Summary
Background
One of the concerns of allergen-specific immunotherapy is the possible boost of inflammatory allergen-specific T lymphocytes. To address this problem, treatment with B cell epitopes devoid of allergen-specific T cell epitopes would be a promising alternative.
Objective
In this study, we examined the therapeutic potency of a single mimotope, mimicking a structural IgE epitope of grass pollen allergen Phl p 5 in an established memory mouse model of acute allergic asthma.
Methods
In the experimental set-up, BALB/c mice were primed with intraperitoneal injections of recombinant Phl p 5a (rPhl p 5a) and subsequently aerosol challenged with the nebulized allergen. Mice developed signs of bronchial asthma including hypereosinophilia around bronchi, goblet cell hyperplasia and enhanced mucus production.
Results
When the mice were subsequently treated with the grass pollen mimotope coupled to keyhole limpet haemocyanin, bronchial eosinophilic inflammation and mucus hypersecretion decreased. Further, a decrease of Th2 cytokines IL-4 and IL-5 could be observed in the bronchoalveolar lavage (BAL). In contrast to rPhl p 5a, the mimotope was in vitro not able to stimulate splenocytes to proliferation or IL-5 production. Despite not affecting the levels of pre-existing IgE, vaccination with the single mimotope thus rendered anti-inflammatory effects in a mouse model of acute asthma.
Conclusion
From our data, we conclude that vaccination with a mimotope peptide representing a single IgE epitope of the allergen Phl p 5a and being devoid of allergen-specific T cell epitopes is able to down-regulate inflammation in acute asthma.
Keywords: epitope-specific immunotherapy, grass pollen allergy, mimotopes
Introduction
To date, specific immunotherapy (SIT) of allergy is the only causative treatment with long-term effects in patients suffering from IgE-mediated allergic disorders. The efficacy of SIT is well documented [1]. It is ascribed to a down-regulation or modulation of the allergen-specific Th2 response and is accompanied by the induction of allergen-specific blocking antibodies [2]. Furthermore, peripheral T cell tolerance is turned on, initiated by the autocrine action of IL-10 and TGF-β which are produced by antigen-specific T cells in health and disease [2, 3].
On the other hand, clinical studies with T cell epitope peptides have indicated that the directed stimulation of allergen-specific regulatory T lymphocytes might be difficult due to the danger of recruitment of inflammatory T cells [4, 5]. Therefore, treatments with B cell epitopes devoid of T cell epitopes could be an attractive option. For this, mimotopes, i.e. small peptides able to imitate structural B cell epitopes by molecular mimicry, could be useful. Indeed, in a recent study, a mimotope cDNA construct has been successfully shown to trigger allergen-specific antibody responses without the activation of allergen-specific T cells [6]. To ensure sufficient immunogenicity of pure B cell epitopes, T cell bystander help is needed and can be provided by the carrier of the peptides [7]. Keyhole limpet haemocyanin (KLH) is an attractive carrier as it allows the dense and rigid display of coupled peptides.
With respect to grass pollen allergens, we have been focusing on major timothy grass allergen Phl p 5a in previous studies and identified mimotopes from different types of phage libraries [8, 9]. Three-dimensional analysis showed structural, discontinuous surface patches of Phl p 5a as being relevant for IgE binding. The mimotopes were able to interfere with the high-affinity interaction of IgE with recombinant Phl p 5a (rPhl p 5a) ranging in the order of 10−11 m [8]. Consequently, the goal of the present study was to examine for the first time the therapeutic efficacy of a decameric peptide mimotope of Phl p 5a, termed here Phl mim 5. In an established memory mouse model of acute allergic asthma [10], we mimicked the human sensitization process with Phl p 5 during the flowering period via inhalative allergen challenges to induce an asthmatic phenotype including inflammation and hypersecretion of mucus. To evaluate whether vaccination with the Phl mim 5-peptide mimotope linked to KLH could protect from acute asthma upon re-exposure to the allergen, mice were, after vaccination therapy, re-challenged with aerosolized allergen.
Materials and methods
Generation of peptide mimotopes
In a previous study, a phage clone encoding the peptide mimotope C-KLGKFGAARV-C (1,12 cyclo), here termed Phl mim 5, could be identified as a specific epitope mimic (mimotope) of the IgE epitope of grass pollen major allergen Phl p 5 [8]. This mimotope [molecular weight (Mw): 1895.1 Da] was synthesized (piCHEM, Graz, Austria) with a purity >95% and coupled to didecameric KLH (Sigma-Aldrich, Sulzfeld, Germany), which has a Mw of approximately 800 kDa with 50–75 potential binding sites. The KLH used in this study is for preclinical purposes and has no GMP quality like its monomeric counterpart used for human clinical trials. The sequence GPGPGK-(S-acetomercaptoacetic acid)-G was used as a linker.
Animals
BALB/c mice (female; 5–6 weeks) were purchased from Charles River Laboratories GmbH (Sulzfeld, Germany) and treated according to European Community rules of animal care [11] with the permission of the Austrian Ministry of Science (number BMBWK-66.009/0233-BrGT/2006).
