Abstract
The regulation of negative emotion through reappraisal has been shown to induce increased prefrontal activity and decreased amygdala activity. Individual differences in dispositional mindfulness reflect differences in typical recognition, detachment and regulation of current experience, thought to also operate as top–down control mechanism. We sought to investigate whether such individual differences would be associated with brain activity elicited during reappraisal of negative emotion. Eighteen healthy participants completed a functional magnetic resonance imaging task that involved attending to or reappraising negative stimuli, and provided emotion experience ratings after each trial. Dispositional mindfulness was assessed with a self-report questionnaire. Reappraisal induced activity in a brain network involving predominantly dorsal portions of the prefrontal cortex, replicating previous studies. A voxelwise regression analysis showed that individual differences in the tendency to be mindful predicted activity in neural regions underlying reappraisal, with dorsomedial prefrontal cortex activation increasing with more mindfulness traits. Notably, this prefrontal activation was inversely correlated with the amygdala response to negative scenes, further supporting its role in down-regulating emotion-generation regions. These findings suggest that individual differences in dispositional mindfulness, which reflect the tendency to recognize and regulate current states, may modulate activity in neural systems involved in the effective cognitive control of negative emotion.
Keywords: emotion regulation, fMRI, individual differences, mindfulness, prefrontal cortex
INTRODUCTION
The ability to regulate negative emotion is crucial to adaptively respond to the distressing experiences we can encounter in our everyday life. Over the past years, the implications of emotion regulation both at clinical and non-clinical levels have stimulated the search for its neural substrates (Gross and Munoz, 1995). Reappraisal is perhaps the most studied of the cognitive processes used to decrease the experience of negative emotion, involving the reinterpretation of affective stimuli to modify their emotional impact (Gross, 1999).
Functional magnetic resonance imaging (fMRI) studies seeking to ascertain the neural basis of reappraisal of negative emotion have consistently reported that it is accomplished through interactions within a distributed cortico-subcortical network (Ochsner and Gross, 2005, 2008). In particular, critical regions for cognitive control such as the prefrontal cortex (PFC) and the anterior cingulate cortex (ACC) would exert down-regulatory influences on subcortical regions such as the amygdala, which has a well-known role in the processing of the emotional significance of external stimuli (LeDoux, 1996; Phelps and LeDoux, 2005). The majority of these fMRI studies have typically scanned subjects who had been instructed to use reappraisal to diminish the negative affect elicited by stimuli of negative valence (Ochsner et al., 2002; Kim and Hamann, 2007; van Reekum et al., 2007; Goldin et al., 2008). Importantly, we also engage in reappraisal spontaneously, in the absence of any specific instructions to regulate our emotions (Gross and John, 2003). A recent fMRI study examined whether individual differences in spontaneous reappraisal use would similarly influence frontolimbic interactions in response to negative stimuli. Drabant and colleagues (2009) used a ‘pure’ reactivity task instead of instructed reappraisal, assuming that subjects would differ in their ability to spontaneously engage in reappraisal. The authors reported that individual differences in spontaneous reappraisal predicted increased activation in the PFC and decreased response in the amygdala. These findings shed new light on the notion that individual differences in the tendency to engage in self-regulatory processes may mediate neural responses to affective stimuli (Jackson et al., 2003; Haas et al., 2007). There is rising scientific interest in the study of the neural correlates of such individual differences given that individuals who tend to use reappraisal more often report experiencing less negative affect, better social functioning and greater psychological well-being (Gross and John, 2003). Furthermore, individual differences in the tendency to regulate emotion may function as protective factors to reduce the risk for psychiatric disorders involving emotion dysregulation (Taylor and Liberzon, 2007).
Mindfulness is conceptualized as a self-regulatory process, thought to operate as a top–down mechanism to reduce negative affect and promote well-being (Lutz et al., 2008). Central to numerous meditation practices, mindfulness is thought to entail a better recognition of and detachment from current emotional patterns, improving the ability to adaptively respond to them (Brown and Ryan, 2003). Dispositional mindfulness refers to the tendency to be mindful in everyday life, in which individuals may differ from one another (Brown and Ryan, 2003) and is considered to imply emotion regulation abilities (Lutz et al., 2008). Interestingly, the available neuroimaging literature on meditation indicates an association with increased activity in brain regions involved in cognitive control and attention, i.e. PFC and ACC (Cahn and Polich, 2006). With regard to the limbic system, although some studies have reported increased activity in amygdala and hippocampus during meditation (Newberg and Iversen, 2003), others have found decreases, which would support the notion that mindfulness training is associated with a significant decrease in emotionally reactive behaviors that are incompatible with stability of concentration and self-regulation (Lutz et al., 2008). Individual differences in dispositional mindfulness may be reliably detected through self-report questionnaires, as mindfulness traits may also be present in meditation-naïve individuals (Baer et al., 2004). Associations between individual difference measures, such as a measure of trait mindfulness, and brain activity may be reliably identified with functional magnetic resonance imaging (fMRI) paradigms, providing appropriate corrections for multiple comparisons are applied (Lieberman et al., in 2009). Such analysis may underscore relationships between brain activity and psychological variables, in this case the tendency to be more mindful in everyday life. To date, only one fMRI study has investigated the effect of individual differences in the tendency to be mindful on affect regulation. Creswell and colleagues reported an association between dispositional mindfulness and enhanced dorsal PFC and decreased amygdala response during an affect-labeling task (Creswell et al., 2007), in line with previous positive associations between mindfulness-based strategies and PFC activation (Farb et al., 2007) and decreased amygdala response during affect labeling (Lieberman et al., 2007).
