Abstract
Natural killer (NK) cells have been implicated in tumour surveillance and in the early control of several microbial infections. In autoimmune disease their involvement in these processes has been evaluated in animal models, with conflicting results. Both a disease-controlling and a disease-promoting role have been suggested. In human autoimmune disease only a few studies, mainly descriptive, have demonstrated qualitative and quantitative modification of NK cells. These changes were observed on blood- or tissue-infiltrating NK cells. Taken together with our expanding knowledge of the genetical variability of NK cell receptors and NK cell physiology, these findings pave the way for the dissection of the role of NK cells in human autoimmune diseases. NK cells may be directly involved in these diseases through their potential autoreactivity or through their interaction with dendritic cells, macrophages or T lymphocytes, thereby inducing excessive inflammation or favouring the adaptive autoimmune response. Thus, NK cells may be implicated in the onset, the maintenance or the progression of autoimmune diseases. Some reports also suggest the involvement of NK cells in the treatment of human autoimmune disease by biotherapies. All these observations suggest that NK cells are involved in the complex processes of autoimmune diseases. Nevertheless, further careful analysis of NK cells at different steps of these diseases, in different tissues and through combined genetical and functional studies will contribute to a better understanding of their role in autoimmune diseases. This knowledge might allow the development of new therapeutic strategies based on NK cells for the treatment of some autoimmune diseases.
Keywords: autoimmune diseases, innate immunity, natural killer cells, systemic lupus erythematosus
Introduction
The treatment of individuals with human autoimmune diseases and the achievement of improvements in their quality of life remain a challenge in 2010, despite our increasing knowledge of the immunological basis of these diseases. Several new molecules, in particular monoclonal antibodies targeting lymphocyte subsets or cytokines, have recently resulted in clear benefits for these patients. Identification of new cellular or molecular targets should allow new therapeutic improvements. Studies on NK cells and NK cell receptors are therefore of great interest.
Autoimmune diseases can be divided into two categories: tissue-specific autoimmune diseases (e.g. diabetes mellitus or multiple sclerosis) and systemic autoimmune diseases (e.g. systemic lupus erythematosus). Despite great diversity in clinical and biological symptoms, they all share the presence of an adaptive B-cell (auto-antibody) and/or T-cell (autoreactive T-cell) response against self. For the majority of these diseases, the auto-antigens have been identified and are the target of this acquired autoimmune ‘response’. Some studies have shown that the acquired B- or T-cell autoimmune response arises a long time before clinical symptoms appear.1 This indicates that the development of an autoimmune disease is a several-year-long ‘multi-step’ process. This process is under the control of the genetic background and as yet poorly defined environmental factors.2 In such a dynamic process the involvement of NK cells should be investigated at both preclinical and clinical stage. Most of our knowledge of NK cells in human autoimmune diseases comes from analyses of NK cells from patients in the clinical stages of disease and is merely correlative.
NK cells have been first described by their ability to kill leukaemic cells without prior sensitization. They represent a small proportion of blood lymphocytes and do not express a specific receptor for antigens, as do T and B cells. NK cells have been implicated in tumour surveillance and in the early control of microbial infections, including infections with viruses (such as herpesvirus) and intracellular parasites (such as Plasmodium falciparum). Their natural cytotoxicity is under the control of natural cytotoxicity receptors (NCRs) and their antibody-dependent cytotoxicity is linked to the engagement of CD16/FcγRIIIa.3 In addition to NCRs, NKG2D activating NK receptor (NKG2D), DNAX accessory molecule-1 and NK receptor of 80kDa are also activatory receptors and some of their ligands are expressed under inflammatory conditions and have been implicated in autoimmune diseases. The identification of a large multigenic and multiallelic family of cell surface receptors specific for human leucocyte antigen (HLA) class I molecules [the killer-cell immunoglobulin-like receptors (KIRs)4] which control NK cell activation has improved our knowledge on the association of genetic background and autoimmune disease. Most studies on KIRs have been based on the missing self theory, and have investigated variation in KIR genotypes together with HLA class I gene polymorphism.
Human NK cells are usually characterized as CD3− CD56+ cells. Blood NK cells can be divided into two major subtypes, CD56bright and CD56dim.5 The former subtype represents about 10% of circulating NK cells; these cells express low levels of CD16 and perforin, produce high amounts of cytokines in response to cytokines such as interleukin (IL)-12 and IL-18, and represent the major fraction of NK cells in lymph nodes.6 CD56dim NK cells express high levels of CD16, perforin and KIRs and can be cytotoxic as well as being cytokine producers.
In studies of mouse models of autoimmune diseases, it has been shown that NK cells have either a disease-promoting or a disease-controlling role.7 Controversial findings regarding NK cell depletion in the experimental autoimmune encephalomyelitis model have complicated the picture.8 These apparently conflicting results may be related to differences in the strains and model systems used. Divergent effects of specialized NK cell subsets have also been suggested to explain these discrepancies. Studies in humans have also produced some contradictory results, although some of these results should be treated with caution as the distinctions among NK, NKT and T cells expressing NK cell receptors were not always clearly addressed. In this review, we focus on current knowledge regarding NK cells and NK cell receptors in human autoimmune diseases using the example of systemic lupus erythematosus (SLE), a prototypic human autoimmune disease.
