Abstract
A neuropeptide was isolated from a frog brain extract by HPLC purification and characterized by mass spectrometry. This 26-aa neuropeptide, which belongs to the RFamide peptide family, was designated 26RFa, and its primary structure was established as VGTALGSLAEELNGYNRKKGGFSFRF-NH2. Research in databases revealed the presence of sequences homologous to frog 26RFa in the human genome and in rat ESTs. On the basis of this sequence information, the cDNAs encoding the human and rat 26RFa precursors were cloned. The two preproteins show a similar organization, with the 26RFa sequence located in the C-terminal region of the precursor. Human preprotein (prepro)-26RFa encodes an additional putative RFamide peptide that is not found in the rat precursor. The primary structures of human, rat, and frog 26RFa exhibit ≈80% identity, and the C-terminal octapeptide has been fully conserved from amphibians to mammals. In situ hybridization histochemistry revealed that, in the rat brain, the 26RFa gene is exclusively expressed in the ventromedial hypothalamic nucleus and in the lateral hypothalamic area. 26RFa induced a dose-dependent stimulation in cAMP production by rat pituitary cells in vitro and markedly increased food intake in mice. The conservation of the primary structure of 26RFa during vertebrate evolution, the discrete localization of the mRNA encoding its precursor in hypothalamic nuclei involved in the control of feeding behavior, and the observation that 26RFa possesses orexigenic properties indicate that this neuropeptide may play important biological functions.
The tetrapeptide Phe-Met-Arg-Phe-NH2 (FMRFamide) was originally isolated from the ganglia of the venus clam (1). Since then, several FMRFamide-related peptides (RFRPs), which all possess the Arg-Phe-NH2 motif at their C terminus, have been isolated in various groups of invertebrates (see ref. 2 for review), and it has been found that a single species may express multiple members of RFRPs. In particular, in Caenorhabditis elegans, 22 genes encoding 59 distinct RFRPs have been identified (3). Although genomic data predict that the human genome encodes a number of proteins encompassing RFamide-related sequences, only a few RFRPs have been isolated so far in vertebrates. Notably, in human, only three genes encoding RFRPs precursors have been characterized to date. One of these genes encodes the precursor for two RFRPs, i.e., neuropeptide FF (NPFF) and neuropeptide AF (4, 5). These two neuropeptides modulate the action of morphine (6) and are thus thought to be involved in the transmission of pain stimuli. However, NPFF is probably implicated in other physiological processes, such as regulation of energy homeostasis (7), arterial blood pressure (8), and hormone secretion (9, 10). A second RFRP gene encodes the precursor for prolactin-releasing peptide (PrRP), which has been originally identified as the endogenous ligand of the orphan G protein-coupled receptor hGR3 (11). PrRP has been initially named because of its ability to stimulate prolactin release (11), but recent studies suggest that this peptide plays a role in the control of food intake (12), stress response, and nociception (13). Finally, database search has led to the identification of a third human gene that encodes three putative RFRPs (14), and one of these peptides, RFRP-1, has been found to stimulate prolactin secretion in vivo (14). The avian counterpart of RFRP-1 has been recently isolated in the Japanese quail; this peptide, which inhibits gonadotropin release, has been called GnIH (15).
The concentration of neuropeptides in the brain of cold-blooded vertebrates is several orders of magnitude higher than in the brain in mammmals (16, 17). Taking advantage of this particularity, we have been able to isolate, from the frog brain, several neuropeptides, including secretoneurin (18), urotensin II (19), and cortistatin (20), which have all been subsequently identified in humans (21–23). In the course of these studies, we have found that the frog brain contains several RFRPs, and we have characterized one of them, the dodecapeptide R-RFa (2), which turned out to be the orthologue of mammalian RFRP-1 (14) and avian GnIH (15).
Here, we report the identification of 26RFa, an RFRP isolated from the frog brain that exhibits no meaningful sequence similarity with any known vertebrate RFRPs. We provide the sequences of the cDNAs encoding the 26RFa precursors in rat and human. We describe the distribution of preprotein (prepro)-26RFa mRNA in the rat brain, and we show that 26RFa is a potent orexigenic peptide in mice.
