Abstract
Background
Animal models are essential for analyzing the allergenic potential of food proteins and for investigating mechanisms underlying food allergy. Based on previous studies revealing acid-suppression medication as risk factor for food allergy induction, we aimed to establish a mouse model mimicking the natural route of sensitization in patients.
Methods
The effect of acid-suppressing medication on murine gastric pH was assessed by intragastric pH measurements after two injections of a proton pump inhibitor (PPI). To investigate dose-dependency, mice were fed different concentrations of ovalbumin (OVA; 0.2, 0.5, 1.0, 2.5 or 5.0 mg) either with or without anti-ulcer medication. Additionally, different routes of exposure (i.p. vs. oral) were compared in a second immunization experiment. Sera were screened for OVA-specific antibody titers (IgG1, IgG2a and IgE) in ELISA and RBL assay. Clinical reactivity was evaluated by measuring rectal temperature after oral challenge and by type I skin tests.
Results
Two intravenous injections of PPI significantly elevated the gastric pH from 2.97 to 5.3. Only oral immunization with 0.2 mg OVA under anti-acid medication rendered elevated IgG1, IgG2a and IgE titers compared to all other concentrations. Protein feeding alone altered antibody titers only marginally. Even though also i.p. immunizations induced high levels of specific IgE, only oral immunizations under anti-acids induced anaphylactic reactions evidenced by a significant decrease of body temperature.
Conclusion
Only low-dosage ovalbumin feedings under anti-acid medication resulted in IgE mediated food allergy. Based on this knowledge we have established a suitable food allergy model for further investigations of food adverse reactions.
Keywords: Acid-suppression, Dose-dependency, Food allergy, Mouse model, Ovalbumin
1. Introduction
Today, population studies indicate a rising prevalence of food allergies in western societies [1,2]. Consequently there is a growing need to investigate in-depth mechanisms and risk factors leading to sensitization towards food compounds. For this purpose, animal food allergy models including rodent, swine and dog models are used and discussed to have the potential to mimic the human disease process [3]. Additionally, in the 2001 joint FAO/WHO meeting animal models were suggested as suitable tools for the analysis of the allergenic potential of novel dietary compounds [4], which should be further evaluated for structural homologies or sequence similarities with known allergens, included in allergy testing and should be assessed for their digestion stability [5].
Despite significant differences between human and mouse immunology [6], mice are the preferentially used organism for food allergy models. A wide range of inbred strains have been characterized as being either high or low IgE responder animals [7]. The easy handling and the possibility to include a larger number of animals per group account for the widespread use. Important for these studies are immunological similarities between mice and humans as both produce IgE antibodies, which bind to the high affinity receptor FcεRI being expressed on mast cells and basophils. In both species gastrointestinal symptoms depend on synthesis and secretion of serotonin and platelet-activating factor [8].
Recently, we have developed a novel oral immunization protocol in BALB/c mice inducing an IgE mediated response by protein feedings under concomitant anti-acid medication [9-11]. In these studies, the normal function of the stomach representing a gate-keeper against food allergens has been determined as an important factor for preventing food allergies [12].
The relevance of this mechanism was demonstrated both in the murine system [9-11] as well as for humans, as 25% of gastroenterological patients being treated with acid-suppression medication for a 3-month period due to dyspeptic disorders revealed formation of specific IgE antibodies and sensitization towards dietary compounds [9,13].
Based on this knowledge we aimed to establish a true mouse food allergy model exploiting the oral route for immunizations under concomitant acid-suppression treatment. The major egg white allergen ovalbumin (OVA), a 45 kDa protein constituting about 54% of all egg white proteins, was chosen as a model antigen due to its wide use in allergy research to enable a better comparison with previously established food allergy models. Moreover, we wanted to standardize our immunization regimen addressing the effect of dose-dependency on food allergy induction.
2. Materials and methods
2.1. The model allergen ovalbumin
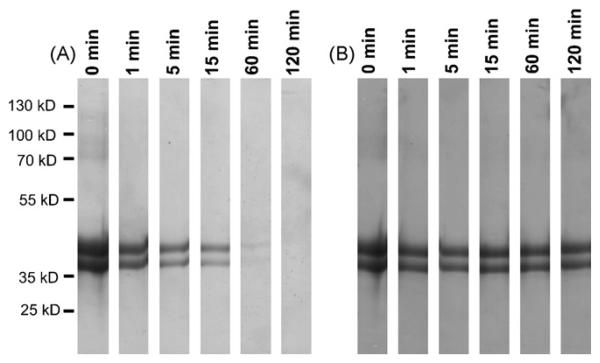
Lyophilized OVA (Sigma, Vienna, Austria, 98% purity) was used for all experiments. Simulated gastric fluid (SGF) was prepared with a pharmaceutical enzyme tablet (Enzynorm® forte, Pharmaselect Handels GmbH, Vienna, Austria) as previously described [14] with slight modifications: one tablet was dissolved in 100 μL 0.9% sterile sodium chloride at pH 2.0 or pH 5.0. For digestion, 500 μL of SGF was incubated with 500 μg OVA. The digestion was quenched with 0.1 N NaOH after 1, 5, 15, 60 and 120 min. The effect of incubation with SGF on protein integrity was evaluated in SDS-PAGE using Coomassie brilliant blue staining [15].