Observed changes in NK cells in human autoimmune diseases
Changes in circulating blood NK cells
Quantitative and qualitative NK cell variations have been reported in human autoimmune diseases. In several cases of autoimmune disease, a reduction in the number of circulating NK cells has been shown.9–13 This quantitative defect is usually paralleled by a decrease in NK cell cytotoxicity.9,14 This defect gave rise to the hypothesis that circulating NK cells are involved in the control of the autoimmune reaction, as has been shown for regulatory T cells. It could also be interpreted as a consequence of chronic NK cell activation associated with excessive NK cell apoptosis. A decrease in NK cell differentiation from haematopoietic stem cells has also been proposed to explain in part the NK cell deficiency.14 Very few changes in NK cell receptor expression have been found in these autoimmune diseases, and these changes do not seem to explain the functional deficiency observed.12,15,16 The findings of these studies underline the difficulties inherent in correlating quantitative and qualitative NK cell variations with autoimmune disorders and in distinguishing between a causative role and a consequence of the disease or associated treatments. For example, severe varicella was reported to be associated with profound NK cell deficiency, which was first thought to be causative but finally found to be transiently caused by the infection.17
Intriguingly, NK-lymphoproliferative disease of granular lymphocytes (LDGL), a rare condition in which there is a chronic excess of circulating NK cells, is also associated with autoimmune manifestations.18,19 In this chronic haematological disease, NK cell cytology is normal but NK cells show profound changes in their inhibitory and activating receptors.20–23 This condition, which is very similar to the T-cell phenotype of LDGL, suggests that NK cell proliferation in humans may ‘induce’ autoimmune diseases. However, the mechanism by which NK-LDGL induces tissue or cell injury has not been elucidated.
Changes in tissue NK cells
NK cells are abundant in the human liver and uterus. Both liver and uterine NK cells are specialized NK cells and differ from circulating blood NK cells. Uterine NK cells are the most important uterine lymphocyte subset during pregnancy.24 Thus, the roles of uterine NK cells in pregnancy, and particularly in manifestations of autoimmune diseases during pregnancy, have been explored. In women with recurrent miscarriage associated with autoimmune diseases, changes in the NK cell compartment have been demonstrated for blood but not tissue NK cells. In contrast to blood NK cells, the roles of tissues NK cells have not been systematically explored in autoimmune diseases. Such studies were hampered for a long time by the lack of specific NK cell markers. However, in inflammatory myopathies, autoimmune diseases characterized by muscle injuries secondary to muscle infiltration by inflammatory cells and an increase in tissue HLA class I expression, NK cells were not found.25 In type I diabetes, NK cells were found to be localized around the pancreatic islets in recently diagnosed patients.26 In a larger post-mortem series, pancreas-infiltrating NK cells were not found.27 This suggests that NK cells could participate in the destruction of pancreatic beta cells before the clinical onset of diabetes.
In rheumatoid arthritis (RA), an autoimmune disease characterized by joint inflammation, synovium-infiltrating NK cells have been documented.28 These NK cells are CD56bright and produce more interferon (IFN)-γ than blood NK cells from the same patients.13 Moreover, these NK cells can induce the differentiation of monocytes into dendritic cells (DCs). Thus, in RA tissue, NK cells are characterized by disease-promoting functions, which is in contradiction to findings that circulating NK cells in patients with RA are reduced in number and have reduced functions.13,14 Such observations underline the importance of studies on tissue NK cells in autoimmune disease in parallel with blood NK cell changes.
Genetic associations
Inhibitory and activating KIRs belong to a family of highly homologous receptors recognizing major histocompatibility complex (MHC) class I molecules. The genes coding for these receptors are all located on 19q13.4.29,30 Interindividual variability is explained by variation in gene number, depending mainly on the set of activating NK cell receptors, and allelic variability in KIR genes.4 This interindividual variation in genes and alleles coding for inhibitory and activating receptors for MHC class I molecules, mainly expressed by NK cells, has raised the question of a genetic KIR/HLA class I background for susceptibility to autoimmune disease. In type 1 diabetes mellitus, an association with the 2DS2/HLA-C1 genotype31 and a decrease in the proportion of inhibitory KIR/HLA genotype combinations were reported.31 In psoriatic arthritis, the 2DS1/2DS2 genotype and HLA-Cw homozygosity were associated with susceptibility to developing the disease.32,33 Similarly, systemic sclerosis was associated with the 2DS2+/2DL2− genotype34 and with the 2DS1+/2DS2− genotype.35 Moreover, KIR 2DS1+ patients with progressive systemic sclerosis displayed a significant decrease in the amount of inhibitory KIR corresponding to the appropriate HLA-C ligand.35 In a Canadian cohort of patients with SLE, an increase in the prevalence of the 2DS1+/2DS2− genotype was documented.35
Thus, genetic data obtained from KIR/MHC class I association studies have identified genotypes associated with enhanced susceptibility to the development of autoimmune diseases or their severe forms (Table 1). Most of these situations seem to be associated with a potential lack of NK cell inhibition or with excessive NK cell activation through MHC class I receptor signalling. These interesting results need to be confirmed in gain or loss of function studies. Moreover, genetic studies on KIR genes should focus not only on NK cells but also on other T-cell subsets expressing these receptors. This model has also to take into account KIR allelic variants and variegation of KIR distribution on NK cells for a given genotype.
