Abstract
Digestion assays with simulated gastric fluid have been introduced for characterization of food proteins to imitate the effect of stomach proteolysis on dietary compounds in vitro. By using these tests, dietary proteins can be categorized as digestion-resistant class 1 (true allergens triggering direct oral sensitization) or as labile class 2 allergens (nonsensitizing elicitors). Thus the results of these digestion assays mirror situations of intact gastric proteolysis. Alterations in the gastric milieu are frequently experienced during a lifetime either physiologically in the very young and the elderly or as a result of gastrointestinal pathologies. Additionally, acid-suppression medications are frequently used for treatment of dyspeptic disorders. By increasing the gastric pH, they interfere substantially with the digestive function of the stomach, leading to persistence of labile food protein during gastric transit. Indeed, both murine and human studies reveal that antiulcer medication increases the risk of food allergy induction. Gastric digestion substantially decreases the potential of food proteins to bind IgE, which increases the threshold dose of allergens required to elicit symptoms in patients with food allergy. Thus antiulcer agents impeding gastric protein digestion have a major effect on the sensitization and effector phase of food allergy.
Keywords: Food allergy, gastric digestion, acid-suppression medication, digestion assay, simulated gastric fluid
Despite being considered a pleasure by most persons, food intake might also represent a health hazard in situations of altered metabolism or if food proteins are recognized as potentially harmful by the immune system. This failure of oral tolerance leading to hyperimmune reactions toward food compounds is termed food allergy1 and is considered to be a major health concern in Western society. Even though population studies indicate that more than 20% of all patients believe themselves to be allergic to food,2 the true prevalence of this disorder ranges between 3% and 4% in the general population.3,4 The number of affected patients peaks in children younger than 3 years,5 and an increasing prevalence of peanut sensitization has been shown.6,7
Not only the rising number of food-allergic patients but also the severity of food-induced adverse reactions accounts for the importance of this disorder. On intake of the offending food, susceptible persons report a large variety of symptoms, ranging from mild local reactions at the first contact sites (oral allergy syndrome) to life-threatening systemic reactions, such as asthma or anaphylactic shock.8-12 Interestingly, food allergy accounts for up to 50% of all anaphylactic episodes resulting in hospitalization and represents the major cause for these hazardous reactions.13-15 Therefore it is obvious that greater knowledge of the underlying mechanisms and characteristics of food allergens is crucial for a better understanding of this disease.
FOOD ANTIGEN ABSORPTION: A DELICATE BALANCE BETWEEN ORAL TOLERANCE AND INDUCTION OF IMMUNE RESPONSES
During human evolution, a sophisticated safety system developed to simultaneously allow immune defense against pathogens and avoidance of hypersensitivity reactions against harmless substances, such as food. The mucosal barrier, consisting of intestinal epithelial cells joined together by apical and basolateral tight junctions and mucus produced by specialized epithelial cells, such as goblet cells, prevents antigen penetration.16 Additionally, immunologic mechanisms, including immune exclusion accomplished by mucosal secretory IgA antibodies, and downregulatory mechanisms contribute to oral tolerance.17 T-cell anergy, clonal deletion, and T regulatory cell induction are induced under normal conditions by orally ingested food proteins.18,19 Here the amount of the administered food antigen plays a decisive role. Relatively low antigen dosage preferentially induces active suppression by regulatory T cells, whereas higher antigen amounts seem to be associated with clonal T-cell anergy or deletion.20,21 Also, intestinal epithelial cells play a decisive role in tolerance induction. They were shown to express MHC class II on their surfaces and might function as antigen-presenting cells.22 These cells do not express costimulatory molecules and therefore induce anergy in T cells responding to the presented antigen.23
Despite the barrier and control function of the gastrointestinal mucosa, immunologically active food proteins can be absorbed and systemically distributed. At the beginning of the 20th century, the topic of food absorption through the gastrointestinal tract was addressed in pioneer studies. Through passive sensitization with sera derived from patients with fish allergy, the absorption of immunologically active proteins after a meal of fish was proved for the first time in healthy individuals.24,25 Part of these proteins might enter the circulation through the oral mucosa, representing the first contact site of food proteins with immune cells of the oropharynx. Recently, the absorption of peanut proteins into the circulation through the buccal mucosa was reported,26 which has been indicated also for fish proteins.27 In the latter study, the time kinetics of protein absorption revealed the presence of the maximal amount of fish proteins in the circulation 1 or 2 hours after the fish meal, correlating with the average gastric transit time determined for drug compounds.28
Regarding the amount of absorbed, intact food proteins, it was shown that between 0.1 and 3 ng of β-lactalbumin per milliliter of serum could be detected in the circulation 30 minutes to 3 hours after consumption of 1.2 L of cow's milk.29 Up to 10 ng of oval-bumin per milliliter of serum was found in the circulation 2 to 3 hours after protein ingestion.30 The quantity of proteins entering the circulation in an intact form through the intestinal mucosa might be increased under certain disease conditions, such as untreated celiac disease,31 or in the perinatal period because of an immature barrier function of the intestinal tract.32
Nevertheless, the majority of ingested food proteins are exposed to the denaturing environment and to digestive enzymes on their travel through the gastrointestinal tract.
PHYSIOLOGIC DIGESTION OF DIETARY PROTEINS
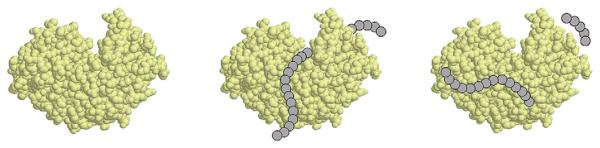
After a relatively quick passage through the esophagus, proteins contained in the macerated food bole enter the gastric lumen. Here the stomach is distended by the entering food, resulting in increased gastrin secretion. Absorbed from the blood stream, gastrin triggers hydrochloric acid production in the parietal cells and, to a lesser extent, digestive enzyme secretion by the chief cells of the gastric glands.33 In the stomach the chyme is not only exposed to hydrochloric acid, mucins, and inorganic salts but also to different pepsins, the major gastric proteases.34 These proteinases are produced and secreted into the gastric lumen as inactive proenzymes, called zymogens or pepsinogens.35 At low pH levels, the acidic amino acid (AA) residues in the active enzyme moieties undergo protonation. The electrostatic interactions between the N-terminal prosegment and the active pepsin are disrupted, which initiates a conformational change in both the prosegment and the active enzyme portion. Thus the removal of the prosegment results in conversion into the enzymatically active form of pepsin.36,37 Only then is the substrate-binding cleft with the 2 active-site aspartates accessible for binding to protein chains, and protein cleavage can take place (Fig 1). Whereas at a pH of greater than 5.0, limited pepsin is activated, the rate of active enzyme increases with decreasing gastric pH.38,39 An acidic milieu is required for the proteolytic activity of pepsins, with an activity optimum between pH 1.8 and 3.2.40 Pepsins have a broad specificity against large molecular peptides, preferentially cleaving proteins at phenylalanine, tyrosine, and leucine residues.41,42
FIG 1.
Physiologic gastric protein digestion by pepsin. After activation of pepsin, the substrate-binding cleft is accessible for proteins, and protein cleavage into peptides takes place. The figure was created with the program Protein Explorer 2.411 Beta (available at: http://proteinexplorer.org) by using the structural information of porcine pepsin provided with the Research Collaboratory for Structural Bioinformatics Protein Data Bank accession number 5PEP.