However, the relationship between individual differences in dispositional mindfulness and brain activity during cognitive emotion control through a complex process such as reappraisal has not been studied to date. This could further our current understanding of the neural dynamics underlying cognitive emotion control through reappraisal, as well as shed new light on the neural correlates of mindfulness traits.
The present study sought to examine whether brain activity elicited during instructed reappraisal would be modulated by differences in dispositional mindfulness, in a sample of meditation-naïve subjects. We first hypothesized that reappraisal would elicit brain activation in a predominantly prefrontal network of brain regions involved in attention, evaluation and reappraisal of emotional stimuli, including the ACC and lateral prefrontal areas (Ochsner and Gross, 2005), in a whole-brain analysis. Second, in light of the dorsomedial PFC (DMPFC) role in reappraisal (Ochsner and Gross, 2005), and on prior neuroimaging literature in mindfulness and meditation (Creswell et al., 2007; Lutz et al., 2008), we envisaged that individual differences in mindfulness traits would modulate activity in dorsal prefrontal emotion control regions during reappraisal. Furthermore, we expected this prefrontal activity to be negatively associated with the amygdala response to negative stimuli, as prior research has suggested that effective down-regulation of negative emotion is attained through such PFC–amygdala interactions (Ochsner and Gross, 2005).
MATERIALS AND METHODS
Participants
Eighteen healthy right-handed subjects (11 males, 7 females; mean age 21.1 ± 2.8 years) from a university sample participated in the study. None of the subjects had a history of psychiatric or neurologic disorders. All participants gave written informed consent after a detailed explanation of the experimental protocol, approved by the Medical Ethical Committee of the University Medical Center Groningen.
Individual differences in dispositional mindfulness
Before scanning, all subjects completed the Kentucky Inventory of Mindfulness Skills (KIMS, Baer et al., 2004). This is a 39-item instrument for the measurement of four aspects of mindfulness: (i) observe (e.g. ‘I intentionally stay aware of my feelings’; ‘I pay attention to how my emotions affect my thoughts and behavior’); (ii) describe (e.g. ‘I’m good at finding the words to describe my feelings’; ‘When I have a sensation in my body, it’s difficult for me to describe it because I can’t find the right words.’); (iii) acting with awareness (e.g. ‘When I’m doing something, I’m only focused on what I’m doing, nothing else’; ‘I don’t pay attention to what I’m doing because I’m daydreaming, worrying, or otherwise distracted’); and (iv) accepting without judgment (e.g. ‘I think some of my emotions are bad or inappropriate and I shouldn’t feel them’; ‘I make judgments about whether my thoughts are good or bad’). Items are rated on a 5-point Likert-type scale (1 = never or very rarely true, 5 = always or almost always true), and a total score is computed by adding the scores on each item (0–195 points). Completion of the KIMS does not require previous experience with meditation (all participants in the study were meditation-naïve) and has been validated as a self-report of good test–retest reliability and internal consistency for the assessment of dispositional mindfulness in student and patient samples (Baer et al., 2004, 2006).
Pre-scan session
In a pre-scanning session immediately before scanning, participants received extensive training in the experimental protocol. Subjects were trained in different reappraisal strategies: (i) positive outcome, e.g. a man in a hospital bed had completely recovered afterward; (ii) transforming the scene into different terms, e.g. a woman crying outside of a church could be crying out of happiness at her daughter’s wedding; (iii) objectifying the scene depicted in the picture, e.g. a woman being attacked by an armed man was only a movie scene. Subjects were advised that no ideal reinterpretation type was applicable to all pictures, so that they were to choose the strategy with which they felt more comfortable. Pilot testing indicated that reappraisal was generally accomplished by generating an interpretation or alternate story of the photo that could explain its content in a less negative way. Previous studies using such reappraisal strategies on pictures eliciting negative affect have indicated the frequent use and success of their application (e.g. Ochsner et al., 2002).