Table 1.
Natural killer (NK) cell changes in blood or tissues and NK cell receptor genes associated with some autoimmune diseases in humans
| Autoimmune disease | Changes in circulating or tissue NK cells | Genetic susceptibility |
|---|---|---|
| SLE | Decreased number and functions | 2DS1+/2DS2− |
| Sjögren's disease | Decreased number | Not analysed |
| Systemic sclerosis | Decreased number and functions | 2DS1/2DL1 2DS1+/2DS2− |
| Antiphospholipid syndrome | Increased number | Not analysed |
| Pemphigus vulgaris | Increased number | Not analysed |
| Rheumatoid arthritis | Decreased number and functions in blood. Increased number in synovial fluid | Some association of KIR genes with clinical manifestations of rheumatoid arthritis. Association with KIR2DS4? |
| Spondylarthropathies | Increased frequency of KIR2DL3 expression | Association with 3DS1 and protective role of 3DL1 |
| Psoriasis/psoriatic arthritis | Decreased number and functions in blood. Increased number in synovial fluid | 2DS1/2DS2, HLA-Cw homozygosity |
| Ulcerative colitis | Unknown | KIR 2DL2/2DS2 frequency. Protective effect of KIR 2DL3 in the presence of its ligand |
| Diabetes | Infiltrating NK cells in pancreas? Decrease in NK cell number and functions in blood | |
| Multiple sclerosis | Association of an increase of a specific NK cell subset with the control of the disease |
HLA, human leucocyte antigen; KIR, killer-cell immunoglobulin-like receptor; SLE, systemic lupus erythematosus.
In addition to studies on KIR/HLA, there have been studies carried out showing clinical associations of autoimmune disease with the FcγRIIIa polymorphism. Polymorphism at position 158 results in either valine (V) or phenylalanine (F) expression of the FcγRIIIa receptor and has been reported to influence human IgG1 binding and antibody dependent cellular cytotoxicity (ADCC) activity. These results strongly suggests a role for NK cells because of their high level of expression of CD16 and their ADCC function. The FcγRIIIa F/F genotype is associated with SLE nephritis.36 Interestingly, genetic polymorphism in the FcγRIIIa gene have been also shown to influence the response to monoclonal therapeutic antibodies such as anti-CD20.37 In haematological malignancies NK cell participate to the therapeutic effect of CD20 depleting the B cell compartment through ADCC.38 The therapeutic use of these monoclonal antibodies has now been extended to the treatment of autoimmune diseases. This raises the question of the influence of quantitative and functional deficiencies in NK cells in the therapeutic response.
Hypothesis of a role of NK cells in autoimmune diseases
‘Rupture’ of NK cell tolerance
Rupture of NK cell tolerance to self can be brought about either by the removal of inhibitory signals or by the addition of excessive activating signals (Fig. 1a). In such a case, NK cells can be responsible for tissue injuries through the effects of their natural cytotoxicity. Patients with transporter associated peptide (tap) deficiency do not express MHC class I molecules and thus correspond to the model of the removal of inhibitory signals. These patients present severe tissue destruction usually following bacterial infections.39 It has been suggested that tissue injuries are at least in part attributable to NK cell cytotoxicity that is not inhibited by MHC class I recognition. However, the absence of MHC class I expression is not enough to induce NK cell cytotoxicity. For example, erythrocytes express very few MHC class I molecules at baseline level, and are not lysed by NK cells in normal conditions. This model is also in disagreement with the recent finding of MHC class I-guided NK cell education. MHC class I molecules influence NK cell education and tolerance by inducing responsiveness of the NK cell upon ligation of the cognate inhibitory receptor. This explains the fact that NK cells lacking the appropriate inhibitory receptor to self (a substantial number of NK cells in normal individuals) are self tolerant because of their hyporesponsiveness.40,41
Figure 1.