Materials and Methods
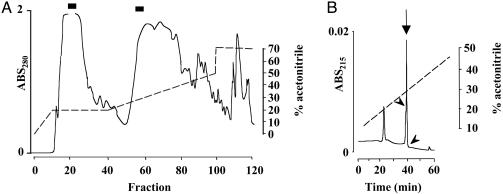
Purification of 26RFa from Frog Brain. Adult male frogs (Rana esculenta) were purchased from a commercial source (Couétard, St-Hilaire de Riez, France). Whole brains from 2,500 animals (235 g wet weight) were collected on dry ice and kept frozen at -80°C. The tissues were boiled in 0.5 M acetic acid for 15 min, homogenized, and centrifuged (4,000 × g, 4°C, 30 min). Purification of the peptide was performed as described (16). Briefly, the supernatant was pumped at a flow rate of 2 ml/min through 10 Sep-Pak C18 cartridges (Waters) connected in series. Bound material was eluted with 70% (vol/vol) acetonitrile in water, and acetonitrile was partially evaporated under reduced pressure. The solution was diluted with 10 ml of water/trifluoroacetic acid (TFA) (99.9:0.1, vol/vol) and pumped onto a 1 × 25-cm Vydac 218TP1010 C18 reverse-phase HPLC column (Touzart et Matignon, Courtaboeuf, France) equilibrated with 0.12% TFA at a flow rate of 2 ml/min by using the gradient indicated in Fig. 1A. Absorbance was measured at 215 and 280 nm, and eluting fractions were assayed for NPFF-like immunoreactivity (NPFFLI) as described (2). Fraction 61 was selected for further purification and successively chromatographed on a 0.46 × 25-cm Shodex C8P-50 4E C8 column, a 0.46 × 25-cm Vydac 218TP54 C18 column, a 0.46 × 25-cm Shodex C8P-50 4E C8 column, and a 0.46 × 25-cm Vydac 218TP54 C18 column, at a flow rate of 1 ml/min, by using the elution conditions indicated in Fig. 1B.
Fig. 1.
Purification of 26RFa from frog brain. (A) After prepurification on Sep-Pak cartridges, the brain extract was analyzed on a semipreparative reverse-phase HPLC column. The fractions denoted by the bars contained NPFF-LI, and fraction 61 was selected for further purification. (B) Final purification step of 26RFa on an analytical Vydac C18 reverse-phase HPLC column. The column was eluted with a linear gradient of increasing acetonitrile concentration (denoted by the dashed line), and individual peaks were collected by hand. The arrow shows the peak containing NPFF-LI. The arrowheads show where peak collection began and ended.
Sequence Analysis of Frog 26RFa. The primary structure of 26RFa was determined by mass spectrometry using a nano-electrospray quadrupole time-of-flight (Q-Tof) instrument (Micromass, Manchester, U.K.). Before analysis, the sample was desalted and concentrated on a C18 ZIP TIP (Millipore), which was eluted with 2 μl of acetonitrile 40% (vol/vol)/formic acid 1% (vol/vol) in water. The eluate was loaded in a disposable borosilicate capillary with two-thirds of the length Au/Pd-coated (Protana Engineering, Odense, Denmark). Capillary voltage was set to 800 V, with the sampling cone at 35 V. Collision voltage was tuned between 15 and 50 V to obtain intelligible peptide fragmentation. MS/MS spectra were deconvoluted and deisotoped with the MaxEnt 3 algorithm and processed with the pepseq sequencing software (Micromass).
In-solution tryptic digestion of the peptide was carried out overnight at 37°C in a 40-μl digestion solution containing 0.1 μg of modified porcine trypsin (Promega), 25 mM NH4CO3, and 5% acetonitrile (vol/vol).
Cloning and Sequencing. PCR amplifications of 26RFa-encoding sequences were carried out on reverse-transcribed RNA isolated from rat hypothalamic tissue, or on human genomic DNA. PCR was performed in a 50-μl volume containing 500 ng of template, 200 μM dNTPs, 1.5 mM MgCl2, 1 unit TaqDNA polymerase (Invitrogen), and 50 pmol of sense and antisense primers in the buffer (pH 9.0) supplied with the enzyme for 30 cycles (1 min at 94°C, 1 min at 50°C, and 1 min at 72°C) in a Perkin–Elmer GeneAmp PCR system 9700. To amplify the DNA sequences encoding human 26RFa, two successive PCR reactions were performed by using oligonucleotides whose sequences were deduced from the human hypothetical mRNA_XM_071001 in GenBank. In the first PCR amplification, the sense primer 5′-ATGCCTCCTGGACGAGGA-3′ and the antisense primer 5′-ATCCTTCCAAGGCGTCCTG-3′ were used. The products obtained were used in a second PCR performed with the sense primer 5′-AGCCTGTCCCGGGGAAACA-3′ and the antisense primer 5′-TCACCGCCGACCGAAGCG-3′, to amplify the full-length ORF encoding human 26RFa. To amplify the rat 26RFa cDNA, a first PCR was performed on reverse-transcribed hypothalamic RNA by using oligonucleotides designed based on a genomic clone (CH230-239D4) identified in the rat High Throughput Genomic sequences (accession no. AC105586). A first PCR was performed by using the sense primer 5′-ATGTGGCCAAATGGTGGC-3′ and the antisense primer 5′-ACAGTCAGGGCAGAGTC CA-3′, followed by another PCR amplification by using the sense primer 5′-TGTGCTCAGAT GAGGTGC-3′ and the antisense primer 5′-TCACCGGCCGAAGCGGAA-3′, to amplify the full reading frame encoding the rat 26RFa. DNA fragments were inserted into the pGEMT vector (Promega) and sequenced on both strands by using fluorescent universal primers and the Thermosequenase kit (Amersham Pharmacia Biosciences) in a Li-Cor 4200 DNA sequencer (ScienceTec, Les Ulis, France).