Stimuli
The stimulus set consisted of 66 color pictures from the International Affective Picture System (IAPS; Lang et al., 1997). Twenty-two neutral (valence M = 5.1 ± 1.7; arousal M = 2.9 ± 2) and 44 negative pictures (valence M = 2.5 ± 1.6; arousal M = 5.8 ± 2.2) were chosen based on normative ratings (Lang et al., 1997). Both sets of pictures (neutral and negative) were matched for general content and their mean arousal and valence ratings were comparable to those reported in previous studies of instructed reappraisal (e.g. Ochsner et al., 2002). All negative pictures depicted complex scenes of burn victims, funeral scenes, people crying and dead animals, which are thought to consistently evoke negative emotions and brain activation in the limbic and paralimbic regions (Phan et al., 2002).
Procedure
The fMRI paradigm involved three main conditions, Neutral (viewing of a neutral picture), Negative (viewing of a negative picture) and Reappraise (reinterpretation of a negative picture), with the first one serving as a control condition. Each trial began with a photo in the center of a black screen, for 2 s, with the instruction VIEW displayed in white letters underneath. During this period subjects were to view the photo and allow themselves to naturally experience any emotional response to it. The photo remained on the screen for an additional 4 s, and the instruction underneath changed from VIEW to either ATTEND (Neutral and Negative trials) or REINTERPRET (Reappraise trials). On Attend trials, either a negative or a neutral photo was presented, and subjects were instructed to attend and naturally experience any feelings elicited by the photo. On Reappraise trails, a negative photo was shown and subjects were instructed to reinterpret its content so that it no longer elicited a negative response. Immediately after, the photo disappeared and, for 3.1 s, subjects saw a black screen where they could continue attending to, or reinterpreting, any feelings that remained after its presentation. A four-point scale was then presented for 3 s, for the subjects to rate the strength of their current negative affect (1 = weak, 4 = strong) using a four-button response box. Finally, an instruction to RELAX in white letters appeared in the center of a black screen for 5 s. A 900 ms interval separated each trial. Hence, the experimental paradigm comprised a total of 66 trials of 18 s, interleaved with four 20 s rest trials consisting of a white fixation cross presented in the center of a black screen. Trial order (Neutral, Negative, Reappraise) was counterbalanced, over two separate runs. Pictures of negative valence were randomly allocated to both negative conditions (Negative, Reappraise) across subjects.
fMRI methods
Scanning
Imaging was performed on a 3-T Philips Intera MR scanner (Philips Medical Systems, Best, The Netherlands). Functional MRI data comprised 634 volumes acquired with T2-weighted gradient echo-planar imaging (EPI) sequences, using a sense-8 head coil, in two functional runs of 317 volumes. Thirty-seven echo planar images per volume sensitive to blood oxygenation level-dependent (BOLD) contrast were obtained (TR = 2000 ms, TE = 35 ms, flip angle = 70°, in-plane resolution = 3.5 × 3.5 mm). Slices were acquired interleaved and parallel to the AC-PC line, with a thickness of 3.5 mm and no gap. High-resolution T1-weighted 3D fast-field echo (FFE) sequences were obtained for anatomical reference (160 slices, TR = 25 ms, TE = 4.6 ms, slice thickness = 1 mm, flip angle = 30°, matrix = 256 × 256, voxel size = 1 × 1 × 1 mm).
fMRI preprocessing and analysis
Data were preprocessed and analyzed using Statistical Parametric Mapping software SPM5 (http://www.fil.ion.ucl.ac.uk), implemented in Matlab (The Mathwork Inc.). Standard pre-processing was applied, with slice time correction, and realignment to the first volume to correct for insterscan motion artifacts. After realignment, a mean EPI image was created, which was co-registered with the structural T1 image. Subsequently, images were spatially normalized to the standard stereotactic space defined by the Montreal Neurological Institute (MNI) template. Functional images were then spatially smoothed with a 3D isotropic 8-mm full-width/half-maximum (FWHM) Gaussian kernel to minimize noise and residual differences in gyral anatomy. Low-frequency noise was removed by applying a high-pass filter (cut-off of 128 s) to the fMRI time series at each voxel. Significant hemodynamic changes for each condition were examined using the General Linear Model. Statistical parametric maps for each contrast of the t-statistic were calculated on a voxel-by-voxel basis. Effects were modeled using a boxcar convolved with a canonical hemodynamic response function for the 4 s trial epoch during which participants reinterpreted or attended each picture.
BOLD response during reappraisal
To confirm that the experimental task induced reappraisal-related activation at a group level, a one-sample t-test was designed with the individual images from Reappraise > Negative contrast. Activations were considered significant after P < 0.05 False Discovery Rate (FDR) correction for multiple comparisons across the whole brain. We performed whole-brain analysis following the objective to replicate previous studies that have successfully identified reappraisal-related brain regions, in order to subsequently explore their relationship with dispositional mindfulness in a voxel-wise regression analysis.