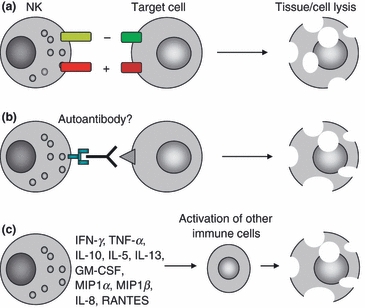
Mechanisms by which natural killer (NK) cells can induce tissue/cell injuries in autoimmune diseases. (a) NK cells can lyse target cells by natural cytotoxicity. Natural cytotoxicity is regulated by the balance of inhibitory [recognition of human leucocyte antigen (HLA) class I molecules] and activating signals. Both changes in major histocompatibility complex (MHC) class I recognition and the expression of activation ligands by the target cell can modulate NK cell cytotoxicity. For example, the expression of MHC class I polypeptide-related sequence (MIC) molecules that are ligands for the activating NKG2D receptor is induced in stressed cells in inflammatory conditions. (b) NK cells are responsible for antibody dependent cellular cytotoxicity. They can bind autoantibodies through the FcγRIIIa. (c) NK cells produce several cytokines. They can induce an inflammatory response and promote the recruitment and activation of immune cells in tissues. GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; MIP, macrophage inflammatory protein; TNF, tumour necrosis factor.
More evidence has been obtained for the disruption of NK cell tolerance by an excessive activatory signal. The ligands of the activating receptor NKG2D are the MHC class I polypeptide-related sequence A (MICA) and MICB proteins and the cytomegalovirus UL-16 binding protein. In the model of celiac disease (gluten-sensitive enteropathy, both the over-expression of MIC by the gut epithelium and local IL-15 production induce a local NKG2D-mediated lysis of gut epithelial cells by intra-epithelial lymphocytes (IELs) independently of T-cell receptor (TCR) specificity.42–44 High levels of MICA have also been found on intestinal epithelial cells from patients with Crohn's disease, and in serum from patients with RA. Thus, the expression of stress signals, such as MIC molecules recognized by NKG2D, can induce autoreactive cell cytotoxicity. Such ‘rupture of tolerance’ mediated by engagement of activatory receptors has been shown for T-cell subsets but not NK cells in human autoimmune diseases.
Control of the autoimmune response by NK cells
Innate immune cells participate in the early stages of immune defence. NK cells are present at inflammatory sites and in lymph nodes where they can co-operate with other immune cells.6 NK cells can also participate in the exposition of autoantigens through cell lysis. Thus, NK cells have been shown to initiate robust adaptive immune responses via killing of antigen-expressing target cells.45 Interaction through cell–cell contact between NK cells and DCs, influenced by the local cytokine environment, can lead to: (i) NK cell-mediated killing of immature monocyte-derived DCs (iDCs); (ii) DC-induced NK cell proliferation through IL-12 production; (iii) NK cell-dependent DC maturation. NK cells can discriminate between myeloid iDCs, which typically under-express MHC class I molecules, and mature DCs, which up-regulate MHC class I expression after antigen uptake.46 The killing of iDCs by NK cells has been interpreted as a control of the quality of DCs, allowing only mature DCs to migrate to the lymph node. Both NK cell deficiency, as observed in several autoimmune diseases, and activated NK cells can potentially modify their interaction with DCs and thus the specific T-cell response (Figs 2 and 3).
Figure 2.
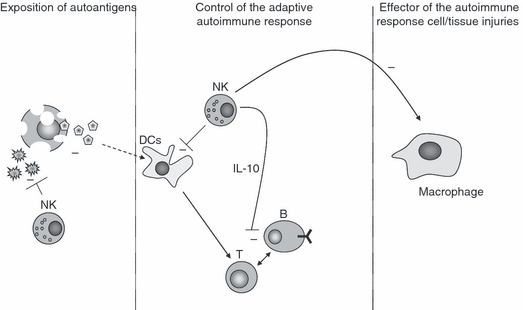
Hypothesis for the protective role of natural killer (NK) cells in autoimmune diseases. NK cells may participate in the control of an autoimmune disease at different stages. First, NK cells could control the release of autoantigens, for example by modulating the control of environmental factors such as viral infections. NK cells may also control the adaptive immune response through its interaction with dendritic cells (DCs) by killing immature DCs that present autoantigens. By producing interleukin (IL)-10, NK cells can also modulate the adaptive immune response. Finally, NK cells can control the activation of macrophages that are responsible for tissue injury through chronic inflammation.
Figure 3.
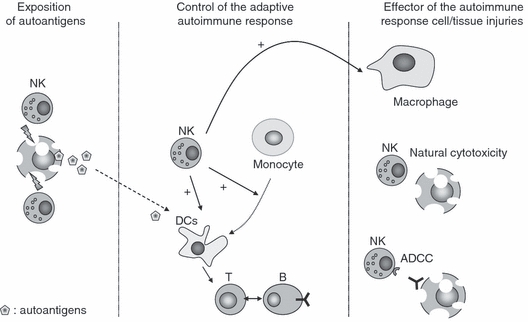
Hypothesis for the detrimental role of natural killer (NK) cells in autoimmune diseases. NK cells may participate in the induction, the maintenance of the autoimmune reaction and the direct cell or tissue injuries in autoimmune diseases. First, NK cell cytotoxicity can lead to abnormal exposure of autoantigens. Secondly, during the adaptive immune response they can induce the maturation of autoantigen-presenting dendritic cells (DCs) and induce the differentiation of monocytes into antigen-presenting cells in inflamed tissues such as the joints of patients with rheumatoid arthritis (RA). Finally, NK cells can directly promote cell or tissue injuries during chronic inflammation in autoimmune diseases by exerting natural cytotoxic effects against cells expressing activating ligands for natural cytotoxicity receptors (NCRs), by ADCC and indirectly through activation of macrophages. ADCC, antibody dependent cellular cytotoxicity.