2.2. Animals
Four to six weeks old female BALB/c mice were purchased from the Institute of Laboratory Animal Science and Genetics, Medical University of Vienna, Austria. All experiments were performed according to European Community rules of animal care (permission numbers GZ 66.009/0039-BrGT/2005 and GZ 66.009/0170-BrGT/2006 of the Austrian Federal Ministry of Science and Research).
2.3. Intragastric pH measurements after acid-suppression
Six weeks old female BALB/c mice were divided into two groups (n = 10). After overnight fasting mice were either left untreated or were injected intravenously (i.v.) with the proton pump inhibitor omeprazole (PPI, Losec®, AstraZeneca GmbH, Wedel, Germany; 116 μg omeprazole diluted in 0.9% sodium chloride), which was followed by a second i.v. injection after 1 h. After 15 min, mice were sacrificed and the stomach was immediately removed and perfused with 150 μL sterile sodium chloride. The pH of this washing fluid was measured using a pH microelectrode.
2.4. Immunization protocol
For investigating the effect of antigen dosage, animals were divided into 10 groups (n = 5 each). Based on the data generated by intragastric pH measurements groups 1–5 were medicated intravenously with the proton pump inhibitor for 3 days (on days 1–3, 16–18 and 29–31). On days 2–3, 17–18 and 30–31 mice were immunized orally with different concentrations of OVA (0.2, 0.5, 1.0, 2.5, 5.0 mg per mouse) mixed with 2 mg sucralfate (Ulcogant®, Merck) 15 min after a repeated i.v. injection of the PPI. Groups 6–10 were fed the allergen at the different concentrations without PPI on the respective days. Blood samples were taken on days 0, 15, 28 and 42.
To compare different routes of exposure, the immunization experiments were repeated with four groups of animals (n =10 each). Group A was immunized intraperitoneally (i.p.) with 2 μg OVA adsorbed to 2% aluminum hydroxide solution (1.3 μg Al(OH)3). Group B (0.2 mg OVA i.g. under acid-suppressing medication) and Group C (0.2 mg OVA i.g.) were immunized following the same protocol with the previously selected concentration. The negative control Group D remained naïve.
All immunizations were performed in two independent sets of experiments.
2.5. Evaluation of OVA-specific antibodies in ELISA, RBL-assay and dot blot experiments
Murine sera were screened for OVA-specific antibody subclasses (IgG1, IgG2a) in an enzyme-linked immunosorbent assay (ELISA). Microtiter plates (Maxisorp, NUNC, Roskild, Denmark) were coated with 1 μg OVA per well. After blocking with TBST (Tris buffered saline with Tween-20) with 1% dried milk powder (DMP), mouse sera diluted 1:100 for IgG1 and IgG2a in TBST/0.1% DMP were incubated overnight at 4 °C. Bound antibodies were detected using rat anti-mouse IgG1 and IgG2a (BD Biosciences, Franklin Lakes, NJ; 1:500) followed by a peroxidase labeled goat anti-rat IgG (Amersham, Buckinhamshire, UK, diluted 1:1000). For detection, TMB (tetramethylbenzidine, BD Bioscience, Vienna, Austria) was added for 15 min and the reaction was stopped with 1.8 M H2SO4. The color reaction was measured at 450–630 nm. Antibody concentrations were calculated according to standard dilution series after subtracting levels detected in pre-immune sera as background values.
To evaluate biologically active OVA-specific IgE, a rat basophil leukemia cell assay (RBL-assay) was performed [16]. RBL-2H3 cells, exclusively expressing the high affinity IgE receptor FcεRI [17], were passively sensitized with murine sera (dilution 1:10) and incubated at 37 °C for 1 h. After washing, 10 μg/mL OVA were added to the appropriate wells. The induced β-hexosaminidase release was detected using 4-methylumbelliferyl β-d-galactopyranoside (4-MUG, Sigma, Vienna, Austria) and the fluorescence was measured at 360–465 nm. Calculation was made by correlating measured values with β-hexosaminidase release of cells lysed with triton-x (Sigma), which was set 100%.
In order to evaluate the binding capacity of OVA-specific IgE antibodies to undigested antigen and to OVA digested for 120 min either at pH 2 or pH 5 conditions, dot blot experiments were performed. Therefore, 1 μg of the appropriate antigen, either OVA or as control α-casein, were dotted onto nitrocellulose membranes (Life Sciences Bio Trace, Pall Corporation, Vienna Austria). After blocking with 1% DMP in TBS 0.1% Tween blot stripes were incubated with serum samples taken before the first and after the last immunization (1:500 diluted in TBS 0.1% Tween) for 2 h. After extensive washing, first rat anti-mouse IgE (1:1000) and after 1 h peroxidase labeled goat anti-rat IgG antibodies (1:3000) were added for detection. The blot stripes were developed by luminescence reaction using the ECL kit (Amersham).
2.6. In vivo read-out by rectal temperature measurement after oral antigen challenge and type I skin testing
On day 49, animals were fed with 2 mg OVA in PBS (oral challenge) for evaluating anaphylactic reactions. Rectal temperature was measured before and 15 min after oral challenge with a thermometer (Thermalert TH5, Clifton, NJ).