Immunization of mice
Mice (n = 5) were immunized subcutaneously (s.c.) with Phl mim 5-KLH [20 μg/100 μL phosphate-buffered saline (PBS); piCHEM] on days 0, 7, 14 and 28. Control mice (n = 5) were immunized s.c. with rPhl p 5a (10 μg/100 μL PBS) (Biomay, Vienna, Austria) on days 0 and 21. Blood samples to be analysed were taken after the last immunization.
For the asthma model, mice (n = 15) were immunized intraperitoneally (i.p.) with rPhl p 5a (10 μg/100 μL PBS) on days 0 and 21 or were sham treated with sterile PBS (100 μL). Ten days later, mice were aerosol challenged with nebulized rPhl p 5a (0.25 mg rPhl p 5a/50 mL PBS/challenge) in a plexiglass chamber by an ultrasonic nebulizer (Kendall, Aerodyne Omega, Oldenburg, Schleswig-Holstein, Germany) for 60 min twice daily at a 4-h interval on 2 consecutive days (days 31 and 32). At this stage, physiologically relevant airway hyperreactivity was proven by respiratory metabolism studies using the Oxylet system (Panlab, Barcelona, Spain).
On days 48, 76, 84, 119, 126 and 150, mice (n = 5) were immunized s.c. with either KLH (10 μg/100 μL PBS; Sigma-Aldrich), Phl mim 5-KLH (20 μg/100 μL PBS; piCHEM) or rPhl p 5a (2 μg/100 μL). During the entire treatment period with the mimotope vaccine, no local or systemic side-effects could be observed in the animals. Unsensitized control mice (n = 5) were sham treated (100 μL PBS). For disease relapse, mice were re-challenged with aerosolized rPhl p 5a on day 172 following treatment (0.25 mg rPhl p 5a/50 mL PBS per aerosol challenge).
Blood samples were drawn on days 0 (preimmune serum), 22 (after systemic sensitization), 47 (after aerosol challenge), 55, 84, 102, 126, 157 (during treatment) and on day 172 (before sacrifice).
Specific serum immunoglobulin G1 antibody detection in immunodot
Analysis of IgG1 from immunized mice was performed in immunodot. Synthetic mimotope Phl mim 5 alone or coupled to KLH, rPhl p 5a, rDer p 2 (kindly provided by Prof. Dr J. M. Saint-Remy, University Leuven, Belgium) as a control allergen and a control peptide (C-AISGGYPV-C cyclo 1–12, a mimotope of profilin [12]) were dotted in triplicates (1 μg/dot) onto a nitrocellulose membrane (Schleicher & Schuell, Dassel, Niedersachsen, Germany). Dot strips were air-dried and blocked with TBS/0.5% Tween-20 (TBST) (Merck, Darmstadt, Germany)/5% dry milk powder (DMP) overnight at 4 °C. Pooled mouse sera were diluted 1 : 10 in 0.5% TBST/1% DMP and incubated for 4 h at 4 °C. Bound IgG1 was detected using IgG1-specific rat anti-mouse antibody (PharMingen, San Diego, CA, USA). The reaction was developed using the ECL Plus Western Blotting Detection System (Amersham, Buckinghamshire, UK).
Specific serum immunoglobulin G1 and E antibody detection in enzyme-linked immunosorbent assay
For measurement of Phl p 5-specific IgE antibodies in an ELISA, microtitre plates (Maxisorp, Nunc, Roskilde, Denmark) were coated overnight at 4 °C with rPhl p 5a (1 μg per well/100 μL 50 mm NaHCO3, pH 9.6), washed with TBST and blocked for 2 h at room temperature (RT) with TBST/1% bovine serum albumin (BSA) (Sigma-Aldrich).
Sera were diluted 1 : 100 in TBST/0.1% BSA for IgG1 and 1 : 10 for IgE and incubated overnight at 4 °C. Plates were washed and the respective isotype-specific rat anti-mouse antibody (PharMingen) 1 : 500 in TBST/0.1% BSA was added for 2 h at RT. After washing, plates were incubated with horseradish peroxidase-conjugated mouse anti-rat IgG antibody (Jackson ImmunoResearch, West Grove, PA, USA) diluted 1 : 1000 in 0.05% TBST/0.1% BSA for 2 h at RT. Standard serial dilutions of the respective purified mouse antibody (PharMingen) served as the standard. The reaction was detected by adding 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulphonic acid) diammonium salt (Sigma-Aldrich) in citric acid and hydrogen peroxide. The optical density was measured at 405–490 nm in a microplate reader (Molecular Devices, München, Germany).
Recovery of the bronchoalveolar lavage and preparation of cytospin slides
The tracheas of lethally anaesthesized mice (naïve, asthmatic or mimotope-treated) were cannulated (BD Venflon, 0.8×25 mm; Heidelberg, Baden Württemberg, Germany), the supernatants were collected individually and cell pellets were resuspended in 500 μL PBS (2×104 cells) after centrifugation at 800 g for 18 min. Total leucocytes were counted with a haemocytometer. Cytospin slides were prepared and stained with May–Grünwald–Giemsa [13] to determine the cell differential. BAL supernatants were individually collected for cytokine measurements.