Subsequently, the remaining peptones and polypeptides present in the chyme are released into the small intestine. Here they are exposed to a variety of proteases and peptidases produced and secreted by the pancreas, such as trypsin, chymotrypsin, or carboxypeptidases, or to brush border peptidase of the intestinal mucosa. Requiring an alkaline pH level, these enzymes catalyze further digestion into single AAs or small peptides of up to 3 AAs in length, which are actively taken up by enterocytes and serve as nutrients for the human body.43-45 This extensive digestion renders small compounds of less than a size of 8 AAs, which are non-reactive with any structure responsible for antigen recognition and presentation and are therefore immunologically ignored.46
CLASSIFICATION OF FOOD ALLERGENS
Only dietary proteins large enough to elicit immune responses are potential food allergens. It has been hypothesized previously that protein epitopes recognized by IgE antibodies are of conformational nature,47,48 which we recently confirmed for the IgE-binding site of the major fish allergen parvalbumin,49 as well as for other allergens.50-52 However, on chronic allergen exposure, such as in milk allergy, linear epitopes might become important in later stages of the disease.53 Additionally, polyvalence has been identified as a general characteristic of allergens that enable cross-linking processes,54 which has also been discussed for food proteins.55
For classification purposes, food allergens have been divided in 2 classes based on their potential to trigger specific IgE antibody formation. The complete or class 1 allergens not only cross-link IgE but are also the primary source of sensitization. These allergens are described as resistant to the denaturating conditions of food processing or of enzymatic digestion in the gastrointestinal transit, thereby enabling direct oral sensitization.56 Prominent examples for these class 1 allergens are β-lactoglobulin in cow's milk and stable peanut proteins.57,58 In contrast, the class 2 or incomplete food allergens are postulated to lack sensitizing capacity. These proteins have the potential to elicit symptoms only after primary sensitization with cross-reactive inhalative allergens and were therefore termed nonsensitizing elicitors.59 Prominent examples are protein homologues of Bet v 1, the major birch pollen allergen, which are present in fruits and vegetables, such as apple, pear, apricot, and cherry. Their susceptibility to peptic digestion has been demonstrated60,61 and might explain why most often local but not systemic symptoms are triggered on ingestion of Bet v 1 homologues. Only stabilization of a Bet v 1 epitope in a mimotope configuration rendered a successful oral sensitization.60
PREDICTING THE ALLERGENIC POTENTIAL OF FOOD PROTEINS BY USING DIGESTION ASSAYS
Based on current knowledge on the relation of gastrointestinal digestion, food allergy, and dietary allergens, digestion experiments have been introduced for assessing the allergenic capacity of novel food proteins. In 1996, Astwood et al62 reported in a cutting-edge study that digestion experiments in simulated gastric fluid (SGF) ideally distinguish between potentially allergenic and nonallergenic food proteins. Their work was triggered by reports on common characteristics of food allergens63 and the emerging need to predict the allergenic potential of novel dietary compounds. The growing number of genetically modified plants entering the market was a challenge for regulatory authorities to ensure consumer safety,64 as indeed potent allergens had previously been transferred into transgenic food.65 Therefore the methodology of testing food for its resistance to pepsin digestion was incorporated in a decision tree protocol that was elaborated in a joint Food and Agriculture Organization/World Health Organization expert meeting in 2001, which was approved by the US Food and Drug Administration in 2004.66,67 Testing proteins for resistance to SGF exposure has since become a tool extensively applied in food allergy research to gain novel insights into food allergen biology. Peanut allergens were discovered to contain large, digestion-stable, allergenic fragments58 or to form aggregates that act as potent triggers of allergic reactions.68 Moreover, a multiphase model of gastrointestinal digestion has been developed to analyze emulsification effects and the effect of food phospholipid content on dietary protein digestibility.69,70
It has become evident that some potent allergens are not stable in SGF, as previously expected.71-73 Even though the outcome of these digestion assays might depend on the applied protein to pepsin ratio,74 digestion experiments with the basal gastric pepsin concentration for SGF or even pharmaceutical enzyme tablets revealed the quick digestibility of potent allergens, such as milk, fish, and hazelnut, by gastric enzymes.27,75-79 Thus these allergens do not show features previously postulated for true class 1 allergens; however, they might still contain peptide fragments recognizable by allergen-specific T cells.80
PHYSIOLOGICALLY AND PATHOLOGICALLY ALTERED GASTRIC DIGESTION CAPACITY
Interestingly, gastric digestion assays only simulate situations in which both the production of digestive enzymes and the acid-secretion capacity of the stomach are intact. It is noteworthy that the secretory capacity of the stomach changes physiologically throughout a lifetime, influencing gastric protein digestion. Early studies indicated that in newborns the intragastric pH ranges from 6.0 to 8.0,81,82 which is followed by a burst of acid secretion leading to adult gastric pH levels (pH 1.0-3.0) 24 to 48 hours after birth. After these first days of life, the gastric acid production remains low during the next months, and adult pH levels in the stomach are not reached until the average age of 2 years.83-87 It is well established that gastric acid secretion decreases with age, resulting in low gastric acidity in more than 50% of all patients aged 60 years and older.88 It has been reported that low gastric acid output is associated with pathologies like atrophic gastritis, celiac disease, diabetes mellitus, rheumatoid arthritis, and Sjögren syndrome.89-91
On the other hand, increase of the gastric pH is the therapeutic goal in patients with dyspepsia, such as gastritis, ulcer, erosions, and reflux symptoms. Approximately 25% to 54% of the adult population in Western countries is affected by dyspeptic disorders per year.92-95 Even though most of them take medication without specialist consultation and adequate diagnosis, dyspeptic symptoms account for up to 5% of all consultations to general practitioners.96-98 Moreover, gastroesophageal reflux (ie, the presence of gastric fluid proximal to the stomach) is one of the most prevalent problems affecting the gastrointestinal tract in infancy,99 being today treated with long-term acid suppression by proton pump inhibitors (PPIs) or H2-receptor blockers.100-102
ACID SUPPRESSION MEDICATION: WORLDWIDE PRESCRIPTION HABITS, MECHANISMS OF ACTION, AND POSSIBLE SIDE EFFECTS
From their broad application in clinics, it is not surprising that antiulcer agents are among the top-selling drugs worldwide. The use of antiulcer medication is rapidly increasing in Western countries and comprises up to 10% of the national medical budget.103-106 In 1996, 2% of the English health authority budget was spent on acid-suppression drugs, with 80% of the costs being caused by repeated prescriptions without further medical consultation.107-109 Despite clear evidence-based guidelines, approximately 60% of acid-suppressive therapy is started inappropriately during hospitalization.110-112 Between 1997 and 2001, antiulcer medication use increased from 9.6% to 15.9% in a Taiwanese cohort, being highest in patients 60 years and older (with a prevalence of 25.9%) in 2001.113 Reflecting the worldwide situation, the sales volume of PPIs has almost doubled in some European countries between 2000 and 2005.114,115
Despite large differences in mechanisms of action between the currently available drug subclasses of antacids, sucralfate, H2-receptor blockers, and PPIs (Table I), all these pharmaceuticals effectively suppress gastric acidity and therefore substantially increase intraluminal pH levels.116-118 Five days of PPI intake was shown to increase the gastric pH to an average pH of 5.0.119
TABLE I.
Drug action mechanisms of acid-suppression medication
| Active substance | Mechanism of gastric acid suppression |
|---|---|
| Antacids | Slight bases neutralizing gastric acid |
| Sucralfate | Aluminum compound acquiring a strong negative charge on aluminum release, binds to positive charges in its environment |
| H2-receptor blocker | Antagonist for the stimulating effect of histamine through its H2-receptor on the basolateral surface of parietal cells |
| PPI | Potent, irreversible blocker of the acid pump function (H+, K+, ATPase) on parietal cells |
Even though long-term use of this medication is generally accepted as safe for infants, adolescents, and adult patients, including pregnant women,120-123 it is important to note that antiulcer agents interfere with the protective function of gastric acidity against bacterial overgrowth, both in the stomach and the gut.124-126 Gastric pH increase has been discussed to be associated with pneumonia development in ventilated intensive care patients,127 as well as with an increased risk for community-acquired pneumonia.128 Persistent hypergastrinemia induced by long-term gastric acid suppression has been suggested as a risk factor for gastric carcinogenesis.129 In the early 1980s, the first correlation between allergic and dyspeptic disorders was reported, even though only the observed drug allergy was interpreted in association with the multipharmaceutical treatment, whereas the food-induced adverse reactions were attributed to genetic and alimentary factors.130 Researchers later reported high IgE levels and food-specific IgE in the gastrointestinal mucosa in patients with peptic ulcers.131-133 IgE against Helicobacter pylori and antacids has been reported in clinical practice, in which acid reduction combined with antibiotics is standard treatment.134-136 This might be due to the fact that aluminum compounds found in antacids are potent adjuvants.137,138 However, none of these studies addressed the question of whether interference with protein digestion by gastric acid suppression because of antiulcer drug intake could have an effect on food allergies.