To evaluate the in vivo relevance of the induced OVA-specific antibodies, type I skin tests were performed on day 50. 100 μL of 0.5% Evans Blue (w/v) were applied intravenously. 0.6 μg of OVA and the control antigen α-casein, both diluted in sterile sodium chloride were injected intradermally into the shaved abdominal skin. The mast cell degranulation compound 48/80 (Sigma, 20 μg) was used as positive and sterile sodium chloride as negative control. After 20 min, animals were sacrificed and the color reaction was evaluated on the inside of the abdominal skin.
2.7. Cytokine determination
As previously described [11], spleen cells were removed under sterile conditions, minced and passed through sterile nylon filters (cell strainer 40 μm Nylon). Erythrocytes were lysed in 0.75% ammonium chloride (3 mL) for exactly 4 min. After stopping the reaction cells were washed for 3 times in medium. Cell number was determined in a Coulter Counter. For spleen cell stimulation, 4 × 105 cells per well were incubated at 37 °C in triplicates with OVA (20 μg/mL), medium (as negative control) or concanavalin A (as positive control; 50 μg/mL) for 4 days. Thereafter, 100 μL spleen cell supernatants were withdrawn for evaluation of cytokine levels and were screened for IL-5 and IFN-γ using a capture ELISA (eBioscience Ready-set-go! IL-5 or IFN-γ Femto HS, San Diego, CA) following the manufacturer's instructions. In short, after coating the appropriate capture antibody, plates were blocked with assay diluent for 1 h. Either standard (recombinant mouse IL-5 or IFN-γ) or mouse spleen cell supernatants were incubated for 2 h. After extensive washing, a biotin-labeled detection antibody was added. After 1 h incubation with avidin-HRP, the reaction was developed using a substrate solution (TMB-solution) for 15 min and the absorbance was read at 450–570 nm. Comparisons were performed by calculating concentrations according to a standard curve after subtracting medium values as background levels.
2.8. Data analysis
Antibody titers, RBL-assay and cytokine results were statistically compared using the non-parametric Mann–Whitney U test. pH measurements and temperature results were compared using the two-tailed Student's t-test with the SPSS 14.0 program. A P value <.05 was considered statistically significant.
3. Results
3.1. Digestion stability of OVA to simulated gastric fluid
In line with previously published data [18], SGF digestion of OVA using a pharmaceutical enzyme tablet revealed that OVA proteins were degraded within 60 min of gastric digestion at pH 2.0 (Fig. 1A). However, by increasing the pH conditions to pH 5.0 the protein bands remained stable up to 120 min (Fig. 1B), representing the average gastric transit time.
Fig. 1.
Impaired OVA digestion at elevated pH levels of SGF. OVA (lane 1) was incubated with pepsin for 1, 5, 15, 60 and 120 min either at pH 2 (panel A) or pH 5 (panel B). All proteins were degraded after 60 min of digestion at pH 2. However, by increasing the pH of the simulated gastric fluid to pH 5 all proteins remain stable up to 120 min.
3.2. Two injections with a proton pump inhibitor significantly increases the gastric pH
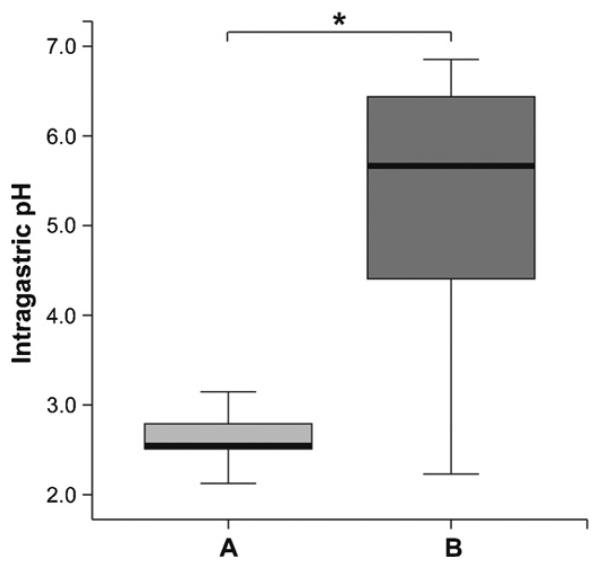
To evaluate the effect of PPI medication on the murine gastric environment, we performed intragastric pH measurements after two i.v. injections of the PPI omeprazole. As depicted in Fig. 2, the gastric pH levels increased significantly from pH 2.97 in the untreated animals (group A) to pH 5.30 measured 15 min after the second PPI injection (group B).
Fig. 2.
Repeated anti-acid medication elevated intragastric pH levels. Compared to naïve animals (group A), mice being medicated twice intravenously with the proton pump inhibitor (group B) showed a significant increase of intragastric pH already 15 min after the second injection. The boxes represent the inner quartiles value range with the median indicated as black line. Brackets indicate the statistically significant difference of intragastric pH (*P < .05).