Peptide Synthesis. Human 26RFa (TSGPLGNLAEELNGYSRKKGGFSFRF-NH2), rat 26RFa (ASGPLGTLAEELSSYSRRKGGFSFRF-NH2), and frog R-RFa (SLKPAANLPLRFNH2) were synthesized (0.1-mmol scale) by the solid phase methodology on a Rink amide 4-methylbenzhydrylamine resin (Biochem, Meudon, France) by using a 433A Applied Biosystems peptide synthesizer and the standard fluorenylmethoxycarbonyl (Fmoc) procedure as described (24). The synthetic peptides were purified by reverse-phase HPLC on a 2.2 × 25-cm Vydac 218TP1022 C18 column (Alltech, Templemars, France) by using a linear gradient (10–50% over 50 min) of acetonitrile/trifluoroacetic acid (99.9:0.1, vol/vol) at a flow rate of 10 ml/min. Analytical HPLC, performed on a Vydac 218TP54 C18 column (0.46 × 25-cm), showed that the purity of the peptide was >99%. The purified peptides were characterized by mass spectrometry on a Micromass Tof-Spec E (Micromass). Bovine NPFF was purchased from Neosystem (Strasbourg, France).
In Situ Hybridization Histochemistry. Adult male Wistar rat brains were dissected, frozen immediately in isopentane (-30°C) and stored at -80°C until use. Frozen coronal sections (18 μm thick) were collected onto poly (l-lysine)-coated slides and fixed in 4% paraformaldehyde. Single-stranded 35S-labeled sense and antisense 26RFa RNA probes were generated by in vitro transcription of the rat cDNA clone, by using a riboprobe kit (Promega). Brain slices were incubated for 5 min in 0.1 M triethanolamine (pH 8.0), rinsed in 2× SSC (0.3 M NaCl and 0.03 M sodium citrate) and covered with prehybridization buffer (pH 7.5) containing 60% formamide, 0.6 M NaCl, 10 mM Tris·HCl (pH 7.5), 0.02% Ficoll, 0.02% polyvinylpyrolidone, 0.02% BSA, 1 mM EDTA, 550 μg/ml denaturated salmon sperm DNA, and 50 μg/ml yeast tRNA. Hybridization was performed overnight at 55°C in the same buffer (except for salmon sperm DNA, whose concentration was lowered to 60 μg/ml) supplemented with 10 mM DTT, 10% dextran sulfate, and 1.5 × 107 cpm/ml heat-denaturated RNA probes. Slices were washed in 2× SSC at 60°C and treated with RNase A (60 μg/ml) at 37°C for 60 min. Five final high-strengency washes were performed in 0.1× SSC containing 14 mM 2-mercaptoethanol and 0.05% sodium pyrophosphate at 60°C. Tissue sections were dehydrated in ethanol and exposed onto Hyperfilm-βmax (Amersham Pharmacia) for 5 days. Slices were subsequently dipped into Kodak NTB2 liquid emulsion at 42°C, exposed for 25 days, and developed. To identify anatomic structures, sections were stained with hematoxylin.
Cell Culture and cAMP Assay. Pituitary glands from 21-day-old male Wistar rats were dissociated in 0.5% trypsin and 2 mM EDTA, followed by mechanical dispersion. Pituitary cells were cultured in DMEM (Invitrogen) supplemented with 2.5% FCS, 10% horse serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 250 ng/ml fungizone, in poly(l-lysine)-coated 24-well plates at a density of 2.5 × 105 cells per well. After 48 h, cells were preincubated for 2 h in Krebs medium (11.8 mM NaCl/0.25 mM CaCl2/0.12 mM KH2PO4/0.12 mM MgSO4/2.4 mM NaHCO3/0.47 mM KCl/1 mM Hepes/0.2 g/liter glucose, pH 7.3) containing 1 mg/ml BSA and 0.2 mM 3-isobutyl-1-methylxanthine (Sigma). The cells were then incubated for an additional 30 min in the same medium containing 0.5 μM forskolin in the absence or presence of graded concentrations of human 26RFa, bovine NPFF, or frog R-RFa. The medium was removed, and the cells were extracted by ice-cold ethanol. Alcohol was evaporated in a speed-Vac concentrator (AES 2000, Savant, Hicksville, NY), and the cAMP content was determined by using a cAMP RIA kit (Amersham Pharmacia Biosciences). Protein concentrations were determined according to the method of Bradford by using BSA as standard. A concentration-response curve was generated by using PRISM software (GraphPad, San Diego). Data are expressed as the mean ± SEM of four determinations.
Feeding Experiments. Male Swiss albino mice CD1 (25–30 g) were maintained under controlled temperature (22°C) and lightning (light on from 7:00 a.m. to 7:00 p.m.) with food and water available ad libitum. Three days before the experiments, the animals were isolated in individual cages, and 18 h before testing (3:00 p.m. to 9:00 p.m.) they were deprived of half of their daily food consumption (3 g instead of 6 g). Intracerebroventricular (i.c.v.) injections of saline or 26RFa (10 μl per mouse) were made free hand in the left ventricle, according to the procedure of Haley and McCormick (25). Ten minutes after the injection, mice had access to a weighed food pellet (5 g). The pellet was briefly (<20 s) removed and weighed at times 30, 60 and 120 min. Data are expressed as mean ± SEM from 20 mice. Differences between groups were assessed by Dunnett's t test.