CD56bright NK cells from patients with RA can induce monocytes to differentiate into DCs, which is initiated by local IL-15 production.47 These DCs are functional antigen-presenting cells and can promote CD4+ T-cell activation and their polarization to Th1 cells. These results suggest that NK cells can sustain joint inflammation in RA. NK cells also promote the Th1 polarization of CD4+ T cells through the production of IFN-γ.48
This picture is complicated by IL-10 production by NK cells under certain conditions.49 The production of the anti-inflammatory cytokine IL-10, in contrast to IFN-γ, can inhibit DCs. Thus, NK cells can also be considered immune regulatory cells that can dampen antigen presentation by antigen-presenting cells and the proliferation of T cells.50 Little is known about IL-10-producing NK cells in human diseases.
To date, the extremely rare cases of selective NK cell deficiencies in humans have not helped to elucidate in vivo the role of NK cells in the onset and/or progression of autoimmune diseases.51
NK cell control of macrophage activation
The rare disease haemophagocytic lymphohistiocytosisis (HLH) is characterized by an intrinsic defect of NK cell and T-cell cytotoxicity related to the perforin/granzyme release pathway. It is sometimes an acquired condition associated with autoimmune diseases and viral infections. In all cases, a marked decrease in NK cell numbers and functions is reported.52 This is associated with a decrease in cytotoxic T-cell counts, an increase in the numbers of some T cells and sustained macrophage activation, with the production of excessive amounts of IFN-γ, tumour necrosis factor (TNF)-α, IL-18, IL-1 and IL-6. Interaction between human NK cells and macrophages is bidirectional and can result in activation of NK cells or in the regulation of macrophage activity through direct killing of macrophages. For example, cytolysis of lipopolysaccharide-activated macrophages is triggered by the recognition of inducible NKG2D ligands by NK cells.53 In HLH, a defective NK cell compartment, reminiscent of observations in other autoimmune diseases, is associated with excessive activation of other immune cells (such as macrophages) capable of a deleterious inflammatory response. This observation suggests the hypothesis that defective NK cells can lead to abnormal inflammation.
The example of SLE, a prototypical autoimmune disease
SLE is characterized by a non-tissue-specific autoimmune reaction.54 Chronic inflammation can lead to organ damage with loss of function. It primarily affects young women and the chronic course of the disease is associated with flare-ups. SLE is characterized by polyclonal B-cell activation and the production of autoantibodies against several nuclear proteins and, more specifically, double-stranded DNA.54 Defective control of apoptosis is thought to induce this autoimmune response through an excessive exposition of nuclear autoantigens. Genetic associations of KIR genotype contents and FcγRIIIA polymorphism have been demonstrated in some SLE populations.35,36 In SLE, blood NK cells show a variable and usually moderate NK cell quantitative deficiency and a decrease in CD4+ CD25+ regulatory T cells.9–11,13 This alteration of NK cell function cannot be related only to the decrease in NK cell counts, as studies on sorted NK cells have confirmed a cytotoxic defect.9,14 Extensive phenotypic characterization of NK cells in SLE patients have shown only mild changes. In a Swedish cohort of lupus patients, an increase in the proportion of CD56bright NK cells was observed without a correlation with the severity of the disease.55 In this study, as in our study,15 the expression of the different NK inhibitory and activating receptors was not statistically different from that of the control group. In Sjögren's syndrome, an autoimmune disease similar to SLE and characterized by inflammation of the exocrine glands, as in RA, circulating NK cell counts were also shown to be reduced and associated with decreased cytotoxic functions.12,14 In SLE it has been shown that the quantitative NK cell deficiency correlates with some clinical manifestations such as nephritis and thrombocytopenia during flare-up of the disease.14 Thus it has been postulated that the decrease in NK cell number and function is implicated in the inflammatory process of SLE. However, this decrease could also be a consequence of the disease, through chronic NK cell activation leading to anergy, through relocalization of NK cells in tissues, or through a defect in NK cell differentiation. SLE NK cells express lower perforin levels (mRNA and protein), suggesting chronic activation. It has also been shown that blood or bone marrow haematopoietic stem cells (HSCs) of SLE patients have lower proliferative indices than those of controls. Moreover, these HSCs generated a lower proportion of NK cells when cultured with IL-15 as compared with the control HSCs.14 This suggests that both a ‘central’ and a ‘peripheral’ mechanism are implicated in the decrease in NK cell numbers in SLE.
However, the question of how NK cell deficiency participates in the control of the course of SLE remains completely unanswered. With regard to the key role of plasmacytoid DCs in SLE, it can be hypothesized that defective control of immature DCs by NK cells is involved.