INTERFERENCE WITH GASTRIC DIGESTIVE CAPACITY REPRESENTS A RISK FACTOR FOR FOOD-INDUCED ALLERGIC REACTIONS
When gastric digestion experiments with digestion-labile food allergens, such as fish, milk, or hazelnut, were performed with SGF pH at 5.0, these allergens remained stable, even for 2 hours.27,77-79 Titration experiments revealed that the enzymatic capacity of pepsin contained in SGF was completely eliminated when the pH was increased to 2.75 in the case of codfish and to 3.0 for milk proteins.77,78 Additionally, RAST inhibition experiments showed the IgE-binding capacity of fish allergens to be reduced up to 10,000-fold, accompanied by a loss of their histamine-releasing capacity after SGF digestion.78
These in vitro data point toward a strong effect of digestive proteolysis on IgE-binding capacity. In vivo studies in BALB/c mice demonstrated for the first time the association between antiulcer drug intake and food allergy induction. Concomitant administration of digestion-labile food allergens, such as caviar, hazelnut, or parvalbumin, and acid-suppression drugs, such as sucralfate, H2-receptor blockers, or PPIs, induced allergen-specific IgE antibodies and positive mucosal and skin reactivities.76,79 These mice also had a pronounced infiltration of eosinophils into the gastric mucosa.139
Interestingly, the effect of the gastric digestive system on acquiring tolerance toward orally administered dietary proteins was suggested based on murine experiments carried out 20 years ago.140 Encapsulation of dietary proteins, preventing degradation during gastrointestinal transit, has been used for oral allergy induction in murine models of food allergy141 and was reported to eliminate previously established oral tolerance.142 The correlation of food allergy induction with gastric acid suppression was found to be not dependent on age, with a major effect for aged individuals,143 as well as with a TH2-biasing potential in the off-spring induced by gastric acid neutralization during pregnancy.144
The effect of these murine findings was confirmed in a human cohort study of 152 gastroenterologic patients with dyspeptic disorders. After a 3-month course of medication with either H2-receptor blockers or PPIs, a boost or de novo IgE formation toward regular constituents of the daily diet was observed in 25% of the followed up patients. Sensitization in these patients could be confirmed by positive skin test results 5 months after discontinuation of antiacid treatment.77 In a group of patients who had hazelnut-specific IgE antibodies during the 3-month antiulcer medication therapy, hazelnut allergy could be diagnosed by means of positive double-blind, placebo-controlled, food challenges.79 Almost 12% of all patients had formed IgE antibodies toward those food allergens that would have been previously interpreted as nonsensitizing elicitors.77
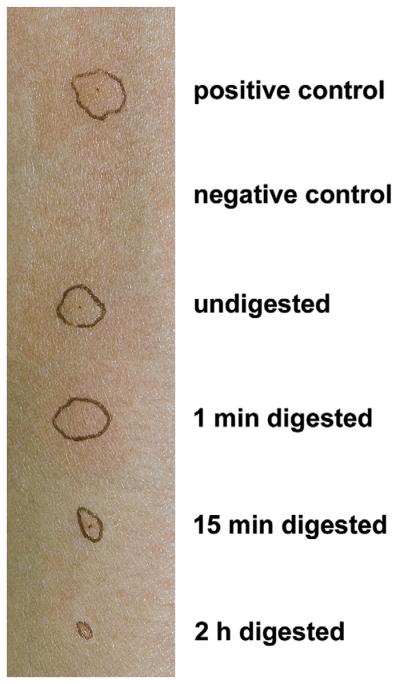
Clinically important, the interference with gastric digestion capacity might also influence allergic responses in already sensitized patients, in whom IgE is already bound to the effector cells of allergy. Decreased skin reactivity to melon extract after different time points of in vitro digestion in a patient with grass pollen and melon allergy showed that gastric digestion substantially decreased the allergenic capacity of these cross-reactive food proteins (Fig 2). Skin testing in patients with fish allergy with SGF-predigested or SGF-undigested codfish allergens showed a significant digestion time-dependent reduction of induced wheal reaction. Moreover, double-blind, placebo-controlled, food challenges in these patients with fish allergy resulted in a 10- to 30-fold higher tolerated allergen dose if the fish proteins were previously subjected to in vitro gastric digestion.27
FIG 2.
Reduced allergenicity of melon allergens after incubation with gastric enzymes. After subjection to SGF, melon allergens induce a smaller wheal reaction in a patient with melon allergy compared with the undigested extract. The alteration of skin reactivity was dependent on the incubation time with the proteolytic enzymes.
SAFETY ISSUES AND CLINICAL IMPLICATIONS FOR ALLERGIC AND NONALLERGIC CONSUMERS
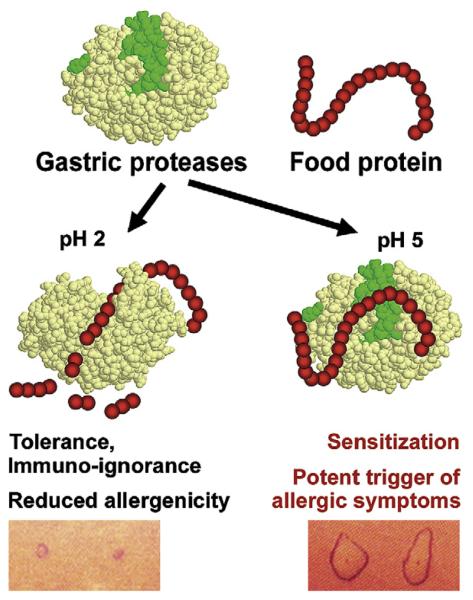
The data reviewed here suggest that the immunologic or clinical outcome after the consumption of a digestion-sensitive dietary protein depends to a certain degree on the gastric digestive capacity. If the food protein is exposed to gastric enzymes during transit, protein cleavage takes place, inducing either oral tolerance or immune ignorance toward the ingested food protein. However, if proteins persist during the gastric transit because of impaired digestion, such as during acid-suppression treatment, IgE-mediated food allergy can be induced. Gastric digestion might also influence the extent of reactivity in already sensitized patients. Physiologic gastric proteolysis substantially decreases the allergenic capacity of ingested food proteins, whereas severe allergic reactions at much lower amounts of ingested food proteins could occur if digestion is impaired (Fig 3).
FIG 3.
The gate-keeping function of the stomach in the sensitization and effector phase of food allergy. The fate of a dietary protein depends on the gastric digestive function mainly accomplished by the gastric protease pepsin. If the protein structure is destroyed because of proteolysis, oral tolerance or immune ignorance and a reduced allergenic potential might be the result. However, when gastric digestion is impaired (eg, under hypoacidic gastric conditions), digestion-labile proteins might trigger oral sensitization or severe allergic symptoms in a previously sensitized individual. The figure was created with the structural information provided from Research Collaboratory for Structural Bioinformatics Protein Data Bank accession numbers 3PSG and 5PEP.
The reviewed data indicate that the current concept of food allergen classification into class 1 (true food allergen) and class 2 (labile food proteins) is misleading and thus should be reconsidered. We suggest introducing the concept of allergen persistence in food allergen terminology. Serious implications for patients' and consumers' safety might be envisaged (Table II). It should be taken into consideration that currently applied safety tests for novel dietary compounds do not account for situations of impaired digestive capacity.145,146 Thus these protocols should be reconsidered to ensure consumers' safety and to prevent novel sensitizations. Additionally, antiulcer treatment might substantially alter the reactivity in patients with food allergy, such that previously diagnosed threshold levels and estimated no-observed-adverse-effect levels147 might not be valid. Strict food allergen labeling, independent of content level, might be the only legislative tool to ensure comprehensive patient safety.148 Most importantly, the interference of antiulcer treatment with the important gate-keeping function of the stomach should be recognized in daily clinical practice, and patients should be advised to limit medication intake to the prescription time period. Dietary recommendations (eg, light meals) during antiulcer therapies combined with repeated allergologic diagnosis of patients on long-term acid-suppression therapy could prevent novel sensitizations or food-induced adverse reactions in sensitized individuals.