Results
Peptide Isolation. The Sep-Pak-prepurified frog brain extract was subjected to reverse-phase HPLC on a semipreparative Vydac C18 column, and all collected fractions (1 min each) were assayed for NPFF-LI. As shown by the bars in Fig. 1 A, NPFF-LI was eluted in two distinct pools of 6 consecutive fractions (fractions 19–24 and fractions 56–61). Fraction 61, which exhibited the highest immunoreactivity among the more hydrophobic pool of fractions, was selected for further purification. The immunoreactive peptide was purified to near homogeneity by successive chromatographies on analytical columns, i.e., a Shodex C8 column, a Vydac C18 column, a Shodex C8 column, and a Vydac C18 column (Fig. 1B). The final yield of pure material was ≈170 pmol.
Structural Characterization. Half of the pure material isolated by reverse-phase HPLC was analyzed in its native state by quadrupole time-of-flight mass spectrometry. A survey mass spectrum from m/z 1,000 to 3,400, taken in the MS mode, revealed the presence of a single peptide component of 2,818.3 Da, which was subsequently subjected to collision-induced dissociation. The resulting MS/MS fragmentation pattern allowed us to identify the amino acid sequence of the N-terminal region as VGTALGSLAEELN. Tryptic digest of the remaining 50% of the peptide generated six fragments, the primary structures of which were established as follows: VGTALGSLAEELNGYNR, VGTALGSLAEELNGYNRK, VGTALLGSLAEELNGYN RKK, GGFSFRF, KGGFSFRF, and KKGGFSFRF. By combining native and digest analyses, it was possible to deduce the complete sequence of the peptide as VGTALGSLAEELNGYNRKKGGFSFRF. The presence of an α-amidated C-terminal residue was confirmed by comparison of the observed molecular mass of the peptide (2,818.3 atomic mass units) with the calculated mass of the amidated form of the proposed sequence (2,818.17 atomic mass units).
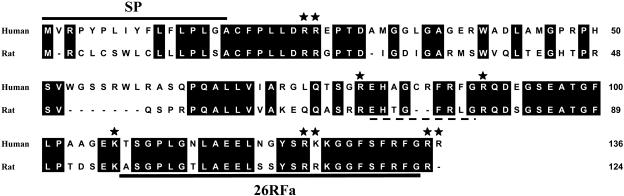
Characterization of the Human and Rat Prepro-26RFa cDNAs. The search for mammalian peptides related to frog 26RFa in the GenBank/European Molecular Biology Laboratory/SwissProt and High Throughput Genomics databases revealed a human hypothetical protein (accession no. XP_071001.1) derived from a genomic DNA sequence (accession no. XM_071001) localized on chromosome 9, locus 138625, and a rat DNA sequence from clone CH230-239D4 (accession no. AC105586), which both encode an RFamide peptide highly homologous to frog 26RFa. On the basis of these sequences, we have isolated by PCR human and rat DNA sequences encoding the full-length coding regions. These human and rat sequences encoded preproteins of 136 and 124 aa, respectively (Fig. 2). Human and rat 26RFa precursors show a similar organization, with a signal peptide of 17 and 16 aa, respectively, as predicted by the Signal PV1.1 algorithm, a fully conserved downstream peptide of six residues followed by a dibasic processing site (Arg-Arg), and the 26RFa sequence located in the C-terminal region of each precursor. Human and rat 26RFa are both composed of 26 aa and are followed by a Gly residue. In both human and rat precursors, 26RFa is flanked, at its N terminus, by a monobasic residue (Lys). Human 26RFa is followed by a dibasic cleavage site (Arg-Arg) whereas, in the rat precursor, a single Arg residue is found at this position. The human precursor contains, upstream of the 26RFa region, an additional putative RFamide peptide of 9 aa flanked by two Arg residues, which is lacking in the rat preprotein.
Fig. 2.
Alignment of the amino acid sequences of human and rat prepro-26RFa deduced from the corresponding cDNAs. The putative signal peptide sequence (SP) is designated by the bold line, and the sequence of 26RFa is underlined. The sequence of a second putative FMRFamide-related peptide in human prepro-26RFa is denoted by a broken line. Potential cleavage sites are marked by stars. Amino acids are numbered on the right, and conserved residues are indicated in black. Gaps, marked by hyphens, have been inserted to achieve optimal alignment.
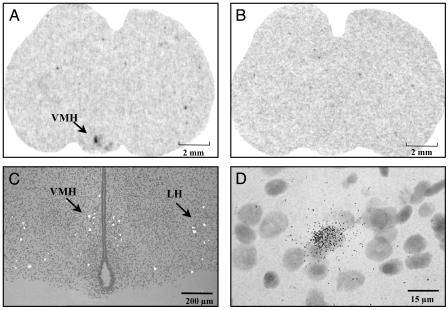
Distribution of Rat Prepro-26RFa mRNA. The distribution of prepro-26RFa mRNAs was studied in the rat brain by in situ hybridization. The antisense probe generated a strong hybridization signal exclusively in the hypothalamic region (Fig. 3A) whereas the sense probe did not produce any staining (Fig. 3B). No hybridization signal has been detected in any extrahypothalamic region including the medulla. Specifically, the prepro-26RFa gene was expressed in the ventromedial hypothalamic nucleus (VMH) and in the lateral hypothalamic area (LH) (Fig. 3C). At a higher magnification, a dense accumulation of silver grains was observed on scattered neurons in the VMH (Fig. 3D) and the LH (data not shown).