Counts of inflammatory cells by Tissue FAXs technology
Following BAL recovery, tracheas were perfused with formalin (4%). Paraffin-embedded lung sections of 4 μm were stained with Luna [14] for eosinophil and with periodic acid-Schiff (PAS) for mucopolysaccharide staining. In each mouse, the total eosinophil numbers surrounding 10 bronchioles positioned in a bordered area in parallel to the central airways were counted by laser scanning microscopy and analysed with the HistoQuest software module (Tissuegnostics, Vienna, Austria). Visual grading of mucus was performed in a blinded fashion in comparison with the maximal release in asthmatic mice. This value was set to 100% and the other samples were compared hereto.
Cytokine measurements
IL-4, IL-5 and IFN-γ measurements were performed by ELISA with anti-mouse cytokine antibodies and standards (Bender MedSystems, Vienna, Austria) in non-diluted BAL samples according to the manufacturer’s instructions.
Isolation of splenocytes for the interleukin -5 cytokine assay and the 3H thymidine proliferation assay
Mice (n = 5) sensitized and aerosol challenged with rPhl p 5a were killed and the spleens were removed. Spleen cell suspensions of each mouse were prepared by cutting, mincing and filtering the spleens through 70 μm nylon meshes (BD Biosciences, Schwechat, Austria). Cells were resuspended in RPMI medium (Gibco Invitrogen, Lofer, Austria) supplemented with 10% fetal calf serum, 1% l-glutamine and 1% penicillin/streptomycin.
Mononuclear cells from each spleen were isolated by density separation (Lympholyte-M; Cedarlane, Hornby, ON, Canada) according to the manufacturer’s instructions. Cells were plated (4×105 cells/well) in triplicates in sterile round-bottom 96-well tissue culture plates (Costar, New York, NY, USA). For stimulation, rPhl p 5a, mimotope Phl mim 5, control allergen Ara h 2 (from peanut; vector kindly provided by Prof. Dr H. Sampson, Mount Sinai School of Medicine, New York) and positive control Con A (Sigma-Aldrich) (2.5 μg/mL each) were added. The cells were cultured for 72 h at 37 °C and 5% CO2. The supernatants were harvested and stored at −20 °C until further use for cytokine determination. Single splenocytes were pulsed with 0.5 μCi/well 3H thymidine diluted in 50 μL RPMI medium for 16 h at 37 °C and 5% CO2. Cells were harvested with a Filter Mate Harvester (Perkin Elmer, Waltham, MA, USA) and radioactivity of DNA was measured in counts per minute and expressed as mean value per mouse.
Measurement of IL-5 was performed by ELISA with anti-mouse cytokine-antibodies and standards (Bender MedSystems) in pooled supernatants of stimulated splenocytes (diluted 1 : 2) according to the manufacturer’s instructions.
Statistical analysis
Statistical comparison between groups was performed by the Mann–Whitney U-test, using the software SPSS (version 14.0 for Windows). Differences were considered statistically significant at P<0.05.
Results
Immunization with the mimotope induced Phl p 5-specific immune responses in naïve mice
For proof of the mimicry potential, naïve mice were immunized s.c. with the mimotope Phl mim 5 linked to KLH. Testing of pooled sera showed that the mimotope-induced IgG antibodies cross-reacted with the orthologue allergen Phl p 5, but not with the control allergen Der p 2 or an unrelated control mimotope of panallergen profilin, termed Prof mim 1 (Fig. 1). As expected, these sera showed IgG reactivity against the carrier KLH, but also to uncoupled synthetic Phl mim 5. Vice versa, rPhl p 5a immunization yielded predominantly anti-Phl p 5 IgG and only slight crossreactivity towards Phl mim 5, which may have to do with the fact that a mimotope only represents a sub-epitope structure.
Fig. 1.
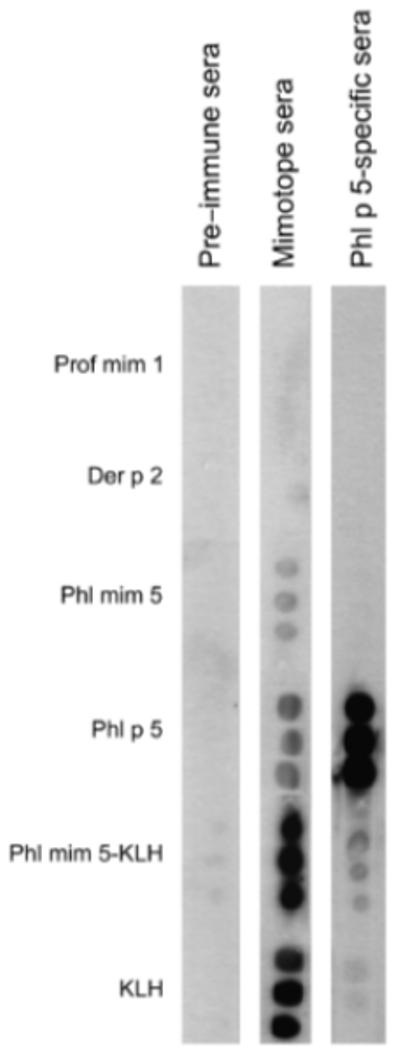
Antigenic cross-reactivity of mimotopes and recombinant allergen Phl p 5a (rPhl p 5a) in immunodot. Pooled sera of mimotope- or of rPhl p 5a-immunized BALB/c mice were tested for IgG1 antibodies towards various antigens and control antigens (1 μg/dot) indicated on the left-hand side. No reactivity could be detected to control allergen Der p 2 from house dust mite and to control peptide Prof mim 1. Preimmune sera represent the sera of naïve mice. Bound IgG was detected with peroxidase-labelled anti-mouse IgG. KLH, keyhole limpet haemocyanin.