TABLE II.
Key concepts and safety implications
| Regulatory authorities might consider a re-evaluation of currently applied safety tests for novel (eg, genetically modified) food compounds. |
| Patients should be advised that threshold levels might vary in situations of altered protein digestion. This problem also affects current efforts to define no-observed-adverse-effect levels for food allergens. |
| Dietary recommendations and repeated allergologic testing of long-term acid-suppressed patients could prevent unexpected allergic reactions on food ingestion. |
INFORMATION FOR CATEGORY 1 CME CREDIT.
Credit can now be obtained, free for a limited time, by reading the review articles in this issue. Please note the following instructions.
Method of Physician Participation in Learning Process: The core material for these activities can be read in this issue of the Journal or online at the JACI Web site: www.jacionline.org. The accompanying tests may only be submitted online at www.jacionline.org. Fax or other copies will not be accepted.
Date of Original Release: June 2008. Credit may be obtained for these courses until May 31, 2010.
Copyright Statement: Copyright © 2008-2010. All rights reserved.
Overall Purpose/Goal: To provide excellent reviews on key aspects of allergic disease to those who research, treat, or manage allergic disease.
Target Audience: Physicians and researchers within the field of allergic disease.
Accreditation/Provider Statements and Credit Designation: The American Academy of Allergy, Asthma & Immunology (AAAAI) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The AAAAI designates these educational activities for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
List of Design Committee Members: Authors: Eva Untersmayr, MD, and Erika Jensen-Jarolim, MD
Activity Objectives
To review the influence of gastric digestion on food protein allergenicity.
To discuss the implications of impaired gastric digestion on the development of food allergy.
Recognition of Commercial Support: This CME activity has not received external commercial support.
Disclosure of Significant Relationships with Relevant Commercial Companies/Organizations: Eva Untersmayr and Erika Jensen-Jarolim have no significant relationships to disclose.
What do we know?
The ability of a food protein to keep gastrointestinal digestion structurally intact increases its potential to be an allergen.
Classification of food proteins with digestion stability as a criterion does not provide sufficient information for safety aspects.
Impairment of gastrointestinal digestion (eg, with antiulcer medication) represents a risk factor for food allergy induction.
Impairment of digestion decreases the threshold dose of food allergens needed to elicit symptoms in sensitized individuals.
What is still unknown?
The exact site of food protein absorption and of food allergy induction in the gastrointestinal tract remains unknown.
Common characteristics of food allergens need to be defined.
The amount of undigested dietary proteins that has to persist gastrointestinal digestion to induce food allergy is unknown.
Acknowledgments
Supported by grant 11375 of the Austrian National bank “Jubiläumsfond” and by grants H220-B13 and SFB F1808-B13 of the Austrian National Science Foundation (FWF).
Abbreviations used
- AA
Amino acid
- PPI
Proton pump inhibitor
- SGF
Simulated gastric fluid
REFERENCES
- 1.Johansson SG, Hourihane JO, Bousquet J, Bruijnzeel-Koomen C, Dreborg S, Haahtela T, et al. A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy. 2001;56:813–24. doi: 10.1034/j.1398-9995.2001.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 2.Young E, Stoneham MD, Petruckevitch A, Barton J, Rona R. A population study of food intolerance. Lancet. 1994;343:1127–30. doi: 10.1016/s0140-6736(94)90234-8. [DOI] [PubMed] [Google Scholar]
- 3.Kanny G, Moneret-Vautrin DA, Flabbee J, Beaudouin E, Morisset M, Thevenin F. Population study of food allergy in France. J Allergy Clin Immunol. 2001;108:133–40. doi: 10.1067/mai.2001.116427. [DOI] [PubMed] [Google Scholar]
- 4.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114:159–65. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Sampson HA. Epidemiology of food allergy. Pediatr Allergy Immunol. 1996;7:42–50. doi: 10.1111/j.1399-3038.1996.tb00394.x. [DOI] [PubMed] [Google Scholar]
- 6.Grundy J, Matthews S, Bateman B, Dean T, Arshad SH. Rising prevalence of allergy to peanut in children: data from 2 sequential cohorts. J Allergy Clin Immunol. 2002;110:784–9. doi: 10.1067/mai.2002.128802. [DOI] [PubMed] [Google Scholar]
- 7.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of peanut and tree nut allergy in the United States determined by means of a random digit dial telephone survey: a 5-year follow-up study. J Allergy Clin Immunol. 2003;112:1203–7. doi: 10.1016/s0091-6749(03)02026-8. [DOI] [PubMed] [Google Scholar]
- 8.Sloane D, Sheffer A. Oral allergy syndrome. Allergy Asthma Proc. 2001;22:321–5. [PubMed] [Google Scholar]
- 9.Wang J, Sampson HA. Food anaphylaxis. Clin Exp Allergy. 2007;37:651–60. doi: 10.1111/j.1365-2222.2007.02682.x. [DOI] [PubMed] [Google Scholar]
- 10.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–20. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 11.Sicherer SH, Sampson HA. 9 Food allergy. J Allergy Clin Immunol. 2006;117(suppl):S470–5. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 12.Roberts G, Lack G. Food allergy and asthma—what is the link? Paediatr Respir Rev. 2003;4:205–12. doi: 10.1016/s1526-0542(03)00058-7. [DOI] [PubMed] [Google Scholar]
- 13.Sampson HA. Food anaphylaxis. Br Med Bull. 2000;56:925–35. doi: 10.1258/0007142001903607. [DOI] [PubMed] [Google Scholar]
- 14.Brown AF, McKinnon D, Chu K. Emergency department anaphylaxis: a review of 142 patients in a single year. J Allergy Clin Immunol. 2001;108:861–6. doi: 10.1067/mai.2001.119028. [DOI] [PubMed] [Google Scholar]
- 15.Smit DV, Cameron PA, Rainer TH. Anaphylaxis presentations to an emergency department in Hong Kong: incidence and predictors of biphasic reactions. J Emerg Med. 2005;28:381–8. doi: 10.1016/j.jemermed.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 16.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73(suppl):1131S–41S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 17.Brandtzaeg P. Mechanisms of gastrointestinal reactions to food. Environ Toxicol Pharmacol. 1997;4:9–24. doi: 10.1016/s1382-6689(97)10036-9. [DOI] [PubMed] [Google Scholar]
- 18.Brandtzaeg P. History of oral tolerance and mucosal immunity. Ann N Y Acad Sci. 1996;778:1–27. doi: 10.1111/j.1749-6632.1996.tb21110.x. [DOI] [PubMed] [Google Scholar]