BOLD response in amygdala to negative stimuli
Given our a priori hypothesis that the amygdala’s response to negative photos would be down-regulated during reappraisal, we performed an exploratory analysis on the amygdala as the functional region of interest (ROI) by applying a pre-defined anatomical mask as provided by the Anatomical Automatic Labeling (AAL) software comprising the amygdala bilaterally, using the WFU-PickAtlas toolbox in SPM5. Effects were considered significant after P < 0.05 FDR correction for multiple comparisons.
BOLD response during reappraisal associated with dispositional mindfulness
Next, to examine the relationship between individual differences in mindfulness traits and the brain activity associated with reappraisal, we carried out a voxel-wise regression analysis using the individual t-maps from the Reappraise > Negative contrast, and KIMS scores as covariate of interest. Given our a priori hypothesis that DMPFC activation elicited during reappraisal would be related to trait mindfulness based on prior literature on meditation and mindfulness (Creswell et al., 2007; Lutz et al., 2008), we performed a ROI analysis by applying a pre-defined anatomical mask as provided by the AAL software, comprising the superior medial frontal gyrus bilaterally, using the WFU-PickAtlas toolbox. Correlations were considered significant after FDR correction for multiple comparisons (P < 0.05).
Behavioral data analysis, correlations between reported emotion experience (ratings) and brain activity, as well as corrections for possible influences of outliers, were carried out in SPSS 14.0 (Chicago, IL, USA). We set the level of significance at <0.05, two-tailed.
RESULTS
Behavioral data
Analysis of reported emotion experience showed stronger negative emotion after Negative trials (M = 2.7 ± 0.4) and diminishment of this affect after Reappraise trials (M = 1.9 ± 0.4), F(1,34) = 28.82, P < 0.001. This significant difference was used as an index for the success of the emotion regulation strategies, as done in previous studies (e.g. Ochsner et al., 2002). Accordingly, both emotion induction and reappraisal were attained on the behavioral level. A ‘reappraisal success’ score was computed subtracting ratings after Reappraise from those after Negative trials (e.g. Wager et al., 2008). Correlation analyses revealed a positive correlation between dispositional mindfulness scores and reappraisal success (Pearson’s r = 0.646, P = 0.005, two-tailed). Correlations for each subscale of the KIMS were as follows: ‘Describe’, r = −0.137, P = 0.601; ‘Observe’, r = 0.361, P = 0.155; ‘Act with awareness’, r = 0.515, P = 0.034; ‘Accept without judgment’, r = 0.250, P = 0.332. There were no significant gender differences in KIMS scores [F(1,16) <1, ns], or in reappraisal success [F(1,16) <1, ns].
Imaging data
BOLD response in reappraisal-related regions
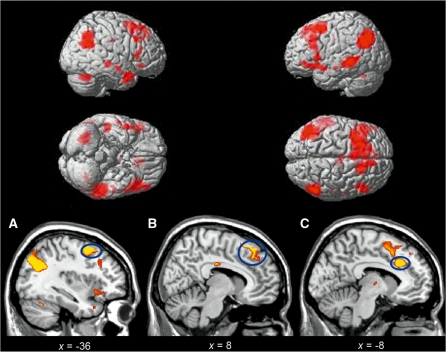
Subtracting activity associated with attending to negative stimuli from that associated with reappraisal (Reappraise > Negative) confirmed significant activation in left dorsolateral PFC (DLPFC) and right DMPFC, bilateral inferior frontal gyrus (IFG) and dorsal ACC. Additional activations included the middle temporal gyrus, angular gyrus and cerebellum (Table 1 and Figure 1). The left and right dorsal PFC activity seen in this contrast was significantly correlated with the reappraisal success (left, Pearson’s r = 0.500, P = 0.034; right, r = 0.472, P = 0.048; two-tailed), in line with previous research (Ochsner et al., 2002; Wager et al., 2008).
Table 1.
Areas with statistically significant blood-oxygenation-level-dependent (BOLD) activation during reappraisal of negative affect
| Hemisphere | Brain region | Coordinates x, y, z MNI | T-value | Z-value | ||
|---|---|---|---|---|---|---|
| Left | Middle frontal gyrus (DLPFC) | −36 | 6 | 52 | 6.62 | 4.60 |
| Right | Superior medial gyrus (DLPFC) | 8 | 34 | 42 | 4.46 | 3.58 |
| Left and | Inferior frontal gyrus | −50 | 40 | −6 | 5.25 | 3.85 |
| Right | 44 | 22 | 8 | 4.83 | 3.78 | |
| Left | ACC | −8 | 26 | 32 | 5.54 | 4.13 |
| Left and | Middle temporal gyrus | −62 | −34 | −6 | 6.00 | 4.34 |
| Right | 50 | −24 | −8 | 5.22 | 3.98 | |
| Left and | Angular gyrus | −42 | −66 | 36 | 6.62 | 4.59 |
| Right | 54 | −54 | 26 | 5.79 | 4.25 | |
| Right | Cerebellum | 32 | −64 | −30 | 4.56 | 3.64 |
Clusters of 20 or more contiguous voxels whose global maxima survived FDR correction for multiple comparisons across the whole brain, P < 0.05. DMPFC = dorsomedial prefrontal cortex. DLFPC = dorsolateral prefrontal cortex. MNI = Montreal Neurological Institute.