Immunoglobulin E antibody titres of asthmatic BALB/c mice were not affected by mimotope and control treatments
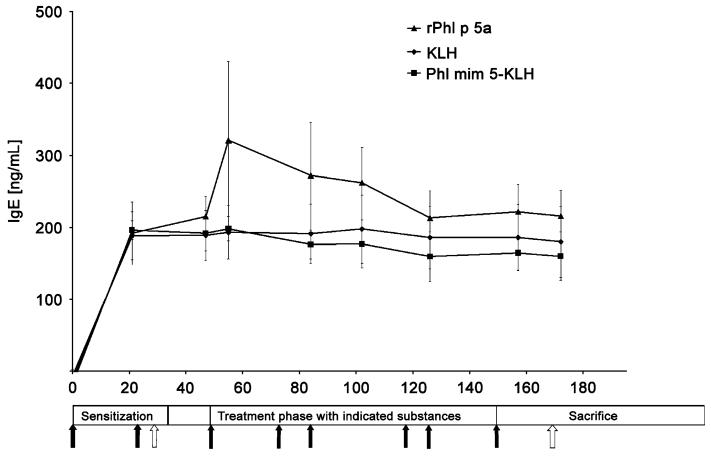
For the induction of acute allergic asthma, mice were sensitized i.p. with rPhl p 5a, followed by an aerosol challenge. The allergen induced increases of specific IgE in all sensitized mice (Fig. 2). Consecutively, groups of mice were treated s.c. either with Phl mim 5 coupled to KLH, with KLH alone or with rPhl p 5a, for control. During the entire treatment period, the IgE (and IgG1, data not shown) antibody titres of mimotope- and control-treated mice remained elevated without a statistical difference (Fig. 2).
Fig. 2.
Recombinant Phl p 5a (rPhl p 5a)-specific IgE titres in sera of mice with grass pollen-specific asthma in ELISA. During treatments, IgE levels in all the groups remained at comparable levels upon treatment with either allergen rPhl p 5a, the keyhole limpet haemocyanin (KLH)-linked mimotope Phl mim 5 or KLH alone. Black arrows represent treatments, i.e. immunizations. White arrows indicate aerosol challenges of mice with the nebulized allergen. Values of sham-treated control animals were below the detection limit.
Reduced eosinophil numbers in bronchoalveolar lavage and peribronchiolar tissue of mimotope-treated mice
As serology did not reveal any differences between the treated mouse groups, we aimed to analyse inflammatory parameters in the lungs. BAL cytology revealed that mimotope vaccination significantly prevented eosinophil infiltration in the airways compared with mice treated with KLH alone (P = 0.032) (Fig. 3). The effect was more pronounced than treatments with the recombinant allergen molecule rPhl p 5a (not significant).
Fig. 3.
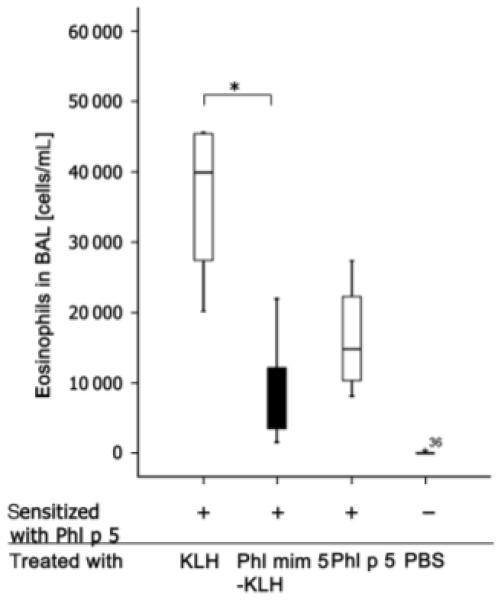
Mimotope treatments of BALB/c mice with grass pollen-specific asthma are associated with a decreased number of eosinophils in bronchoalveolar lavage (BAL) cytospins. Mice were intraperitoneally and aerosol sensitized with rPhl p 5a (+) and subsequently vaccinated with substances indicated on the x-axis. Finally, mice were aerosol re-challenged with rPhl p 5a. Calculation of eosinophils was performed by BAL cytometry. P<0.05. BAL samples showing more than a threefold deviation from the end of the box were defined as extremes and marked with asterisks.