- 19.Brandtzaeg PE. Current understanding of gastrointestinal immunoregulation and its relation to food allergy. Ann N Y Acad Sci. 2002;964:13–45. doi: 10.1111/j.1749-6632.2002.tb04131.x. [DOI] [PubMed] [Google Scholar]
- 20.Faria AM, Weiner HL. Oral tolerance and TGF-beta-producing cells. Inflamm Allergy Drug Targets. 2006;5:179–90. doi: 10.2174/187152806778256034. [DOI] [PubMed] [Google Scholar]
- 21.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumberg RS, Lencer WI, Zhu X, Kim HS, Claypool S, Balk SP, et al. Antigen presentation by intestinal epithelial cells. Immunol Lett. 1999;69:7–11. doi: 10.1016/s0165-2478(99)00093-0. [DOI] [PubMed] [Google Scholar]
- 23.Bland PW. Gut epithelium: food processor for the mucosal immune system? Gut. 1998;42:455–6. doi: 10.1136/gut.42.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brunner M, Walzer M. Absorption of undigested proteins in human beings: the absorption of unaltered fish protein in adults. Arch Intern Med. 1928;42:173–9. [Google Scholar]
- 25.Brunner M, Walzer M. The absorption of undigested proteins in human beings: the absorption of unaltered fish protein in adults. Arch Intern Med. 1928;42:173–9. [Google Scholar]
- 26.Dirks CG, Pedersen MH, Platzer MH, Bindslev-Jensen C, Skov PS, Poulsen LK. Does absorption across the buccal mucosa explain early onset of food-induced allergic systemic reactions? J Allergy Clin Immunol. 2005;115:1321–3. doi: 10.1016/j.jaci.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 27.Untersmayr E, Vestergaard H, Malling HJ, Jensen LB, Platzer MH, Boltz-Nitulescu G, et al. Incomplete digestion of codfish represents a risk factor for anaphylaxis in patients with allergy. J Allergy Clin Immunol. 2007;119:711–7. doi: 10.1016/j.jaci.2006.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura T, Higaki K. Gastrointestinal transit and drug absorption. Biol Pharm Bull. 2002;25:149–64. doi: 10.1248/bpb.25.149. [DOI] [PubMed] [Google Scholar]
- 29.Paganelli R, Levinsky RJ. Solid phase radioimmunoassay for detection of circulating food protein antigens in human serum. J Immunol Methods. 1980;37:333–41. doi: 10.1016/0022-1759(80)90319-1. [DOI] [PubMed] [Google Scholar]
- 30.Husby S, Jensenius JC, Svehag SE. Passage of undegraded dietary antigen into the blood of healthy adults. Quantification, estimation of size distribution, and relation of uptake to levels of specific antibodies. Scand J Immunol. 1985;22:83–92. doi: 10.1111/j.1365-3083.1985.tb01862.x. [DOI] [PubMed] [Google Scholar]
- 31.Husby S, Foged N, Host A, Svehag SE. Passage of dietary antigens into the blood of children with coeliac disease. Quantification and size distribution of absorbed antigens. Gut. 1987;28:1062–72. doi: 10.1136/gut.28.9.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer L. Mucosal immunity. Pediatrics. 2003;111:1595–600. [PubMed] [Google Scholar]
- 33.Schubert ML. Gastric secretion. Curr Opin Gastroenterol. 2007;23:595–601. doi: 10.1097/MOG.0b013e3282f03462. [DOI] [PubMed] [Google Scholar]
- 34.Etherington DJ, Taylor WH. The pepsins from human gastric mucosal extracts. Biochem J. 1970;118:587–94. doi: 10.1042/bj1180587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang J, Wong RN. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987;33:53–63. doi: 10.1002/jcb.240330106. [DOI] [PubMed] [Google Scholar]
- 36.Richter C, Tanaka T, Yada RY. Mechanism of activation of the gastric aspartic proteinases: pepsinogen, progastricsin and prochymosin. Biochem J. 1998;335(suppl):481–90. doi: 10.1042/bj3350481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan AR, James MN. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 1998;7:815–36. doi: 10.1002/pro.5560070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McPhie P. A spectrophotometric investigation of the pepsinogen-pepsin conversion. J Biol Chem. 1972;247:4277–81. [PubMed] [Google Scholar]
- 39.al-Janabi J, Hartsuck JA, Tang J. Kinetics and mechanism of pepsinogen activation. J Biol Chem. 1972;247:4628–32. [PubMed] [Google Scholar]
- 40.Samloff IM. Peptic ulcer: the many proteinases of aggression. Gastroenterology. 1989;96:586–95. doi: 10.1016/s0016-5085(89)80054-x. [DOI] [PubMed] [Google Scholar]
- 41.Oka T, Morihara K. Specificity of pepsin: Size and property of the active site. FEBS Lett. 1970;10:222–4. doi: 10.1016/0014-5793(70)80633-0. [DOI] [PubMed] [Google Scholar]
- 42.Trout GE, Fruton JS. The side-chain specificity of pepsin. Biochemistry. 1969;8:4183–90. doi: 10.1021/bi00838a041. [DOI] [PubMed] [Google Scholar]
- 43.Erickson RH, Kim YS. Digestion and absorption of dietary protein. Annu Rev Med. 1990;41:133–9. doi: 10.1146/annurev.me.41.020190.001025. [DOI] [PubMed] [Google Scholar]
- 44.Gray GM, Cooper HL. Protein digestion and absorption. Gastroenterology. 1971;61:535–44. [PubMed] [Google Scholar]
- 45.Sleisenger MH, Kim YS. Protein digestion and absorption. N Engl J Med. 1979;300:659–63. doi: 10.1056/NEJM197903223001207. [DOI] [PubMed] [Google Scholar]
- 46.York IA, Goldberg AL, Mo XY, Rock KL. Proteolysis and class I major histocompatibility complex antigen presentation. Immunol Rev. 1999;172:49–66. doi: 10.1111/j.1600-065x.1999.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 47.Laver WG, Air GM, Webster RG, Smith-Gill SJ. Epitopes on protein antigens: misconceptions and realities. Cell. 1990;61:553–6. doi: 10.1016/0092-8674(90)90464-p. [DOI] [PubMed] [Google Scholar]
- 48.Aalberse RC. Structural biology of allergens. J Allergy Clin Immunol. 2000;106:228–38. doi: 10.1067/mai.2000.108434. [DOI] [PubMed] [Google Scholar]
- 49.Untersmayr E, Szalai K, Riemer AB, Hemmer W, Swoboda I, Hantusch B, et al. Mimotopes identify conformational epitopes on parvalbumin, the major fish allergen. Mol Immunol. 2006;43:1454–61. doi: 10.1016/j.molimm.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 50.Ganglberger E, Grunberger K, Sponer B, Radauer C, Breiteneder H, Boltz-Nitulescu G, et al. Allergen mimotopes for 3-dimensional epitope search and induction of antibodies inhibiting human IgE. FASEB J. 2000;14:2177–84. doi: 10.1096/fj.99-1000com. [DOI] [PubMed] [Google Scholar]
- 51.Hantusch B, Krieger S, Untersmayr E, Scholl I, Knittelfelder R, Flicker S, et al. Mapping of conformational IgE epitopes on Phl p 5a by using mimotopes from a phage display library. J Allergy Clin Immunol. 2004;114:1294–300. doi: 10.1016/j.jaci.2004.06.048. [DOI] [PubMed] [Google Scholar]
- 52.Szalai K, Fuhrmann J, Pavkov T, Scheidl M, Wallmann J, Bramswig KH, et al. Mimotopes identify conformational B-cell epitopes on the two major house dust mite allergens Der p 1 and Der p 2. Mol Immunol. 2008;45:1308–17. doi: 10.1016/j.molimm.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Jarvinen KM, Beyer K, Vila L, Chatchatee P, Busse PJ, Sampson HA. B-cell epitopes as a screening instrument for persistent cow's milk allergy. J Allergy Clin Immunol. 2002;110:293–7. doi: 10.1067/mai.2002.126080. [DOI] [PubMed] [Google Scholar]