3.3. OVA feeding under acid-suppression induces antigen-specific antibodies in a dose-dependent manner
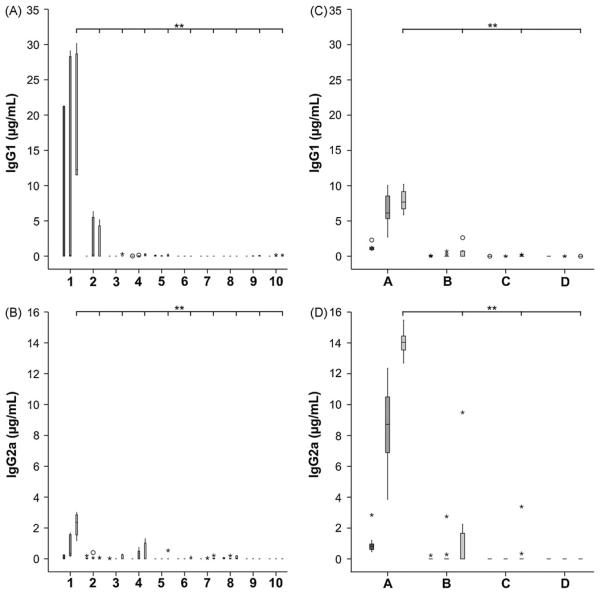
Based on the results from intragastric pH measurements, we have medicated mice intravenously with the PPI on 3 consecutive days for 3 times and further immunized orally with OVA (group 1–5). For an additional Th2 biasing effect [19], the aluminum containing anti-acid sucralfate was added to the solution, which was fed the animals. To further address the question of dose-dependency, we have used different OVA concentrations (0.2, 0.5, 1.0, 2.5 and 5.0 mg per mouse) for the feedings. As controls, mice were fed the respective OVA concentration without acid-suppression (group 6–10). In the medicated animals, the IgG1 and IgG2a levels were significantly increased only in group 1 being fed 0.2 mg OVA under acid-suppression (Fig. 3A and B). Interestingly, immunizations with all other allergen concentrations (0.5, 1.0, 2.5 or 5.0 mg per gavage) did not elevate antibody titers even under concomitant anti-acid therapy. In the second set of experiments, comparing different routes of immunization, especially the positive control group A (OVA i.p.) developed high levels of both IgG subtypes and to a lesser extent also the acid-suppressed animals of group B. Nevertheless, differences in IgG1 levels were observed between groups 1 and B in the two sets of experiments. Animals being fed the OVA proteins alone (Group C) and naïve mice (Group D) showed no altered antibody levels (Fig. 3C and D).
Fig. 3.
OVA-specific antibody induction in acid-suppressed mice. Acid-suppressed animals (1–5) or BALB/c mice without anti-acid medication (group 6–7) were fed different concentrations of OVA (0.2 mg: groups 1 and 6; 0.5 mg: groups 2 and 7; 1.0 mg: groups 3 and 8; 2.5 mg: groups 4 and 9; 5.0 mg OVA: groups 5 and 10). Only acid-suppressed mice receiving 0.2 mg OVA showed significantly increased levels of IgG1 and IgG2a (panel A and B). Comparing the effect of i.p. immunization with oral feeding (panel C and D) higher levels of IgG1 and IgG2a were observed in the positive controls (group A; OVA i.p.) and to a lesser extent in the acid-suppressed animals (group B; OVA i.g. + PPI). No changes in antibody titers were observed in mice fed the protein alone (group C) and in the naïve animals (group D). The boxes (first immune serum, dark grey; second immune serum, middle grey; third immune serum, light grey) represent the inner quartiles value range with the median indicated. Sera with signals showing more than 1.5-fold interquartile range deviation from the end of the box were defined as outliers and marked as circles. Sera with titers lying more than 3-fold interquartile range away were defined as extremes and marked with asterisks. Brackets indicate statistically significant differences of antibody concentrations (**P < .01).
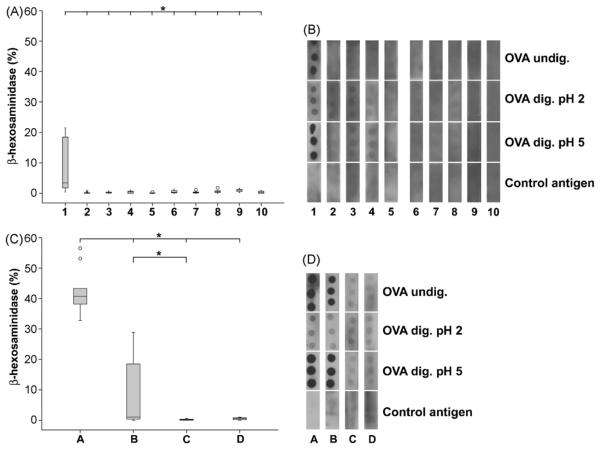
In a RBL assay, murine sera taken after the last immunization were assessed for functional antigen-specific IgE antibodies. Only the acid-suppressed animals being fed 0.2 mg OVA showed significantly increased β-hexosaminidase release compared to all other immunization concentrations (Fig. 4A). These findings were confirmed in the second set of experiments, where a significantly elevated β-hexosaminidase release was observed in the positive control group A and after oral immunizations with OVA under acid-suppression (Group B) (Fig. 4C).
Fig. 4.