Accordingly, we evaluated the eosinophil numbers in peribronchiolar regions of the paraffin-embedded lungs by Luna-staining highlighting the eosinophil granules in red.
The results indicate that Phl mim 5-KLH vaccination, but not KLH treatment alone, protected mice from eosinophilic infiltration (P = 0.029), similar to treatments with rPhl p 5a (P = 0.024) (Figs 4a–c).
Fig. 4.
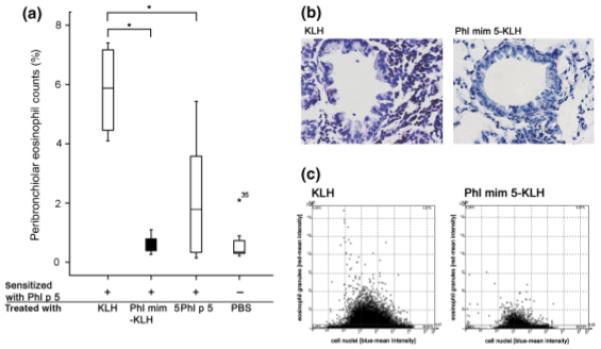
Peribronchiolar eosinophil counts in mimotope-vaccinated mice. Mice were sensitized with recombinant Phl p 5a (rPhl p 5a) (+) and treated with substances indicated on the x-axis. After treatment, they were aerosol re-challenged with rPhl p 5a (a). Peribronchiolar eosinophil counts were determined and samples lying more than threefold away from the end of the box were defined as extremes and marked with asterisks (b). Representative histological Luna stainings of lungs from mice treated with keyhole limpet haemocyanin (KLH) or Phl mim 5-KLH (c). Respective scattergram by Tissue FAXs; x-axis: counted cells according to their size and the staining of their nuclei; y-axis: the number of eosinophils according to their granules stained in red. PBS, phosphate-buffered saline.
Mimotope treatments reduce T-helper type 2 cytokine levels in bronchoalveolar lavage
The influence of the mimotope vaccine on the cytokines IFN-γ, IL-4 and IL-5 in BAL was investigated by ELISA. As depicted in Fig. 5, treatment with Phl mim 5-KLH before re-challenge with the allergen did not affect IFN-γ production (Fig. 5a), but slightly reduced IL-4 (P = 0.3) (Fig. 5b), although no significance could be achieved. With respect to IL-5 levels in BAL, the effect of the mimotope vaccine was comparable to treatment with rPhl p 5a, again without a significant difference (P = 0.4) (Fig. 5c).
Fig. 5.
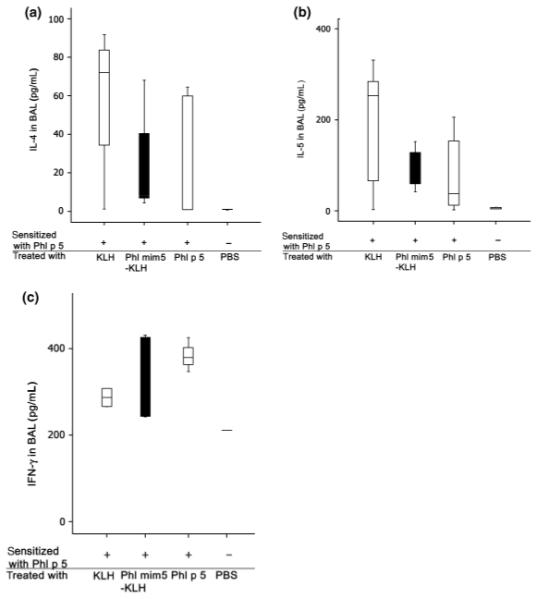
Th2 cytokines in the bronchoalveolar lavage (BAL) fluid of mimotope-treated asthmatic mice in ELISA. Mice were sensitized either with rPhl p 5a (+) or treated with substances indicated below the x-axis. After aerosol re-challenge with rPhl p 5a, ELISA measurements revealed that the IL-4 (a) and IL-5 (b) levels in BAL were reduced in Phl mim 5-treated mice, whereas the IFN-γ (C) levels remained unchanged. PBS, phosphate-buffered saline; rPhl p 5a, recombinant Phl p 5a.
Mimotope treatments diminish mucus hypersecretion within bronchioles
To determine whether mucus hypersecretion could be modulated by mimotope vaccination, lung sections were stained with PAS. Mucus production as a direct indicator for goblet cell numbers was graded for each mouse group, showing that Phl mim 5-KLH and rPhl p 5a but not KLH vaccinations, induced a reduction of the goblet cell numbers (Fig. 6a). Also, less mucus was visible in mice immunized with the Phl mim 5-KLH and rPhl p5 a in comparison with mice receiving KLH alone (Fig. 6b) or PBS (data not shown).
Fig. 6.