Conclusion
While inappropriate activation of cells of the adaptative immune response was mainly reported in autoimmune diseases, numerous observations suggest a role for NK cells in these disorders. To date, NK cells have not been specifically targeted by treatments of autoimmune diseases. However, some biotherapies are associated with changes in the circulating NK compartment. For example, daclizumab (anti-IL-2Rα) in multiple sclerosis is associated with an increase in the number of CD56bright NK cells.56 The same change is observed in the treatment of uveitis with daclizumab.57 Interestingly, the expansion of blood NK cells has been found to be correlated with the suppression of disease activity, and NK cells isolated from patients during treatment were able to kill autologous-activated T cells.56 From another point of view, NK cells play an important role, through ADCC, in the efficacy of monoclonal antibodies used to treat human diseases.38 Thus, identifying the modifications of NK cells and the role of NK cells in autoimmune diseases will help to define new therapeutic targets and to optimize the efficiency of current biotherapies in such indications.
It has been suggested that NK cells have a disease-promoting or a disease-controlling role in autoimmune diseases, depending on the disease and the NK cell subset analysed. A disease-controlling role is clearly suggested by studies in SLE patients in which circulating NK cells are reduced in number and functions. These abnormalities have been shown to correlate with some clinical manifestations of the disease. Such modifications of NK cells mirror the protective role of NK cells proposed in the model of multiple sclerosis where an increase in the numbers of circulating NK cells correlates with remission of the disease. However, despite clinical correlative evidence, the mechanisms by which NK cells control autoimmune disease remain unknown. Moreover, it is difficult to reconcile the dual NK cell roles in different autoimmune diseases. Perhaps NK cells can have either a protective or a disease-promoting role in a given autoimmune disease at different stages of the disease (e.g. onset and/or progression) in a several-year-long process. Thus, forthcoming studies should focus on tissue NK cells and NK cell interactions with other immune cells at sites of inflammation at different phases of the development of autoimmune diseases.
References
- 1.Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–33. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 2.Shmerling RH. Autoantibodies in systemic lupus erythematosus--there before you know it. N Engl J Med. 2003;349:1499–500. doi: 10.1056/NEJMp038142. [DOI] [PubMed] [Google Scholar]
- 3.Moretta L, Moretta A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 2004;23:255–9. doi: 10.1038/sj.emboj.7600019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middleton D, Meenagh A, Moscoso J, Arnaiz-Villena A. Killer immunoglobulin receptor gene and allele frequencies in Caucasoid, Oriental and Black populations from different continents. Tissue Antigens. 2008;71:105–13. doi: 10.1111/j.1399-0039.2007.00973.x. [DOI] [PubMed] [Google Scholar]
- 5.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 6.Fehniger TA, Cooper MA, Nuovo GJ, Cella M, Facchetti F, Colonna M, Caligiuri MA. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101:3052–7. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 7.Johansson S, Berg L, Hall H, Hoglund P. NK cells: elusive players in autoimmunity. Trends Immunol. 2005;26:613–8. doi: 10.1016/j.it.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Lunemann A, Lunemann JD, Munz C. Regulatory NK-cell functions in inflammation and autoimmunity. Mol Med. 2009;15:352–8. doi: 10.2119/molmed.2009.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabuhara A, Yang FC, Nakazawa T, Iwasaki Y, Mori T, Koike K, Kawai H, Komiyama A. A killing defect of natural killer cells as an underlying immunologic abnormality in childhood systemic lupus erythematosus. J Rheumatol. 1996;23:171–7. [PubMed] [Google Scholar]
- 10.Erkeller-Yuksel FM, Lydyard PM, Isenberg DA. Lack of NK cells in lupus patients with renal involvement. Lupus. 1997;6:708–12. doi: 10.1177/096120339700600905. [DOI] [PubMed] [Google Scholar]
- 11.Erkeller-Yusel F, Hulstaart F, Hannet I, Isenberg D, Lydyard P. Lymphocyte subsets in a large cohort of patients with systemic lupus erythematosus. Lupus. 1993;2:227–31. doi: 10.1177/096120339300200404. [DOI] [PubMed] [Google Scholar]
- 12.Izumi Y, Ida H, Huang M, et al. Characterization of peripheral natural killer cells in primary Sjogren's syndrome: impaired NK cell activity and low NK cell number. J Lab Clin Med. 2006;147:242–9. doi: 10.1016/j.lab.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Aramaki T, Ida H, Izumi Y, et al. A significantly impaired natural killer cell activity due to a low activity on a per-cell basis in rheumatoid arthritis. Mod Rheumatol. 2009;19:245–52. doi: 10.1007/s10165-009-0160-6. [DOI] [PubMed] [Google Scholar]