Biologically active IgE was induced in mice under anti-ulcer medication. Gavages of 0.2 mg OVA under anti-acid treatment (lane 1) induced significantly higher IgE levels compared to other groups (lanes 2–10) (panel A). Intraperitoneal immunizations (group A) and oral injection under acid-suppression (group B) revealed significantly higher values of specific IgE compared to feeding OVA alone (group C) and naïve animals (group D) (panel C). In dot blot experiments (panels B and D) only the i.p. immunized mice (group A) and the acid-suppressed animals being fed 0.2 mg OVA (lanes 1 and B) revealed IgE binding capacity to undigested OVA and OVA digested at pH 5.0. In all other groups only background reactivity was observed. The IgE binding was diminished when testing with OVA digested at pH 2.0. Representative dot blots are shown.
Dot blot experiments were performed to evaluate the binding capacity of specific IgE antibodies to undigested OVA and further to OVA digested either at pH 2.0 or pH 5.0. In accordance with the RBL assay results, only IgE of the animals being fed 0.2 mg OVA under acid-suppression and the mice being injected OVA i.p. bound specifically to undigested OVA and to the protein being digested at elevated pH conditions. Only background reactivity was observed with proteins digested at pH 2 for 120 min. Neither sera obtained from other groups (Fig. 4B and D) nor preimmune sera (data not shown) revealed binding reactivity with the dotted OVA samples.
3.4. Decreased body temperature and positive skin testing reveal food allergy in animals
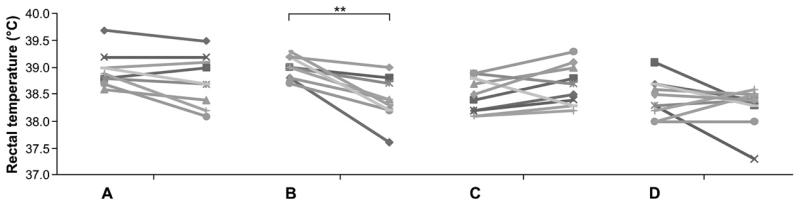
To assess shock symptoms causing blood centralization and, therefore, a decline of body temperature, the rectal temperature was measured in immunized animals before and after oral provocation with OVA. Only the animals which were immunized orally with 0.2 mg OVA under anti-acid medication revealed a significant decline in body temperature 15 min after the oral challenge (P = 0.046), whereas all other groups receiving the antigen via the oral route showed only a marginal decrease or even an increase of body temperature (data not shown). These data were confirmed in the second set of experiments. Despite the high levels of IgE antibodies detected in the sera of animals of group A (OVA i.p.) a significant decrease of body temperature was detected only in group B immunized with OVA under anti-ulcer medication (Fig. 5).
Fig. 5.
Acid-suppressed animals revealed a significant decrease of body temperature after oral OVA challenge. Compared to the positive control (OVA i.p., group A), the animals fed OVA alone (group C) and the naïve animals (group D), only group B (0.2 mg OVA i.g. with anti-acids) revealed a significant decrease of rectal temperature. Brackets indicate the statistically significant differences of rectal temperature before and 15 min after oral OVA challenge (**P < .01) within group B.
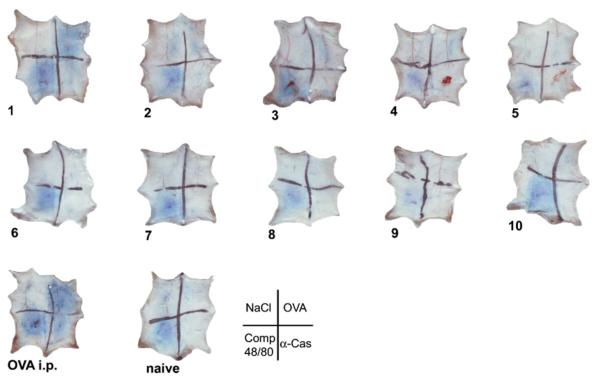
To evaluate the in vivo relevance of detected antibodies, type I skin tests were performed in the immunized animals. Only animals immunized with 0.2 mg OVA under acid-suppression showed positive skin reactions to OVA, whereas no other oral immunization protocol revealed any reactivity to the tested allergens. Intraperitoneal immunization resulted in positive skin reactions only to OVA and to the positive control (compound 48/80), whereas naïve animals did not develop any skin reactivity except to the positive control antigen (Fig. 6).
Fig. 6.
Feedings of OVA under acid-suppression induced positive type I skin tests. Group 1 (0.2 mg OVA i.g. with anti-acids) and the i.p. immunized animals revealed positive skin reactions to OVA (top right) and the positive control compound 48/80 (bottom left), but no reactions to sodium chloride (top left) and the control antigen (α-casein). No skin reactions were found in the acid-suppressed animals fed OVA with other concentrations (2–5), in the mice fed the protein alone (6–10) or in the naïve animals. Representative skin tests are shown. The skin test injection scheme is depicted in the right corner.
3.5. Antigen feeding under gastric acid-suppression induces Th2 cytokine production
In order to evaluate a possible Th2 switch induced by oral immunization under anti-ulcer medication, spleen cell supernatants of mice from the second experiment were screened for IL-5 as a Th2 marker, and IFN-γ as a Th1 marker. Compared to the animals being fed OVA alone, the i.p. immunized mice developed a 20-fold increase and the acid-suppressed animals revealed a 12-fold increase of mean IL-5 levels, although differences were not statistically significant. The IL-5 levels of naïve animals were below detection limits. In contrast, the IFN-γ levels of the animals being injected OVA i.p. and of the mice being medicated with anti-ulcer drugs were twofold increased compared to animals being fed the antigen alone and to naïve animals (Table 1).