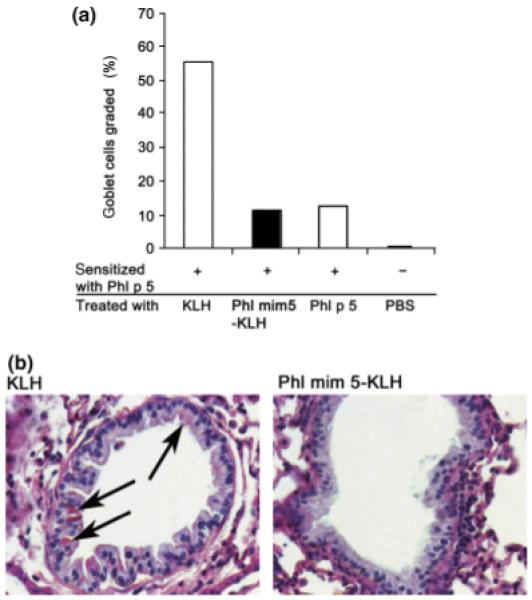
Mimotope-treated mice show diminished mucus secretion within bronchioles. Mice sensitized with recombinant Phl p 5a (rPhl p 5a) (+) were treated with the substances indicated. Following an aerosol re-challenge with rPhl p 5a, mucus secretion in bronchioles was evaluated by periodic-acid-Schiff staining. (a) Diagram showing the relative percentage of mucus production in treated mice as compared with the level in untreated control, which was set to 100%. (b) Representative lung stainings from mice treated with keyhole limpet haemocyanin (KLH) or Phl mim 5-KLH (arrows: mucus-positive cells). PBS, phosphate-buffered saline.
Proliferation and interleukin-5 production of splenocytes upon allergen or mimotope stimulation
To prove whether a mimotope, being devoid of allergen-specific T cell epitopes, is able to activate allergen-specific T cells, specific proliferation of splenocytes was analysed in a 3H thymidine incorporation assay. Stimulation of murine splenocytes with rPhl p 5a resulted in more specific proliferation than treatment with mimotope Phl mim 5 (P = 0.4) or irrelevant allergen Ara h 2 (P = 0.1) (Fig. 7a). In parallel, IL-5 production was monitored in pooled supernatants of these stimulated splenocytes. In accordance with the proliferation assay, more IL-5 production could also be observed in the supernatants of rPhl p 5a-stimulated splenocytes, whereas there was hardly any response to Phl mim 5 (Fig. 7b).
Fig. 7.
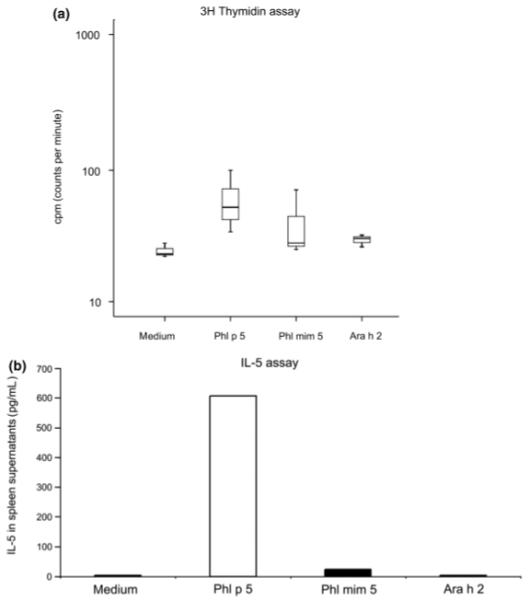
Mice were sensitized and nebulized with recombinant Phl p 5a (rPhl p 5a) to induce allergic asthma. Splenocytes of these mice were isolated and stimulated with either rPhl p 5a, peptide mimotope Phl mim 5 or control allergen Ara h 2. (a) Less proliferation of splenocytes was observed in response to stimulation with mimotope Phl mim 5 and irrelevant allergen Ara h 2 than after addition of rPhl p 5a. The y-axis indicates the amount of 3H thymidine incorporated into DNA of stimulated splenocytes. Splenocytes were stimulated with substances indicated below the x-axis. (b) Stimulation with rPhl p 5a, but not with control allergen Ara h 2 leads to IL-5 synthesis. The concentration of IL-5 levels is given in pg/mL, indicated on the y-axis. Substances used for stimulation are indicated below the x-axis.
Discussion
In a previous study, decameric peptides have been defined as IgE-epitope mimics (mimotopes) of major grass pollen allergen rPhl p 5a [8]. The mimotope with the highest specificity and binding capacity to rPhl p 5a-specific IgE (termed here Phl mim 5) was chosen for synthesis and coupling to KLH as an antigenic carrier in the present study. Although previously less effective for immunization, due to the low copy number of peptides when displayed by phage protein pIII, we demonstrate here that the same mimotope induced rPhl p 5a-reactive IgG in naïve BALB/c mice when displayed at a high density on KLH. When manufacturing the KLH-mimotope conjugate, we intended to approach an equimolar dose with rPhl p 5a. According to our calculation, the mimotope in this conjugate exceeded the molarity of rPhl p 5a per applied dose by a factor of 5. Still, the levels of the mimotope-induced IgG were lower and the immunodot showed that it exhibited a higher degree of cross-reactivity with the allergen than vice versa. Both findings may have to do with the fact that the mimotope represents only a subunit of the original allergen epitope.