Fig. 1.
Statistical parametric maps during response Reappraisal > Negative. (A) DLPFC, (B) DMPFC, (C) dorsal ACC. All activations are reported after FDR correction for multiple comparisons across the whole brain (P < 0.05). Coordinates for activated regions are presented in Table 1.
BOLD response in amygdala to negative stimuli
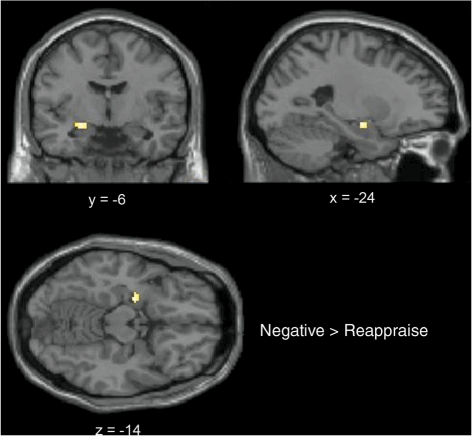
The exploratory analysis using a pre-defined anatomical mask showed significant activation of the left amygdala. The peak of activation was centered at −24, −6, −14, encompassing 28 voxels, T-value = 3.43, Z-value = 2.95, P < 0.05 FDR corrected) (Figure 2).
Fig. 2.
Group-averaged cluster of activation in the left amygdala in the contrast Negative > Reappraise, shown on the coronal, axial and sagittal planes. Activation is reported after FDR correction for multiple comparisons (P < 0.05).
BOLD response during reappraisal associated with dispositional mindfulness
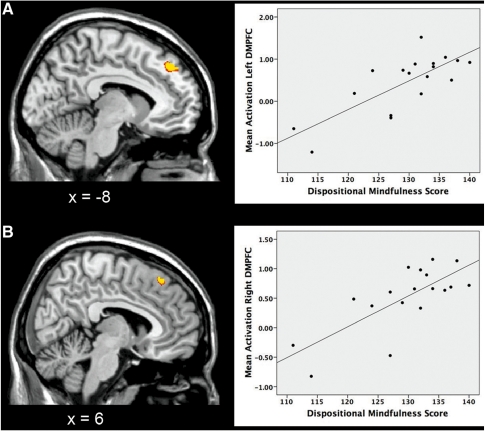
As hypothesized, we observed a positive correlation between individual differences in mindfulness traits and BOLD response in our prefrontal ROIs, bilaterally (Brodmann’s areas (BA) 8/9) (Table 2 and Figure 3). For completeness, we applied Cook’s D-test in order to rule out the influence of potential outliers. This showed that the correlations remained significant after removing the outliers (P < 0.05, two-tailed; one outlier detected for each region). Furthermore, the DMPFC activity was positively correlated with reappraisal success (left, Pearson’s r = 0.510, P = 0.030; right, r = 0.501, P = 0.034; two-tailed).
Table 2.
Areas with statistically significant blood-oxygenation-level-dependent (BOLD) activation during reappraisal that were modulated by individual differences in dispositional mindfulness
| Hemisphere | Brain region | Coordinates x, y, z MNI | T-value | Z-value | ||
|---|---|---|---|---|---|---|
| Left and | Superior medial gyrus | −8 | 42 | 44 | 5.87 | 4.23 |
| Right | (DMPFC) | 6 | 34 | 54 | 5.10 | 3.88 |
Clusters of five or more contiguous voxels whose global maxima survived the false discovery rate correction for multiple comparisons, P < 0.05. DMPFC = dorsomedial prefrontal cortex; MNI = Montreal Neurological Institute.
Fig. 3.
Positive correlation between brain activity in our prefrontal regions of interest during reappraisal and individual differences in dispositional mindfulness. (A) Left and (B) right DMPFC. Each point in the scatterplots represents data for a single subject. All activations are reported after FDR correction for multiple comparisons (P < 0.05). Coordinates for significantly correlated regions are presented in Table 2.
We followed up these significant associations by testing for correlations between the individual activation parameters in each of these regions and the four subscales of the KIMS (Describe, Observe, Act with awareness and Accept without judgment). There were no significant correlations, indicating that it was rather the total dispositional mindfulness score that accounted for the differential activation observed in the right DMPFC.