Table 1.
Cytokine levels measured in OVA stimulated spleen cell supernatants
| Group A OVA i.p. | Group B i.g. + antacid | Group C OVA i.g. | Group D naïve | |
|---|---|---|---|---|
| IL-5 | ||||
| Mean | 69.47 | 40.62 | 3.34 | 0 |
| 95% confidence interval | 12.30–126.65 | 0–101.08 | 0–8.51 | 0–0 |
| IFN-γ | ||||
| Mean | 109.34 | 140.43 | 67.37 | 68.64 |
| 95% confidence interval | 0–271.87 | 0–348.31 | 0–177.17 | 0–195.49 |
Cytokine concentrations are given as mean and 95% confidence interval in pg/mL.
4. Discussion
Recently, we were able to show that acid-suppression treatment interfering with gastric digestion of proteins in murine as well as human studies leads to IgE induction and food allergy [9-11,13]. The intake of anti-acid medication, e.g. sucralfate or proton pump inhibitors, results in an elevation of the gastric pH. Under these circumstances, normally digestion-labile proteins remain stable and their conformation intact, which enhances the possibility for de novo sensitization and formation of antigen-specific IgE [12].
The aim of the current study was to experimentally investigate underlying mechanisms and to compare our murine model of food allergy with other immunization protocols and further to evaluate the dose-dependency of antigen under acid-suppression.
For this purpose, we have chosen OVA as a model antigen. Even though OVA is generally accepted as a typical class 1 allergen being of high stability to thermal and enzymatic degradation [20], it was previously reported to be degraded within 60 min in simulated gastric fluid experiments [18]. Indeed, also in our SGF assays using a pharmaceutical gastric enzyme tablet these data could be confirmed. However, if physiological low pH conditions (pH 2.0) were changed to pH 5.0 which is found after 5 days of treatment with proton pump inhibitors [21] OVA proteins remain stable up to 120 min representing the normal gastric transit time. Interestingly, apart from protein fragmentation, gastric digestion was additionally described to substantially change the immunological behavior of OVA by altering its biochemical features [22] and was shown to have impact on antigen presentation [23]. Therefore, we suggest OVA as suitable and highly relevant model food protein for our immunization protocol.
In a first experiment we wanted to confirm the acid-suppressing effect of PPIs on the murine gastric environment. Already two cycles of i.v. PPI injections significantly elevated the intragastric pH of BALB/c mice from 2.97 to 5.30. Thus, having a similar effect as on the human pH levels [21] we have used repeated injections of PPI in our immunization protocol. We additionally combined it with sucralfate, as aluminum-compounds are known to establish a pronounced Th2 response [24], which was recently also evidenced for sucralfate [19,25]. As both drugs were previously reported to induce a Th2 biasing effect only in combination with the respective allergen [9,11,19], we expected a profound Th2 induction by this immunization protocol.
In a first set of murine immunizations we addressed the question of dose-dependency on allergy induction. It has been previously reported that high doses of allergens are known to induce tolerance rather than sensitization [26]. Even though results might be influenced by chosen mouse strains, it was demonstrated that feedings of high concentrations of OVA (20–100 mg) suppressed antigen-specific IgG1, IgG2a and IgE antibody levels [27]. However, it was further described that rather low doses of OVA (0.25 mg) may also render a reduction in IgE levels [28]. Furthermore, low doses of OVA feedings (0.4–0.5 mg) did not induce specific IgE production when given without adjuvant [29]. In line with these results, feedings of OVA alone did not induce sensitization at any concentration. Whereas recent data revealed feedings of low concentrations of OVA (0.2 and 1 mg) with cholera toxin as adjuvant preferentially induced antigen-specific IgG1 [30], we could show that gavages of the lowest dose of OVA (0.2 mg) under concomitant acid-suppression resulted in significantly higher formation of antigen-specific IgG1, IgG2a and IgE compared to exclusive OVA feedings. Furthermore, only this immunization protocol with the lowest antigen dose revealed a significant drop of body temperature after oral OVA challenge.
We applied the concentration of 0.2 mg OVA in a second set of murine immunizations for comparing different routes of immunization. Therefore, we included a positive control of i.p. immunization and compared the induced antibodies with the levels observed in naïve animals. Here we could confirm the humoral immune response upon OVA feeding under anti-acid treatment, underlined by elevated IgG1 titers and biologically functional IgE. Also the positive controls developed high levels of all antibody subclasses, which is in line with previous studies [31,32]. Comparable to this route, even subcutaneous immunizations with OVA adsorbed to aluminum hydroxide were reported to induce high levels of IgG1 and IgE [33,34]. Furthermore, in our experiments both immunization regimens, i.p. injection of OVA and feeding under acid-suppression rendered formation of the Th2 cytokine IL-5 and additionally revealed positive type I skin tests to OVA, whereas mice being fed OVA alone and naïve animals did not show skin reactions upon allergen testing.