- 54.Schöll I, Kalkura N, Shedziankova Y, Bergmann A, Verdino P, Knittelfelder R, et al. Dimerization of the major birch pollen allergen Bet v 1 is important for its in vivo IgE-cross-linking potential in mice. J Immunol. 2005;175:6645–50. doi: 10.4049/jimmunol.175.10.6645. [DOI] [PubMed] [Google Scholar]
- 55.Lehrer SB, Ayuso R, Reese G. Current understanding of food allergens. Ann N Y Acad Sci. 2002;964:69–85. doi: 10.1111/j.1749-6632.2002.tb04133.x. [DOI] [PubMed] [Google Scholar]
- 56.Bannon GA. What makes a food protein an allergen? Curr Allergy Asthma Rep. 2004;4:43–6. doi: 10.1007/s11882-004-0042-0. [DOI] [PubMed] [Google Scholar]
- 57.Maier I, Okun VM, Pittner F, Lindner W. Changes in peptic digestibility of bovine beta-lactoglobulin as a result of food processing studied by capillary electrophoresis and immunochemical methods. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;841:160–7. doi: 10.1016/j.jchromb.2006.06.040. [DOI] [PubMed] [Google Scholar]
- 58.Sen M, Kopper R, Pons L, Abraham EC, Burks AW, Bannon GA. Protein structure plays a critical role in peanut allergen stability and may determine immunodominant IgE-binding epitopes. J Immunol. 2002;169:882–7. doi: 10.4049/jimmunol.169.2.882. [DOI] [PubMed] [Google Scholar]
- 59.Aalberse RC. Food allergens. Environ Toxicol Pharm. 1997;4:55–60. doi: 10.1016/s1382-6689(97)10042-4. [DOI] [PubMed] [Google Scholar]
- 60.Jensen-Jarolim E, Wiedermann U, Ganglberger E, Zurcher A, Stadler BM, Boltz-Nitulescu G, et al. Allergen mimotopes in food enhance type I allergic reactions in mice. FASEB J. 1999;13:1586–92. doi: 10.1096/fasebj.13.12.1586. [DOI] [PubMed] [Google Scholar]
- 61.Vieths S, Scheurer S, Ballmer-Weber B. Current understanding of cross-reactivity of food allergens and pollen. Ann N Y Acad Sci. 2002;964:47–68. doi: 10.1111/j.1749-6632.2002.tb04132.x. [DOI] [PubMed] [Google Scholar]
- 62.Astwood JD, Leach JN, Fuchs RL. Stability of food allergens to digestion in vitro. Nat Biotechnol. 1996;14:1269–73. doi: 10.1038/nbt1096-1269. [DOI] [PubMed] [Google Scholar]
- 63.Hefle SL, Nordlee JA, Taylor SL. Allergenic foods. Crit Rev Food Sci Nutr. 1996;36(suppl):S69–89. doi: 10.1080/10408399609527760. [DOI] [PubMed] [Google Scholar]
- 64.Taylor SL, Hefle SL. Will genetically modified foods be allergenic? J Allergy Clin Immunol. 2001;107:765–71. doi: 10.1067/mai.2001.114241. [DOI] [PubMed] [Google Scholar]
- 65.Nordlee JA, Taylor SL, Townsend JA, Thomas LA, Bush RK. Identification of a Brazil-nut allergen in transgenic soybeans. N Engl J Med. 1996;334:688–92. doi: 10.1056/NEJM199603143341103. [DOI] [PubMed] [Google Scholar]
- 66.Evaluation of genetically modified foods Available at: www.who.int/foodsafety/publications/biotech/en/ec_jan2001.pdf. Accessed February 10, 2008.
- 67.Food safety: regulating plant agricultural biotechnology in the United States Available at: http://usinfo.state.gov/products/pubs/archive/biotech. Accessed February 10, 2008.
- 68.Eiwegger T, Rigby N, Mondoulet L, Bernard H, Krauth MT, Boehm A, et al. Gastro-duodenal digestion products of the major peanut allergen Ara h 1 retain an allergenic potential. Clin Exp Allergy. 2006;36:1281–8. doi: 10.1111/j.1365-2222.2006.02565.x. [DOI] [PubMed] [Google Scholar]
- 69.Burnett GR, Wickham M, Fillery-Travis A, Robertson JA, Belton PS, Gilbert SM, et al. Interaction between protein allergens and model gastric emulsions. Biochem Soc Trans. 2002;30:916–8. doi: 10.1042/bst0300916. [DOI] [PubMed] [Google Scholar]
- 70.Moreno FJ, Mackie AR, Mills EN. Phospholipid interactions protect the milk allergen alpha-lactalbumin from proteolysis during in vitro digestion. J Agric Food Chem. 2005;53:9810–6. doi: 10.1021/jf0515227. [DOI] [PubMed] [Google Scholar]
- 71.Yagami T, Haishima Y, Nakamura A, Osuna H, Ikezawa Z. Digestibility of allergens extracted from natural rubber latex and vegetable foods. J Allergy Clin Immunol. 2000;106:752–62. doi: 10.1067/mai.2000.109171. [DOI] [PubMed] [Google Scholar]
- 72.Fu TJ, Abbott UR, Hatzos C. Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid—a comparative study. J Agric Food Chem. 2002;50:7154–60. doi: 10.1021/jf020599h. [DOI] [PubMed] [Google Scholar]
- 73.Fu TJ. Digestion stability as a criterion for protein allergenicity assessment. Ann N Y Acad Sci. 2002;964:99–110. doi: 10.1111/j.1749-6632.2002.tb04135.x. [DOI] [PubMed] [Google Scholar]
- 74.Taylor SL. Comment on digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid—a comparative study. J Agric Food Chem. 2003;51:5183–7. doi: 10.1021/jf030375e. [DOI] [PubMed] [Google Scholar]
- 75.Vieths S, Reindl J, Müller U, Hoffmann A, Haustein D. Digestibility of peanut and hazelnut allergens investigated by a simple in vitro procedure. Eur Food Res Technol. 1999;209:379–88. [Google Scholar]
- 76.Untersmayr E, Schöll I, Swoboda I, Beil WJ, Förster-Waldl E, Walter F, et al. Antacid medication inhibits digestion of dietary proteins and causes food allergy: a fish allergy model in BALB/c mice. J Allergy Clin Immunol. 2003;112:616–23. doi: 10.1016/s0091-6749(03)01719-6. [DOI] [PubMed] [Google Scholar]
- 77.Untersmayr E, Bakos N, Schöll I, Kundi M, Roth-Walter F, Szalai K, et al. Anti-ulcer drugs promote IgE formation toward dietary antigens in adult patients. FASEB J. 2005;19:656–8. doi: 10.1096/fj.04-3170fje. [DOI] [PubMed] [Google Scholar]
- 78.Untersmayr E, Poulsen LK, Platzer MH, Pedersen MH, Boltz-Nitulescu G, Skov PS, et al. The effects of gastric digestion on codfish allergenicity. J Allergy Clin Immunol. 2005;115:377–82. doi: 10.1016/j.jaci.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 79.Schöll I, Untersmayr E, Bakos N, Roth-Walter F, Gleiss A, Boltz-Nitulescu G, et al. Antiulcer drugs promote oral sensitization and hypersensitivity to hazelnut allergens in BALB/c mice and humans. Am J Clin Nutr. 2005;81:154–60. doi: 10.1093/ajcn/81.1.154. [DOI] [PubMed] [Google Scholar]
- 80.Schimek EM, Zwolfer B, Briza P, Jahn-Schmid B, Vogel L, Vieths S, et al. Gastrointestinal digestion of Bet v 1-homologous food allergens destroys their mediator-releasing, but not T cell-activating, capacity. J Allergy Clin Immunol. 2005;116:1327–33. doi: 10.1016/j.jaci.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 81.Ebers DW, Gibbs GE, Smith DI. Gastric acidity on the first day of life. Pediatrics. 1956;18:800–2. [PubMed] [Google Scholar]
- 82.Avery GB, Randolph JG, Weaver T. Gastric acidity in the first day of life. Pediatrics. 1966;37:1005–7. [PubMed] [Google Scholar]
- 83.Euler AR, Byrne WJ, Cousins LM, Ament ME, Walsh JH. Increased serum gastrin concentrations and gastric acid hyposecretion in the immediate newborn period. Gastroenterology. 1977;72:1271–3. [PubMed] [Google Scholar]