Fig. 3.
X-ray auroradiographs illustrating the localization of prepro-26RFa mRNA in coronal sections of the rat brain at the level of the hypothalamus. (A) Sections hybridized with the antisense riboprobe. Labeling is observed in the VMH. (B) Control section adjacent to A hybridized with the sense riboprobe. (C) Polarized-field illumination photomicrograph showing the presence of hybridization signal in the periventricular region of the VMH and in the LH. (D) After counterstaining with hematoxylin, accumulation of silver grains was observed over scattered cells in the VMH.
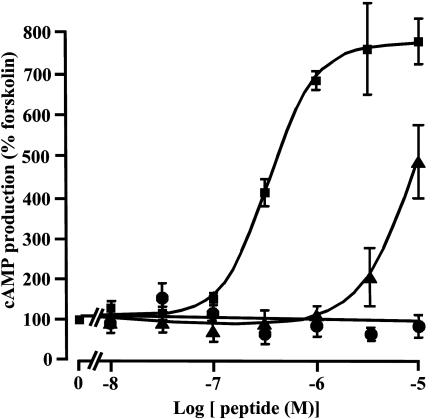
Effect of 26RFa on cAMP Production by Rat Pituitary Cells. Incubation of cultured rat anterior pituitary cells, preincubated with forskolin (5 × 10-7 M), with graded doses of human 26RFa (10-8 M to 10-5 M) resulted in a concentration-dependent increase of cAMP production (Fig. 4). In contrast, bovine NPFF produced only a modest stimulation at high concentration, and frog R-RFa had no effect on cAMP formation. At the maximum effective concentration (3 × 10-6 M), 26RFa produced a 680% increase in cAMP concentration (Fig. 4).
Fig. 4.
Effects of 26RFa and related peptides on cAMP formation by rat pituitary cells. Human 26RFa (squares) induced a dose-dependent increase in cAMP formation. Bovine NPFF (triangles) only induced a moderate increase at high concentrations and frog R-RFa (dots) had no effect on cAMP formation. Values are mean ± SEM of four determinations. Three experiments were performed with similar results.
Effect of 26RFa on Feeding Behavior. In mice partially deprived of food for 18 h, intracerebroventricular administration of 26RFa elicited a dose-dependent increase in food intake during the subsequent 2 h (Table 1). At the lowest dose tested (10 ng), 26RFa provoked a slight but not significant elevation of food consumption. At higher doses (100 and 1,000 ng), a significant increase in food intake was observed at all times investigated (Table 1).
Table 1. Time course of the effect of 26RFa on food intake in food-restricted mice.
| Time of food presentation, min
|
|||
|---|---|---|---|
| 30 | 60 | 120 | |
| Saline | 273 ± 42 | 316 ± 47 | 324 ± 47 |
| 10 ng of 26RFa | 319 ± 40 | 377 ± 42 | 403 ± 42 |
| 100 ng of 26RFa | 394 ± 45* | 458 ± 52* | 491 ± 63* |
| 1,000 ng of 26RFa | 458 ± 50** | 519 ± 47** | 527 ± 48** |
Mice, previously deprived (18 h before testing) of half of their daily food consumption, were injected intracerebroventricularly with either saline or increasing doses of 26RFa (10–1,000 ng per mouse). Ten minutes after the injection, mice had access to a weighed food pellet, and their food consumption was measured during the periods indicated. Data are means ± SEM food intake (mg) from 20 mice per group. Dunnett's t test; *, P < 0.05; **, P < 0.01.
Discussion
Up to now, three cDNAs encoding RFRPs have been identified in human (4, 5, 11, 14). In the present study, we have characterized a fourth cDNA encoding a neuropeptide designated 26RFa that exhibits weak structural similarity with the other RFRPs described so far.
Structural Characteristics of 26RFa. Comparison of the amino acid sequence of frog 26RFa with those deduced from the rat and human prepro-26RFa cDNAs shows that the primary structure of the peptide has been strongly conserved from amphibian to mammals, with 77% identity between the frog and human sequences, 65% between the frog and rat sequences, and 81% between the rat and human sequences (Table 2). In particular, strong evolutionary pressure has acted to conserve the sequence of the C-terminal octapeptide of 26RFa, suggesting that this region is crucial for the biological activity of the neuropeptide. Consistent with this notion, structure-activity studies have recently shown the importance of the C-terminal domain of two other RFRPs, i.e., NPFF and RFRP-3, in determining the binding affinity and biological potency of these neuropeptides (26, 27). Although the primary structure of the N-terminal domain is more variable, the sequence of the pentapeptide Leu-Ala-Glu-Glu-Leu has been fully conserved, and the sequence of the octapeptide Leu-Ala-Glu-Glu-Leu-Asn-Gly-Tyr is identical in frog and human. In contrast, 26RFa exhibits very low structural similarity with the other RFRPs identified to date in human (Table 2), the only consistent identity being the Arg-Phe-NH2 motif present in 26RFa as in other RFRPs. As a matter of fact, 26RFa is the single representative of the RFRP family that possesses the Phe-Arg-Phe-NH2 sequence, the other vertebrate RFRPs exhibiting a Gln-Arg-Phe-NH2 (e.g., RFRP-3, NPFF, and neuropeptide AF), a Gly-Arg-Phe-NH2 (e.g., PrRP), or a Leu-Arg-Phe-NH2 (e.g., RFRP-1, GnIH and R-RFa). These data clearly indicate that 26RFa is a member of a previously uncharacterized subfamily of RFRPs identified in vertebrates.