Most importantly we observed a significant reduction of body temperature after oral antigen challenge only in the animals immunized with low dosage of OVA under concomitant acid-suppression (Fig. 5). The measurement of body temperature has since long been applied for the read-out of anaphylactic reactions in numerous studies [35-37]. Interestingly, the induction of an anaphylactic reaction upon oral antigen challenge exclusively in the acid-suppressed animals might result from the induced titers of different allergen-specific antibody isotypes. It has been hypothesized that the lack of a clinical reactivity following i.p. immunization with aluminum as an adjuvant could be due to existence of blocking antibodies of the IgA and IgG subclass [8].
Based on the current data we suggest our murine model to represent an experimental food allergy model mimicking the situation in allergic patients and confirming the dose-dependent sensitization capacity of allergens, again under acid-suppression [9,13]. Thus, we may have a valid model in our hand for further investigations of mechanisms in food allergy and for safety testing of novel dietary compounds.
Acknowledgments
This study was supported by grants H220-B13, SFB F1808-B13 (E.J.-J.) and T283-B13 (I.P.-S.) of the Austrian Science Funds and the Austrian National Bank “Jubiläumsfond” grant Nr. 11375.
Abbreviations
- OVA
ovalbumin
- PPI
proton pump inhibitors
- SGF
simulated gastric fluid
- SDS-PAGE
sodium dodecylsulfate polyacrylamide gel electrophoresis
- i.v.
intravenous
- i.p.
intraperitoneal
- i.g.
intragastric
- Al(OH)3
aluminum hydroxide
- ELISA
enzyme-linked immunosorbent assay
- TBST
Tris buffered saline with Tween-20
- DMP
dried milk powder
- TMB
tetramethylbenzidine
- RBL
rat basophil leukaemia
- 4-MUG
4-methylumbelliferyl β-d-galactopyranoside
- HRP
horseradish peroxidase
References
- 1.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–19. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110:784–9. doi: 10.1067/mai.2002.128802. [DOI] [PubMed] [Google Scholar]
- 3.McClain S, Bannon GA. Animal models of food allergy: opportunities and barriers. Curr Allergy Asthma Rep. 2006;6:141–4. doi: 10.1007/s11882-006-0052-1. [DOI] [PubMed] [Google Scholar]
- 4.Evaluation of genetically modified foods. Available at: www.who.int/foodsafety/publications/biotech/en/ec_jan2001.pdf (last access: 08/11/2008)
- 5.Taylor SL, Hefle SL. Will genetically modified foods be allergenic? J Allergy Clin Immunol. 2001;107:765–71. doi: 10.1067/mai.2001.114241. [DOI] [PubMed] [Google Scholar]
- 6.Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–8. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 7.Helm RM. Food allergy animal models. Ann NY Acad Sci. 2002;964:139–50. doi: 10.1111/j.1749-6632.2002.tb04139.x. [DOI] [PubMed] [Google Scholar]
- 8.Finkelmann FD, Rothenberg ME, Brandt EB, Morris SC, Strait RT. Molecular mechanisms of anaphylaxis: lessons from studies with murine models. J Allergy Clin Immunol. 2005;115:449–57. doi: 10.1016/j.jaci.2004.12.1125. [DOI] [PubMed] [Google Scholar]
- 9.Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, et al. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–60. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- 10.Untersmayr E, Ellinger A, Beil WJ, Jensen-Jarolim E. Eosinophils accumulate in the gastric mucosa of food-allergic mice. Int Arch Allergy Immunol. 2004;135:1–2. doi: 10.1159/000080035. [DOI] [PubMed] [Google Scholar]
- 11.Untersmayr E, Schöll I, Swoboda I, Beil WJ, Förster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in Balb/c mice. J Allergy Clin Immunol. 2003;112:616–23. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 12.Untersmayr E, Jensen-Jarolim E. The effect of gastric digestion on food allergy. Curr Opin Allergy Clin Immunol. 2006;6:214–9. doi: 10.1097/01.all.0000225163.06016.93. [DOI] [PubMed] [Google Scholar]
- 13.Untersmayr E, Bakos N, Schöll I, Kundi M, Roth-Walter F, Szalai K, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656–8. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- 14.Vieths S, Reindl J, Müller U, Hoffmann A, Haustein D. Digestibility of peanut and hazelnut allergens investigated by a simple in vitro procedure. Eur Food Res Technol. 1999;209:379–88. [Google Scholar]
- 15.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann A, Jamin A, Foetisch K, May S, Aulepp H, Haustein D, et al. Determination of the allergenic activity of birch pollen and apple prick test solutions by measurement of β-hexosaminidase release from RBL-2H3 cells. Comparison with classical methods in allergen standardization. Allergy. 1999;54:446–54. doi: 10.1034/j.1398-9995.1999.00917.x. [DOI] [PubMed] [Google Scholar]