As a continuation of our previous efforts, the present study was designed to evaluate for the first time the therapeutic capacity of Phl mim 5, taking advantage of a memory mouse model of acute allergic asthma [15]. The induction of allergic asthma in mice is characterized by airway hyperreactivity, eosinophilia, increased IgE levels and mucus hypersecretion, which makes it a relevant model of human allergic disease and useful for testing novel therapeutics. In the BALB/c mouse model, a sustained elevation of allergen-specific IgE can be induced by rPhl p 5a sensitization; however, aerosol challenge with the grass pollen allergen is needed to induce the asthmatic phenotype in the bronchi. To determine whether immunological memory was generated, mice were allowed to recover from acute disease before a re-challenge, which should mimic re-exposure in the pollen season. As intermittent exposure to seasonal allergens already induces allergic symptoms, the rapid reaction to these allergens in sensitized human patients or in mice refers to immunological memory [10]. It has been demonstrated previously that up to 800 days after the onset of acute disease, mice were still susceptible to the development of characteristic features of allergic asthma by a secondary aerosol challenge using ovalbumin as a specific antigen [15]. One predominant parameter of acute allergic asthma in mice is the eosinophilic airway inflammation that resolves within 11 days after onset of acute disease. Indeed, we observed recurrent eosinophilic inflammation in BAL fluids 48 h upon re-challenge with the allergen rPhl p 5a. Even though representing a subunit vaccine as outlined above, the mimotope vaccination inhibited peribronchiolar eosinophilic infiltration and exsudation of eosinophils into BAL, indicating the anti-inflammatory capacity of this intervention. This effect seemed to be antigen-specific, as treatments with the carrier KLH alone had no effect.
IFN-γ is described to promote cell-mediated immune responses, and in case of asthma, inhibits the development of airway hyperreactivity in the lungs induced by Th2 responses [16]. In our model, IFN-γ levels in the BAL were not changed; however, the Th2 cytokines IL-4 and IL-5 showed a downward trend in the mimotope-treated group, pointing towards an immunomodulation. IL-4 plays a major role in the induction of IgE, including the IgE switch and airway hyperreactivity [17]. Similarly, IL-5 plays an important role in airway hyperresponsiveness and is a central factor mediating eosinophil expansion, priming, recruitment and prolonged tissue survival in response to allergic stimuli [18]. IL-4 and IL-5 are thus key regulators of airway inflammation and hyperreactivity in asthma. In our study, the relatively lower IL-5 levels observed after mimotope therapy were in accordance with lower counts of eosinophils. However, the somehow reduced IL-4 levels in the mimotope-treated group did not parallel IgE antibody titres, which remained unchanged during the whole experiment. This may possibly be due to long-lived plasma cells [19] and/or ‘tissue-memory’ as receptor-bound IgE prolong the survival time of effector cells in the periphery (reviewed in [20]). Under healthy conditions, the columnar epithelial surface in the bronchi comprises a small percentage of goblet cells and a majority of ciliated cells, whereas in chronic airway diseases like asthma, 20–25% of airway epithelial cells transform mucus-producing goblet cells [21]. After an acute onset of asthma, mucus within goblet cells of allergic mice normally decreases to baseline levels within 30 days after acute disease. In contrast, upon repeated allergen exposure, mucus production can be readily restored and visualized on PAS-stained lungs sections. The fact that mucus hypersecretion by goblet cells was abrogated in the mimotope-treated animals supports our hypothesis that B cell epitope mimics could have a potency in abating allergic asthma. In this context, the fact that the Phl mim 5 mimotope used here is devoid of T cell epitopes may be advantageous [6] because allergen-specific T lymphocytes cannot be re-activated through the treatment. We believe that the absence of allergen-specific T cell epitopes might have contributed to the observed anti-inflammatory effects of the mimotope vaccine in our mouse model. The duration of the vaccine effect remains to be determined, but might be comparable to the effect of other subunit vaccines. Another advantage is that mimotope peptides can be easily produced synthetically on a large scale and at relatively low costs.
Taken together, the results from this study suggest that there are several arguments in favour of the presented mimotope vaccine. It down-regulates bronchial inflammation including Th2 cytokines, eosinophil counts and mucus production without the activation of allergen-specific T cells, and may thus relieve the symptoms of grass pollen-induced asthma.
Acknowledgements
We are obliged to Dr Rupert Ecker, Dipl. Ing. Radu Rogojanu and Katja Österreicher from Tissuegnostics for providing technical support with respect to the Tissue FAXs laser scanning microscope technology and automated readout. Further, we would like to thank Dr Werner Sallegger and Dr Fritz Andreae, piChem, Graz, Austria, for their excellent support in peptide design. We also like to thank Ing. Magdolna Vermes, Silke Gruber and Philipp Starkl for their excellent technical assistance. This work was supported by SFB project F1808-B13, and I.P-S. by Hertha-Firnberg stipend T283-B13, both from the Austrian Science Fund.