Table 2. Comparison of the primary structures of 26RFa (human, rat, and frog sequences) with those of other RFamide-related peptides (human sequences).
| Peptide | Amino acid sequence |
|---|---|
| Human 26RFa | TSGPLGNLAEELNGYSRKKGGFSFRF-NH2 |
| Rat 26RFa | ASGPLGTLAEELSSYSRRKGGFSFRF-NH2 |
| Frog 26RFa | VGTALGSLAEELNGYNRKKGGFSFRF-NH2 |
| Human RFRP-1 | MPHSFANLPLRF-NH2 |
| Human RFRP-2 | SAGATANLPLRS-NH2 |
| Human RFRP-3 | VPNLPQRF-NH2 |
| Human NPFF | SQAFLFQPQRF-NH2 |
| Human NPAF | AGEGLNSQFWSLAAPQRF-NH2 |
| Human PrRP | TPDINPAWYAGRGIRPVGRF-NH2 |
Bold letters denote residue identity.
Characterization of the Prepro-26RFa cDNA. Molecular cloning of the cDNAs encoding the 26RFa precursors in rat and human revealed that the two proteins have a similar organization with the 26RFa sequence at the C terminus. The overall identity of rat and human prepro-26RFa is 59%, indicating that the sequence of the whole precursor has been relatively well conserved. The isolation and characterization of 26RFa from the frog brain strongly suggests that the rat and human precursors are also processed to generate 26RFa. In the rat and human precursors, a single Lys residue is found upstream the sequence of 26RFa. Although an individual lysine is not regarded as a preferential cleavage site, a few regulatory peptides are actually processed at this site (28, 29). The occurrence of an internal dibasic motif (Arg-Lys or Arg-Arg) within the frog, rat, and human 26RFa sequences suggests that the peptide could be further processed to generate the highly conserved C-terminal peptide (Lys)-Gly-Gly-Phe-Ser-Phe-Arg-Phe-NH2. However, the fact that authentic 26RFa has been isolated from the frog brain indicates that this dibasic site is probably not efficiently cleaved.
The human 26RFa precursor encompasses another RFRP of 9 aa that is delimited by single Arg residues at its N- and C-terminal extremities. The C-terminal tripeptide sequence of this nonapeptide is identical to that of 26RFa, indicating that the two peptides likely arose from exon duplication of an ancestral DNA sequence. Interestingly, several other RFRPs, including PrRP (11), GnIH (30), and RFRP-1 to -3 (31, 32), are also flanked by individual Arg residues, suggesting that processing of the 26RFa precursor by prohormone-convertases may actually generate the nonapeptide Glu-His-Ala-Gly-Cys-Arg-Phe-Arg-Phe-NH2, in addition to 26RFa.
Distribution of Prepro-26RFa mRNA and Biological Activity of 26RFa. In situ hybridization studies have shown that, in the rat brain, prepro-26RFa mRNA is exclusively present in the VMH and the LH, two hypothalamic regions known to be involved in the regulation of food intake and energy expenditure. Besides the brain, we cannot exclude that the 26RFa gene could be also expressed in peripheral tissues, notably in the gut and adrenal gland. We also observed that intracerebroventricular injection of 26RFa in mice provoked a dose-dependent stimulation of food consumption. Taken together, these data strongly suggest that this neuropeptide is involved in the control of feeding behavior.
It has been previously shown that several RFRPs regulate pituitary function. For instance, in mammals, NPFF, PrRP, and RFRP-1 stimulate prolactin secretion (9, 11, 14); in birds, GnIH inhibits luteinizing hormone release (15); in amphibians, R-RFa activates growth hormone secretion (33); and in fish, Carassius RFamide increases prolactin and somatolactin secretion (34). Here, we show that 26RFa provokes a massive increase in cAMP formation by rat pituitary cells whereas NPFF and frog R-RFa have little or no effect. These data indicate that 26RFa, like other RFRPs, may act as a hypophysiotropic neurohormone.
While this study was submitted, the putative ligand for the orphan G protein-coupled receptor GPR103 (also referred to as SP9155 and AQ27) was identified through a search in the human genome database (35). The predicted sequence of the mature peptide (termed P518), deduced from the cDNA sequence, turned out to be identical to that of 26RFa (ref. 35 and this study). The fact that GPR103 is actively expressed in the hypothalamus and pituitary (35, 36) is consistent with the notion that 26RFa is involved in the control of feeding behavior and pituitary hormone secretion.