- 17.Barsumian EL, Isersky C, Petrino MG, Siraganian RP. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and non-releasing clones. Eur J Immunol. 1981;11:317–23. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- 18.Fu TJ, Abbott UR, Hatzos C. Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid-a comparative study. J Agric Food Chem. 2002;50:7154–60. doi: 10.1021/jf020599h. [DOI] [PubMed] [Google Scholar]
- 19.Brunner R, Wallmann J, Szalai K, Karagiannis P, Kopp T, Scheiner O, et al. The impact of aluminium in acid-suppressing drugs on the immune response of BALB/c mice. Clin Exp Allergy. 2007;37:1566–73. doi: 10.1111/j.1365-2222.2007.02813.x. [DOI] [PubMed] [Google Scholar]
- 20.Elsayed S, Hammer AS, Kalvenes MB, Florvaag E, Apold J, Vik H. Antigenic and allergenic determinants of ovalbumin. I. Peptide mapping, cleavage at the methionyl peptide bonds and enzymic hydrolysis of native and carboxymethyl OA. Int Arch Allergy Appl Immunol. 1986;79:101–7. [PubMed] [Google Scholar]
- 21.Prichard PJ, Yeomans ND, Mihaly GW, Jones DB, Smallwood RA, Louis WJ. Effect of daily oral omeprazole on 24 h intragastric acidity. Br Med J. 1983;287:1378–9. doi: 10.1136/bmj.287.6402.1378-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain SL, Michael JG. The influence of antigen digestion on orally induced immunity and tolerance. Adv Exp Med Biol. 1995;371B:1245–50. [PubMed] [Google Scholar]
- 23.Dearman RJ, Caddick H, Stone S, Kenna JG, Basketter DA, Kimber I. Immunogenic properties of rapidly digested food proteins following gavage exposure of mice: a comparison of ovalbumin with a potato acid phosphatase preparation. Food Chem Toxicol. 2002;40:625–33. doi: 10.1016/s0278-6915(01)00132-6. [DOI] [PubMed] [Google Scholar]
- 24.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL4- or IL14-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 25.Schöll I, Ackermann U, Ozdemir C, Blümer N, Dicke T, Sel S, et al. Anti-ulcer treatment during pregnancy induces food allergy in mouse mothers and a Th2-bias in their offspring. FASEB J. 2007;21:1264–70. doi: 10.1096/fj.06-7223com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helm RM, Ermel RW, Frick OL. Nonmurine animal models of food allergy. Environ Health Perspect. 2003;11:239–44. doi: 10.1289/ehp.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strid J, Thomson M, Hourihane J, Kimber I, Strobel S. A novel model of sensitization and oral tolerance to peanut protein. Immunology. 2004;113:293–303. doi: 10.1111/j.1365-2567.2004.01989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wakabayashi A, Utsuyama MH, Hosoda T, Sato K, Takahashi H, Hirokawa K. Induction of immunological tolerance by oral, but not intravenous and intra-portal, administration of ovalbumin and the difference between young and old mice. J Nutr Health Aging. 2006;10:183–91. [PubMed] [Google Scholar]
- 29.Ito K, Inagaki-Ohara K, Murosaki S, Nishimura H, Shimokata T, Torii S, et al. Murine model of IgE production with a predominant Th2-response by feeding protein antigen without adjuvants. Eur J Immunol. 1997;27:3427–37. doi: 10.1002/eji.1830271243. [DOI] [PubMed] [Google Scholar]
- 30.Snider DP, Marshall JS, Perdue MH, Liang H. Production of IgE antibody and allergic sensitization of intestinal and peripheral tissues after oral immunization with protein Ag and cholera toxin. J Immunol. 1994;153:647–57. [PubMed] [Google Scholar]
- 31.Faquim-Mauro EL, Macedo MS. Induction of IL-4-dependent, anaphylactic-type and IL-4-independent, non-anaphylactic-type IgG1 antibodies is modulated by adjuvants. Int Immunol. 2000;12:1733–40. doi: 10.1093/intimm/12.12.1733. [DOI] [PubMed] [Google Scholar]
- 32.Hilton J, Dearman RJ, Sattar N, Basketter DA, Kimber I. Characteristics of antibody response induced in mice by protein allergens. Food Chem Toxicol. 1997;35:1209–18. doi: 10.1016/s0278-6915(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 33.Saldanha JCS, Gargiulo DL, Silva SS, Carmo-Pinto FH, Andrade MC, Alvarez-Leite JI, et al. A model of chronic IgE-mediated food allergy in ovalbumin-sensitized mice. Braz J Med Biol Res. 2004;37:809–16. doi: 10.1590/s0100-879x2004000600005. [DOI] [PubMed] [Google Scholar]
- 34.Mine Y, Yang M. Epitope characterization of ovalbumin in BALB/c mice using different entry routes. Biochim Biophys Acta. 2007;1774:200–12. doi: 10.1016/j.bbapap.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Kind LS. Fall in rectal temperature as an indication of anaphylactic shock in the mouse. J Immunol. 1954;74:387–90. [PubMed] [Google Scholar]
- 36.von Garnier C, Astori M, Kettner A, Dufour N, Corradin G, Spertini F. In vivo kinetics of the immunoglobulin E response to allergen: bystander effect of coimmunization and relationship with anaphylaxis. Clin Exp Allergy. 2002;32:401–10. doi: 10.1046/j.1365-2222.2002.01304.x. [DOI] [PubMed] [Google Scholar]
- 37.Strait RT, Morris SC, Yang M, Qu XW, Finkelmann FD. Pathways of anaphylaxis in the mouse. J Allergy Clin Immunol. 2002;109:658–68. doi: 10.1067/mai.2002.123302. [DOI] [PubMed] [Google Scholar]