- 14.Park YW, Kee SJ, Cho YN, et al. Impaired differentiation and cytotoxicity of natural killer cells in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1753–63. doi: 10.1002/art.24556. [DOI] [PubMed] [Google Scholar]
- 15.Schleinitz N, Chiche L, Guia S, et al. Pattern of DAP12 expression in leukocytes from both healthy and systemic lupus erythematosus patients. PLoS ONE. 2009;4:e6264. doi: 10.1371/journal.pone.0006264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schepis D, Gunnarsson I, Eloranta ML, Lampa J, Jacobson SH, Karre K, Berg L. Increased proportion of CD56bright natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126:140–6. doi: 10.1111/j.1365-2567.2008.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vossen MT, Biezeveld MH, de Jong MD, Gent MR, Baars PA, von Rosenstiel IA, van Lier RA, Kuijpers TW. Absence of circulating natural killer and primed CD8+ cells in life-threatening varicella. J Infect Dis. 2005;191:198–206. doi: 10.1086/426866. [DOI] [PubMed] [Google Scholar]
- 18.Bareau B, Rey J, Hamidou M, et al. Analysis of the french LGL leukemia cohort: report on 229 cases. Haematologica. 2010;95:1534–41. doi: 10.3324/haematol.2009.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamy T, Loughran TP., Jr Clinical features of large granular lymphocyte leukemia. Semin Hematol. 2003;40:185–95. doi: 10.1016/s0037-1963(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 20.Pascal V, Schleinitz N, Brunet C, et al. Comparative analysis of NK cell subset distribution in normal and lymphoproliferative disease of granular lymphocyte conditions. Eur J Immunol. 2004;34:2930–40. doi: 10.1002/eji.200425146. [DOI] [PubMed] [Google Scholar]
- 21.Gattazzo C, Miorin M, Scquizzato E, et al. Lack of expression of inhibitory KIR3DL1 receptor in patients with NK-type lymphoproliferative disease of granular lymphocytes. Haematologica. 2010 doi: 10.3324/haematol.2010.023358. Apr 21. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scquizzato E, Teramo A, Miorin M, et al. Genotypic evaluation of killer immunoglobulin-like receptors in NK-type lymphoproliferative disease of granular lymphocytes. Leukemia. 2007;21:1060–9. doi: 10.1038/sj.leu.2404634. [DOI] [PubMed] [Google Scholar]
- 23.Zambello R, Falco M, Della Chiesa M, et al. Expression and function of KIR and natural cytotoxicity receptors in NK-type lymphoproliferative diseases of granular lymphocytes. Blood. 2003;102:1797–805. doi: 10.1182/blood-2002-12-3898. [DOI] [PubMed] [Google Scholar]
- 24.Parham P. NK cells and trophoblasts: partners in pregnancy. J Exp Med. 2004;200:951–5. doi: 10.1084/jem.20041783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleinitz N, Cognet C, Guia S, et al. Expression of the CD85j (leukocyte Ig-like receptor 1, Ig-like transcript 2) receptor for class I major histocompatibility complex molecules in idiopathic inflammatory myopathies. Arthritis Rheum. 2008;58:3216–23. doi: 10.1002/art.23871. [DOI] [PubMed] [Google Scholar]
- 26.Dotta F, Censini S, van Halteren AG, et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci U S A. 2007;104:5115–20. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–81. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalbeth N, Callan MF. A subset of natural killer cells is greatly expanded within inflamed joints. Arthritis Rheum. 2002;46:1763–72. doi: 10.1002/art.10410. [DOI] [PubMed] [Google Scholar]
- 29.Trowsdale J, Barten R, Haude A, Stewart CA, Beck S, Wilson MJ. The genomic context of natural killer receptor extended gene families. Immunol Rev. 2001;181:20–38. doi: 10.1034/j.1600-065x.2001.1810102.x. [DOI] [PubMed] [Google Scholar]
- 30.Barrow AD, Trowsdale J. The extended human leukocyte receptor complex: diverse ways of modulating immune responses. Immunol Rev. 2008;224:98–123. doi: 10.1111/j.1600-065X.2008.00653.x. [DOI] [PubMed] [Google Scholar]
- 31.van der Slik AR, Alizadeh BZ, Koeleman BP, Roep BO, Giphart MJ. Modelling KIR-HLA genotype disparities in type 1 diabetes. Tissue Antigens. 2007;69(Suppl. 1):101–5. doi: 10.1111/j.1399-0039.2006.762_5.x. [DOI] [PubMed] [Google Scholar]
- 32.Martin MP, Nelson G, Lee JH, et al. Cutting edge: susceptibility to psoriatic arthritis: influence of activating killer Ig-like receptor genes in the absence of specific HLA-C alleles. J Immunol. 2002;169:2818–22. doi: 10.4049/jimmunol.169.6.2818. [DOI] [PubMed] [Google Scholar]
- 33.Nelson GW, Martin MP, Gladman D, Wade J, Trowsdale J, Carrington M. Cutting edge: heterozygote advantage in autoimmune disease: hierarchy of protection/susceptibility conferred by HLA and killer Ig-like receptor combinations in psoriatic arthritis. J Immunol. 2004;173:4273–6. doi: 10.4049/jimmunol.173.7.4273. [DOI] [PubMed] [Google Scholar]