In conclusion, we have identified a hypothalamic neuropeptide, 26RFa, that is an additional representative of the RFamide superfamily of regulatory peptides. 26RFa exhibits several characteristics of biologically active peptides: (i) it is C-terminally α-amidated; (ii) it is located at the C-terminal extremity of the precursor like many other neuropeptides such as somatostatin, corticotropin-releasing factor, β-endorphin, and melaninconcentrating hormone; (iii) its structure has been strongly conserved during evolution; (iv) it is expressed in discrete hypothalamic nuclei involved in the control of feeding behavior; (v) it activates adenylyl cyclase activity in rat pituitary cells; and (vi) it stimulates food intake in mice. Altogether, these data suggest that 26RFa may play important biological functions.
Acknowledgments
This research was supported by the Institut National de la Santé et de la Recherche Médicale U-413, the Ministère délégué à la Recherche et aux Nouvelles Technologies, an exchange program from the Institut National de la Santé et de la Recherche Médicale-Ministerie van de Vlaamse Gemeenschap (to F.V. and H.V.), and the Conseil Régional de Haute-Normandie.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: GnIH, gonadotropin-inhibiting hormone; LH, lateral hypothalamic area; NPFF, neuropeptide FF; NPFF-LI, NPFF-like immunoreactivity; PrRP, prolactin-releasing peptide; RFRP, FMRFamide-related peptide; R-RFa, Rana RFamide; VMH, ventromedial hypothalamic nucleus; prepro, preprotein.
Data deposition: The sequences reported in this paper have been deposited in the SWISS-PROT/TrEMBL database [accession no. P83683 (frog 26RFa)], and in the GenBank database [accession nos. AY438326 (human prepro-26RFa) and AY438327 (rat prepro-26RFa)].
References
- 1.Price, D. A. & Greenberg, M. (1977) Science 197, 670-671. [DOI] [PubMed] [Google Scholar]
- 2.Chartrel, N., Dujardin, C., Leprince, J., Desrues, L., Tonon, M. C., Cellier, E., Cosette, P., Jouenne, T., Simonnet, G. & Vaudry, H. (2002) J. Comp. Neurol. 448, 111-127. [DOI] [PubMed] [Google Scholar]
- 3.Li, C., Kyuhyung, K. & Nelson, L. S. (1999) Brain Res. 848, 26-34. [DOI] [PubMed] [Google Scholar]
- 4.Perry, S. J., Yi-Kung Huang, E., Cronk, D., Bagust, J., Sharma, R., Walker, R. J., Wilson, S. & Burke, J. F. (1997) FEBS Lett. 409, 426-430. [DOI] [PubMed] [Google Scholar]
- 5.Vilim, F. S., Aarnisalo, A. A., Nieminen, M. L., Lintunen, M., Karlstedt, K., Kontinen, V. K., Kalso, E., States, B., Panula, P. & Ziff, E. (1999) Mol. Pharmacol. 55, 804-811. [PubMed] [Google Scholar]
- 6.Yang, H.-Y. T., Fratta, W., Majane, E. A. & Costa, E. (1985) Proc. Natl. Acad. Sci. USA 82, 7757-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sunter, D., Hewson, A. K., Lynam, S. & Dickson, S. L. (2001) Neurosci. Lett. 313, 145-148. [DOI] [PubMed] [Google Scholar]
- 8.Roth, B. L., Disimone, J., Majane, E. A. & Yang, H.-Y. T. (1987) Neuropeptides 10, 37-42. [DOI] [PubMed] [Google Scholar]
- 9.Aarnisalo, A. A., Tuominen, R. K., Nieminen, M., Vainio, P. & Panula, P. (1997) Neuroendocrinol. Lett. 18, 191-196. [Google Scholar]
- 10.Labrouche, S., Laulin, J. P., Le Moal, M., Tramu, G. & Simonnet, G. (1998) J. Neuroendocrinol. 10, 59-565. [DOI] [PubMed] [Google Scholar]
- 11.Hinuma, S., Habata, Y., Fujii, R., Kawamata, Y., Hosoya, M., Fukusumi, S., Kitada, C., Masuo, Y., Asano, T., Matsumoto, H., et al. (1998) Nature 393, 272-276. [DOI] [PubMed] [Google Scholar]
- 12.Lawrence, C. B., Celsi, F., Brennand, J. & Luckman, S. M. (2000) Nat. Neurosci. 7, 645-646. [DOI] [PubMed] [Google Scholar]
- 13.Lin, S. H. S., Leslie, F. M. & Civelli, O. (2002) Brain Res. 952, 15-30. [DOI] [PubMed] [Google Scholar]