- 84.Agunod M, Yamaguchi N, Lopez R, Luhby AL, Glass GB. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. Am J Dig Dis. 1969;14:400–14. doi: 10.1007/BF02239360. [DOI] [PubMed] [Google Scholar]
- 85.Miller RA. Observations on the gastric acidity during the first month of life. Arch Dis Child. 1941;16:22–30. doi: 10.1136/adc.16.85.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deren JS. Development of structure and function in the fetal and newborn stomach. Am J Clin Nutr. 1971;24:144–59. doi: 10.1093/ajcn/24.1.144. [DOI] [PubMed] [Google Scholar]
- 87.Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55:667–86. doi: 10.1016/s0169-409x(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 88.Davies D, James TG. An investigation into the gastric secretion of a hundred normal persons over the age of sixty. Brit J Med. 1930;1:1–14. [Google Scholar]
- 89.de Witte TJ, Geerdink PJ, Lamers CB, Boerbooms AM, van der Korst JK. Hypochlorhydria and hypergastrinaemia in rheumatoid arthritis. Ann Rheum Dis. 1979;38:14–7. doi: 10.1136/ard.38.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hosking DJ, Moody F, Stewart IM, Atkinson M. Vagal impairment of gastric secretion in diabetic autonomic neuropathy. BMJ. 1975;2:588–90. doi: 10.1136/bmj.2.5971.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Howitz J, Schwartz M. Vitiligo, achlorhydria, and pernicious anaemia. Lancet. 1971;1:1331–4. doi: 10.1016/s0140-6736(71)91889-7. [DOI] [PubMed] [Google Scholar]
- 92.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., 3rd Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–68. [PubMed] [Google Scholar]
- 93.Kay L, Jorgensen T. Epidemiology of upper dyspepsia in a random population. Prevalence, incidence, natural history, and risk factors. Scand J Gastroenterol. 1994;29:2–6. [PubMed] [Google Scholar]
- 94.Talley NJ, Silverstein MD, Agreus L, Nyren O, Sonnenberg A, Holtmann G. AGA technical review: evaluation of dyspepsia. American Gastroenterological Association. Gastroenterology. 1998;114:582–95. doi: 10.1016/s0016-5085(98)70542-6. [DOI] [PubMed] [Google Scholar]
- 95.Westbrook JI, McIntosh JH, Talley NJ. The impact of dyspepsia definition on prevalence estimates: considerations for future researchers. Scand J Gastroenterol. 2000;35:227–33. doi: 10.1080/003655200750024065. [DOI] [PubMed] [Google Scholar]
- 96.Lydeard S, Jones R. Factors affecting the decision to consult with dyspepsia: comparison of consulters and non-consulters. J R Coll Gen Pract. 1989;39:495–8. [PMC free article] [PubMed] [Google Scholar]
- 97.Warndorff DK, Knottnerus JA, Huijnen LG, Starmans R. How well do general practitioners manage dyspepsia? J R Coll Gen Pract. 1989;39:499–502. [PMC free article] [PubMed] [Google Scholar]
- 98.Heikkinen M, Pikkarainen P, Takala J, Julkunen R. General practitioners' approach to dyspepsia. Survey of consultation frequencies, treatment, and investigations. Scand J Gastroenterol. 1996;31:648–53. doi: 10.3109/00365529609009144. [DOI] [PubMed] [Google Scholar]
- 99.Sunku B, Marino RV, Sockolow R. A primary care approach to pediatric gastroesophageal reflux. J Am Osteopath Assoc. 2000;100(suppl):S11–5. [PubMed] [Google Scholar]
- 100.Tolia V, Wuerth A, Thomas R. Gastroesophageal reflux disease: review of presenting symptoms, evaluation, management, and outcome in infants. Dig Dis Sci. 2003;48:1723–9. doi: 10.1023/a:1025486710231. [DOI] [PubMed] [Google Scholar]
- 101.Gold BD. Review article: epidemiology and management of gastro-oesophageal reflux in children. Aliment Pharmacol Ther. 2004;19(suppl 1):22–7. doi: 10.1111/j.0953-0673.2004.01832.x. [DOI] [PubMed] [Google Scholar]
- 102.Gold BD, Scheiman JM, Sabesin SM, Vitat P. Updates on the management of upper gastrointestinal disorders in the primary care setting: NSAID-related gastropathies and pediatric reflux diseases. J Fam Pract. 2007;56(suppl):S1–11. [PubMed] [Google Scholar]
- 103.Bashford JN, Norwood J, Chapman SR. Why are patients prescribed proton pump inhibitors? Retrospective analysis of link between morbidity and prescribing in the General Practice Research Database. BMJ. 1998;317:452–6. doi: 10.1136/bmj.317.7156.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Westbrook JI, Duggan AE, McIntosh JH. Prescriptions for antiulcer drugs in Australia: volume, trends, and costs. BMJ. 2001;323:1338–9. doi: 10.1136/bmj.323.7325.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Marshall JK, Grootendorst PV, O'Brien BJ, Dolovich LR, Holbrook AM, Levy AR. Impact of reference-based pricing for histamine-2 receptor antagonists and restricted access for proton pump inhibitors in British Columbia. CMAJ. 2002;166:1655–62. [PMC free article] [PubMed] [Google Scholar]
- 106.Lassen A, Hallas J, Schaffalitzky De Muckadell OB. Use of anti-secretory medication: a population-based cohort study. Aliment Pharmacol Ther. 2004;20:577–83. doi: 10.1111/j.1365-2036.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- 107.Ryder SD, O'Reilly S, Miller RJ, Ross J, Jacyna MR, Levi AJ. Long term acid suppressing treatment in general practice. BMJ. 1994;308:827–30. doi: 10.1136/bmj.308.6932.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McCullagh M, Brown C, Bell D, Powell K. Long term acid suppressing treatment. Survey shows variation in practice. BMJ. 1994;308:1238. doi: 10.1136/bmj.308.6938.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Boutet R, Wilcock M, MacKenzie I. Survey on repeat prescribing for acid suppression drugs in primary care in Cornwall and the Isles of Scilly. Aliment Pharmacol Ther. 1999;13:813–7. doi: 10.1046/j.1365-2036.1999.00524.x. [DOI] [PubMed] [Google Scholar]
- 110.Gullotta R, Ferraris L, Cortelezzi C, Minoli G, Prada A, Comin U, et al. Are we correctly using the inhibitors of gastric acid secretion and cytoprotective drugs? Results of a multicentre study. Ital J Gastroenterol Hepatol. 1997;29:325–9. [PubMed] [Google Scholar]
- 111.Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95:3118–22. doi: 10.1111/j.1572-0241.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- 112.Zink DA, Pohlman M, Barnes M, Cannon ME. Long-term use of acid suppression started inappropriately during hospitalization. Aliment Pharmacol Ther. 2005;21:1203–9. doi: 10.1111/j.1365-2036.2005.02454.x. [DOI] [PubMed] [Google Scholar]
- 113.Chen TJ, Chou LF, Hwang SJ. Prevalence of anti-ulcer drug use in a Chinese cohort. World J Gastroenterol. 2003;9:1365–9. doi: 10.3748/wjg.v9.i6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Statistical registry of pharmaceuticals, Danish Medicines Agency Available at: http://www.laegemiddelstyrelsen.dk/1024/visLSArtikel.asp?artikelID=8479. Accessed January 28, 2008.