To further characterize the increases in prefrontal activation during reappraisal that were modulated by individual differences in mindfulness traits, we related the extracted mean parameter estimates from the peak foci/voxel in the prefrontal ROIs to those in the left amygdala (from the Negative > Reappraise contrast). This analysis showed that the right DMPFC activity increase was inversely correlated with the left amygdala activity in response to Negative>Reappraise (Pearson’s r = −0.495, P = 0.037, two-tailed), indicating greater down-regulation of activity in emotion-experience regions with more mindfulness traits. In sum, this suggests lessened reactivity due to better recruitment of prefrontal resources.
DISCUSSION
The present study revealed that individual differences in the disposition to be mindful modulated brain activity in cortical regions involved in the cognitive control of emotion. Thus, the ability to be open to and detach from current affective experience was associated with DMPFC activity during instructed reappraisal, which was in turn inversely correlated with the amygdala response to negative stimuli. Our results support the notion that reappraisal operates through interplay between frontolimbic brain regions (Ochsner and Gross, 2005), and suggest that individual differences in typical emotion regulation as indexed by mindfulness traits may influence these interactions.
Activity in reappraisal-related regions
On the behavioral level, reported emotion experience confirmed that participants experienced significantly less negative affect after reappraisal trials. On the brain level, as predicted, reappraisal induced activation in a predominantly frontal network of brain regions encompassing the DLPFC and DMPFC, including the ACC.
Our findings are consistent with prior studies on the down-regulation of negative emotion that have repeatedly indicated a prominent involvement of the PFC, especially its dorsal medial and lateral portions (Ochsner et al., 2002, 2004; Goldin et al., 2008; Wager et al., 2008). In particular, lateral PFC portions have been related to mediating interactions between self-referential processing and higher-order processing (Northoff et al., 2006). Aside from reappraisal, the DMPFC has been traditionally associated with evaluation of self-referential stimuli (Gusnard et al., 2001) and judgment of emotional stimuli (Northoff et al., 2004), processes on which subjects may presumably draw when reappraising their own negative emotions. Some of the pictures used in the present study depicted other people in suffering that would tap into theory of mind processing, in which the dorsal PFC also plays a critical role (Frith and Frith, 2003; Northoff and Bermpohl, 2004). As for the dorsal ACC, this region has been involved in monitoring and control functions primarily associated with self-referential stimuli, regardless of the sensory modality of the task involved (Northoff and Bermpohl, 2004). The dorsal ACC was also engaged during reappraisal in the present study, supporting its role in cognitive control (Mohanty et al., 2007) and emotion regulation (Lane et al., 1998).
Amygdala response to negative stimuli
Increased left amygdala activity was identified when attending to negative pictures compared to reappraisal. The amygdala was not significantly activated during reappraisal even when lowering the threshold at a much lenient level (P < 0.001 uncorrected). This finding supports the notion that the natural amygdala response to negative stimuli is down-regulated during reappraisal of negative affect (Ochsner et al., 2002, 2004; Schaefer et al., 2002; Kim and Hamann, 2007; van Reekum et al., 2007).
Neural activity during reappraisal associated with dispositional mindfulness
The tendency to engage in self-regulatory processes as indexed by individual differences in mindfulness traits was significantly associated with brain activity in the DMPFC. Of note, the regions of peak activation reported herein were anatomically close to those reported in prior reappraisal studies (Ochsner et al., 2002, 2004; Hutcherson et al., 2005; Goldin et al., 2008). Current conceptualizations of emotion regulation propose that instructed and spontaneous processes, although empirically separable, are likely to interact both during an emotional experience and over longer periods of time (Jackson et al., 2003). Our results may be related to recent findings on the neural correlates of individual differences in spontaneous reappraisal during a pure reactivity task (Drabant et al., 2009), while extending them to neural activity elicited during instructed reappraisal use, and to individual differences in the tendency to be mindful. This was further supported by the positive correlation between reappraisal success and mindfulness traits. Furthermore, it appeared that the ‘Act with awareness’ subscale was significantly contributing to this association.
Our results suggest how dispositional mindfulness might be related to brain activity. The prefrontal area on the right hemisphere (BA8) whose activity correlated with dispositional mindfulness is also known for its role in attention and working memory (Smith and Jonides, 1999). Mindfulness-based strategies have been indicated to modulate attentional systems (Jha et al., 2007). Prefrontal activity has been previously associated with mindfulness training (Farb et al., 2007; Siegel, 2007), as well as with meditation practices in general (Cahn and Polich, 2006), and with individual differences in mindfulness traits (Creswell et al., 2007). Since tasks that require sustained attention are thought to involve the PFC and ACC, increased activity in these regions would be associated with meditation as it entails focusing attention (Newberg and Iversen, 2003). The tendency to be mindful can be trained through mindfulness-based training, and a number of studies have reported its benefits for the enhancement of psychological well-being (Brown and Ryan, 2003), as well as for the treatment of psychological disorders involving deficits in emotion regulation (Baer, 2003). Thus, our findings tentatively suggest a neural mechanism by which mindfulness-based strategies may help promote psychological well-being, in light of the relevance of effective emotion regulation for mental health (Gross and Munoz, 1995).