Footnotes
Cite this as: J. Wallmann, M. M. Epstein, P. Singh, R. Brunner, K. Szalai, L. El-Housseiny, I. Pali-Schöll and E. Jensen-Jarolim, Clinical & Experimental Allergy, 2010 (40) 650-658.
References
- 1.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 2.Akdis M. Healthy immune response to allergens: T regulatory cells and more. Curr Opin Immunol. 2006;18:738–44. doi: 10.1016/j.coi.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Jutel M, Akdis M, Budak F, et al. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003;33:1205–14. doi: 10.1002/eji.200322919. [DOI] [PubMed] [Google Scholar]
- 4.Ali FR, Oldfield WL, Higashi N, Larche M, Kay AB. Late asthmatic reactions induced by inhalation of allergen-derived T cell peptides. Am J Respir Crit Care Med. 2004;169:20–6. doi: 10.1164/rccm.200305-690OC. [DOI] [PubMed] [Google Scholar]
- 5.Smith TR, Larche M. Investigating T cell activation and tolerance in vivo: peptide challenge in allergic asthmatics. Cytokine. 2004;28:49–54. doi: 10.1016/j.cyto.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Wallmann J, Proell M, Stepanoska T, et al. A mimotope gene encoding the major IgE epitope of allergen Phl p 5 for epitope-specific immunization. Immunol Lett. 2009;122:68–75. doi: 10.1016/j.imlet.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scholl I, Wiedermann U, Forster-Waldl E, et al. Phage-displayed Bet mim 1, a mimotope of the major birch pollen allergen Bet v 1, induces B cell responses to the natural antigen using bystander T cell help. Clin Exp Allergy. 2002;32:1583–8. doi: 10.1046/j.1365-2222.2002.01527.x. [DOI] [PubMed] [Google Scholar]
- 8.Hantusch B, Krieger S, Untersmayr E, et al. Mapping of conformational IgE epitopes on Phl p 5a by using mimotopes from a phage display library. J Allergy Clin Immunol. 2004;114:1294–300. doi: 10.1016/j.jaci.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 9.Hantusch B, Knittelfelder R, Wallmann J, et al. Internal images: human anti-idiotypic Fab antibodies mimic the IgE epitopes of grass pollen allergen Phl p 5a. Mol Immunol. 2006;43:2180–7. doi: 10.1016/j.molimm.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 10.Mojtabavi N, Dekan G, Stingl G, Epstein MM. Long-lived Th2 memory in experimental allergic asthma. J Immunol. 2002;169:4788–96. doi: 10.4049/jimmunol.169.9.4788. [DOI] [PubMed] [Google Scholar]
- 11.Waldegrave W. Directive CEE 86/609. J Officiel Communautés. 1986;L358:1–28. [Google Scholar]
- 12.Leitner A, Vogel M, Radauer C, et al. A mimotope defined by phage display inhibits IgE binding to the plant panallergen profilin. Eur J Immunol. 1998;28:2921–7. doi: 10.1002/(SICI)1521-4141(199809)28:09<2921::AID-IMMU2921>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Paseyro P, Varela Lopez M, Prodanov E, Villar H, Ghiggino CW. Simple technic for the cytologic study of sputum using the May Grunwald-Giemsa stain. Med Panam. 1957;9:63–6. [PubMed] [Google Scholar]
- 14.Luna L. Manual of histologic staining methods of the armed forces. Institute of Pathology; New York: 1968. pp. 114–5. [Google Scholar]
- 15.Epstein MM. Are mouse models of allergic asthma useful for testing novel therapeutics? Exp Toxicol Pathol. 2006;57(Suppl. 2):41–4. doi: 10.1016/j.etp.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Ford JG, Rennick D, Donaldson DD, et al. Il-13 andIFN-gamma: interactions in lung inflammation. J Immunol. 2001;167:1769–77. doi: 10.4049/jimmunol.167.3.1769. [DOI] [PubMed] [Google Scholar]
- 17.Foster PS, Ming Y, Matthei KI, et al. Dissociation of inflammatory and epithelial responses in a murine model of chronic asthma. Lab Invest. 2000;80:655–62. doi: 10.1038/labinvest.3780068. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–10. doi: 10.1016/j.jaci.2007.03.048. quiz 1311-2. [DOI] [PubMed] [Google Scholar]
- 19.McHeyzer-Williams MG, Ahmed R. B cell memory and the long-lived plasma cell. Curr Opin Immunol. 1999;11:172–9. doi: 10.1016/s0952-7915(99)80029-6. [DOI] [PubMed] [Google Scholar]
- 20.Jensen-Jarolim E, Achatz G, Turner MC, et al. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63:1255–66. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohn L. Mucus in chronic airway diseases: sorting out the sticky details. J Clin Invest. 2006;116:306–8. doi: 10.1172/JCI27690. [DOI] [PMC free article] [PubMed] [Google Scholar]