- 115.Untersmayr E, Diesner SC, Jensen-Jarolim E. Acid-suppression: a risk to develop food allergy. In: Koletzko S, editor. Food allergy in childhood. Causes and consequences. SPS Publications; Heilbronn: 2007. pp. 52–65. [Google Scholar]
- 116.McCarthy DM. Sucralfate. N Engl J Med. 1991;325:1017–25. doi: 10.1056/NEJM199110033251407. [DOI] [PubMed] [Google Scholar]
- 117.Richardson P, Hawkey CJ, Stack WA. Proton pump inhibitors. Pharmacology and rationale for use in gastrointestinal disorders. Drugs. 1998;56:307–35. doi: 10.2165/00003495-199856030-00002. [DOI] [PubMed] [Google Scholar]
- 118.Aihara T, Nakamura E, Amagase K, Tomita K, Fujishita T, Furutani K, et al. Pharmacological control of gastric acid secretion for the treatment of acid-related peptic disease: past, present, and future. Pharmacol Ther. 2003;98:109–27. doi: 10.1016/s0163-7258(03)00015-9. [DOI] [PubMed] [Google Scholar]
- 119.Prichard PJ, Yeomans ND, Mihaly GW, Jones DB, Buckle PJ, Smallwood RA, et al. Omeprazole: a study of its inhibition of gastric pH and oral pharmacokinetics after morning or evening dosage. Gastroenterology. 1985;88:64–9. doi: 10.1016/s0016-5085(85)80133-5. [DOI] [PubMed] [Google Scholar]
- 120.Tolia V, Fitzgerald J, Hassall E, Huang B, Pilmer B, Kane R., 3rd Safety of lansoprazole in the treatment of gastroesophageal reflux disease in children. J Pediatr Gastroenterol Nutr. 2002;35(suppl 4):S300–7. doi: 10.1097/00005176-200211004-00002. [DOI] [PubMed] [Google Scholar]
- 121.Gold BD, Gunasekaran T, Tolia V, Wetzler G, Conter H, Traxler B, et al. Safety and symptom improvement with esomeprazole in adolescents with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2007;45:520–9. doi: 10.1097/MPG.0b013e318148c17c. [DOI] [PubMed] [Google Scholar]
- 122.van Zyl J, van Rensburg C, Vieweg W, Fischer R. Efficacy and safety of pantoprazole versus ranitidine in the treatment of patients with symptomatic gastroesophageal reflux disease. Digestion. 2004;70:61–9. doi: 10.1159/000080130. [DOI] [PubMed] [Google Scholar]
- 123.Richter JE. Review article: the management of heartburn in pregnancy. Aliment Pharmacol Ther. 2005;22:749–57. doi: 10.1111/j.1365-2036.2005.02654.x. [DOI] [PubMed] [Google Scholar]
- 124.Williams C. Occurrence and significance of gastric colonization during acid-inhibitory therapy. Best Pract Res Clin Gastroenterol. 2001;15:511–21. doi: 10.1053/bega.2001.0191. [DOI] [PubMed] [Google Scholar]
- 125.Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol. 2005;96:94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x. [DOI] [PubMed] [Google Scholar]
- 126.Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102:2047–57. doi: 10.1111/j.1572-0241.2007.01275.x. [DOI] [PubMed] [Google Scholar]
- 127.Inglis TJ, Sherratt MJ, Sproat LJ, Gibson JS, Hawkey PM. Gastroduodenal dys-function and bacterial colonisation of the ventilated lung. Lancet. 1993;341:911–3. doi: 10.1016/0140-6736(93)91208-4. [DOI] [PubMed] [Google Scholar]
- 128.Laheij RJ, Sturkenboom MC, Hassing RJ, Dieleman J, Stricker BH, Jansen JB. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–60. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 129.Waldum HL, Brenna E, Sandvik AK. Long-term safety of proton pump inhibitors: risks of gastric neoplasia and infections. Expert Opin Drug Saf. 2002;1:29–38. doi: 10.1517/14740338.1.1.29. [DOI] [PubMed] [Google Scholar]
- 130.Budagovskaia VN, Voitko NE. Allergic reactions in patients with peptic ulcer; incidence of food and drug allergy. Vopr Pitan. 1984;3:30–3. [PubMed] [Google Scholar]
- 131.De Lazzari F, Mancin O, Plebani M, Venturi C, Battaglia G, Vianello F, et al. High IgE serum levels and “peptic” ulcers: clinical and functional approach. Ital J Gastroenterol. 1994;26:7–11. [PubMed] [Google Scholar]
- 132.De Lazzari F, Venturi C, Fregona I, Galliani EA, Bortolami M, Violato D, et al. Specific IgE in the gastric and duodenal mucosa. An epiphenomenon or pathogenetic mechanism of some forms of “peptic” ulcer? Minerva Gastroenterol Dietol. 1994;40:1–9. [PubMed] [Google Scholar]
- 133.Mancin O, De Lazzari F, Pasqualetti P, Venturi C, Faggian D, Plebani M, et al. Raised levels of IgE and allergic diseases in patients subjected to esophagogastroduodenoscopy. Minerva Dietol Gastroenterol. 1988;34:171–5. [PubMed] [Google Scholar]
- 134.Permin H, Norgaard A, Norn S, Andersen LP, Nielsen H. IgE-mediated immune response to Helicobacter pylori examined by basophil histamine release in patients with dyspepsia. Inflamm Res. 2000;49(suppl 1):S29–30. doi: 10.1007/PL00000168. [DOI] [PubMed] [Google Scholar]
- 135.Liutu M, Kalimo K, Uksila J, Savolainen J. Extraction of IgE-binding components of Helicobacter pylori by immunoblotting analysis in chronic urticaria patients. Int Arch Allergy Immunol. 2001;126:213–7. doi: 10.1159/000049516. [DOI] [PubMed] [Google Scholar]
- 136.Lauerma AI, Petman L, Makinen-Kiljunen S. IgE-mediated anaphylaxis to antacid. Allergy. 2001;56:580. doi: 10.1034/j.1398-9995.2001.056006580.x. [DOI] [PubMed] [Google Scholar]
- 137.Brewer JM, Conacher M, Hunter CA, Mohrs M, Brombacher F, Alexander J. Aluminium hydroxide adjuvant initiates strong antigen-specific Th2 responses in the absence of IL-4- or IL-13-mediated signaling. J Immunol. 1999;163:6448–54. [PubMed] [Google Scholar]
- 138.Brunner R, Wallmann J, Szalai K, Karagiannis P, Kopp T, Scheiner O, et al. The impact of aluminium in acid-suppressing drugs on the immune response of BALB/c mice. Clin Exp Allergy. 2007;37:1566–73. doi: 10.1111/j.1365-2222.2007.02813.x. [DOI] [PubMed] [Google Scholar]
- 139.Untersmayr E, Ellinger A, Beil WJ, Jensen-Jarolim E. Eosinophils accumulate in the gastric mucosa of food-allergic mice. Int Arch Allergy Immunol. 2004;135:1–2. doi: 10.1159/000080035. [DOI] [PubMed] [Google Scholar]
- 140.Michael JG. The role of digestive enzymes in orally induced immune tolerance. Immunol Invest. 1989;18:1049–54. doi: 10.3109/08820138909030606. [DOI] [PubMed] [Google Scholar]
- 141.Hogan SP, Mishra A, Brandt EB, Royalty MP, Pope SM, Zimmermann N, et al. A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2001;2:353–60. doi: 10.1038/86365. [DOI] [PubMed] [Google Scholar]
- 142.Barone KS, Reilly MR, Flanagan MP, Michael JG. Abrogation of oral tolerance by feeding encapsulated antigen. Cell Immunol. 2000;199:65–72. doi: 10.1006/cimm.1999.1603. [DOI] [PubMed] [Google Scholar]
- 143.Untersmayr E, Diesner SC, Bramswig KH, Knittelfelder R, Bakos N, Gundacker C, et al. Characterization of intrinsic and extrinsic risk factors for celery allergy in immunosenescence. Mech Ageing Dev. 2008;129:120–8. doi: 10.1016/j.mad.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 144.Schöll I, Ackermann U, Ozdemir C, Blumer N, Dicke T, Sel S, et al. Anti-ulcer treatment during pregnancy induces food allergy in mouse mothers and a Th2-bias in their offspring. FASEB J. 2007;21:1264–70. doi: 10.1096/fj.06-7223com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jensen-Jarolim E, Untersmayr E. Food safety: in vitro digestion tests are non-predictive for allergenic potential of food in stomach insufficiency. Immunol Lett. 2006;102:118–9. doi: 10.1016/j.imlet.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 146.Jensen-Jarolim E, Untersmayr E. In vitro tests for the determination of allergenic potency of “novel foods” and genetically modified organisms: relevance in vivo? Wien Klin Wochenschr. 2005;117:437–9. doi: 10.1007/s00508-005-0397-9. [DOI] [PubMed] [Google Scholar]
- 147.Taylor SL, Hefle SL, Bindslev-Jensen C, Atkins FM, Andre C, Bruijnzeel-Koomen C, et al. A consensus protocol for the determination of the threshold doses for allergenic foods: how much is too much? Clin Exp Allergy. 2004;34:689–95. doi: 10.1111/j.1365-2222.2004.1886.x. [DOI] [PubMed] [Google Scholar]
- 148.Carreno I. The new European Community rules on the labeling of allergen ingredients in foodstuffs. Food Drug Law J. 2005;60:375–91. [PubMed] [Google Scholar]