR5+/+ CD11c+ cells is necessary for the adjuvant activity of flagellin. J Immunol. 2009;182:7539–47. doi: 10.4049/jimmunol.0804225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Huleatt JW, Nakaar V, Desai P, Huang Y, Hewitt D, Jacobs A, et al. Potent immunogenicity and efficacy of a universal influenza vaccine candidate comprising a recombinant fusion protein linking influenza m2e to the TLR5 ligand flagellin. Vaccine. 2008;26:201–14. doi: 10.1016/j.vaccine.2007.10.062. [DOI] [PubMed] [Google Scholar]
- [101].Huleatt JW, Jacobs AR, Tang J, Desai P, Kopp EB, Huang Y, et al. Vaccination with recombinant fusion proteins incorporating toll-like receptor ligands induces rapid cellular and humoral immunity. Vaccine. 2007;25:763–75. doi: 10.1016/j.vaccine.2006.08.013. [DOI] [PubMed] [Google Scholar]
- [102].Skountzou I, Martin MDP, Wang B, Ye L, Koutsonanos D, Weldon W, et al. Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine. 2009 doi: 10.1016/j.vaccine.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Le Moigne V, Robreau G, Mahana W. Flagellin as a good carrier and potent adjuvant for Th1 response: study of mice immune response to the p27 (rv2108) mycobacterium tuberculosis antigen. Mol Immunol. 2008;45:2499–507. doi: 10.1016/j.molimm.2008.01.005. [DOI] [PubMed] [Google Scholar]
- [104].Uematsu S, Fujimoto K, Jang MH, Yang BG, Jung YJ, Nishiyama M, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing toll-like receptor 5. Nat Immunol. 2008;9:769–76. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- [105]. http://clinicaltrials.gov/ct2/show/nct00921947.
- [106].Crosbie EJ, Kitchener HC. Cervarix—a bivalent l1 virus-like particle vaccine for prevention of human papillomavirus type 16- and 18-associated cervical cancer. Expert Opin Biol Ther. 2007;7:391–6. doi: 10.1517/14712598.7.3.391. [DOI] [PubMed] [Google Scholar]
- [107].Van Herck K, Leroux-Roels G, Van Damme P, Srinivasa K, Hoet B. Ten-year antibody persistence induced by hepatitis A and B vaccine (Twinrix) in adults. Travel Med Infect Dis. 2007;5:171–5. doi: 10.1016/j.tmaid.2006.07.003. [DOI] [PubMed] [Google Scholar]
- [108].Chen SC, Jones DH, Fynan EF, Farrar GH, Clegg JC, Greenberg HB, et al. Protective immunity induced by oral immunization with a rotavirus DNA vaccine encapsulated in microparticles. J Virol. 1998;72:5757–61. doi: 10.1128/jvi.72.7.5757-5761.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Sundling C, Schön K, Mörner A, Forsell MN, Wyatt RT, Thorstensson R, et al. CTA1-DD adjuvant promotes strong immunity against human immunodeficiency virus type 1 envelope glycoproteins following mucosal immunization. J Gen Virol. 2008;89:2954–64. doi: 10.1099/vir.0.2008/005470-0. [DOI] [PubMed] [Google Scholar]
- [110].Belmusto-Worn VE, Sanchez JL, McCarthy K, Nichols R, Bautista CT, Magill AJ, et al. Randomized, double-blind, phase III, pivotal field trial of the comparative immunogenicity, safety, and tolerability of two yellow fever 17D vaccines (ARILVAX and YF-VAX) in healthy infants and children in peru. Am J Trop Med Hyg. 2005;72:189–97. [PubMed] [Google Scholar]
- [111].Carter PB, Collins FM. Assessment of typhoid vaccines by using the intraperitoneal route of challenge. Infect Immun. 1977;17:555–60. doi: 10.1128/iai.17.3.555-560.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