The amygdala response to negative pictures was inversely correlated with the DMPFC region whose activity was associated with dispositional mindfulness. The concept that reappraisal is accomplished through frontolimbic interactions has been supported by brain connectivity analysis. A recent study by Wager and colleagues showed that reappraisal success might operate through fronto-subcortical pathways, by which regulatory effects from prefrontal regions to the amygdala are exerted when decreasing negative affect (Wager et al., 2008). We found a positive correlation between dispositional mindfulness and reappraisal success, indicating that more mindful individuals reported more reappraisal success. With regard to mindfulness strategies, prior studies have suggested that these practices would exert regulatory influences from prefrontal regions to attenuate the response in limbic regions in order to disengage from aversive emotional stimuli following mindfulness training (Lutz et al., 2008). As mentioned earlier, the DMPFC is critical in self-reflective thought and judgments that depend on inferred social and emotional content (e.g. Gusnard et al. 2001; Kelley et al. 2002). Furthermore, this region has been implicated in meta-cognitive tasks that require thinking about and rating one’s own feelings (Amodio and Frith, 2006). Together with these previous findings, and in light of our findings of an inverse relationship between activity in DMPFC and activity in the amygdala, the results presented herein suggest that individuals with more mindful traits showed increased modulation of frontolimbic networks. That is, activity in DMPFC regions that are thought to support meta-cognitive reflective awareness of internal states (Olsson and Ochsner, 2008) is associated with the ability of being more aware of and detached from current experience, as indexed by mindfulness skills.
The present study sought to examine specific associations between individual differences in mindfulness traits and DMPFC activation, based on prior neuroimaging literature on mindfulness and meditation (Creswell et al., 2007; Lutz et al., 2008). We report that mindfulness influenced the DMFPC response during reappraisal, which in turn was negatively associated with the amygdala reactivity to negative stimuli. The amygdala response was not directly related to trait mindfulness, as assessed by Pearson’s product–moment correlation between KIMS scores and the parameter estimates extracted from the amygdala (alpha level set at <0.05, two-tailed). Further research with functional connectivity measures may help expand the nature of the DMPFC–amygdala–mindfulness relationship, insofar as the other structures, such as the ACC, could also have a role in mediating the observed associations. Evidence from animal work suggests that the DMPFC may have connections to the rodent amygdala, where the DMPFC exerts an up-regulatory effect on amygdala activity during fear response (e.g. Burgos-Robles et al., 2009). Mapping rodent anatomy to the human brain is not straightforward (see, e.g. Milad et al., 2007). However, activity in DMPFC could exert down- or up-regulatory effects on the amygdala depending on the context. In line with this is a recent study (Ochsner et al., in 2009) showing that DMPFC activity increases when individuals generate negative appraisals about neutral images. Therefore, the content of the representations processed could have up- or down-regulatory effects, depending on the context. In the context of the present study, in which DMPFC seemed to be important for processes related to mindfulness, more mindful individuals may be better at deploying effective top–down appraisals that down-regulate amygdala activity.
Interpretational limitations
The present study had some limitations. The study could have benefited from the inclusion of another measure of automatic reappraisal to support the premise that subjects with more mindful traits do engage in reappraisal more frequently. We based our assumptions on the literature on mindfulness and the implications of being more mindful, which strongly indicate that they imply being more aware and regulative of current affective states (Brown and Ryan, 2003), thus theoretically related to how emotion regulation processes operate. Further supporting this idea, dispositional mindfulness was positively associated with reappraisal success. The present study did not include a measure of general psychological adjustment, which could in part influence the results as it tends to be significantly correlated with dispositional mindfulness. Finally, the sample size in the present study was relatively small. Potential limitations on inference with sample sizes of the sort that are used in fMRI studies must be acknowledged. However, individual differences in mindfulness scores were clearly present between our subjects as may be seen in Figure 3, and their effect on brain activity was statistically significant after correction for multiple comparisons was applied in all analyses. Furthermore, recent reviews have stated that typical fMRI sample sizes (N = 15–20) will only rarely produce statistically significant correlations in the absence of any true effect. Thus, correlations identified with similar fMRI studies are likely to represent true underlying relationships between brain activity and psychological variables (Lieberman et al., in 2009) and the formulation of prior anatomical hypotheses limit false positive findings, as done in the present study.