- 14.Hinuma, S., Shintani, Y., Fukusumi, S., Iijima, N., Matsumoto, Y., Hosoya, M., Fujii, R., Watanabe, T., Kikuchi, K., Terao, Y., et al. (2000) Nat. Cell Biol. 2, 703-708. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui, K., Saigoh, E., Ukena, K., Teranishi, H., Fujisawa, Y., Kikuchi, M., Ishii, S. & Sharp, P. J. (2000) Biochem. Biophys. Res. Commun. 275, 661-667. [DOI] [PubMed] [Google Scholar]
- 16.Chartrel, N., Conlon, J. M., Danger, J. M., Fournier, A., Tonon, M. C. & Vaudry, H. (1991) Proc. Natl. Acad. Sci. USA 88, 3862-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chartrel, N., Tonon, M. C., Vaudry, H. & Conlon, J. M. (1991) Endocrinology 129, 3367-3371. [DOI] [PubMed] [Google Scholar]
- 18.Vaudry, H. & Conlon, J. M. (1991) FEBS Lett. 284, 31-33. [DOI] [PubMed] [Google Scholar]
- 19.Conlon, J. M., O'Harte, F., Smith, D. D., Tonon M. C. & Vaudry, H. (1992) Biochem. Biophys. Res. Commun. 188, 578-583. [DOI] [PubMed] [Google Scholar]
- 20.Vaudry, H., Chartrel, N. & Conlon, J. M. (1992) Biochem. Biophys. Res. Commun. 188, 477-482. [DOI] [PubMed] [Google Scholar]
- 21.Schmid, K. W., Kunk, B., Kirchmair, R., Tötsch, M., Böcker, W. & Fischer-Colbrie, R. (1995) Histochem. J. 27, 473-481. [PubMed] [Google Scholar]
- 22.Coulouarn, Y., Lihrmann, I., Jegou, S., Anouar, Y., Tostivint, H., Beauvillain, J. C., Conlon, J. M., Bern H. A. & Vaudry, H. (1998) Proc. Natl. Acad. Sci. USA 95, 15803-15808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Lecea, L., Criado, J. R., Prospero-Garcia, O., Gautvik, K. M., Schweitzer, P., Danielson, P. E., Dunlop, C. L. M., Siggins, G. R., Henriksen, S. G. & Sutcliffe, J. G. (1996) Nature 381, 242-245. [DOI] [PubMed] [Google Scholar]
- 24.Leprince, J., Oulyadi, H., Vaudry, D., Masmoudi, O., Gandolfo, P., Patte, C., Costentin, J., Fauchère, J. L., Davoust, D., Vaudry, H. & Tonon, M. C. (2001) Eur. J. Biochem. 268, 6045-6047. [DOI] [PubMed] [Google Scholar]
- 25.Haley, T. J. & McCormick, W. G. (1957) Br. J. Pharmacol. 12, 12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazarguil, H., Gouardères, C., Tafani, J. A. M., Marcus, D., Kotani, M., Mollereau, C., Roumy, M. & Zajac, J. M. (2001) Peptides 22, 1471-1478. [DOI] [PubMed] [Google Scholar]
- 27.Yoshida, H., Habata, Y., Hosoya, M., Kawamata, Y., Kitada, C. & Hinuma, S. (2003) Biochim. Biophys. Acta 1593, 151-157. [DOI] [PubMed] [Google Scholar]
- 28.Viereck, J. C. & Beinfeld, M. C. (1992) J. Biol. Chem. 267, 19475-19481. [PubMed] [Google Scholar]
- 29.Rourke, I. J., Johnsen, A. H., Din, N., Petersen, J. G. L. & Rehfeld, J. F. (1997) J. Biol. Chem. 272, 9720-9727. [DOI] [PubMed] [Google Scholar]
- 30.Satake, H., Hisada, M., Kawada, T., Minakata, H., Ukena, K. & Tsutsui, K. (2001) Biochem. J. 354, 379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukusumi, S., Habata, Y., Yoshida, H., Iijima, N., Kamamata, Y., Hosoya, M., Fujii, R., Hinuma, S., Kitada, C., Shintani, Y., et al. (2001) Biochim. Biophys. Acta 1540, 221-232. [DOI] [PubMed] [Google Scholar]
- 32.Ukena, K., Imakoshi, E., Minakata, H. & Tsutsui, K. (2002) FEBS Lett. 512, 255-258. [DOI] [PubMed] [Google Scholar]
- 33.Koda, A., Ukena, K., Teranishi, H., Ohta, S., Yamamoto, K., Kikuyama, S. & Tsutsui, K. (2002) Endocrinology 143, 411-419. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama, S., Ito, T., Takahashi, A., Amano, M., Somer, S. A., Hirano, T., Yamamori K. & Kawouchi, H. (2002) Endocrinology 143, 2071-2079. [DOI] [PubMed] [Google Scholar]
- 35.Jiang, Y., Luo, L., Gustafson, E. L., Yadav, D., Laverty, M., Murgolo, N., Vassileva, G., Zeng, M., Laz, T. M., Behan, J., et al. (2003) J. Biol. Chem. 278, 27652-27657. [DOI] [PubMed] [Google Scholar]
- 36.Lee, D. K., Nguyen, T., Lynch, K. R., Cheng, R., Vanti, W. B., Arkhitko, O., Lewis, T., Evans, J. F., George S. R. & O'Dowd, B. F. (2001) Gene 275, 83-91. [DOI] [PubMed] [Google Scholar]