- 34.Momot T, Koch S, Hunzelmann N, Krieg T, Ulbricht K, Schmidt RE, Witte T. Association of killer cell immunoglobulin-like receptors with scleroderma. Arthritis Rheum. 2004;50:1561–5. doi: 10.1002/art.20216. [DOI] [PubMed] [Google Scholar]
- 35.Pellett F, Siannis F, Vukin I, Lee P, Urowitz MB, Gladman DD. KIRs and autoimmune disease: studies in systemic lupus erythematosus and scleroderma. Tissue Antigens. 2007;69(Suppl. 1):106–8. doi: 10.1111/j.1399-0039.2006.762_6.x. [DOI] [PubMed] [Google Scholar]
- 36.Jonsen A, Gunnarsson I, Gullstrand B, et al. Association between SLE nephritis and polymorphic variants of the CRP and FcgammaRIIIa genes. Rheumatology (Oxford) 2007;46:1417–21. doi: 10.1093/rheumatology/kem167. [DOI] [PubMed] [Google Scholar]
- 37.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754–8. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 38.Beum PV, Lindorfer MA, Taylor RP. Within peripheral blood mononuclear cells, antibody-dependent cellular cytotoxicity of rituximab-opsonized Daudi cells is promoted by NK cells and inhibited by monocytes due to shaving. J Immunol. 2008;181:2916–24. doi: 10.4049/jimmunol.181.4.2916. [DOI] [PubMed] [Google Scholar]
- 39.Moins-Teisserenc HT, Gadola SD, Cella M, et al. Association of a syndrome resembling Wegener's granulomatosis with low surface expression of HLA class-I molecules. Lancet. 1999;354:1598–603. doi: 10.1016/s0140-6736(99)04206-3. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–23. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anfossi N, Andre P, Guia S, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 42.Hue S, Mention JJ, Monteiro RC, et al. A direct role for NKG2D/MICA interaction in villous atrophy during celiac disease. Immunity. 2004;21:367–77. doi: 10.1016/j.immuni.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Mention JJ, Ben Ahmed M, Begue B, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–45. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 44.Meresse B, Chen Z, Ciszewski C, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–66. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Krebs P, Barnes MJ, Lampe K, Whitley K, Bahjat KS, Beutler B, Janssen E, Hoebe K. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood. 2009;113:6593–602. doi: 10.1182/blood-2009-01-201467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Della Chiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing CD94/NKG2A, but lacking inhibitory killer Ig-like receptors. Eur J Immunol. 2003;33:1657–66. doi: 10.1002/eji.200323986. [DOI] [PubMed] [Google Scholar]
- 47.Zhang AL, Colmenero P, Purath U, et al. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007;110:2484–93. doi: 10.1182/blood-2007-02-076364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajenoff M, Breart B, Huang AY, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–31. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perona-Wright G, Mohrs K, Szaba FM, et al. Systemic but not local infections elicit immunosuppressive IL-10 production by natural killer cells. Cell Host Microbe. 2009;6:503–12. doi: 10.1016/j.chom.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Deniz G, Erten G, Kucuksezer UC, Kocacik D, Karagiannidis C, Aktas E, Akdis CA, Akdis M. Regulatory NK cells suppress antigen-specific T cell responses. J Immunol. 2008;180:850–7. doi: 10.4049/jimmunol.180.2.850. [DOI] [PubMed] [Google Scholar]
- 51.Eidenschenk C, Jouanguy E, Alcais A, et al. Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes. J Immunol. 2006;177:8835–43. doi: 10.4049/jimmunol.177.12.8835. [DOI] [PubMed] [Google Scholar]
- 52.Mazodier K, Marin V, Novick D, et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood. 2005;106:3483–9. doi: 10.1182/blood-2005-05-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nedvetzki S, Sowinski S, Eagle RA, et al. Reciprocal regulation of human natural killer cells and macrophages associated with distinct immune synapses. Blood. 2007;109:3776–85. doi: 10.1182/blood-2006-10-052977. [DOI] [PubMed] [Google Scholar]
- 54.Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358:929–39. doi: 10.1056/NEJMra071297. [DOI] [PubMed] [Google Scholar]
- 55.Schepis D, Gunnarsson I, Eloranta ML, Lampa J, Jacobson SH, Karre K, Berg L. Increased proportion of CD56(bright) natural killer cells in active and inactive systemic lupus erythematosus. Immunology. 2009;126:140–6. doi: 10.1111/j.1365-2567.2008.02887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bielekova B, Catalfamo M, Reichert-Scrivner S, et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci U S A. 2006;103:5941–6. doi: 10.1073/pnas.0601335103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Z, Lim WK, Mahesh SP, Liu B, Nussenblatt RB. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56(bright) regulatory NK cells in patients with active uveitis. J Immunol. 2005;174:5187–91. doi: 10.4049/jimmunol.174.9.5187. [DOI] [PubMed] [Google Scholar]