CONCLUSIONS
These results suggest that individual differences in dispositional mindfulness are positively associated with brain activity in the DMPFC during reappraisal of negative emotion. This prefrontal activity was shown to be involved in down-regulating activity in emotion-generation regions by its inverse association with the amygdala response to negative stimuli, as reported in prior studies on the reappraisal of a negative effect. Our results align with the notion that individual differences in processes involving emotion regulation may influence the neural dynamics underlying the cognitive control of emotion, and extend this idea to individual differences in dispositional mindfulness.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Kevin Ochsner for the critical comments and helpful discussions, and Anita Kuiper for assistance with MRI scanning. This work was supported by European Science Foundation EURYI grant [Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) number 044035001 to A.A.].
REFERENCES
- Amodio D, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews. Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Baer RA. Mindfulness training as a clinical intervention: a conceptual and empirical review. Clinical Psychology: Science and Practice. 2003;10:125–43. [Google Scholar]
- Baer RA, Smith GT, Allen KB. Assessment of mindfulness by self-report: the Kentucky inventory of mindfulness skills. Assessment. 2004;11:191–206. doi: 10.1177/1073191104268029. [DOI] [PubMed] [Google Scholar]
- Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13:27–45. doi: 10.1177/1073191105283504. [DOI] [PubMed] [Google Scholar]
- Brown KW, Ryan RM. The benefits of being present: mindfulness and its role in psychological well-being. Journal of Personality and Social Psychology. 2003;84:822–48. doi: 10.1037/0022-3514.84.4.822. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. Journal of Neuroscience. 2009;29:8474–82. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn BR, Polich J. Meditation states and traits: EEG, ERP, and Neuroimaging studies. Psychological Bulletin. 2006;132:180–211. doi: 10.1037/0033-2909.132.2.180. [DOI] [PubMed] [Google Scholar]
- Creswell JD, Way BM, Eisenberger NI, Lieberman MD. Neural correlates of dispositional mindfulness during affect labeling. Psychosomatic Medicine. 2007;69:560–5. doi: 10.1097/PSY.0b013e3180f6171f. [DOI] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. 2009;65:367–73. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith U, Frith CD. Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London: Series B: Biological Sciences. 2003;358:459–73. doi: 10.1098/rstb.2002.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: past, present, future. Cognition and Emotion. 1999;13:551–73. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85:348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Munoz FR. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–64. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self/referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:4259–62. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas BW, Omura K, Constable RT, Canli T. Is automatic emotion regulation associated with agreeableness? A perspective using a social neuroscience approach. Psychological Science. 2007;18:130–2. doi: 10.1111/j.1467-9280.2007.01861.x. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ. Attention and emotion: does rating emotion alter neural responses to amusing and sad films? Neuroimage. 2005;27:656–68. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Jackson DC, Mueller CJ, Dolski I, et al. Now you feel it, now you don’t: frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science. 2003;14:612–7. doi: 10.1046/j.0956-7976.2003.psci_1473.x. [DOI] [PubMed] [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive Affective and Behavioral Neuroscience. 2007;7:109–19. doi: 10.3758/cabn.7.2.109. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 1998;10:525–35. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainsville, FL: NIMH Center for the Study of Emotion and Attention, University of Florida; 1997. [Google Scholar]
- LeDoux JE. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York: Simon and Schuster; 1996. [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Berkman ET, Wager TD. Correlations in social neuroscience aren't; voodoo: A reply to Vul et al. Perspectives on Psychological Science. 2009;4:299–307. doi: 10.1111/j.1745-6924.2009.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends in Cognitive Sciences. 2008;12:163–9. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulated cortex in fear expression. Biological Psychiatry. 2007;62:1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional functions. Psychophysiology. 2007;44:343–51. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Newberg AB, Iversen J. The neural basis of the complex mental task of meditation: neurotransmitter and neurochemical considerations. Medical Hypotheses. 2003;61:282–91. doi: 10.1016/s0306-9877(03)00175-0. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, Bermpohl F, et al. Reciprocal modulation and attenuation in the prefrontal cortex: an fMRI study on emotional-cognitive interaction. Human Brain Mapping. 2004;21:202–12. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. Cognitive emotion regulation: insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–8. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:121529. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray R, Robertson E, Cooper J, Gross JJ, Gabrieli J.DE. Bottom-up and top-down processes in emotion generation. Psychological Science. 2009;20:1322–31. doi: 10.1111/j.1467-9280.2009.02459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson A, Ochsner KN. The role of social cognition in emotion. Trends in Cognitive Sciences. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience. 2002;14:913–21. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Siegel DJ. Mindfulness training and neural integration: differentiation of distinct streams of awareness and the cultivation of well-being. Social Cognitive and Affective Neuroscience. 2007;2:259–63. [Google Scholar]
- Smith E, Jonides J. Storage and executive processes in the frontal lobes. Science. 1999;283:657–60. doi: 10.1126/science.283.5408.1657. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends of Cognitive Sciences. 2007;11:413–8. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Van Reekum CM, Urry HL, Johnstone T, et al. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience. 2007;19:237–48. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]